
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 31 August 2023
Sec. Aquatic Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1248999
This article is part of the Research TopicEndocrine Regulation and Physiological Adaptation of Stress Response in Aquatic OrganismsView all 18 articles
 Tingting Shu1,2*
Tingting Shu1,2* Yan Chen1,2,3
Yan Chen1,2,3 Kan Xiao1,2
Kan Xiao1,2 Hongtao Huang1,2
Hongtao Huang1,2 Jingyi Jia4
Jingyi Jia4 Zhaoxi Yu1,2
Zhaoxi Yu1,2 Wei Jiang1,2
Wei Jiang1,2 Jing Yang1,2*
Jing Yang1,2*Since 2011, ecological operation trials of the Three Gorges Reservoir (TGR) have been continuously conducted to improve the spawning quantity of the four major Chinese carp species below the Gezhouba Dam. In particular, exploring the effects of short-term water velocity stimulation on ovarian development in grass carp (Ctenopharyngodon idellus) is essential to understand the response of natural reproduction to ecological flows. We performed ovary histology analysis and biochemical assays among individuals with or without stimulation by running water. Although there were no obvious effects on the ovarian development characteristics of grass carp under short-term water velocity stimulation, estradiol, progesterone, follicle-stimulating hormone (FSH), and triiodothyronine (T3) concentrations were elevated. Then, we further explored the ovarian development of grass carp under short-term water velocity stimulation by RNA sequencing of ovarian tissues. In total, 221 and 741 genes were up- or downregulated under short-term water velocity stimulation, respectively, compared to the control group. The majority of differentially expressed genes (DEGs) were enriched in pathways including ABC transporters, cytokine-cytokine receptor interaction, ECM-receptor interaction, and steroid hormone biosynthesis. Important genes including gpr4, vtg1, C-type lectin, hsd17b1, cyp19a1a, cyp17a1, and rdh12 that are involved in ovarian development were regulated. Our results provide new insights and reveal potential regulatory genes and pathways involved in the ovarian development of grass carp under short-term water velocity stimulation, which may be beneficial when devising further ecological regulation strategies.
Construction of hydraulic engineering not only significantly alters the natural hydrologic regime and water quality in the upstream and downstream of the dam (Poff and Schmidt, 2016; Chen et al., 2020), but also has undesirable ecological effects on aquatic species (Stone, 2016), and finally reduces the aquatic biodiversity (Liu et al., 2021). As the most severely affected biota at the top of the aquatic food chain, fish are often chosen as indicator species for determining the health status of the riverine ecosystems (Gao et al., 2009; She et al., 2023). Dam operations affect the spawning activity of native fish species and thus threaten aquatic populations and communities (Barbarossa et al., 2020; Mouchlianitis et al., 2021), and have been reported on rivers all over the world, such as the Madeira River, the Snake River, and the Colorado River in foreign basins (Finch et al., 2015; Cella-Ribeiro et al., 2017; McClure et al., 2020), as well as the Yangtze River, the Jinsha River, the Pearl River, the Han River, the Gan River, and the Lancang-Mekong River in domestic basins (Zhang C. et al., 2019; Zhang P. et al., 2019).
Fish reproduction is likely triggered by a variety of environmental factors, including flow velocity, water temperature, photoperiod, and dissolved oxygen (King et al., 2016; Buddendorf et al., 2017; Fellman et al., 2019). These environmental factors have a complex impact on fish spawning behaviors and gonadal development through adjusting the biological process of hormone synthesis and secretion. Among these environmental factors, water flow velocity is a key environmental factor that affects spawning and fertilization for fishes delivering drifting eggs (Lechner et al., 2014). Moreover, water flow velocity plays a crucial role in determining nutrient retention and oxygen delivery during fish spawning (McDonnell, 2000). Additionally, water flow velocity also affects gonadal development. Stimulated by water flows, fish generate impulses into the hypothalamus through sensory organs, and stimulate gonadotropin-releasing hormone (GnRH) release that direct acts on GnRH nerve terminals (Liu et al., 2021). Besides, the weakening of the water flow stimulus will lead to a decline in gonadal development among fish, resulting in a reduction of spawning quantity in watersheds, and potentially causing long-term cumulative differences in the population structure of fish stocks (Zhang W. et al., 2019).
The four major Chinese carp species, including grass carp (Ctenopharyngodon idellus), silver carp (Hypophthalmichthys molitrix), bighead carp (Hypophthalmichthys nobilis), and black carp (Mylopharyngodon piceus), play important roles in Chinese aquaculture and capture fisheries (Cao et al., 2015). A spawning site from Yichang to Chenglingji, situated at the middle reaches of the Yangtze River, is one of the most important natural reproduction zones of these Chinese carps, which accounting for 42.7% of the total spawning capacity along the river (Guo et al., 2011; Li et al., 2013a). However, in recent decades, due to the remarkable changes in hydrological conditions caused by the impoundment of the Three Gorges Reservoir (TGR), spawning behaviors and sexual maturation process of the four carp species have been severely hindered (Chen et al., 2021). In 2003, the number of fish eggs and larvae was only 10% of that in 2002, when the TGR began operation (Xie and Chen, 2001; Li et al., 2013b). Previous studies showed that water flow velocity suitable for grass carp spawning and sexual maturation mainly ranged from 0.33 to 1.50 m/s (Lin et al., 2022). Thus, providing essential ecological flows is widely recognized as an effective means of maintaining ecological integrity and restoring habitats for the spawning of major fish species in rivers (Stamou et al., 2018; Yang et al., 2019). However, the physiological mechanism of the response of natural reproduction to ecological flows is still unclear.
In this study, we used sexually mature female grass carp to conduct laboratory experiments to explore the effect of flow velocity on gonadal development in fish. The objective of the present study was to understand how flow velocity affect the gonadal development of grass carp. Specifically, we sought to a) explore the effect of short-term flow velocity on biochemical response, and b) analyze the possible regulatory mechanism of short-term flow velocity on the gonadal development of female grass carp. Our findings will provide a scientific basis for riverine ecosystem protection of the Yangtze River.
Sexually mature females (n = 30, body length of 70.56 ± 3.13 cm, body weight of 5.16 ± 0.85 kg) and males (n = 30, body length of 68.40 ± 3.03 cm, body weight of 4.45 ± 0.70 kg) of grass carp were collected from Tengda Ecological Agriculture Development Co., Ltd. in Zhijiang City, Hubei Province, China. The grass carps were domesticated in Chinese Sturgeon Research Institute for 2 weeks, kept in a recirculating aquaculture system under controlled temperature conditions (21°C ± 0.5°C) with constant aeration, and fed in excess duckweed twice a day prior to the experimental trials.
The experiment was performed in 20,000 L PVC circular tanks. Sixty grass carps were randomly divided into three groups with 10 females and 10 males, including the water velocity stimulation group, the hormone injection group, and the control group, labelled ZS, JS, and NS, respectively. Previous studies showed that suitable water flow velocity for grass carp spawning and sexual maturation mainly ranged from 0.33 to 1.50 m/s. However, even though ecological flows are provided, water mobility will weaken significantly in the middle and lower reaches of the Yangtze River. It was found that the flow velocity is rarely exceeding 0.5 m/s from April to June (Zhou et al., 2009; Wang et al., 2016; Liu et al., 2021). In some reservoir areas, the flow velocity cannot even reach 0.2 m/s (Xu et al., 2017). Therefore, we set a water velocity of 0.5 m/s in the ZS group. In the JS group, the females were injected with 2 mg/kg domperidone and 2.5 μg/kg LHRH-A2, at the base of the pectoral fin.
After 3 h, females were selected for sampling, including blood and ovaries. To avoid catching stress, fish were anesthetized by immersion with benzocaine (200 mg/L) (Geraylou et al., 2012; Wang et al., 2015), and then killed by a sharp blow to the head based on a previously described procedure (Hultmann et al., 2012). Blood samples were collected quickly from the caudal vein of each fish, then centrifuged at 3,000 g for 15 min at 4°C. The obtained serum samples were then transferred to −80°C for storage until enzyme linked immunosorbent assay (ELISA). Ovary tissues were also collected, and some of them were quickly fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at room temperature for 24 h for histological sections, and other ovaries were rapidly frozen in liquid nitrogen, then stored at −80°C until ELISA and RNA extraction.
