
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol., 09 October 2023
Sec. Avian Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1285938
This article is part of the Research TopicThe Association between Avian Physiology and Meat QualityView all 12 articles
Meat-type (broiler) and egg-type (layer) chickens were bred by intensive selection over the years, resulting in more numbers and larger sizes of myofibers. Although the characteristics are important parameters in muscle growth and meat quality, muscle bundle characteristics have not been studied in poultry. Therefore, this study aimed to compare the histological characteristics of myofibers and muscle bundles in muscles between male broiler (Ross broiler breed) chickens and layer (Hy-Line) chickens. Chicken muscles, pectoralis major (PM) and gastrocnemius (GM), were sampled at the age of 49 days and stained to analyze histological characteristics. Expectedly, body weights (BWs) and weights of PM and GM muscles in 49-day-old broilers were significantly heavier than those in layers. Within PM, broilers exhibited greater number and cross-sectional area (CSA) of myofibers than layers (3.3- and 3.3-fold, respectively). The total number and CSA of PM muscle bundles were approximately 1.5 and 6.6 times greater, respectively, in broilers than layers. Moreover, broilers exhibited 2 times greater number of myofibers per bundle of PM muscle than layers. Within GM, myofiber number and CSA were 2.3- and 2.4-fold greater, respectively, in broilers than layers. In addition, the total number of muscle bundles and bundle CSA were 2.5- and 2.1-fold greater, respectively, in broilers than in the layers. The novel findings of the current study provide evidence that greater muscle mass of broilers occurs by both hyperplasia and hypertrophy of muscle bundles and myofibers.
Poultry meat and egg consumption are steadily increasing as consumers face increasing health concerns related to red meat consumption. As a meat-type poultry breed, broilers have been genetically selected to have a fast-growing performance and high meat yield, whereas layers selected for egg production and backyard chickens, such as Hubbard JA57, have a slow-growing performance. As a result, they show significant differences in body and muscle weight, especially in the breast muscle, and these differences most likely involve the quantity or size of myofibers (Scheuermann et al., 2004).
Muscle bundle characteristics have been compared and related with growth characteristics of muscle among different breeds of livestock species (Albrecht et al., 2006). Greater muscle mass in fast-growing animals is generally associated with increased number (hyperplasia), increased size (hypertrophy), or both of myofibers and bundles, which can be attributed to various factors, such as animals, species, body weight, breed, age, sex, growth rate, and physical activity (Kiessling, 1977; Scheuermann et al., 2004; Albrecht et al., 2006; Choi and Kim, 2009; Choi et al., 2013a; Choi et al., 2013b; Choi et al., 2014; Kokoszyński et al., 2018; Kokoszyński et al., 2022; Kim et al., 2022; Weng et al., 2022). The low-weight quail line exhibited a lower number, but similar size of myofibers, compared to the random bred control (RBC) quail line, providing a unique muscle hypoplasia model in avian species (Choi et al., 2014). In contrast, the heavy-weight quail line has a greater size of myofibers in the breast muscle, but no difference in total fiber number compared to the RBC quail line (Choi et al., 2013b). Additionally, myofiber hypertrophy appeared in the fast-growing duck line compared to ducks in the slow-growing line (Huo et al., 2021). In chickens, commercial broiler lines with higher breast yield compared to Leghorn egg-type chickens of the same age and sex showed myofiber hyperplasia and hypertrophy (Scheuermann et al., 2004). Although muscle bundle characteristics are important parameters contributing to muscle growth and meat quality in livestock animals (Scheuermann et al., 2004; Albrecht et al., 2006; Chandraratne et al., 2006; Lee et al., 2018; Choi et al., 2019), these factors have not been extensively studied in chickens. Therefore, the objective of this study was to compare histological traits of myofiber and muscle bundle in the pectoralis major (PM) and gastrocnemius (GM) muscles between male broiler chickens and layer chickens.
Commercially available chickens (broiler and layer; Ross broiler breed and Hy-Line, respectively) and experiments were approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC; protocol no. 2020A00000094). All animals were raised under the same environmental conditions such as room temperature and the size of brooder cages. In addition, we fed the same diet to both layer and broiler chickens to eliminate diet effects (Table 1). Chickens were euthanized by cervical dislocation after CO2 inhalation according to the IACUC protocol.
