- 1Department of Cellular and Integrative Physiology, University of Nebraska Medical Center, Omaha, NE, United States
- 2Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE, United States
- 3US Department of Veterans Affairs Nebraska-Western Iowa Healthcare System, Omaha, NE, United States
The corpus luteum is a transient ovarian endocrine gland that produces the progesterone necessary for the establishment and maintenance of pregnancy. The formation and function of this gland involves angiogenesis, establishing the tissue with a robust blood flow and vast microvasculature required to support production of progesterone. Every steroidogenic cell within the corpus luteum is in direct contact with a capillary, and disruption of angiogenesis impairs luteal development and function. At the end of a reproductive cycle, the corpus luteum ceases progesterone production and undergoes rapid structural regression into a nonfunctional corpus albicans in a process initiated and exacerbated by the luteolysin prostaglandin F2α (PGF2α). Structural regression is accompanied by complete regression of the luteal microvasculature in which endothelial cells die and are sloughed off into capillaries and lymphatic vessels. During luteal regression, changes in nitric oxide transiently increase blood flow, followed by a reduction in blood flow and progesterone secretion. Early luteal regression is marked by an increased production of cytokines and chemokines and influx of immune cells. Microvascular endothelial cells are sensitive to released factors during luteolysis, including thrombospondin, endothelin, and cytokines like tumor necrosis factor alpha (TNF) and transforming growth factor β 1 (TGFB1). Although PGF2α is known to be a vasoconstrictor, endothelial cells do not express receptors for PGF2α, therefore it is believed that the angioregression occurring during luteolysis is mediated by factors downstream of PGF2α signaling. Yet, the exact mechanisms responsible for angioregression in the corpus luteum remain unknown. This review describes the current knowledge on angioregression of the corpus luteum and the roles of vasoactive factors released during luteolysis on luteal vasculature and endothelial cells of the microvasculature.
1 Introduction
Vascular remodeling occurs in various disease states such as myocardial infarction, traumatic spinal cord injury (Olive et al., 2003; Benton et al., 2008), neurovascular disease (Planas, 2020), and various hypertensive disorders (Intengan and Schiffrin, 2001). Degradation of blood vessels, or angioregression, also occurs in disease states such as chronic graft nephropathy (Ishii et al., 2005) and muscle degeneration (Dedkov et al., 2002; Malek et al., 2010; Olenich et al., 2014). Vast non-pathological angioregression occurs during normal development (Yin and Pacifici, 2001; McKeller et al., 2002; Nayak et al., 2018); however, it is not prevalent in most adult tissues. There are some instances where physiological angioregression occurs, such as in uterine remodeling during pregnancy (Henry et al., 2006), mammary gland involution (Djonov et al., 2001), and during luteolysis of the ovarian corpus luteum during the female reproductive cycle (Augustin, 2000).
The corpus luteum is a temporary, dynamic endocrine gland formed from the remnants of the ovulated follicle (Stocco et al., 2007). It produces the progesterone that is necessary for successful establishment and maintenance of pregnancy. Following ovulation, the remaining granulosa and theca cells of the ovulated follicle undergo rapid, but limited, division and then differentiation as the ovary shifts from making estradiol to making progesterone in a process known as luteinization (Smith et al., 1994; Fraser and Duncan, 2009). Notably, endothelial cells from the theca layer of the follicle undergo rapid division, comparable to that of tumor angiogenesis, forming the vast microvasculature of the corpus luteum, with around 50% of cells in the mature corpus luteum comprised of endothelial cells (Rodgers et al., 1984; O’shea et al., 1989). In addition to the steroidogenic and endothelial cells, the corpus luteum contains small populations of fibroblasts, mesenchymal-like cells that produce and regulate extracellular matrix, and immune cells (O’shea et al., 1989; Van Linthout et al., 2014). The luteal vasculature contains mostly capillaries, and nearly every steroidogenic cell is in contact with a microvascular endothelial cell (Zheng et al., 1993; Fraser and Duncan, 2009). As a result, the corpus luteum has one of the highest blood supplies, per unit of any organ system (Smith et al., 1994), as well as high blood flow and low vascular resistance compared to the surrounding ovarian stroma (Wiltbank et al., 1990). The microvasculature is crucial for luteal function, as blockage of angiogenesis during luteal formation results in dysfunctional corpora lutea (Yamashita et al., 2008). The luteal vasculature is important for both transport of progesterone from steroidogenic cells to the rest of the body and for nourishment of the corpus luteum (Woad and Robinson, 2016). Early studies in rabbits demonstrated the importance of the corpus luteum for the production of progesterone and pregnancy (Mesiano, 2022). In women, luteal function is crucial for pregnancy, as removal of the corpus luteum within the first 7 weeks of pregnancy results in termination of said pregnancy (Csapo et al., 1974). Progesterone secreted from the corpus luteum is crucial for creating optimal conditions for embryo implantation, a crucial point in pregnancy (Kolatorova et al., 2022).
In a reproductive cycle that does not result in pregnancy, the corpus luteum must cease progesterone production so the next reproductive cycle can begin. In many species including bovines (Knickerbocker et al., 1988), ovines (Goding, 1974), guinea pigs (Evans, 1987), and rats (Wang et al., 1993), luteal regression (luteolysis) is initiated by pulses of prostaglandin F2α (PGF2α) produced by the uterus. In primates, it is believed to be initiated by the lack of gonadotropin support and a concomitant rise in intra-ovarian PGF2α production (Stouffer et al., 2013). Luteolysis occurs in two steps: (1) functional regression, in which progesterone production declines, and (2) structural regression, in which the corpus luteum dissipates into a fibrotic corpus albicans within the ovarian stroma. Structural regression involves various pathways of tissue remodeling, including immune cell infiltration, breakdown and buildup of extracellular matrix, and regression of the microvasculature. Although it is known that blood flow is impaired and the vascular endothelial cells are sensitive to various factors released during luteolysis, the exact mechanism of angioregression in the corpus luteum remains unknown. This review will highlight current knowledge on angioregression of the corpus luteum: from macrovascular changes, dilation and constriction of luteal vasculature and subsequent impedance of blood flow, to microvascular changes, such as the effects of growth factors, cytokines, and thrombospondins on microvascular endothelial cells.
2 Blood flow
Electron microscopy of regressing guinea pig and bovine corpora lutea revealed that endothelial cells detach from the basement membrane, slough off the capillaries, and clog the vessels; yet the capillary walls remain intact (Azmi and O'Shea, 1984; Modlich et al., 1996). However, luteal capillaries eventually disappear throughout regression. Twelve hours after PGF2α-induced regression, there were noticeably fewer capillaries in ovine corpora lutea (Nett et al., 1976), but more arterial vessels were present (Bauer et al., 2003). These vessels contain thicker walls of smooth muscle (Bauer et al., 2003), which is further evidenced by increased smooth muscle actin positive cells surrounding arterioles in regressing bovine corpora lutea (Hojo et al., 2009). The diameter of these vessels was demonstrated to be smaller compared to fully functional mid-cycle corpora lutea (Lei et al., 1991; Nio-Kobayashi et al., 2016), indicating that vasoconstriction may be occurring in luteal regression. Similarly, arterial wall thickening is associated with various cardiovascular diseases including atherosclerosis, thrombosis, and hypertension (Burke et al., 1995; Corinaldesi and Corinaldesi, 2008). Changes in blood flow during luteal regression as a result of vasoactive factors are illustrated in Figure 1.
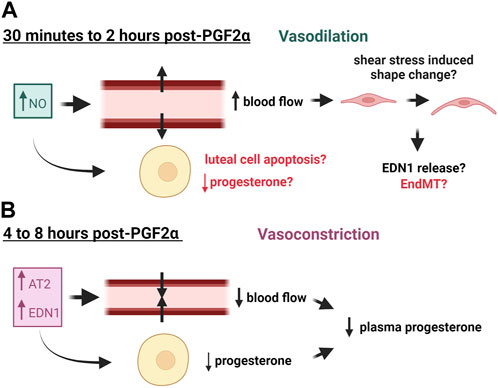
FIGURE 1. Effects of Vasoactive Factors released in response to PGF2α on blood flow of the corpus luteum. (A) Nitric Oxide (NO) is released early in luteal regression and causes vasodilation and subsequent increased blood flow, which may consequently alter endothelial cell phenotype. (B) Later in luteal regression, vasoconstrictors such as Angiotensin II (AT2) and endothelin 1 (EDN1) are released, causing decreased blood flow and decreased plasma progesterone. These factors may also impair progesterone production of luteal steroidogenic cells. This figure was made with Biorender.com.
Early studies in the rabbit indicate that luteal blood flow is highly correlated to progesterone levels in pseudopregnant rabbit corpora lutea (Janson et al., 1981). Due to their size and prominent vascular blood supply, corpora lutea from larger species such as ruminants and humans can be identified via ultrasonography. Doppler ultrasound has been utilized to study corpora lutea during natural and PGF2α-induced regression. Doppler readings in human corpora lutea have identified that blood flow drops in the days nearing menstruation, which correlates to the reduction in progesterone production in the late luteal phase (Miyazaki et al., 1998), and pregnant cattle exhibited a much higher luteal blood flow than non-pregnant cattle (Kanazawa et al., 2022). Additionally, in vivo studies of cows injected with PGF2α to induce luteolysis have identified that there is an early increase in blood flow after 2 h of treatment that ultimately decreases below baseline levels after 8 h of treatment (Acosta et al., 2002; Miyamoto et al., 2005; Jonczyk et al., 2021). A study in the ovine suggested that ovarian blood flow to the luteal ovary decreased 4 h post-PGF2α treatment, followed by a decline in progesterone 6 h post-PGF2α treatment (Nett et al., 1976). Similar findings were reported in the equine and the donkey (Ginther et al., 2007; Miró et al., 2015). Also, luteal blood flow and plasma progesterone followed a more similar trend in the cycling bovine corpus luteum than did luteal size, which is commonly used to identify luteal stage (Herzog et al., 2010), thus implicating blood flow as an accurate measure of luteal function. However, the opposite has been determined for the midcycle in lactating dairy cows (Lüttgenau et al., 2011). The reason for the discrepancy is likely due to the enhanced hepatic metabolism of progesterone in lactating dairy cows (Wiltbank et al., 2006).
The transient increase in blood flow seen early in luteal regression is believed to be a result of the increased synthesis of the vasodilator, nitric oxide (NO) (Miyamoto et al., 2005; Shirasuna et al., 2008). Evidence across various species has shown that shear stress from increased blood flow changes the shape of vascular endothelial cells from their signature cobblestone shape to one that is more spindle-like and oriented towards the direction of flow (reviewed in (Masuda et al., 2003; Campinho et al., 2020)). This has been observed in endothelial cells derived from the carotid arteries of rabbits (Masuda et al., 1999) and mouse cardiac and pulmonary endothelial cells (Merna et al., 2018). High stretch of cultured endothelial cells induces a similar phenotype in which the cells acquire a spindle-like shape via phalloidin remodeling and produce increased reactive oxygen species (Girão-Silva et al., 2021). This change in endothelial cell phenotype is suggested to be mediated by calcium signaling and tyrosine kinase phosphorylation in cultured bovine aortic endothelial cells (Malek and Izumo, 1996). More recent studies show that increased shear stress causes retinal endothelial cells cultured in 3D (Luu et al., 2023) and human aortic endothelial cells (Kidder et al., 2023) to adopt a more mesenchymal phenotype. There are no reports to our knowledge of this phenomenon occurring in luteal endothelial cells; however, shear stress from a compensatory blood flow increase early in luteal regression may contribute to the changes in capillary basement membrane that are seen during regression, which could result in the release of endothelial cells from the capillary. Another hypothesis is that the shear stress could cause the endothelial cells to transition to a mesenchymal phenotype, as increased matrix stiffness is associated with fibrosis and endothelial cell destabilization (Yu et al., 2023). It is also important to note that endothelial cells are sensitive to many factors released during luteal regression as discussed later; therefore, changes in blood flow are likely not the sole contributor to luteal angioregression.
3 Effect of vasoactive factors on luteal vasculature and endothelial cells
Outside of the corpus luteum, PGF2α is more commonly known as a vasoconstrictor, in which it does so by activating calcium signaling and inducing contraction of smooth muscle cells (Watts, 2007). In addition to PGF2α, various other vasoactive agents are increased in luteal regression, such as NO, angiotensin II (AT2), and endothelin 1 (EDN1) (Davis et al., 2003; Skarzynski et al., 2008; Shirasuna et al., 2012b). The known changes in vasoactive factors contributing to blood flow in luteal regression are summarized in Table 1.
