- 1Division of Nephrology, Wuhu Hospital, East China Normal University, Wuhu, China
- 2Health Science Center, East China Normal University, Shanghai, China
The kidney is the key organ responsible for maintaining the body’s water and electrolyte homeostasis. About 99% of the primary urine filtered from the Bowman’s capsule is reabsorbed along various renal tubules every day, with only 1–2 L of urine excreted. Aquaporins (AQPs) play a vital role in water reabsorption in the kidney. Currently, a variety of molecules are found to be involved in the process of urine concentration by regulating the expression or activity of AQPs, such as antidiuretic hormone, renin-angiotensin-aldosterone system (RAAS), prostaglandin, and several nuclear receptors. As the main bile acid receptors, farnesoid X receptor (FXR) and membrane G protein-coupled bile acid receptor 1 (TGR5) play important roles in bile acid, glucose, lipid, and energy metabolism. In the kidney, FXR and TGR5 exhibit broad expression across all segments of renal tubules, and their activation holds significant therapeutic potential for numerous acute and chronic kidney diseases through alleviating renal lipid accumulation, inflammation, oxidative stress, and fibrosis. Emerging evidence has demonstrated that the genetic deletion of FXR or TGR5 exhibits increased basal urine output, suggesting that bile acid receptors play a critical role in urine concentration. Here, we briefly summarize the function of bile acid receptors in renal water reabsorption and urine concentration.
Introduction
The kidney is a vital organ that receives 20%–25% of an adult human’s cardiac output and controls the metabolism of salt and water. About 180 L of filtrate are produced daily by an adult human kidney, but only 1–2 L of urine are expelled, with 99% of the primary urine being reabsorbed along various renal tubules.
Many factors are involved in the progress of urine concentration. Firstly, the hypertonic environment of the inner medullary is a necessary condition for urine concentration, which promotes the reabsorption of water. The hypertonic environment is mainly due to the accumulation of urea and sodium chloride in the inner medullary, where Na+/K+/2Cl–cotransporter (NKCC2) and urea transporters play an important role in this process. Secondly, aquaporins also play important roles in this process. Eight aquaporins, including AQP1-7 and AQP11, have been identified in the kidney (Noda et al., 2010). Among them, AQP1 is expressed on both the apical and basolateral membrane of the proximal tubule, as well as the thin descending limb of the loop of Henle and the vasa recta (Chou et al., 1999), responsible for the reabsorption of about 80% of the water in primary urine. AQP2 is highly expressed in the apical membrane and subapical vesicles of principal cells in the renal collecting duct, responsible for about 20% of water reabsorption (Kwon et al., 2013). Mice that do not have AQP1, AQP2, AQP3, or AQP4 genes exhibit a significant increase in urine production (Yang et al., 2001). Finally, The Epithelial sodium channel (ENaC) is important for sodium and water reabsorption, which is located in the apical membrane of the collecting ducts. ENaC facilitates Na⁺ reabsorption and then the movement of Na+ creates an osmotic gradient, allowing water to follow in the same direction.
Currently, it has been reported that a variety of molecules are involved in regulating the reabsorption of water in the kidney. Antidiuretic hormone (ADH) or arginine vasopressin (AVP) is a critical hormone synthesized in the hypothalamus that plays a key role in water homeostasis. In the renal collecting duct, AVP binds to the V2 receptor (V2R) and increases the phosphorylation of AQP2 by the cAMP-PKA pathway. Recently, increasing evidence has demonstrated that AVP played a critical role in facilitating urinary concentration via activating ENaC (Mironova et al., 2012; Mironova et al., 2015; Stockand et al., 2022; Wang et al., 2022). Moreover, AVP also rapidly increased water and urea transport in the terminal inner medullary collecting duct (IMCD) by increasing the expression and apical membrane trafficking of the urea transporter A1 (UT-A1) (Sands et al., 2011). The renin-angiotensin-aldosterone system (RAAS) additionally contributes to the regulation of water and sodium reabsorption in the kidney. Angiotensin II has different effects on different parts of the kidneys. In the proximal tubules, it heightens the activity of sodium/hydrogen exchanger and sodium-bicarbonate cotransporter through binding to the Angiotensin II receptor type 1 (AT1) receptor. In the distal tubule and collecting ducts, Angiotensin II further amplifies the activity of sodium-chloride cotransporter (NCC) and ENaC, which increases the reabsorption of water and sodium. Additionally, Angiotensin II increases aldosterone levels, which promotes sodium reabsorption by binding to the mineralocorticoid receptor (Harrison-Bernard, 2009; Zaika et al., 2013). Prostaglandin E2 (PGE2) is the main cyclooxygenase metabolite of arachidonic acid (AA). It is primarily synthesized in the medullary collecting tubule of the kidney (Bonvalet et al., 1987). EP1-4 are the 4 G protein-coupled receptors (GPCRs) of PGE2. Among these, EP1 and EP3 have high expression in the basolateral membrane of the collecting duct, respectively. When these receptors are activated, less sodium chloride and water are absorbed, which increases the excretion of sodium ions and urine (Ando and Asano, 1995; Guan et al., 1998; Breyer and Breyer, 2001; Nasrallah et al., 2018). In the kidney, EP2 expression is low, however, the activation of EP2 increased AQP2 membrane targeting (Olesen et al., 2016). The primary function of EP4, which is mostly expressed in glomeruli, is to control the release of renin (Breyer and Breyer, 2001). In the collecting ducts, disruption of EP4 impaired urinary concentration via decreasing AQP2 through the cAMP/PKA pathway (Gao et al., 2015). Several nuclear receptors have been implicated in regulating water homeostasis. Activation of peroxisome proliferator-activated receptor γ (PPARγ) increased the water and sodium reabsorption through the ENaC and AQP2 (Guan et al., 2005; Zhou et al., 2015). The activation of the glucocorticoid receptor (GR) enhanced the AQP2 gene expression induced by AVP in the collecting duct cell (Su et al., 2020). Liver X receptor β (LXRβ) knockout mice also exhibited polyuria due to decreased AVP and AQP1 (Gabbi et al., 2012), and increased ubiquitination of AQP2 protein (Su et al., 2017). The administration of estradiol decreased the expression of AQP2 by binding to estrogen receptor α, consequently leading to an increase in urine output in ovariectomized rats (Cheema et al., 2015).