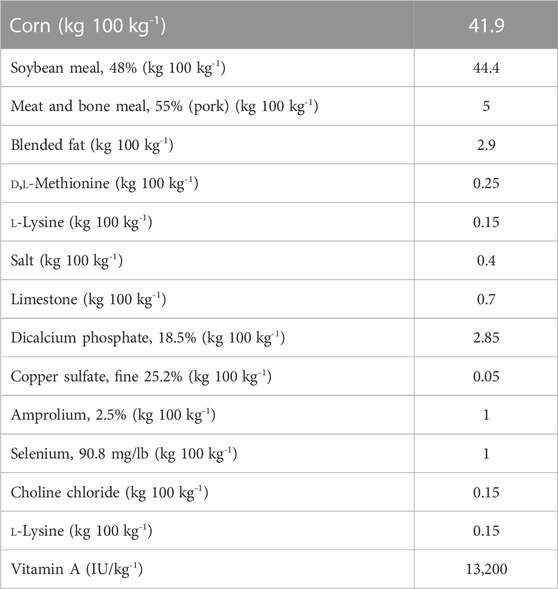
TABLE 1. Ingredient and calculated nutrient composition of the dry matter of diets fed to both layer and broiler chickens.
A total of 12 male chickens (age, 49 days; broiler, n = 6; layer, n = 6) were used in this study. Body weight (BW), PM muscle weight (PMW), and GM muscle weight (GMW) were measured, and percentages of PMW and GMW were calculated in relation to BW. After measurement of PMW, CSA of the left PM muscle was measured in an area cut from the lower left to the upper right at the half-point of the muscle (Scheuermann et al., 2004). Whole right GM muscles were fixed and then cut in the middle of the muscle to prepare paraffin blocks.
PM and GM muscles fixed with 10% neutral-buffered formalin were embedded in paraffin and then cross-sectioned into 10-µm slices. The sections were stained using a hematoxylin and eosin stain method following our previous study (Kim et al., 2022). All stained sections were assessed in terms of myofiber and muscle bundle characteristics, including total number, average CSA, and myofiber number per bundle, using image analysis (Image-Pro Plus software, Media Cybernetics, Silver Spring, MD). For each sample, at least 500 different fibers and 30 bundles were randomly selected and measured to determine these parameters at ×10 and ×40 magnification. Average CSA and total number of myofibers were calculated according to previous studies (Scheuermann et al., 2004; Kim et al., 2022). Total bundle number was calculated by dividing the PM muscle CSA by the mean bundle area of each sample. The average of the bundle CSA was determined by dividing the total bundle area by the total bundle number measured.
To compare carcass traits and histological characteristics between broiler and layer chickens, the data were analyzed by t-tests using GraphPad Prism software, version 6.02. All data were expressed as means ± SEM. The results with p < 0.05 were considered significant.
As expected, at 49 days post-hatch, broilers exhibited heavier body weight than layers (4,097 vs 512.3 g, p < 0.001) (Table 2). PMW (946.7 vs 36.5 g, p < 0.001) and percentage of PM (23.1% vs 7.06%, p < 0.001) were 25.9- and 3.3-fold greater in broilers than in layers, respectively. GMW of the broiler was approximately 8.3 times greater than that of the layer (41.3 g vs 4.98 g, p < 0.001), although there was no difference in the percentage of the GM muscle between the breeds (1.01% vs 0.97%, p > 0.05).