The fixed ovaries were dehydrated in graded ethanol solutions, and infiltrated with xylene. The sectioning and staining procedures were performed as described in a previous study (Lau et al., 2016). Briefly, the samples were embedded and processed for paraffin sectioning using a Leica RM2235 microtome (Leica Biosystems, Germany). Paraffin sections of 5 µm in thickness were mounted on slides, deparaffinized, rehydrated, and washed with ultrapure water. After staining with H&E, dehydrated, and mounted, the sections were observed and imaged by a Nikon Eclipse Ni-U microscope (Nikon, Japan). Scale bars are provided in the lower right corner of each image.
The concentrations of testosterone, estradiol, progesterone, and 17α,20β-dihydroxy-4-pregnen-3-one (DHP), as well as triiodothyronine (T3), thyroxine (T4), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) in serum and ovaries were measured using commercial ELISA kits (mlbio, Shanghai). Briefly, 300 µL of serum was diluted to 500 µL with PBS, while 0.1 g ovary samples were isolated and homogenized in 1 mL PBS in a TGrinder H24R Tissue Homogenizer (TIANGEN, China). Following homogenization, the hormones were extracted with an organic solvent four times according to the manufacturer’s instructions. The layers were allowed to separate by vortex and centrifugation. Then the organic phase was transferred to a fresh tube and evaporated by heating to 30°C under a gentle stream of nitrogen. Finally, the extracts were dissolved in 200 µL ELISA buffer and the hormone concentrations were measured according to the manufacturer’s instructions.
The ovary samples from grass carp were isolated and homogenized in a TGrinder H24R Tissue Homogenizer (TIANGEN, China), and total RNA was extracted by TRIzol reagent (Ambion, America) following the manufacturer’s instructions. RNA concentration was determined using NanoDrop One (Thermo Scientific, America), and the integrity and quality were assessed by RNA denaturing gel electrophoresis. High-quality RNA samples were selected for library construction. Using an Illumina NovaSeq 6000 system, RNA-Seq reads were generated by sequencing. Fastp (version 0.19.7) was used to generate clean reads. Then de novo assembly of clean reads was conducted with Trinity software (v2.6.6) and all assembled full-length sequences were named unigenes. All the unigenes were predicted and used for Blastx search and annotation against the NR, NT, KOG, SwissProt, PFAM, KEGG, and GO databases. After assembly, gene expression abundance was calculated using the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values. Differential expression analysis was performed using the DESeq2 package (v1.20.0) with a log2 fold change (FC) of 1.5 and a p-value cutoff of 0.05 (|log2FC|≥1.5, p < 0.05). GO function enrichment analysis and KEGG pathway enrichment analysis of differential gene sets were implemented using the Bioconductor R package clusterProfiler (v 3.18.1).
Independent RNA samples were extracted and used for cDNA synthesis for qRT-PCR to confirm the transcriptome results. RNA template with a content of 1.5 μg was used for reverse transcription to synthesize cDNA using the EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing) according to the manufacturer’s guidelines. All primers were designed using Primer-BLAST in the National Center for Biotechnology Information (NCBI), and the sequences are listed in Table 1. The primers for qRT-PCR were validated by agarose gel electrophoresis and DNA sequencing of PCR products. For amplification, the TransStart® Tip Green qPCR SuperMix (TransGen Biotech, Beijing) and StepOnePlus™ real-time system (ABI, America) were used. All mRNA levels were calculated as the fold expression relative to the housekeeping gene, β-actin, ef1a, and gapdh and expressed as a fold change compared to the control group. Each sample was run in triplicate repeats and data analysis was performed using the ΔΔCt method (Schmittgen and Livak, 2008).
Statistical analysis was performed with GraphPad Prism 8.0 software (GraphPad software, America). All results were presented as mean ± standard deviation (SD) in each experimental group. Differences were determined using one-way ANOVA followed by Fisher’s least significant difference (LSD) test for multiple comparisons. For all statistical comparisons, p < 0.05 was considered statistically significant.
To determine the maturation level of grass carp gonads, we examined dissected ovaries by histological sections with H&E staining. Well-differentiated ovaries were observed in all groups, occupied by many full-grown follicles with normal reproductive characteristics (Figure 1). After short-term water velocity stimulation and the hormone injection, respectively, the development characteristics were similar to those of the control group. Specifically, the yolk filled the ovaries. Oocytes were easily identified by their large spherical nucleoli, each of which contained numerous nucleoli and a large cytoplasmic region bordered by a visible cell membrane. Each oocyte was a different size and was surrounded by a thin follicular cell layer. Most follicular cells contained ovoid nuclei and were stained with black, indicating that the fish used was in the pre-spawning period. No substantial effect was observed in the histological sections of the JS and ZS groups when compared to the NS group.
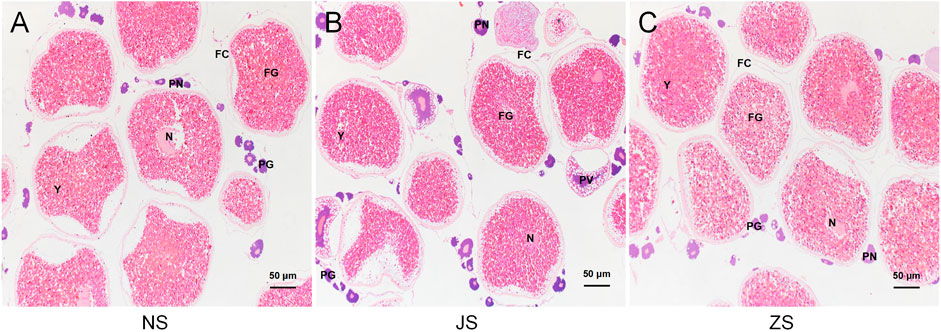
FIGURE 1. Histological sections of the ovarian status of grass carp. (A) NS group. (B) JS group. (C) ZS group. PG, primary growth follicle; PN, perinucleolar follicle; PV, previtellogenic follicle; FG, full-grown follicle; N, nucleus; Y, yolks; FC, follicle cells.
The concentrations of several important sex steroids were measured in female grass carp. Serum and ovary testosterone concentrations did not differ among the three groups (Figures 2A,E). Serum and ovary estradiol, progesterone, as well as DHP concentrations were significantly higher in the JS group than those in the NS group (Figures 2B–D,F–H). However, only ovary estradiol and progesterone concentrations in the ZS group were significantly elevated (Figures 2F,G). Serum estradiol, progesterone, and DHP concentrations, as well as ovary DHP concentration in the ZS group were slightly increased, although there were not statistically significant (Figures 2B–D,H).
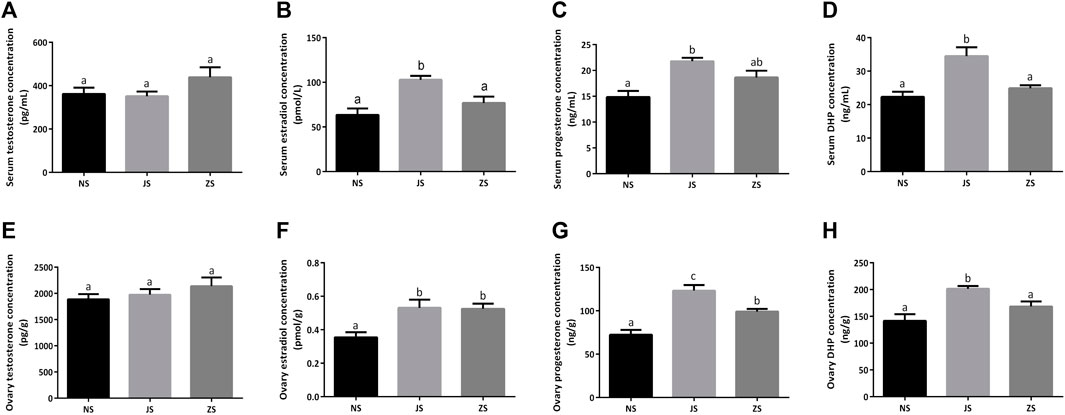
FIGURE 2. The sex steroid hormones measurements. Serum concentrations of testosterone (A), estradiol (B), progesterone (C), and DHP (D) in the NS, JS, and ZS groups. Ovary concentrations of testosterone (E), estradiol (F), progesterone (G), and DHP (H) in the NS, JS, and ZS groups. The letters in the bar charts represent significant differences.
