- 1Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
- 2Centre for Applied Biomedical Research—CRBA, St. Orsola Hospital, University of Bologna, Bologna, Italy
- 3Department of Experimental, Diagnostic and Specialty Medicines, University of Bologna, Bologna, Italy
- 4Department of Neurological Surgery, Oregon Health and Science University, Portland, OR, United States
A Corrigendum on
Synthetic torpor triggers a regulated mechanism in the rat brain, favoring the reversibility of Tau protein hyperphosphorylation
by Squarcio F, Hitrec T, Piscitiello E, Cerri M, Giovannini C, Martelli D, Occhinegro A, Taddei L, Tupone D, Amici R and Luppi M (2023). Front. Physiol. 14:1129278. doi: 10.3389/fphys.2023.1129278
In the published article, there was an error in Figure 4E as published. The only histogram bars represented in Panel E left side (referred to P-Cx [i.e., Parietal cortex]) are wrong, together with the relative “y” scale. However, the representative Western blot bands depicted at the bottom of the histograms are correct, as described in the original caption that is also reported below. The corrected Figure 4 and its correct original caption appear below.
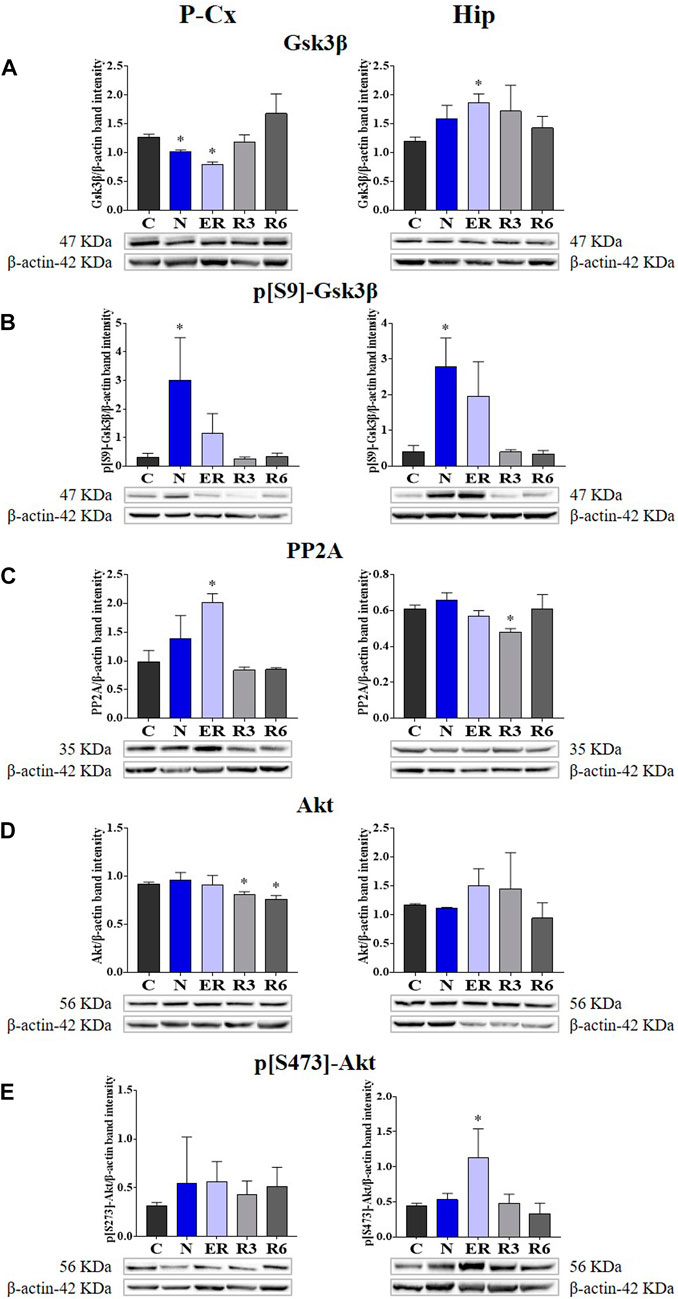
FIGURE 4. Western blot detection of the main enzymes involved in phosphorylation and dephosphorylation of Tau, determined in brain extracts of the parietal cortex (P-Cx) and hippocampus (Hip). Below each histogram, WB representative samples are shown for each experimental condition. (A) glycogen-synthase kinase-3β (GSK3β), the main kinase targeting Tau; (B) p[S9]-GSK3β (inactive form of GSK3β, phosphorylated at Ser9); (C) protein phosphatase-2A (PP2A), the main phosphatase targeting Tau; (D) different isoforms of Akt (protein kinase-B; Akt 1/2/3), kinases targeting GSK3β at Ser9 and antiapoptotic factors; (E) p[S473] Akt, the active form of Akt 1/2/3, phosphorylated at Ser473. Data are normalized by β-actin and expressed as means ± S.E.M., n = 3. *: p < 0.05 vs. C. Experimental groups (see Figure 1): C, control; N, samples taken at nadir of hypothermia, during ST; ER, early recovery, samples taken when Tb reached 35.5°C following ST; R3, samples taken 3 h after ER; R6, samples taken 6 h after ER.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: deep hypothermia, microtubules, melatonin, glycogen synthase kinase 3β, hippocampus, parietal cortex
Citation: Squarcio F, Hitrec T, Piscitiello E, Cerri M, Giovannini C, Martelli D, Occhinegro A, Taddei L, Tupone D, Amici R and Luppi M (2023) Corrigendum: Synthetic torpor triggers a regulated mechanism in the rat brain, favoring the reversibility of Tau protein hyperphosphorylation. Front. Physiol. 14:1256251. doi: 10.3389/fphys.2023.1256251
Received: 10 July 2023; Accepted: 13 July 2023;
Published: 25 July 2023.
Edited and reviewed by:
Jérémy Terrien, Muséum National d’Histoire Naturelle, FranceCopyright © 2023 Squarcio, Hitrec, Piscitiello, Cerri, Giovannini, Martelli, Occhinegro, Taddei, Tupone, Amici and Luppi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Luppi, bWFyY28ubHVwcGlAdW5pYm8uaXQ=
†These authors have contributed equally to this work and share first authorship
 Fabio Squarcio1†
Fabio Squarcio1† Marco Luppi
Marco Luppi