- Department of Biological Sciences, St. Edward’s University, Austin, TX, United States
Collapsin response mediator protein-2 (CRMP2) in humans, UNC-33 in C. elegans, is a molecule that mediates axonal outgrowth and stability. UNC-33/CRMP2 has been hypothesized as a potential drug target for treating Alzheimer’s and other neurodegenerative diseases, which can often be attributed in part to aging. In aging, CRMP2 becomes hyperphosphorylated, which decreases the protein’s functionality, destabilizes the cellular skeleton, and contributes to neurodegeneration. In C. elegans, aging can be slowed by entering dauer diapause; a non-aging developmental stage turned on when the DAF-7/TGFβ signaling pathway is silenced in response to environmental stressors. In our laboratory, we discovered that unc-33 mutants are unable to form dauers in response to environmental stressors, but the mechanism behind this is still unknown. Here, we present a study that investigates whether a mutation in the daf-7 gene which leads to a temperature sensitive constitutive dauer phenotype can rescue phenotypes characteristic of unc-33 mutants. To this end, we created unc-33; daf-7 double mutants and quantified proper dauer formation after exposure to unfavorable environmental conditions. In addition, we tested how the introduction of the daf-7 mutation would affect the locomotion of the double mutants on an agar plate and a liquid medium. Furthermore, we examined axonal elongation of the double mutants using a transgene, juIs76, which expresses GFP in GABAergic motor neurons. Our analysis of unc-33; daf-7 double mutants showed that introducing the daf-7 mutation into an unc-33 mutant rescued dauer formation. However, further studies revealed that the unc-33; daf-7 double mutants had defects in axonal outgrowth of their D-type motor neuron which had been previously seen in unc-33 single mutants and impaired locomotion. Based on these results, we concluded that unc-33 mutants might have a problem suppressing DAF-7 signaling under unfavorable environmental conditions, leading to the activation of reproductive programs and the development of adults instead of dauers.
1 Introduction
The unc-33 gene in Caenorhabditis elegans (C. elegans) is the ortholog of the human collapsin response mediator protein 2 or CRMP2/DPYSL2. This gene encodes for three different isoforms through alternative splicing, which are UNC-33S, UNC-33M, and UNC-33L (Tsuboi et al., 2005). In vertebrates, CRMP2 is vital for the polarity of neurons, growth of axons, strength of the synapse, neuronal migration and differentiation, release of neurotransmitters, and survival of neurons (Na et al., 2017). Hyperphosphorylation and inactivation of CRMP2 has been detected in many neurological disorders, including Alzheimer’s and Parkinson’s disease, ALS, and chronic pain, and could thus be a promising drug target for the treatment of neurological diseases (Williamson et al., 2011; Moutal et al., 2019; Togashi et al., 2019). As for C. elegans, UNC-33 is fundamental for the growth and guidance of the axons in sensory neurons, interneurons, and motor neurons. Research conducted by Maniar and colleagues has shown that UNC-33 has a primary role in supporting the asymmetry of the two different compartments in neurons, axons, and dendrites (Mainar et al., 2011). UNC-33 is also needed for normal body movements, laying eggs, and defecation. Characterization of various unc-33 mutations resulted in identifying null or hypomorph mutants. The null mutant, allele mn407, possesses a 500 base pair deletion resulting in an early stop codon and no unc-33 gene products, but is viable and fertile (Li et al., 1992; Tsuboi et al., 2005). The hypomorph mutant, allele e204, has a single nucleotide change that produces the substitution of a conserved Asp amino acid to Asn (D 389 N) (Li et al., 1992; Tsuboi et al., 2005). Studies have found that unc-33(e204) mutants mislocalize UNC-33 proteins to the cell body of neurons instead of the ventral nerve cord and nerve ring where the proteins normally accumulate (Tsuboi et al., 2005). As a result, unc-33 mutants have numerous axonal outgrowth defects, suggesting that the localization of UNC-33 is vital for the outgrowth of axons (Li et al., 1992; Tsuboi et al., 2005). Furthermore, both unc-33(e204) and unc-33(mn407) mutants are short and dumpy, exhibit uncoordinated locomotion, and have defects in axonal guidance of sensory and motor neurons (Brenner et al., 1974; Hedgecock et al., 1985; Li et al., 1992).
More recently, studies reported that unc-33 mutants are incapable of forming dauers (Lopez et al., 2022). The formation of dauers is an alternative developmental non-aging stage in the C. elegans life cycle that is induced by harsh conditions sensed by larvae at the L1 life stage. The persistence of these environmental conditions leads to the formation of an L2d followed by the entry into dauer diapause (Karp, 2018). C. elegans entry into the dauer diapause is mainly regulated by two different pathways: the insulin/DAF-2 signaling cascade and the TGFβ/DAF-7 signaling pathway (Fielenbach and Antebi, 2008). Upon inactivating of the DAF-2 or DAF-7 pathways, mTOR is repressed, autophagy is induced, and L1, L2 respond by becoming dauers (Fielenbach and Antebi, 2008). DAF-7 is the C. elegans homolog of transforming growth factor beta (TGFβ). The TGFβ signaling pathway enables processes such as development, regulation of the immune system, and differentiation of adult stem cells in humans (Wang and Levy, 2006). In the case of C. elegans, the DAF-7 signaling pathway regulates reproductive development, and when disabled, it leads to dauer diapause. The expression of daf-7 is enriched in ASI ciliated neurons, and the secretion of DAF-7 is regulated by environmental signals (Wang and Levy, 2006). In favorable environmental conditions, DAF-7 is secreted from ASI neurons. This secreted factor binds to DAF-1/4 serine-threonine kinase receptors, which then phosphorylate DAF-8 and DAF-14, translocating them to the nucleus, inhibiting DAF-3 and DAF-5, leading to the development of adults. In unfavorable environmental conditions, DAF-7 is not secreted, allowing for the formation of dauers (Fielenbach and Antebi, 2008). Moreover, mutations in the genes that regulate dauer formation can either result in a defective dauer formation phenotype or constitutive dauer formation phenotype (Murakami et al., 2001). As such, the daf-7(e1372) point mutation results in a temperature sensitive phenotype producing 100% dauers at 25°C (Ren et al., 1996).