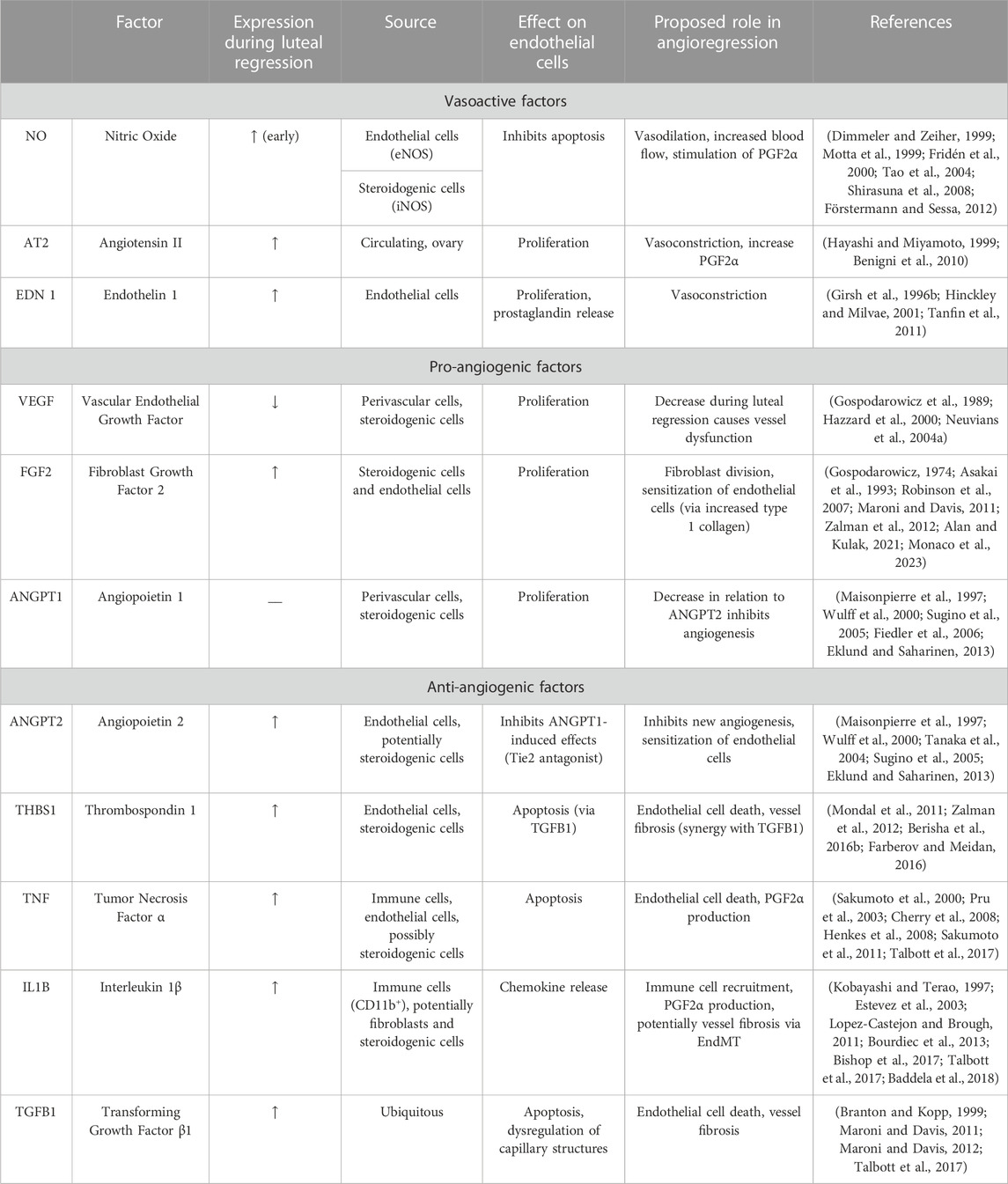
TABLE 1. Summary of Vasoactive, Pro-angiogenic, and Anti-angiogenic factors that are regulated during luteal angioregression.
3.1 Nitric oxide (NO)
Nitric oxide (NO) is commonly known as a vasodilator that is produced by nitric oxide synthase (NOS) enzymes. There are three identified NOS enzymes (NOS1-3), two of the three being uniquely expressed in specific cell types, neuronal NOS (nNOS or NOS1) and endothelial NOS (eNOS or NOS3). The third NOS enzyme is inducible NOS (iNOS or NOS2) (Förstermann and Sessa, 2012). Both eNOS and iNOS have been identified in luteal tissue, with eNOS primarily being localized to the endothelial cells and iNOS exhibiting weak localization to the steroidogenic cells (Fridén et al., 2000; Tao et al., 2004). One study identified increased eNOS in naturally regressing bovine corpora and in corpora lutea induced to regress following treatment with PGF2α (Shirasuna et al., 2008). Studies in the human report increased iNOS at mid and late luteal phase (Vega et al., 2000). However, others reported decreased eNOS immunolocalization and transcription during the bovine late luteal phase and luteal regression (Rosiansky-Sultan et al., 2006). Such differences can be attributed to differences in staging of the corpus luteum as opposite effects of PGF2α treatment were seen in early corpora lutea compared to midcycle corpora lutea, as well as whether the tissue sample was in the middle or periphery of the corpus luteum, as eNOS was elevated only in the periphery of midcycle corpora lutea in the bovine (Shirasuna et al., 2008). Direct intra-luteal delivery of an NO donor temporarily increased blood flow but then caused both blood flow and progesterone to fall to levels below baseline (Shirasuna et al., 2008). Similar results have been reported in other species. For instance, NO donor treatment decreased serum progesterone but increased ovarian PGF2α production in pseudopregnant rats, and the same has been reported in cultured human luteal cells (Motta et al., 1999; Fridén et al., 2000). Such differences may be due to pharmacologic administration of NO donors in vivo and species-to-species variations.
In addition to its ability to stimulate PGF2α, it has been suggested that NO may have a direct effect on luteal cells. L-arginine, an NOS substrate, increased apoptosis of cultured human luteal tissue, which was suppressed by treatment with an NO inhibitor (Vega et al., 2000). Similarly, treatment of bovine luteal cells with the NO donor, NONOate, increased levels of cleaved caspase 3, a marker of apoptosis (Korzekwa et al., 2006; Kowalczyk-Zieba et al., 2014). Conversely, treatment of luteinized goat granulosa cells with NO donor increased progesterone production, whereas treatment with an NOS inhibitor decreased progesterone and increased apoptosis (Guo et al., 2019). Such differences may be due to differences in culture conditions and the presence of multiple cell types (endothelial cells and immune cells) in the cultures. Collectively, these data indicate that NO may contribute to luteolysis beyond the vasculature and may have a direct effect on steroidogenesis. Although there is some evidence that NO may be an inhibitor of apoptosis in endothelial cells (Dimmeler and Zeiher, 1999, this phenomenon has yet to be studied in luteal endothelial cells.
3.2 Angiotensin II (AT2)
Angiotensin II (AT2) is a potent vasoconstrictor that is derived from angiotensinogen produced by the liver. Angiotensinogen is converted to angiotensin I by the enzyme renin, and angiotensin I is converted to angiotensin II by angiotensin converting enzyme (ACE) (Ng and Vane, 1967). AT2 has pleiotropic effects that include production of aldosterone, which increases blood pressure, or it can act as an independent vasoactive factor or act on other systems such as the kidney to raise blood pressure (Fyhrquist et al., 1995; Santos, 2014). Such effects, along with pro-inflammatory and pro-fibrotic effects are mediated by the angiotensin receptor 1 (AGTR1) (Benigni et al., 2010). Angiotensin II is also capable of binding to the AGTR2, which induces vasodilation and anti-inflammatory and anti-fibrotic effects (Benigni et al., 2010). Interestingly, AGTR1 levels remain consistent throughout the luteal phase, but AGTR2 levels are decreased at midcycle (days 8–12) but are increased during the late luteal phase (days 13–17) and regression (> day 18) in bovine corpora lutea, but were also found to be elevated to the same level in the corpus luteum of pregnancy (Hayashi et al., 2000; Kobayashi et al., 2001; Schams et al., 2003). Although this seems contradictory to the increased inflammation and fibrosis seen during luteal regression, it is possible that (1) these studies only accounted for AGTR2 mRNA and not for desensitization or endocytosis of the AGTR2, or (2) AGTR2 actions ensure that the regression is tightly controlled.
The ovary is capable of producing renin and AT2 (Yoshimura, 1997; Palumbo et al., 2016). Additionally, ACE has been identified in endothelial cells of the bovine corpus luteum during early luteal phase (Hayashi et al., 2000). The luteal renin-angiotensin-aldosterone system (RAAS) may serve an important role in pregnancy in women, as increased corpus luteum number was associated with differential levels of RAAS components, but there were no differences in birth outcomes (Wiegel et al., 2021).
PGF2α has been shown to increase AT2 levels in microdialyzed bovine corpora lutea in vitro (Hayashi and Miyamoto, 1999), and AT2 has been demonstrated to increase PGF2α levels but also progesterone levels alone and in conjunction with PGF2α treatment in vitro (Kobayashi et al., 2001). In that study, AT2 increased secretion of oxytocin, another luteolytic factor. In rat luteal cells, AT2 had no effect on basal progesterone, but inhibition of AGTR2 slightly decreased progesterone secretion (Pepperell et al., 2006). However, in another study, AT2 decreased progesterone after 14 h alone and after 4 h in conjunction with PGF2α in microdialyzed bovine corpora lutea (Hayashi and Miyamoto, 1999). It is possible that such effects are mediated by angiotensin reactive proteins in the luteal endothelial cells, as ACE is present in bovine luteal endothelial cells, and treatment with an ACE inhibitor, captopril, decreased AT2 secretion from luteal endothelial cells (Hayashi et al., 2000). Therefore, it is possible that endothelial-derived AT2 may aid in decreasing progesterone either alone or through the release of luteolytic factors.
3.3 Endothelin-1 (EDN1)
Endothelin 1 (EDN1) is a 21 amino acid protein derived from endothelial cells that is known to be a potent vasoconstrictor (Yanagisawa et al., 1988). It is produced in endothelial cells as a larger pro-endothelin which is then cleaved to active endothelin by endothelin converting enzyme (ECE) (Sawamura et al., 1989; Tanfin et al., 2011). Studies in the cow and the ewe have identified that luteal endothelin 1 levels increase during natural and PGF2α-induced luteal regression (Girsh et al., 1996b; Ohtani et al., 1998; Hinckley and Milvae, 2001), and PGF2α induces secretion of EDN1 from microdialysed bovine corpora lutea (Miyamoto et al., 1997). Furthermore, tumor necrosis factor α (TNF), an inflammatory cytokine that is elevated in luteal regression, increased EDN1 production by bovine aorta endothelial cells (Marsden and Brenner, 1992) therefore EDN1 secretion may be regulated by PGF2α, inflammatory cytokines, and other secondary responses to PGF2α.
Although EDN1 is well known for its role as a vasoconstrictor, it may have some direct effects on steroidogenic cells in the corpus luteum. In vitro, EDN1 decreased progesterone secretion of ovine luteal minces and decreased basal and LH-induced progesterone production by large, but not small bovine luteal cells (Girsh et al., 1996a; Doerr et al., 2008). In vivo, EDN1 decreased progesterone in a synergistic manner with PGF2α in the bovine (Shirasuna et al., 2006). Given this information, the luteolytic role of EDN1 may involve both vasoconstriction and direct action on luteal steroidogenic cells.
4 Pro- and anti-angiogenic factors in luteolysis
In addition to the presence of factors affecting vessel dilation or contraction during luteal regression, luteolysis is associated with decreased expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and increased expression of anti-angiogenic factors, such as thrombospondin 1 (THBS1), EDN1, transforming growth factor β 1 (TGFB1) and pro-inflammatory cytokines (Skarzynski et al., 2008; Shirasuna et al., 2012b; Berisha et al., 2016a; Talbott et al., 2017). The proposed effects of these factors on luteal vasculature are illustrated in Figure 2. These events are triggered by a luteolytic dose of PGF2α in vivo; however, multiple studies have demonstrated that endothelial cells do not express the receptor for PGF2α (Liptak et al., 2005; Maroni and Davis, 2011). Therefore, the effects of the luteolytic cascade on microvascular endothelial cells must be from release of factors following the initial PGF2α pulses. Furthermore, luteal endothelial cells are known to be sensitive to such factors, and thus are believed to be the first to die during luteal regression (Azmi and O'Shea, 1984). Additionally, the luteal rescue signal, interferon tau, increases endothelial cell survival and decreases transcription of luteolytic factors like THBS1, EDN1, and TGFB1 in bovine luteal slices (Basavaraja et al., 2017). Therefore, the presence or absence of secreted factors relating to angiogenesis may contribute to luteal angioregression.
