- 1Department of Cardiovascular Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Acupuncture and Moxibustion School of Teaching, Hospital of Chengdu, University of Traditional Chinese Medicine, Tianjin, China
- 3Key Laboratory of Emergency and Trauma, Ministry of Education, Hainan Medical University, Haikou, China
Colchicine is a widely used drug that was originally used to treat gout and rheumatic diseases. In recent years, colchicine has shown high potential in the cardiovascular field. Atrial fibrillation (AF) is a cardiovascular disease with a high incidence. One of the most frequent complications following cardiovascular surgery is postoperative atrial fibrillation (POAF), which affects patient health and disease burden. This article reviews the research status of colchicine in AF and summarizes the relevant progress.
1 Introduction
Colchicine is an ancient drug. It has been extensively used to treat gout and rheumatic diseases for centuries (Roubille et al., 2013). In recent years, colchicine has become a good candidate drug for cardiac protection and plays a variety of roles, including inhibiting arrhythmia (Imazio and Nidorf, 2021). Approximately one-quarter of adults may have Atrial fibrillation (AF) during their lifetime (Baman and Passman, 2021). One of the most frequent complications of cardiovascular surgery is POAF, which can result in heart failure and stroke (Echahidi et al., 2008). This article reviews the literature on colchicine and AF and summarizes the potential mechanism of its application in AF.
2 Background and pharmacology of colchicine
Colchicine is a microtubule disassembling agent that is extracted from cum autumnale and has anti-inflammatory properties (Deftereos et al., 2013a; Roubille et al., 2013). It is a widely accessible, reasonably priced medicine with good safety and few adverse drug reactions (Aimo et al., 2021a). Colchicine can inhibit microtubule polymerization and mitosis, stop neutrophils from becoming activated, degranulating, and migrating, and have anti-inflammatory effects (Finkelstein et al., 2010). Colchicine is frequently used to treat and prevent gout and rheumatic diseases, Behcet’s disease, pericarditis and familial Mediterranean fever (FMF) (Terkeltaub, 2009).
The bioavailability of colchicine is approximately 44%, and it is absorbed through the digestive system and reaches its highest plasma concentration in less than 1 h (Deftereos et al., 2013b). Colchicine binds to albumin and undergoes glucuronidation and cytochrome CYP3A4 metabolism in the liver (Hemkens et al., 2016). Approximately 10%–20% of the drug is cleared by P-glycoprotein (P-gp) transporters in the kidney (Hemkens et al., 2016). Therefore, P-glycoprotein inhibitors or potent CYP3A4 inhibitors cannot be used in conjunction with colchicine. The half-life of colchicine in healthy volunteers is 27–31 h (Pascart and Richette, 2018). After the termination of treatment, the biological effect lasts 24–48 h, and the final elimination half-life is 16 h. The half-life might be two or three times longer in people with renal or hepatic dysfunction (Phelps, 1970).
Symptoms of the digestive system, including nausea, vomiting, and diarrhoea, are the most frequent adverse effects of colchicine (Abrantes et al., 2021). Excessive serum concentrations may lead to serious adverse reactions such as muscle toxicity or cytopenia (Pascart and Richette, 2018). Currently, numerous studies have demonstrated the safety of administering low-dose colchicine over an extended period of time in patients with healthy liver and kidney function or long-term use of 0.5–1.0 mg colchicine in patients with FMF, gout, pericarditis and coronary heart disease (Ozen et al., 2016; Imazio et al., 2020; Nidorf et al., 2020; Sammaritano et al., 2020; Andreis et al., 2021a). However, a few small case reports have reported the toxicity of colchicine, such as neuromyopathy (Altiparmak et al., 2002). Therefore, colchicine toxicity should be determined even in patients receiving long-term standard doses of colchicine. At present, a specific antidote of colchicine has been developed for use in preclinical trials (Eddleston et al., 2018). In addition, colchicine is lipophilic, can cross the placenta and is eliminated in breast milk; however, research has shown that taking colchicine while pregnant has no impact on the risk of miscarriage or foetal deformity (Brucato et al., 2019).
3 Use of colchicine in cardiovascular disease
Colchicine was first used 40 years ago to demonstrate the role of antimicrotubule proteins as anti-atherosclerotic agents, and since then, LoDoCo has been proposed for the treatment and prevention of recurrent pericarditis initially (Chaldakov, 1982; Chaldakov, 2018; Andreis et al., 2021b). The unique properties of colchicine give it remarkable potential in cardiovascular diseases such as pericardial syndrome recurrence, atherosclerotic plaque inflammation, atrial fibrillation recurrence and ventricular remodelling (Andreis et al., 2021a). With continued research progress, colchicine has been used to prevent coronary atherosclerosis, POAF, and heart failure (Imazio and Nidorf, 2021). A meta-analysis of 22 studies determined the effectiveness and safety of a low dose (0.5 mg daily) of colchicine given for a long period of time (greater than 6 months) to treat cardiac disease (Schattner, 2022). This study further confirmed the role of colchicine in reducing the risk of arrhythmia recurrence, recurrent pericarditis, cardiogenic death, stroke, coronary revascularization or hospitalization, and myocardial infarction (Schattner, 2022). Schattner et al. systematically reviewed and summarized 126 RCTs published in the past 20 years. These studies supported the anti-inflammatory effects of colchicine in the treatment and prevention of acute pericarditis, postcardiac injury syndrome after cardiac surgery, POAF, acute coronary syndrome and stable coronary artery disease (Guindo et al., 1990). Furthermore, most studies have shown that in addition to diarrhoea and myalgia, which may lead to drug withdrawal, there are almost no serious adverse events related to colchicine, which confirms the safety of LoDoCo (Guindo et al., 1990).
Colchicine is a second-line therapeutic option for pericarditis (Dainese et al., 2011; Deftereos et al., 2013b). Adding colchicine to standard treatments can reduce the recurrence rate of patients with pericarditis. Additionally, colchicine can be used to treat postoperative recurrent pericardial effusion and reduce the incidence of postoperative pericardial incision syndrome (Imazio et al., 2013a; Verma et al., 2015; Imazio and Nidorf, 2021). Colchicine decreased the incidence of recurrent pericarditis, pericardial incision syndrome following surgery and perioperative AF in an RCT by Verma et al. (Imazio et al., 2014). The COPPS-2 study by Imazio et al. obtained the same conclusion in terms of postoperative pericardial incision syndrome, but the incidence of POAF or postoperative pericardial/pleural effusion was not significantly decreased by colchicine (Robinson et al., 2022).
Long-term LoDoCo can be used for secondary prevention of ischaemic stroke and myocardial infarction (Tardif et al., 2019). The COLchicine Cardiovascular Outcomes Trial (COLCOT) of 4,745 patients demonstrated that a daily dose of 0.5 mg of colchicine to treat patients with myocardial infarction within 30 days could effectively reduce ischaemic cardiovascular events (Bo et al., 2020). Using LoDoCo as soon as possible after myocardial infarction can significantly reduce the incidence of ischaemic cardiovascular events (Hennessy et al., 2019). Studies have also demonstrated that low-dose colchicine was safe and tolerable after acute myocardial infarction (Deftereos et al., 2015). Colchicine has been proven in systematic reviews to lower the risk of myocardial infarction, but it has no discernible impact on all-cause mortality (Hemkens et al., 2016). In patients with myocardial infarction, colchicine can decrease the infarct area and the concentration of the creatine kinase muscle-brain (CK-MB) fraction in cardiac MRI (Bresson et al., 2021; Li et al., 2022). Furthermore, colchicine can inhibit cardiac inflammation after acute myocardial infarction and reduce cardiac remodelling (Sgouropoulou et al., 2019). Low-dose colchicine treatment, however, did not appreciably lower C-reactive protein levels within 30 days of acute myocardial infarction (Deftereos et al., 2015). Studies have shown that the cardiovascular effect of colchicine may not be related to its anti-inflammatory effect (Kukuy et al., 2017; Grajek et al., 2021).