To better understand the inability of unc-33 mutants to enter dauer diapause in response to environmental stress, we created two double mutants, unc-33 (mn407); daf-7 (e1372) and unc-33(e204); daf-7(e1372), to examine their phenotypes. First, we assessed whether the introduction of the daf-7 mutation restores dauer formation by isolating dauers via a treatment with 1% SDS. Next, we characterized the locomotion and axonal outgrowth of these double mutants. Collectively, our results show that the introduction of the daf-7(e1372) mutation successfully restores the production of dauers in unc-33 mutants exposed to unfavorable environmental conditions. Furthermore, we found that adding daf-7(e1372) mutation to an unc-33 mutant background does not rescue the uncoordinated movements in unc-33 mutants, nor does it rescue the axonal elongation defects.
2 Materials and methods
2.1 Strains
Strains Wild-type N2, CB204 unc-33(e204) IV, CB1372 daf-7(e1372) III, SP1382 unc-33(mn407) IV, CZ13799 juIs76 [unc-25p::GFP + lin-15(+)] II, and DR466 him-5 (e1490) V were provided by Caenorhabditis Genetics Center (CGC). Strains AMH113 daf-7(e1372) III; unc-33(mn407) IV, AMH115 daf-7(e1372) III; unc-33(e204) IV, AMH32 juIs76 II; unc-33(mn407) IV, AMH34 juIs76 II; unc-33(e204) IV, AMH145 juIs76 II; daf-7(e1372) III; unc-33(mn407) IV, AMH151 juIs76 II; daf-7(e1372) III, AMH155 juIs76 II; daf-7(e1372) III; unc-33(e204) IV were generated in our laboratory.
All strains were grown and maintained on standard NGM agar plates at 20°C on an Escherichia coli OP50 lawn (Brenner, 1974). Strains containing daf-7(e1372) mutation were subject to incubation of 25°C to induce dauer formation. According to Ren and colleagues, daf-7(e1372) produce 100% dauers at 25°C and 5% at 20°C (Ren et al., 1996).
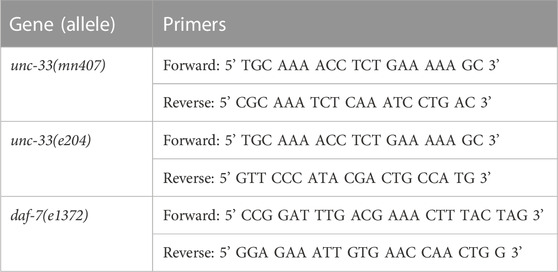
2.2 Genotyping
10–20 worms per strain were put into PCR tubes containing 10 µL of worm lysis buffer (10 mM Tris pH 8.2, 2.5 mM MgCl2, 50 mM KCl, 0.45% NP40, 0.45% Tween 20) with 100 ug/ml of proteinase K. Tubes were then placed at −80°C for 20 min, and worm lysis was finalized by incubating tubes for 90 min at 62°C and 15 min at 95°C. Worm lysates were then combined with the appropriate primers (see table 1), PCR master mix at a final concentration of 1X (Apex Bioresearch), and placed in a thermocycler for amplification. Fragments of the unc-33 gene were amplified using the following parameters: 5 min at 94°C, then 25 cycles of 30 s at 94°C, 30 s at 47°C, 1 min at 72 °C, 7 min at 72°C, and stored at 4°C. Fragments of the daf-7 gene were amplified using the following parameters: 5 min at 94°C, then 25 cycles of 30 s at 94°C, 30 s at 49°C, 1 min at 72 °C, 7 min at 72°C, stored at 4°C. PCR amplicons were then evaluated using 1% agarose gel electrophoresis. After evaluation, PCR amplicons were cleaned using the QIAGEN QIAquick PCR Kit and following manufacturer recommendations. Cleaned PCR fragments were sent to be sequenced.
