
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 24 March 2023
Sec. Aquatic Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.989442
This article is part of the Research TopicAquatic Physiology, Environmental Pollution, Nanotoxicology and Phytoremediation, Volume IIIView all 5 articles
Schizothorax esocinus, commonly known as snow trout, is one of the main contributors of food and livelihood in the colder zone of Himalayan region. The comprehensive information on its hematological and serum biochemical reference intervals is not reported yet. In the present study an attempt has been made to elucidate the hematological and serum biochemical reference intervals of S. esocinus from River Jhelum using protocols of the American Society of Veterinary Clinical Pathology (ASVCP). Wild fish were sampled over a period of 2 years from the pollution free sites of river Jhelum. Fish blood was harvested through caudal venipuncture and hemato-biochemical analysis performed thereof. Data values from a total of healthy 432 adult fish specimens (216 male, 216 female) were systematically recorded. The reference intervals for hematological and serum biochemical parameters of S. esocinus were established using Reference Value Advisor software v 2.1. RIs for hematological and serum analytes ranged as: hemoglobin (Hb) 78.38–116.35 (g/L); white blood cells (WBC) 10–20 (×109/L); red blood cells (RBC) 1.30–2.15 (×1012/L); packed cell volume 27.00–39.45 (%); total protein 39.21–61.62 (g/L); albumin 8.20–22.02 (g/L); globulin 27.58–49.55 (g/L); glucose 3.25–7.18 (mmol/L); urea 0.96—2.38 (mmol/L); cholesterol 3.80–6.90 (mmol/L). The study also depicted that certain blood measurands were influenced with respect to sex. Significantly (p < 0.05) higher values of Hb, red blood cells count and serum glucose were noted in male as compared to female which, on the other hand, registered higher white blood cells count and serum cholesterol level (Mann Whitney U test, p < 0.05). The work, therefore, provides baseline information on hematological and serum biochemical analytes of this species which holds high commercial importance. RIs reported here can help monitor the health status of fish by improving the use of non-lethal diagnostic methods in piscine medicine.
Blood is a vital fluid consisting of a variety of cells in the plasma medium. Analysis of blood is a common tool to evaluate physiological status of an organism including fish. Recent research shows that hematological parameters are important indicators of fish health status and are used in innovative methods (Fazio, 2019; Fazio et al., 2019). Among all the diagnostic tools used to evaluate the health of organism including fish, hematological and serum biochemical assessments are recognized as easy and reliable methods for analyzing the health status of aquatic animals as these indices provide speedy and reliable information on metabolic disorders, diseases, deficiencies and chronic stress status (Ahmed et al., 2020; Zakes et al., 2016). A multitude of endogenous and exogenous factors such as age, sex, season, breeding cycle, biological behavior, environment, health status, habitat as well as external factors like water temperature, environmental quality, seasonal dynamics, food and stress influence the hematological parameters of fish while the quantitative determinations of various serum analytes facilitate in assessment of the functional status of the vital body organs like the kidney, liver, heart, pancreas, etc., and can predict the degree of organ damage as well (Ahmed et al., 2020; Ahmad et al., 2021a; Fawole et al., 2023; Khan and Khan, 2021; Reshi and Ahmed, 2022; Seibel et al., 2021). Although, blood is considered as an immediate reflector of health condition of fish but still hematological as well as serum data in piscine species are not optimally utilized due to the lack of reference ranges and appropriate interpretive skills (Nabi et al., 2022).
One of the intricacies in assessment of the state of health of natural fish populations has been the paucity of reliable reference ranges in the normal condition. In contrast to fish, where it is mainly understudied, this field has widely been expanded in veterinary medicine (Reshma et al., 2020). Development of database of normal hemato-biochemical ranges in fishes is of critical importance for assessment of health and successful running of aquaculture systems as well as maintaining the natural stocks of the fish (Reshi and Ahmed, 2022). Apart from hematological parameters, serum biochemical values act as reflectors of internal milieu of the fish and also help in detection and diagnosis of metabolic disturbances and diseases in fishes (Ferrari et al., 2007). Total protein, albumin and globulin levels in fish blood are used as basic indices of health status and condition (Ahmad et al., 2021a; Svoboda et al., 2001). These parameters along with other analytes are being used consistently in healthcare of humans and domestic animals as well.
Among all the aquatic ecosystems of Kashmir, river Jhelum, locally known as ‘Vyeth’ is the chief riverine system of the Himalayan region. It is one of the largest fisheries resources of the region and it supports a wide variety of fish fauna (Ahmad et al., 2021b; Reshi and Ahmed, 2020). It caters to considerable percentage of the fish demand of the human population. Of the various fishes found in the river, Cyprinids are predominant with main representatives from family Cyprinidae which include Schizothoracines. Among different Schizothorax species, Schizothorax esocinus (Heckel, 1838) is commonly called as snow trout and locally known as ‘Chirruh’. It is one of the endemic and economically important cold water food fishes of Indian Himalayan colder zone in general and Kashmir region in particular (Reshi and Ahmed, 2020). The fish species found in most of Asian countries like India, Pakistan, China, Afghanistan and Nepal (Mir et al., 2014). It fetches high commercial price due to its gratifying taste and high food value (Ahmed, 2018). The work on aspects of S. esocinus has been reported in the past but these are mainly focused on its length-weight relationship, fecundity, growth, genome organization, however, the normal hematological and serum biochemical reference values of S. esocinus are not reported from any part of the world yet. Despite having high commercial importance, the fish is available only in the wild and its culture in the farms is not developed up to the mark yet. Therefore, the current study was undertaken to define the presently lacking RIs for hematological and serum biochemical parameters of S. esocinus using ASVCP protocol (Friedrichs et al., 2012). The study draws significance in terms of the lack of hematological data of this particular fish species which needs to be established. This has relevance in improving the applications of clinical diagnosis for benefitting the currently lacking culture of Schizothorax spp. Due to anthropogenic activities, the population of Schizothorax species are declining day-by-day and in order to start proper conservation and management of these important food fish species, monitoring of health status of the species is imperative which warrant the conscious study for establishment of reference values of the species, which could be instrumental for the assessment of health of these species both in natural and culture condition.
For the establishment of hematological and serum biochemical reference values, live adult specimens of S. esocinus (Average size = 33.47 ± 2.41 cm; Average weight = 400.68 ± 90.54 g) were captured by using cast nets with the help of local fishermen from three sites, i.e., Kadlabal (34°00′39″N, 74°54′38.43″E), Chattbal (34°05′23.89″N, 74°47′06.87″E) and Shadipora (34°10′26.85″N, 74°41′05.02″E) of River Jhelum, during the study period April 2017- March 2019. Length of river Jhelum in Kashmir valley is 129 km. The map showing study sites is given in Figure 1. Distance between site one and site two was nearly 15 km and the distance between site two and site three was nearly 13 km. Site selection was based on thorough survey of the study area by considering the various ecological (physico-chemical characteristic features including occurrence of contaminants or pollutants), biological [(availability of fishes, availability of food for fishes, quality and density of plankton) and micro-biological] factors (species and quantity of parasites). Fish were examined for gross morphology and necropsy, those with signs of injury, lesions and abnormal behaviour were eliminated from the study. All the fish specimens were adult and of nearly similar sizes. Sample collection was done on monthly basis for 2 years. Data values from a total of healthy 432 adult fish specimens (216 male, 216 females) were recorded. Sampled fishes were immediately transferred in plastic containers containing water and transported to wet laboratory and stocked in plastic tanks having continuous flow through water system facility for overnight acclimation of fishes. The fish were maintained in natural 12:12 h light dark photoperiod at a density of three fish per 70 L tank. The sex of the fish was identified by macroscopic examination of gonads. Besides, the morphological features such as body shape, size, anal fin length, dorsal fin spines were also observed to determine sex (Rai et al., 2002; Sharma and Mehta, 2010).