A number of meta-analyses have demonstrated the positive effects of LoDoCo on the prognosis of patients with coronary heart disease, such as a significant reduction in cardiovascular mortality, myocardial infarction, and stroke (Samuel et al., 2021a; Samuel et al., 2021b; Xia et al., 2021; Ma et al., 2022). The systematic review by Samuel et al. also supported this result (Fiolet et al., 2021). However, after administering colchicine, there was no discernible difference in all-cause mortality (Nidorf and Thompson, 2007). Colchicine has been associated with a reduction in inflammation in people with stable coronary disease and has been proven to have antiatherosclerotic properties (Wójcicki et al., 1986; Akrami et al., 2021).
Taken regularly, low-dose colchicine can greatly improve the survival rates of individuals with acute and chronic coronary syndrome and reduce the frequency of serious adverse cardiac events (Giannopoulos et al., 2015; Imazio et al., 2020). The overall safety is satisfactory, and its therapeutic and preventive value for chronic coronary artery disease has been recognized to some extent (Aimo et al., 2021b). A meta-analysis that included 12 studies confirmed its effects. Colchicine decreased the risk of cardiovascular events in CCS patients by approximately 50% and the risk of ACS by 23% compared to the placebo (Yasgur, 2023). Not long ago a new pilot study demonstrated that colchicine could be added the day after PCI to reduce a patient’s risk of ischemic events and to mitigate the increased risk of aspirin-related bleeding (Papageorgiou et al., 2017).
However, the frequent complications such as gastrointestinal reactions caused by colchicine treatment often leads to withdrawal from the study, especially in postoperative patients (Salih et al., 2017; Andreis et al., 2021c; Robinson et al., 2022). The efficacy of colchicine is significantly affected by the interruption rate of treatment. A study confirmed that using colchicine to treat cardiovascular disease increased the risk of adverse gastrointestinal reactions and myalgia but has no significant relationship with other adverse reactions (Frommeyer et al., 2017). In addition, acute infusion of colchicine can increase the incidence of ventricular tachyarrhythmias in cardiac models (Kirchhof et al., 2016).
In summary, colchicine has been shown to prevent cardiovascular disease, but before routine use of colchicine in practice, more research is required to further explore the effectiveness and safety of colchicine and to find appropriate concomitant therapies to improve and reduce gastrointestinal complications.
4 Application and research of colchicine in AF
AF is a common arrhythmia with a prevalence of approximately 3% (Brundel et al., 2022). AF causes rapid irregular abnormal contraction of atrial myocardial cells, which leads to palpitations, dizziness and other symptoms (Zimetbaum, 2017). The most serious consequences of AF are heart failure and stroke (Baman and Passman, 2021). A number of variables, including age, smoking, obesity, hypertension, and diabetes, are linked to the occurrence of AF (Fuster et al., 2011). The main pathogenesis of AF is affected by many factors, such as blood pressure, atrial remodelling, increased autonomic nervous tension, inflammation, oxidative stress, and atrial electrical abnormalities (Frendl et al., 2014).
The use of colchicine to treat AF has been focused on the prevention of POAF. With an incidence of 20%–50%, POAF is the most prevalent arrhythmia after cardiovascular surgery (Echahidi et al., 2008; Haghjoo et al., 2012; Yadava et al., 2014). Definition, monitoring duration and technical methods can significantly affect the detection rate. POAF is generally considered to be a self-limiting disease, but data show that the occurrence of POAF is related to the incidence of stroke, acute renal infarction, congestive heart failure, and death (Raiten et al., 2015). As the population has aged over the past few years, there has been an increase in the number of elderly patients undergoing cardiac surgery, and the hospitalization time and medical burden of patients can increase (Imazio et al., 2011; Rezaei et al., 2020).
Age, race, and severe cardiovascular risk factors may contribute to POAF (Ishii et al., 2005). In addition, perioperative factors can also lead to POAF, such as pericarditis, autonomic nerve imbalance, catecholamine overdose or fluid transfer (Abdelhadi et al., 2004; Davis et al., 2010), which can change the atrial refractory period and create an environment that triggers the occurrence of AF (Echahidi et al., 2008; January et al., 2014; Rezaei et al., 2020).
At present, the main drugs used to prevent AF include β-blockers, antiarrhythmic drugs, cardiac glycosides and a variety of anti-inflammatory drugs. Generally, β-blockers are the first choice, and preoperative amiodarone can effectively lower the occurrence of AF. In the ACC/AHA guidelines, colchicine is recommended in Class IIb (Lu et al., 2016; Janu et al., 2019).
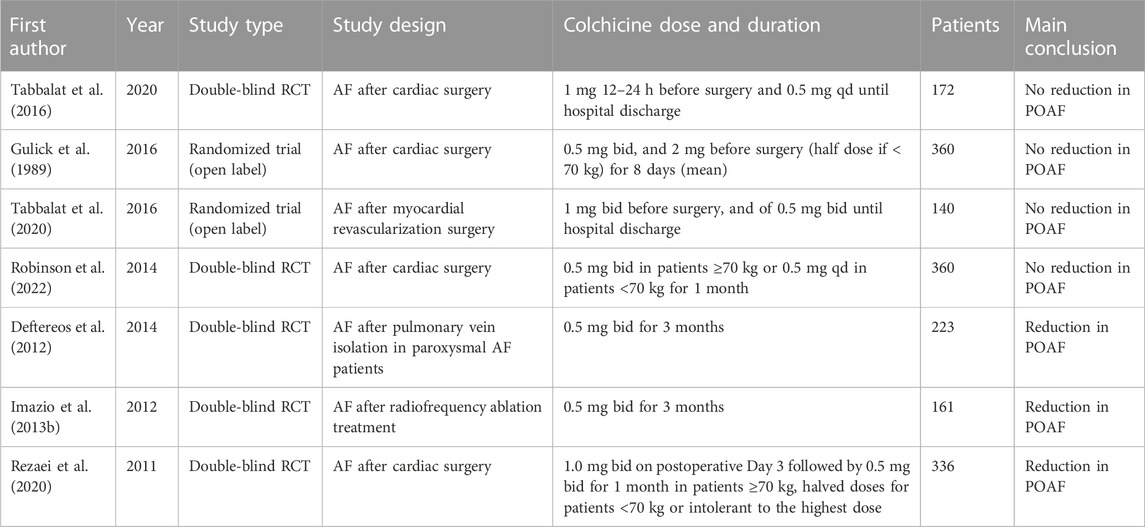
The recurrence of AF may be caused by atrial inflammation or atrial remodelling (Koyama et al., 2009; L et al., 2016). The role of colchicine in avoiding AF after cardiac surgery and reducing hospitalization time has been confirmed to some extent (Deftereos et al., 2014; Trivedi and Sadadia, 2014; Rezaei et al., 2020). The data showed that the use of colchicine for 3 months after ablation could reduce the possibility of AF recurrence by 37% after 15 months (Deftereos et al., 2012; Imazio et al., 2013b). Colchicine could considerably lower the incidence of postoperative pericardial effusion and POAF in the COPPS test and COPPS-POAF substudy (Rezaei et al., 2020; Shvartz et al., 2022a; Robinson et al., 2022). A double-blind RCT involving 240 subjects showed that short-term administration of colchicine after cardiac surgery significantly prevented POAF and systemic inflammation, but the incidence of diarrhoea and abdominal pain increased significantly (Zhao et al., 2022). A meta-analysis showed that colchicine could prevent the occurrence of POAF, its efficacy had no significant relationship with the prolongation of treatment time, and there were fewer adverse reactions (Lennerz et al., 2017). Another meta-analysis obtained similar results, but the use of colchicine significantly increased adverse gastrointestinal reactions (Ge et al., 2022). A number of meta-analyses have noted that colchicine could effectively prevent the occurrence of AF. Although it does not increase the incidence of major adverse events, it increases the likelihood of gastrointestinal side effects such as diarrhoea and abdominal pain. These side effects may lead to the interruption of treatment so that a complete therapeutic effect cannot be achieved. Therefore, further research on the dose and duration of colchicine is needed (Andreis et al., 2021c; Shvartz et al., 2022b; Kommu and Arepally, 2023). Some studies have shown that the prevalence of POAF may not be significantly impacted by colchicine because of the limited sample size (Zarpelon et al., 2016; Tabbalat et al., 2020). Clinical studies on the prevention of AF by colchicine in recent years are shown in Table 1. At present, there are 27 ongoing clinical trials for the use of colchicine in AF, of which 8 are under recruitment. The efficacy and safety of colchicine are expected to be further confirmed in the future.