2.3 1% sodium dodecyl sulfate (SDS) treatment
Twenty gravid hermaphrodites were used to synchronize nematodes from strains containing the daf-7(e1372) mutation. The hermaphrodites were placed on NGM agar plates containing an OP50 lawn and incubated at 25°C for 6 hours. After 6 hours, gravid hermaphrodites were removed, and the synchronous eggs were incubated at 25°C for 4 days. After 4 days, nematodes were resuspended with 1 mL DI water and transferred into 1.5 mL tubes. Worms were centrifuged for 1 min at 9,221 x g and pellet worms were treated with 1 mL of 1% SDS for 20 min at room temperature on an orbital shaker. After the 20-min incubation, worms were centrifuged for 1 min at 9,221 x g and the supernatant was removed. Pelleted worms were washed 3 times using 1 mL of M9 buffer and centrifuged for 1 min at 9,221 x g at 20°C after each wash. After the last wash, 100 uL of pellet worms were transferred onto an NGM plate with no bacteria and the percent survival was quantified (Lopez et al., 2022). Strains that did not contain the daf-7(e1372) mutation were grown on NGM agar plates containing a spot of OP50 at 25°C until all OP50 was consumed. Nematodes were then starved for 7 days at 25°C. After starvation, nematodes were exposed to 1% SDS treatment as described, and survival was calculated.
2.4 Locomotion on agar
In order to measure locomotion, an animal in the L3 or dauer stage was placed onto an NGM agar plate containing an OP50 lawn and was allowed to move for 10 min at room temperature (Zhang and Koch, 2017). The distance traveled was imaged using a Leica Stereo Microscope, and the LASX software was used to measure the distance traveled by the animal. The same protocol was followed for assessing locomotion on agar for adults that developed after exiting the dauer stage. These adults were obtained after incubating dauers at 20°C for a minimum of 2 days. Locomotion was measured as distance traveled after 10 min. The locomotion of a total of 60 hermaphrodites per strain was analyzed. dedevlop.
2.5 Locomotion on liquid
The motility in liquid of L3 nematodes, dauers, as well as adults that developed after exiting the L3 or the dauer stage was quantified after following these steps. First, 1 mL of deionized water was placed in each well on a 24-well plate at room temperature. Next, individual nematodes were picked from the plate and suspended in one of the wells. After a 10–15 s acclimation period, the body movements within 30 s were recorded. One isolated body movement of head, torso, or tail was considered one thrash. A total of 60 hermaphrodites per strain was quantified.
2.6 Axonal outgrowth
In order to assess axonal outgrowth, nematodes of the L3 stage or dauer stage were placed on an agar pad containing 5 μL of 200 mM Levamisole to immobilize them. These nematodes were then imaged using an Olympus fluoview confocal microscope at a total magnification of 600X, and z-stacks of the dorsal nerve cord were captured. Each animal’s whole dorsal nerve cord was recorded using two z-stack images, one containing the head to the midsection of the worm and the other focusing on the midsection to the tail. Once the z-stacks were captured, the images were then turned into a projection using the Olympus CellSens software, and the projections were then stitched together using a Multiple Image Alignment. Axonal outgrowth in the dorsal nerve cord was analyzed by measuring the number and length of the gaps within the dorsal nerve cord using an ROI polyline in CellSens.
2.7 Statistical analysis
Statistical analysis of data collected that followed a normal distribution was performed using a one-way ANOVA with Tukey’s multiple comparison post hoc test after testing for homogeneity of variance using the Brown-Forsythe Test. Data that did not pass the Shapiro-Wilk test for normal distribution were analyzed using Kruskal–Wallis’s test with Dunn’s multiple comparison test. All statistical analyses and graphs were generated with Prism, Grand GraphPad.
3 Results
3.1 A mutation in daf-7/TGFβ rescues dauer formation defects in unc-33 mutants
Previous studies have shown that unc-33 mutants fail to form dauers under starvation conditions (Lopez et al., 2022); however, the mechanism behind this inability is still unknown. To test the hypothesis that the inability of unc-33 mutants to enter dauer diapause stems from an issue within the DAF-7/TGFβ pathway, strains containing the unc-33 mutation (allele mn407 or e204) and the temperature-sensitive mutation daf-7(e1372) were generated. Once these double mutant strains were confirmed via genotyping, strains grown in dauer-inducing conditions were exposed to 1% SDS treatment, and their survival was quantified. Strains with the daf-7 mutation were synchronized and grown at 25°C, then exposed to 1% SDS treatment. The results confirmed that unc-33 mutants die after the SDS treatment suggesting that they do not form dauers (Figure 1). Wildtype nematodes exposed to starvation for 7 days at 25°C produced 58% ± 4 dauers while daf-7(e1372) incubated at 25°C for 4 days formed 93% ± 8 dauers. Additionally, these results revealed that the double mutant strains unc-33(mn407); daf-7(e1372) and unc-33(e204); daf-7(e1372) form dauers as they are resistant to 1% SDS (Figure 1). One-way ANOVA with Tukey’s multiple comparisons shows that the percent survival of double mutants unc-33(mn407); daf-7(e1372) and unc-33(e204); daf-7(e1372) to 1% SDS is not significantly different from wildtype nematodes (p = 0.331 and p = 0.346, respectively). However, the percent survival of double mutants unc-33(mn407); daf-7(e1372) and unc-33(e204); daf-7(e1372) is different from daf-7(e1372) (p < 0.0001). Together, this data suggests that unc-33 mutants could have a defect that prevents them from inhibiting the synthesis or secretion of DAF-7 in response to environmental stressors, thus failing to form dauers under these conditions.