Increasing evidence suggests that bile acid receptors play a crucial role in regulating renal water and sodium reabsorption. In this article, we will review the general functions of two bile acid receptors, nuclear receptor FXR, and membrane receptor TGR5, and finally focus on their roles in renal water and sodium homeostasis, offering novel strategies for addressing disorders related to water and salt metabolism, such as diabetes insipidus.
Classification and general function of bile acid receptors
The liver is responsible for the synthesis of primary bile acids, specifically cholic acid (CA) and chenodeoxycholic acid (CDCA). These primary bile acids are subsequently conjugated and excreted into the intestine. Within the gut, they were metabolized by the gut microbiota, leading to the formation of secondary bile acids, namely, lithocholic acid (LCA) and deoxycholic acid (DCA) (Wang et al., 1999). In the intestine, roughly 95% of bile acids are reabsorbed into hepatocytes, with only a small fraction entering the bloodstream. Initially, these circulating bile acids go through glomerular filtration but are nearly completely reabsorbed in the proximal renal tubules, facilitated by the apical sodium-dependent bile acid transporter (ASBT) and the basolateral Organic Solute Transporter α/β (OSTα/β). Consequently, only about 5% of the filtered bile acids end up in the urine each day (Stiehl, 1974; Herman-Edelstein et al., 2018). Bile acids play crucial roles in various physiological and pathophysiological processes by binding and activating the nuclear receptor FXR and the membrane G protein-coupled receptor TGR5.
FXR is a member of the nuclear receptor (NR) superfamily that controls the transcription of specific target genes. It exhibits prominent expression in organs such as the liver, kidney, and small intestine. As the endogenous ligand of FXR, bile acids activate FXR in the following order: CDCA > DCA > LCA > CA (Wang et al., 1999). Upon activation, FXR assumes critical roles in the regulation of various metabolic pathways, including bile acid, glucose, and lipid metabolism (Guo et al., 2023). Notably, it acts to inhibit the production and accumulation of bile acids in the liver and intestines (Liu et al., 2003; Boyer et al., 2006; Landrier et al., 2006). Additionally, the activation of FXR increases glycogen synthesis and reduces glycolysis (Zhang et al., 2006; Caron et al., 2013; Dong et al., 2019). Furthermore, it reduced the accumulation of lipids in the kidney in insulin-resistance animal models (Nakahara et al., 2002; Zhang et al., 2006; Lai et al., 2022). Recently, several studies reported that overexpression of FXR in the kidney substantially alleviated hypertension and elevated renal nitric oxide (NO) levels, which was achieved by stimulating the expression of endothelial nitric oxide synthase (eNOS) in a mouse model of hypertension induced by an 8-week regimen of 20% fructose in drinking water combined with a 4% sodium chloride diet (referred to as HFS) (Ghebremariam et al., 2013; Li et al., 2015). In the kidney, activation of FXR attenuated acute kidney injury caused by cisplatin and renal ischemia–reperfusion (I/R) through regulating apoptosis, ferroptosis, and autophagy (Bae et al., 2014; Gai et al., 2017; Luan et al., 2021; Zhang et al., 2022). Additionally, FXR agonists also improved renal inflammation, fibrosis, lipid accumulation, and glucose metabolism disorders, which prevented the progression of chronic kidney disease (Evans et al., 2009; Wang et al., 2010; Zhou et al., 2016; Marquardt et al., 2017).