Meanwhile, we examined serum and ovary gonadotropins, FSH and LH, in female grass carp. Both serum and ovary FSH and LH concentrations in the JS group were significantly increased compared to the NS group (Figures 3A,B,E,F). However, only enhanced serum and ovary FSH concentrations were observed in the ZS group (Figures 3A,E). LH concentrations were also compared, but did not show any significant differences. (Figures 3B,F).
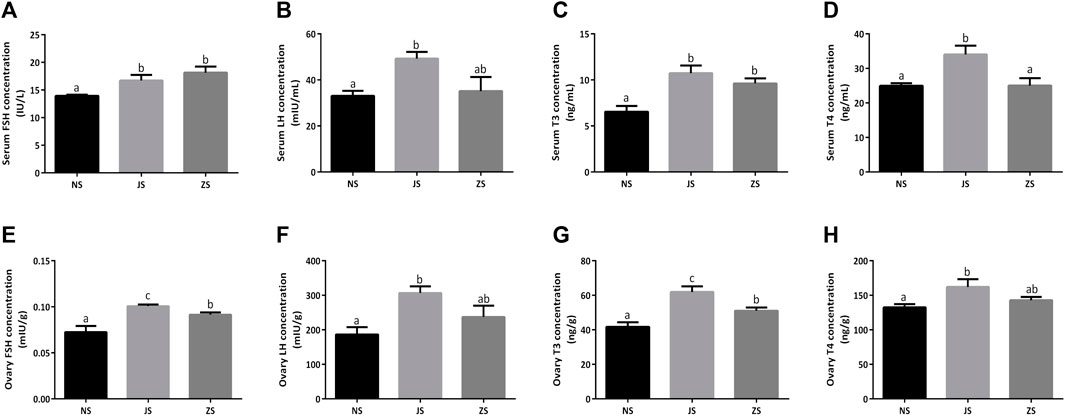
FIGURE 3. The gonadotropins and thyroid hormones measurements. Serum concentrations of FSH (A), LH (B), T3 (C), and T4 (D) in the NS, JS, and ZS groups. Ovary concentrations of FSH (E), LH (F), T3 (G), and T4 (H) in the NS, JS, and ZS groups. The letters in the bar charts represent significant differences.
Serum and ovary T3 and T4 concentrations were also evaluated in female grass carp. We observed that serum and ovary T3 concentrations in the JS and ZS groups were significantly higher than that in the NS group while elevated serum and ovary T4 concentrations were only observed in the JS group (Figures 3C,D,G,H).
To identify the underlying molecular signaling pathways of short-term water velocity stimulation on the ovary transcriptional profile in grass carp, nine mRNA libraries were constructed and sequenced from NS, JS, and ZS ovary tissues using the Illumina NovaSeq 6000 system. All datasets from the Illumina sequencing platform are available in the NCBI Short Read Archive (SRA) database with the accession number (PRJNA977722). The main sequencing characteristics are listed in Table 2. A total length of 206,508,378 bp raw reads were obtained from the nine samples, and an average length of 21,593,012 bp clean reads were obtained from each sample after strict filtering. The Q20, Q30, and mapping rates for each group were within 96.90%–97.31%, 92.29%–93.18%, and 83.94%–85.97%, respectively, indicating that sequencing quality was acceptable for further analysis. Totally, 37,976 unigenes were acquired from de novo assembly. All unigenes were annotated by seven databases, including NR, NT, KOG, SwissProt, PFAM, KEGG, and GO databases (Table 3).
RNA sequencing analysis showed that the transcriptome profiles of JS and ZS groups were distinct from the NS group. Out of 21,248 annotated genes, we identified 561 differentially regulated genes between the JS and NS groups. Among the 561 regulated genes, 287 genes were highly expressed in the NS group while 274 genes were upregulated in the JS group (Figures 4A,C). Compared with expression levels in the NS group, 962 genes were differentially expressed in the ZS group, of which 221 and 741 were up- or downregulated in the ovary of treated fish (Figures 4B,C). Genes including G protein-coupled receptor 4 (gpr4), caspase a (caspa), solute carrier family 12, member 2 (slc12a2), sterile alpha motif domain containing 9 like (samd9l), SRY-box transcription factor 4 (sox4), forkhead box B1 (foxb1), collagen type IV alpha 1 chain (col4a1), early growth response 1 (egr1), vitellogenin 1 (vtg1), and cytochrome P450 family 17, subfamily A member 1 (cyp17a1) were significantly regulated (Table 4). These results suggest that short-term water velocity stimulation had a significant effect on transcription in the ovary.
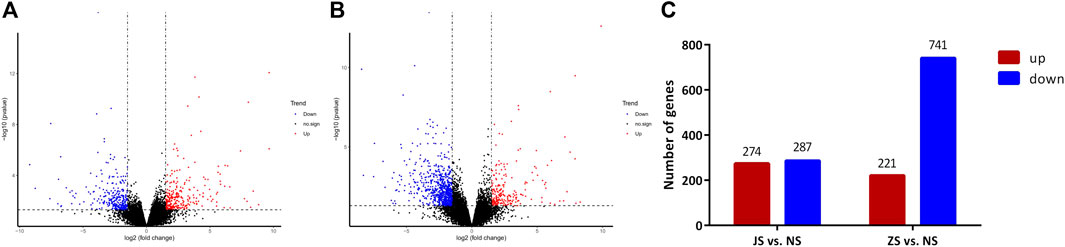
FIGURE 4. Volcano diagram of differential expression genes and numbers of DEGs in each comparison. (A) Volcano diagram of differential expression genes in JS vs. NS group. (B) Volcano diagram of differential expression genes in ZS vs. NS group. Significantly upregulated and downregulated genes are indicated in red and blue, respectively, and those not significantly different are in black. (C) The number of differentially expressed genes between different groups.
We evaluated DEGs between the NS and JS groups, as well as the NS and ZS groups by GO and KEGG functional enrichment analyses. GO analysis revealed that genes from different signaling networks were significantly affected in the JS and ZS groups compared to the NS group. The important gene ontology upregulated in the JS and ZS groups included signaling receptor activator activity (cxcl11.6, slc12a2, abcg5), signaling receptor binding (cxcl11.6, slc12a2, abcg5), transmembrane signaling receptor activity (gpr4, or131-2, tas1r1, cd84), and nucleoside binding (rab9a, rab1a, abcc2) (Figures 5A,B, Supplemental Table S1, S2). Significantly downregulated gene ontology in the JS and ZS groups contained tetrapyrrole binding (ba1, lama1, cyp17a1, cyp19a1a), cell wall macromolecule metabolic process (col4a1, pc), DNA polymerase activity (pol, znf180), and ubiquitin-like protein transferase activity (znf180, znf333, birc6, ercc6, rnf114) (Figures 5C,D, Supplemental Table S1, S2).