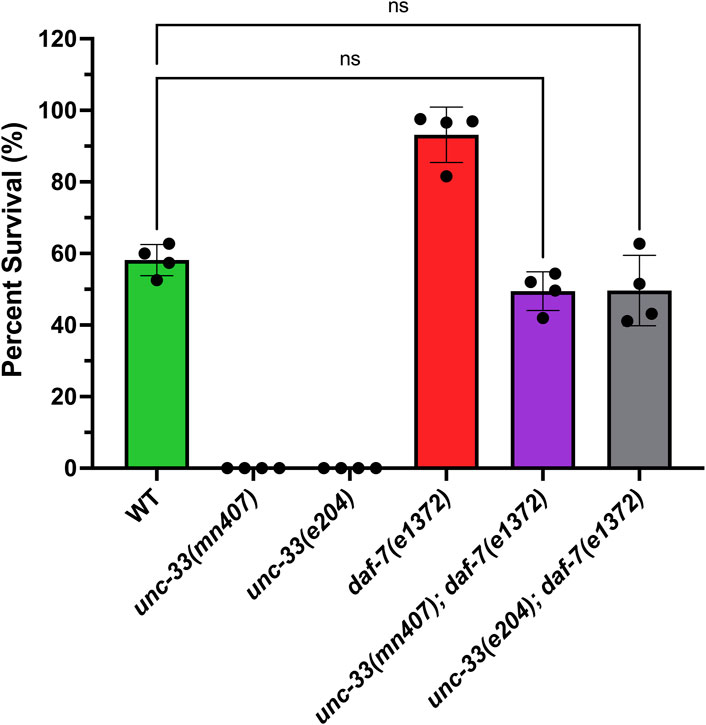
FIGURE 1. Addition of the daf-7 mutation into unc-33 mutants restores dauer formation. Percent survival of all strains after stimulation of dauer formation followed by 1% SDS treatment was quantified. unc-33(mn407) and unc-33(e204) had no detectable survival to 1% SDS confirming that unc-33 mutants are incapable of forming dauers. Analysis of one-way ANOVA with Tukey’s multiple comparisons test shows that the percent survival of double mutants unc-33; daf-7 was not statistically different from wildtype. However, unc-33(mn407) and unc-33(e204) were statistically different from wildtype (p < 0.0001). Plotted is the mean percent survival +/- standard deviation of 4 independent replicas per strain. ns = not significant.
3.2 Dauers unc-33; daf-7 double mutants have uncoordinated movements
Besides defects in dauer formation, unc-33 mutants have severely uncoordinated movements (Tsuboi et al., 2005). To study whether the introduction of the daf-7(e1372) mutation into unc-33 mutants rescues uncoordinated locomotion, the movement of all strains was analyzed on two media: NGM plates seeded with E. coli OP50 and wells with liquid media. Strains with the daf-7 mutation were synchronized as dauers, while the other strains without the daf-7 mutation were synchronized to be at the L3 life stage. Analysis of nematodes’ locomotion on NGM plates showed that the severely uncoordinated locomotion characteristics of unc-33 mutants was not rescued by introducing the daf-7(e1372) mutation (Figure 2A). Statistical evaluations of distance traveled after 10 min revealed no significant difference (p > 0.9999) between the single mutants unc-33(mn407), unc-33(e204), and double mutants unc-33(mn407); daf-7(e1372), and unc-33(e204); daf-7(e1372). In contrast, the distance traveled by wildtype nematodes was significantly more than double mutants unc-33(mn407); daf-7(e1372) (p < 0.0001), and unc-33(e204); daf-7(e1372) (p < 0.0172).
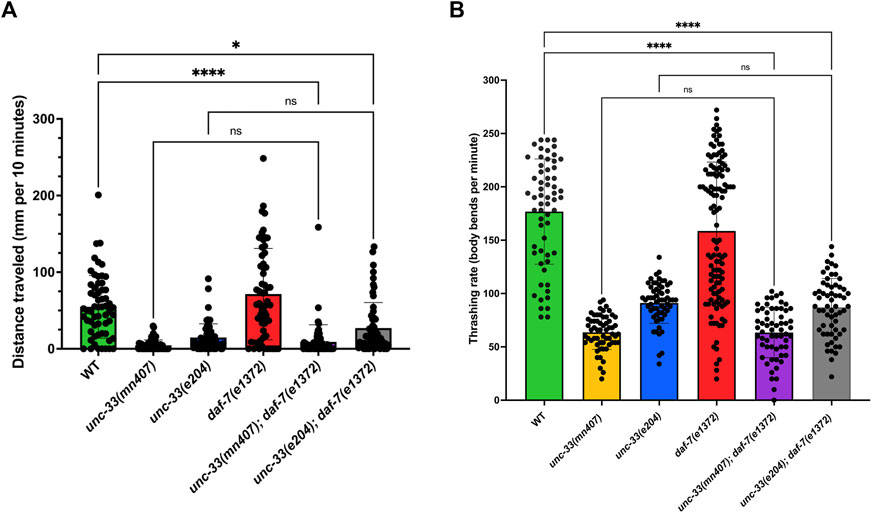
FIGURE 2. Double mutant unc-33; daf-7 dauers have similar rates of locomotion to unc-33 single mutant nematodes (A) The average distance traveled in millimeters per 10 minutes for each strain on a plate seeded with E. coli OP50 is plotted. (B) Average thrashing rate (body bends per minute) of each strain placed in liquid media. Statistical analyses using Kruskal–Wallis’s tests followed by Dunn’s multiple comparisons show no significant differences (ns) between double mutants unc-33; daf-7 and their unc-33 single mutant counterpart. However, unc-33(mn407) and unc-33(e204) were statistically different from wildtype (p < 0.0001). n = 60 animals per strain.