Water samples from the three study sites were collected each month of the 2-year study and assessed for physico-chemical parameters. i.e., temperature was recorded using mercury thermometer on spot; pH was recorded using digital pH meter (pHep-HI 98107, United States of America) on spot. The pH meter was standardized using suitable buffer solutions; dissolved oxygen was estimated manually by Winklerʼs method, fixed on the spot and estimated in laboratory; free carbon dioxide was estimated manually by titrimetric method using sodium hydroxide as titrant and phenolphthalein as an indicator in the laboratory; total alkalinity was estimated manually by titrimetric method using sulphuric acid as titrant, phenolphthalein and methyl orange as indicators in the laboratory (APHA, 1998).
In the laboratory, the fish were maintained for 1 day under normal photoperiod (Zhao et al., 2018). Blood was drawn from the caudal vein of the live fish using a sterile syringe and needle after using anesthesia (MS-222 at 0.3 gL−1). Acquisition of blood samples was quickly followed by immediate hematological and serum biochemical analysis. 2–3 mL blood sample was taken in heparin (anticoagulant) coated vials (AcCuvet-PLUS; Lithium Heparin; 25 I.U mL-1) and immediately used for hematological analysis. 2 mL of the blood directly from the syringe was immediately (within 1 min) taken in Eppendorf tubes, centrifuged at 5000 g for 5 min in centrifuge (Tarsons, Spinwin, MC-02) to obtain serum for biochemical analysis. Hemoglobin (Hb) was estimated by mixing 20 µL of blood in 5 mL of Drabkins reagent and left to stand for 15 min, then concentration was measured spectrophotometrically at 540 nm (Thermoscientific- Genesys 10 S UV-VIS) (Drabkin, 1946). The white blood cells (WBCs) and red blood cells (RBCs) were counted manually using Natt and Herrick’s diluent (1:200) and a Neubauer-ruled hemocytometer (Marienfeld-Superior, Lauda-Konigshofen, Germany) (Khan and Maqbool, 2017). The microhematocrit technique was used to determine packed cell volume (PCV) values after the capillary tubes containing blood had been spun in centrifuge (REMI RM-12C India) at 15300 g for 5 min and expressed as percentage (Khan and Maqbool, 2017). Erythrocyte sedimentation rate (ESR) was recorded by following Wintrobe Tube method wherein the anticoagulated blood was filled in a Wintrobe tube upto zero mark on top and kept undisturbed in vertical position in a rack. This allows the sedimentation of erythrocytes. After 1 h level of fall of the column of sediment was noted as ESR and expressed in mm per hour (Wedemeyer et al., 1983). These data were used to calculate the following erythrocyte indices: mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and mean corpuscular volume (MCV) according to Dacie and Lewis (1991). The serum was immediately analyzed in Biochemistry Auto Analyzer (Robert Riele GmbH and Co. KG, Germany) using standard commercial kits -(Erba Diagnostic Kits, Transasia Bio-medicals Ltd., Solan; In technical collaboration with: ERBA diagnostics Mannheim GmbH, Germany) wherein the total protein estimation was based on Biuret method; Albumin estimation was based on BCG (bromocresol green) Dye Method; The concentration of globulins was calculated (total protein minus albumin concentrations); Serum glucose estimation was based on GOD-POD (glucose oxidase-peroxidase) method; serum urea estimation was based on glutamate-urease method; cholesterol concentration was based by CHOD-PAP (cholesterol oxidase phenol 4-aminoantipyrine peroxidase) method (Ratnakar, 2016). Analysis was carried by the authors after proper guidance from the experts/service providers.
Reference intervals for hematological and serum biochemical analytes were determined according to the published guidelines of American Society for Veterinary Clinical Pathology (ASVCP) which follow recommendations of Clinical Laboratory and Standards Institute (CLSI) (Friedrichs et al., 2012). Normality was assessed for each measurand by the Anderson-Darling test, where the p-value threshold limit of 0.05 was used. Mann Whitney U test was used to compare male versus female blood results, where the p-value threshold limit was 0.05. Data was analyzed statistically using Reference value Advisor software V 2.1 (Geffre et al., 2011). Data was screened for outliers using Tukeys/Dixon Reed outlier test by the software. After eliminating the outliers, the data was reanalyzed. Seasonal variations in hematological and serum biochemical analytes were assessed by one way ANOVA using SPSS software.
In laboratory, the average values of physico-chemical parameters were noted as temperature = 14.03°C ± 4.95 °C; pH = 8.11 ± 0.34; dissolved oxygen = 6.83 ± 1.17 mg/L; free carbon dioxide = 5.15 ± 2.42 mg/L; total alkalinity = 135.79 ± 26.64 mg/L. The results of physico-chemical parameters of water samples are summarized in Table 1. There was no significant variation in the parameters of river Jhelum and laboratory.
The values of mean, SD, coefficient of variation, minimum, maximum and reference intervals (90% Confidence Interval) for hematological and serum biochemical parameters of S. esocinus (combined sexes) are presented in Table 2. Among all the measured hematological variables, Hb and RBC were found to be significantly (p < 0.05) higher in male fish in comparison to female fish while WBC count was noted significantly (p < 0.05) higher in female fish as compared to the male fish (Table 3). In case of serum parameters, glucose was observed significantly (p < 0.05) higher in male fish and cholesterol values were significantly (p < 0.05) higher in female fish as presented in Table 3. Distribution curve of hematological and serum biochemical reference values of S. esocinus are given in Figures 2, 3. Overall, statistical analysis depicted significant (p < 0.05) differences in some blood analytes between the sexes. Our findings show that the values of Hb, RBC and glucose in male fish were significantly (p < 0.05) higher than that of female fish. Female fish showed higher values of WBC and serum cholesterol. Correlation matrix of blood analytes of S. esocinus and physico-chemical parameters of River Jhelum is given in Table 4, while seasonal variations in hematological parameters of male and female S. esocinus are presented in Table 5 and Table 6, respectively. Significant variations were observed in analytes with respect to seasons. Moreover, it was observed that all these values were found within the reference range.