TGR5 is a membrane receptor of bile acids, which can be bound and activated by various endogenous bile acids, especially LCA. TGR5 is expressed in various tissues, such as the kidney, liver, digestive tract, and central nervous system (Poole et al., 2010). Many studies have demonstrated that it plays a significant role in multiple physiological processes, as well as the pathogenesis of various metabolic diseases (Reich et al., 2021; Tian et al., 2022; Chen et al., 2023). Activation of TGR5 results in coupling with a stimulatory G-alpha-protein (Gαs) which, in turn, activates the cAMP-PKA signaling pathway. This cascade of events results in the phosphorylation of the cAMP response element binding protein (CREB) and its subsequent nuclear import, ultimately leading to the activation of the target genes. When activated, TGR5 promotes GLP-1 secretion from enteroendocrine L cells (Li et al., 2017), mitochondrial thermogenesis in adipocytes (Velazquez-Villegas et al., 2018), and protected against lipopolysaccharide (LPS)-induced liver inflammation by decreasing inflammatory cytokine secretion (Wang et al., 2011). In the kidney, TGR5 activation has been found to alleviate renal I/R injury by reducing inflammation and macrophage migration (Zhang et al., 2019). Furthermore, it has also been observed that the activation of TGR5 can prevent renal inflammation and fibrosis by inhibiting the NF-κB pathway in diabetic mice induced by streptozotocin (STZ) (Xiao et al., 2020). Moreover, a selective TGR5 agonist INT-777 has been shown to ameliorate proteinuria and podocyte injury in diabetic db/db mice (Wang et al., 2016). In addition, TGR5 activation also decreased the high glucose-induced fibrosis in glomerular mesangial cells (GMCs) (Yang et al., 2016). Recently studies have also revealed that the deubiquitination of TGR5 at K306 residue also restored TGR5 expression and protected db/db mice from diabetic nephropathy (Lin et al., 2023).
Currently, there are some studies on dual FXR and TGR5 agonists in the kidney. Diabetic mice treated with the dual FXR/TGR5 agonist INT-767 showed an improvement in proteinuria and prevention of podocyte injury, mesangial expansion, and tubulointerstitial fibrosis (Wang et al., 2016). The same dual agonist reduced the proteinuria and fibronectin accumulation in aging mice (Wang et al., 2017). These findings indicate a potential role of dual FXR/TGR5 agonists in the regulation of many kidney diseases.
Bile acid receptors and kidney water homeostasis
As mentioned above, about 95% of the bile acids synthesized from the liver are recycled through the enterohepatic circulation. In the kidney, approximately 100 μmol bile acids are filtered in the glomeruli per day, and almost all of them are reabsorbed in the proximal tubule. Only 1–2 μmol/day is excreted in the urine (Dawson et al., 2009). The renal tubules, especially the collecting duct, express high levels of bile acid receptors FXR and TGR5, where water is reabsorption through aquaporins to complete the final step of urine concentration, suggesting that FXR and TGR5 may play an important role in the regulation of water homeostasis. Whether the activation of the bile acid receptor in collecting ducts plays a significant role in water homeostasis is worth studying.
Farnesoid X receptor and renal water reabsorption
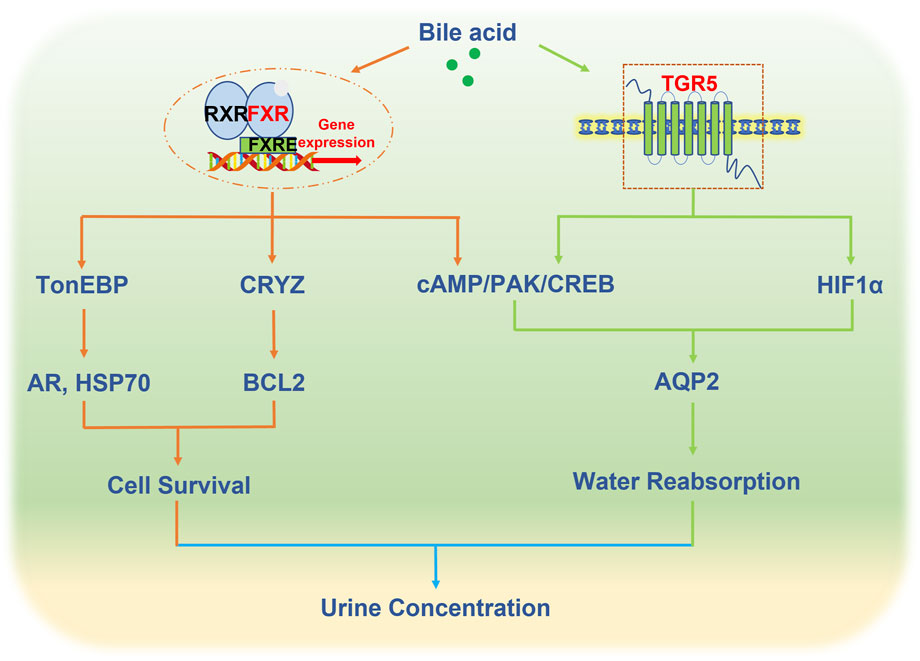
In the kidney, FXR exhibits high expression levels in various renal tubules, especially the collecting duct. However, the current study of FXR in water reabsorption only reveals its role in collecting ducts. FXR plays a pivotal role in the regulation of urine volume. FXR knockout mice displayed diminished urine concentrating ability in comparison to WT mice, and its activation by binding with CDCA or a synthetic agonist GW4064 increased urinary concentrating capacity, mainly by increasing renal AQP2 expression (Zhang et al., 2014). Moreover, MCDs are exposed to a massive hypertonic environment, which is critical in regulating urine concentration. FXR can prevent hypertonic-induced apoptosis of MCDs by activating tonicity response enhancer-binding protein (TonEBP), a critical transcription factor responsible for facilitating