5 Mechanism of colchicine in AF
5.1 Anti-inflammatory effects
Inflammation is one of the important pathogeneses of AF, and it is closely related to an increase in white blood cells (Davis et al., 2010). Inflammatory mediators play an important role in AF. Through different mechanisms, IL-6 can control cardiovascular function and alter the responsiveness of adrenergic receptors (Pagani et al., 1992), promote left ventricular remodelling (Yokoyama et al., 1993) or lead to myocardial systolic dysfunction (Wan and Yim, 1999). IL-8 can activate leukocytes and neutrophils (Chandrasekar et al., 1999), activate the proapoptotic pathway in endothelial cells, and continuously increase heart damage (Liu et al., 2007). Chronic inflammation, including elevated serum levels of C-reactive protein, IL-1β, IL-6, and tumour necrosis factor (TNF), can contribute to the development and maintenance of AF (Chappey et al., 1993).
Colchicine can exert anti-inflammatory effects by binding to free tubulin dimers, which is the basic mechanism of colchicine (Imazio and Nidorf, 2021). Low concentrations of colchicine can prevent cytoplasmic microtubule polymerization, and LoDoCo have therapeutic effects and remain effective within a few days of administration (Leung et al., 2015). High concentrations of colchicine can promote microtubule depolymerization, thereby preventing immune cell activation and reducing the inflammatory response (Chaldakov and Vankov, 1986). Originally described in arterial smooth muscle cells, microtubules have been found to be closely associated with organelles involved in protein synthesis, particularly the Golgi apparatus, especially in vascular smooth muscle cells of the synthetic phenotype. (Bhattacharyya et al., 2008). Since then, there has been increasing evidence that microtubules are important components of many cytoskeletons, participate in intracellular transport activities, affect cytokine and chemokine release, and control ion channels and cell division (Andreu and Timasheff, 1982; Van Wagoner, 2011). Colchicine forms a soluble complex with tubulin with high affinity, which affects the formation of microtubules (Martinon et al., 2006; Van Wagoner, 2011).
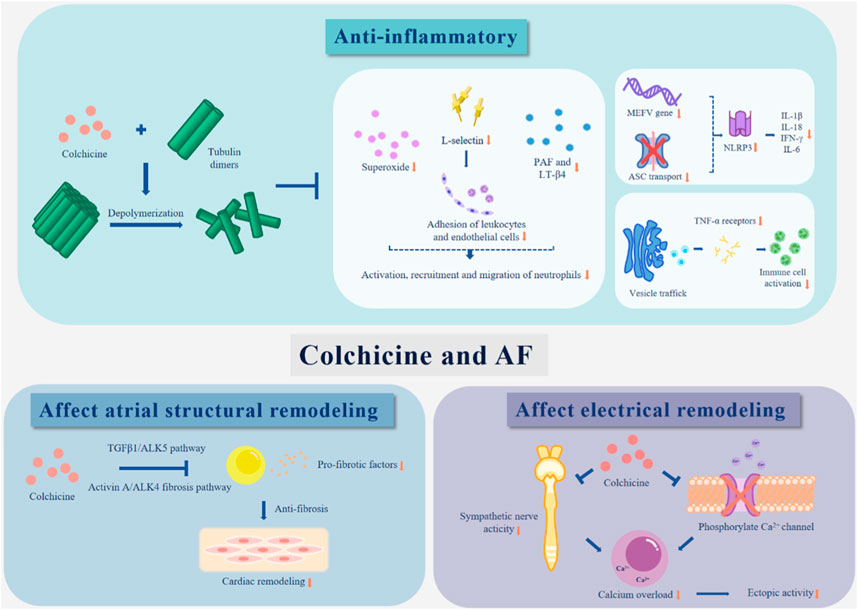
Thus, colchicine can directly reduce inflammation by blocking inflammatory signalling networks (Bonaventura and Montecucco, 2019). Colchicine mainly changes the adhesion of endothelial cells and leukocytes, thereby inhibiting the activation, recruitment and migration of neutrophils (Chia et al., 2008; Terkeltaub, 2009). Superoxide is an important factor in neutrophil activation, and colchicine inhibits superoxide production (Cronstein et al., 1995). Colchicine also reduces the expression of E-selectin in endothelial cells and L-selectin in neutrophils, which affects the recruitment of neutrophils (Asako et al., 1992). Furthermore, colchicine reduces platelet activating factor (PAF) and leukotriene-β4 (LT-β4), which weakens the induction of neutrophil adhesion and inhibits neutrophil migration (Dalbeth et al., 2014). In addition, colchicine attenuates vesicle trafficking, reduces the expression of TNF-α receptors on macrophages and blocks mast cell degranulation (Marques-da-Silva et al., 2011). Colchicine was shown to reduce the levels of the proinflammatory cytokines IL-1β, IFN-γ, IL-18, and IL-6 in vivo and in vitro (Martínez et al., 2018).
The NOD-like receptor pyrin containing domain 3 (NLRP3) inflammasome is associated with the antiinflammatory effects of colchicine. Colchicine affects the activity of NLRP3, which decreases the release of IL-1 and IL-18 (Yao et al., 2018). The impact of NLRP3 was amplified in the atrial cardiomyocytes of an AF model (Triantafilou et al., 2016). NLRP3 is a cytoplasmic complex present in neutrophils, monocytes, eosinophils and cardiomyocytes (Martinon et al., 2009; Buckley and Abbate, 2018). The NLRP3 inflammasome consists of the Toll-like receptor NLRP3, adaptor protein apoptosis-related spot-like protein (ASC) and the cysteine protease caspase-1 (Campbell et al., 2016). Inflammasome activation triggers an increase in the expression of inflammatory components, which in turn widely stimulates the activation of caspase-1 and produces the activated inflammatory factors interleukin-1β (IL-1β) and IL-18, which are important mediators of the inflammatory cascade (Schenone and Menon, 2018; D'Amario et al., 2021). By blocking activation of the NLRP3 inflammasome, colchicine blocks this activation process and inhibits the release of IL-1 and IL-18 (Martínez et al., 2015; Fiolet et al., 2020). Research has shown that short-term colchicine treatment in ACS patients significantly reduces the levels of inflammatory markers such as IL-1, IL-18, and IL-6 (Martinon et al., 2002; Bhattacharyya et al., 2008; Otani et al., 2016). According to recent research, colchicine can inhibit NLRP3 activation in the following ways. Colchicine can prevent the MEFV gene from being expressed, which prevents the production of NLRP3 (Li et al., 1999; Misawa et al., 2013; Nidorf et al., 2014). In addition, colchicine can inhibit pore formation, resulting in a decrease in intracellular K+, thereby reducing ROS and IL-1β levels (Martínez et al., 2018). Nidoret et al. found that colchicine decreased the risk of cholesterol crystallization and disrupted neutrophil function, thereby preventing related inflammatory responses (Misawa et al., 2013).
5.2 Colchicine affects atrial structural remodelling
Atrial fibrosis can slow the conduction of local areas, resulting in increased heterogeneity of conduction, which in turn contributes to arrhythmia (Goette et al., 2002). The mechanism and clinical importance of atrial structural remodelling have been supported by numerous investigations. Left atrial dilatation and left atrial fibrosis can lead to atrial structural remodelling. In AF patients, one of the main causes of cardiac remodelling is the development of myocardial fibrosis (Nikolaidis et al., 2006; Galea et al., 2014).
