- 1Department of Physiology and Pharmacology, Des Moines University Medicine and Health Sciences, Des Moines, IA, United States
- 2Department of Biology, Simpson College, Indianola, IA, United States
- 3Laboratory of Cardiorespiratory Control, Department of Physiology, Pontificia Universidad Católica de Chile, Santiago, Chile
- 4Facultad de Ciencias de la Salud, Instituto de Ciencias Biomédicas, Universidad Autónoma de Chile, Santiago, Chile
- 5Centro de Excelencia en Biomedicina de Magallanes (CEBIMA), Universidad de Magallanes, Punta Arenas, Chile
- 6Department of Kinesiology, Iowa State University, Ames, IA, United States
Introduction: Sleep apnea (SA) is highly prevalent in patients with chronic kidney disease and may contribute to the development and/or progression of this condition. Previous studies suggest that dysregulation of renal hemodynamics and oxygen flux may play a key role in this process. The present study sought to determine how chronic intermittent hypoxia (CIH) associated with SA affects regulation of renal artery blood flow (RBF), renal microcirculatory perfusion (RP), glomerular filtration rate (GFR), and cortical and medullary tissue PO2 as well as expression of genes that could contribute to renal injury. We hypothesized that normoxic RBF and tissue PO2 would be reduced after CIH, but that GFR would be increased relative to baseline, and that RBF, RP, and tissue PO2 would be decreased to a greater extent in CIH vs. sham during exposure to intermittent asphyxia (IA, FiO2 0.10/FiCO2 0.03). Additionally, we hypothesized that gene programs promoting oxidative stress and fibrosis would be activated by CIH in renal tissue.
Methods: All physiological variables were measured at baseline (FiO2 0.21) and during exposure to 10 episodes of IA (excluding GFR).
Results: GFR was higher in CIH-conditioned vs. sham (p < 0.05), whereas normoxic RBF and renal tissue PO2 were significantly lower in CIH vs. sham (p < 0.05). Reductions in RBF, RP, and renal tissue PO2 during IA occurred in both groups but to a greater extent in CIH (p < 0.05). Pro-oxidative and pro-fibrotic gene programs were activated in renal tissue from CIH but not sham.
Conclusion: CIH adversely affects renal hemodynamic regulation and oxygen flux during both normoxia and IA and results in changes in renal tissue gene expression.
Introduction
Sleep apnea (SA) is observed in 50%–60% of patients with chronic kidney disease (CKD) and is associated with increased risk of cardiovascular events, end stage renal disease (ESRD), and mortality (Huang et al., 2017; Centers for Disease Control and Prevention, 2021). In support of the notion that SA is injurious to renal health, treatment of SA with continuous positive airway pressure or adaptive servo ventilation results in improvements in renal function (Kinebuchi et al., 2004; Owada et al., 2013; Puckrin et al., 2015; Li et al., 2019). Because of these established clinical relationships, the pathophysiology underlying sleep apnea and renal dysfunction is an area of significant research interest.
Studies in SA patients have shown persistent increases in sympathetic nerve activity (SNA) (Maier et al., 2022), decreased renal plasma flow (Kinebuchi et al., 2004), glomerular hyperfiltration (Kinebuchi et al., 2004), increased activity of the renin angiotensin aldosterone system (RAAS) (Di Murro et al., 2010; Zalucky et al., 2015), as well as systemic inflammation (Unnikrishnan et al., 2015; Perrini et al., 2017; Orrù et al., 2020) and evidence of oxidative stress (Orrù et al., 2020; Hu et al., 2022). Previous studies have shown that the chronic intermittent hypoxia (CIH) associated with SA results in tonic increases in afferent carotid body chemoreflex (CBC) activity (Peng et al., 2003; Rey et al., 2004; Roy et al., 2018; Prabhakar et al., 2023) as well as changes in central chemoreflex sensitivity, both of which contribute to elevated efferent SNA (Prabhakar et al., 2023; Ostrowski et al., 2023; Gilmartin et al., 2010; Huang et al., 2009; Marcus et al., 2010; Molkov et al., 2011). Acute activation of the CBC with hypoxia has previously been shown to increase renal SNA and decrease renal blood flow (RBF) in healthy animals (Huang et al., 2009; Marcus et al., 2015; Pügge et al., 2016; Kious et al., 2022), an effect exacerbated by conditions with enhanced chemoreceptor sensitivity (Huang et al., 2009; Marcus et al., 2015; Pügge et al., 2016; Kious et al., 2022). Recent studies have also suggested that tonic increases in chemoreflex activation in the absence of hypoxic stimulation can exert a significant influence on resting normoxic RBF (Marcus et al., 2015; Kious et al., 2022). The potential clinical significance of these findings should not be underestimated given the aforementioned high prevalence of co-morbid SA in patients with CKD or ESRD. CKD patients with high chemosensitivity and SA might suffer chronic CBC-mediated increases in renal SNA and reductions in RBF which are then compounded by further reductions following apneas or hypopneas. This represents a potentially important link between sleep apnea and renal dysfunction as chemoreflex-mediated increases in renal SNA would be expected to result in chronic reductions in RBF and renal tissue oxygenation (Lin et al., 2020).
A recent study found that CIH conditioning causes reduced renal cortical oxygen tension under normoxic conditions even in the absence of significant reductions in RBF (O’Neill et al., 2019). It is important that these studies be extended to identify how CIH affects renal hemodynamic regulation and oxygen flux during repeated bouts of asphyxia as this is analogous to what a person with SA experiences at night when asleep. The importance of altered hemodynamics and decreased tissue PO2 is underscored by the fact that tissue hypoxia is theorized to be an important driver of renal inflammation, oxidative stress, fibrosis, and subsequent declines in kidney function (Wang et al., 2022).
Therefore, we sought to assess the effect of CIH on RBF, renal microcirculatory perfusion (RP), and cortical and medullary PO2 (CPO2, MPO2) at baseline and during repeated bouts of chemoreflex stimulation with intermittent asphyxia (IA). Because of the potential influence of glomerular hyperfiltration and additional solute load on renal oxygen consumption, we also did experiments to determine the effects of CIH on glomerular filtration rate (GFR) measured in conscious animals after conditioning with CIH. Finally, we explored changes in renal tissue gene expression that could link CIH conditioning, hemodynamic dysregulation, and tissue hypoxia with renal injury and fibrosis, potentially leading to reductions in renal function and development of CKD.
We hypothesized that reductions in RBF, RP, and CPO2/MPO2 during IA would be exacerbated in rats previously conditioned with CIH, and that GFR measured in un-anesthetized animals would be increased after CIH. Furthermore, we also hypothesized that CIH would result in activation of pro-oxidative pro-fibrotic gene programs. Our results indicate that CIH may facilitate development of renal dysfunction through exaggerated reduction in RBF, RP, CPO2, and MPO2 during episodes of asphyxia characteristic of SA. We also observed changes in renal tissue gene expression with CIH conditioning that may contribute to renal damage.
Materials and methods
Ethical approval and statement of compliance
The experimental protocols were approved by the Des Moines University Medicine and Health Sciences Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the American Physiological Society’s Guide for the Care and Use of Laboratory Animals. The authors wish to declare that our work complies with the animal ethics checklist outlined by Grundy (Grundy, 2015).
Experimental groups
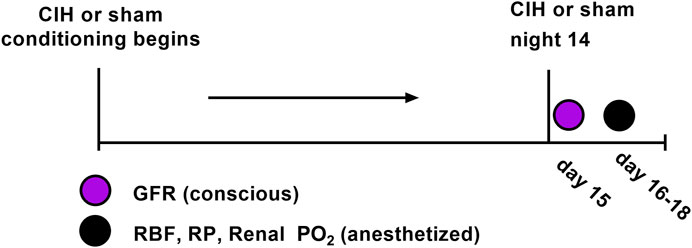
Adult male Sprague-Dawley rats (Envigo, Madison, WI) of similar age (8–10 weeks) and weight (250–300 g) were used for these experiments. All rats were group-housed under controlled temperature (23°C) and humidity conditions and kept on a 12:12 h light-dark cycle and fed standard rat chow with water available ad libitum. At the beginning of the study 30 animals were randomly assigned to the following groups (n = 15 per group): Sham (animals exposed to air-air cycling), CIH (animals exposed to intermittent hypoxia-air cycling). In certain cases we were unable to use a given data stream or in other cases not all animals were used for all experiments. For each experimental variable, the n values are given in the results section as well as the figure legends. Both groups followed identical experimental timelines (shown in Figure 1).
Chronic intermittent hypoxia and sham conditioning
Both experimental groups were exposed to 14 sequential days of chronic intermittent hypoxia (CIH, 60 s FiO2 0.10, 120 s FiO2 0.21) or normoxia/normoxia cycling (sham, 60 s FiO2 0.21, 120 s FiO2 0.21), as previously described (Marcus et al., 2009; Marcus et al., 2010; Philippi et al., 2010; Marcus et al., 2012). The CIH protocol ran for 8 h per day (from 07:30-15:30) and took place in a pair of adjacent Plexiglas chambers (Coy Laboratory Products Inc., Glass Lake, MI) in a designated room in the animal care facility. All animals remained in their home cages which were placed in the Plexiglas chambers during daily exposures.