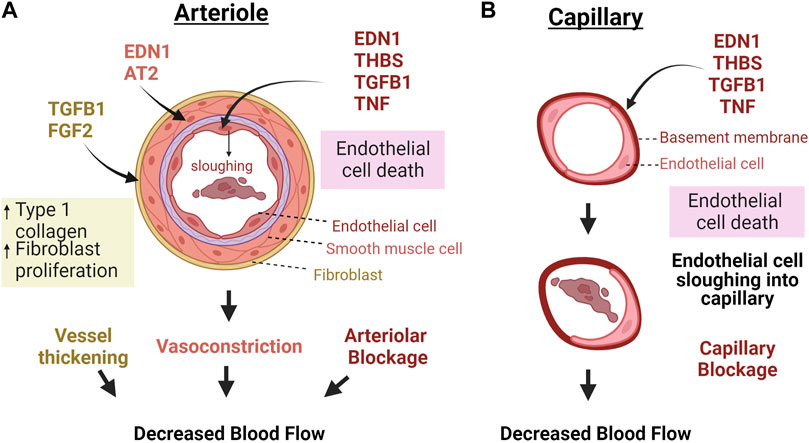
FIGURE 2. Macro vs. microvascular changes in response to factors secreted during luteal regression. (A) Because the luteal microvasculature also contains smooth muscle and pericytes, vasoactive factors cause contraction of vascular smooth muscle cells and consequent vasoconstriction, in addition to TGFB1 and FGF2 activating fibroblasts to produce more collagen, thereby thickening and stiffening the vessels. Capillaries do not have these bordering cells (B). Therefore, these vessels would mostly be impacted by factors that directly impact endothelial cells. In both cases, death of endothelial cells causes sloughing into the capillaries which can clog vessels and impair blood flow. Abbreviations: Endothelin 1 = EDN1, Angiotensin II = AT2, Fibroblast growth factor 2 = FGF2, Transforming growth factor β-1 = TGFB1, Thrombospondin 1 = THBS1, Tumor necrosis factor α = TNF. The figure was made using Biorender.com.
4.1 Pro-angiogenic factors
4.1.1 Vascular endothelial growth factor (VEGF)
Vascular endothelial growth factor (VEGF) is growth factor that acts as a potent mitogen on endothelial cells (Gospodarowicz et al., 1989). There are five VEGF isoforms which can bind to three receptors, VEGFR1 (Flt1), VEGFR2 (KDR or Flk1) and VEGFR3 (Flk4) (Geva and Jaffe, 2000). Because rapid angiogenesis is necessary to build the luteal microvasculature, VEGF is highly prominent in the developing corpus luteum (Redmer et al., 1996; Woad and Robinson, 2016). Interruption of VEGF during ovulation results in decreased luteal formation (Duncan et al., 2008). In the late luteal phase, and in PGF2α-induced regression across multiple species, luteal VEGF mRNA decreases (Otani et al., 1999; Hazzard et al., 2000; Neuvians et al., 2004a; Vonnahme et al., 2006). The rapid reduction in VEGF expression indicates that no active angiogenesis is occurring during luteal regression, a finding confirmed by studies across various species, including the rat (Tamura and Greenwald, 1987), sheep (Reynolds and Redmer, 1999) and pig (Ricke et al., 1999), indicating that little endothelial cell proliferation occurs during luteal regression. The reduction in VEGF may reduce support for the luteal vasculature, leading to vascular regression. Such a phenomenon has been witnessed in tumors where withdrawal of VEGF will cause regression of the vessels that had built up in that tumor (Benjamin and Keshet, 1997; Baffert et al., 2006), and this phenomenon is a current target for anti-cancer therapeutics (Meadows and Hurwitz, 2012; Frezzetti et al., 2017). However, this has yet to be confirmed in the regressing corpus luteum.
4.1.2 Fibroblast growth factor 2 (FGF2)
Fibroblast growth factor 2, or basic fibroblast growth factor (FGF2) is a heparin binding protein, discovered for its ability to induce proliferation of NIH 3T3 fibroblast cell lines (Gospodarowicz, 1974). FGF2 is a prominent angiogenic factor, potentially even more so than VEGF, and it is necessary for luteal formation, as blockade of FGF2 at the time of ovulation results in corpora lutea that have malformed blood vessels and lower progesterone (Robinson et al., 2007; Yamashita et al., 2008). Immunohistochemical studies reveal FGF2 localization in endothelial cells and steroidogenic cells of the corpus luteum (Asakai et al., 1993; Alan and Kulak, 2021).
Unlike VEGF, FGF2 expression is increased during luteal regression (Neuvians et al., 2004a; Neuvians et al., 2004b; Zalman et al., 2012; Monaco et al., 2023). These findings indicate that an elevation in luteal FGF2 is not sufficient to prevent the loss of endothelial cells and capillaries that occurs during early luteal regression, nor does FGF2 cotreatment affect progesterone levels of cows treated with PGF2α in vivo (Piotrowska-Tomala et al., 2021). An explanation for this phenomenon could be that the increased FGF2 transcription is a compensatory effect and slows down the luteolytic process (Neuvians et al., 2004b). Another explanation is that FGF2 acts on stromal or fibrotic components of the corpus luteum. In support of this idea, using smooth muscle actin as a marker, Reynolds and Redmer observed that fibroblast number was elevated in the corpus luteum during the late luteal phase of sheep, and collagen production was also elevated following PGF2α treatment (Reynolds and Redmer, 1999; Vonnahme et al., 2006). A recent study showed that FGF2 induced both proliferation and collagen production of bovine luteal fibroblasts (Monaco et al., 2023). Furthermore, in vitro studies reveal that the presence of type 1 collagen causes endothelial cells to become more sensitive to TGFB1 (Maroni and Davis, 2011). Therefore, FGF2 in the regressing corpus luteum may be acting as a luteal pro-fibrotic factor that contributes to sensitizing endothelial cells to luteolytic ligands.
4.1.3 Angiopoietins 1 & 2 (ANGPT1/2)
Angiopoietins (ANGPTs) are growth factors that regulate angiogenesis through binding to tyrosine kinase receptors Tie1 and Tie2. ANGPT1 is known to be secreted by pericytes and smooth muscle cells adjacent to endothelial cells, and ANGPT2 is believed to be exclusive to endothelial cells (Eklund and Saharinen, 2013). However, in situ hybridization and immunohistochemistry of the human corpus luteum revealed ANGPT 1 and 2 distribution in both steroidogenic and endothelial cells (Wulff et al., 2000; Sugino et al., 2005), and a similar result was revealed in the buffalo corpus luteum (Mishra et al., 2016). ANGPT1 binds to Tie2 on endothelial cells to promote angiogenesis by inducing proliferation and migration. In contrast, ANGPT2 acts as a Tie2 receptor antagonist to ANGPT1 and plays a role in vessel stabilization rather than new vessel formation (Maisonpierre et al., 1997). The ANGPT2/ANGPT1 ratio was reported to be elevated in regressing corpora lutea, suggesting that angiogenesis does not occur during luteal regression (Goede et al., 1998). Expression of ANGPT1 transcripts in corpora lutea may be species-dependent, as it was highest at mid-luteal phase in bubaline (Mishra et al., 2016) and bovine (Tanaka et al., 2004) corpora lutea, and in corpora lutea of pregnancy in human (Sugino et al., 2005), but highest at late cycle in rhesus monkey corpora lutea (Hazzard et al., 2000). ANGPT2 expression was lowest at midluteal phase in human corpora lutea and decreased in corpora lutea of pregnancy, when the corpus luteum was fully functional (Sugino et al., 2005), whereas it was highest in the late luteal phase in the rhesus monkey (Hazzard et al., 2000) and bubaline corpus luteum (Mishra et al., 2016). In the bovine corpus luteum, no changes in luteal ANGPT2 mRNA were detected throughout the luteal phase but ANGPT2 mRNA was transiently elevated 2 h post-PGF2α injection, and then decreased after 4 h of PGF2α treatment (Tanaka et al., 2004). Although there are no reports of the effects of ANGPT2 on luteal endothelial cells, ANGPT2 has been shown to sensitize endothelial cells to the pro-apoptotic effects of TNF (Fiedler et al., 2006). Tanaka et al. found that ANGPT2 decreased progesterone secretion of bovine corpora lutea at 4–8 h of treatment at higher concentrations (100 ng/mL) in an in vitro microdialysis system (Tanaka et al., 2004). This is inconsistent with what was reported in the buffalo, in which ANGPT1 and 2 increased progesterone production in cultured bubaline luteal cells after 24 h of treatment at 100 ng/mL (Mishra et al., 2016). Because ANGPTs are known to act primarily on endothelial cells (Eklund and Saharinen, 2013), more studies using primary luteal endothelial cells are needed to elucidate their role in angioregression.
4.2 Anti angiogenic factors
4.2.1 Thrombospondin 1 (THBS1)
Thrombospondins are a family of calcium-binding glycoproteins that influence a variety of functions, including angiogenesis, vessel biology, and wound healing. They are known to regulate several factors such as AT2, TGFB1, and even matrix metalloproteinases (Adams and Lawler, 2011), but the well-established function of thrombospondins 1 and 2 is dysregulation of angiogenesis (Armstrong and Bornstein, 2003). The role of thrombospondins in the corpus luteum has been reviewed in detail (Farberov et al., 2019). Localization of THBS1 was identified in endothelial cells and steroidogenic cells in the corpus luteum (Berisha et al., 2016b), and THBS1 expression is increased in the regressing corpus luteum and is reduced in the corpus luteum of pregnancy (Mondal et al., 2011; Zalman et al., 2012; Romero et al., 2013; Farberov and Meidan, 2016). Treatment of luteal tissue slices with interferon tau, the maternal signal for luteal rescue in bovines, decreased THBS1 mRNA and protein (Basavaraja et al., 2017). It is believed that THBS1 exerts anti-angiogenic effects on the regressing corpus luteum, as it has been demonstrated to decrease numbers of bovine and rabbit luteal endothelial cells in culture (Bagavandoss and Wilks, 1990; Farberov and Meidan, 2016). Thrombospondin 1 may act in part through TGFB1 signaling, as THBS1-treated luteal cells exhibited increased downstream TGFB1 signaling, which was decreased with THBS1 knockdown; however, inhibition of TGFB1 signaling did not ameliorate THBS1-induced endothelial cell death (Farberov and Meidan, 2016). Therefore, other signaling factors must come into play. For instance, a TGFB superfamily member, Nodal, which is increased in hypoxic conditions, elevated THBS1 and CD36 and downregulated CD31 (an endothelial cell marker) in equine luteal explants (Walewska et al., 2019). Furthermore, despite its elevation in luteolysis, THBS1 has been shown to be upregulated after ovulation and induced migration of monkey ovarian endothelial cells (Bender et al., 2019). Therefore, the action of THBS1 must depend on multiple signaling pathways and the presence of other factors in the corpus luteum, as the pro or anti-angiogenic effect of THBS1 is dependent on the target receptor. Thrombospondin 1 acts as an anti-angiogenic factor when bound to CD36 (Dawson et al., 1997), and as a pro-angiogenic factor when bound to the low density lipoprotein receptor-related protein-1 (LRP1) receptor (Orr et al., 2003). CD36 was elevated in PGF2α-induced luteolysis of the bovine corpus luteum (Berisha et al., 2016b). However, it has been shown in the ovary that THBS1 can inhibit VEGF through LRP1 binding (Greenaway et al., 2007). THBS1 has also been demonstrated to sequester FGF2 by binding to its heparin-binding site (Farberov et al., 2019). The ability of THBS1 to bind to many targets including angiogenic factors and cytokines such as TGFB1, makes it an ideal candidate for further investigation of factors that can influence cytokine levels and angioregression in the corpus luteum.
5 Effects of cytokines on endothelial cells
5.1 Tumor necrosis factor α (TNF)
In most cells, TNF binding to its receptor (TNFR1) results in inflammatory responses and/or programmed cell death. TNF protein is elevated in the pseudopregnant mouse corpus luteum following treatment with a luteolytic dose of PGF2α (Henkes et al., 2008). In the bovine, mRNA for TNF is upregulated early in PGF2α induced luteolysis (Talbott et al., 2017) and in the natural cycle (Friedman et al., 2000; Sakumoto et al., 2000). TNF has been shown to be localized to immune cells and faintly in steroidogenic cells in the bovine corpus luteum (Sakumoto et al., 2011); however, others have found TNF to be localized in the vascular immune cells of the porcine corpus luteum (Hehnke-Vagnoni et al., 1995). TNF affects steroidogenesis directly by decreasing LH-stimulated progesterone production in luteinized murine granulosa cells (Adashi et al., 1990) and in porcine (Pitzel et al., 1993) and bovine (Benyo and Pate, 1992; Sakumoto et al., 2000) luteal cells. Furthermore, TNF can induce production of PGF2α, thus creating a positive feedback loop within the regressing corpus luteum (Sakumoto et al., 2000).