the cellular accumulation of organic osmolytes to resist the hyperosmotic stress through increasing the expression of the target genes, including aldose reductase (AR) and heat shock protein 70 (HSP70) (Xu et al., 2018). Recently, studies revealed that crystallin zeta (CRYZ), a direct target gene of FXR, increased the NKCC2 expression to help maintain medullary hyperosmotic gradient. Additionally, overexpressing CRYZ reduced the cell death caused by hypertonicity by elevating the expression of B-cell lymphoma 2 (BCL2). These data demonstrated that FXR plays a critical role in the regulation of urine volume by increasing the expression of AQP2 and promoting the survival of MCDs in a dehydrated state. The above results proved the physiological function of FXR in water reabsorption, but its role in the pathophysiological state such as diabetes insipidus is still unclear. Moreover, recent evidence also showed that enhanced urinary excretion of bile acids in some conditions such as cholestasis may cause the injury of tubular epithelial cells, and FXR agonist obeticholic acid (OCA) ameliorated the renal tubular damage in bile duct ligation (BDL) induced hepatorenal syndrome (HRS) (Tsai et al., 2020). Low urine volume also existed in HRS mainly caused by a reduction in renal blood flow, it is not known the effect of increased bile acids in renal tubules on the expression of AQPs and water reabsorption.
TGR5 and renal water reabsorption
In normal kidney tissue, TGR5 exhibited high expression in collecting ducts, distal convoluted tubules, and the thin loop of Henle, with minimal or sporadic weak staining in the proximal tubules (Zhao et al., 2018). Different from the study of FXR in water reabsorption focusing on physiological levels, the study of TGR5 in AQP2 regulation is mainly carried out in kidney diseases. Lithium is a frequently prescribed medication for managing bipolar disorder, which may cause multiple endocrinopathies including nephrogenic diabetes insipidus (NDI). In a mouse model of lithium-induced nephrogenic diabetes insipidus, the activation of TGR5 by INT-777 or INT-767 elevated the expression of AQP2 through the cAMP/PKA signaling pathway (Li et al., 2018). Acute kidney injury is a common clinical disease, accompanied by changes in urine output. With the progression of the disease, oliguria, anuria, and polyuria can occur. In the I/R-induced AKI rat model, urinary output was significantly decreased, accompanied by the loss of renal AQP1, AQP2, and AQP3 in the cortex and outer medulla (Hussein et al., 2012; Asvapromtada et al., 2018; Liu et al., 2021). While the activation of TGR5 by LCA or INT-777 effectively prevented the downregulation of renal AQP2 in I/R-induced kidney injury through activating HIF-1α signaling (Han et al., 2021). These findings support the potential role of TGR5 in the regulation of renal water reabsorption.
Perspectives
FXR and TGR5, as bile acid receptors, play an important role in renal water homeostasis through regulating the expression and trafficking of AQP2, which provides a novel therapeutic for the treatment of water and salt metabolism disorders such as diabetes insipidus (Figure 1). However, the mechanism of bile acids in the regulation of water homeostasis is largely unknown. Firstly, there are 8 types of aquaporins in the kidney, in addition to affecting AQP2, whether the activation of bile acid receptors affects other aquaporins is worth exploring. Secondly, AVP synthesized in the hypothalamus regulates AQP2 expression. Recently studies revealed that FXR and TGR5 were expressed in the hypothalamus (Castellanos-Jankiewicz et al., 2021; Deckmyn et al., 2021), and circulating bile acids can also reach the hypothalamus (Xu, 2018). Whether bile acid receptors affect AVP secretion remains to be investigated. Thirdly, in addition to aquaporins, other ion channels such as ENac can also indirectly affect water reabsorption. At present, research has not delved into the direct regulation of ENaC by either FXR or TGR5 in the kidney, but several studies have reported that ENaC is regulated by bile acids in overexpressed human ENaC Xenopus laevis oocytes (Ilyaskin et al., 2016; Wang et al., 2019).
