
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 07 August 2023
Sec. Renal Physiology and Pathophysiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1231448
This article is part of the Research TopicThe Immune System, Gut Microbiota, and Cross-Talk in Diabetic Kidney DiseaseView all 6 articles
Background: The Controlled Nutritional Status (CONUT) score, calculated from albumin, total cholesterol, and lymphocyte count, is a useful indicator for immune-nutritional assessment and is associated with the prognosis of various diseases. However, its relationship with renal outcomes, cardiovascular disease (CVD), and all-cause mortality in patients with diabetic kidney disease is unclear.
Methods: This retrospective single-center study enrolled 336 patients with biopsy-confirmed diabetic kidney disease from August 2009 to December 2018. The outcomes were progression to end-stage renal disease (ESRD), CVD events, and death. Univariate and multivariate Cox regression analyses were performed to estimate the association between confounding factors and outcomes. The Kaplan-Meier curve was used to compare the outcomes of the patients according to the median CONUT score. The area under the curve (AUC) evaluated with time-dependent receiver operating characteristics was used to test discriminative power of COUNT score.
Results: During a median follow-up period of 5.1 years. The Kaplan-Meier analysis showed that patients in the high CONUT group (CONUT score > 3) had a significantly higher incidence of ESRD, CVD events, and all-cause mortality than those in the low CONUT group (CONUT score ≤ 3). The multivariate COX regression analysis indicated that, The CONUT score was an independent predictor of ESRD (hazards ration [HR] = 1.129, 95% confidence interval [CI] 1.037-1.228, p = 0.005), CVD events (HR = 1.159, 95% CI 1.057-1.271, p = 0.002), and all-cause mortality (HR = 1.299, 95% CI 1.143-1.478, p < 0.001).
Conclusion: The CONUT score is an independent risk factor for ESRD, CVD events, and overall death in patients with diabetic kidney disease.
Diabetic kidney disease (DKD) is a common complication of diabetes that affects up to 50% of diabetic patients and is the leading cause of end-stage renal disease (ESRD) globally. It is also associated with a significant increase in cardiovascular disease (CVD) morbidity and mortality, making it a major public health concern (Selby and Taal, 2020). Traditional risk factors such as metabolic abnormalities and hemodynamic stress are well-known contributors to these unfavorable clinical outcomes (Giunti et al., 2006). Renin-angiotensin system (RAS) inhibitors, sodium-glucose cotransporter-2 inhibitors (SGLT-2is), glucagon-like peptide-1 receptor agonists (GLP-1RAs), and mineralocorticoid receptor antagonist (MRA) have been proven to effectively mitigate the impact of these risk factors on diabetic kidney disease patients (Yang et al., 2022; Filippatos et al., 2023; Giglio et al., 2023). However, a growing body of research indicates that chronic inflammation, immune dysregulation, and nutritional status also play crucial roles in the progression of diabetic kidney disease (Yang and Mou, 2017; Zhang et al., 2022; Liu et al., 2023; Zhang et al., 2023). As such, early identification and management of these non-traditional risk factors may be critical for optimizing the prognosis of diabetic kidney disease.
The Controlled Nutritional Status (CONUT) score is an immune-nutritional index developed by Ulibari et al. in 2005 for early monitoring of the nutritional status of hospitalized patients. It is calculated based on the serum albumin level, total cholesterol concentration, and total lymphocyte count (Ignacio de Ulíbarri et al., 2005). These three indicators represent information on nutritional status, immune function, and lipid metabolism, respectively, and can be obtained through routine blood analysis, making the CONUT score feasible and reproducible. Increasingly, studies have shown that the CONUT score can predict the prognosis of solid tumors and hematologic diseases (Xue et al., 2021; Zhou et al., 2021; Liu et al., 2022). Additionally, the CONUT score is an independent predictor of all-cause mortality and CVD in patients with coronary artery disease (Arero et al., 2022). In kidney diseases, the CONUT score is a strong predictor of CVD morbidity and all-cause mortality in peritoneal dialysis patients (Yang et al., 2020; Zhou et al., 2020), and is independently associated with previous CVD in patients with chronic kidney disease (Tsuda et al., 2023). Furthermore, the CONUT score has been shown to predict all-cause mortality in patients on hemodialysis (Takagi et al., 2022), and is significantly associated with disease activity in lupus nephropathy (Ahn et al., 2019). However, the potential role and prognosis of the CONUT score in diabetic kidney disease are rarely reported.