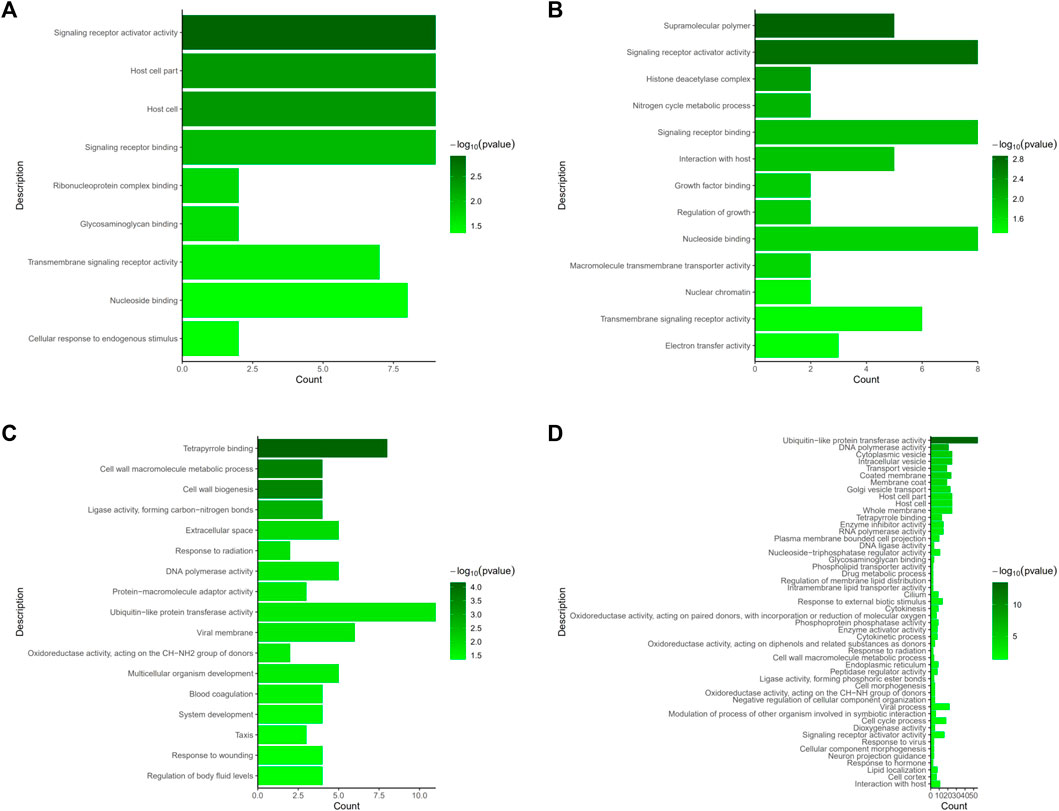
FIGURE 5. GO terms enrichment analysis of the differentially expressed genes. (A) Upregulated GO terms in JS vs. NS group. (B) Upregulated GO terms in ZS vs. NS group. (C) Downregulated GO terms in JS vs. NS group. (D) Downregulated GO terms in ZS vs. NS group.
By comparing the DEGs to the KEGG pathway enrichment database, potential functions of the significant DEGs were analyzed to further understand the ovarian development of grass carp under short-term water velocity stimulation. For short-term water velocity stimulation treatment, 30 KEGG pathways (7 upregulated and 23 downregulated pathways) were significantly enriched in the ZS group (Figures 6B,D). Besides, compared with the NS group, 37 KEGG pathways (11 upregulated and 26 downregulated pathways) were significantly enriched in the JS group (Figures 6A,C). The KEGG enrichment analysis showed that genes involved in different pathways, such as ABC transporters (abcc2, abcg5), bile secretion (abcc2, abcg5), cytosolic DNA-sensing pathway (caspa, cxcl11.6), legionellosis (caspa, rab1a), ECM-receptor interaction (lama1, col1a1, col4a1, col6a2, col1a2, col6a1), protein digestion and absorption (mme, col1a1, col4a1, col6a2, col1a2, col6a1), ovarian steroidogenesis (cyp17a1, cyp19a1a, hsd17b1), focal adhesion (lama1, col1a1, col4a1, col6a2, col1a2, col6a1), steroid hormone biosynthesis (cyp17a1, cyp19a1a, hsd17b1), and AGE-RAGE signaling pathway in diabetic complications (col1a1, col4a1, col1a2) were differentially regulated in the JS and ZS groups (Figures 6A–D; Supplemental Table S3, S4).
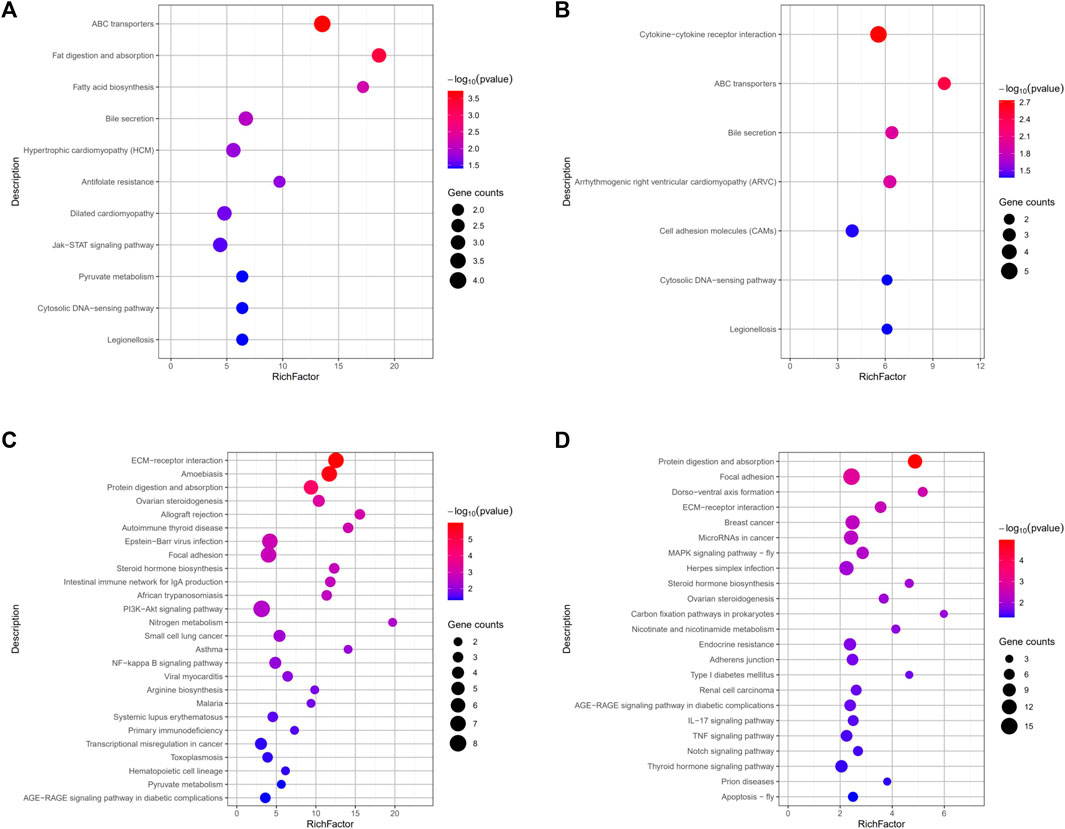
FIGURE 6. KEGG pathways enrichment analysis of the differentially expressed genes. (A) Upregulated pathways in JS vs. NS group. (B) Upregulated pathways in ZS vs. NS group. (C) Downregulated pathways in JS vs. NS group. (D) Downregulated pathways in ZS vs. NS group.
Four DEGs (cyp17a1, hsd17b1, slc12a2, and vtg1) were randomly selected to further validate the reliability of DEGs identified by RNA-Seq. The qRT-PCR results were consistent with those of RNA-Seq (Figure 7; Supplemental Figure S1, S2), indicating that the RNA-Seq data was accurate.
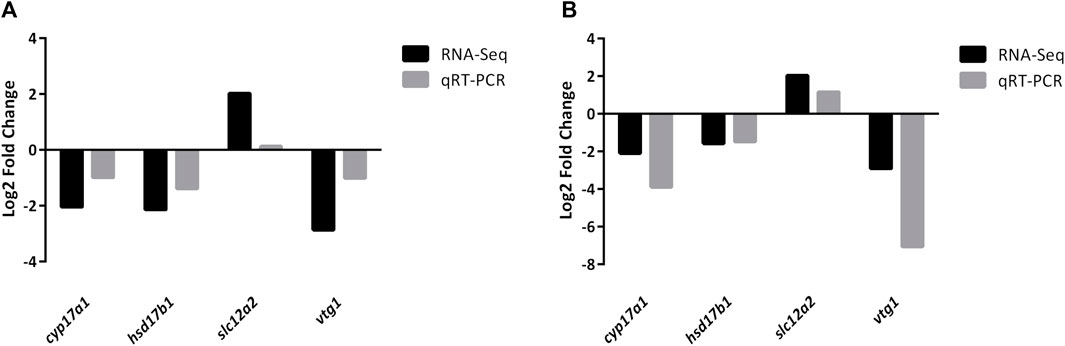
FIGURE 7. Validation of randomly selected four DEGs of RNA-Seq results using qRT-PCR analysis. All mRNA levels were calculated as the fold expression relative to the housekeeping gene β-actin. (A) Relative fold change of DEGs between qRT-PCR and RNA-Seq results in JS vs. NS group. (B) Relative fold change of DEGs between qRT-PCR and RNA-Seq results in ZS vs. NS group. Relative expression levels from the RNA-Seq results were calculated as log2FC values.