Author contributions
YG: Writing–original draft. TL: Writing–original draft. GX: Writing–original draft. XZ: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The following funding sources provided support for this project: Grants 82270703, 81970606, and 81970595 from the National Natural Science Foundation of China, as well as the East China Normal University Medicine and Health Joint Fund (2022JKXYD03001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ando Y., Asano Y. (1995). Luminal prostaglandin E2 modulates sodium and water transport in rabbit cortical collecting ducts. Am. J. Physiology-Renal Physiology 268, F1093–F1101. doi:10.1152/ajprenal.1995.268.6.F1093
Asvapromtada S., Sonoda H., Kinouchi M., Oshikawa S., Takahashi S., Hoshino Y., et al. (2018). Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am. J. Physiol. Ren. Physiol. 314, F584-F601–f601. doi:10.1152/ajprenal.00184.2017
Bae E. H., Choi H. S., Joo S. Y., Kim I. J., Kim C. S., Choi J. S., et al. (2014). Farnesoid X receptor ligand prevents cisplatin-induced kidney injury by enhancing small heterodimer partner. PLoS One 9, e86553. doi:10.1371/journal.pone.0086553
Bonvalet J. P., Pradelles P., Farman N. (1987). Segmental synthesis and actions of prostaglandins along the nephron. Am. J. Physiol. 253, F377–F387. doi:10.1152/ajprenal.1987.253.3.F377
Boyer J. L., Trauner M., Mennone A., Soroka C. J., Cai S.-Y., Moustafa T., et al. (2006). Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiology-Gastrointestinal Liver Physiology 290, G1124–G1130. doi:10.1152/ajpgi.00539.2005
Breyer M. D., Breyer R. M. (2001). G protein-coupled prostanoid receptors and the kidney. Annu. Rev. Physiol. 63, 579–605. doi:10.1146/annurev.physiol.63.1.579
Caron S., Huaman Samanez C., Dehondt H., Ploton M., Briand O., Lien F., et al. (2013). Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol. Cell Biol. 33, 2202–2211. doi:10.1128/MCB.01004-12
Castellanos-Jankiewicz A., Guzmán-Quevedo O., Fénelon V. S., Zizzari P., Quarta C., Bellocchio L., et al. (2021). Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 33, 1483–1492.e10. doi:10.1016/j.cmet.2021.04.009
Cheema M. U., Irsik D. L., Wang Y., Miller-Little W., Hyndman K. A., Marks E. S., et al. (2015). Estradiol regulates AQP2 expression in the collecting duct: a novel inhibitory role for estrogen receptor α. Am. J. Physiol. Ren. Physiol. 309, F305–F317. doi:10.1152/ajprenal.00685.2014
Chen B., Bai Y., Tong F., Yan J., Zhang R., Zhong Y., et al. (2023). Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes 15, 2192155. doi:10.1080/19490976.2023.2192155
Chou C. L., Knepper M. A., Hoek A. N., Brown D., Yang B., Ma T., et al. (1999). Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J. Clin. Invest. 103, 491–496. doi:10.1172/JCI5704
Dawson P. A., Lan T., Rao A. (2009). Bile acid transporters. J. Lipid Res. 50, 2340–2357. doi:10.1194/jlr.R900012-JLR200
Deckmyn B., Domenger D., Blondel C., Ducastel S., Nicolas E., Dorchies E., et al. (2021). Farnesoid X receptor activation in brain alters Brown adipose tissue function via the sympathetic system. Front. Mol. Neurosci. 14, 808603. doi:10.3389/fnmol.2021.808603
Dong R., Yang X., Wang C., Liu K., Liu Z., Ma X., et al. (2019). Yangonin protects against non-alcoholic fatty liver disease through farnesoid X receptor. Phytomedicine 53, 134–142. doi:10.1016/j.phymed.2018.09.006
Evans M. J., Mahaney P. E., Borges-Marcucci L., Lai K., Wang S., Krueger J. A., et al. (2009). A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G543–G552. doi:10.1152/ajpgi.90585.2008
Gabbi C., Kong X., Suzuki H., Kim H. J., Gao M., Jia X., et al. (2012). Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor β. Proc. Natl. Acad. Sci. U. S. A. 109, 3030–3034. doi:10.1073/pnas.1200588109
Gai Z., Chu L., Xu Z., Song X., Sun D., Kullak-Ublick G. A. (2017). Farnesoid X receptor activation protects the kidney from ischemia-reperfusion damage. Sci. Rep. 7, 9815. doi:10.1038/s41598-017-10168-6
Gao M., Cao R., Du S., Jia X., Zheng S., Huang S., et al. (2015). Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc. Natl. Acad. Sci. U. S. A. 112, 8397–8402. doi:10.1073/pnas.1509565112