Luteal endothelial cells are sensitive to inflammatory cytokines, as TNF induces apoptosis of luteal endothelial cells (Friedman et al., 2000; Pru et al., 2003; Cherry et al., 2008; Henkes et al., 2008). Endothelial cells are a rich source of acid sphingomyelinase (ASMase), a common mediator of cytokine signaling. A report by Henkes et al. shows that TNF, but not PGF2α, activated ASMase and cell death in murine ovarian endothelial cells, a response that was not present in endothelial cells lacking ASMase (Henkes et al., 2008). In vivo studies revealed that mice treated with Etanercept, a TNF receptor inhibitor, were resistant to PGF2α-induced luteal regression. Additionally, mice lacking ASMase were protected from PGF2α-induced luteal regression. There was no gross evidence of PGF2α-induced disruption of the corpus luteum in the ASMase deficient mice, findings supported by the maintenance of progesterone levels (Henkes et al., 2008). These results suggest that TNF-mediated activation of ASMase serves a pivotal role in altering the luteal vasculature during PGF2α-induced luteal regression. The absence of direct cytotoxic effects of PGF2α on isolated luteal endothelial cells argues that luteal regression in response to PGF2α requires cytokines like TNF to disrupt vascular integrity. In vitro studies with bovine luteal endothelial cells show that TNF also induces apoptosis by inducing ASMase, production of ceramide, and activation of Jun-N-terminal Kinase (JNK) MAPK signaling (Pru et al., 2003). TNF-induced endothelial cell death may increase vascular permeability, allowing for increased infiltration of immune cells, which release more TNF, thereby exacerbating angioregression. However, further studies need to be performed to determine whether TNF affects luteal vascular permeability in vivo.
5.2 Interleukin 1 β (IL1B)
Interleukin 1 β (IL1B) is a pro-inflammatory cytokine that, like TNF, is upregulated early in luteal regression (Talbott et al., 2017). Also like TNF, IL1B is produced in immune cells, based on previous studies (Lopez-Castejon and Brough, 2011), although some studies indicate that fibroblasts may be able to secrete inflammatory cytokines like IL1B (Kobayashi and Terao, 1997; Bartok and Firestein, 2010), and a transcriptomic study identified the presence of IL1B mRNA in small steroidogenic cells of the bovine corpus luteum (Baddela et al., 2018). Although the exact localization of IL1B has not been determined in the corpus luteum, flow cytometry of the primate corpus luteum indicated that IL1B is produced by CD11b+ cells in the late luteal phase (Bishop et al., 2017). Unlike TNF, the role of IL1B has not been investigated in luteal endothelial cells; however, in the corpus luteum, IL1B has been demonstrated to impair steroidogenesis and increase cyclooxygenase and subsequent PGF2α production in cultured rat ovarian tissue (Estevez et al., 2003), indicating cytokines like IL1B can potentially contribute to a positive-feedback loop to enhance PGF2α production and action.
In studies of human umbilical vein endothelial cells (HUVECs), IL1B induces matrix metalloprotease 9 and decreases levels of tissue inhibitor of matrix metalloprotease 1, suggesting that IL1B induces a net breakdown of matrix (Qin et al., 2012). In the same study, it was demonstrated that IL1B also increased endothelial cell permeability and altered expression of membrane cadherins and claudins, which could implicate increased immune cell recruitment (Qin et al., 2012). In agreement with this possibility, both TNF and IL1B increased neutrophil recruitment to HUVECs. Furthermore, the effect of IL1B was increased slightly in conditions of shear stress (Sheikh et al., 2005). A potential mechanism could be through the secretion of chemokines, which attract immune cells like neutrophils. For instance, IL1B increased secretion of IL8, a chemokine known to recruit neutrophils, in immortalized human microvascular endothelial cells (Bourdiec et al., 2013). Additionally, the receptor for IL1B, IL1R, has been implicated in shear-stressed induced EndMT, where endothelial cells transdifferentiate into myofibroblast-like cells (Kidder et al., 2023). Therefore, IL1B action in angioregression may be beyond generalized inflammation, and may even induce further inflammation through recruitment of immune cells and increased production of PGF2α.
5.3 Transforming growth factor β1 (TGFB1)
Similar to TNF and IL1B, TGFB1 is increased very early during luteal regression (Hou et al., 2008; Talbott et al., 2017). TGFB1 is a ubiquitously expressed cytokine that binds to tyrosine kinase TGFB receptors (TGFBRs) and activates a family of transcription factors called mothers against decapentaplegic, commonly known as SMADs, by phosphorylation (Branton and Kopp, 1999; Tzavlaki and Moustakas, 2020). One of the classic functions of TGFB1 is activating fibroblasts into myofibroblasts, in which they gain more actin fibers and produce more collagen (Vallée and Lecarpentier, 2019). Maroni and Davis demonstrated that TGFB1 induces collagen and laminin production by bovine luteal fibroblasts in vitro (Maroni and Davis, 2012). In luteal endothelial cells, TGFB1 decreased DNA incorporation, cell migration, and endothelial sprouting (Maroni and Davis, 2011). In that same study, TGFB1 increased caspase-3/7 activity of bovine luteal endothelial cells, disrupted the distribution of VE-cadherin, and increased endothelial cell permeability (Maroni and Davis, 2011). These findings demonstrate that TGFB1 has a direct effect on luteal angioregression by elimination of endothelial cells, increased vascular permeability, and fibrosis of the vasculature.
6 Summary and future directions
Although the exact mechanism of vascular regression of the corpus luteum remains unclear, it is known that blood flow transiently increases then is impaired. The early increase in blood flow may be from the release of vasodilators like NO during luteal regression. Based on observations in other tissues, shear stress may alter endothelial cell morphology and consequent function (Malek and Izumo, 1996; Watts, 2007). As mentioned in the review, endothelial cells themselves are also sensitive to growth factors and cytokines released during luteal regression. Increased production of cytokines in the luteal tissue microenvironment during regression may contribute to such changes in morphology as well as death of endothelial cells. The cytokine-mediated events, in conjunction with the loss of growth factors that typically signal endothelial cell survival and proliferation, contribute to angioregression in the corpus luteum. Endothelial cell apoptosis may result in sloughing of cells into capillaries and increased permeability of the vessels.
In this review, we summarized the current knowledge on changes in blood flow, presence of vasoactive factors and inflammatory cytokines, which all may contribute to angioregression of the corpus luteum. However, endothelial cells themselves may also be acting as luteolytic agents. Luteal endothelial cells can produce cytokines such as TNF as well as chemokines like monocyte chemoattractant 1 protein, which attracts immune cells to the site of luteolysis (Liptak et al., 2005; Cherry et al., 2008). Furthermore, in vivo studies revealed that PGF2α rapidly induced P-selectin, a leukocyte adhesion molecule, in bovine endothelial cells and recruited polymorphonuclear neutrophils into the corpus luteum (Shirasuna et al., 2012a). Studies in the primate indicate infiltration of macrophages, neutrophils, and natural killer cells in the regressing corpus luteum (Bishop et al., 2017). Endothelial-immune cell interactions may exacerbate luteolysis, as in vitro studies revealed that contact co-culture of endothelial cells with peripheral blood mononuclear cells was required to synergistically increase monocyte chemoattractant protein 1 (Liptak et al., 2005). Endothelial cells also express class II major histocompatibility (MHC) proteins, which allow binding and subsequent activation of T lymphocytes (Cannon et al., 2007a). Luteal endothelial cells also express proteins that stabilize MHC II binding to T-cells as well as the costimulatory molecule CD80, which induces T-cell survival and proliferation (Cannon et al., 2006; Cannon et al., 2007b). However, most of the current research focuses on the influence of endothelial cells on immune cells rather than vice versa. Studies are needed to determine how immune cell binding to luteal endothelial cells affects vascular permeability, recruitment of other immune cells and viability.
This review has centered on the actions of a number of growth factors and cytokines, but newly developing research implicates additional factors like adipokines and neuropeptides in luteal regression. The potential role of many of these factors is the subject of a recent review (Mlyczyńska et al., 2022). Many of these factors impact metabolic pathways in cells that ultimately control cellular fate. How adipokines and neuropeptides contribute to luteal angioregression is a question requiring further investigation.
It is well established that structural regression of the corpus luteum involves programmed cell death of endothelial cells and steroidogenic cells. However, recent research indicates that apoptosis, autophagic cell death, and necroptosis may all contribute to regression of the bovine corpus luteum (Hojo et al., 2022). It is not known whether and how each of these processes contribute to the demise of endothelial cells. Additionally, non-apoptotic pathways of angioregression may also occur in the regressing corpus luteum, such as a change in endothelial cell phenotype. Senescence of vascular endothelial cells has been associated with vascular dysfunction in variety of disease states by impairing new angiogenesis and endothelial-mediated changes in vascular tone (Shosha et al., 2018; Kiss et al., 2020; Cohen et al., 2021; Bloom et al., 2023a; Bloom et al., 2023b; Han and Kim, 2023). Senescent cells exhibit a pro-inflammatory phenotype (Birch and Gil, 2020); thus, if endothelial cell senescence is present in the regressing corpus luteum, then these senescent endothelial cells may be producing the inflammatory mediators that contribute to angioregression and the viability of steroidogenic cells. Research is needed to determine the phenotypes of luteal endothelial cells before and during luteal regression.
Another possibility is that some endothelial cells are changing phenotype by transdifferentiation or EndMT. This phenomenon has been implicated in vascular dysfunction of various disease states such as cancer, vascular fibrosis, sclerosis, pulmonary hypertension, and atherosclerosis (Zeisberg et al., 2007; Manetti et al., 2017; Alvandi and Bischoff, 2021; Cai et al., 2021; Gorelova et al., 2021). EndMT is known to be initiated by TGFB1, and is exacerbated by TNF in cancer-associated fibroblasts (Yoshimatsu et al., 2020); therefore, it is possible that the increases in content of TNF and TGFB1 during luteal regression can contribute to EndMT. Because luteal endothelial cells exhibit a variety of phenotypes (Spanel-Borowski and van der Bosch, 1990; Spanel-Borowski, 1991; Fenyves et al., 1994; Davis et al., 2003), it is possible that different endothelial cells undergo different forms of regression, for instance cell death compared to senescence or EndMT. Lineage tracing models using a transgenic animal model can be used to study EndMT in vivo (Sánchez-Duffhues et al., 2018), but has yet to be done in the corpus luteum to track whether EndMT occurs or when it occurs in the regression process. It is possible that EndMT may appear later in regression, when fibrosis occurs, following inflammation.
Furthermore, many of these previous studies are done in cultured endothelial cells from whole digested luteal tissue. Thus, arteriolar, venular, and microvascular endothelial cells are all considered to be the same. However, studies in other tissues indicate that the type of vessel from which the endothelial cell belonged can have an effect of its responsiveness to certain environmental stressors. For instance, Polk et al. demonstrated that human dermal microvascular cells slightly oriented themselves in response to shear stress, and Reinitz et al. demonstrated that human brain microvascular endothelial cells did not change shape as HUVECs did in response to shear stress (Reinitz et al., 2015; Polk et al., 2022). It was also demonstrated that rat endothelial cells from different vessels respond differently to vasoactive factors, such as acetylcholine-induced relaxation (Abukabda et al., 2017). In that study, it was identified that titanium dioxide nanoparticles impaired acetylcholine-induced relaxation in endothelial cells from the aorta, third-, and fourth-order mesenteric arteries, whereas it had no effect in endothelial cells from the femoral artery (Abukabda et al., 2017). Additionally, human dermal microvascular endothelial cells exhibited less damage in response to Candida albicans compared to HUVECs but more damage in response to Staphylococcus aureus (Seidl et al., 2012). The human microvascular endothelial cells also released less IL-8 compared to HUVECs in response to either pathogen (Seidl et al., 2012). Because of such differences between macro- and microvascular endothelial cell responses to pathogens and shear stress, it is important to decipher the macro- and microvascular responses separately when studying luteal angioregression. Various changes in the vasculature and causes of endothelial cell death have been identified in the corpus luteum (summarized in Table 1). However, a unifying mechanism of vascular regression has yet to be established. The current knowledge revolves around changes in blood flow and secreted factors that may affect endothelial cell viability; yet, neither the exact transcriptome or proteome of regressing vascular endothelial cells nor their varying phenotypes has yet to be studied. Single-cell proteomic or transcriptomic studies conducted during luteal regression could provide new insight on how changes in the luteal microenvironment in response to PGF2α lead to angioregression.