Measurement of glomerular filtration rate
At the conclusion of the CIH or sham protocol, GFR was assessed non-invasively in conscious, unrestrained rats by measuring clearance of the fluorescent tracer FITC-sinistrin as described previously (Schock-Kusch et al., 2009; Schock-Kusch et al., 2011; Friedemann et al., 2016; Herrera Pérez et al., 2016; Kious et al., 2022). Briefly, FITC-sinistrin clearance was measured using the preclinical transdermal GFR monitor (Medibeacon, St. Louis, MO). On the day prior to the study, a site on the back was shaved and prepared with a depilatory agent (i.e. Nair). On the day of the study the rats were briefly anesthetized (1.5% Isoflurane in air) and the depilated area was cleaned. The monitor was affixed to the back with an adhesive patch and a bolus injection of FITC-sinistrin (5 mg/100 g BW) (Herrera Pérez et al., 2016) was given through one of the lateral tail veins. Measurements of FITC-sinistrin fluorescence were made once per second for 120 min. At the conclusion of the experiment, the monitor and battery pack were removed, and the data was downloaded for later analysis with MPD Lab software (Medibeacon, St Louis, MO) as described previously (Friedemann et al., 2016).
Anesthetic protocol and monitoring procedures
Experiments to measure RBF, RVC, RP, CPO2, and MPO2 were conducted 24 h after GFR measurements. For surgical physiological experiments, anesthesia was induced with 4% inhalation isoflurane in air after which time anesthesia was reduced to 1.5%–2.0% as necessary to maintain a surgical plane of anesthesia. In order to determine appropriate anesthetic depth was reached, a toe pinch and/or tail pinch was administered after induction and every 15 min thereafter. The absence of paw withdrawal, movement of the tail, vocalization, or marked increase in respirations after a pinch was taken as evidence of adequate anesthetic depth. Body temperature was measured using a rectal thermometer and monitored using a Physiosuite w/RightTemp Temperature Monitoring and Homeothermic Control system (Kent Scientific, Torrington, CT), and was maintained at 37°C using a far infrared heating pad integrated with a Surgisuite surgical platform (Kent Scientific, Torrington, CT).
Measurement of renal blood flow, perfusion, and tissue PO2
After appropriate anesthetic depth was reached, the left femoral artery was cannulated with a Millar Mikro-Cath pressure catheter (Millar, Houston, TX) connected to a bridge amp (AD Instruments, Colorado Springs, CO) for measurement of arterial pressure. Then a ventral mid-line incision was made, and the kidney was approached in the peritoneal space. Once the renal artery and vein were identified the renal artery was gently separated from the vein and a transit-time flow probe (Transonic, Ithaca, NY) was placed around the renal artery. After the flow probe was secured, two needle probes were inserted 0.5–1 mm (cortical) and 3–4 mm (medullary) into the kidney to measure tissue PO2 using the Oxylite Pro (Oxford Optronix, United Kingdom). In a sub-set of CIH and sham animals (n = 7 per group), a laser speckle contrast imager (Moor FLPI-2, Moor Instruments, Devon United Kingdom) was positioned with the whole kidney in the field of view for assessment of renal microcirculatory perfusion (RP) (in addition to RBF, CPO2, and MPO2). After a 60-min equilibration period, a 5-min baseline period of RBF, arterial pressure, RP, and cortical and medullary PO2 was recorded for analysis. After measurement of baseline values, a series of ten 30 s episodes of intermittent asphyxia (IA) were initiated. Each IA episode was characterized by an FiO2 of 0.10 and FiCO2 0.03 with the remaining balance made up of nitrogen. Each IA episode was followed by a 60 s recovery period breathing medical grade air (FiO2 0.21, balance nitrogen). All variables were measured continuously during the IA protocol.
Method of euthanasia
At the conclusion of the experimental protocol all rats were humanely euthanized. In accordance with standards set forth by the American Veterinary Medical Association and the Institutional Animal Care and Use Committee at Des Moines University Medicine and Health Sciences, all rats were euthanized via anesthetic overdose (5% isoflurane) combined with the addition of a secondary physical method of euthanasia.
Measurement of renal mRNA expression
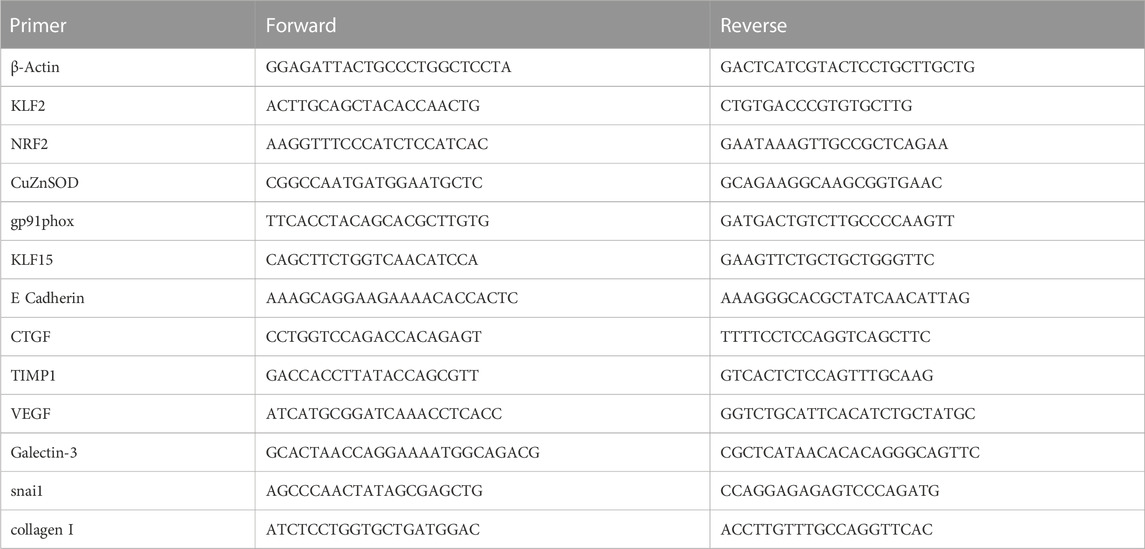
At the conclusion of physiological experiments and after anesthetic overdose, rat kidneys were excised and dissected, flash frozen in liquid nitrogen, and stored at −80°C for later analysis. Studies of renal mRNA expression were performed as previously described (Kious et al., 2022). Briefly, RNA isolation and cDNA synthesis were performed according to manufacturer instructions using the Direct-zol RNA MiniPrep Plus (Zymo, Irvine, CA) and High-Capacity cDNA Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA) kits, respectively. Gene expression was assessed by Sso Advanced SYBR green chemistry (Bio-Rad, Hercules, CA) real-time PCR following reverse transcription of total RNA. Real-time PCR was performed on a CFX Connect Real Time PCR Detection System (Bio-Rad, Hercules, CA). β-Actin mRNA was quantified as an internal control for each sample and quantifications were performed using the ΔΔCt method and expressed as fold change. Forward and Reverse primer sequences are show in Table 1. The following genes were probed in our samples: Krüppel-like factor 2 (KLF2), nuclear factor erythroid 2-related factor 2 (NRF2), copper zinc superoxide dismutase (CuZn SOD), NAD(P) H oxidase catalytic subunit (gp91phox), Krüppel-like Factor 15 (KLF15), E Cadherin, connective tissue growth factor (CTGF), tissue inhibitor of metalloproteinase 1 (TIMP), vascular endothelial growth factor (VEGF), Galectin-3, snail family zinc finger 1 (snail), and collagen I.
Data analysis
All physiological parameters except GFR (MPD Lab, Medibeacon, St Louis, MO) and RP (moorFLPI2 Research Software, Moor Instruments, Devon, United Kingdom) were recorded with a Powerlab data acquisition and analysis system (AD Instruments, Colorado Springs, CO). Mean values for baseline (B1) RBF, RP, and CPO2 and MPO2 were calculated from 5-min of data immediately following a 60-min post-instrumentation equilibration period (pre-IA baseline). In addition to computing baseline values, we quantified the RBF, RP, and CPO2/MPO2 response to IA using the following approaches. Baseline (b2-b10) and nadir (n1-n10) measurements occurring during IA were based on 10 s periods centered around appropriate peaks and nadirs. First, we analyzed the change (if any) in normoxic baseline RBF, RP, CPO2, and MPO2 relative to the pre-IA baseline (e.g. B1-B2, B1-B3, B1-B4, etc.). Then we analyzed the average change in RBF, RP, and CPO2/MPO2 during the IA protocol (e.g. B1-N1, B2-N2, B3-N3, etc.). A graphical representation of the time points analyzed is shown in Figure 2. Group mean data was analyzed using unpaired t-tests. All data was analyzed using GraphPad Prism version 9.5.1 for Windows software (San Diego, CA), and the threshold for statistical significance was set at p < 0.05. Values in the results section are expressed as mean ± standard deviation, and in the figures as median and quartiles with violin plots.