In this study, we aim to determine the prognostic value of CONUT scores in individuals with diabetic kidney disease for ESRD, CVD, and all-cause mortality.
This retrospective study included 336 patients with biopsy-confirmed DKD from Xinqiao Hospital of the Army Medical University in China between August 2009 and December 2018. DKD was diagnosed based on criteria established by the Renal Pathology Society in 2010 (Tervaert et al., 2010). All participants were followed up from the screening date until 31 December 2021, or until their death. The study protocol was approved by the ethical committee of Xinqiao Hospital (No. 2018-006-02).
Inclusion criteria were: 1) biopsy-confirmed diabetic kidney disease (including pure diabetic nephropathy and diabetic nephropathy combined with non-diabetic nephropathy); 2) adults aged 18 years or older; 3) complete medical information and follow-up data. Exclusion criteria were: 1) ESRD, CVD events, or all-cause death took place within 1 month of follow-up after enrollment; 2) patients with incomplete pathological information or blood routine examination; 3) patients with malignancies (e.g., breast, lung, gastrointestinal, hematologic cancers). (Figure 1).
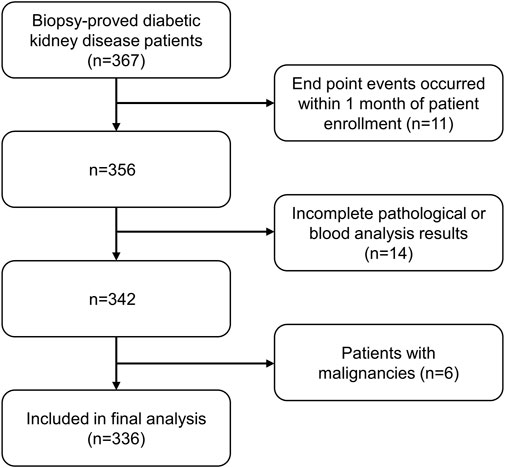
FIGURE 1. Flowchart of included patients in this study. ESRD: end-stage renal disease. CVD: Cardiovascular disease.
We extracted baseline demographic characteristics and laboratory values from the Electronic Medical Record System of Xinqiao Hospital at the time of the patient’s first renal biopsy. This included demographic data such as age and gender, medical history including hypertension and history of coronary heart disease, and laboratory data such as lymphocyte count, hemoglobin, serum creatinine, blood urea nitrogen (BUN), uric acid, intact parathyroid hormone (iPTH), calcium, magnesium, phosphate, albumin, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), proteinuria, and pathological information. We determined estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation. We calculated body mass index (BMI) by dividing weight in kilograms by height in meters squared based on the obtained height and weight measurements. Additionally, we calculated the CONUT score based on three laboratory variables: serum albumin concentration, total cholesterol concentration, and total peripheral lymphocyte count. The range of CONUT scores is 0–12, where a higher score indicates worse nutritional status (Ignacio de Ulíbarri et al., 2005) (Supplementary Table S1).
The study evaluated three outcomes: renal events, CVD events, and all-cause mortality, each of which was considered separately. Renal events were defined as an eGFR less than 15 mL/min/1.73 m2 or the need for maintenance renal replacement therapy due to irreversible deterioration of renal function, including hemodialysis, peritoneal dialysis, or kidney transplantation. CVD events were defined as the occurrence of new CVD events, such as coronary heart disease, heart failure, cerebrovascular events, and severe arrhythmia. All-cause mortality was defined as death from any cause. The study obtained clinical outcomes primarily through telephone follow-up or patient medical record reports.
The data analysis involved the use of SPSS (version 26.0) or R version 4.1.3. All the data used were checked for normality of distribution using the Kolmogorov-Smirnov test. Normally distributed data were expressed as mean ± standard deviation while non-normally distributed data were expressed as median (interquartile range). The differences between groups were tested using t-tests, Mann Whitney U tests, and chi-square tests. The Kaplan-Meier curve was used to compare the outcomes of the patients according to the median CONUT score. Additionally, Spearman rank correlation analysis was used to analyze the correlation between CONUT score and selected characteristics or parameters. Furthermore, the independent relationships between CONUT score and ESRD, CVD events, and all-cause mortality were investigated by univariate and multivariate Cox regression models. Hazard ratios (HRs) and 95% confidence intervals (CIs) are provided. Morevoer, subgroup analysis based on different clinical characteristics and sensitive analysis using renal pathology was also conducted to investigate the potential role of CONUT in specific populations. Furthermore, the prediction of CONUT score at different times for clinical outcomes was evaluated with time-dependent receiver operating characteristic (td-ROC) curve, and the area under the curve (AUC) was calculated for different times. It is important to note that a p value of < 0.05 was considered statistically significant during the analysis.