Furthermore, the fibrosis of various luteal vessels has yet to be explored. Studies show that while the microvasculature is disappearing the walls of larger vessels become thicker during luteal regression (Bauer et al., 2003). The presence of myofibroblast-like cells in the regressing corpus luteum (Nio-Kobayashi et al., 2016) may contribute to a fibrotic response. As reviewed above, many endothelial-reactive factors may contribute to fibrosis. Fibroblast activation by TGFB1 and FGF2 (Monaco et al., 2023) and deposition of matrix may be a major contributor to this process. EDN1 has been implicated in pulmonary fibrosis and TNF-induced EndMT in cardiac fibrosis (Swigris and Brown, 2010; Hu et al., 2023). Thrombospondins may also contribute to vascular fibrosis through TGFB1 (Sweetwyne and Murphy-Ullrich, 2012). Given that the end stage of luteal regression is a fibrotic corpus albicans, more studies are needed to elucidate the mechanism of luteal vascular regression beyond blood flow and apoptotic cell death of endothelial cells.
Author contributions
CM: Writing–original draft, Writing–review and editing. JD: Writing–original draft, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by an American Heart Association Predoctoral Fellowship (https://doi.org/10.58275/AHA.23PRE1018741.pc.gr.161093) (CM), USDA NIFA Grants 2017-67015-26450 and 2023-67015-40795 (JD), NIH grants R03HD112585 and R01 HD092263 (JD), and Department of Veterans Affairs I01 BX004272 (JD). This work was also supported by The Olson Center for Women’s Health. JD is the recipient of VA Senior Research Career Scientist Award (IK6BX005797).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abukabda A. B., Stapleton P. A., Mcbride C. R., Yi J., Nurkiewicz T. R. (2017). Heterogeneous vascular bed responses to pulmonary titanium dioxide nanoparticle exposure. Front. Cardiovasc Med. 4, 33. doi:10.3389/fcvm.2017.00033
Acosta T. J., Yoshizawa N., Ohtani M., Miyamoto A. (2002). Local changes in blood flow within the early and midcycle corpus luteum after prostaglandin F(2 alpha) injection in the cow. Biol. reproduction 66, 651–658. doi:10.1095/biolreprod66.3.651
Adams J. C., Lawler J. (2011). The thrombospondins. Cold Spring Harb. Perspect. Biol. 3, a009712. doi:10.1101/cshperspect.a009712
Adashi E. Y., Resnick C. E., Packman J. N., Hurwitz A., Payne D. W. (1990). Cytokine-mediated regulation of ovarian function: tumor necrosis factor alpha inhibits gonadotropin-supported progesterone accumulation by differentiating and luteinized murine granulosa cells. Am. J. Obstet. Gynecol. 162, 889–896. doi:10.1016/0002-9378(90)91289-o
Alan E., Kulak Y. (2021). The immunoexpression patterns of fibroblast growth factors in the pregnant and postpartum rat ovary. Reprod. Fertil. Dev. 33, 817–830. doi:10.1071/RD21025
Alvandi Z., Bischoff J. (2021). Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 41, 2357–2369. doi:10.1161/ATVBAHA.121.313788
Armstrong L. C., Bornstein P. (2003). Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 22, 63–71. doi:10.1016/s0945-053x(03)00005-2
Asakai R., Tamura K., Eishi Y., Iwamoto M., Kato Y., Okamoto R. (1993). Basic fibroblast growth factor (bFGF) receptors decrease with luteal age in rat ovarian luteal cells: colocalization of bFGF receptors and bFGF in luteal cells. Endocrinology 133, 1074–1084. doi:10.1210/endo.133.3.7689947
Augustin H. G. (2000). Vascular morphogenesis in the ovary. Baillieres Best. Pract. Res. Clin. Obstet. Gynaecol. 14, 867–882. doi:10.1053/beog.2000.0132
Azmi T. I., O'Shea J. D. (1984). Mechanism of deletion of endothelial cells during regression of the corpus luteum. Lab. Invest. 51, 206–217.
Baddela V. S., Koczan D., Viergutz T., Vernunft A., Vanselow J. (2018). Global gene expression analysis indicates that small luteal cells are involved in extracellular matrix modulation and immune cell recruitment in the bovine corpus luteum. Mol. Cell. Endocrinol. 474, 201–213. doi:10.1016/j.mce.2018.03.011
Baffert F., Le T., Sennino B., Thurston G., Kuo C. J., Hu-Lowe D., et al. (2006). Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiology-Heart Circulatory Physiology 290, H547–H559. doi:10.1152/ajpheart.00616.2005
Bagavandoss P., Wilks J. (1990). Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem. biophysical Res. Commun. 170, 867–872. doi:10.1016/0006-291x(90)92171-u
Bartok B., Firestein G. S. (2010). Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255. doi:10.1111/j.0105-2896.2009.00859.x
Basavaraja R., Przygrodzka E., Pawlinski B., Gajewski Z., Kaczmarek M. M., Meidan R. (2017). Interferon-tau promotes luteal endothelial cell survival and inhibits specific luteolytic genes in bovine corpus luteum. Reproduction 154, 559–568. doi:10.1530/REP-17-0290
Bauer M., Schilling N., Spanel-Borowski K. (2003). Development and regression of non-capillary vessels in the bovine corpus luteum. Cell tissue Res. 311, 199–205. doi:10.1007/s00441-002-0640-x
Bender H. R., Campbell G. E., Aytoda P., Mathiesen A. H., Duffy D. M. (2019). Thrombospondin 1 (THBS1) promotes follicular angiogenesis, luteinization, and ovulation in primates. Front. Endocrinol. (Lausanne) 10, 727. doi:10.3389/fendo.2019.00727
Benigni A., Cassis P., Remuzzi G. (2010). Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol. Med. 2, 247–257. doi:10.1002/emmm.201000080
Benjamin L. E., Keshet E. (1997). Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc. Natl. Acad. Sci. U. S. A. 94, 8761–8766. doi:10.1073/pnas.94.16.8761
Benton R. L., Maddie M. A., Worth C. A., Mahoney E. T., Hagg T., Whittemore S. R. (2008). Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J. Cereb. Blood Flow. Metab. 28, 1771–1785. doi:10.1038/jcbfm.2008.76
Benyo D. F., Pate J. L. (1992). Tumor necrosis factor-alpha alters bovine luteal cell synthetic capacity and viability. Endocrinology 130, 854–860. doi:10.1210/endo.130.2.1733731
Berisha B., Schams D., Rodler D., Pfaffl M. W. (2016a). Angiogenesis in the ovary - the most important regulatory event for follicle and corpus luteum development and function in cow - an overview. Anat. Histol. Embryol. 45, 124–130. doi:10.1111/ahe.12180
Berisha B., Schams D., Rodler D., Sinowatz F., Pfaffl M. W. (2016b). Expression and localization of members of the thrombospondin family during final follicle maturation and corpus luteum formation and function in the bovine ovary. J. Reprod. Dev. 62, 501–510. doi:10.1262/jrd.2016-056
Birch J., Gil J. (2020). Senescence and the SASP: many therapeutic avenues. Genes Dev. 34, 1565–1576. doi:10.1101/gad.343129.120
Bishop C. V., Xu F., Steinbach R., Ficco E., Hyzer J., Blue S., et al. (2017). Changes in immune cell distribution and their cytokine/chemokine production during regression of the rhesus macaque corpus luteum. Biol. Reprod. 96, 1210–1220. doi:10.1093/biolre/iox052
Bloom S. I., Islam M. T., Lesniewski L. A., Donato A. J. (2023a). Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 20, 38–51. doi:10.1038/s41569-022-00739-0
Bloom S. I., Liu Y., Tucker J. R., Islam M. T., Machin D. R., Abdeahad H., et al. (2023b). Endothelial cell telomere dysfunction induces senescence and results in vascular and metabolic impairments. Aging Cell 22, e13875. doi:10.1111/acel.13875
Bourdiec A., Bédard D., Rao C. V., Akoum A. (2013). Human chorionic gonadotropin regulates endothelial cell responsiveness to interleukin 1 and amplifies the cytokine-mediated effect on cell proliferation, migration and the release of angiogenic factors. Am. J. Reprod. Immunol. 70, 127–138. doi:10.1111/aji.12080
Branton M. H., Kopp J. B. (1999). TGF-beta and fibrosis. Microbes Infect. 1, 1349–1365. doi:10.1016/s1286-4579(99)00250-6
Burke G. L., Evans G. W., Riley W. A., Sharrett A. R., Howard G., Barnes R. W., et al. (1995). Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 26, 386–391. doi:10.1161/01.str.26.3.386
Cai W., Sun X., Jin F., Xiao D., Li H., Sun H., et al. (2021). PERK-eIF2α-ERK1/2 axis drives mesenchymal-endothelial transition of cancer-associated fibroblasts in pancreatic cancer. Cancer Lett. 515, 86–95. doi:10.1016/j.canlet.2021.05.021
Campinho P., Vilfan A., Vermot J. (2020). Blood flow forces in shaping the vascular system: A focus on endothelial cell behavior. Front. Physiology 11, 552. doi:10.3389/fphys.2020.00552
Cannon M. J., Davis J. S., Pate J. L. (2007b). Expression of costimulatory molecules in the bovine corpus luteum. Reprod. Biol. Endocrinol. 5, 5. doi:10.1186/1477-7827-5-5
Cannon M. J., Davis J. S., Pate J. L. (2006). Presence and regulation of messenger ribonucleic acids encoding components of the class II major histocompatibility complex-associated antigen processing pathway in the bovine corpus luteum. Reproduction 131, 689–698. doi:10.1530/rep.1.00906
Cannon M. J., Davis J. S., Pate J. L. (2007a). The class II major histocompatibility complex molecule BoLA-DR is expressed by endothelial cells of the bovine corpus luteum. Reproduction 133, 991–1003. doi:10.1530/REP-06-0362
Cherry J. A., Hou X., Rueda B. R., Davis J. S., Townson D. H. (2008). Microvascular endothelial cells of the bovine corpus luteum: A comparative examination of the estrous cycle and pregnancy. J. Reproduction Dev. 54, 183–191. doi:10.1262/jrd.19182
Cohen C., Le Goff O., Soysouvanh F., Vasseur F., Tanou M., Nguyen C., et al. (2021). Glomerular endothelial cell senescence drives age-related kidney disease through PAI-1. EMBO Mol. Med. 13, e14146. doi:10.15252/emmm.202114146
Corinaldesi G., Corinaldesi C. (2008). Arterial wall thickness: marker of atherosclerosis or risk factor for thrombosis. Blood 112, 5470. doi:10.1182/blood.v112.11.5470.5470
Csapo A. I., Pulkkinen M. O., Kaihola H. L. (1974). The relationship between the timing of luteectomy and the incidence of complete abortions. Am. J. Obstet. Gynecol. 118, 985–989. doi:10.1016/0002-9378(74)90671-1
Davis J. S., Rueda B. R., Spanel-Borowski K. (2003). Microvascular endothelial cells of the corpus luteum. Reprod. Biol. Endocrinol. 1, 89. doi:10.1186/1477-7827-1-89
Dawson D. W., Pearce S. F. A., Zhong R., Silverstein R. L., Frazier W. A., Bouck N. P. (1997). CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 138, 707–717. doi:10.1083/jcb.138.3.707
Dedkov E., Kostrominova T., Borisov A., Carlson B. (2002). Resistance vessel remodeling and reparative angiogenesis in the microcirculatory bed of long-term denervated skeletal muscles. Microvasc. Res. 63, 96–114. doi:10.1006/mvre.2001.2372
Dimmeler S., Zeiher A. M. (1999). Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 6, 964–968. doi:10.1038/sj.cdd.4400581
Djonov V., Andres A. C., Ziemiecki A. (2001). Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc. Res. Tech. 52, 182–189. doi:10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M
Doerr M. D., Goravanahally M. P., Rhinehart J. D., Inskeep E. K., Flores J. A. (2008). Effects of endothelin receptor type-A and type-B antagonists on prostaglandin F2alpha-induced luteolysis of the sheep corpus luteum. Biol. reproduction 78, 688–696. doi:10.1095/biolreprod.107.064105
Duncan W. C., Van Den Driesche S., Fraser H. M. (2008). Inhibition of vascular endothelial growth factor in the primate ovary up-regulates hypoxia-inducible factor-1alpha in the follicle and corpus luteum. Endocrinology 149, 3313–3320. doi:10.1210/en.2007-1649
Eklund L., Saharinen P. (2013). Angiopoietin signaling in the vasculature. Exp. Cell Res. 319, 1271–1280. doi:10.1016/j.yexcr.2013.03.011
Estevez A., Tognetti T., Luchetti C., Sander V., Motta A. (2003). Sequence of interleukin 1beta actions on corpus luteum regression: relationship with inducible cyclooxygenase and nitric oxide synthase expression. Reproduction 126, 639–645. doi:10.1530/rep.0.1260639
Evans J. J. (1987). The effect of prostaglandin F2 alpha administration on progesterone after hysterectomy of Guinea-pigs. Prostaglandins 33, 561–566. doi:10.1016/0090-6980(87)90279-6
Farberov S., Basavaraja R., Meidan R. (2019). Thrombospondin-1 at the crossroads of corpus luteum fate decisions. Reproduction 157, R73–R83. doi:10.1530/REP-18-0530
Farberov S., Meidan R. (2016). Thrombospondin-1 affects bovine luteal function via transforming growth factor-beta1-dependent and independent actions. Biol. Reproduction 94, 25. doi:10.1095/biolreprod.115.135822
Fenyves A. M., Saxer M., Spanel-Borowski K. (1994). Bovine microvascular endothelial cells of separate morphology differ in growth and response to the action of interferon-gamma. Experientia 50, 99–104. doi:10.1007/BF01984942
Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G., et al. (2006). Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 12, 235–239. doi:10.1038/nm1351
Förstermann U., Sessa W. C. (2012). Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837. doi:10.1093/eurheartj/ehr304
Fraser H. M., Duncan W. C. (2009). SRB reproduction, fertility and development award lecture 2008. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod. Fertil. Dev. 21, 377–392. doi:10.1071/rd08272
Frezzetti D., Gallo M., Maiello M. R., D’Alessio A., Esposito C., Chicchinelli N., et al. (2017). VEGF as a potential target in lung cancer. Expert Opin. Ther. Targets 21, 959–966. doi:10.1080/14728222.2017.1371137
Fridén B. E., Runesson E., Hahlin M., Brannstrom M. (2000). Evidence for nitric oxide acting as a luteolytic factor in the human corpus luteum. Mol. Hum. Reprod. 6, 397–403. doi:10.1093/molehr/6.5.397
Friedman A., Weiss S., Levy N., Meidan R. (2000). Role of tumor necrosis factor alpha and its type I receptor in luteal regression: induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol. Reproduction 63, 1905–1912. doi:10.1095/biolreprod63.6.1905
Fyhrquist F., Metsärinne K., Tikkanen I. (1995). Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J. Hum. Hypertens. 9 (5), S19–S24.