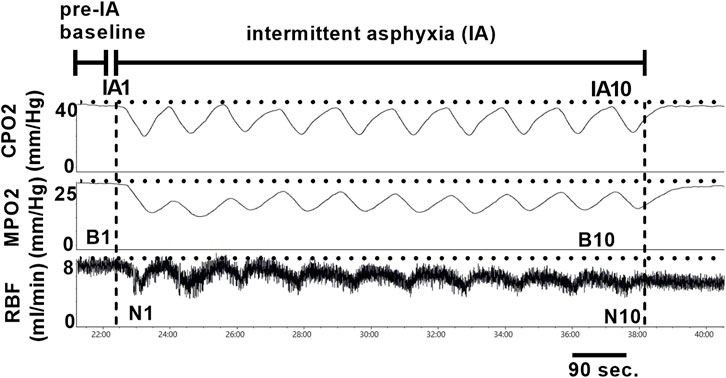
FIGURE 2. Glomerular filtration rate in sham and CIH rats after CIH conditioning. Shown in panel (A) are representative decay curves of transcutaneous fluorescence of FITC-Sinistrin in one CIH and one sham animal. Panel (B) illustrates mean data for GFR at the post-exposure time point. GFR was significantly increased in CIH relative to sham. Data analyzed by unpaired t-test and displayed as violin plots with median and quartiles, n = 10 animals per group. *p < 0.05 vs. sham.
Results
Effects of CIH on baseline hemodynamic variables, GFR, and renal tissue PO2
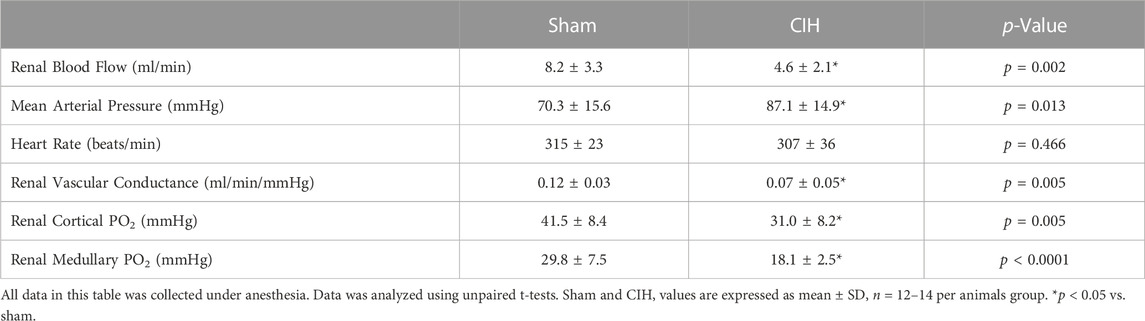
As shown in Figure 3, after 2 weeks of CIH or sham conditioning, glomerular filtration rate (GFR) in conscious unrestrained rats was significantly higher in the CIH group than the sham group (1.09 ± 0.23 ml/min/g BW sham vs. 1.39 ± 0.34 ml/min/g BW CIH, t 2.29 df 18, n = 10 sham and CIH, p = 0.03). Baseline hemodynamic, CPO2, and MPO2 values are shown in Table 2. RBF (8.2 ± 3.3 ml/min sham vs. 4.6 ± 2.1 ml/min CIH, t 3.45 df 27, n = 15 sham, n = 14 CIH, p = 0.002), RVC (0.12 ± 0.03 ml/min/mmHg sham vs. 0.07 ± 0.05 ml/min/mmHg CIH, t 2.81 df 23, n = 13 sham, n = 12 CIH, p = 0.005), CPO2 (41.5 ± 8.4 mmHg sham vs. 31.0 ± 8.2 mmHg CIH, t 3.14 df 23, n = 14 sham, n = 11 CIH, p = 0.005), and MPO2 (29.8 ± 7.5 mmHg sham vs. 18.1 ± 2.5 mmHg CIH, t 5.71 df 25, n = 12 sham, n = 15 CIH, p = 0.005) were all lower in CIH vs sham. In contrast, arterial pressure was significantly higher in CIH vs sham animals (73.2 ± 14.3 mmHg sham vs. 87.1 ± 14.9 mmHg CIH, t 2.45 df 25, n = 15 sham, n = 12 CIH, p = 0.021). Heart rate was not significantly different between groups at baseline (315.3.2 ± 5.9 mmHg sham vs. 307.0 ± 35.6 mmHg CIH, t 0.74 df 25, n = 15 sham, n = 12 CIH, p = 0.021).
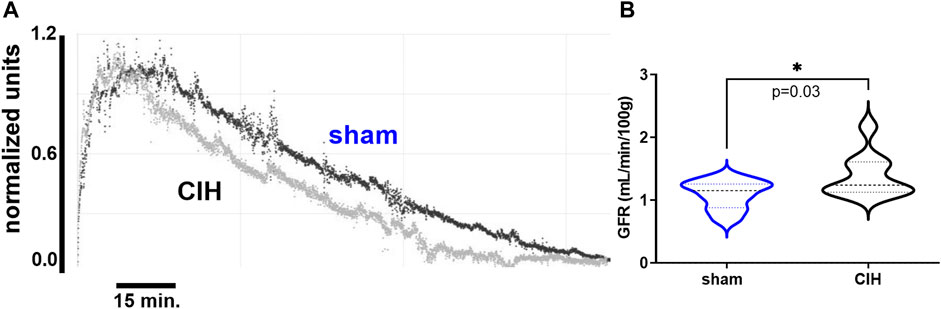
FIGURE 3. Representative tracings of renal tissue PO2 and renal artery blood flow during ten episodes of intermittent asphyxia. Time points for analysis are indicated in the figure and are as follows: 1. Pre-IA baseline (i.e., B1) 2. IA FiO2 0.21 baseline deltas (e.g. B2 vs B1, B3 vs B1, etc.) 3. IA FiO2 0.10/FiCO2 0.03 deltas (e.g. B1 vs N1, B2 vs N2, B3 vs N3, etc.). IA = intermittent asphyxia, B = baseline, N = nadir.
Effects of IA on hemodynamic variables and tissue PO2 in CIH-conditioned rats
Hemodynamic and tissue PO2 responses to the IA protocol are shown in Figures 4–6. Normoxic RBF (-0.43 ± 0.54 ml/min sham vs. -0.98 ± 0.59 ml/min CIH, t 2.2 df 18, n = 10 per group, p = 0.04), RVC (-0.0006 ± 0.008 ml/min/mmHg sham vs. -0.006 ± 0.006 ml/min/mmHg CIH, t 1.6 df 18, n = 10 per group, p = 0.06), RP (-0.84% ± 3.6% sham vs. -4.33 ± 1.9% CIH, t 2.26 df 12, n = 7 per group, p = 0.04) CPO2 (-2.4 ± 1.4 mmHg sham vs. -4.1 ± 2.2 mmHg CIH, t 2.2 df 20, n = 11 per group, p = 0.04) and MPO2 (-2.8 ± 3.7 mmHg sham vs. -6.5 ± 3.6 mmHg CIH, t 2.2 df 18, n = 10 per group, p = 0.04) baselines during IA decreased relative to pre-IA baseline in both groups, but to a greater extent in CIH. Similarly, RBF (-0.41 ± 0.22 ml/min sham vs. -0.75 ± 0.45 ml/min CIH, t 2.2 df 13, n = 10 per group, p = 0.04), RVC (-0.002890 ± 0.006101 ml/min/mmHg sham vs. -0.01488 ± 0.01473 ml/min/mmHg CIH, t 2.4 df 18, n = 10 per group, p = 0.03), RP (-4.6 ± 1.3% sham vs. -8.4 ± 1.3% CIH, t 5.7 df12, n = 7 per group, p = 0.0001) and CPO2 (-9.0 ± 3.2 mmHg sham vs. -13.3 ± 5.0 mmHg CIH, t 2.4 df 20, n = 11 per group, p = 0.02) MPO2 (-5.8 ± 3.2 mmHg sham vs. -10.7 ± 5.0 mmHg CIH, t 2.6 df 18, n = 10 per group, p < 0.02) decreased during asphyxia in both groups, but to a greater extent in CIH vs sham.