Ghebremariam Y. T., Yamada K., Lee J. C., Johnson C. L., Atzler D., Anderssohn M., et al. (2013). FXR agonist INT-747 upregulates DDAH expression and enhances insulin sensitivity in high-salt fed Dahl rats. PLoS One 8, e60653. doi:10.1371/journal.pone.0060653
Guan Y., Hao C., Cha D. R., Rao R., Lu W., Kohan D. E., et al. (2005). Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat. Med. 11, 861–866. doi:10.1038/nm1278
Guan Y., Zhang Y., Breyer R. M., Fowler B., Davis L., Hébert R. L., et al. (1998). Prostaglandin E2 inhibits renal collecting duct Na+ absorption by activating the EP1 receptor. J. Clin. Invest. 102, 194–201. doi:10.1172/JCI2872
Guo Y., Xie G., Zhang X. (2023). Role of FXR in renal Physiology and kidney diseases. Int. J. Mol. Sci. 24, 2408. doi:10.3390/ijms24032408
Han M., Li S., Xie H., Liu Q., Wang A., Hu S., et al. (2021). Activation of TGR5 restores AQP2 expression via the HIF pathway in renal ischemia-reperfusion injury. Am. J. Physiology-Renal Physiology 320, F308–F321. doi:10.1152/ajprenal.00577.2020
Harrison-Bernard L. M. (2009). The renal renin-angiotensin system. Adv. Physiology Educ. 33, 270–274. doi:10.1152/advan.00049.2009
Herman-Edelstein M., Weinstein T., Levi M. (2018). Bile acid receptors and the kidney. Curr. Opin. Nephrol. Hypertens. 27, 56–62. doi:10.1097/MNH.0000000000000374
Hussein A. A., El-Dken Z. H., Barakat N., Abol-Enein H. (2012). Renal ischaemia/reperfusion injury: possible role of aquaporins. Acta Physiol. (Oxf) 204, 308–316. doi:10.1111/j.1748-1716.2011.02372.x
Ilyaskin A. V., Diakov A., Korbmacher C., Haerteis S. (2016). Activation of the human epithelial sodium channel (ENaC) by bile acids involves the degenerin site. J. Biol. Chem. 291, 19835–19847. doi:10.1074/jbc.M116.726471
Kwon T. H., Frøkiær J., Nielsen S. (2013). Regulation of aquaporin-2 in the kidney: a molecular mechanism of body-water homeostasis. Kidney Res. Clin. Pract. 32, 96–102. doi:10.1016/j.krcp.2013.07.005
Lai C. R., Tsai Y. L., Tsai W. C., Chen T. M., Chang H. H., Changchien C. Y., et al. (2022). Farnesoid X receptor overexpression decreases the migration, invasion and angiogenesis of human bladder cancers via AMPK activation and cholesterol biosynthesis inhibition. Cancers (Basel) 14, 4398. doi:10.3390/cancers14184398
Landrier J.-F., Eloranta J. J., Vavricka S. R., Kullak-Ublick G. A. (2006). The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am. J. Physiology-Gastrointestinal Liver Physiology 290, G476–G485. doi:10.1152/ajpgi.00430.2005
Li C., Li J., Weng X., Lan X., Chi X. (2015). Farnesoid X receptor agonist CDCA reduces blood pressure and regulates vascular tone in spontaneously hypertensive rats. J. Am. Soc. Hypertens. 9, 507–516. doi:10.1016/j.jash.2015.04.006
Li S., Qiu M., Kong Y., Zhao X., Choi H. J., Reich M., et al. (2018). Bile acid G protein-coupled membrane receptor TGR5 modulates aquaporin 2-mediated water homeostasis. J. Am. Soc. Nephrol. 29, 2658–2670. doi:10.1681/ASN.2018030271
Li Y., Cheng K. C., Niu C. S., Lo S. H., Cheng J. T., Niu H. S. (2017). Investigation of triamterene as an inhibitor of the TGR5 receptor: identification in cells and animals. Drug Des. Devel Ther. 11, 1127–1134. doi:10.2147/DDDT.S131892
Lin Z., Li S., Xiao H., Xu Z., Li C., Zeng J., et al. (2023). The degradation of TGR5 mediated by Smurf1 contributes to diabetic nephropathy. Cell Rep. 42, 112851. doi:10.1016/j.celrep.2023.112851
Liu Q., Kong Y., Guo X., Liang B., Xie H., Hu S., et al. (2021). GSK-3β inhibitor TDZD-8 prevents reduction of aquaporin-1 expression via activating autophagy under renal ischemia reperfusion injury. Faseb J. 35, e21809. doi:10.1096/fj.202100549R
Liu Y., Binz J., Numerick M. J., Dennis S., Luo G., Desai B., et al. (2003). Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra-and extrahepatic cholestasis. J. Clin. investigation 112, 1678–1687. doi:10.1172/JCI18945
Luan Z. L., Ming W. H., Sun X. W., Zhang C., Zhou Y., Zheng F., et al. (2021). A naturally occurring FXR agonist, alisol B 23-acetate, protects against renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 321, F617–f628. doi:10.1152/ajprenal.00193.2021