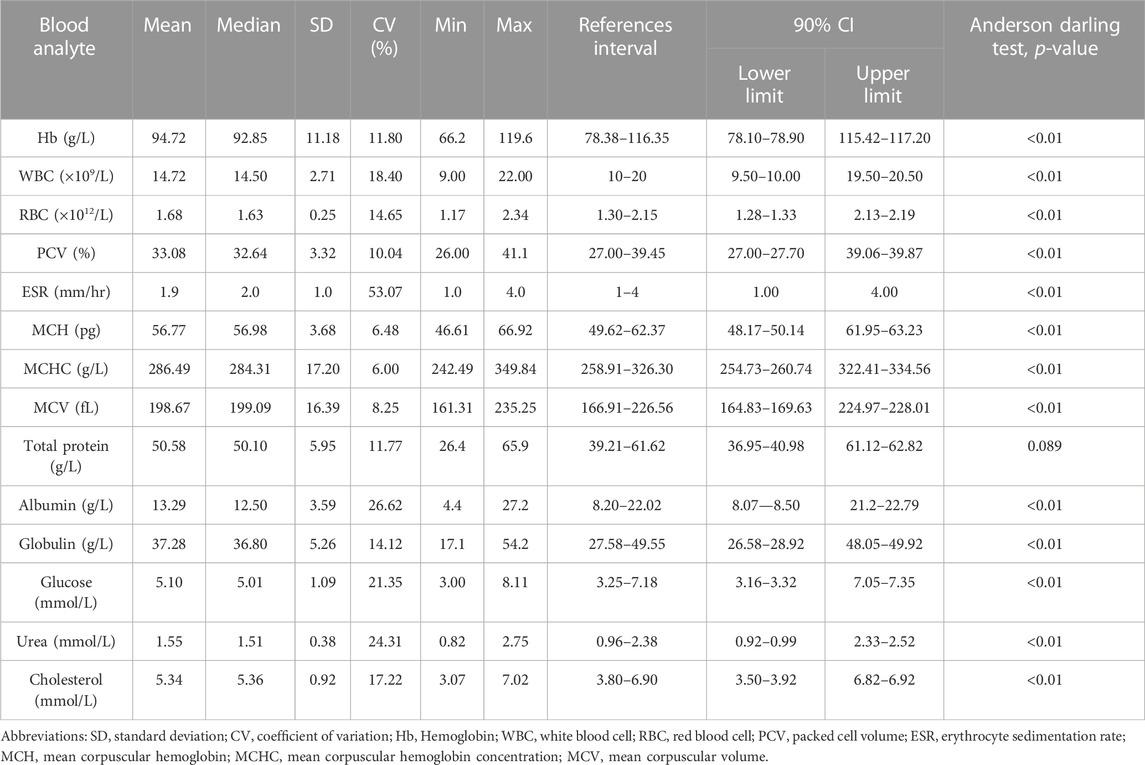
TABLE 2. Hematology and serum biochemical reference intervals for snow trout, Schizothorax esocinus from River Jhelum. Reference intervals were determined using reference value advisor software following non-parametric method for all measurands (p < 0.05) and robust method (p > 0.05) for total protein.

TABLE 3. Sex based hematological and serum biochemical reference intervals for Schizothorax esocinusa Reference intervals were determined using reference value advisor software following non-parametric method (p < 0.05) for all measurands and robust method (p > 0.05) for WBC of female and glucose of male.
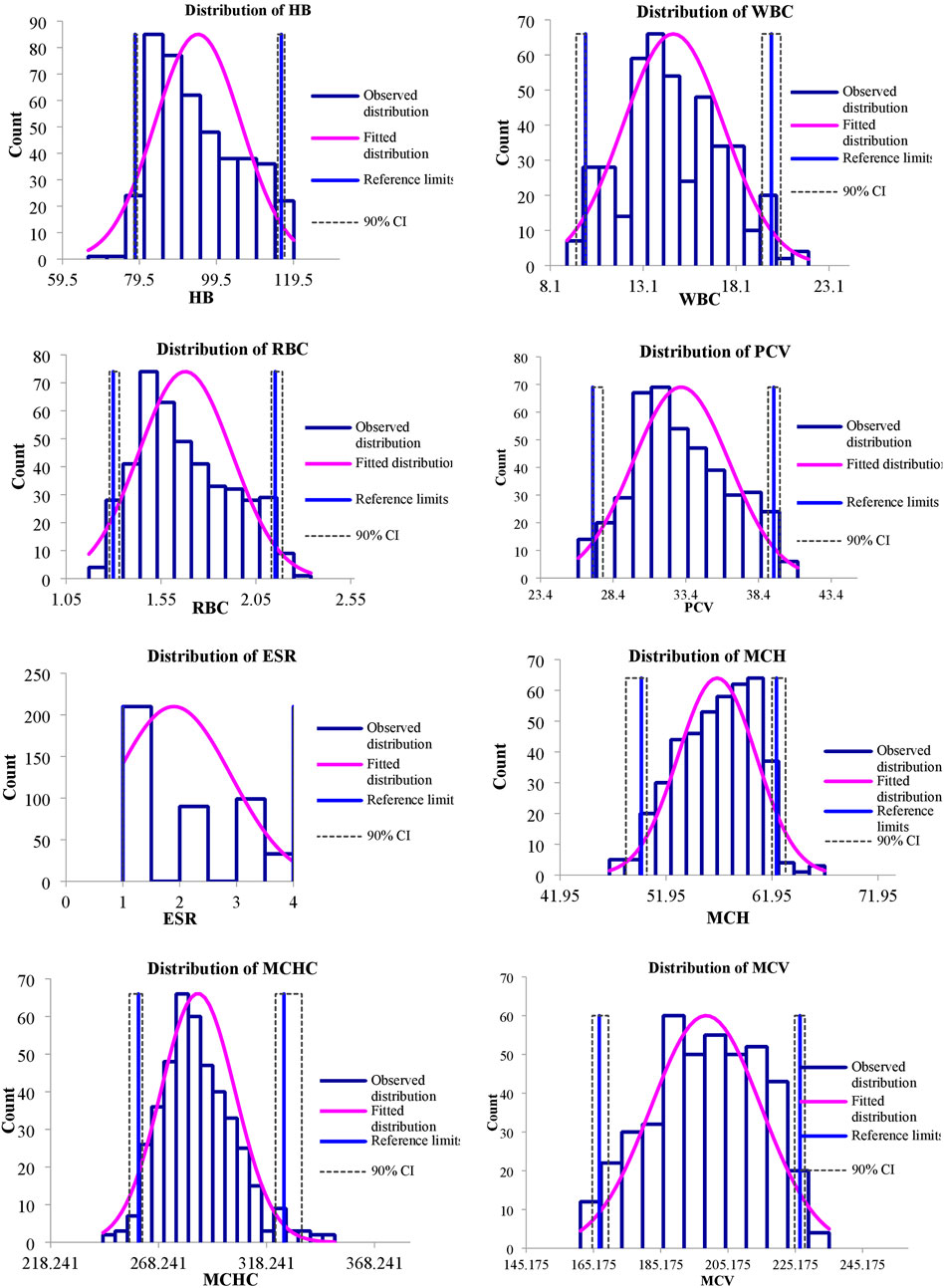
FIGURE 2. Representing distribution of hematological reference intervals of S. esocinus from River Jhelum.