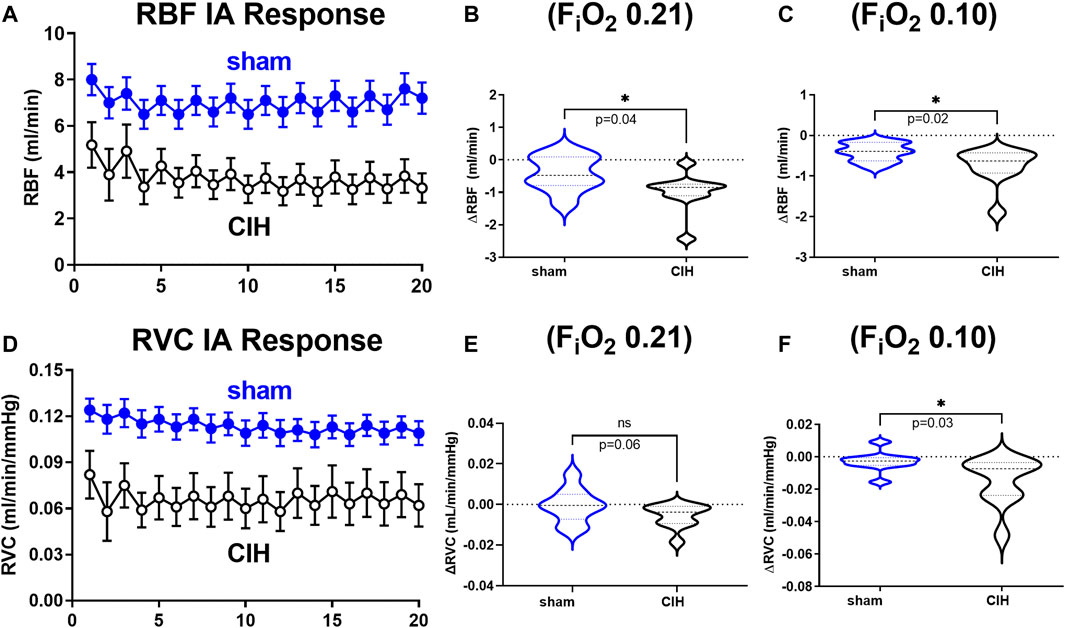
FIGURE 4. Renal artery blood flow and renal vascular conductance during intermittent asphyxia. Shown in panel (A) and (D) are composite traces of the renal artery blood flow (RBF) and renal vascular conductance (RVC) responses to ten episodes of intermittent asphyxia (IA, FiO2 0.10, FiCO2 0.03) in sham and CIH groups. The panels to the right show mean data for the 1) change in RBF (B) and RVC (E) during normoxia (FiO2 0.21) relative to pre-IA baseline and 2) the change in RBF (C) and RVC (F) during asphyxia (FiO2 0.10, FiCO2 0.03). Data analyzed by unpaired t-test and displayed as violin plots with median and quartiles, n = 10 animals per group. *p < 0.05 vs. sham.
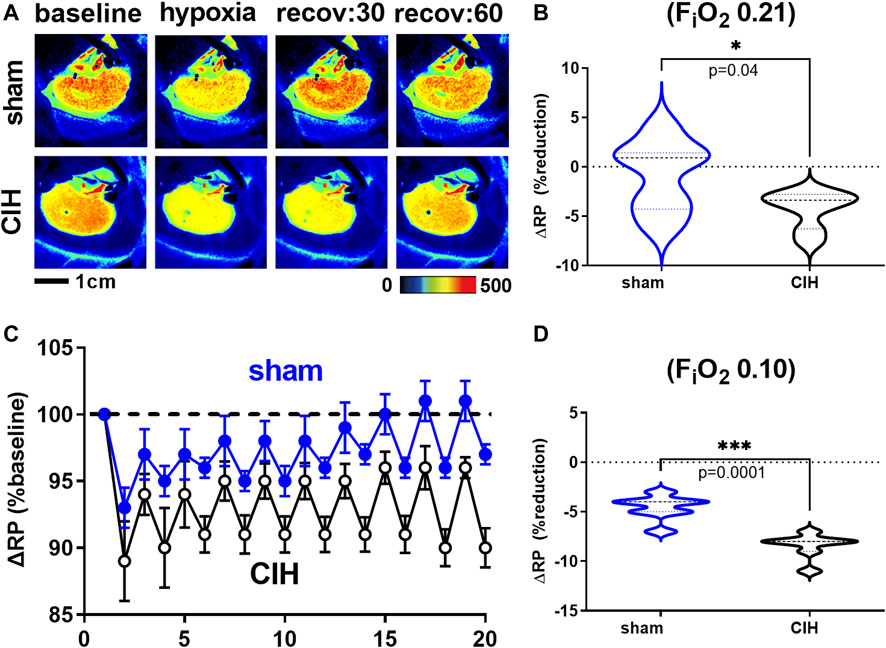
FIGURE 5. Renal microcirculatory perfusion during intermittent asphyxia. Shown in panel (A) are representative images of renal microcirculatory perfusion (RP) measured with laser speckle contrast imaging during exposure to a single episode of intermittent asphyxia (IA, FiO2 0.10, FiCO2 0.03) in one sham and one CIH animal. Shown in panel (C) are composite traces of the RP response to ten episodes of IA in sham and CIH groups. Panel (B) and (D) show mean data for the 1) change in RP during normoxia (FiO2 0.21) relative to pre-IA baseline and 2) the change in RP during asphyxia (FiO2 0.10, FiO2 0.03), respectively. Data analyzed by unpaired t-test and displayed as violin plots with median and quartiles, n = 7 per group. ***p < 0.001, ****p < 0.0001 vs. sham.
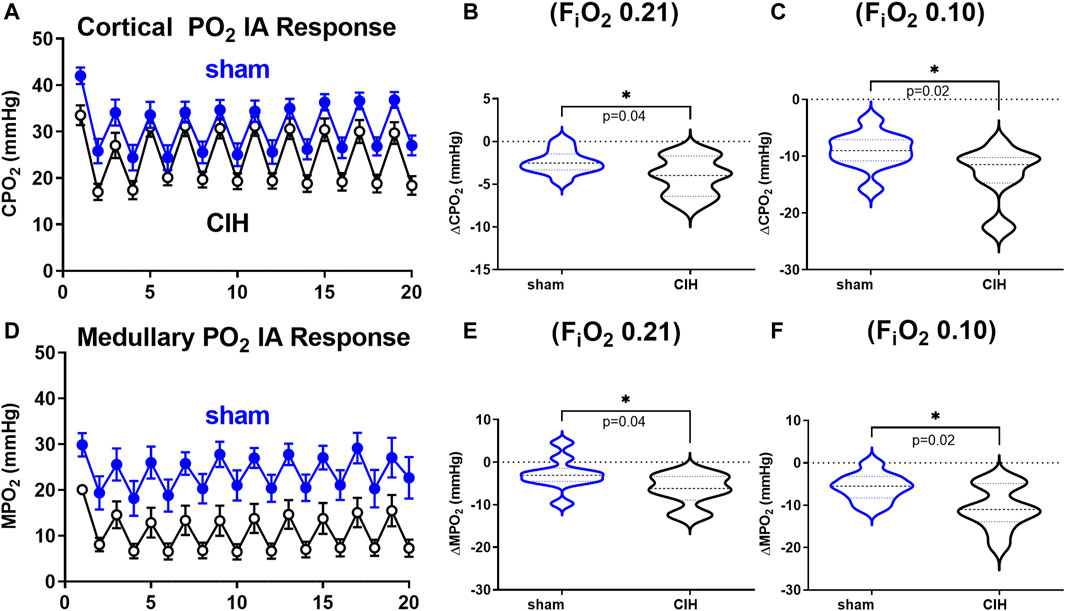
FIGURE 6. Cortical and medullary PO2 during intermittent asphyxia. Shown in panel (A) and (D) are composite traces of the cortical and medullary PO2 response to ten episodes of intermittent asphyxia (IA, FiO2 0.10, FiCO2 0.03) in sham and CIH groups. The panels to the right show mean data for the 1) change in cortical (B) and medullary (E) tissue PO2 during normoxia (FiO2 0.21) relative to pre-IA baseline and 2) the change in cortical (C) and medullary (F) PO2 during IA exposures. Data analyzed by unpaired t-test and displayed as violin plots with median and quartiles, n = 10–11 per group. **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. sham.
Effect of CIH on renal cortical mRNA expression
Results of qRT-PCR studies on renal cortical tissue are shown in Figure 7. Expression of genes related to antioxidant defense (KLF2 (2.81 ± 1.9-fold change sham vs. 1.46 ± 0.89-fold change CIH, t 2.2 df 19, n = 9–12 per group, p = 0.04) NRF2 (0.59 ± 0.26-fold change sham vs. 0.28 ± 0.19-fold change CIH, t 2.5 df 12, n = 6–8 per group, p = 0.03), CuZnSOD (0.93 ± 0.33-fold change sham vs. 0.55 ± 0.30-fold change CIH, t 2.2 df 13, n = 5–10 per group, p = 0.04) were downregulated while genes associated with the pro-oxidative catalytic subunit of NADPH oxidase (gp91phox (1.44 ± 1.8-fold change sham vs. 4.54 ± 4.2-fold change CIH, t 1.9 df 14, n = 8 per group, p = 0.04) were upregulated in CIH vs. sham. Expression of anti-fibrotic genes KLF15 (1.10 ± 0.35-fold change sham vs. 0.62 ± 0.44-fold change CIH, t 2.4 df 15, n = 8–9 per group, p = 0.02), and E-Cadherin (1.03 ± 0.54-fold change sham vs. 0.32 ± 0.25-fold change CIH, t 2.8 df 9, n = 5–6 per group, p = 0.02) were lower in CIH vs sham, whereas expression of pro-fibrotic/fibrosis-associated genes (CTGF (1.29 ± 0.51-fold change sham vs. 2.10 ± 0.79-fold change CIH, t 2.3 df 13, n = 7–8 per group, p = 0.04), TIMP (1.13 ± 0.22-fold change sham vs. 2.04 ± 0.83-fold change CIH, t 2.4 df 14, n = 7–9 per group, p = 0.03), VEGF (1.22 ± 1.13-fold change sham vs. 2.35 ± 1.62-fold change CIH, t 1.8 df 18, n = 9–11 per group, p = 0.04), Galectin 3 (0.81 ± 0.54-fold change sham vs. 2.08 ± 1.74-fold change CIH, t 1.9 df 12, n = 7 per group, p = 0.04), snail (0.91 ± 0.37-fold change sham vs. 2.02 ± 1.38-fold change CIH, t 2.2 df 14, n = 8 per group, p = 0.04), and collagen I (0.91 ± 0.60-fold change sham vs. 1.86 ± 1.05-fold change CIH, t 2.2 df 15, n = 7–10 per group, p = 0.04) were higher in CIH vs. sham.