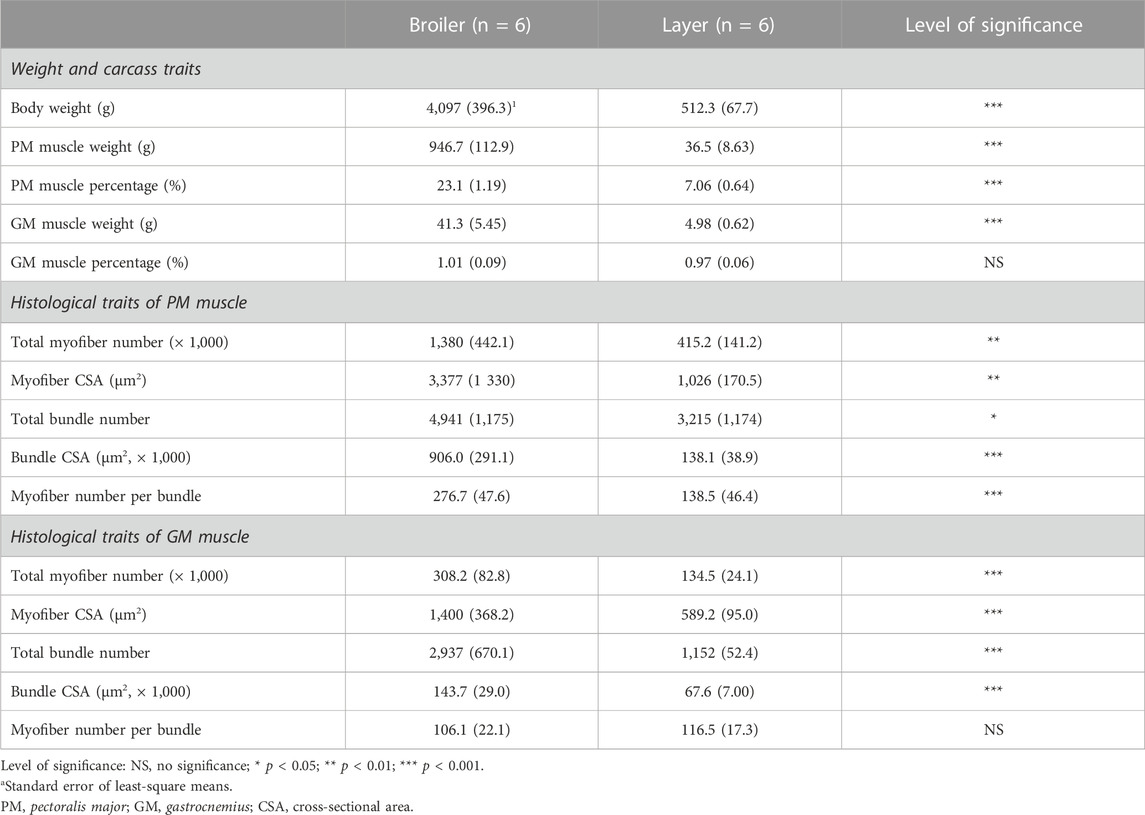
TABLE 2. Comparison of body weight, carcass traits, and histological traits of the pectoralis major and gastrocnemius muscles between broiler and layer chickens at 49 days post-hatch.
PM muscles of broiler chickens had a 3.3-fold greater total number (1,380,000 vs 415,200, p < 0.01) and CSA (3,377 vs 1,026 μm2, p < 0.01) of myofibers compared to those of layer chickens. In the GM muscle, broilers showed more number (308,200 vs 134,500, p < 0.001) and greater size (1,400 vs 589.2 μm2, p < 0.001) of myofibers compared to layers. PM muscles of broilers were 1.5-, 6.5-, and 2.0-fold greater in total bundle number (4,941 vs 3,215, p < 0.05), bundle CSA (906,000 vs 138,100 μm2, p < 0.001), and myofiber number per bundle (276.7 vs 138.5, p < 0.001), respectively, compared to those of layers. Similar to PM muscles, greater number (2,937 vs 1,152, p < 0.001) and CSA (143,700 vs 67,600 μm2, p < 0.001) of GM muscle bundles were found in broilers compared to layers; whereas two breeds did not differ in myofiber number per bundle (106.1 vs 116.5, p > 0.05). Their representative images are presented in Figure 1.
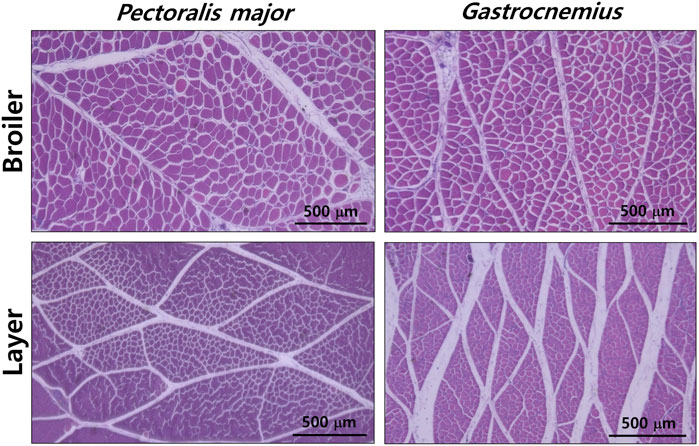
FIGURE 1. Histological differences in pectoralis major and gastrocnemius muscles between male broiler and layer chickens at 49 days post-hatch. Scale bar: 500 μm.