Finally, a total of 336 patients with DKD was included for analysis. The mean age was 50.5 (45-59) years, and 35.1% (118) were female. Among the patients, 35.4% (119) were smokers, and 21.7% (73) had a history of coronary heart disease. A total of 70.5% (237) had hypertension. The median eGFR and proteinuria were 65.78 (39-98.70) mL/min/1.73 m2 and 2.26 (0.72-5.37) g/day, respectively. Then, the CONUT score was calculated with a median of 3 (IQR 1-5). Table 1 lists the baseline demographic and clinical characteristics of the study population. In addition, we also analyzed the renal pathological parameters of all patients, as show in Supplementary Table S2. Then, patients were divided into two groups based on median CONUT score: the low CONUT score group (CONUT score ≤ 3) and the high CONUT score group (CONUT score > 3). According to our findings, patients in the high CONUT group were older and more likely to have coronary heart disease, as well as higher levels of proteinuria, BUN, serum creatinine, phosphate, and LDL. They also had lower levels of hemoglobin, eGFR, albumin, and lymphocyte count. Additionally, a higher proportion of individuals in the high CONUT score group were using insulin, statins, and anti-platelet drugs. Next, we also analyzed the renal pathological parameters between the two groups and found significant differences in glomerular class, interstitial fibrosis and tubular atrophy (IFTA), interstitial inflammation, and arteriolar hyalinosis. These results suggest that patients in the high CONUT group experienced more severe renal pathological changes compared to the low CONUT score group.
Next, we analyzed the correlations between the CONUT score and selected characteristics or laboratory variables. Spearman’s rank correlation analysis revealed that age, serum creatinine, BUN, cystatin C, LDL, and proteinuria levels were positively correlated with the CONUT score. Conversely, hemoglobin, eGFR, lymphocyte count, and albumin were negatively correlated with the CONUT score. Furthermore, with respect to renal pathological changes, the CONUT score was positively correlated with glomerular lesions (r = 0.228, p < 0.001), IFTA (r = 0.225, p < 0.001), interstitial inflammation (r = 0.222, p < 0.001), arteriolar hyalinosis (r = 0.149, p = 0.006), and arteriosclerosis (r = 0.155, p = 0.005) (Table 2).
During a median follow-up period of 5.1 years, 129 patients (38.4%) reached ESRD, while CVD events occurred among 112 individuals (33.3%), and 69 patients (20.5%) died. The Kaplan-Meier curve comparing patient’s outcomes according to median CONUT scores are shown in Figures 2A–C. The high CONUT group (CONUT score > 3) had significantly higher risk of ESRD (p < 0.001), CVD events (p < 0.01), and death (p < 0.01) than the low CONUT group (CONUT score ≤ 3). We also investigated the association of CONUT score with ESRD in subgroups of patients with different clinical characteristics. The results demonstrated that high CONUT group was still significantly associated with an increased risk of ESRD in males and females (Figure 3A), in patients with or without anemia (hemoglobin level < 120 g/L) (Figure 3B), in patients with BMI 25 < kg/m2 or not (Figure 3C), in patients with eGFR < 60 mL/min/1.73 m2 or not (Figure 3D), and in patients with or without hypertension (Figure 3E). Furthermore, to mitigate heterogeneity among the included patients, sensitivity analyses were conducted specifically for patients with biopsy-confirmed pure diabetic nephropathy. The Kaplan-Meier curve demonstrated that patients in the high CONUT group remained a significantly higher risk of ESRD, CVD events, and mortality (Supplementary Figure S1).