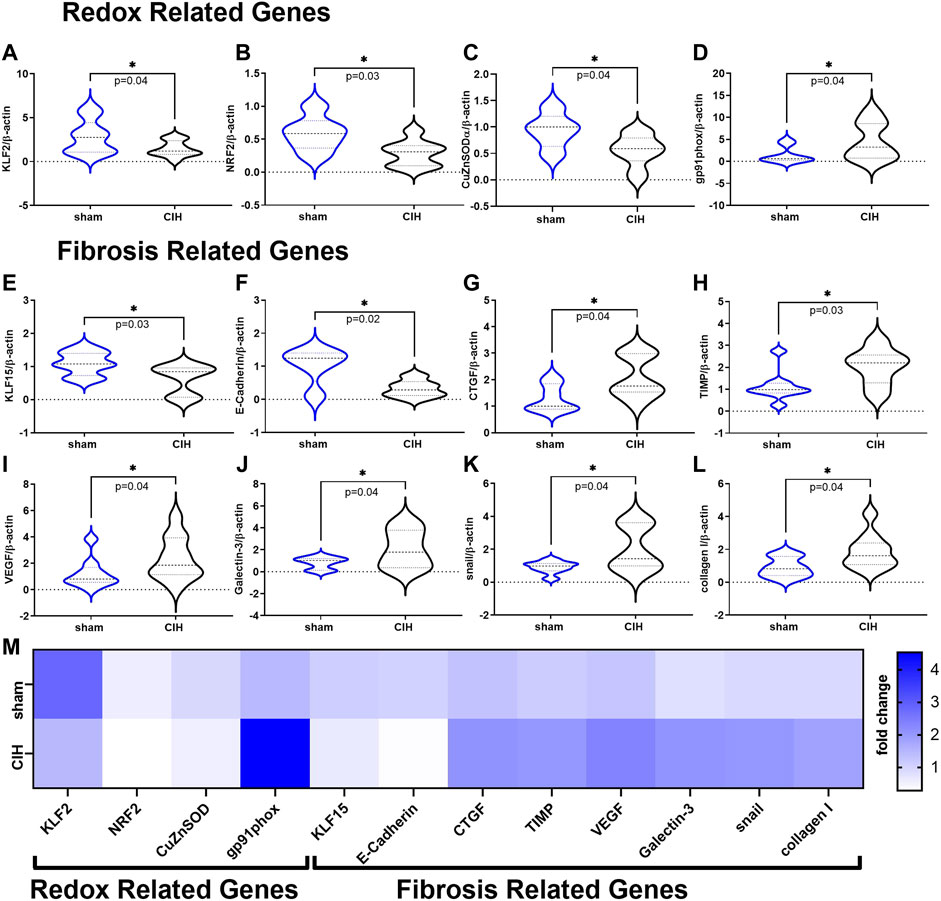
FIGURE 7. Redox and Fibrosis related mRNA expression in renal cortical tissue. Panels (A–D) show mean mRNA expression for redox genes (A) Krüppel-like factor 2 (KLF2), (B) nuclear erythroid 2-related factor 2 (NRF2), (C) copper zinc super oxide dismutase (CuZnSOD), and (D) the NADPH oxidase catalytic subunit (gp91phox). Panels (E–L) show mean mRNA expression for fibrosis related genes (E) Krüppel-like factor 15 (KLF15), (F) E-Cadherin (G) connective tissue growth factor (CTGF), (H) tissue inhibitor of metalloproteinase 1 (TIMP), (I) vascular endothelial growth factor (VEGF), (J) Galectin-3 (K) snail family zinc finger 1 (snail), and (L) collagen I. Panel M is a heat map showing differences in mean mRNA expression. All data is expressed as fold change relative to housekeeping gene β-actin. Data analyzed by unpaired t-test and displayed as violin plots with median and quartiles, n = 6–10 samples per group. *p < 0.05 vs. sham.
Discussion
In this study we sought to determine the effect of CIH conditioning on renal blood flow (RBF), renal vascular conductance (RVC), renal microcirculatory perfusion (RP), glomerular filtration rate (GFR), and renal cortical and medullary tissue PO2 (CPO2, MPO2) at rest and during exposure to intermittent asphyxia (IA). The main findings of our study are (I) GFR measured in the conscious state was significantly increased in CIH rats vs sham rats, (II) baseline RBF, RVC, CPO2, and MPO2 were significantly reduced in CIH rats vs sham rats, (III) normoxic RBF, RVC, RP, CPO2, and MPO2 decreased below baseline during the course of IA exposure, (IV) RBF, RVC, CPO2, and MPO2 decreased to a greater extent in CIH rats vs sham rats during the hypoxic/hypercapnic (asphyxia) portion of the IA protocol, (V) CIH was associated with activation of pro-oxidative and pro-fibrotic gene programs. Our results indicate that the CIH has significant effects on control of renal hemodynamics and tissue oxygenation during normoxia and exposure to asphyxia.
Effects of chronic intermittent hypoxia on GFR
We observed that GFR was higher in CIH relative to sham, an observation consistent with the hyperfiltration observed in patient populations with SA (Kinebuchi et al., 2004; Adeseun and Rosas, 2010). In contrast, O’Neil et al. found a lower GFR in CIH conditioned rats relative to sham (O'Neill et al., 2019), an observation which is also consistent with clinical studies showing reduced GFR in patients with SA (Li et al., 2019). Given the similarities of the intensity and duration of the CIH paradigms used in our study and that of O’Neil et al. it is difficult to reconcile these differences. It may be that our findings represent a hyperfiltration state which precedes eventual decreases in GFR associated with prolonged or more severe CIH exposure. Progression from hyperfiltration to reduced GFR occurs in diabetic nephropathy (Palatini, 2012), so this phenomenon is not without precedent.
Another explanation may be the physiological state under which GFR was measured in these two studies. In our study, GFR was measured in conscious unrestrained animals, whereas the study by O’Neil et al. measured GFR in pentobarbital anesthetized rats (O'Neill et al., 2019). Previous studies have shown that pentobarbital anesthesia in rats causes a marked reduction in mean arterial pressure, heart rate, GFR, and effective renal plasma flow (Walker et al., 1986). Determining whether or not the differences in the effect of CIH on GFR observed between studies can be attributed to anesthesia will require future studies to perform both techniques in the same animals. While our studies do not directly address mechanisms of glomerular hyperfiltration after CIH conditioning there are multiple physiological changes occurring with CIH that may contribute. Vascular and autonomic dysfunction associated with CIH are mediated in part by chronic RAAS activation (Marcus et al., 2010; Marcus et al., 2012), which has been shown to elicit constriction of the efferent arterioles and increase GFR (Cortinovis et al., 2022). Further study is needed to fully address the nature and time course of changes in GFR with CIH conditioning.
Effects of chronic intermittent hypoxia on RBF, RVC, and RP
The present study shows significantly lower baseline RBF, RVC, and RP as well as exaggerated hypoxia-evoked reductions in RBF, RVC, and RP in CIH relative to sham. The only study that has previously explored the effect of CIH conditioning on baseline RBF found no significant difference in RBF or renal vascular resistance (RVR) between CIH and sham groups (O'Neill et al., 2019). This unexpected finding may be explained by the nature of CIH related changes in control of organ blood flow. CIH contributes to enhanced baseline sympathetic nerve activity and increased arterial pressure (Huang et al., 2009; Gilmartin et al., 2010; Marcus et al., 2010; Del Rio et al., 2016), as well as enhanced sympathetic responses to hypoxic events (Huang et al., 2009; Gilmartin et al., 2010; Marcus et al., 2010). CIH specifically augments chemoreflex control of renal sympathetic nerve activity (Huang et al., 2009) (RSNA), and previous work has established that conditions characterized by chemoreflex-mediated increases in RSNA have the potential to cause reductions in RBF and RVC, and increases in RVR (Clayton et al., 2011; Marcus et al., 2015; Pügge et al., 2016; Kious et al., 2022). Thus in a CIH model it would be logical to hypothesize reductions in RBF and RVC. Both our study and the study by O’Neil et al. measured RBF under anesthesia, however as mentioned previously, O’Neil et al. used pentobarbital anesthesia which has been shown to obtund cardiovascular reflex function (Peiss and Manning, 1964). Therefore, it is possible that pentobarbital suppressed chemoreflex control of RSNA and for this reason the expected reductions in RBF after CIH were not observed by O’Neil et al. In addition to changes in autonomic function that affect control of blood flow, vascular dysfunction also likely plays a role in the dysregulation of integrated hemodynamic control we observed. We have previously shown that CIH is associated with vascular endothelial dysfunction (Philippi et al., 2010; Marcus et al., 2012) and oxidative stress (Marcus et al., 2010; Philippi et al., 2010), however these parameters have not previously been assessed in the renal vasculature in the CIH model. ROS may be generated in CIH animals as a results of mitochondrial dysfunction (Prabhakar et al., 2007), or acutely in response to hypoxia, angiotensin II (ANGII), and/or proinflammatory cytokines (Liu et al., 2017). CIH is associated with RAAS activation, renal inflammation, and oxidative damage in the kidney (Sun et al., 2012). ROS in the kidney has previously been shown to reduce renal medullary blood flow and to induce afferent arteriolar constriction in presence of ANGII (Ratliff et al., 2016). Thus, the multiple pathophysiological insults associated with CIH are consistent with established causes of autonomic and vascular dysfunction, and the observed dysregulation of renal hemodynamic control observed in our studies.