Geva E., Jaffe R. B. (2000). Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil. Steril. 74, 429–438. doi:10.1016/s0015-0282(00)00670-1
Ginther O., Gastal E., Gastal M., Utt M., Beg M. (2007). Luteal blood flow and progesterone production in mares. Animal Reproduction Sci. 99, 213–220. doi:10.1016/j.anireprosci.2006.05.018
Girão-Silva T., Fonseca-Alaniz M. H., Ribeiro-Silva J. C., Lee J., Patil N. P., Dallan L. A., et al. (2021). High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells. Sci. Rep. 11, 13493. doi:10.1038/s41598-021-93081-3
Girsh E., Milvae R. A., Wang W., Meidan R. (1996a). Effect of endothelin-1 on bovine luteal cell function: role in prostaglandin F2alpha-induced antisteroidogenic action. Endocrinology 137, 1306–1312. doi:10.1210/endo.137.4.8625904
Girsh E., Wang W., Mamluk R., Arditi F., Friedman A., Milvae R. A., et al. (1996b). Regulation of endothelin-1 expression in the bovine corpus luteum: elevation by prostaglandin F 2 alpha. Endocrinology 137, 5191–5196. doi:10.1210/endo.137.12.8940334
Goding J. R. (1974). The demonstration that PGF2alpha is the uterine luteolysin in the Ewe. J. Reprod. Fertil. 38, 261–271. doi:10.1530/jrf.0.0380261
Goede V., Schmidt T., Kimmina S., Kozian D., Augustin H. G. (1998). Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab. Invest. 78, 1385–1394.
Gorelova A., Berman M., Al Ghouleh I. (2021). Endothelial-to-Mesenchymal transition in pulmonary arterial hypertension. Antioxid. Redox Signal 34, 891–914. doi:10.1089/ars.2020.8169
Gospodarowicz D., Abraham J. A., Schilling J. (1989). Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc. Natl. Acad. Sci. U. S. A. 86, 7311–7315. doi:10.1073/pnas.86.19.7311
Gospodarowicz D. (1974). Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature 249, 123–127. doi:10.1038/249123a0
Greenaway J., Lawler J., Moorehead R., Bornstein P., Lamarre J., Petrik J. (2007). Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J. Cell. physiology 210, 807–818. doi:10.1002/jcp.20904
Guo Y. X., Zhang G. M., Yao X. L., Tong R., Cheng C. Y., Zhang T. T., et al. (2019). Effects of nitric oxide on steroidogenesis and apoptosis in goat luteinized granulosa cells. Theriogenology 126, 55–62. doi:10.1016/j.theriogenology.2018.12.007
Han Y., Kim S. Y. (2023). Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 55, 1–12. doi:10.1038/s12276-022-00906-w
Hayashi K., Miyamoto A. (1999). Angiotensin II interacts with prostaglandin F2alpha and endothelin-1 as a local luteolytic factor in the bovine corpus luteum in vitro. Biol. Reproduction 60, 1104–1109. doi:10.1095/biolreprod60.5.1104
Hayashi K., Miyamoto A., Berisha B., Kosmann M. R., Okuda K., Schams D. (2000). Regulation of angiotensin II production and angiotensin receptors in microvascular endothelial cells from bovine corpus luteum. Biol. Reproduction 62, 162–167. doi:10.1095/biolreprod62.1.162
Hazzard T., Christenson L., Stouffer R. (2000). Changes in expression of vascular endothelial growth factor and angiopoietin-1 and-2 in the macaque corpus luteum during the menstrual cycle. Mol. Hum. Reprod. 6, 993–998. doi:10.1093/molehr/6.11.993
Hehnke-Vagnoni K. E., Clark C. L., Taylor M. J., Ford S. P. (1995). Presence and localization of tumor necrosis factor alpha in the corpus luteum of nonpregnant and pregnant pigs. Biol. Reproduction 53, 1339–1344. doi:10.1095/biolreprod53.6.1339
Henkes L. E., Sullivan B. T., Lynch M. P., Kolesnick R., Arsenault D., Puder M., et al. (2008). Acid sphingomyelinase involvement in tumor necrosis factor alpha-regulated vascular and steroid disruption during luteolysis in vivo. Proc. Natl. Acad. Sci. U. S. A. 105, 7670–7675. doi:10.1073/pnas.0712260105
Henry F., Quatresooz P., Valverde-Lopez J. C., Piérard G. E. (2006). Blood vessel changes during pregnancy: A review. Am. J. Clin. Dermatol 7, 65–69. doi:10.2165/00128071-200607010-00006
Herzog K., Brockhan-Lüdemann M., Kaske M., Beindorff N., Paul V., Niemann H., et al. (2010). Luteal blood flow is a more appropriate indicator for luteal function during the bovine estrous cycle than luteal size. Theriogenology 73, 691–697. doi:10.1016/j.theriogenology.2009.11.016
Hinckley S., Milvae R. (2001). Endothelin-1 mediates prostaglandin F(2alpha)-induced luteal regression in the Ewe. Biol. reproduction 64, 1619–1623. doi:10.1095/biolreprod64.6.1619
Hojo T., Al-Zi'Abi M. O., Skarzynski D. J., Acosta T. J., Okuda K. (2009). Changes in the vasculature of bovine corpus luteum during the estrous cycle and prostaglandin F2alpha-induced luteolysis. J. Reproduction Dev. 55, 512–517. doi:10.1262/jrd.20257
Hojo T., Skarzynski D. J., Okuda K. (2022). Apoptosis, autophagic cell death, and necroptosis: different types of programmed cell death in bovine corpus luteum regression. J. Reprod. Dev. 68, 355–360. doi:10.1262/jrd.2022-097
Hou X., Arvisais E. W., Jiang C., Chen D.-B., Roy S. K., Pate J. L., et al. (2008). Prostaglandin F2alpha stimulates the expression and secretion of transforming growth factor B1 via induction of the early growth response 1 gene (EGR1) in the bovine corpus luteum. Mol. Endocrinol. 22, 403–414. doi:10.1210/me.2007-0272
Hu H., Huang J., Zhang S., Zhang B., Li W., Sun K. (2023). Tumor necrosis factor-α stimulation endothelial-to-mesenchymal transition during cardiac fibrosis via endothelin-1 signaling. J. Biochem. Mol. Toxicol. 37, e23411. doi:10.1002/jbt.23411
Intengan H. D., Schiffrin E. L. (2001). Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38, 581–587. doi:10.1161/hy09t1.096249
Ishii Y., Sawada T., Kubota K., Fuchinoue S., Teraoka S., Shimizu A. (2005). Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 67, 321–332. doi:10.1111/j.1523-1755.2005.00085.x
Janson P. O., Damber J. E., Axén C. (1981). Luteal blood flow and progesterone secretion in pseudopregnant rabbits. J. Reprod. Fertil. 63, 491–497. doi:10.1530/jrf.0.0630491
Jonczyk A. W., Piotrowska-Tomala K. K., Skarzynski D. J. (2021). Comparison of intra-CL injection and peripheral application of prostaglandin F(2α) analog on luteal blood flow and secretory function of the bovine corpus luteum. Front. Vet. Sci. 8, 811809. doi:10.3389/fvets.2021.811809
Kanazawa T., Seki M., Iga K. (2022). Early pregnancy diagnosis based on luteal morphology and blood flow on Days 17-21 post-artificial insemination in Japanese Black cattle. Theriogenology 181, 69–78. doi:10.1016/j.theriogenology.2022.01.002
Kidder E., Pea M., Cheng S., Koppada S.-P., Visvanathan S., Henderson Q., et al. (2023). The interleukin-1 receptor type-1 in disturbed flow-induced endothelial mesenchymal activation. Front. Cardiovasc. Med. 10, 1190460. doi:10.3389/fcvm.2023.1190460
Kiss T., Nyúl-Tóth Á., Balasubramanian P., Tarantini S., Ahire C., Delfavero J., et al. (2020). Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42, 429–444. doi:10.1007/s11357-020-00177-1
Knickerbocker J. J., Wiltbank M. C., Niswender G. D. (1988). Mechanisms of luteolysis in domestic livestock. Domest. Anim. Endocrinol. 5, 91–107. doi:10.1016/0739-7240(88)90011-2
Kobayashi H., Terao T. (1997). Hyaluronic acid-specific regulation of cytokines by human uterine fibroblasts. Am. J. Physiol. 273, C1151–C1159. doi:10.1152/ajpcell.1997.273.4.C1151
Kobayashi S., Berisha B., Amselgruber W., Schams D., Miyamoto A. (2001). Production and localisation of angiotensin II in the bovine early corpus luteum: A possible interaction with luteal angiogenic factors and prostaglandin F2alpha. J. Endocrinol. 170, 369–380. doi:10.1677/joe.0.1700369
Kolatorova L., Vitku J., Suchopar J., Hill M., Parizek A. (2022). Progesterone: A steroid with wide range of effects in physiology as well as human medicine. Int. J. Mol. Sci. 23, 7989. doi:10.3390/ijms23147989
Korzekwa A. J., Okuda K., Woclawek-Potocka I., Murakami S., Skarzynski D. J. (2006). Nitric oxide induces apoptosis in bovine luteal cells. J. Reprod. Dev. 52, 353–361. doi:10.1262/jrd.17092
Kowalczyk-Zieba I., Boruszewska D., Sinderewicz E., Skarzynski D. J., Woclawek-Potocka I. (2014). Influence of lysophosphatidic acid on nitric oxide-induced luteolysis in steroidogenic luteal cells in cows. Biol. Reprod. 90, 17. doi:10.1095/biolreprod.113.113357
Lei Z., Chegini N., Rao C. V. (1991). Quantitative cell composition of human and bovine corpora lutea from various reproductive states. Biol. reproduction 44, 1148–1156. doi:10.1095/biolreprod44.6.1148
Liptak A. R., Sullivan B. T., Henkes L. E., Wijayagunawardane M. P. B., Miyamoto A., Davis J. S., et al. (2005). Cooperative expression of monocyte chemoattractant protein 1 within the bovine corpus luteum: evidence of immune cell-endothelial cell interactions in a coculture system. Biol. Reproduction 72, 1169–1176. doi:10.1095/biolreprod.104.032953
Lopez-Castejon G., Brough D. (2011). Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 22, 189–195. doi:10.1016/j.cytogfr.2011.10.001
Lüttgenau J., Ulbrich S. E., Beindorff N., Honnens A., Herzog K., Bollwein H. (2011). Plasma progesterone concentrations in the mid-luteal phase are dependent on luteal size, but independent of luteal blood flow and gene expression in lactating dairy cows. Animal Reproduction Sci. 125, 20–29. doi:10.1016/j.anireprosci.2011.02.002
Luu R. J., Hoefler B. C., Gard A. L., Ritenour C. R., Rogers M. T., Kim E. S., et al. (2023). Fibroblast activation in response to TGFβ1 is modulated by co-culture with endothelial cells in a vascular organ-on-chip platform. Front. Mol. Biosci. 10, 1160851. doi:10.3389/fmolb.2023.1160851
Maisonpierre P. C., Suri C., Jones P. F., Bartunkova S., Wiegand S. J., Radziejewski C., et al. (1997). Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60. doi:10.1126/science.277.5322.55
Malek A. M., Izumo S. (1996). Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 109, 713–726. doi:10.1242/jcs.109.4.713
Malek M. H., Olfert I. M., Esposito F. (2010). Detraining losses of skeletal muscle capillarization are associated with vascular endothelial growth factor protein expression in rats. Exp. Physiol. 95, 359–368. doi:10.1113/expphysiol.2009.050369
Manetti M., Romano E., Rosa I., Guiducci S., Bellando-Randone S., De Paulis A., et al. (2017). Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann. Rheum. Dis. 76, 924–934. doi:10.1136/annrheumdis-2016-210229