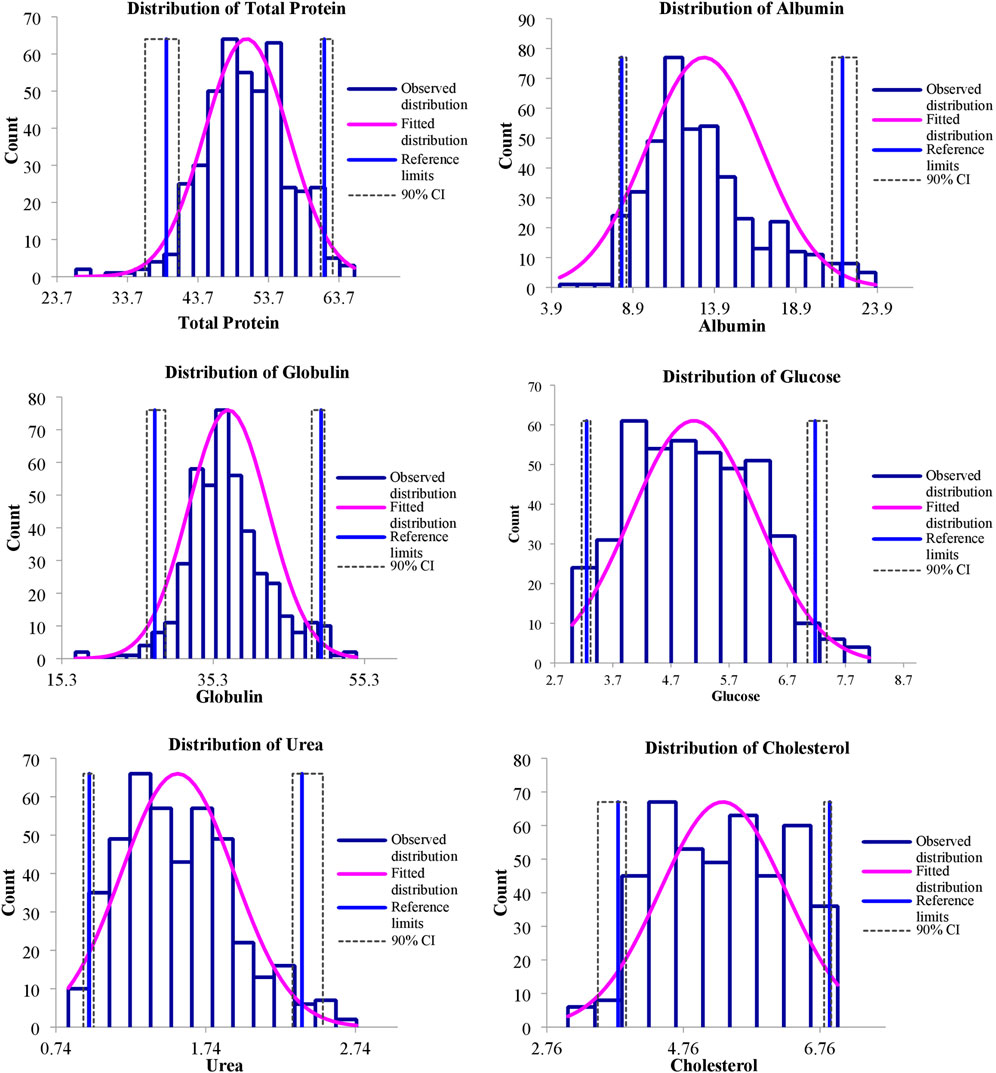
FIGURE 3. Representing distribution of serum biochemical reference intervals of S. esocinus from River Jhelum.
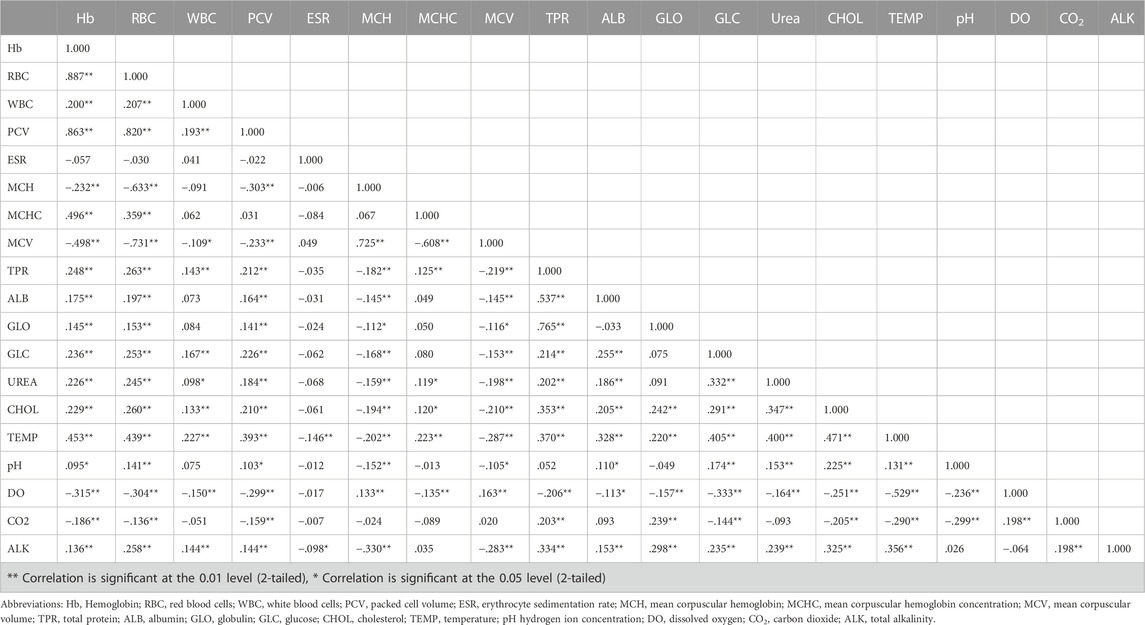
TABLE 4. Correlation matrix of blood analytes of S. esocinus and physico-chemical parameters from River Jhelum.