In addition, locomotion in liquid was assessed in dauers or the L3 life stage of nematodes. Strains were given a 10-s or 15-s acclimation period followed by a 30-s recording of thrashes. The thrashing rate was then multiplied by two to provide a total recorded time of 1 min. In liquid medium, the uncoordinated locomotion of unc-33 mutants was not rescued either. Double mutants unc-33(mn407); daf-7(e1372) and unc-33(e204); daf-7(e1372) dauers showed jerky locomotion and diminished frequencies of body bends which were indistinguishable from those quantified for unc-33 single mutants (Figure 2B). In the case of daf-7(e1372) dauers, we noticed that after leaving the animals in liquid media for several minutes, they displayed a different behavior. First, dauers were lethargic in liquid media and then they became active by producing significantly more body bends per minute after the initial 30-s recording of thrashes. This phenomenon of two different behaviors in liquid was not observed in wildtype, unc-33 mutants, or any of the unc-33; daf-7 double mutants.
3.3 The daf-7(e1372) mutation does not restore axonal outgrowth defects in unc-33 mutants
Research has also shown that unc-33 mutants have defects in axonal outgrowth which result in the uncoordinated movements in unc-33 mutants (Tsuboi et al., 2005). To assess if the introduction of the daf-7 mutation to an unc-33 mutant has the ability to restore axonal elongation, the dorsal nerve cord for each strain was imaged in dauers or the L3 life stage using a transgene that expresses GFP in D-type motor neurons (juIs76) (Figure 3A). Multiple images for each individual animal were captured for total visualization of the dorsal nerve cord and then the images were merged together using the Multiple Image Alignment algorithm in cellSens (Olympus). The average number of gaps and the average length of each gap were recorded for each strain. Statistical analysis of the number of gaps showed no significant difference between the unc-33 single mutants and the unc-33; daf-7 double mutants (p > 0.9999, Figure 3B). Additionally, there was no significant difference in the average length of gap for the unc-33 single mutants and the unc-33; daf-7 double mutants further indicating that the introduction of daf-7 mutation to an unc-33 mutant background does not restore axonal elongation in dauer unc-33; daf-7 double mutants (Figure 3C). The lack of restoration of axonal outgrowth in unc-33; daf-7 double mutants aligns with the previous findings denoting that locomotion is still impaired in unc-33; daf-7 double mutants (Figure 2).

FIGURE 3. The introduction of daf-7 mutation into an unc-33 mutant background does not rescue axonal outgrowth defects seen in unc-33 mutants (A) Images taken of the dorsal (top) and ventral (bottom) nerve cords of all the strains as dauers or the L3 larval stage. White Arrows indicate a gap within the dorsal nerve cord. (B) Average gaps within the dorsal nerve cord per animal for each strain. (C) Average length of the gaps per animal for each strain. Statistical analysis was performed using the Kruskal–Wallis’s test and the Dunn’s multiple comparisons test. However, unc-33(mn407) and unc-33(e204) were statistically different from wildtype (p < 0.001). n = 20 animals per strain. ns = not significant. Scale bar: 50 µm.
3.4 Adult unc-33; daf-7 double mutants have defects in locomotion
After analyses revealed that unc-33; daf-7 double mutants dauers still have defects in locomotion, another study was conducted to determine if adult double mutants recovering from the dauer stage would have the same abnormalities. We evaluated the locomotion of adults that developed after exiting the dauer stage for plate locomotion and locomotion in liquid. Strains with the daf-7 mutation were selected by synchronizing them at 25°C to promote dauer formation and then shifted to 20°C to induce them to leave dauer diapause to develop into adults. Strains without the daf-7 mutation were chosen as they entered adulthood. The strains were then placed either on an NGM agar plate or in a liquid medium.
Strains that were placed on an NGM agar plate were given a total of 10 minutes to travel on the plate. Images were then taken and quantified using LASX software. The analysis revealed that unc-33; daf-7 double mutants traveled the same distance as their unc-33 single mutant counterparts (Figure 4A). For liquid locomotion, results revealed that unc-33; daf-7 double mutants had partial rescue as denoted by the increase of thrashing rates when compared to the unc-33 single mutants (p < 0.022 for the unc-33(mn407) allele and p < 0.0036 for unc-33(e204), Figure 4B). However, the locomotion of unc-33; daf-7 double mutants showed a significantly lower thrashing rate in comparison to wildtype animals (p < 0.0001, Figure 4B) which implies the double mutants are severely uncoordinated in locomotion. Together, these results further suggest that the introduction of daf-7 mutation to an unc-33 background does not fully restore locomotion defects seen in unc-33 single mutants as it does for the case of dauer formation defects. However, it is important to note that adult nematodes that recovered from dauer are older than the unc-33 single mutants that never entered dauer diapause.