Because of the influence of large reservoirs distributed in the Yangtze River, the flow regime has been severely altered and flow velocity in the middle and lower river reaches is rarely exceeding 0.5 m/s from April to June (Zhou et al., 2009; Wang et al., 2016; Liu et al., 2021). In some reservoir areas, the flow velocity cannot even reach 0.2 m/s (Xu et al., 2017). To explore the effect of flow velocity on the ovarian development of female grass carp, we evaluated ovarian histology, hormone concentrations, and transcription levels of genes related to the ovaries. Our results revealed that even if ovarian development characteristics were not affected by short-term water velocity stimulation, the concentrations of sex steroids, gonadotropins, and thyroid hormones, as well as the transcriptional levels were significantly altered in female grass carp. These findings provide fundamental knowledge for technical support for ecological protection and restoration of hydraulic engineering.
Normal oocyte development and maturation are critical for successful reproduction in fish (Nakayama et al., 2004). However, no spawning activity was observed in the ZS group at a velocity of 0.5 m/s. Previous studies have shown that the required flow velocity for spawning differs greatly among different fish species. The determined triggering velocity of female silver carp was about 1.0 m/s in flume experiments (Chen et al., 2021), and female Atlantic salmon (Salmo salar)) constructs spawning redds in areas with an averaged flow velocity of 0.53 m/s (Beland et al., 1982). Generally, for the four major Chinese carp species, the velocity during the spawning period was 0.6–1.3 m/s, and the most appropriate velocity was 0.9–1.0 m/s (Dai et al., 2022).
The hypothalamus-pituitary-gonad (HPG) axis is responsible for fundamental regulation of all developmental stages of ovarian follicles, including progression to maturation or follicular atresia. And the pituitary gonadotropins, FSH and LH, which subsequently act on the ovary and regulate ovarian follicular development, maturity, steroidogenesis, and growth factor production, are the key players in the HPG axis (Patino et al., 2001; Nagahama and Yamashita, 2008; Zhang D. et al., 2022). In this study, we used serum and ovary testosterone, estradiol, progesterone, and DHP, as well as FSH, LH, T3 and T4 levels to represent gonadal development (Gadekar, 2014; Tucker et al., 2020), and measured these levels using ELISA. Normal reproductive functions in female fish are attributed to the sex steroid hormones, which mainly include testosterone, estradiol, progesterone, and DHP. By triggering germinal vesicle breakdown during final oocyte maturation, testosterone may contribute to oocyte growth and development (So et al., 1985). Moreover, testosterone is also involved in female steroidogenesis and acts as a substrate for aromatase during estradiol synthesis, which concentration is not stable in female fish (Barannikova et al., 2004). Estradiol is a crucial sex steroid hormone, and plays a significant role in stimulating the liver to produce the yolk precursor protein, vitellogenin, which is subsequently incorporated into the developing oocyte (Barannikova et al., 2004). Estradiol levels increase dramatically in the oocytes during vitellogenesis and decrease when vitellogenesis is complete (Amiri et al., 1996; Barannikova, 1999). Progesterone is a vital steroidogenic mediator for oocyte growth and maturation in female fish (Al-Hasawi, 2022). Our study showed that ovary estradiol and progesterone concentrations were all upregulated in the ZS and JS groups compared to the NS group (Figures 2F,G), indicating a positive effect of flow stimulation on fish gonad development. DHP acts as the most potent maturation-inducing steroid (MIS) in stimulating final oocyte maturation in fish (Amiri et al., 1999). In our study, serum and ovary DHP concentration in the ZS group was slightly increased (Figures 2D,H), although this was not statistically significant. Pituitary gonadotropins, FSH and LH, are major regulators of steroidogenesis by the ovary, resulting in the synthesis of sex steroid hormones that play critical roles in the orderly progression of growth and development of ovarian follicles (Harding et al., 2023). It is well known that thyroid hormones, T3 and T4, play a dominant role in oocyte development and final maturation and are well established in fish (Weber et al., 1992). We found that serum and ovary FSH and T3 concentrations in the ZS group were significantly elevated, as well as in the JS group (Figures 3A,C,E,G). However, no significant changes in LH and T4 levels were observed in the ZS group (Figures 3B,D,F,H). The underlying mechanism is unknown and requires further exploration. However, these data still show that short-term water velocity stimulation has an important influence on the ovarian development in female grass carp.
To understand the impact of short-term water velocity stimulation on gene expression of selected endocrine pathways and explore their underlying molecular mechanisms in fish, ovary samples from the NS, JS, and ZS groups were analyzed. Transcriptomic analysis of ovaries from the NS, JS, and ZS groups provided evidence that the water flow velocity is important for regulating genes from different signaling pathways. G protein-coupled receptors (GPRs), the largest membrane receptor family in eukaryotes, play a pivotal role in regulating various essential physiological and biochemical processes, including sexual maturation and reproduction (Flaherty et al., 2008; Nguyen et al., 2018). Ovarian development and maturation are controlled by many important factors, such as hormones and their receptors, which predominantly bind and activate GPRs on the cell surface, thereby initiating multiple downstream cascades (Zhang X. et al., 2022). We showed that gpr4 was significantly upregulated in the ZS group, suggesting that it may regulate the ovarian development of grass carp under short-term water velocity stimulation. In fish oocytes, lectin may prevent polyspermy fertilization and participate in the formation of fertilization shell through binding with glycoproteins. Furthermore, lectin and vitellin are closely bound to ovomucin to form the basic structure of the vitellin outer membrane (Kido et al., 1992). In our study, the expression of C-type lectin was upregulated in the ZS group, suggesting that lectin affects ovarian development in grass carp under short-term water velocity stimulation.
Vitellogenin 1 (Vtg1) which plays a very important role in oocytes development was downregulated in the ZS group (Table 4). A large proportion of energy-related biomolecules from the liver, such as vitellogenin and lipids, are absorbed and utilized by the reproductive system (Della Torre et al., 2014; Zhang D. et al., 2022). In the turbot (Scophthalmus maximus), Xue et al. found that the ovary displayed a higher estradiol level and lower vtg expression, indicating that some other factors limit high vtg expression (Xue et al., 2018). Similar to the results of the present experiment, previous work in conger eel (Conger myriaster) also reported that flowing water could inhibit the gene expression of liver vtg and reduce VTG synthesis in the liver, which may promote lipid accumulation (Liu et al., 2022). In our study, flowing water stimulation may inhibit yolk accumulation during the ovarian development of grass carp. Retinol and its derivatives are known to play important roles in female reproductive processes, including follicular development, ovarian steroidogenesis, and oocyte maturation (Wang et al., 2022). Retinol dehydrogenase 12 (rdh12), a novel member of the microsomal short-chain dehydrogenase/reductase protein superfamily, has been identified as a key component in steroid metabolism (Keller and Adamski, 2007). In our results, rdh12 expression was also decreased in the ZS group. Researchers have identified several genes that encode crucial enzymes in the steroidogenesis pathway, including cyp19a1a, cyp17a1, and hsd17b1, which could synthesize estradiol and progesterone, and play an important role in ovarian development and reproduction in fish (Fang et al., 2019; Li et al., 2021). Transcriptomic analysis showed that cyp19a1a, cyp17a1, and hsd17b1 were also downregulated in the ZS group compared to the NS group. It has been proven that cyp19a1a, cyp17a1, and hsd17b1 mRNAs showed a significant decrease when oocytes matured. Moreover, gene set enrichment analysis (GSEA) showed that the steroid hormone biosynthesis pathway was downregulated and that cyp19a1a, cyp17a1, and hsd17b1 were core genes in this pathway (Dong et al., 2021). These findings revealed that these genes and this pathway play key roles in oocyte maturation. As previously described in ovoviviparous black rockfish (Sebastes schlegeli), the transcription level of cyp19a1a in ovary declined when the ovary developed from vitellogenic stage to ovulation stage during the reproductive cycle (Wen et al., 2014). A decreased cyp19a1a expression was also reported in amago salmon (Oncorhynchus rhodurus), rainbow trout (Oncorhynchus mykiss), and spotted scat (Scatophagus argus) during final maturation (Young et al., 1983; Gohin et al., 2011; Liu et al., 2015). In Japanese eel (Anguilla japonica), the expression of related transcripts hsd17b1 and cyp19a1a declined at the migratory nucleus stage (Lai et al., 2022). These suggest that they can promote gonadal maturation in grass carp.