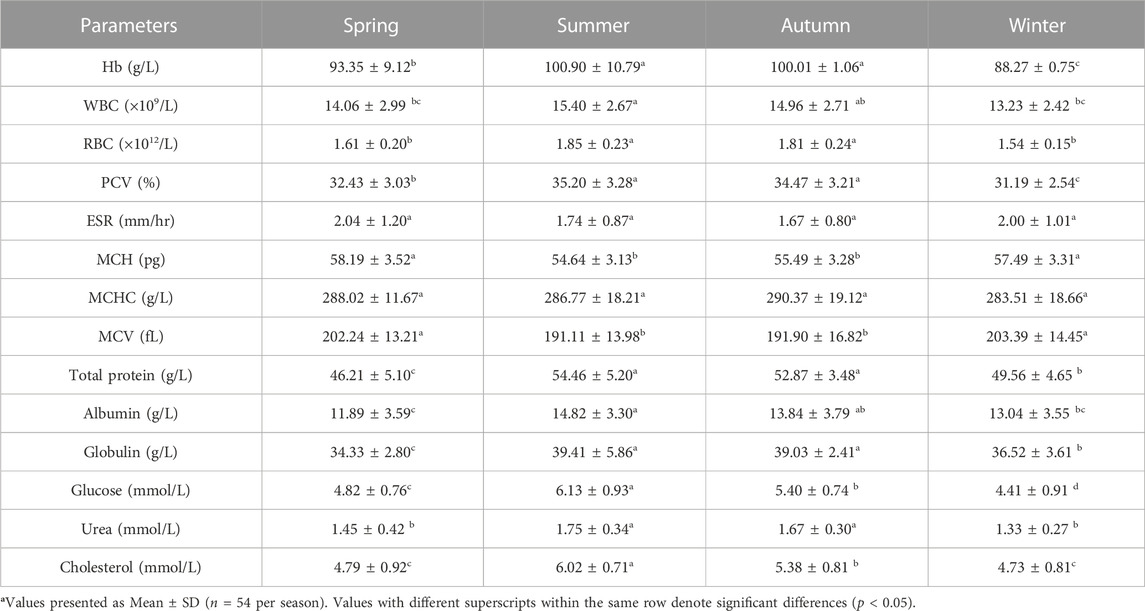
TABLE 5. Seasonal variations in hematological and serum biochemical parameters of male S. esocinus from River Jheluma.
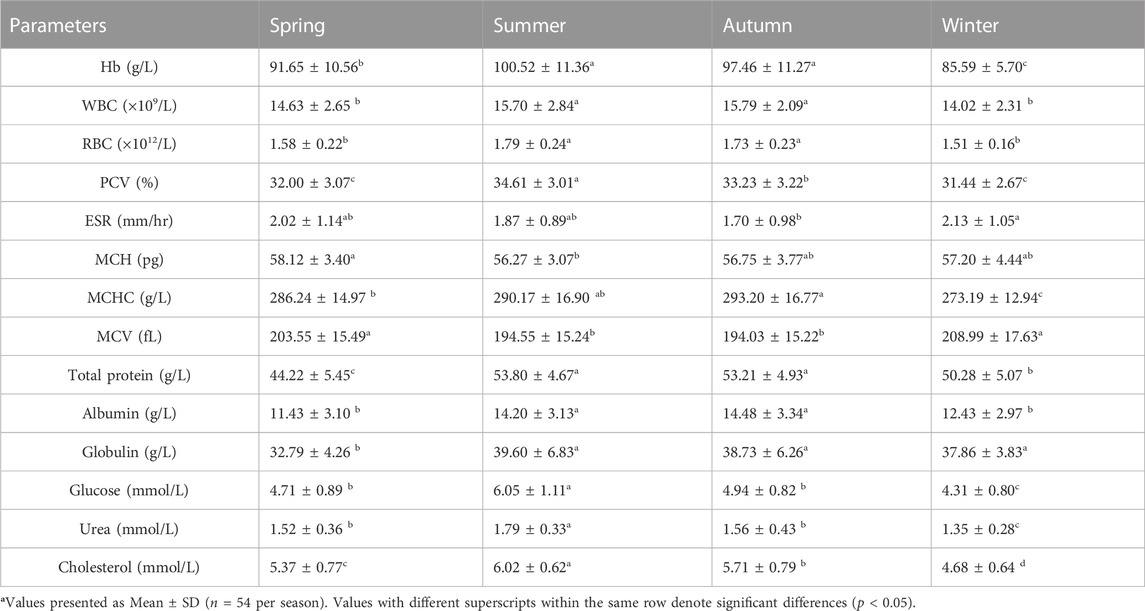
TABLE 6. Seasonal variations in hematological and serum biochemical parameters of female S. esocinus from River Jheluma.
Hematological studies reflect the functional status of the fish body (Sidiq and Ahmed, 2020). Among hematological constituents, Hb concentration in fish blood reflects its oxygen supply and the fish strives to uphold the values in as much as steady condition as possible. During the present work, Hb values were significantly higher in male than female fish, similar trend has been observed in Sander lucioperca (Zakes et al., 2016). The higher mean hematological values of Hb and RBC observed in male as compared to that of the female could be due to higher physiological activeness as well as metabolic activity in male than the female fish (Sharma et al., 2017). The higher values of hematological parameters in the male are attributed to the action of glycoprotein hormone known as erythropoietin which is known to stimulate erythrocyte synthesis that transport oxygen (Zakes et al., 2016). Since erythropoietin is known to amplify under the influence of the sex hormones, particularly testosterone, this could explain the reason of higher hematological values in male (Golemi et al., 2013). Higher values of Hb, RBC and PCV were noted during summer as compared to other seasons. Similar results have been reported in other freshwater fish species such as Catla and Schizothorax niger (Pradhan et al. 2012; Sheikh and Ahmed, 2019). The pattern of relatively higher values of RBC and PCV observed in S. esocinus during warmer seasons (summer and autumn) as compared to colder seasons was also observed in another fish, Tinca and the result was interpreted as an adjustment for increased oxygen intake by the fish body due to the depletion in dissolved oxygen concentration of water bodies during warm months, as rising temperature reduces solubility of oxygen in water bodies (Guijarro et al., 2003; Sharma et al. 2017). Higher levels of WBC in a fish make them proficient to fight infection more effectively than that of others, because of direct correlation between the WBC counts and the immune responses (Douglass and Jane, 2010; Ahmadifar et al., 2019). With respect to sex, female fish had higher WBC values as compared to male. Similar observation of higher WBC in female than the male was recorded in Heteroclarias hybrid (Okorie-Kanu and Unakalamba, 2015). Also, the large energy requirements for the growth and maturation of oocytes influence the condition of females and also cause lowered immunity (Kapila et al., 2000; Zakes et al., 2016). This gap could be compensated for by the elevated production of leucocytes in female than in male, as has been previously noted in Tor putitora (Kapila et al., 2000). The maximum WBC count was found in the summer and the lowest values were recorded during winter months of the year, similar observations has also been observed in Cirrhinus mrigala (Pradhan et al., 2014). While the Mann Whitney U test between male and female snow trout showed statistically significant (p < 0.05) differences for Hb, WBC, RBC, glucose and cholesterol, the differences in reference intervals are slight but may have some clinical relevance (Table 3). RBC is vital for the transport of oxygen in animals. PCV is useful to check anaemic condition in fishes and is therefore, a reliable guide in aquaculture systems as well as fishery management for assessing fish health from various aspects like nutrition, stress and diseases (Brill et al., 2008; Sharma and Chadha, 2021). The PCV values in fishes usually fall in the range of 20%–45% (Hrubec and Smith, 2000). The observed values of PCV in our study fall within this range suggesting fairly good health of the fishes. Similar values of PCV have also been reported in Heteroclarias hybrid (Okorie-Kanu and Unakalamba, 2015). ESR is a common hematological test used as a non-specific measure of inflammation and variations in blood plasma or stress condition may affect ESR level in the fish therefore, this factor can be used as an indicator for fish health (Mozanzadeh et al., 2015). The ESR values in fishes depicted no significant difference between the sexes or seasons. ESR values of current study were similar to those found in S. davidi (Zhu et al., 2017). On average, the MCH values in fishes range from 30 to 100 pg, MCV values varies between 150 and 350 fL and MCHC in fishes range from 18 to 30 g/dL (Hrubec and Smith, 2000). The observations of our study do fall within this range. Also, no significant differences were observed in any of the erythrocyte indices with respect to sex. Since the erythrocyte indices are dependent on Hb, RBC and PCV, so the values of erythrocyte indices also varied seasonally. The study of erythrocyte indices in fish is of paramount importance for diagnosing several patho-physiological abnormalities, however, their interpretation demands in-depth information about the multiple number of controlling factors such as age, gender, water quality, capture, handling, and use of anesthetics involved (Clauss et al., 2008).