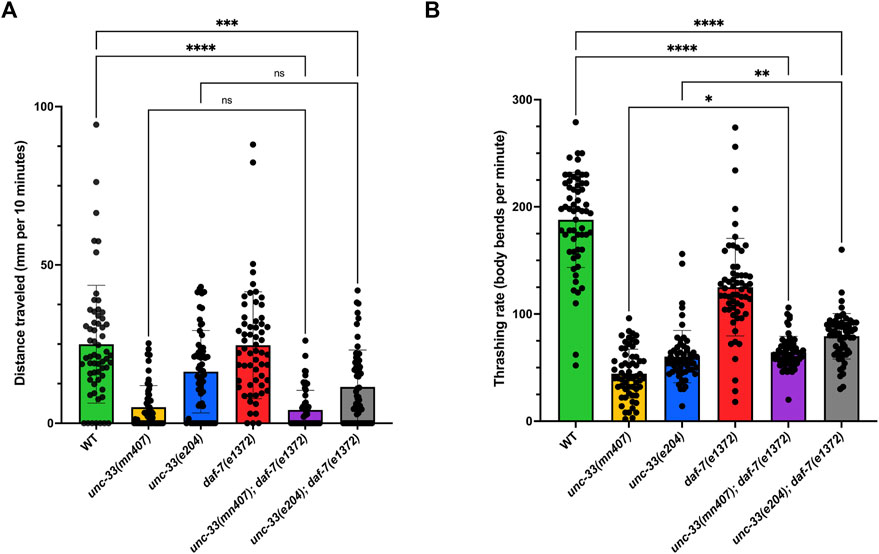
FIGURE 4. unc-33; daf-7 mutant adults that were previously dauers show that locomotion defect persists (A) Average distance traveled in millimeters per 10 minutes for each strain on a plate seeded with OP50 E. coli. (B) Average thrashing rate (body bends per minute) for each strain in liquid media. Statistical analyses using Kruskal–Wallis’s tests followed by Dunn’s multiple comparisons show no significant differences (ns) between double mutants unc-33; daf-7 and their unc-33 single mutant counterpart. However, unc-33(mn407) and unc-33(e204) were statistically different from wildtype (p < 0.001). n = 60 animals per strain.
4 Discussion
In C. elegans, TGFβ/DAF-7 helps regulate growth and development and allows entry into dauer arrest (Fielenbach and Antebi, 2008; Karp, 2018). Dauer diapause is a developmental stage entered under unfavorable environmental conditions such as overcrowding, lack of resources, and high temperatures, thus enabling C. elegans to survive up to several months without feeding (Fielenbach and Antebi, 2008). C. elegans interpret environmental cues through chemosensory neurons which initiate neuro-endocrine signaling to influence development and behaviors (Pandey et al., 2021). Previous research has found that unc-33 mutants are incapable of making dauers (Lopez et al., 2022), however, the mechanism behind this inability to enter dauer diapause is still unknown. Our research shows that dauer formation defects in unc-33 mutants can be rescued by the introduction of the daf-7(e1372) mutation indicating that the inability of unc-33 mutants to enter dauer diapause could be related to a defect within the DAF-7 pathway in unc-33 mutants. Studies have shown that UNC-33 is essential for neuronal polarity, microtubule assembly, and formation of axons (Tsuboi et al., 2005). It has been documented that loss of unc-33 alters microtubule polarity in axon and dendrites, suggesting that UNC-33 regulates microtubule orientation in neuronal processes (Harterink et al., 2018). UNC-33/CRMP2 binds to tubulin heterodimers to promote microtubule assembly and stability (Kodama et al., 2004; He et al., 2020). Research has shown that unc-33(mn407) mutants prematurely terminate the axonal process of ASI neurons which would normally extend to the nerve ring (Tsuboi et al., 2005). Additionally, studies of unc-33(mn407) mutants found a mislocalization of the sensory chemoreceptor protein ODR-10 from the cilium to axons (Maniar et al., 2012). The neuro-endocrine signal DAF-7 is predominantly expressed in ASI neurons, chemosensory neurons that detect environmental cues and regulate the progression through the life cycle of the nematode (Wang and Levy, 2006). The improper axonal formation of the ASI neurons and the mislocalization of ODR-10 in unc-33 mutants could explain the defects in the DAF-7 signaling pathway and the inability to form dauers in unfavorable conditions. We postulate that under unfavorable environmental conditions wildtype nematodes inhibit the production and release of DAF-7, activating programs that lead to dauer formation (Figure 5A) (Fielenbach and Antebi, 2008). In contrast, we hypothesize that unc-33 mutants fail to form dauers under unfavorable environmental conditions because as a cytoskeletal protein it may indirectly interferes with the inhibition of DAF-7 release or may contain defects in channels that regulate membrane potentials in ASI neurons or may affect neurons acting upstream of the ASI neurons (Figure 5B). Therefore, the introduction of the daf-7(e1372) mutation into an unc-33 mutant background restores dauer formation by conditionally inhibiting DAF-7 and impeding the activation of its downstream pathway (Figure 5C).