Effects of chronic intermittent hypoxia on renal tissue PO2
Previous studies have shown a reduction in resting normoxic renal cortical but not medullary tissue PO2 after conditioning with a similar CIH paradigm (O’Neill et al., 2019). Interestingly, this occurred in the presence of a reduction in GFR and sodium transport without a reduction in renal artery blood flow. In addition, the authors noted that CIH animals exhibited higher renal oxygen consumption at any given level of sodium transport. Thus, in this study decreases in cortical tissue PO2 were driven by changes in tissue metabolism rather than as a result of decreased oxygen delivery. In the current study, both cortical and medullary PO2 were lower in CIH exposed animals at baseline and during the IA protocol. While the reason for this discrepancy is unclear, one potential explanation might center around differences in GFR between the studies. In our study we observed increased GFR and decreased RBF in CIH animals under normoxic conditions, whereas O’Neil et al. observed decreased GFR and sodium transport in CIH animals relative to sham. While O’Neil et al. observed no differences in oxygen consumption between CIH and sham, this was in the face of lower GFR and sodium transport. It is possible that a higher GFR in our study drove greater sodium transport and oxygen consumption contributing to lower tissue PO2. We did not measure tissue oxygen consumption or sodium transport, so our data can neither directly support nor refute this possibility, but it is a plausible explanation based on the data we collected.
Under normal circumstances, renal tissue PO2 is maintained at stable levels by a unique interaction between RBF, GFR, and oxygen consumption (QO2) (Brezis et al., 1994). Renal QO2 is primarily driven by the metabolic demands associated with tubular solute reabsorption, however this relationship can become dysregulated as a result of mitochondrial dysfunction, RAAS activation, and hypoxic conditions (Brezis et al., 1994; Haase, 2013). Disease states such as diabetes and hypertension are often associated with increased QO2 and decreased renal tissue PO2 (Hansell et al., 2013). In these diseases, elevated kidney QO2 is commonly mediated by increased angiotensin receptor tonus and oxidative stress which contributes to progression of renal damage and reduced function (Hansell et al., 2013). Given the well-known RAAS activation and oxidative stress in CIH models of SA, these factors likely contribute to the decreases we observed in renal tissue PO2. Thus, the combination of oscillations in RSNA and RBF/RP combined with persistent RAAS activation and intermittent hypoxemia (associated with individual episodes of asphyxia) act synergistically to create a state of tissue hypoxia.
Effect of CIH on renal gene expression
Because we observed notable decreases in RBF/RVC/RP in CIH conditioned animals, we sought to determine how this might affect gene expression of transcription factors related to blood flow. We measured expression of the shear stress-sensitive transcription factor Krüppel-like factor 2 (KLF2), as KLF2 controls expression of mediators of antioxidant defense, nitric oxide production, and tissue fibrosis (Dekker et al., 2005; Boon et al., 2007; Fledderus et al., 2008) and has previously been shown to be downregulated in animal models characterized by reduced RBF (Haack et al., 2014; Del Rio et al., 2017; Marcus et al., 2018; Kious et al., 2022). As shown in Figure 7, we observed that the gene expression of renal cortical KLF2 and the master antioxidant transcription factor NRF2 (a downstream target of KLF2) were significantly downregulated in CIH relative to sham. To determine if NRF2 downregulation resulted in decreased gene expression of antioxidant enzymes under its control, we measured mRNA expression of CuZn SOD, which was also significantly decreased in CIH vs sham. In contrast, gene expression of the catalytic subunit of NADPH oxidase (gp91phox) was significantly increased in CIH vs sham, consistent with findings in other studies (Prabhakar et al., 2007; Sun et al., 2012).
Tissue fibrosis has been theorized to be a common pathway to renal dysfunction (Wang et al., 2022) and may resist oxygen diffusion and contribute to tissue hypoxia (Edwards and Kurtcuoglu, 2022), so we examined gene expression of fibrosis related pathways in renal cortical tissue. In particular we focused on pathways related to tissue hypoxia because of its relevance to our physiological measurements. KLF15, and E-Cadherin, which have fibrosis-suppressing activity and have been shown to be downregulated by ANG II and hypoxia (Esteban et al., 2006; Lu et al., 2019), were decreased in cortical tissue from CIH relative to sham. Expression of pro-fibrotic CTGF, TIMP, Galectin 3, Snail, and VEGF (all of which are induced by hypoxia and/or ANG II (Liu et al., 2017; Boutin et al., 2022; Khalaji et al., 2023)), was significantly higher in cortical tissue from CIH vs sham, suggesting that CIH exposure promotes activation of pro-oxidative, pro-fibrotic signaling that may contribute to tissue damage and organ dysfunction.
Strengths and limitations
Often, discerning the effects of multiple-concurrent co-morbidities or conditions can make clear interpretation of data difficult. The common presence of obesity, metabolic syndrome, and advanced age alongside sleep apnea, can confound interpretation of the relative contribution of any given variable. Our animal model examines the physiological changes that occur in response to CIH conditioning in the absence of common co-morbidities, giving a clearer picture of the contribution of CIH per se. These findings yield significant insight into the potential for the CIH associated with sleep apnea to precipitate or hasten the decline of renal function and to identify the mechanisms by which this might occur. In addition to the specificity of our model, our approach also has significant advantages. In this study we combine measurement of renal artery blood flow using a transit time flow probe with measurement of renal microcirculatory perfusion using laser speckle contrast imaging (LSCI). The larger imaging field used in LSCI allows for a more comprehensive quantification of ‘whole organ’ regulation of microcirculatory perfusion.
In this study direct measurement of RBF, RVC, RP, CPO2, and MPO2 is made at the conclusion of the experimental period under anesthesia. The use of anesthesia during invasive experiments is a limitation, however, any changes induced by anesthesia would be expected to affect both experimental and control animals, thus we do not feel this confounds interpretation of differences observed between groups. Measurement of physiological variables under anesthesia has inherent limitations regardless of anesthetic, but we feel that the difference in anesthetics used in similar studies (O’Neill et al., 2019) should be considered when comparing data. In addition, we acknowledge that the length of our CIH exposure (2 weeks) is short relative to the amount of time that patients with sleep apnea are typically exposed to CIH associated with their condition. Previous studies in rats and mice have shown that short-term CIH conditioning is sufficient to elicit enhanced chemoreflex control of sympathetic nerve activity (Huang et al., 2009; Marcus et al., 2010), however longer-term studies might reveal more profound effects of CIH on renal perfusion, tissue PO2, and injury. Indeed, studies focused on dose response of CIH exposure time show marked changes in tissue redox regulation and fibrosis with longer exposure times (Sun et al., 2012).
Finally, in the studies presented we only assessed GFR, renal hemodynamic regulation, and tissue PO2 in male animals, therefore inferences based on our study should be limited to the male sex. It is unclear how female sex steroid hormones interact with the insults associated with CIH, and therefore the effect of CIH on renal function and hemodynamic control in females deserves study.
Contribution to the field
Renal dysfunction is a prominent independent risk factor for cardiovascular mortality (Centers for Disease Control and Prevention, 2021), and despite well-established epidemiological relationships between SA and renal dysfunction (Owada et al., 2013; Puckrin et al., 2015; Huang et al., 2017; Li et al., 2019), there is a paucity of mechanistic physiological studies in this area. A better understanding of the underlying physiological and biochemical changes that occur in the kidney in SA is important to address existing knowledge gaps. These studies provide new mechanistic insights into the pathophysiology of renal dysfunction in the context of SA. Because SA is a common co-morbidity in many cardio-metabolic diseases, our studies have the potential to promote greater understanding of the pathophysiology of renal dysfunction in clinical populations including type II diabetes, hypertension, and heart failure.
Conclusion
Cumulatively, our results underscore the importance of CIH experienced during SA and its potential deleterious effects on regulation of renal function. These findings suggest that SA may contribute to renal damage and dysfunction as a result of promoting persistent renal hypoperfusion and tissue hypoxia both during wakefulness and during sleep when apneic episodes are occurring. Therapeutic modalities that reduce SA incidence and/or severity or that attenuate the deleterious effects of reductions in renal blood flow and chronic tissue hypoxia have the potential to improve renal health and function in patients with primary or co-morbid sleep apnea.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Des Moines University Medicine and Health Sciences Animal Care and Use Committee.