In addition to hematological parameters, serum biochemical parameters are also used as biomarkers to assess fish health. The plasma or serum protein concentration levels also serve as clinical indicators of health, stress and nutritional condition in the fishes and are often used as a diagnostic tool in evaluating the general physiological status of fish (Zakes et al., 2016). The total protein values are affected by nutritional state, reproductive cycle, habitat and disease or toxins (Coskun et al., 2016). Albumin plays a role in transportation of lipid and general metabolism in fishes while the globulin levels are related to the innate immune response of fish (Andreeva, 1999; Domingo-Roura et al., 2001). The values of total protein, albumin and globulin levels are similar to the values recorded in C. gariepinus (Mozanzadeh et al., 2015). In our study, serum total protein, albumin and globulin levels did not exhibit significant variations (p < 0.05) with respect to sex. Similar trend was also noted in Heteroclarias hybrid (Okorie-Kanu and Unakalamba, 2015). Total protein, globulin and albumin values showed seasonal variations such that higher levels were observed in summer and autumn, while comparatively lower values were recorded in winter and spring seasons. Seasonal variations in these analytes were also noted in Barilius bendelisis and Schizothorax labiatus (Sharma et al. 2017; Jan and Ahmed, 2020). Glucose is considered as the principal source of energy for the cells of the body. It is transported from the intestines or liver to body cells through bloodstream. Glucose levels are regarded as one of the specific indicators of sympathetic activation during stress conditions and the homeostatic mechanism of the body keeps blood glucose levels within a range (Svoboda et al., 2001; Kulkarni and Bedjargi, 2016). The sex based comparison of serum glucose revealed significantly higher (p < 0.05) values in male as compared to female. The plausible explanation of high glucose level in male is due to high deposition of glucose in the cells and ovarian tissues of female that use considerably larger reserves of this monosacchride than do the tissues of the male testes (Zakes et al., 2016). Serum glucose values observed in our study are somewhat similar to the values recorded in a related species S. niger (Ruqaya et al., 2012). Seasonally, the glucose content of S. esocinus was found highest in summer and lowest values were recorded during winter season, similar trend on serum glucose content has also been reported in Oncorhynchus mykiss (Coskun et al,. 2016). Serum urea levels in the present study are similar to those reported in Clarias gariepinus (Mozanzadeh et al., 2015). Serum cholesterol is an important steroid metabolite in the cell membranes and transported in the blood plasma of all animals, needed for proper body functions and functions as precursors for the synthesis of sexual hormones (Ruqaya et al., 2012). It serves as the substrate for the synthesis of various important, biologically active compounds, such sex hormones and corticosteroids (Zakes et al., 2016). Moreover, sex based comparison showed that cholesterol levels in female are higher than the values in male. Similar trend was also reported in S. lucioperca (Zakes et al., 2016). The results achieved in the present study express the higher reproductive cost of female. In both sexes, the values of urea and cholesterol contents were highest in summer and lowest in winter season of the year. Our findings are conformity with the finding on C. mrigala by Pradhan et al. (2014). Among all the serum biochemical parameters significant differences were recorded in case of cholesterol and glucose values between male and female. The variations in hematological and plasma chemistry reference intervals with respect to sex has also been reported in Alburnus chalcoides (Moshfegh et al., 2018). Thus, the values observed in S. esocinus are consistent with the values found in other freshwater fishes. The current study has implications in terms of addressing the health management of this particular species especially in wild conditions, but its applications in aquaculture are limited due to its currently unexplored culture.
This work offers a baseline data of hemato-biochemical profiling of S. esocinus and how these values fluctuate seasonally. The information may improve the application of clinical chemistry in fish medicine such as health assessment, diagnosis of various subclinical as well as clinical diseases, reference point for future comparative studies, thus enabling better conservation and management strategies, which in turn could assist in rearing this species on commercial basis thereby paving way for better gains to fish farmers and enriching the economy as well.
The original contribution presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study and protocols have been approved by Animal Ethical Committee registered under R.No. 801/Go/RE/S/2003/CPCSEA.
QR: data collection, chemical and statistical analysis, manuscript writing; IA: study design, review and editing, and supervision; KA-A: drafting of manuscript and corrections; MF: drafting of manuscript and corrections. All authors contributed and approved the submitted version.
This work was supported by the Researchers Supporting Project number (RSP2023R154), King Saud University, Riyadh, Saudi Arabia.
The authors are grateful to the Head, Department of Zoology, University of Kashmir, Hazratbal, for providing necessary laboratory facilities. The authors also thank the fishing community of River Jhelum for their co-operation. The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2023R154), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, I., Ahmed, I., Fatma, S., and Peres, H. (2021a). Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquac. Nutr. 27, 1270–1289. doi:10.1111/anu.13267
Ahmad, I., Ahmed, I., Reshi, Q. M., Jan, K., Gupta, A., Dar, S. A., Molnar, K., Kaur, H., Malla, B. A., and Andrabi, S.M. (2021b). Morphological, histopathological and molecular characterization of Myxobolus szekelyianus n. sp.(Cnidaria: Myxosporea: Myxobolidae) causing acute gill disease in Schizothorax esocinus (Heckel, 1838) from River Jhelum of Kashmir Himalayan region, India. Aquac. Res. 52, 6537–6549. doi:10.1111/are.15524
Ahmadifar, E., Moghadam, M. S., Dawood, M. A., and Hoseinifar, S. H. (2019). Lactobacillus fermentum and/or ferulic acid improved the immune responses, antioxidative defence and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol 94, 916–923. doi:10.1016/j.fsi.2019.10.019
Ahmed, I. (2018). “Nutritional status of some commercially important food fish species of Kashmir Valley,” in Aquaculture for Nutritional and Livelihood Security. Editors A.S. Ninwae, J. R. Dhanze, and R. Dhanze (Narendra Publishing House Pvt. Ltd.), 124–134. ISBN: 978-93-86110-74-9.