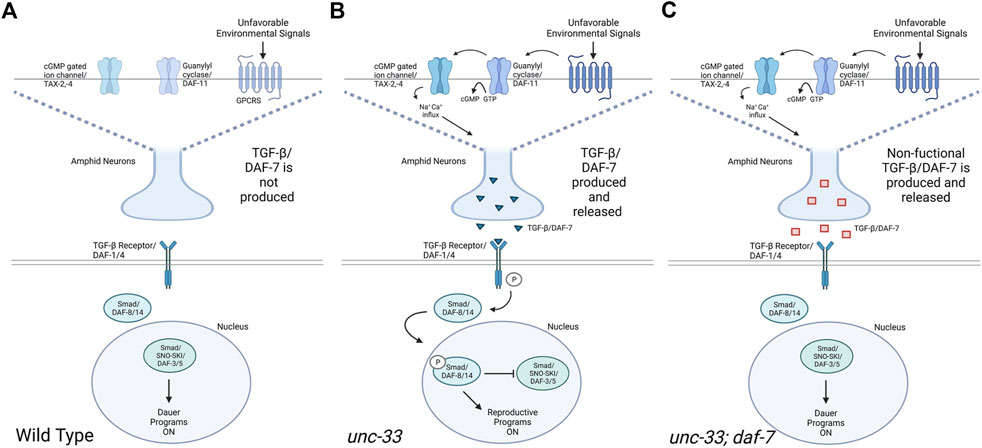
FIGURE 5. TGFβ/DAF-7-dependent regulation of dauer formation under unfavorable environmental conditions. Schematic models depicting proposed molecular pathways regulating dauer formation in wildtype, unc-33 mutants, and unc-33; daf-7 double mutants exposed to unfavorable environmental conditions. (A) When unfavorable environmental signals are detected by wildtype animals, GPCRs are not activated, the production TGFβ/DAF-7 is inhibited, and Smad/DAF-8/14 is unable to translocate into the nucleus. In the absence of nuclear DAF-8/14, Smad/SNO-SKI/DAF-3/5 inhibits the biosynthesis of hormones and promotes dauer formation. (B) When unfavorable environmental signals are detected by unc-33 mutants, GPCRs remain active inducing the synthesis of cGMP. High levels of cGMP open cGMP gated ion channels TAX-2,-4 depolarizing amphid neurons which release TGFβ/DAF-7. The ligand TGFβ/DAF-7 binds to the TGFβ receptor which phosphorylates Smad/DAF-8/14. Phosphorylated Smad/DAF-8/14 translocates into the nucleus and inhibits Smad/SNO-SKI/DAF-3/5 which promotes reproductive programs to remain active. (C) Similarly, to unc-33 mutants, when unc-33; daf-7 double mutants are exposed to unfavorable environmental signals, GPCRs remain active leading to the release of the mutant TGFβ/DAF-7. However, the mutant TGFβ/DAF-7 does not bind to the TGFβ receptor, Smad/DAF-8/14 is not phosphorylated rendering Smad/SNO-SKI/DAF-3/5 active, and dauer formation programs ON. Diagram created with BioRender.com.
Besides its inability to regulate dauer diapause, unc-33 mutants have \numerous defects in axonal outgrowth of sensory and motor neurons as well as uncoordinated movements (Li et al., 1992; Tsuboi et al., 2005). In this study, our findings revealed that the introduction of the daf-7(e1372) mutation into unc-33 mutants does not restore the uncoordinated locomotion in dauer and adult animals. Further analysis of the dorsal nerve cord of unc-33; daf-7 double mutants demonstrated that axonal outgrowth defects persisted. These findings suggest that knocking out the DAF-7 pathway affects dauer formation but does not impact the restoration of axonal outgrowth or other neuron-specific developmental features impacted by the mutation in the unc-33 locus. These results also suggest that DAF-7 does not play a significant role in the regulation of axonal outgrowth in C. elegans motor neurons. In contrast, expression and axonal localization of UNC-33 was sufficient for restoring coordinated movements and rescuing axonal outgrowth defects in an unc-33(mn407) mutant (Tsuboi et al., 2005). Therefore, we conclude that UNC-33 is necessary for proper axonal outgrowth and motility and these defects found in unc-33 mutants cannot be resolved through the introduction of daf-7 mutation. However, while the mutation in the unc-33 locus may lead to neurodevelopmental defects that disrupt chemosensation and dauer regulation, the addition of the daf-7(e1372) mutation to an unc-33 animal is sufficient to conditionally restore dauer formation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://drive.google.com/drive/folders/1GYokdZ2WRH5PKRBbG19aHgjC_3VGUSt9?usp=share_link.
Author contributions
AS: Generation of Strains, Performed Experiments, Data Analysis, Writing- initial draft. AC: Performed Experiments, Data Analysis, Writing- initial draft. AR: Generation of Strains, Performed Experiment- 1% SDS Treatment, Data Analysis, Writing- initial draft. RV: Performed Experiments, Data Analysis, Writing- initial draft. SB: Performed Experiments, Data Analysis, Writing- initial draft. JC: Performed Experiments- Locomotion Assays. MM: Performed Experiments- Locomotion Assays, Data Analysis. AV: Performed Experiments- Locomotion Assays. OCM: Performed Experiments, Data Analysis. AH: Designed and Supervised Experiments, Data analysis, Writing, Secured Funding.
Funding
This work was supported by The National Science Foundation award #1748523 and the Department of Biological Sciences at St. Edward’s University.