Marquardt A., Al-Dabet M. M., Ghosh S., Kohli S., Manoharan J., Elwakiel A., et al. (2017). Farnesoid X receptor agonism protects against diabetic tubulopathy: potential add-on therapy for diabetic nephropathy. J. Am. Soc. Nephrol. 28, 3182–3189. doi:10.1681/ASN.2016101123
Mironova E., Bugaj V., Roos K. P., Kohan D. E., Stockand J. D. (2012). Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc. Natl. Acad. Sci. U. S. A. 109, 10095–10100. doi:10.1073/pnas.1201978109
Mironova E., Chen Y., Pao A. C., Roos K. P., Kohan D. E., Bugaj V., et al. (2015). Activation of ENaC by AVP contributes to the urinary concentrating mechanism and dilution of plasma. Am. J. Physiol. Ren. Physiol. 308, F237–F243. doi:10.1152/ajprenal.00246.2014
Nakahara M., Fujii H., Maloney P. R., Shimizu M., Sato R. (2002). Bile acids enhance low density lipoprotein receptor gene expression via a MAPK cascade-mediated stabilization of mRNA. J. Biol. Chem. 277, 37229–37234. doi:10.1074/jbc.M206749200
Nasrallah R., Zimpelmann J., Eckert D., Ghossein J., Geddes S., Beique J. C., et al. (2018). PGE(2) EP(1) receptor inhibits vasopressin-dependent water reabsorption and sodium transport in mouse collecting duct. Lab. Invest. 98, 360–370. doi:10.1038/labinvest.2017.133
Noda Y., Sohara E., Ohta E., Sasaki S. (2010). Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 6, 168–178. doi:10.1038/nrneph.2009.231
Olesen E. T., Moeller H. B., Assentoft M., Macaulay N., Fenton R. A. (2016). The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am. J. Physiol. Ren. Physiol. 311, F935-F944–f944. doi:10.1152/ajprenal.00559.2015
Poole D. P., Godfrey C., Cattaruzza F., Cottrell G. S., Kirkland J. G., Pelayo J. C., et al. (2010). Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol. Motil. 22 (814-25), 814–825. doi:10.1111/j.1365-2982.2010.01487.x
Reich M., Spomer L., Klindt C., Fuchs K., Stindt J., Deutschmann K., et al. (2021). Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J. Hepatol. 75, 634–646. doi:10.1016/j.jhep.2021.03.029
Sands J. M., Blount M. A., Klein J. D. (2011). Regulation of renal urea transport by vasopressin. Trans. Am. Clin. Climatol. Assoc. 122, 82–92.
Stiehl A. (1974). Bile salt sulphates in cholestasis. Eur. J. Clin. Invest. 4, 59–63. doi:10.1111/j.1365-2362.1974.tb00373.x
Stockand J. D., Mironova E. V., Xiang H., Soares A. G., Contreras J., Mccormick J. A., et al. (2022). Chronic activation of vasopressin-2 receptors induces hypertension in Liddle mice by promoting Na(+) and water retention. Am. J. Physiol. Ren. Physiol. 323, F468–f478. doi:10.1152/ajprenal.00384.2021
Su S.-H., Ho C.-H., Yu M.-J. (2020). Glucocorticoid receptor maintains vasopressin-regulated water reabsorption pathway in the kidney collecting duct cells. FASEB J. 34, 1. doi:10.1096/fasebj.2020.34.s1.05129
Su W., Huang S. Z., Gao M., Kong X. M., Gustafsson J., Xu S. J., et al. (2017). Liver X receptor β increases aquaporin 2 protein level via a posttranscriptional mechanism in renal collecting ducts. Am. J. Physiol. Ren. Physiol. 312, F619-F628–f628. doi:10.1152/ajprenal.00564.2016
Tian F., Xu W., Chen L., Chen T., Feng X., Chen J., et al. (2022). Ginsenoside compound K increases glucagon-like peptide-1 release and L-cell abundance in db/db mice through TGR5/YAP signaling. Int. Immunopharmacol. 113, 109405. doi:10.1016/j.intimp.2022.109405
Tsai Y. L., Liu C. W., Hsu C. F., Huang C. C., Lin M. W., Huang S. F., et al. (2020). Obeticholic acid ameliorates hepatorenal syndrome in ascitic cirrhotic rats by down-regulating the renal 8-iso-PGF2α-activated COX-TXA2 pathway. Clin. Sci. (Lond) 134, 2055–2073. doi:10.1042/CS20200452
Velazquez-Villegas L. A., Perino A., Lemos V., Zietak M., Nomura M., Pols T. W. H., et al. (2018). TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 9, 245. doi:10.1038/s41467-017-02068-0
Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999). Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553. doi:10.1016/s1097-2765(00)80348-2
Wang X. P., Im S. J., Balchak D. M., Montalbetti N., Carattino M. D., Ray E. C., et al. (2019). Murine epithelial sodium (Na(+)) channel regulation by biliary factors. J. Biol. Chem. 294, 10182–10193. doi:10.1074/jbc.RA119.007394