Previous studies have suggested that fibrosis in atherosclerosis and hypertension may be based on the coupling between the microtubule cytoskeleton and protein secretion, and people have begun to look for ways to control fibrosis using microtubules as an entry point (Bhattacharyya et al., 2008). Colchicine has potential as a drug that can control microtubule polymerization. The exact mechanism of the antifibrotic effect of colchicine is still unclear, but its effect has been confirmed in multiple models (Clare and Troughton, 2007; Wu et al., 2020). Colchicine prevents AF in SP rats by inhibiting atrial fibrosis (Rennard et al., 1988). In an in vivo model, colchicine exerted an indirect antifibrotic effect by inhibiting the release of profibrotic factors (Singhal et al., 2014). In a rabbit model of heart failure, colchicine reduced left atrial fibrosis (Yue et al., 2019). Studies have shown that colchicine has acute cardiac protective effects and a positive effect on cardiac haemodynamics. After the application of colchicine, left ventricular remodelling was reduced, and the commonly used parameters for evaluating left ventricular diameter (LVEF) were improved (Bresson et al., 2021). Transcriptomics analysis showed that the mechanism of colchicine involved a pathway closely related to myocardial fibrosis (Yue et al., 2022). Current research shows that colchicine can inhibit the TGFβ1/ALK5 pathway and activin A/ALK4 fibrosis pathways, thereby exerting an antifibrotic effect (Christ et al., 2004).
5.3 Colchicine affects electrical remodelling
AF is related to the electrical remodelling of cardiac ion channels, which include Ca2+ and K+ channels (Nattel et al., 2007; Nattel et al., 2020). AF is caused by ectopic activity, which is the spontaneous depolarization of atrial tissue away from the sinus node (Zimetbaum, 2017). Studies have confirmed that its main mechanism involves changing the function of the ryanodine receptor 2 (RyR2) Ca2+ release channel and ion channel so that the diastolic sarcoplasmic reticulum (SR) releases more Ca2+, thereby shortening the duration of the action potential (Workman et al., 2006). Ion channels also play a role in POAF. According to previous studies, people with POAF had more L-type Ca2+ channels in their atrial cardiomyocytes, which is indicative of the role excess calcium plays in AF (Van Wagoner et al., 1999; Swartz et al., 2009). In POAF patients and non-POAF patients, K+ channel transcripts were not significantly changed (Kerfant et al., 2001).
Colchicine directly regulates calcium (Ca2+) homeostasis in cardiomyocytes (137). Colchicine can weaken sympathetic nerve activity and responses; furthermore, it can phosphorylate Ca2+ ion channels, which can reduce calcium overload, reduce ectopic activity, and thus reduce the possibility of inducing AF (Andreu and Timasheff, 1982).
6 Future prospects
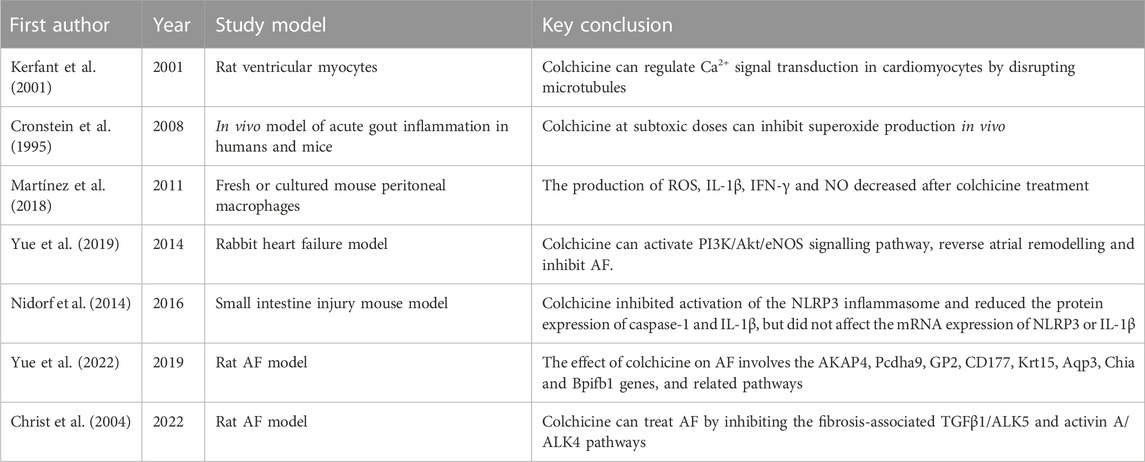
Colchicine has good application prospects in clinical practice. Its preventive and therapeutic effects on AF have been found in many studies. This article summarized the possible mechanisms of colchicine in the treatment of AF (Table 2; Figure 1), but its specific mechanism has not been clearly determined and needs further study. Furthermore, considering that there are many cases of drug withdrawal due to adverse reactions in clinical trials, future research is required to determine the timing and dose of colchicine that will have the greatest effectiveness and tolerance to further improve the medication regimen. In addition, colchicine has been examined for its ability to prevent AF in people who have undergone cardiac surgery. Future confirmatory studies on the efficacy, safety, and precise timing of colchicine treatment to avoid POAF are urgently needed. In summary, more exploration is needed before colchicine is fully applied in clinical practice.
Author contributions
YZ: Validation, Visualization, Writing–original draft, Writing–review and editing. HY: Funding acquisition, Supervision, Validation, Writing–original draft, Writing–review and editing. XZ: Supervision, Writing–review and editing. JT: Writing–review and editing. ZW: Supervision, Validation, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China, Grant number 82370319; the National Natural Science Foundation of China, Grant number 82172060; Department of Science and Technology of Sichuan Province, Grant numbers 2022YFS0362 and 2022YFS0363; Post-Doctor Research Project, West China Hospital, Sichuan University, Grant number 2021HXBH070; and Key Laboratory of Emergency and Trauma of Ministry of Education (Hainan Medical University), Grant number KLET-202114.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhadi R. H., Gurm H. S., Van Wagoner D. R., Chung M. K. (2004). Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am. J. Cardiol. 93 (9), 1176–1178. doi:10.1016/j.amjcard.2004.01.053
Abrantes A. M., Nogueira-Garcia B., Alves M., Teixeira Passos D., Brito D., Pinto F. J., et al. (2021). Low-Dose Colchicine in Coronary Artery Disease - Systematic Review and Meta-Analysis. Circ. Rep. 3 (8), 457–464. doi:10.1253/circrep.CR-21-0065
Aimo A., Pascual Figal D. A., Bayes-Genis A., Emdin M., Georgiopoulos G. (2021b). Effect of low-dose colchicine in acute and chronic coronary syndromes: A systematic review and meta-analysis. Eur. J. Clin. Invest. 51 (4), e13464. doi:10.1111/eci.13464
Aimo A., Pascual-Figal D. A., Barison A., Cediel G., Vicente Á H., Saccaro L. F., et al. (2021a). Colchicine for the treatment of coronary artery disease. Trends Cardiovasc Med. 31 (8), 497–504. doi:10.1016/j.tcm.2020.10.007
Akrami M., Izadpanah P., Bazrafshan M., Hatamipour U., Nouraein N., Drissi H. B., et al. (2021). Effects of colchicine on major adverse cardiac events in next 6-month period after acute coronary syndrome occurrence; a randomized placebo-control trial. BMC Cardiovasc Disord. 21 (1), 583. doi:10.1186/s12872-021-02393-9
Altiparmak M. R., Pamuk O. N., Pamuk G. E., Hamuryudan V., Ataman R., Serdengecti K. (2002). Colchicine neuromyopathy: a report of six cases. Clin. Exp. Rheumatol. 20 (4), S13–S16.