Author contributions
All experiments were performed in the Department of Physiology and Pharmacology at Des Moines University. KK collected, analyzed, and interpreted data, and contributed to drafting and revision of the manuscript. KS, ST, AH, AP, and SC contributed to data collection. HD, RDR, and JL contributed to analysis and interpretation of data, and revision of the manuscript. NM and SC were responsible for conception and design of the experiments, supervised the study, procured the resources to perform the study, collected, analyzed, and interpreted data, and drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the Heart, Lung, and Blood Institute (R15 HL138600-01, to NJM) and by the Iowa Osteopathic Education Research Fund (IOER#12-22-05, to NJM). Funding for open access publication fees were provided by the Department of Physiology and Pharmacology at Des Moines University Medicine and Health Sciences. RDR received funding from Fondecyt grant #1220950.
Acknowledgments
The authors thank Brock Pope and Reagan Sesker for technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeseun, G. A., and Rosas, S. E. (2010). The impact of obstructive sleep apnea on chronic kidney disease. Curr. Hypertens. Rep. 12 (5), 378–383. doi:10.1007/s11906-010-0135-1
Boon, R. A., Fledderus, J. O., Volger, O. L., van Wanrooij, E. J., Pardali, E., Weesie, F., et al. (2007). KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler. Thromb. Vasc. Biol. 27 (3), 532–539. doi:10.1161/01.ATV.0000256466.65450.ce
Boutin, L., Dépret, F., Gayat, E., Legrand, M., and Chadjichristos, C. E. (2022). Galectin-3 in kidney diseases: From an old protein to a new therapeutic target. Int. J. Mol. Sci. 23 (6), 3124. doi:10.3390/ijms23063124
Brezis, M., Agmon, Y., and Epstein, F. H. (1994). Determinants of intrarenal oxygenation. I. Effects of diuretics. Am. J. Physiol. 267 (6), F1059–F1062. doi:10.1152/ajprenal.1994.267.6.F1059
Centers for Disease Control and Prevention (2021). Chronic kidney disease in the United States, 2021. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention.
Clayton, S. C., Haack, K. K., and Zucker, I. H. (2011). Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am. J. Physiol. Ren. Physiol. 300 (1), F31–F39. doi:10.1152/ajprenal.00088.2010
Cortinovis, M., Perico, N., Ruggenenti, P., Remuzzi, A., and Remuzzi, G. (2022). Glomerular hyperfiltration. Nat. Rev. Nephrol. 18 (7), 435–451. doi:10.1038/s41581-022-00559-y
Dekker, R. J., van Thienen, J. V., Rohlena, J., de Jager, S. C., Elderkamp, Y. W., Seppen, J., et al. (2005). Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am. J. Pathol. 167 (2), 609–618. doi:10.1016/S0002-9440(10)63002-7
Del Rio, R., Andrade, D. C., Lucero, C., Arias, P., and Iturriaga, R. (2016). Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension 68 (2), 436–445. doi:10.1161/HYPERTENSIONAHA.116.07255
Del Rio, R., Andrade, D. C., Toledo, C., Diaz, H. S., Lucero, C., Arce-Alvarez, A., et al. (2017). Carotid body-mediated chemoreflex drive in the setting of low and high output heart failure. Sci. Rep. 7 (1), 8035. doi:10.1038/s41598-017-08142-3
Di Murro, A., Petramala, L., Cotesta, D., Zinnamosca, L., Crescenzi, E., Marinelli, C., et al. (2010). Renin-angiotensin-aldosterone system in patients with sleep apnoea: Prevalence of primary aldosteronism. J. Renin Angiotensin Aldosterone Syst. 11 (3), 165–172. doi:10.1177/1470320310366581
Edwards, A., and Kurtcuoglu, V. (2022). Renal blood flow and oxygenation. Pflugers Arch. 474 (8), 759–770. doi:10.1007/s00424-022-02690-y
Esteban, M. A., Tran, M. G., Harten, S. K., Hill, P., Castellanos, M. C., Chandra, A., et al. (2006). Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 66 (7), 3567–3575. doi:10.1158/0008-5472.CAN-05-2670
Fledderus, J. O., Boon, R. A., Volger, O. L., Hurttila, H., Ylä-Herttuala, S., Pannekoek, H., et al. (2008). KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28 (7), 1339–1346. doi:10.1161/ATVBAHA.108.165811
Friedemann, J., Heinrich, R., Shulhevich, Y., Raedle, M., William-Olsson, L., Pill, J., et al. (2016). Improved kinetic model for the transcutaneous measurement of glomerular filtration rate in experimental animals. Kidney Int. 90 (6), 1377–1385. doi:10.1016/j.kint.2016.07.024
Gilmartin, G. S., Lynch, M., Tamisier, R., and Weiss, J. W. (2010). Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am. J. Physiol. Heart Circ. Physiol. 299 (3), H925–H931. doi:10.1152/ajpheart.00253.2009
Grundy, D. (2015). Principles and standards for reporting animal experiments in the journal of Physiology and experimental Physiology. J. Physiol. 593 (12), 2547–2549. doi:10.1113/JP270818
Haack, K. K., Marcus, N. J., Del Rio, R., Zucker, I. H., and Schultz, H. D. (2014). Simvastatin treatment attenuates increased respiratory variability and apnea/hypopnea index in rats with chronic heart failure. Hypertension 63 (5), 1041–1049. doi:10.1161/HYPERTENSIONAHA.113.02535
Haase, V. H. (2013). Mechanisms of hypoxia responses in renal tissue. J. Am. Soc. Nephrol. 24 (4), 537–541. doi:10.1681/ASN.2012080855
Hansell, P., Welch, W. J., Blantz, R. C., and Palm, F. (2013). Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin. Exp. Pharmacol. Physiol. 40 (2), 123–137. doi:10.1111/1440-1681.12034
Herrera Pérez, Z., Weinfurter, S., and Gretz, N. (2016). Transcutaneous assessment of renal function in conscious rodents. J. Vis. Exp. (109), e53767. doi:10.3791/53767
Hu, Y., Mai, L., Luo, J., Shi, W., Xiang, H., Song, S., et al. (2022). Peripheral blood oxidative stress markers for obstructive sleep apnea-a meta-analysis. Sleep. Breath. 26 (4), 2045–2057. doi:10.1007/s11325-021-02557-z
Huang, H. C., Walters, G., Talaulikar, G., Figurski, D., Carroll, A., Hurwitz, M., et al. (2017). Sleep apnea prevalence in chronic kidney disease - association with total body water and symptoms. BMC Nephrol. 18 (1), 125. doi:10.1186/s12882-017-0544-3
Huang, J., Lusina, S., Xie, T., Ji, E., Xiang, S., Liu, Y., et al. (2009). Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir. Physiol. Neurobiol. 166 (2), 102–106. doi:10.1016/j.resp.2009.02.010
Khalaji, A., Amirkhani, N., Sharifkashani, S., and Behnoush, A. H. (2023). Role of galectin-3 as a biomarker in obstructive sleep apnea: A systematic review and meta-analysis. Sleep. Breath. [Epub ahead of print] doi:10.1007/s11325-023-02842-z
Kinebuchi, S., Kazama, J. J., Satoh, M., Sakai, K., Nakayama, H., Yoshizawa, H., et al. (2004). Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin. Sci. (Lond) 107 (3), 317–322. doi:10.1042/CS20040074
Kious, K. W., Philipose, A., Smith, L. J., Kemble, J. P., Twohey, S. C. E., Savage, K., et al. (2022). Peripheral chemoreflex modulation of renal hemodynamics and renal tissue PO2 in chronic heart failure with reduced ejection fraction. Front. Physiol. 13, 955538. doi:10.3389/fphys.2022.955538
Li, X., Liu, C., Zhang, H., Zhang, J., Zhao, M., Sun, D., et al. (2019). Effect of 12-month nasal continuous positive airway pressure therapy for obstructive sleep apnea on progression of chronic kidney disease. Med. Baltim. 98 (8), e14545. doi:10.1097/MD.0000000000014545
Lin, C. H., Lurie, R. C., and Lyons, O. D. (2020). Sleep apnea and chronic kidney disease: A state-of-the-art review. Chest 157 (3), 673–685. doi:10.1016/j.chest.2019.09.004
Liu, M., Ning, X., Li, R., Yang, Z., Yang, X., Sun, S., et al. (2017). Signalling pathways involved in hypoxia-induced renal fibrosis. J. Cell. Mol. Med. 21 (7), 1248–1259. doi:10.1111/jcmm.13060
Lu, Y. Y., Li, X. D., Zhou, H. D., Shao, S., He, S., Hong, M. N., et al. (2019). Transactivation domain of Krüppel-like factor 15 negatively regulates angiotensin II-induced adventitial inflammation and fibrosis. FASEB J. 33 (5), 6254–6268. doi:10.1096/fj.201801809R