Utilizing KEGG pathway enrichment, the main biochemical metabolism and signal transduction pathways involved in genes can be identified. Generally, follicle development strongly depends on communication between germ cells and surrounding somatic cells through cytokine-cytokine receptor interaction, such as Kit and KitL (Matzuk et al., 2002; Saatcioglu et al., 2016). We observed a great number of genes in cytokine-cytokine receptor interaction obviously upregulated in ovaries of the ZS group (Figure 6B; Supplemental Table S4), indicating that the normal conservation between germ cells and somatic cells was already activated. Pathway analysis results indicated that 7 genes, including lama1, col1a1, col1a2, col4a1, col6a1, col6a2, and col6a3, enriched in ECM-receptor interaction were down regulated under short-term water velocity stimulation (Figure 6D; Supplemental Table S4). The pathways associated with ECM-receptor interaction play crucial roles in various biological processes, including cell migration, proliferation, follicle growth, and oocyte maturation (Berkholtz et al., 2006; Reing et al., 2009; Deng et al., 2022). Therefore, we speculate that these genes might have significant implications in the transition from the follicular development stage to the oocyte maturation stage. However, the specific mechanism remains unknown, and the functions of these genes in the reproductive cycle require further study.
We investigated the ovarian development of grass carp under short-term water velocity stimulation by histology analysis, biochemical assays, and RNA-Seq technology. Although there was no obvious effect on the ovarian development characteristics of grass carp under short-term water velocity stimulation, estradiol, progesterone, FSH, and T3 concentrations were elevated. Totally, 962 DEGs with 741 downregulated genes and 221 upregulated genes were identified in transcriptome data. The key genes identified were enriched in ABC transporters, cytokine-cytokine receptor interaction, ECM-receptor interaction, and steroid hormone biosynthesis, which play an essential role in the response of the ovaries in grass carp to short-term water velocity stimulation. This study provides new insights into the ovarian development of grass carp under short-term water velocity stimulation. However, these transcriptomic data are still preliminary, and the function of the DEGs in reproductive cycle of fish species requires further investigation.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA977722.
The animal study was approved by Animal ethics committee of Institute of Hydrobiology, Chinese Academy of Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
TS conducted most of the experiments for this work. YC, KX, and HH provided help with fish rearing and sampling. JJ, ZY, and WJ gave valuable suggestion and discussion, TS and JY performed training and provided insights for this work. TS wrote the paper and prepared all of the figures. All authors contributed to the article and approved the submitted version.
This study received funding from Hubei Provincial Natural Science Foundation of China (2022CFB738), and Director’s Fund of the Hubei Key Laboratory of Three Gorges Project for Conservation of Fishes, China Three Gorges Corporation (25901-2022-27). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
We would like to thank Xin Zhu, Binzhong Wang, Dezhi Zhang, and Wei Wang from Chinese Sturgeon Research Institute, China Three Gorges Corporation for the support of the culture system in this research work.
TS, YC, KX, HH, ZY, WJ, and JY are employed by China Three Gorges Corporation.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1248999/full#supplementary-material
Al-Hasawi, Z. M. M. (2022). Adverse impacts of toxic metal pollutants on sex steroid hormones of Siganus rivulatus (teleostei: siganidae) from the red sea. Fishes 7 (6), 367. doi:10.3390/fishes7060367
Amiri, B. M., Maebayashi, M., Adachi, S., Moberg, G. P., Doroshov, S. I., and Yamauchi, K. (1999). In vitro steroidogenesis by testicular fragments and ovarian follicles in a hybrid sturgeon, Bester. Fish Physiology Biochem. 21 (1), 1–14. doi:10.1023/a:1007706128184
Amiri, B. M., Maebayashi, M., Hara, A., Adachi, S., and Yamauchi, K. (1996). Ovarian development and serum sex steroid and vitellogenin profiles in the female cultured sturgeon hybrid, the bester. J. fish Biol. 48, 1164–1178. doi:10.1111/j.1095-8649.1996.tb01812.x
Barannikova, I. A., Bayunova, L. V., and Semenkova, T. B. (2004). Serum levels of testosterone, 11-ketotestosterone and oestradiol-17beta in three species of sturgeon during gonadal development and final maturation induced by hormonal treatment. J. Fish Biol. 64 (5), 1330–1338. doi:10.1111/j.0022-1112.2004.00395.x
Barannikova, I. A. (1999). Sex steroids in the serum of Caspian sturgeons and their specific cytosol binding in brain and gonads during the migratory cycle. J. Appl. Ichthyology-Zeitschrift Fur Angewandte Ichthyologie 15 (4-5), 193–195. doi:10.1111/j.1439-0426.1999.tb00232.x
Barbarossa, V., Schmitt, R. J. P., Huijbregts, M. A. J., Zarfl, C., King, H., and Schipper, A. M. (2020). Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci. U. S. A. 117 (7), 3648–3655. doi:10.1073/pnas.1912776117
Beland, K. F., Jordan, R. M., and Meister, A. L. (1982). Water depth and velocity preferences of spawning Atlantic salmon in Maine rivers. North Am. J. Fish. Manag. 2(1), 11–13.
Berkholtz, C. B., Shea, L. D., and Woodruff, T. K. (2006). Extracellular matrix functions in follicle maturation. Seminars Reproductive Med. 24 (4), 262–269. doi:10.1055/s-2006-948555
Buddendorf, W. B., Malcolm, I. A., Geris, J., Fabris, L., Millidine, K. J., Wilkinson, M. E., et al. (2017). Spatio-temporal effects of river regulation on habitat quality for Atlantic salmon fry. Ecol. Indic. 83, 292–302. doi:10.1016/j.ecolind.2017.08.006
Cao, L., Naylor, R., Henriksson, P., Leadbitter, D., Metian, M., Troell, M., et al. (2015). Global food supply. China's aquaculture and the world's wild fisheries. Science 347 (6218), 133–135. doi:10.1126/science.1260149
Cella-Ribeiro, A., da Costa Doria, C. R., Dutka-Gianelli, J., Alves, H., and Torrente-Vilara, G. (2017). Temporal fish community responses to two cascade run-of-river dams in the Madeira River, Amazon basin. Ecohydrology 10 (8), e1889. doi:10.1002/eco.1889
Chen, Q., Shi, W., Huisman, J., Maberly, S. C., Zhang, J., Yu, J., et al. (2020). Hydropower reservoirs on the upper Mekong River modify nutrient bioavailability downstream. Natl. Sci. Rev. 7 (9), 1449–1457. doi:10.1093/nsr/nwaa026
Chen, Q., Zhang, J., Chen, Y., Mo, K., Wang, J., Tang, L., et al. (2021). Inducing flow velocities to manage fish reproduction in regulated rivers. Engineering 7 (2), 178–186. doi:10.1016/j.eng.2020.06.013
Dai, L. Q., Wang, Y., Dai, H. C., Li, W., Zheng, T. G., and Zhang, Q. S. (2022). Assessment of environmental flow requirements for four major Chinese carps in the lower reaches of the Jinsha River, southwest China. Front. Ecol. Evol. 10. doi:10.3389/fevo.2022.810889
Della Torre, S., Benedusi, V., Fontana, R., and Maggi, A. (2014). Energy metabolism and fertility-a balance preserved for female health. Nat. Rev. Endocrinol. 10 (1), 13–23. doi:10.1038/nrendo.2013.203
Deng, H., Sun, A., and Yang, S. (2022). A characterization and comparative analysis of ovary transcriptome in locally adopted xiangxi and angus cattle. Pak. J. Agric. Sci. 59 (2), 341–347. doi:10.21162/pakjas/22.765
Dong, Y., Lyu, L., Zhang, D., Li, J., Wen, H., and Shi, B. (2021). Integrated lncRNA and mRNA transcriptome analyses in the ovary of Cynoglossus semilaevis reveal genes and pathways potentially involved in reproduction. Front. Genet. 12, 671729. doi:10.3389/fgene.2021.671729
Fang, D.-A., Yang, X.-j., Feng, X., Zhou, Y.-F., Xu, D.-P., Zhang, M.-Y., et al. (2019). FoxL2 combined with Cyp19a1a regulate the spawning upstream migration in Coilia nasus. Gene 710, 307–315. doi:10.1016/j.gene.2019.05.037
Fellman, J. B., Hood, E., Nagorski, S., Hudson, J., and Pyare, S. (2019). Interactive physical and biotic factors control dissolved oxygen in salmon spawning streams in coastal Alaska. Aquat. Sci. 81 (1), 2. doi:10.1007/s00027-018-0597-9
Finch, C., Pine, W., and Limburg, K. (2015). Do hydropeaking flows alter juvenile fish growth rates? A test with juvenile humpback chub in the Colorado river river. River Res. Appl. 31 (2), 156–164. doi:10.1002/rra.2725
Flaherty, P., Radhakrishnan, M. L., Dinh, T., Rebres, R. A., Roach, T. I., Jordan, M. I., et al. (2008). A dual receptor crosstalk model of G-protein-coupled signal transduction. Plos Comput. Biol. 4 (9), e1000185. doi:10.1371/journal.pcbi.1000185
Gadekar, G. P. (2014). Studies on the seasonal histomorphological changes in the ovary of Indian major carp, Labeo Rohita (HAM). Bioscan 9 (3), 1037–1042.