FIGURE 2. Kaplan-Meier curves for outcomes in patients with biopsy-confirmed DKD across different controlling nutritional status score (CONUT) groups. Divide patients into two groups based on the median of CONUT. the low CONUT group (CONUT≤3), the high CONUT group (CONUT>3). (A) End-stage renal disease (ESRD). (B) Cardiovascular disease. (C) All-cause mortality.
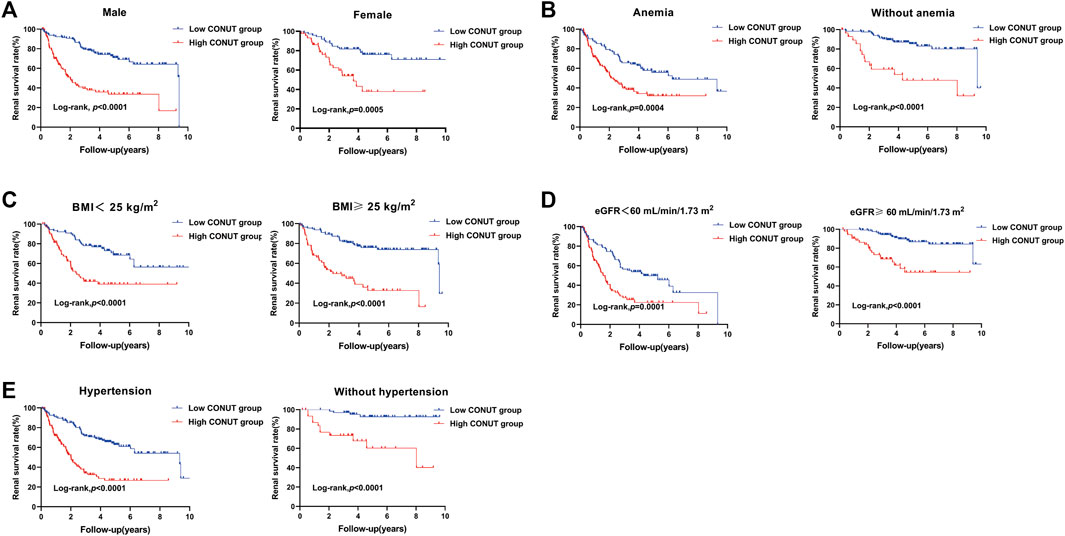
FIGURE 3. Kaplan-Meier curves for subgroup analysis in patients with biopsy-confirmed DKD with different types of clinical manifestations. Divide patients into two groups based on the median of controlling nutritional status (CONUT) score. The low CONUT group (CONUT≤3). The high CONUT group (CONUT>3). (A) Renal survival rate of male or female patients with different CONUT groups. (B) Renal survival rate of diabetic kidney disease patients with or without anemia in different CONUT groups. (C) Renal survival rate of diabetic kidney disease patients with serum body mass index (BMI) < 25 kg/m2 or ≥25 kg/m2 in different CONUT groups. (D) Renal survival rate of diabetic kidney disease patients with eGFR <60 mL/min/1.73 m2 or ≥60 mL/min/1.73 m2 in different CONUT groups. (E) Renal survival rate of diabetic kidney disease patients with hypertension or without hypertension in different CONUT groups.
Cox proportional hazards models were used to examine the relationship between the CONUT score and renal events, CVD events, and all-cause mortality. In univariate analysis, we found that higher CONUT scores were associated with higher risk of ESRD (HR = 1.253, 95% CI 1.174-1.337, p < 0.001), CVD events (HR = 1.251, 95% CI 1.163-1.345, p < 0.001), and all-cause mortality (HR = 1.424, 95% CI 1.295-1.566, p < 0.001). Moreover, hypertension, hemoglobin, eGFR, iPTH, LDL, glomerular class, IFTA, and interstitial inflammation were also associated with clinical outcomes (Table 3). After adjustment for baseline age, hypertension, hemoglobin, eGFR, iPTH, LDL, glomerular class, IFTA, and interstitial inflammation in a multivariate Cox regression model, CONUT score was still an independent predictor of ESRD (HR = 1.129, 95% CI 1.037-1.228, p = 0.005), CVD events (HR = 1.159, 95% CI 1.057-1.271, p = 0.002), and all-cause mortality (HR = 1.299, 95% CI 1.143-1.478, p < 0.001) (Table 4).