Maier, L. E., Matenchuk, B. A., Vucenovic, A., Sivak, A., Davenport, M. H., and Steinback, C. D. (2022). Influence of obstructive sleep apnea severity on muscle sympathetic nerve activity and blood pressure: A systematic review and meta-analysis. Hypertension 79 (9), 2091–2104. doi:10.1161/HYPERTENSIONAHA.122.19288
Marcus, N. J., Del Rio, R., Ding, Y., and Schultz, H. D. (2018). KLF2 mediates enhanced chemoreflex sensitivity, disordered breathing, and autonomic dysregulation in heart failure. J. Physiol. 596 (15), 3171–3185. doi:10.1113/JP273805
Marcus, N. J., Li, Y. L., Bird, C. E., Schultz, H. D., and Morgan, B. J. (2010). Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: Role of the angiotensin II type 1 receptor. Respir. Physiol. Neurobiol. 171 (1), 36–45. doi:10.1016/j.resp.2010.02.003
Marcus, N. J., Olson, E. B., Bird, C. E., Philippi, N. R., and Morgan, B. J. (2009). Time-dependent adaptation in the hemodynamic response to hypoxia. Respir. Physiol. Neurobiol. 165 (1), 90–96. doi:10.1016/j.resp.2008.10.013
Marcus, N. J., Philippi, N. R., Bird, C. E., Li, Y. L., Schultz, H. D., and Morgan, B. J. (2012). Effect of AT1 receptor blockade on intermittent hypoxia-induced endothelial dysfunction. Respir. Physiol. Neurobiol. 183 (2), 67–74. doi:10.1016/j.resp.2012.05.025
Marcus, N. J., Pügge, C., Mediratta, J., Schiller, A. M., Del Rio, R., Zucker, I. H., et al. (2015). Exercise training attenuates chemoreflex-mediated reductions of renal blood flow in heart failure. Am. J. Physiol. Heart Circ. Physiol. 309 (2), H259–H266. doi:10.1152/ajpheart.00268.2015
Molkov, Y. I., Zoccal, D. B., Moraes, D. J., Paton, J. F., Machado, B. H., and Rybak, I. A. (2011). Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J. Neurophysiol. 105 (6), 3080–3091. doi:10.1152/jn.00070.2011
O'Neill, J., Jasionek, G., Drummond, S. E., Brett, O., Lucking, E. F., Abdulla, M. A., et al. (2019). Renal cortical oxygen tension is decreased following exposure to long-term but not short-term intermittent hypoxia in the rat. Am. J. Physiol. Ren. Physiol. 316 (4), F635–F645. doi:10.1152/ajprenal.00254.2018
Orrù, G., Storari, M., Scano, A., Piras, V., Taibi, R., and Viscuso, D. (2020). Obstructive Sleep Apnea, oxidative stress, inflammation, and endothelial dysfunction-An overview of predictive laboratory biomarkers. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 6939–6948. doi:10.26355/eurrev_202006_21685
Ostrowski, D., Heesch, C. M., Kline, D. D., and Hasser, E. M. (2023). Nucleus tractus solitarii is required for the development and maintenance of phrenic and sympathetic long-term facilitation after acute intermittent hypoxia. Front. Physiol. 14, 1120341. doi:10.3389/fphys.2023.1120341
Owada, T., Yoshihisa, A., Yamauchi, H., Iwaya, S., Suzuki, S., Yamaki, T., et al. (2013). Adaptive servoventilation improves cardiorenal function and prognosis in heart failure patients with chronic kidney disease and sleep-disordered breathing. J. Card. Fail 19 (4), 225–232. doi:10.1016/j.cardfail.2013.03.005
Palatini, P. (2012). Glomerular hyperfiltration: A marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol. Dial. Transpl. 27 (5), 1708–1714. Epub 2012 Mar 19. doi:10.1093/ndt/gfs037
Peiss, C. N., and Manning, J. W. (1964). Effects of sodium pentobarbital on electrical and reflex activation of the cardiovascular system. Circ. Res. 14, 228–235. doi:10.1161/01.res.14.3.228
Peng, Y. J., Overholt, J. L., Kline, D., Kumar, G. K., and Prabhakar, N. R. (2003). Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proc. Natl. Acad. Sci. U. S. A. 100 (17), 10073–10078. doi:10.1073/pnas.1734109100
Perrini, S., Cignarelli, A., Quaranta, V. N., Falcone, V. A., Kounaki, S., Porro, S., et al. (2017). Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight 2 (17), e94379. doi:10.1172/jci.insight.94379
Philippi, N. R., Bird, C. E., Marcus, N. J., Olson, E. B., Chesler, N. C., and Morgan, B. J. (2010). Time course of intermittent hypoxia-induced impairments in resistance artery structure and function. Respir. Physiol. Neurobiol. 170 (2), 157–163. doi:10.1016/j.resp.2009.12.003
Prabhakar, N. R., Kumar, G. K., Nanduri, J., and Semenza, G. L. (2007). ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid. Redox Signal 9 (9), 1397–1403. doi:10.1089/ars.2007.1732
Prabhakar, N. R., Peng, Y. J., and Nanduri, J. (2023). Carotid body hypersensitivity in intermittent hypoxia and obtructive sleep apnoea. J. Physiol. Epub ahead of print. PMID: 37029496. doi:10.1113/JP284111
Puckrin, R., Iqbal, S., Zidulka, A., Vasilevsky, M., and Barre, P. (2015). Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int. Urol. Nephrol. 47 (11), 1839–1845. doi:10.1007/s11255-015-1113-y
Pügge, C., Mediratta, J., Marcus, N. J., Schultz, H. D., Schiller, A. M., and Zucker, I. H. (2016). Exercise training normalizes renal blood flow responses to acute hypoxia in experimental heart failure: Role of the α1-adrenergic receptor. J. Appl. Physiol. (1985) 120 (3), 334–343. doi:10.1152/japplphysiol.00320.2015
Ratliff, B. B., Abdulmahdi, W., Pawar, R., and Wolin, M. S. (2016). Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal 25 (3), 119–146. doi:10.1089/ars.2016.6665
Rey, S., Del Rio, R., Alcayaga, J., and Iturriaga, R. (2004). Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J. Physiol. 560 (2), 577–586. doi:10.1113/jphysiol.2004.072033
Roy, A., Farnham, M. M. J., Derakhshan, F., Pilowsky, P. M., and Wilson, R. J. A. (2018). Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naïve carotid bodies. J. Physiol. 596 (15), 3149–3169. doi:10.1113/JP275001
Schock-Kusch, D., Sadick, M., Henninger, N., Kraenzlin, B., Claus, G., Kloetzer, H. M., et al. (2009). Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol. Dial. Transpl. 24 (10), 2997–3001. doi:10.1093/ndt/gfp225
Schock-Kusch, D., Xie, Q., Shulhevich, Y., Hesser, J., Stsepankou, D., Sadick, M., et al. (2011). Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 79 (11), 1254–1258. doi:10.1038/ki.2011.31
Sun, W., Yin, X., Wang, Y., Tan, Y., Cai, L., Wang, B., et al. (2012). Intermittent hypoxia-induced renal antioxidants and oxidative damage in male mice: Hormetic dose response. Dose Response 11 (3), 385–400. doi:10.2203/dose-response.12-027.Cai
Unnikrishnan, D., Jun, J., and Polotsky, V. (2015). Inflammation in sleep apnea: An update. Rev. Endocr. Metab. Disord. 16 (1), 25–34. doi:10.1007/s11154-014-9304-x
Walker, L. A., Gellai, M., and Valtin, H. (1986). Renal response to pentobarbital anesthesia in rats: Effect of interrupting the renin-angiotensin system. J. Pharmacol. Exp. Ther. 236 (3), 7–8. doi:10.1097/00132586-198702000-00008
Wang, B., Li, Z. L., Zhang, Y. L., Wen, Y., Gao, Y. M., and Liu, B. C. (2022). Hypoxia and chronic kidney disease. EBioMedicine 77, 103942. doi:10.1016/j.ebiom.2022.103942
Keywords: sleep apnea, chemoreflex, GFR, renal blood flow, renal PO2
Citation: Kious KW, Savage KA, Twohey SCE, Highum AF, Philipose A, Díaz HS, Del Rio R, Lang JA, Clayton SC and Marcus NJ (2023) Chronic intermittent hypoxia promotes glomerular hyperfiltration and potentiates hypoxia-evoked decreases in renal perfusion and PO2. Front. Physiol. 14:1235289. doi: 10.3389/fphys.2023.1235289
Received: 06 June 2023; Accepted: 27 June 2023;
Published: 06 July 2023.
Edited by:
Irving H. Zucker, University of Nebraska Medical Center, United StatesReviewed by:
Zaid Abassi, Technion Israel Institute of Technology, IsraelJulia Shanks, The University of Auckland, New Zealand
Copyright © 2023 Kious, Savage, Twohey, Highum, Philipose, Díaz, Del Rio, Lang, Clayton and Marcus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noah J. Marcus, bm9haC5tYXJjdXNAZG11LmVkdQ==
 Kiefer W. Kious
Kiefer W. Kious Kalie A. Savage
Kalie A. Savage Stephanie C. E. Twohey1,2
Stephanie C. E. Twohey1,2 Andrew Philipose
Andrew Philipose Hugo S. Díaz
Hugo S. Díaz Rodrigo Del Rio
Rodrigo Del Rio James A. Lang
James A. Lang Sarah C. Clayton
Sarah C. Clayton Noah J. Marcus
Noah J. Marcus