Gao, X., Zhao, S., Zhang, C., and XiangyangTu, (2009). Index system and method for assessing the health status of river. J. Hydraulic Eng. 40 (8), 962–968. doi:10.3321/j.issn:0559-9350.2009.08.010
Geraylou, Z., Souffreau, C., Rurangwa, E., D'Hondt, S., Callewaert, L., Courtin, C. M., et al. (2012). Effects of arabinoxylan-oligosaccharides (AXOS) on juvenile Siberian sturgeon (Acipenser baerii) performance, immune responses and gastrointestinal microbial community. Fish Shellfish Immunol. 33 (4), 718–724. doi:10.1016/j.fsi.2012.06.010
Gohin, M., Bodinier, P., Fostier, A., Chesnel, F., and Bobe, J. (2011). Aromatase is expressed and active in the rainbow trout oocyte during final oocyte maturation. Mol. Reproduction Dev. 78 (7), 510–518. doi:10.1002/mrd.21335
Guo, W., Wang, H., Xu, J., Xia, Z., Ma, X. H., Wang, Y. C., et al. (2011). Gender-specific interactions between alcohol metabolism genes and severity of quantitative alcohol-related-traits in a Tibetan population. J. Hydroelectr. Eng. 30(3), 22–25. doi:10.1016/j.neulet.2011.03.020
Harding, L., Schultz, I. R., Young, G., and Swanson, P. (2023). Salmonid pituitary cells as a test system for identifying endocrine disrupting compounds. Environ. Toxicol. Chem. 42, 1730–1742. doi:10.1002/etc.5644
Hultmann, L., Phu, T. M., Tobiassen, T., Aas-Hansen, O., and Rustad, T. (2012). Effects of pre-slaughter stress on proteolytic enzyme activities and muscle quality of farmed Atlantic cod (Gadus morhua). Food Chem. 134 (3), 1399–1408. doi:10.1016/j.foodchem.2012.03.038
Keller, B., and Adamski, J. (2007). RDH12, a retinol dehydrogenase causing Leber's congenital amaurosis, is also involved in steroid metabolism. J. Steroid Biochem. Mol. Biol. 104 (3-5), 190–194. doi:10.1016/j.jsbmb.2007.03.015
Kido, S., Morimoto, A., Kim, F., and Doi, Y. K. (1992). Isolation of a novel protein from the outer layer of the vitelline membrane. Biochem. J. 286, 17–22. doi:10.1042/bj2860017
King, A. J., Gwinn, D. C., Tonkin, Z., Mahoney, J., Raymond, S., and Beesley, L. (2016). Using abiotic drivers of fish spawning to inform environmental flow management. J. Appl. Ecol. 53 (1), 34–43. doi:10.1111/1365-2664.12542
Lai, X. J., Peng, S., and Wang, Y. L. (2022). Dynamic transcriptome analysis of ovarian follicles in artificial maturing Japanese eel (Anguilla japonica). Theriogenology 180, 176–188. doi:10.1016/j.theriogenology.2021.12.031
Lau, E. S.-W., Zhang, Z., Qin, M., and Ge, W. (2016). Knockout of zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Sci. Rep. 6, 37357. doi:10.1038/srep37357
Lechner, A., Keckeis, H., Schludermann, E., Humphries, P., McCasker, N., and Tritthart, M. (2014). Hydraulic forces impact larval fish drift in the free flowing section of a large European river. Ecohydrology 7 (2), 648–658. doi:10.1002/eco.1386
Li, J., Xia, Z., Dai, H., Yin, W., Zhang, F., Liu, M. F., et al. (2013a). Immunosuppression and the infection caused by gut mucosal barrier dysfunction in patients with early severe acute pancreatitis. J. Hydraulic Eng. 44 (8), 892–900. doi:10.2741/4150
Li, J., Xia, Z., Wang, Y., Zhang, W., Duan, M. H., Liu, Y. T., et al. (2013b). Pulmonary hypertension in POEMS syndrome. Ecohydrology 6 (3), 393–398. doi:10.3324/haematol.2012.073031
Li, Z., Ren, X., Guo, Y., Ru, X., Tian, C., Shi, H., et al. (2021). Identification and ovarian developmental regulation of Insulin-like growth factor 3 in spotted scat (Scatophagus argus). Aquac. Rep. 21, 100866. doi:10.1016/j.aqrep.2021.100866
Lin, J., Li, Y., Liu, Y., Peng, Q., Zhang, D., and Jin, T. (2022). Recent progress in ecological operation and adaptive management for stimulating fish natural spawning. J. Hydraulic Eng. 53 (4), 483–495. doi:10.13243/j.cnki.slxb.20210774
Liu, H., Mu, X., Gui, L., Su, M., Li, H., Zhang, G., et al. (2015). Characterization and gonadal expression of FOXL2 relative to Cyp19a genes in spotted scat Scatophagus argus. Gene 561 (1), 6–14. doi:10.1016/j.gene.2014.12.060
Liu, H., Yin, X. A., Qiu, X., Qin, J., Yang, W., and Zhang, J. (2021). Coupled influence of flow velocity and water temperature on grass carp swimming behaviour and gonad development. Hydrol. Process. 35 (4). doi:10.1002/hyp.14052
Liu, R. C., Li, K., Wang, G. X., Jiang, Z. X., Ba, X. B., and Liu, L. P. (2022). Effect of swimming on the induction of vitellogenin in Conger eel (Conger myriaster). Front. Mar. Sci. 9. doi:10.3389/fmars.2022.887074
Matzuk, M. M., Burns, K. H., Viveiros, M. M., and Eppig, J. J. (2002). Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296 (5576), 2178–2180. doi:10.1126/science.1071965
McClure, C., Quist, M. C., Kozfkay, J. R., Peterson, M. P., and Schill, D. J. (2020). Movement dynamics of smallmouth bass in a large western river system. North Am. J. Fish. Manag. 40 (1), 154–162. doi:10.1002/nafm.10389
McDonnell, R. A. (2000). Hierarchical modelling of the environmental impacts of river impoundment based on a GIS. Hydrol. Process. 14 (11-12), 2123–2142. doi:10.1002/1099-1085(20000815/30)14:11/12<2123:Aid-hyp59>3.0.Co;2-z
Mouchlianitis, F. A., Bobori, D., Tsakoumis, E., Sapounidis, A., Kritikaki, E., and Ganias, K. (2021). Does fragmented river connectivity alter the reproductive behavior of the potamodromous fish Alburnus vistonicus? Hydrobiologia 848 (17), 4029–4044. doi:10.1007/s10750-021-04621-x
Nagahama, Y., and Yamashita, M. (2008). Regulation of oocyte maturation in fish. Dev. Growth and Differ. 50, S195–S219. doi:10.1111/j.1440-169X.2008.01019.x
Nakayama, K., Oshima, Y., Yamaguchi, T., Tsuruda, Y., Kang, I. J., Kobayashi, M., et al. (2004). Fertilization success and sexual behavior in male medaka, Oryzias latipes, exposed to tributyltin. Chemosphere 55 (10), 1331–1337. doi:10.1016/j.chemosphere.2003.11.050
Nguyen, T. V., Rotllant, G. E., Cummins, S. F., Elizur, A., and Ventura, T. (2018). Insights into sexual maturation and reproduction in the Norway lobster (Nephrops norvegicus) via in silico prediction and characterization of neuropeptides and G protein-coupled receptors. Front. Endocrinol. 9, 430. doi:10.3389/fendo.2018.00430
Patino, R., Yoshizaki, G., Thomas, P., and Kagawa, H. (2001). Gonadotropic control of ovarian follicle maturation: the two-stage concept and its mechanisms. Comp. Biochem. Physiology B-Biochemistry Mol. Biol. 129 (2-3), 427–439. doi:10.1016/s1096-4959(01)00344-x