Acknowledgments
Some strains were provided by the CGC, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). NSF provided support for AH to contribute to this project through her Independent Research and Development program. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77 (1), 71–94. doi:10.1093/genetics/77.1.71
Fielenbach N., Antebi A. (2008). C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22 (16), 2149–2165. doi:10.1101/gad.1701508
Harterink M., Edwards S. L., De Haan B., Yau K. W., Van Den Heuvel S., Kapitein L. C., et al. (2018). Local microtubule organization promotes cargo transport in C. elegans dendrites. J. Cell. Sci. 131 (20), jcs223107. doi:10.1242/jcs.223107
He L., Kooistra R., Das R., Oudejans E., van Leen E., Ziegler J., et al. (2020). Cortical anchoring of the microtubule cytoskeleton is essential for neuron polarity. Elife 9, e55111. doi:10.7554/eLife.55111
Hedgecock E. M., Culotti J. G., Thomson J. N., Perkins L. A. (1985). Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111 (1), 158–170. doi:10.1016/0012-1606(85)90443-9
Karp X. (2018). “Working with dauer larvae. WormBook,” in The C. elegans research community, Wormbook. doi:10.1895/wormbook.1.180.1Available at: http://www.wormbook.org.
Kodama Y., Murakumo Y., Ichihara M., Kawai K., Shimono Y., Takahashi M. (2004). Induction of CRMP-2 by GDNF and analysis of the CRMP-2 promoter region. Biochem. Biophys. Res. Commun. 320 (1), 108–115. doi:10.1016/j.bbrc.2004.05.139
Li W., Herman R. K., Shaw J. E. (1992). Analysis of the Caenorhabditis elegans axonal guidance and outgrowth gene unc-33. Genetics 132 (3), 675–689. doi:10.1093/genetics/132.3.675
Lopez M. E., Vacio A. M., Cantu J., Holgado A. (2022). UNC-33L partially rescues life span and locomotion defects in unc-33 mutants but fails to rescue dauer formation defects. Micropubl. Biol. 2022. doi:10.17912/micropub.biology.00051510.17912/micropub.biology.000515
Maniar T. A., Kaplan M., Wang G. J., Shen K., Wei L., Shaw J. E., et al. (2011). UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat. Neurosci. 15 (1), 48–56. doi:10.1038/nn.2970
Moutal A., Kalinin S., Kowal K., Marangoni N., Dupree J., Lin S. X., et al. (2019). Neuronal conditional knockout of collapsin response mediator protein 2 ameliorates disease severity in a mouse model of multiple sclerosis. ASN Neuro 11, 1759091419892090. doi:10.1177/1759091419892090
Murakami M., Koga M., Ohshima Y. (2001). DAF-7/TGF-β expression required for the normal larval development in C. elegans is controlled by a presumed guanylyl cyclase DAF-11. Mech. Dev. 109 (1), 27–35. doi:10.1016/s0925-4773(01)00507-x
Na E. J., Nam H. Y., Park J., Chung M. A., Woo H. A., Kim H. J. (2017). PI3K-mTOR-S6K signaling mediates neuronal viability via collapsin response mediator protein-2 expression. Front. Mol. Neurosci. 10, 288. doi:10.3389/fnmol.2017.00288
Ren P., Lim C. S., Johnsen R., Albert P. S., Pilgrim D., Riddle D. L. (1996). Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science 274 (5291), 1389–1391. doi:10.1126/science.274.5291.1389
Togashi K., Hasegawa M., Nagai J., Tonouchi A., Masukawa D., Hensley K., et al. (2019). Genetic suppression of collapsin response mediator protein 2 phosphorylation improves outcome in methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's model mice. Genes cells. 24 (1), 31–40. doi:10.1111/gtc.12651
Tsuboi D., Hikita T., Qadota H., Amano M., Kaibuchi K. (2005). Regulatory machinery of UNC-33 Ce-CRMP localization in neurites during neuronal development in Caenorhabditis elegans. J. Neurochem. 95 (6), 1629–1641. doi:10.1111/j.1471-4159.2005.03490.x
Wang Y., Levy D. E. (2006). C. elegans STAT cooperates with DAF-7/TGF-β signaling to repress dauer formation. Curr. Biol. 16 (1), 89–94. doi:10.1016/j.cub.2005.11.061
Williamson R., van Aalten L., Mann D. M., Platt B., Plattner F., Bedford L., et al. (2011). CRMP2 hyperphosphorylation is characteristic of Alzheimer's disease and not a feature common to other neurodegenerative diseases. J. Alzheimer’s Dis. 27 (3), 615–625. doi:10.3233/JAD-2011-110617
Keywords: dauer, TGF-β, UNC-33, C. elegans, locomotion, axonal outgrowth
Citation: Samaro A, Cristancho A, Rivas A, Valtierra R, Beck S, Cantu J, Miranda M, Vacio A, Cardenas Muedano O and Holgado A (2023) The daf-7(e1372) mutation rescues dauer formation defects seen in C. elegans unc-33 mutants. Front. Physiol. 14:975878. doi: 10.3389/fphys.2023.975878
Received: 22 June 2022; Accepted: 12 January 2023;
Published: 06 February 2023.
Edited by:
Sylvia Anton, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Janet Duerr, Ohio University, United StatesRachel Arey, Baylor College of Medicine, United States
Copyright © 2023 Samaro, Cristancho, Rivas, Valtierra, Beck, Cantu, Miranda, Vacio, Cardenas Muedano and Holgado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Holgado, YWhvbGdhZG9Ac3RlZHdhcmRzLmVkdQ==
 Alexia Samaro
Alexia Samaro Alejandra Cristancho
Alejandra Cristancho Andrea Holgado
Andrea Holgado