Wang X. P., Tomilin V., Nickerson A. J., Tian R., Ertem M., Mckernan A., et al. (2022). Bile acids regulate the epithelial Na(+) channel in native tissues through direct binding at multiple sites. J. Physiol. 600, 4695–4711. doi:10.1113/JP283318
Wang X. X., Edelstein M. H., Gafter U., Qiu L., Luo Y., Dobrinskikh E., et al. (2016). G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J. Am. Soc. Nephrol. 27, 1362–1378. doi:10.1681/ASN.2014121271
Wang X. X., Jiang T., Shen Y., Caldas Y., Miyazaki-Anzai S., Santamaria H., et al. (2010). Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59, 2916–2927. doi:10.2337/db10-0019
Wang X. X., Luo Y., Wang D., Adorini L., Pruzanski M., Dobrinskikh E., et al. (2017). A dual agonist of farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5, INT-767, reverses age-related kidney disease in mice. J. Biol. Chem. 292, 12018–12024. doi:10.1074/jbc.C117.794982
Wang Y. D., Chen W. D., Yu D., Forman B. M., Huang W. (2011). The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 54, 1421–1432. doi:10.1002/hep.24525
Xiao H., Sun X., Liu R., Chen Z., Lin Z., Yang Y., et al. (2020). Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol. Res. 151, 104559. doi:10.1016/j.phrs.2019.104559
Xu A. W. (2018). Hypothalamic sensing of bile acids, a gut feeling. Trends Endocrinol. Metab. 29, 363–366. doi:10.1016/j.tem.2018.02.001
Xu S., Huang S., Luan Z., Chen T., Wei Y., Xing M., et al. (2018). Farnesoid X receptor is essential for the survival of renal medullary collecting duct cells under hypertonic stress. Proc. Natl. Acad. Sci. U. S. A. 115, 5600–5605. doi:10.1073/pnas.1803945115
Yang B., Ma T., Verkman A. S. (2001). Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3. J. Biol. Chem. 276, 624–628. doi:10.1074/jbc.M008664200
Yang Z., Xiong F., Wang Y., Gong W., Huang J., Chen C., et al. (2016). TGR5 activation suppressed S1P/S1P2 signaling and resisted high glucose-induced fibrosis in glomerular mesangial cells. Pharmacol. Res. 111, 226–236. doi:10.1016/j.phrs.2016.05.035
Zaika O., Mamenko M., Staruschenko A., Pochynyuk O. (2013). Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr. Hypertens. Rep. 15, 17–24. doi:10.1007/s11906-012-0316-1
Zhang L., Fu X., Gui T., Wang T., Wang Z., Kullak-Ublick G. A., et al. (2019). Effects of farnesiferol B on ischemia-reperfusion-induced renal damage, inflammation, and NF-κB signaling. Int. J. Mol. Sci. 20, 6280. doi:10.3390/ijms20246280
Zhang L., Li A., Huang Z., Wang Y., Yi B. (2022). Knockout of farnesoid X receptor gene aggravates cisplatin-induced kidney injury. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 47, 174–182. doi:10.11817/j.issn.1672-7347.2022.210423
Zhang X., Huang S., Gao M., Liu J., Jia X., Han Q., et al. (2014). Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc. Natl. Acad. Sci. U. S. A. 111, 2277–2282. doi:10.1073/pnas.1323977111
Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., et al. (2006). Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. 103, 1006–1011. doi:10.1073/pnas.0506982103
Zhao C. L., Amin A., Hui Y., Yang D., Cao W. (2018). TGR5 expression in normal kidney and renal neoplasms. Diagn. Pathol. 13, 22. doi:10.1186/s13000-018-0700-5
Zhou B., Feng B., Qin Z., Zhao Y., Chen Y., Shi Z., et al. (2016). Activation of farnesoid X receptor downregulates visfatin and attenuates diabetic nephropathy. Mol. Cell. Endocrinol. 419, 72–82. doi:10.1016/j.mce.2015.10.001
Keywords: FXR, tgr5, aquaporin, kidney, water homeostasis
Citation: Guo Y, Luo T, Xie G and Zhang X (2023) Bile acid receptors and renal regulation of water homeostasis. Front. Physiol. 14:1322288. doi: 10.3389/fphys.2023.1322288
Received: 16 October 2023; Accepted: 07 November 2023;
Published: 15 November 2023.
Edited by:
Belisario Enrique Fernandez, University Institute of Health Sciences, ArgentinaReviewed by:
Silvana Lorena Della Penna, University of Twente, NetherlandsCopyright © 2023 Guo, Luo, Xie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Zhang, eHl6aGFuZ0Boc2MuZWNudS5lZHUuY24=
 Yanlin Guo
Yanlin Guo Taotao Luo1
Taotao Luo1 Xiaoyan Zhang
Xiaoyan Zhang