Poff, N. L., and Schmidt, J. C. (2016). How dams can go with the flow. Science 353 (6304), 1099–1100. doi:10.1126/science.aah4926
Reing, J. E., Zhang, L., Myers-Irvin, J., Cordero, K. E., Freytes, D. O., Heber-Katz, E., et al. (2009). Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. Part A 15 (3), 605–614. doi:10.1089/ten.tea.2007.0425
Saatcioglu, H. D., Cuevas, I., and Castrillon, D. H. (2016). Control of oocyte reawakening by kit. Plos Genet. 12 (8), e1006215. doi:10.1371/journal.pgen.1006215
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi:10.1038/nprot.2008.73
She, Z., Tang, Y., Chen, L., Nong, X., and Li, X. (2023). Determination of suitable ecological flow regimes for spawning of four major Chinese carps: a case study of the hongshui river, China. Ecol. Inf. 76, 102061. doi:10.1016/j.ecoinf.2023.102061
So, Y. P., Idler, D. R., Truscott, B., and Walsh, J. M. (1985). Progestogens, androgens and their glucuronides in the terminal stages of oocyte maturation in landlocked Atlantic salmon. J. steroid Biochem. Mol. Biol. 23 (5-), 583–591. part-P1). doi:10.1016/0022-4731(85)90008-1
Stamou, A., Polydera, A., Papadonikolaki, G., Martinez-Capel, F., Munoz-Mas, R., Papadaki, C., et al. (2018). Determination of environmental flows in rivers using an integrated hydrological-hydrodynamic-habitat modelling approach. J. Environ. Manag. 209, 273–285. doi:10.1016/j.jenvman.2017.12.038
Stone, R. (2016). Dam-building threatens Mekong fisheries. Science 354 (6316), 1084–1085. doi:10.1126/science.354.6316.1084
Tucker, E. K., Zurliene, M. E., Suski, C. D., and Nowak, R. A. (2020). Gonad development and reproductive hormones of invasive silver carp (Hypophthalmichthys molitrix) in the Illinois River. Biol. Reproduction 102 (3), 647–659. doi:10.1093/biolre/ioz207
Wang, B., Liu, Y., Feng, L., Jiang, W.-D., Kuang, S.-Y., Jiang, J., et al. (2015). Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 167, 91–99. doi:10.1016/j.foodchem.2014.06.091
Wang, J., Li, J., Ge, Q., Li, W., and Li, J. (2022). Full-length transcriptome sequencing and comparative transcriptomic analysis provide insights into the ovarian maturation of exopalaemon carinicauda. Front. Mar. Sci. 9. doi:10.3389/fmars.2022.906730
Wang, Y., Rhoads, B. L., and Wang, D. (2016). Assessment of the flow regime alterations in the middle reach of the Yangtze River associated with dam construction: potential ecological implications. Hydrol. Process. 30 (21), 3949–3966. doi:10.1002/hyp.10921
Weber, G. M., Okimoto, D. K., Richman, N. H., and Grau, E. G. (1992). Patterns of thyroxine and triiodothyronine in serum and follicle-bound oocytes of the tilapia, Oreochromis mossambicus, during oogenesis. General Comp. Endocrinol. 85 (3), 392–404. doi:10.1016/0016-6480(92)90084-w
Wen, H. S., Mu, W. J., Yang, Y. P., Shi, D., He, F., and Li, J. F. (2014). Molecular physiology mechanism of cytochrome P450 aromatase-regulating gonad development in ovoviviparous black rockfish (Sebastes schlegeli). Aquac. Res. 45 (10), 1685–1696. doi:10.1111/are.12114
Xie, P., and Chen, Y. Y. (2001). Invasive carp in China's plateau lakes. Science 294 (5544), 999–1000. doi:10.1126/science.294.5544.999c
Xu, Z., Yin, X., Sun, T., Cai, Y., Ding, Y., Yang, W., et al. (2017). Labyrinths in large reservoirs: an invisible barrier to fish migration and the solution through reservoir operation. Water Resour. Res. 53 (1), 817–831. doi:10.1002/2016wr019485
Xue, R., Wang, X., Xu, S., Liu, Y., Feng, C., Zhao, C., et al. (2018). Expression profile and localization of vitellogenin mRNA and protein during ovarian development in turbot (Scophthalmus maximus). Comp. Biochem. Physiology B-Biochemistry Mol. Biol. 226, 53–63. doi:10.1016/j.cbpb.2018.08.002
Yang, Z., Hu, P., Wang, J., Zhao, Y., and Zhang, W. (2019). Ecological flow process acknowledging different spawning patterns in the Songhua River. Ecol. Eng. 132, 56–64. doi:10.1016/j.ecoleng.2018.12.034
Young, G., Kagawa, H., and Nagahama, Y. (1983). Evidence for a decrease in aromatase activity in the ovarian granulosa cells of amago salmon (Oncorhynchus rhodurus) associated with final oocyte maturation. Biol. Reproduction 29 (2), 310–315. doi:10.1095/biolreprod29.2.310
Zhang, C., Ding, C. Z., Ding, L. Y., Chen, L. Q., Hu, J. M., Tao, J., et al. (2019a). Ratcheting behavior of intervertebral discs under cyclic compression: experiment and prediction. Rev. Fish Biol. Fish. 29 (4), 895–902. doi:10.1111/os.12530
Zhang, D., Shi, B., Shao, P., Shao, C., Wang, C., Li, J., et al. (2022a). The identification of miRNAs that regulate ovarian maturation in Cynoglossus semilaevis. Aquaculture 555, 738250. doi:10.1016/j.aquaculture.2022.738250
Zhang, P., Qiao, Y., Schineider, M., Chang, J. B., Mutzner, R., Fluixa-Sanmartin, J., et al. (2019b). Using a hierarchical model framework to assess climate change and hydropower operation impacts on the habitat of an imperiled fish in the Jinsha River, China. Sci. Total Environ. 646, 1624–1638. doi:10.1016/j.scitotenv.2018.07.318
Zhang, W., Peng, H., Jia, Y., Ni, G., Yang, Z., and Zeng, Q. (2019c). Investigating the simultaneous ecological operation of dam gates to meet the water flow requirements of fish spawning migration. Pol. J. Environ. Stud. 28 (3), 1967–1980. doi:10.15244/pjoes/89983
Zhang, X., Wang, J., Wang, C., Li, W., Ge, Q., Qin, Z., et al. (2022b). Effects of long-term high carbonate alkalinity stress on the ovarian development in exopalaemon carinicauda. Water 14 (22), 3690. doi:10.3390/w14223690
Keywords: grass carp, water velocity, ovary, hormones, transcriptome
Citation: Shu T, Chen Y, Xiao K, Huang H, Jia J, Yu Z, Jiang W and Yang J (2023) Effects of short-term water velocity stimulation on the biochemical and transcriptional responses of grass carp (Ctenopharyngodon idellus). Front. Physiol. 14:1248999. doi: 10.3389/fphys.2023.1248999
Received: 28 June 2023; Accepted: 14 August 2023;
Published: 31 August 2023.
Edited by:
Yi-Feng Li, Shanghai Ocean University, ChinaReviewed by:
Chunyan Zhao, Qingdao Agricultural University, ChinaCopyright © 2023 Shu, Chen, Xiao, Huang, Jia, Yu, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Shu, MTg3NzgwMDQ4NThAMTYzLmNvbQ==; Jing Yang, eWFuZ19qaW5nN0BjdGcuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.