Andreis A., Imazio M., Avondo S., Casula M., Paneva E., Piroli F., et al. (2021c). Adverse events of colchicine for cardiovascular diseases: a comprehensive meta-analysis of 14 188 patients from 21 randomized controlled trials. J. Cardiovasc Med. Hagerst. 22 (8), 637–644. doi:10.2459/JCM.0000000000001157
Andreis A., Imazio M., Casula M., Avondo S., De Ferrari G. M. (2021b). Colchicine efficacy and safety for the treatment of cardiovascular diseases. Intern Emerg. Med. 16 (6), 1691–1700. doi:10.1007/s11739-021-02654-7
Andreis A., Imazio M., De Ferrari G. M. (2021a). Colchicine for the treatment of cardiovascular diseases: old drug, new targets. J. Cardiovasc Med. Hagerst. 22 (1), 1–8. doi:10.2459/JCM.0000000000001079
Andreu J. M., Timasheff S. N. (1982). Tubulin bound to colchicine forms polymers different from microtubules. Proc. Natl. Acad. Sci. U. S. A. 79 (22), 6753–6756. doi:10.1073/pnas.79.22.6753
Asako H., Kubes P., Baethge B. A., Wolf R. E., Granger D. N. (1992). Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation 16 (1), 45–56. doi:10.1007/BF00917514
Baman J. R., Passman R. S. (2021). Atrial Fibrillation. Jama 325 (21), 2218. doi:10.1001/jama.2020.23700
Bhattacharyya B., Panda D., Gupta S., Banerjee M. (2008). Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 28 (1), 155–183. doi:10.1002/med.20097
Bouabdallaoui N., Tardif J. C., Waters D. D., Pinto F. J., Maggioni A. P., Diaz R., et al. (2020). Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 41 (42), 4092–4099. doi:10.1093/eurheartj/ehaa659
Bonaventura A., Montecucco F. (2019). Inflammation and pericarditis: are neutrophils actors behind the scenes? J. Cell Physiol. 234 (5), 5390–5398. doi:10.1002/jcp.27436
Bresson D., Roubille F., Prieur C., Biere L., Ivanes F., Bouleti C., et al. (2021). Colchicine for Left Ventricular Infarct Size Reduction in Acute Myocardial Infarction: A Phase II, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Study Protocol - The COVERT-MI Study. Cardiology 146 (2), 151–160. doi:10.1159/000512772
Brucato A., Pluymaekers N., Tombetti E., Rampello S., Maestroni S., Lucianetti M., et al. (2019). Management of idiopathic recurrent pericarditis during pregnancy. Int. J. Cardiol. 282, 60–65. doi:10.1016/j.ijcard.2019.02.003
Brundel B., Ai X., Hills M. T., Kuipers M. F., Lip G. Y. H., de Groot N. M. S. (2022). Atrial fibrillation. Nat. Rev. Dis. Prim. 8 (1), 21. doi:10.1038/s41572-022-00347-9
Buckley L. F., Abbate A. (2018). Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur. Heart J. 39 (22), 2063–2069. doi:10.1093/eurheartj/ehy128
Campbell L., Raheem I., Malemud C. J., Askari A. D. (2016). The Relationship between NALP3 and Autoinflammatory Syndromes. Int. J. Mol. Sci. 17 (5), 725. doi:10.3390/ijms17050725
Chaldakov G. N. (1982). Antitubulins-a new therapeutic approach for atherosclerosis? Atherosclerosis 44 (3), 385–390. doi:10.1016/0021-9150(82)90013-2
Chaldakov G. N. (2018). Colchicine, a microtubule-disassembling drug, in the therapy of cardiovascular diseases. Cell Biol. Int. 42 (8), 1079–1084. doi:10.1002/cbin.10988
Chaldakov G. N., Vankov V. N. (1986). Morphological aspects of secretion in the arterial smooth muscle cell, with special reference to the Golgi complex and microtubular cytoskeleton. Atherosclerosis 61 (3), 175–192. doi:10.1016/0021-9150(86)90137-1
Chandrasekar B., Mitchell D. H., Colston J. T., Freeman G. L. (1999). Regulation of CCAAT/Enhancer binding protein, interleukin-6, interleukin-6 receptor, and gp130 expression during myocardial ischemia/reperfusion. Circulation 99 (3), 427–433. doi:10.1161/01.cir.99.3.427
Chappey O. N., Niel E., Wautier J. L., Hung P. P., Dervichian M., Cattan D., et al. (1993). Colchicine disposition in human leukocytes after single and multiple oral administration. Clin. Pharmacol. Ther. 54 (4), 360–367. doi:10.1038/clpt.1993.161
Chia E. W., Grainger R., Harper J. L. (2008). Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: a rationale for use of low-dose colchicine. Br. J. Pharmacol. 153 (6), 1288–1295. doi:10.1038/bjp.2008.20
Christ T., Boknik P., Wöhrl S., Wettwer E., Graf E. M., Bosch R. F., et al. (2004). L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 110 (17), 2651–2657. doi:10.1161/01.CIR.0000145659.80212.6A
Clare G. C., Troughton R. W. (2007). Management of constrictive pericarditis in the 21st century. Curr. Treat. Options Cardiovasc Med. 9 (6), 436–442. doi:10.1007/s11936-007-0038-x
Cronstein B. N., Molad Y., Reibman J., Balakhane E., Levin R. I., Weissmann G. (1995). Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Invest. 96 (2), 994–1002. doi:10.1172/JCI118147
D'Amario D., Cappetta D., Cappannoli L., Princi G., Migliaro S., Diana G., et al. (2021). Colchicine in ischemic heart disease: the good, the bad and the ugly. Clin. Res. Cardiol. 110 (10), 1531–1542. doi:10.1007/s00392-021-01828-9
Dainese L., Cappai A., Biglioli P. (2011). Recurrent pericardial effusion after cardiac surgery: the use of colchicine after recalcitrant conventional therapy. J. Cardiothorac. Surg. 6, 96. doi:10.1186/1749-8090-6-96
Dalbeth N., Lauterio T. J., Wolfe H. R. (2014). Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 36 (10), 1465–1479. doi:10.1016/j.clinthera.2014.07.017
Davis E. M., Packard K. A., Hilleman D. E. (2010). Pharmacologic prophylaxis of postoperative atrial fibrillation in patients undergoing cardiac surgery: beyond beta-blockers. Pharmacotherapy 30 (7), 274e–318e. doi:10.1592/phco.30.7.749
Deftereos S., Giannopoulos G., Papoutsidakis N., Panagopoulou V., Kossyvakis C., Raisakis K., et al. (2013b). Colchicine and the heart: pushing the envelope. J. Am. Coll. Cardiol. 62 (20), 1817–1825. doi:10.1016/j.jacc.2013.08.726
Deftereos S., Giannopoulos G., Angelidis C., Alexopoulos N., Filippatos G., Papoutsidakis N., et al. (2015). Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation 132 (15), 1395–1403. doi:10.1161/CIRCULATIONAHA.115.017611
Deftereos S., Giannopoulos G., Efremidis M., Kossyvakis C., Katsivas A., Panagopoulou V., et al. (2014). Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart rhythm. 11 (4), 620–628. doi:10.1016/j.hrthm.2014.02.002
Deftereos S., Giannopoulos G., Kossyvakis C., Efremidis M., Panagopoulou V., Kaoukis A., et al. (2012). Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J. Am. Coll. Cardiol. 60 (18), 1790–1796. doi:10.1016/j.jacc.2012.07.031
Deftereos S., Giannopoulos G., Raisakis K., Kossyvakis C., Kaoukis A., Panagopoulou V., et al. (2013a). Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J. Am. Coll. Cardiol. 61 (16), 1679–1685. doi:10.1016/j.jacc.2013.01.055
Echahidi N., Pibarot P., O'Hara G., Mathieu P. (2008). Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 51 (8), 793–801. doi:10.1016/j.jacc.2007.10.043
Eddleston M., Fabresse N., Thompson A., Al Abdulla I., Gregson R., King T., et al. (2018). Anti-colchicine Fab fragments prevent lethal colchicine toxicity in a porcine model: a pharmacokinetic and clinical study. Clin. Toxicol. (Phila). 56 (8), 773–781. doi:10.1080/15563650.2017.1422510