It is generally reported that fast-growing broiler chickens are 2- to 3-fold greater in growth rate, and in particular, their breast muscles grow 8-fold faster than those in layer-type chickens (Buzala and Janicki, 2016). The findings that greater percentage of PM muscle in broilers, but similar percentage of GM muscle between two breeds, clearly provide evidence for the general selection for greater yield of breast muscle but not for leg muscle. Our previous study reported that 33-day-old broiler chickens had 2.7-fold greater myofiber CSA of PM muscle than layer chickens at the same age (Kim et al., 2022). In addition, myostatin knock-out chickens showing a rapid growth rate exhibited heavier body weight and greater myofiber CSA of the semitendinosus muscle than wild-type chickens with a slower growth rate (Kim et al., 2020). Similar to these results, in this study, broilers showing a heavier body weight had a greater total number and CSA of myofibers of the PM and GM muscles compared to layers showing a lighter body weight. This suggests that greater PMW and GMW of broilers could have resulted from both myofiber hyperplasia and hypertrophy.
A muscle fascicle is a bundle of different numbers of myofibers surrounded by connective tissue (Albrecht et al., 2006). The bundle characteristics, especially bundle size and fiber number per bundle, are related to muscle growth and meat quality of cattle (Albrecht et al., 2006; Choi et al., 2019). Clear differences in the number and size of muscle bundles and myofiber number per bundle were observed among the cattle breeds that have different muscle characteristics (Albrecht et al., 2006). In our previous study, we reported that a specific line of broiler chickens had a larger bundle CSA of PM muscle with myofiber hypertrophy rather than myofiber number per bundle than a specific line of layers (Kim et al., 2022). As total bundle numbers have not been investigated for both PM and GM muscles of avian species, this is the first study reporting bundle characteristics, including number, size, and myofiber number per bundle, of both PM and GM muscles of broiler and layer chickens. Broilers used in this study had a greater number and CSA of both the muscles, and PM muscle bundles of broilers showed a greater number of myofibers than those of layers (p < 0.05). Therefore, PM muscle bundles of broilers are characterized by a greater number and size of muscle bundles with a higher number of myofibers per bundle due to myofiber hyperplasia and hypertrophy. Greater GM muscle mass of broilers is caused by the greater number and size of muscle bundles rather than myofiber number per bundle.
Taken together, broiler and layer chickens show clear differences in histological characteristics of myofibers and muscle bundles of PM and GM muscles. These findings support that fast-growing broilers have greater muscle mass due to both hyperplasia and hypertrophy of myofibers and muscle bundles. Further investigations are needed to identify factors regulating the size and number of muscle bundles and to expand the possible influence of muscle bundles on the understanding of poultry meat quality.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The animal study was approved by The Ohio State University Institutional Animal Care and Use Committee (protocol no. 2020A00000094). The study was conducted in accordance with the local legislation and institutional requirements.
BL: investigation, methodology, visualization, and writing–original draft. D-HK: conceptualization, formal analysis, methodology, validation, visualization, and writing–original draft. JL: conceptualization, investigation, methodology, validation, and writing–original draft. MC: methodology, resources, and writing–review and editing. YC: investigation, methodology, resources, and writing–review and editing. KL: conceptualization, funding acquisition, project administration, supervision, validation, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the United States Department of Agriculture National Institute of Food and Agriculture Grant (project no. 2022-67015-36482).