Maroni D., Davis J. S. (2011). TGFB1 disrupts the angiogenic potential of microvascular endothelial cells of the corpus luteum. J. Cell Sci. 124, 2501–2510. doi:10.1242/jcs.084558
Maroni D., Davis J. S. (2012). Transforming growth factor Beta 1 stimulates profibrotic activities of luteal fibroblasts in cows. Biol. Reprod. 87, 127. doi:10.1095/biolreprod.112.100735
Marsden P. A., Brenner B. M. (1992). Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am. J. Physiol. Cell Physiol. 262 (4), C854–C861. doi:10.1152/ajpcell.1992.262.4.C854
Masuda H., Kawamura K., Nanjo H., Sho E., Komatsu M., Sugiyama T., et al. (2003). Ultrastructure of endothelial cells under flow alteration. Microsc. Res. Tech. 60, 2–12. doi:10.1002/jemt.10237
Masuda H., Zhuang Y. J., Singh T. M., Kawamura K., Murakami M., Zarins C. K., et al. (1999). Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler. Thromb. Vasc. Biol. 19, 2298–2307. doi:10.1161/01.atv.19.10.2298
Mckeller R. N., Fowler J. L., Cunningham J. J., Warner N., Smeyne R. J., Zindy F., et al. (2002). The Arf tumor suppressor gene promotes hyaloid vascular regression during mouse eye development. Proc. Natl. Acad. Sci. 99, 3848–3853. doi:10.1073/pnas.052484199
Meadows K. L., Hurwitz H. I. (2012). Anti-VEGF therapies in the clinic. Cold Spring Harb. Perspect. Med. 2, a006577. doi:10.1101/cshperspect.a006577
Merna N., Wong A. K., Barahona V., Llanos P., Kunar B., Palikuqi B., et al. (2018). Laminar shear stress modulates endothelial luminal surface stiffness in a tissue-specific manner. Microcirculation 25, e12455. doi:10.1111/micc.12455
Mesiano S. (2022). Progesterone – historical perspective. J. Steroid Biochem. Mol. Biol. 223, 106157. doi:10.1016/j.jsbmb.2022.106157
Miró J., Vilés K., Anglada O., Marín H., Jordana J., Crisci A. (2015). Color Doppler provides a reliable and rapid means of monitoring luteolysis in female donkeys. Theriogenology 83, 485–490. doi:10.1016/j.theriogenology.2014.10.007
Mishra S., Parmar M., Yadav V., Reshma R., Bharati J., Bharti M., et al. (2016). Expression and localization of angiopoietin family in corpus luteum during different stages of oestrous cycle and modulatory role of angiopoietins on steroidogenesis, angiogenesis and survivability of cultured buffalo luteal cells. Reproduction Domest. Animals 51, 855–869. doi:10.1111/rda.12739
Miyamoto A., Kobayashi S., Arata S., Ohtani M., Fukui Y., Schams D. (1997). Prostaglandin F2 alpha promotes the inhibitory action of endothelin-1 on the bovine luteal function in vitro. J. Endocrinol. 152, R7–R11. doi:10.1677/joe.0.152r007
Miyamoto A., Shirasuna K., Wijayagunawardane M. P., Watanabe S., Hayashi M., Yamamoto D., et al. (2005). Blood flow: A key regulatory component of corpus luteum function in the cow. Domest. Anim. Endocrinol. 29, 329–339. doi:10.1016/j.domaniend.2005.03.011
Miyazaki T., Tanaka M., Miyakoshi K., Minegishi K., Kasai K., Yoshimura Y. (1998). Power and colour Doppler ultrasonography for the evaluation of the vasculature of the human corpus luteum. Hum. Reprod. Oxf. Engl. 13, 2836–2841. doi:10.1093/humrep/13.10.2836
Mlyczyńska E., Kieżun M., Kurowska P., Dawid M., Pich K., Respekta N., et al. (2022). New aspects of corpus luteum regulation in physiological and pathological conditions: involvement of adipokines and neuropeptides. Cells 11, 957. doi:10.3390/cells11060957
Modlich U., Kaup F. J., Augustin H. G. (1996). Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab. Invest. 74, 771–780.
Monaco C. F., Plewes M. R., Przygrodzka E., George J. W., Qiu F., Xiao P., et al. (2023). Basic fibroblast growth factor induces proliferation and collagen production by fibroblasts derived from the bovine corpus luteum. Biol. Reproduction 109, 367–380. doi:10.1093/biolre/ioad065
Mondal M., Schilling B., Folger J., Steibel J. P., Buchnick H., Zalman Y., et al. (2011). Deciphering the luteal transcriptome: potential mechanisms mediating stage-specific luteolytic response of the corpus luteum to prostaglandin F₂α. Physiol. Genomics 43, 447–456. doi:10.1152/physiolgenomics.00155.2010
Motta A. B., Estevez A., De Gimeno M. F. (1999). The involvement of nitric oxide in corpus luteum regression in the rat: feedback mechanism between prostaglandin F(2alpha) and nitric oxide. Mol. Hum. Reprod. 5, 1011–1016. doi:10.1093/molehr/5.11.1011
Nayak G., Odaka Y., Prasad V., Solano A. F., Yeo E.-J., Vemaraju S., et al. (2018). Developmental vascular regression is regulated by a Wnt/β-catenin, MYC and CDKN1A pathway that controls cell proliferation and cell death. Development 145, dev154898. doi:10.1242/dev.154898
Nett T., Mcclellan M., Niswender G. (1976). Effects of prostaglandins on the ovine corpus luteum: blood flow, secretion of progesterone and morphology. Biol. reproduction 15, 66–78. doi:10.1095/biolreprod15.1.66
Neuvians T. P., Berisha B., Schams D. (2004a). Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) expression during induced luteolysis in the bovine corpus luteum. Mol. Reprod. Dev. 67, 389–395. doi:10.1002/mrd.20032
Neuvians T. P., Schams D., Berisha B., Pfaffl M. W. (2004b). Involvement of pro-inflammatory cytokines, mediators of inflammation, and basic fibroblast growth factor in prostaglandin F2alpha-induced luteolysis in bovine corpus luteum. Biol. Reprod. 70, 473–480. doi:10.1095/biolreprod.103.016154
Ng K. K. F., Vane J. R. (1967). Conversion of angiotensin I to angiotensin II. Nature 216, 762–766. doi:10.1038/216762a0
Nio-Kobayashi J., Miyazaki K., Hashiba K., Okuda K., Iwanaga T. (2016). Histological analysis of arteriovenous anastomosis-like vessels established in the corpus luteum of cows during luteolysis. J. Ovarian Res. 9, 67. doi:10.1186/s13048-016-0277-0
O'Shea J., Rodgers R., D'Occhio M. (1989). Cellular composition of the cyclic corpus luteum of the cow. Reproduction 85, 483–487. doi:10.1530/jrf.0.0850483
Ohtani M., Kobayashi S., Miyamoto A., Hayashi K., Fukui Y. (1998). Real-time relationships between intraluteal and plasma concentrations of endothelin, oxytocin, and progesterone during prostaglandin F2alpha-induced luteolysis in the cow. Biol. Reprod. 58, 103–108. doi:10.1095/biolreprod58.1.103
Olenich S. A., Audet G. N., Roberts K. A., Olfert I. M. (2014). Effects of detraining on the temporal expression of positive and negative angioregulatory proteins in skeletal muscle of mice. J. Physiol. 592, 3325–3338. doi:10.1113/jphysiol.2014.271213
Olive J. L., Dudley G. A., Mccully K. K. (2003). Vascular remodeling after spinal cord injury. Med. Sci. Sports Exerc. 35, 901–907. doi:10.1249/01.MSS.0000069755.40046.96
Orr A. W., Elzie C. A., Kucik D. F., Murphy-Ullrich J. E. (2003). Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 116, 2917–2927. doi:10.1242/jcs.00600
Otani N., Minami S., Yamoto M., Shikone T., Otani H., Nishiyama R., et al. (1999). The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J. Clin. Endocrinol. Metab. 84, 3845–3851. doi:10.1210/jcem.84.10.6025
Palumbo A., Ávila J., Naftolin F. (2016). The ovarian renin-angiotensin system (ovras): A major factor in ovarian function and disease. Reprod. Sci. 23, 1644–1655. doi:10.1177/1933719116672588
Pepperell J. R., Nemeth G., Yamada Y., Naftolin F., Merino M. (2006). Localized accumulation of angiotensin II and production of angiotensin-(1-7) in rat luteal cells and effects on steroidogenesis. Am. J. Physiol. Endocrinol. Metab. 291, E221–E233. doi:10.1152/ajpendo.00205.2005
Piotrowska-Tomala K. K., Jonczyk A. W., Kordowitzki P., Jalali B. M., Skarzynski D. J. (2021). The effect of basic fibroblast growth factor 2 on the bovine corpus luteum depends on the stage of the estrous cycle and modulates prostaglandin F2α action. Animal 15, 100048. doi:10.1016/j.animal.2020.100048
Pitzel L., Jarry H., Wuttke W. (1993). Effects and interactions of prostaglandin F2 alpha, oxytocin, and cytokines on steroidogenesis of porcine luteal cells. Endocrinology 132, 751–756. doi:10.1210/endo.132.2.8425493
Planas A. M. (2020). Top ten discoveries of the year: neurovascular disease. Free Neuropathol. 1, 1-5. doi:10.17879/freeneuropathology-2020-2615
Polk T., Schmitt S., Aldrich J. L., Long D. S. (2022). Human dermal microvascular endothelial cell morphological response to fluid shear stress. Microvasc. Res. 143, 104377. doi:10.1016/j.mvr.2022.104377
Pru J. K., Lynch M. P., Davis J. S., Rueda B. R. (2003). Signaling mechanisms in tumor necrosis factor alpha-induced death of microvascular endothelial cells of the corpus luteum. Reprod. Biol. Endocrinol. 1, 17. doi:10.1186/1477-7827-1-17
Qin W., Lu W., Li H., Yuan X., Li B., Zhang Q., et al. (2012). Melatonin inhibits IL1β-induced MMP9 expression and activity in human umbilical vein endothelial cells by suppressing NF-κB activation. J. Endocrinol. 214, 145–153. doi:10.1530/JOE-12-0147
Redmer D., Dai Y., Li J., Charnock-Jones D., Smith S., Reynolds L., et al. (1996). Characterization and expression of vascular endothelial growth factor (VEGF) in the ovine corpus luteum. Reproduction 108, 157–165. doi:10.1530/jrf.0.1080157
Reinitz A., Destefano J., Ye M., Wong A. D., Searson P. C. (2015). Human brain microvascular endothelial cells resist elongation due to shear stress. Microvasc. Res. 99, 8–18. doi:10.1016/j.mvr.2015.02.008
Reynolds L., Redmer D. (1999). Growth and development of the corpus luteum. J. Reprod. Fertil. Suppl. 54, 181–191.
Ricke W. A., Redmer D. A., Reynolds L. P. (1999). Growth and cellular proliferation of pig corpora lutea throughout the oestrous cycle. J. Reprod. Fertil. 117, 369–377. doi:10.1530/jrf.0.1170369
Romero J. J., Antoniazzi A. Q., Smirnova N. P., Webb B. T., Yu F., Davis J. S., et al. (2013). Pregnancy-associated genes contribute to antiluteolytic mechanisms in ovine corpus luteum. Physiol. Genomics 45, 1095–1108. doi:10.1152/physiolgenomics.00082.2013
Robinson R. S., Nicklin L. T., Hammond A. J., Schams D., Hunter M. G., Mann G. E. (2007). Fibroblast growth factor 2 is more dynamic than vascular endothelial growth factor A during the follicle-luteal transition in the cow. Biol. Reproduction 77, 28–36. doi:10.1095/biolreprod.106.055434
Rodgers R. J., O'Shea J. D., Bruce N. W. (1984). Morphometric analysis of the cellular composition of the ovine corpus luteum. J. Anat. 138, 757–770.