Finkelstein Y., Aks S. E., Hutson J. R., Juurlink D. N., Nguyen P., Dubnov-Raz G., et al. (2010). Colchicine poisoning: the dark side of an ancient drug. Clin. Toxicol. (Phila). 48 (5), 407–414. doi:10.3109/15563650.2010.495348
Fiolet A. T. L., Opstal T. S. J., Mosterd A., Eikelboom J. W., Jolly S. S., Keech A. C., et al. (2021). Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur. Heart J. 42 (28), 2765–2775. doi:10.1093/eurheartj/ehab115
Fiolet A. T. L., Silvis M. J. M., Opstal T. S. J., Bax W. A., van der Horst F. A. L., Mosterd A., et al. (2020). Short-term effect of low-dose colchicine on inflammatory biomarkers, lipids, blood count and renal function in chronic coronary artery disease and elevated high-sensitivity C-reactive protein. PLoS One 15 (8), e0237665. doi:10.1371/journal.pone.0237665
Frendl G., Sodickson A. C., Chung M. K., Waldo A. L., Gersh B. J., Tisdale J. E., et al. (2014). 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J. Thorac. Cardiovasc Surg. 148 (3), e153–e193. doi:10.1016/j.jtcvs.2014.06.036
Frommeyer G., Krawczyk J., Dechering D. G., Kochhäuser S., Leitz P., Fehr M., et al. (2017). Colchicine Increases Ventricular Vulnerability in an Experimental Whole-Heart Model. Basic Clin. Pharmacol. Toxicol. 120 (5), 505–508. doi:10.1111/bcpt.12702
Fuster V., Rydén L. E., Cannom D. S., Crijns H. J., Curtis A. B., Ellenbogen K. A., et al. (2011). 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 57 (11), e101–e198. doi:10.1016/j.jacc.2010.09.013
Galea R., Cardillo M. T., Caroli A., Marini M. G., Sonnino C., Narducci M. L., et al. (2014). Inflammation and C-reactive protein in atrial fibrillation: cause or effect? Tex Heart Inst. J. 41 (5), 461–468. doi:10.14503/THIJ-13-3466
Ge P., Fu Y., Su Q., Jin M., Guo L., Miao C., et al. (2022). Colchicine for prevention of post-operative atrial fibrillation: meta-analysis of randomized controlled trials. Front. Cardiovasc Med. 9, 1032116. doi:10.3389/fcvm.2022.1032116
Giannopoulos G., Angelidis C., Papoutsidakis N., Panagopoulou V., Cleman M. W., Lekakis J., et al. (2015). Colchicine in Coronary Artery Disease: an Old Acquaintance in New Attire? Curr. Med. Chem. 22 (36), 4177–4188. doi:10.2174/0929867322666151015120458
Goette A., Juenemann G., Peters B., Klein H. U., Roessner A., Huth C., et al. (2002). Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 54 (2), 390–396. doi:10.1016/s0008-6363(02)00251-1
Grajek S., Michalak M., Urbanowicz T., Olasińska-Wiśniewska A. (2021). A Meta-Analysis Evaluating the Colchicine Therapy in Patients With Coronary Artery Disease. Front. Cardiovasc Med. 8, 740896. doi:10.3389/fcvm.2021.740896
Guindo J., Rodriguez de la Serna A., Ramió J., de Miguel Diaz M. A., Subirana M. T., Perez Ayuso M. J., et al. (1990). Recurrent pericarditis. Relief with colchicine. Circulation 82 (4), 1117–1120. doi:10.1161/01.cir.82.4.1117
Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. (1989). Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc. Natl. Acad. Sci. U. S. A. 86 (17), 6753–6757. doi:10.1073/pnas.86.17.6753
Haghjoo M., Heidarali M., Nikfarjam S., Peighambari M., Alizadeh-Ghavidel A., Hosseini S., et al. (2012). Very late effects of postoperative atrial fibrillation on outcome of coronary artery bypass graft surgery. Res. Cardiovasc Med. 1 (1), 23–27. doi:10.5812/cardiovascmed.4584
Hemkens L. G., Ewald H., Gloy V. L., Arpagaus A., Olu K. K., Nidorf M., et al. (2016). Colchicine for prevention of cardiovascular events. Cochrane Database Syst. Rev. 2016 (1), Cd011047. doi:10.1002/14651858.CD011047.pub2
Hennessy T., Soh L., Bowman M., Kurup R., Schultz C., Patel S., et al. (2019). The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study: A pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am. Heart J. 215, 62–69. doi:10.1016/j.ahj.2019.06.003
Imazio M., Andreis A., Brucato A., Adler Y., De Ferrari G. M. (2020). Colchicine for acute and chronic coronary syndromes. Heart 106 (20), 1555–1560. doi:10.1136/heartjnl-2020-317108
Imazio M., Belli R., Brucato A., Ferrazzi P., Patrini D., Martinelli L., et al. (2013b). Rationale and design of the COlchicine for Prevention of the Post-pericardiotomy Syndrome and Post-operative Atrial Fibrillation (COPPS-2 trial): a randomized, placebo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative effusions, and postoperative atrial fibrillation. Am. Heart J. 166 (1), 13–19. doi:10.1016/j.ahj.2013.03.025
Imazio M., Brucato A., Cemin R., Ferrua S., Maggiolini S., Beqaraj F., et al. (2013a). A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 369 (16), 1522–1528. doi:10.1056/NEJMoa1208536
Imazio M., Brucato A., Ferrazzi P., Pullara A., Adler Y., Barosi A., et al. (2014). Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. Jama 312 (10), 1016–1023. doi:10.1001/jama.2014.11026
Imazio M., Brucato A., Ferrazzi P., Rovere M. E., Gandino A., Cemin R., et al. (2011). Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 124 (21), 2290–2295. doi:10.1161/CIRCULATIONAHA.111.026153
Imazio M., Nidorf M. (2021). Colchicine and the heart. Eur. Heart J. 42 (28), 2745–2760. doi:10.1093/eurheartj/ehab221
Ishii Y., Schuessler R. B., Gaynor S. L., Yamada K., Fu A. S., Boineau J. P., et al. (2005). Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation 111 (22), 2881–2888. doi:10.1161/CIRCULATIONAHA.104.475194
January C. T., Wann L. S., Calkins H., Chen L. Y., Cigarroa J. E., Cleveland J. C., et al. (2019). 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 140 (2), e125–e151. doi:10.1161/CIR.0000000000000665
January C. T., Wann L. S., Alpert J. S., Calkins H., Cigarroa J. E., Cleveland J. C., et al. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130 (23), 2071–2104. doi:10.1161/CIR.0000000000000040
Kerfant B. G., Vassort G., Gómez A. M. (2001). Microtubule disruption by colchicine reversibly enhances calcium signaling in intact rat cardiac myocytes. Circ. Res. 88 (7), E59–E65. doi:10.1161/hh0701.090462
Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., et al. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37 (38), 2893–2962. doi:10.1093/eurheartj/ehw210
Kommu S., Arepally S. (2023). The Effect of Colchicine on Atrial Fibrillation: A Systematic Review and Meta-Analysis. Cureus 15 (2), e35120. doi:10.7759/cureus.35120
Koyama T., Sekiguchi Y., Tada H., Arimoto T., Yamasaki H., Kuroki K., et al. (2009). Comparison of characteristics and significance of immediate versus early versus no recurrence of atrial fibrillation after catheter ablation. Am. J. Cardiol. 103 (9), 1249–1254. doi:10.1016/j.amjcard.2009.01.010
Kukuy O., Livneh A., Mendel L., Benor A., Giat E., Perski O., et al. (2017). Normal arterial stiffness in familial Mediterranean fever. Evidence for a possible cardiovascular protective role of colchicine. Clin. Exp. Rheumatol. 35 (6), 32–37.