Ahmed, I., Reshi, Q. M., and Fazio, F. (2020). The influence of the endogenous and exogenous factors on hematological parameters in different fish species: a review. Aquacult. Int. 28, 869–899. doi:10.1007/s10499-019-00501-3
Andreeva, A.M. (1999). Structural and Functional Organization of the Blood Albumin System in Fish. Vopr. Ikhtiol. 39, 825–832.
APHA (American Public Health Association) (1998). Standard methods for the examination of water and waste water. 20th edition. Washington, DC: American Public Health Association.
Brill, R., Bushnell, P., Schroff, S., Seifert, R., and Galvin, M. (2008). Effects of anaerobic exercise accompanying catch-and-release fishing on blood–oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J. Exp. Mar. Biol. Ecol. 354, 132–143. doi:10.1016/j.jembe.2007.10.011
Coskun, O. F., Aydin, D., and Duman, F. (2016). Comparison of some blood parameters of rainbow trout (Oncorhynchus mykiss) living in running and still water. Iran J. Fish. Sci. 15, 497–507.
Clauss, T. M., Dove, A. D., and Arnold, J. E. (2008). Hematologic disorders of fish. Vet Clin North Am Exot Anim Pract 11, 445. doi:10.1016/j.cvex.2008.03.007
Dacie, S., and Lewis, S. (1991). Practical haematology. 7th edn. London: Churchill Livingstone, 633.
Domingo-Roura, X., Newman, C., Calafell, F., and Macdonald, D. W. (2001). Blood biochemistry reflects seasonal nutritional and reproductive constraints in the Eurasian Badger (Meles meles). Physiol. Biochem. Zool. 74, 450–460. doi:10.1086/320417
Douglass, J. W., and Jane, K. W. (2010). Schalms Veterinary Hematology. John Wiley and Sons, Blackwell Publishing Ltd, 1232.
Drabkin, D. L. (1946). Spectrophotometric studies; the crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. J. Biol. Chem. 164, 703–23. doi:10.1016/S0021-9258(17)41272-5
Fawole, F. J., Shamna, N., Memudu, H. A., Abdullahi, N., Hassaan, M. S., and Gbadamosi, O. K. (2023). Housefly maggot meal complement soybean meal in a fish-free diet for hybrid catfish (Clarias gariepinus♀ x Heterobranchus longifilis♂): Effect on growth, body composition, blood biochemistry and antioxidant enzyme activity. Anim. Feed Sci. Technol. 295, 115543. doi:10.1016/j.anifeedsci.2022.115543
Fazio, F. (2019). Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 500, 237–242. doi:10.1016/j.aquaculture.2018.10.030
Fazio, F., Saoca, C., Costa, G., Zumbo, A., Piccione, G., and Parrino, V. (2019). Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): a new hematological approach. Aquaculture 513, 734398. doi:10.1016/j.aquaculture.2019.734398
Ferrari, A., Venturino, A., Pechen, A. M., and D’Angelo, (2007). Effects of carbaryl and azinphos methyl on juvenile rainbow trout (Oncorhynchus mykiss) detoxifying enzymes. Pestic. Biochem. Phys. 88, 134–142. doi:10.1016/j.pestbp.2006.10.005
Friedrichs, K. R., Harr, K. E., Freeman, K. P., Szladovits, B., Walton, R. M., Barnhart, K. F., and Blanco-Chavez, J. (2012). ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 41, 441–453. doi:10.1111/vcp.12006
Geffre, A., Concordet, D., Braun, J. P., and Trumel, C. (2011). Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol. 40, 107–112. doi:10.1111/j.1939-165X.2011.00287.x
Golemi, S., Medja, N., and Lacej, D. (2013). Influence of sex on the hematological and morphometric parameters of Cyprinus carpio (Linnaeus, 1758) from Shkodra Lake. Acad. J. Interdiscipl. S. 2, 45. doi:10.5901/ajis.2013.v2n8p45
Guijarro, A. I., Lopez-Patiño, M. A., Pinillos, M. L., Isorna, E., De Pedro, N., Alonso-Gómez, A. L, Alonso-Bedate, M., and Delgado, M. J. (2003). Seasonal changes in haematology and metabolic resources in the tench. J. Fish Biol. 62, 803–815. doi:10.1046/j.1095-8649.2003.00066.x
Heckel, J. J. (1838). Fische aus Caschmir gesammelt und herausgegeben von Carl Freiherrn von Hugel, beschrieben von JJ Heckel, 1–112. Wien.
Hrubec, T. C., and Smith, S. A. (2000). “Hematology of fish,” in Schalm’s Veterinary Hematology, 5th edn. Editors B. F. Feldman, J. G. Zinkl, and N. C. Jain (Philadelphia): Lippincott Williams and Wilkins), 1120–1125.
Jan, K., and Ahmed, I. (2020). The influence of sex and season on some haematological and biochemical parameters of snow trout Schizothorax labiatus in the Indian Himalayan region. Fish. Sci. 87, 1–16. doi:10.1007/s12562-020-01469-3
Kapila, R., Kapila, S., and Basade, Y. (2000). Sex related haematological variations in Himalayan golden mahseer, Tor putitora (Ham.). Indian J. Fish. 47, 81–85.
Khan, I. A., and Maqbool, A. (2017). Effects of dietary protein levels on the growth, feed utilization and haemato-biochemical parameters of freshwater fish, Cyprinus carpio Var. Specularis. Fish. Aqua. J. 8, 1–3. doi:10.4172/2150-3508.1000187
Khan, Y. M., and Khan, M. A. (2021). Optimization of dietary pyridoxine improved growth performance, hematological indices, antioxidant capacity, intestinal enzyme activity, non-specific immune response, and liver pyridoxine concentration of fingerling major carp Catla catla (Hamilton). Aquaculture 541, 736815. doi:10.1016/j.aquaculture.2021.736815
Kulkarni, R., and Bedjargi, P. (2016). Serum Biochemical Parameters of Four Fresh Water Indian Carps from a Local Aquatic Body. Int. J. Innov. Stud. Aquat. Biol. Fish. 2, 15–20.