Rosiansky-Sultan M., Klipper E., Spanel-Borowski K., Meidan R. (2006). Inverse relationship between nitric oxide synthases and endothelin-1 synthesis in bovine corpus luteum: interactions at the level of luteal endothelial cell. Endocrinology 147, 5228–5235. doi:10.1210/en.2006-0795
Sakumoto R., Berisha B., Kawate N., Schams D., Okuda K. (2000). Tumor necrosis factor-alpha and its receptor in bovine corpus luteum throughout the estrous cycle. Biol. Reproduction 62, 192–199. doi:10.1095/biolreprod62.1.192
Sakumoto R., Vermehren M., Kenngott R. A., Okuda K., Sinowatz F. (2011). Localization of gene and protein expressions of tumor necrosis factor-{alpha} and tumor necrosis factor receptor types I and II in the bovine corpus luteum during the estrous cycle. J. Animal Sci. 89, 3040–3047. doi:10.2527/jas.2010-3479
Sánchez-Duffhues G., García De Vinuesa A., Ten Dijke P. (2018). Endothelial-to-mesenchymal transition in cardiovascular diseases: developmental signaling pathways gone awry. Dev. Dyn. 247, 492–508. doi:10.1002/dvdy.24589
Santos R. A. (2014). Angiotensin-(1–7). Hypertension 63, 1138–1147. doi:10.1161/HYPERTENSIONAHA.113.01274
Sawamura T., Kimura S., Shinmi O., Sugita Y., Yanagisawa M., Masaki T. (1989). Analysis of endothelin related peptides in culture supernatant of porcine aortic endothelial cells: evidence for biosynthetic pathway of endothelin-1. Biochem. Biophys. Res. Commun. 162, 1287–1294. doi:10.1016/0006-291x(89)90813-9
Schams D., Berisha B., Neuvians T., Amselgruber W., Kraetzl W. D. (2003). Real-time changes of the local vasoactive peptide systems (angiotensin, endothelin) in the bovine corpus luteum after induced luteal regression. Mol. Reprod. Dev. 65, 57–66. doi:10.1002/mrd.10257
Seidl K., Solis N. V., Bayer A. S., Hady W. A., Ellison S., Klashman M. C., et al. (2012). Divergent responses of different endothelial cell types to infection with Candida albicans and Staphylococcus aureus. PLoS One 7, e39633. doi:10.1371/journal.pone.0039633
Sheikh S., Rahman M., Gale Z., Luu N. T., Stone P. C., Matharu N. M., et al. (2005). Differing mechanisms of leukocyte recruitment and sensitivity to conditioning by shear stress for endothelial cells treated with tumour necrosis factor-alpha or interleukin-1beta. Br. J. Pharmacol. 145, 1052–1061. doi:10.1038/sj.bjp.0706281
Shirasuna K., Jiemtaweeboon S., Raddatz S., Nitta A., Schuberth H. (2012a). Rapid accumulation of polymorphonuclear neutrophils in the corpus.
Shirasuna K., Nitta A., Sineenard J., Shimizu T., Bollwein H., Miyamoto A. (2012b). Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest. Anim. Endocrinol. 43, 198–211. doi:10.1016/j.domaniend.2012.03.007
Shirasuna K., Watanabe S., Asahi T., Wijayagunawardane M. P., Sasahara K., Jiang C., et al. (2008). Prostaglandin F2alpha increases endothelial nitric oxide synthase in the periphery of the bovine corpus luteum: the possible regulation of blood flow at an early stage of luteolysis. Reproduction 135, 527–539. doi:10.1530/REP-07-0496
Shirasuna K., Watanabe S., Oki N., Wijayagunawardane M. P. B., Matsui M., Ohtani M., et al. (2006). A cooperative action of endothelin-1 with prostaglandin F(2alpha) on luteal function in the cow. Domest. Anim. Endocrinol. 31, 186–196. doi:10.1016/j.domaniend.2005.10.004
Shosha E., Xu Z., Narayanan S. P., Lemtalsi T., Fouda A. Y., Rojas M., et al. (2018). Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int. J. Mol. Sci. 19, 1215. doi:10.3390/ijms19041215
Skarzynski D. J., Ferreira-Dias G., Okuda K. (2008). Regulation of luteal function and corpus luteum regression in cows: hormonal control, immune mechanisms and intercellular communication. Reprod. Domest. Anim. 43 (2), 57–65. doi:10.1111/j.1439-0531.2008.01143.x
Smith M. F., Mcintush E. W., Smith G. W. (1994). Mechanisms associated with corpus luteum development. J. Anim. Sci. 72, 1857–1872. doi:10.2527/1994.7271857x
Spanel-Borowski K. (1991). Diversity of ultrastructure in different phenotypes of cultured microvessel endothelial cells isolated from bovine corpus luteum. Cell Tissue Res. 266, 37–49. doi:10.1007/BF00678709
Spanel-Borowski K., Van Der Bosch J. (1990). Different phenotypes of cultured microvessel endothelial cells obtained from bovine corpus luteum. Study by light microscopy and by scanning electron microscopy (SEM). Cell Tissue Res. 261, 35–47. doi:10.1007/BF00329436
Stocco C., Telleria C., Gibori G. (2007). The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 28, 117–149. doi:10.1210/er.2006-0022
Stouffer R. L., Bishop C. V., Bogan R. L., Xu F., Hennebold J. D. (2013). Endocrine and local control of the primate corpus luteum. Reprod. Biol. 13, 259–271. doi:10.1016/j.repbio.2013.08.002
Sugino N., Suzuki T., Sakata A., Miwa I., Asada H., Taketani T., et al. (2005). Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J. Clin. Endocrinol. Metabolism 90, 6141–6148. doi:10.1210/jc.2005-0643
Sweetwyne M. T., Murphy-Ullrich J. E. (2012). Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol. 31, 178–186. doi:10.1016/j.matbio.2012.01.006
Swigris J. J., Brown K. K. (2010). The role of endothelin-1 in the pathogenesis of idiopathic pulmonary fibrosis. BioDrugs 24, 49–54. doi:10.2165/11319550-000000000-00000
Talbott H., Hou X., Qiu F., Zhang P., Guda C., Yu F., et al. (2017). Early transcriptome responses of the bovine midcycle corpus luteum to prostaglandin F2α includes cytokine signaling. Mol. Cell Endocrinol. 452, 93–109. doi:10.1016/j.mce.2017.05.018
Tamura H., Greenwald G. S. (1987). Angiogenesis and its hormonal control in the corpus luteum of the pregnant rat. Biol. Reproduction 36, 1149–1154. doi:10.1095/biolreprod36.5.1149
Tanaka J., Acosta T. J., Berisha B., Tetsuka M., Matsui M., Kobayashi S., et al. (2004). Relative changes in mRNA expression of angiopoietins and receptors tie in bovine corpus luteum during estrous cycle and prostaglandin F2alpha-induced luteolysis: A possible mechanism for the initiation of luteal regression. J. Reprod. Dev. 50, 619–626. doi:10.1262/jrd.50.619
Tanfin Z., Leiber D., Robin P., Oyeniran C., Breuiller-Fouché M. (2011). Endothelin-1: physiological and pathological roles in myometrium. Int. J. Biochem. Cell Biol. 43, 299–302. doi:10.1016/j.biocel.2010.10.009
Tao Y., Fu Z., Zhang M., Xia G., Yang J., Xie H. (2004). Immunohistochemical localization of inducible and endothelial nitric oxide synthase in porcine ovaries and effects of NO on antrum formation and oocyte meiotic maturation. Mol. Cell. Endocrinol. 222, 93–103. doi:10.1016/j.mce.2004.04.014
Vallée A., Lecarpentier Y. (2019). TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell & Biosci. 9, 98. doi:10.1186/s13578-019-0362-3
Van Linthout S., Miteva K., Tschöpe C. (2014). Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 102, 258–269. doi:10.1093/cvr/cvu062
Vega M., Urrutia L., Iñiguez G., Gabler F., Devoto L., Johnson M. C. (2000). Nitric oxide induces apoptosis in the human corpus luteum in vitro. Mol. Hum. Reprod. 6, 681–687. doi:10.1093/molehr/6.8.681
Vonnahme K. A., Redmer D. A., Borowczyk E., Bilski J. J., Luther J. S., Johnson M. L., et al. (2006). Vascular composition, apoptosis, and expression of angiogenic factors in the corpus luteum during prostaglandin F2alpha-induced regression in sheep. Reproduction 131, 1115–1126. doi:10.1530/rep.1.01062
Walewska E., Wołodko K., Skarzynski D., Ferreira-Dias G., Galvão A. (2019). The interaction between nodal, hypoxia-inducible factor 1 alpha, and thrombospondin 1 promotes luteolysis in equine corpus luteum. Front. Endocrinol. (Lausanne) 10, 667. doi:10.3389/fendo.2019.00667
Wang F., Riley J. C. M., Behrman H. R. (1993). Immunosuppressive levels of glucocorticoid block extrauterine luteolysins in the rat. Biol. Reproduction 49, 66–73. doi:10.1095/biolreprod49.1.66
Watts S. W. (2007). “Intrinsic regulation of the vasculature,” in xPharm: The comprehensive pharmacology reference. Editors S. J. ENNA, and D. B. BYLUND (New York: Elsevier).
Wiegel R. E., Karsten M. J. H., Reijnders I. F., Van Rossem L., Willemsen S. P., Mulders A., et al. (2021). Corpus luteum number and the maternal renin-angiotensin-aldosterone system as determinants of utero-placental (vascular) development: the rotterdam periconceptional cohort. Reprod. Biol. Endocrinol. 19, 164. doi:10.1186/s12958-021-00843-9
Wiltbank M., Gallagher K., Christensen A., Brabec R., Keyes P. (1990). Physiological and immunocytochemical evidence for a new concept of blood flow regulation in the corpus luteum. Biol. Reproduction 42, 139–149. doi:10.1095/biolreprod42.1.139
Wiltbank M., Lopez H., Sartori R., Sangsritavong S., Gümen A. (2006). Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 65, 17–29. doi:10.1016/j.theriogenology.2005.10.003
Woad K. J., Robinson R. S. (2016). Luteal angiogenesis and its control. Theriogenology 86, 221–228. doi:10.1016/j.theriogenology.2016.04.035
Wulff C., Wilson H., Largue P., Duncan W. C., Armstrong D. G., Fraser H. M. (2000). Angiogenesis in the human corpus luteum: localization and changes in angiopoietins, tie-2, and vascular endothelial growth factor messenger ribonucleic acid. J. Clin. Endocrinol. Metabolism 85, 4302–4309. doi:10.1210/jcem.85.11.6942
Yamashita H., Kamada D., Shirasuna K., Matsui M., Shimizu T., Kida K., et al. (2008). Effect of local neutralization of basic fibroblast growth factor or vascular endothelial growth factor by a specific antibody on the development of the corpus luteum in the cow. Mol. Reproduction Dev. 75, 1449–1456. doi:10.1002/mrd.20878
Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., et al. (1988). A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415. doi:10.1038/332411a0
Yin M., Pacifici M. (2001). Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev. Dyn. 222, 522–533. doi:10.1002/dvdy.1212
Yoshimatsu Y., Wakabayashi I., Kimuro S., Takahashi N., Takahashi K., Kobayashi M., et al. (2020). TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation. Cancer Sci. 111, 2385–2399. doi:10.1111/cas.14455
Yoshimura Y. (1997). The ovarian renin–angiotensin system in reproductive physiology. Front. Neuroendocrinol. 18, 247–291. doi:10.1006/frne.1997.0152
Yu Y., Leng Y., Song X., Mu J., Ma L., Yin L., et al. (2023). Extracellular matrix stiffness regulates microvascular stability by controlling endothelial paracrine signaling to determine pericyte fate. Arterioscler. Thromb. Vasc. Biol. doi:10.1161/ATVBAHA.123.319119
Zalman Y., Klipper E., Farberov S., Mondal M., Wee G., Folger J. K., et al. (2012). Regulation of angiogenesis-related prostaglandin f2alpha-induced genes in the bovine corpus luteum. Biol. Reproduction 86, 92. doi:10.1095/biolreprod.111.095067
Zeisberg E. M., Potenta S., Xie L., Zeisberg M., Kalluri R. (2007). Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 67, 10123–10128. doi:10.1158/0008-5472.CAN-07-3127
Keywords: corpus luteum, angioregression, endothelial cells, PGF2α, luteolysis, ovary
Citation: Monaco CF and Davis JS (2023) Mechanisms of angioregression of the corpus luteum. Front. Physiol. 14:1254943. doi: 10.3389/fphys.2023.1254943
Received: 17 July 2023; Accepted: 18 September 2023;
Published: 28 September 2023.
Edited by:
William C. W. Chen, University of South Dakota, United StatesReviewed by:
Marta Tesone, University of Buenos Aires, ArgentinaMahita Kadmiel, Allegheny College, United States
Copyright © 2023 Monaco and Davis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John S. Davis, anNkYXZpc0B1bm1jLmVkdQ==
 Corrine F. Monaco
Corrine F. Monaco John S. Davis
John S. Davis