Lee J. Z., Singh N., Howe C. L., Low S. W., Huang J. J., Ortega G., et al. (2016). Colchicine for Prevention of Post-Operative Atrial Fibrillation: A Meta-Analysis. JACC Clin. Electrophysiol. 2 (1), 78–85. doi:10.1016/j.jacep.2015.09.016
Lennerz C., Barman M., Tantawy M., Sopher M., Whittaker P. (2017). Colchicine for primary prevention of atrial fibrillation after open-heart surgery: systematic review and meta-analysis. Int. J. Cardiol. 249, 127–137. doi:10.1016/j.ijcard.2017.08.039
Leung Y. Y., Yao Hui L. L., Kraus V. B. (2015). Colchicine-Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 45 (3), 341–350. doi:10.1016/j.semarthrit.2015.06.013
Li D., Fareh S., Leung T. K., Nattel S. (1999). Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 100 (1), 87–95. doi:10.1161/01.cir.100.1.87
Li Y. W., Chen S. X., Yang Y., Zhang Z. H., Zhou W. B., Huang Y. N., et al. (2022). Colchicine Inhibits NETs and Alleviates Cardiac Remodeling after Acute Myocardial Infarction. Cardiovasc Drugs Ther. doi:10.1007/s10557-022-07326-y
Liu T., Li G., Li L., Korantzopoulos P. (2007). Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J. Am. Coll. Cardiol. 49 (15), 1642–1648. doi:10.1016/j.jacc.2006.12.042
Lu Y. Y., Chen Y. C., Kao Y. H., Lin Y. K., Yeh Y. H., Chen S. A., et al. (2016). Colchicine modulates calcium homeostasis and electrical property of HL-1 cells. J. Cell Mol. Med. 20 (6), 1182–1190. doi:10.1111/jcmm.12818
Ma Z., Chen J., Jin K., Chen X. (2022). Colchicine and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front. Cardiovasc Med. 9, 947959. doi:10.3389/fcvm.2022.947959
Marques-da-Silva C., Chaves M. M., Castro N. G., Coutinho-Silva R., Guimaraes M. Z. (2011). Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br. J. Pharmacol. 163 (5), 912–926. doi:10.1111/j.1476-5381.2011.01254.x
Martínez G. J., Celermajer D. S., Patel S. (2018). The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 269, 262–271. doi:10.1016/j.atherosclerosis.2017.12.027
Martínez G. J., Robertson S., Barraclough J., Xia Q., Mallat Z., Bursill C., et al. (2015). Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J. Am. Heart Assoc. 4 (8), e002128. doi:10.1161/JAHA.115.002128
Martinon F., Burns K., Tschopp J. (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10 (2), 417–426. doi:10.1016/s1097-2765(02)00599-3
Martinon F., Mayor A., Tschopp J. (2009). The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265. doi:10.1146/annurev.immunol.021908.132715
Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440 (7081), 237–241. doi:10.1038/nature04516
Misawa T., Takahama M., Kozaki T., Lee H., Zou J., Saitoh T., et al. (2013). Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14 (5), 454–460. doi:10.1038/ni.2550
Nattel S., Heijman J., Zhou L., Dobrev D. (2020). Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 127 (1), 51–72. doi:10.1161/CIRCRESAHA.120.316363
Nattel S., Maguy A., Le Bouter S., Yeh Y. H. (2007). Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 87 (2), 425–456. doi:10.1152/physrev.00014.2006
Nidorf M., Thompson P. L. (2007). Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am. J. Cardiol. 99 (6), 805–807. doi:10.1016/j.amjcard.2006.10.039
Nidorf S. M., Eikelboom J. W., Thompson P. L. (2014). Targeting cholesterol crystal-induced inflammation for the secondary prevention of cardiovascular disease. J. Cardiovasc Pharmacol. Ther. 19 (1), 45–52. doi:10.1177/1074248413499972
Nidorf S. M., Fiolet A. T. L., Mosterd A., Eikelboom J. W., Schut A., Opstal T. S. J., et al. (2020). Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 383 (19), 1838–1847. doi:10.1056/NEJMoa2021372
Nikolaidis N., Kountouras J., Giouleme O., Tzarou V., Chatzizisi O., Patsiaoura K., et al. (2006). Colchicine treatment of liver fibrosis. Hepatogastroenterology 53 (68), 281–285.
Otani K., Watanabe T., Shimada S., Takeda S., Itani S., Higashimori A., et al. (2016). Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome. Sci. Rep. 6, 32587. doi:10.1038/srep32587
Ozen S., Demirkaya E., Erer B., Livneh A., Ben-Chetrit E., Giancane G., et al. (2016). EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 75 (4), 644–651. doi:10.1136/annrheumdis-2015-208690
Pagani F. D., Baker L. S., Hsi C., Knox M., Fink M. P., Visner M. S. (1992). Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J. Clin. Invest. 90 (2), 389–398. doi:10.1172/JCI115873
Papageorgiou N., Briasoulis A., Lazaros G., Imazio M., Tousoulis D. (2017). Colchicine for prevention and treatment of cardiac diseases: A meta-analysis. Cardiovasc Ther. 35 (1), 10–18. doi:10.1111/1755-5922.12226
Pascart T., Richette P. (2018). Colchicine in Gout: an Update. Curr. Pharm. Des. 24 (6), 684–689. doi:10.2174/1381612824999180115103951
Phelps P. (1970). Polymorphonuclear leukocyte motility in vitro. IV. Colchicine inhibition of chemotactic activity formation after phagocytosis of urate crystals. Arthritis Rheum. 13 (1), 1–9. doi:10.1002/art.1780130101
Raiten J. M., Ghadimi K., Augoustides J. G., Ramakrishna H., Patel P. A., Weiss S. J., et al. (2015). Atrial fibrillation after cardiac surgery: clinical update on mechanisms and prophylactic strategies. J. Cardiothorac. Vasc. Anesth. 29 (3), 806–816. doi:10.1053/j.jvca.2015.01.001
Rennard S. I., Bitterman P. B., Ozaki T., Rom W. N., Crystal R. G. (1988). Colchicine suppresses the release of fibroblast growth factors from alveolar macrophages in vitro. The basis of a possible therapeutic approach ot the fibrotic disorders. Am. Rev. Respir. Dis. 137 (1), 181–185. doi:10.1164/ajrccm/137.1.181
Rezaei Y., Peighambari M. M., Naghshbandi S., Samiei N., Ghavidel A. A., Dehghani M. R., et al. (2020). Postoperative Atrial Fibrillation Following Cardiac Surgery: from Pathogenesis to Potential Therapies. Am. J. Cardiovasc Drugs 20 (1), 19–49. doi:10.1007/s40256-019-00365-1
Robinson P. C., Terkeltaub R., Pillinger M. H., Shah B., Karalis V., Karatza E., et al. (2022). Consensus Statement Regarding the Efficacy and Safety of Long-Term Low-Dose Colchicine in Gout and Cardiovascular Disease. Am. J. Med. 135 (1), 32–38. doi:10.1016/j.amjmed.2021.07.025
Roubille F., Kritikou E., Busseuil D., Barrere-Lemaire S., Tardif J. C. (2013). Colchicine: an old wine in a new bottle? Antiinflamm. Antiallergy Agents Med. Chem. 12 (1), 14–23. doi:10.2174/1871523011312010004
Salih M., Smer A., Charnigo R., Ayan M., Darrat Y. H., Traina M., et al. (2017). Colchicine for prevention of post-cardiac procedure atrial fibrillation: meta-analysis of randomized controlled trials. Int. J. Cardiol. 243, 258–262. doi:10.1016/j.ijcard.2017.04.022
Sammaritano L. R., Bermas B. L., Chakravarty E. E., Chambers C., Clowse M. E. B., Lockshin M. D., et al. (2020). 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 72 (4), 529–556. doi:10.1002/art.41191
Samuel M., Tardif J. C., Bouabdallaoui N., Khairy P., Dubé M. P., Blondeau L., et al. (2021a). Colchicine for Secondary Prevention of Cardiovascular Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Can. J. Cardiol. 37 (5), 776–785. doi:10.1016/j.cjca.2020.10.006