The authors are grateful to Michelle Milligan for her invaluable assistance in proofreading this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Albrecht E., Teuscher F., Ender K., Wegner J. (2006). Growth- and breed-related changes of muscle bundle structure in cattle. J. Anim. Sci. 84, 2959–2964. doi:10.2527/jas.2006-345
Buzala M., Janicki B. (2016). Review: effects of different growth rates in broiler breeder and layer hens on some productive traits. Poult. Sci. 95, 2151–2159. doi:10.3382/ps/pew173
Choi Y. M., Sarah D., Shin S., Wick M. P., Kim B. C., Lee K. (2013a). Comparative growth performance in different Japanese quail lines: the effect of muscle DNA content and fiber morphology. Poult. Sci. 92, 1870–1877. doi:10.3382/ps.2012-02892
Chandraratne M. R., Samarasinghe S., Kulasiri D., Bickerstaffe R. (2006). Prediction of lamb tenderness using image surface texture features. J. Food Eng. 77, 492–499. doi:10.1016/j.jfoodeng.2005.06.063
Choi Y. M., Garcia L. G., Lee K. (2019). Correlations of sensory quality characteristics with intramuscular fat content and bundle characteristics in bovine longissimus thoracis muscle. Food Sci. Anim. Resour. 39, 197–208. doi:10.5851/kosfa.2019.e15
Choi Y. M., Kim B. C. (2009). Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 122, 105–118. doi:10.1016/j.livsci.2008.08.015
Choi Y. M., Shin S., Wick M. P., Choe J. H., Lee K. (2013b). Muscle fiber characteristics of pectoralis major muscle as related to muscle mass in different Japanese quail lines. Animal 7, 1665–1670. doi:10.1017/S1751731113001298
Choi Y. M., Suh Y., Shin S., Lee K. (2014). Skeletal muscle characterization of Japanese quail line selectively bred for lower body weight as an avian model of delayed muscle growth with hypoplasia. PLoS One 9, e95932. doi:10.1371/journal.pone.0095932
Huo W., Weng K., Gu T., Zhang Y., Zhang Y., Chen G., et al. (2021). Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 100, 101264. doi:10.1016/j.psj.2021.101264
Kiessling K. H. (1977). Muscle structure and function in the goose, quail, pheasant, Guinea hen, and chicken. Comp. Biochem. Physiol. B 57, 287–292. doi:10.1016/0305-0491(77)90055-4
Kim D.-H., Choi Y. M., Lee J., Shin S., Kim S., Suh Y., et al. (2022). Differential expression of MSTN isoforms in muscle between broiler and layer chickens. Animals 12, 539. doi:10.3390/ani12050539
Kim G. D., Lee J. H., Song S., Kim S. W., Han J. S., Shin S. P., et al. (2020). Generation of myostatin-knockout chickens mediated by D10A-Cas9 nickase. FASEB J. 34, 5688–5696. doi:10.1096/fj.201903035R
Kokoszyński D., Saleh M., Bernacki Z., Kotowicz M., Sobczak M., Żochowska-Kujawska J., et al. (2018). Digestive tract morphometry and breast muscle microstructure in spent breeder ducks maintained in a conservation programme of genetic resources. Arch. Anim. Breed. 61, 373–378. doi:10.5194/aab-61-373-2018
Kokoszyński D., Żochowska-Kujawska J., Kotowicz M., Skoneczny G., Kostenko S., Włodarczyk K., et al. (2022). The composition of the carcass, physicochemical properties, texture and microstructure of the meat of D11 dworka and P9 pekin ducks. Anim. open access J. MDPI 12, 1714. doi:10.3390/ani12131714
Lee Y., Lee B., Kim H. K., Yun Y. K., Kang S. J., Kim K. T., et al. (2018). Sensory quality characteristics with different beef quality grades and surface texture features assessed by dented area and firmness, and the relation to muscle fiber and bundle characteristics. Meat Sci. 145, 195–201. doi:10.1016/j.meatsci.2018.06.034
Scheuermann G. N., Bilgili S. F., Tuzun S., Mulvaney D. R. (2004). Comparison of chicken genotypes: myofiber number in pectoralis muscle and myostatin ontogeny. Poult. Sci. 83, 1404–1412. doi:10.1093/ps/83.8.1404
Keywords: broiler, layer, muscle hyperplasia, muscle hypertrophy, muscle bundle
Citation: Lee B, Kim D-H, Lee J, Cressman MD, Choi YM and Lee K (2023) Greater numbers and sizes of muscle bundles in the breast and leg muscles of broilers compared to layer chickens. Front. Physiol. 14:1285938. doi: 10.3389/fphys.2023.1285938
Received: 30 August 2023; Accepted: 26 September 2023;
Published: 09 October 2023.
Edited by:
Yuwares Malila, National Center for Genetic Engineering and Biotechnology (BIOTEC), ThailandReviewed by:
Alejandro Bielli, University of the Republic, UruguayCopyright © 2023 Lee, Kim, Lee, Cressman, Choi and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kichoon Lee, bGVlLjI2MjZAb3N1LmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.