Mir, J. I., Mir, F. A., and Patiyal, R. S. (2014). Phenotypic Variations among three populations of Chirruh Snowtrout, Schizothorax esocinus (Heckel, 1838) in Kashmir Himalaya with Insights from truss network system. Proc. Natl. Acad. Sci. 84, 105–111. doi:10.1007/s40011-013-0194-6
Mir, R. A., Jeelani, G., and Dar, F. A. (2016). Spatio-temporal patterns and factors controlling the hydrogeochemistry of the river Jhelum basin, Kashmir Himalaya. Environ. Monit. Assess. 188, 438–24. doi:10.1007/s10661-016-5429-6
Moshfegh, A., Setorki, M., Bahrpeyma, V., Tehranifard, A., and Rahimibashar, M. (2018). Hematology and plasma chemistry reference intervals for wild population of Alburnus chalcoides (Guldenstadt, 1772): influence of sex, habitat and seasonal variation. Int. J. Aquat. Biol. 6, 162–169. doi:10.22034/ijab.v6i3.377
Mozanzadeh, M. T., Yaghoubi, M., Yavari, V., Agh, N., Marammazi, J. G., and Popovic, N. T. (2015). Reference intervals for haematological and plasma biochemical parameters in sobaity sea bream juveniles (Sparidentex hasta, Valenciennes 1830). Comp. Clin. Path. 24, 1501–1507. doi:10.1007/s00580-015-2107-y
Nabi, N., Ahmed, I., and Wani, G. B. (2022). Hematological and serum biochemical reference intervals of rainbow trout, Oncorhynchus mykiss cultured in Himalayan aquaculture: Morphology, morphometrics and quantification of peripheral blood cells. Saudi J. Biol. Sci. 29, 2942–2957. doi:10.1016/j.sjbs.2022.01.019
Okorie-Kanu, C. O., and Unakalamba, N. J. (2015). Normal haematological and blood biochemistry values of cultured Heteroclarias hybrid in South East Nigeria. Comp. Clin. Path. 24, 1015–1020. doi:10.1007/s00580-014-2021-8
Pradhan, S. C., Patra, A. K., Mohanty, K. C., and Pal, A. (2014). Hematological and plasma biochemistry in Cirrhinus mrigala (Hamilton 1822). Comp. Clin. Path. 23, 509–518. doi:10.1007/s00580-012-1642-z
Pradhan, S. C., Patra, A. K., Sarkar, B., and Pal, A. (2012). Seasonal changes in hematological parameters of Catla catla (Hamilton 1822). Comp. Clin. Path. 21, 1473–1481. doi:10.1007/s00580-011-1316-2
Rai, A. K., Pradhan, B. R., Basnet, S. R., and Sawr, D. B. (2002). Present status of snow trout in Nepal. FAO Fisheries Technical Paper, 213–220.
Ratnakar, S. (2016). Haematological and biochemical changes in Cirrhinus mrigala (Nain) in relation to season, sex and reproductive status. Doctoral dissertation. Pantnagar, Uttarakhand: GB Pant University of Agriculture and Technology.
Reshi, Q. M., and Ahmed, I. (2020). Seasonal variation in length-weight relationship, condition factor and biological indices of snow trout, Schizothorax esocinus (Heckel, 1838) inhabiting River Jhelum of Kashmir Himalaya. J. Ecophysiol. Occup. Health. 20, 232–238. doi:10.18311/jeoh/2020/25930
Reshi, Q. M., and Ahmed, I. (2022). Seasonal variations in hematological and serum biochemical analytes of snow trout, Schizothorax esocinus inhabiting Dal Lake. Comp. Clin. Pathol. 31, 303–311. doi:10.1007/s00580-022-03333-5
Reshma, K. J., Sumithra, T. G., Vishnu, B., Jyothi, R., Ratheesh Kumar, R., Pootholathil, S., and Sanil, N. K. (2020). Indexing serum biochemical attributes of Lutjanus argentimaculatus (Forsskal, 1775) to instrument in health assessment. Aquac. Res. 51, 2590–2602. doi:10.1111/are.14601
Ruqaya, Y., Mir, S. H., Syed, T., Chishti, M. Z., Darzi, M. M., and Mir, M. S. (2012). Comparative biochemical evaluation of Schizothorax niger and Cyprinus carpio from River Jhelum of Kashmir Valley. Res. J. Pharm. Biol. Chem. Sci. 3, 116–131.
Seibel, H., Baßmann, B., and Rebl, A. (2021). Blood will tell: What hematological analyses can reveal about fish welfare. Front. Vet. Sci. 8. doi:10.3389/fvets.2021.616955
Sharma, P., and Chadha, P. (2021). Bisphenol A induced toxicity in blood cells of freshwater fish Channa punctatus after acute exposure. Saudi J. Biol. Sci. 28, 4738–4750.
Sharma, I., and Mehta, H. S. (2010). Studies on Snow Trout Schizothorax richardsonii (Gray) in River Beas and its Tributaries (Himachal Pradesh). Zool Surv India 323, 1–69.
Sharma, N. K., Akhtar, M. S., Pandey, N. N., Singh, R., and Singh, A. K. (2017). Sex specific seasonal variation in hematological and serum biochemical indices of Barilius bendelisis from Central Himalaya, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 87, 1185–1197. doi:10.1007/s40011-015-0692-9
Sheikh, Z. A., and Ahmed, I. (2019). Impact of environmental changes on plasma biochemistry and hematological parameters of Himalayan snow trout, Schizothorax plagiostomus. Comp. Clin. Path. 28, 793–804. doi:10.1007/s00580-019-02914-1
Sidiq, M., and Ahmed, I. (2020). Comparative study of hematological profile of three forage fish species habiting in Dal Lake of Kashmir Himalaya, India. Comp. Clin. Pathol. 29, 913–920. doi:10.1007/s00580-020-03143-7
Svoboda, M., Kouril, J., Hamackova, J., Kalab, P., Savina, L., Svobodova, Z., and Vykusova, B. (2001). Biochemical profile of blood plasma of tench (Tinca tinca L.) during pre-and postspawning period. Acta Vet. Brno. 70, 259–68. doi:10.2754/avb200170030259
Wedemeyer, G. A., Gould, R. W., and Yasutake, W. T. (1983). Some potentials and limits of the leucocrit test as a fish health assessment method. J. Fish Biol. 23, 711–716. doi:10.1111/j.1095-8649.1983.tb02948.x
Zakes, Z., Demska-Zakes, K., Szczepkowski, M., Rozynski, M., and Ziomek, E. (2016). Impact of sex and diet on hematological and blood plasma biochemical profiles and liver histology of pikeperch (Sander lucioperca (L.)). Fish. Aquat. Life. 24, 61–68. doi:10.1515/aopf-2016-0007
Zhao, H., Panase, P., Zhang, Z., Yao, P., Zhang, Y., and Suwannapoom, C. (2018). Hematological and plasma biochemical values for Rhinogobio ventralis in the Yangtze River, China. Comp. Clin. Path. 27, 741–745. doi:10.1007/s00580-018-2660-2
Keywords: fish, reference intervals, hematology, serum biochemistry, Schizothorax
Citation: Reshi QM, Ahmed I, Al-Anazi KM and Farah MA (2023) Indexing hematological and serum biochemical reference intervals of Himalayan snow trout, Schizothorax esocinus to instrument in health assessment. Front. Physiol. 14:989442. doi: 10.3389/fphys.2023.989442
Received: 08 July 2022; Accepted: 13 March 2023;
Published: 24 March 2023.
Edited by:
Vincenzo Parrino, University of Messina, ItalyReviewed by:
Firas Rashad Al-Samarai, University of Baghdad, IraqCopyright © 2023 Reshi, Ahmed, Al-Anazi and Farah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imtiaz Ahmed, aW10aWF6YW11MUB5YWhvby5jb20=
†ORCID: Quseen Mushtaq Reshi, orcid.org/0000-0001-9832-9928; Imtiaz Ahmed, orcid.org/0000-0003-4821-1707
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.