Samuel M., Tardif J. C., Khairy P., Roubille F., Waters D. D., Grégoire J. C., et al. (2021b). Cost-effectiveness of low-dose colchicine after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. Qual. Care Clin. Outcomes 7 (5), 486–495. doi:10.1093/ehjqcco/qcaa045
Schattner A. (2022). Colchicine - new horizons for an ancient drug. Review based on the highest hierarchy of evidence. Eur. J. Intern Med. 96, 34–41. doi:10.1016/j.ejim.2021.10.002
Schenone A. L., Menon V. (2018). Colchicine in Pericardial Disease: from the Underlying Biology and Clinical Benefits to the Drug-Drug Interactions in Cardiovascular Medicine. Curr. Cardiol. Rep. 20 (8), 62. doi:10.1007/s11886-018-1008-5
Sgouropoulou V., Stabouli S., Trachana M. (2019). Arterial stiffness in Familial Mediterranean Fever: correlations with disease-related parameters and colchicine treatment. Clin. Rheumatol. 38 (9), 2577–2584. doi:10.1007/s10067-019-04601-6
Shvartz V., Le T., Enginoev S., Sokolskaya M., Ispiryan A., Shvartz E., et al. (2022a). Colchicine in Cardiac Surgery: the COCS Randomized Clinical Trial. J. Cardiovasc Dev. Dis. 9 (10), 363. doi:10.3390/jcdd9100363
Shvartz V., Le T., Kryukov Y., Sokolskaya M., Ispiryan A., Khugaeva E., et al. (2022b). Colchicine for Prevention of Atrial Fibrillation after Cardiac Surgery in the Early Postoperative Period. J. Clin. Med. 11 (5), 1387. doi:10.3390/jcm11051387
Singhal R., Chang S. L., Chong E., Hsiao Y. W., Liu S. H., Tsai Y. N., et al. (2014). Colchicine suppresses atrial fibrillation in failing heart. Int. J. Cardiol. 176 (3), 651–660. doi:10.1016/j.ijcard.2014.07.069
Swartz M. F., Fink G. W., Lutz C. J., Taffet S. M., Berenfeld O., Vikstrom K. L., et al. (2009). Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart rhythm. 6 (10), 1415–1422. doi:10.1016/j.hrthm.2009.06.018
Tabbalat R. A., Alhaddad I., Hammoudeh A., Khader Y. S., Khalaf H. A., Obaidat M., et al. (2020). Effect of Low-dose ColchiciNe on the InciDence of Atrial Fibrillation in Open Heart Surgery Patients: END-AF Low Dose Trial. J. Int. Med. Res. 48 (7), 300060520939832. doi:10.1177/0300060520939832
Tabbalat R. A., Hamad N. M., Alhaddad I. A., Hammoudeh A., Akasheh B. F., Khader Y. (2016). Effect of ColchiciNe on the InciDence of Atrial Fibrillation in Open Heart Surgery Patients: END-AF Trial. Am. Heart J. 178, 102–107. doi:10.1016/j.ahj.2016.05.006
Tardif J. C., Kouz S., Waters D. D., Bertrand O. F., Diaz R., Maggioni A. P., et al. (2019). Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 381 (26), 2497–2505. doi:10.1056/NEJMoa1912388
Terkeltaub R. A. (2009). Colchicine update: 2008. Semin. Arthritis Rheum. 38 (6), 411–419. doi:10.1016/j.semarthrit.2008.08.006
Triantafilou M., Hughes T. R., Morgan B. P., Triantafilou K. (2016). Complementing the inflammasome. Immunology 147 (2), 152–164. doi:10.1111/imm.12556
Trivedi C., Sadadia M. (2014). Colchicine in prevention of atrial fibrillation following cardiac surgery: systematic review and meta-analysis. Indian J. Pharmacol. 46 (6), 590–595. doi:10.4103/0253-7613.144905
Van Wagoner D. R. (2011). Colchicine for the prevention of postoperative atrial fibrillation: a new indication for a very old drug? Circulation 124 (21), 2281–2282. doi:10.1161/CIRCULATIONAHA.111.057075
Van Wagoner D. R., Pond A. L., Lamorgese M., Rossie S. S., McCarthy P. M., Nerbonne J. M. (1999). Atrial L-type Ca2+ currents and human atrial fibrillation. Circ. Res. 85 (5), 428–436. doi:10.1161/01.res.85.5.428
Verma S., Eikelboom J. W., Nidorf S. M., Al-Omran M., Gupta N., Teoh H., et al. (2015). Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 15, 96. doi:10.1186/s12872-015-0068-3
Wan S., Yim A. P. (1999). Cytokines in myocardial injury: impact on cardiac surgical approach. Eur. J. Cardiothorac. Surg. 16 (1), S107–S111. doi:10.1016/s1010-7940(99)00200-6
Wójcicki J., Hinek A., Jaworska M., Samochowiec L. (1986). The effect of colchicine on the development of experimental atherosclerosis in rabbits. Pol. J. Pharmacol. Pharm. 38 (4), 343–348.
Workman A. J., Pau D., Redpath C. J., Marshall G. E., Russell J. A., Kane K. A., et al. (2006). Post-operative atrial fibrillation is influenced by beta-blocker therapy but not by pre-operative atrial cellular electrophysiology. J. Cardiovasc Electrophysiol. 17 (11), 1230–1238. doi:10.1111/j.1540-8167.2006.00592.x
Wu Q., Liu H., Liao J., Zhao N., Tse G., Han B., et al. (2020). Colchicine prevents atrial fibrillation promotion by inhibiting IL-1β-induced IL-6 release and atrial fibrosis in the rat sterile pericarditis model. Biomed. Pharmacother. 129, 110384. doi:10.1016/j.biopha.2020.110384
Xia M., Yang X., Qian C. (2021). Meta-analysis Evaluating the Utility of Colchicine in Secondary Prevention of Coronary Artery Disease. Am. J. Cardiol. 140, 33–38. doi:10.1016/j.amjcard.2020.10.043
Yadava M., Hughey A. B., Crawford T. C. (2014). Postoperative atrial fibrillation: incidence, mechanisms, and clinical correlates. Cardiol. Clin. 32 (4), 627–636. doi:10.1016/j.ccl.2014.07.002
Yao C., Veleva T., Scott L., Cao S., Li L., Chen G., et al. (2018). Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 138 (20), 2227–2242. doi:10.1161/CIRCULATIONAHA.118.035202
Yasgur B. S. (2023). Could Colchicine Replace Aspirin After PCI for ACS? J. Acc. Cardiovasc. Interv.
Yokoyama T., Vaca L., Rossen R. D., Durante W., Hazarika P., Mann D. L. (1993). Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J. Clin. Invest. 92 (5), 2303–2312. doi:10.1172/JCI116834
Yue H., Liang W., Gu J., Zhao X., Zhang T., Qin X., et al. (2019). Comparative transcriptome analysis to elucidate the therapeutic mechanism of colchicine against atrial fibrillation. Biomed. Pharmacother. 119, 109422. doi:10.1016/j.biopha.2019.109422
Yue H., Liang W., Zhan Y., Zhang Z., Qin X., Bian L., et al. (2022). Colchicine: emerging therapeutic effects on atrial fibrillation by alleviating myocardial fibrosis in a rat model. Biomed. Pharmacother. 154, 113573. doi:10.1016/j.biopha.2022.113573
Zarpelon C. S., Netto M. C., Jorge J. C., Fabris C. C., Desengrini D., Jardim Mda S., et al. (2016). Colchicine to Reduce Atrial Fibrillation in the Postoperative Period of Myocardial Revascularization. Arq. Bras. Cardiol. 107 (1), 4–9. doi:10.5935/abc.20160082
Zhao H., Chen Y., Mao M., Yang J., Chang J. (2022). A meta-analysis of colchicine in prevention of atrial fibrillation following cardiothoracic surgery or cardiac intervention. J. Cardiothorac. Surg. 17 (1), 224. doi:10.1186/s13019-022-01958-9
Keywords: colchicine, atrial fibrillation, postoperative atrial fibrillation, inflammation, NLRP3 inflammasome
Citation: Zhan Y, Yue H, Zhao X, Tang J and Wu Z (2023) Colchicine in atrial fibrillation: are old trees in bloom?. Front. Physiol. 14:1260774. doi: 10.3389/fphys.2023.1260774
Received: 18 July 2023; Accepted: 26 September 2023;
Published: 17 October 2023.
Edited by:
Liu Mei, University of North Carolina at Chapel Hill, United StatesReviewed by:
Tarik Kivrak, Firat University, TürkiyeGeorge Nikov Chaldakov, Medical University of Varna, Bulgaria
Copyright © 2023 Zhan, Yue, Zhao, Tang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Wu, d3V6aG9uZzcxQHNjdS5lZHUuY24=
†These authors have contributed equally to this work
 Yujia Zhan
Yujia Zhan Honghua Yue
Honghua Yue Xueshan Zhao1
Xueshan Zhao1