The prediction of clinical outcomes by the CONUT score was evaluated using the time-dependent receiver operating characteristic (td-ROC) of the subjects. For the prediction of ESRD, the area under curve (AUC) was 0.728 at 1 year, 0.758 at 2 years, and 0.749 at 3 years, respectively (Figure 4A). For the prediction of CVD events, the AUC was 0.706 at 1 year, 0.666 at 2 years, and 0.682 at 3 years, respectively (Figure 4B). And the prediction of all-cause mortality, the AUC was 0.810 at 1 year, 0.804 at 2 years, and 0.771 at 3 years, respectively (Figure 4C).
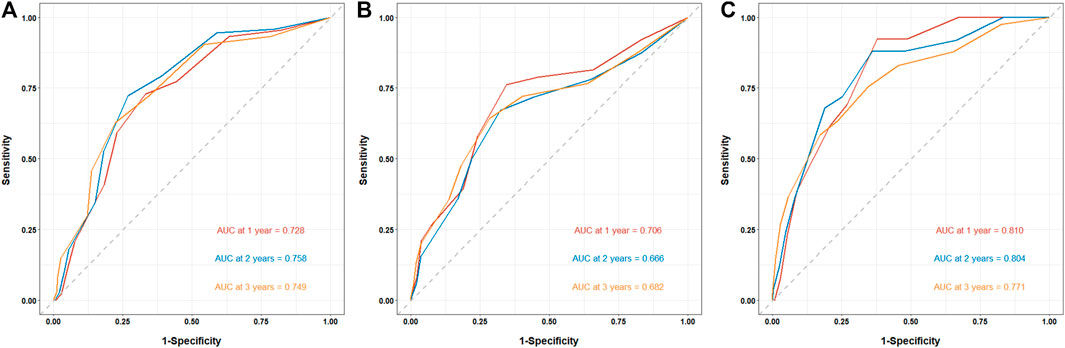
FIGURE 4. The prediction of CONUT score for clinical outcomes in patients with biopsy-confirmed DKD. The prediction of clinical outcomes by the CONUT score was evaluated using the time-dependent receiver operating characteristic (td-ROC) of the subjects. (A) Prediction of ESRD by CONUT score. (B) Prediction of CVD events by CONUT score. (C) Prediction of all-cause mortality by CONUT score.
In this study, we investigated the relationship between the CONUT score and outcomes in patients with diabetic kidney disease. Our study is the first to show that the CONUT score can be used as a clinical predictor of ESRD, CVD events, and all-cause mortality in patients with diabetic kidney disease.
In our study, a high CONUT score was found to be associated with an increased risk of ESRD in patients with diabetic kidney disease, which is the most common complication in these patients. Research has suggested that immune inflammation and nutritional status may be closely related to the progression of renal function in diabetic kidney disease patients (Zhang et al., 2022; Liu et al., 2023). Among the components of CONUT score, albumin serves as a cornerstone for nutritional assessment. Zhang et al. have confirmed that hypoalbuminemia is associated with the progression of ESRD in patients with diabetic kidney disease (Zhang et al., 2019). Lymphocytes in the CONUT score, which are immune cells involved in mediating the inflammatory response. A previous retrospective study showed that the platelet-lymphocyte ratio (PLR) is an independent risk factor for progression to ESRD in patients with diabetic kidney disease (Duan et al., 2021), suggesting that low lymphocyte counts may lead to ESRD. In our study, Cox regression analysis showed that high CONUT scores (indicating low albumin and lymphocyte counts) were significantly associated with an increased risk of diabetic kidney disease progression to ESRD, which is consistent with the aforementioned research results. In addition, there is evidence that the neutrophil-lymphocyte ratio (NLR) is significantly associated with decreased eGFR and histopathological changes in diabetic nephropathy patients (Zhang et al., 2019a). Similarly, we found that high CONUT scores were associated with lower eGFR and more severe pathological damage. To more accurately assess the effect of CONUT scores on ESRD, we performed different subgroup analyses, and the results further showed that the higher CONUT score, the higher the risk of progression to ESRD in different subgroups of diabetic kidney disease patients. Therefore, the CONUT score, as a combined index of nutrition, immunity, and inflammation, may be a more comprehensive predictor of diabetic kidney disease progression to ESRD. Routine assessment of the CONUT score can assist physicians in developing appropriate treatment plans for patients with diabetic kidney disease, thereby slowing their kidney progression.
We observed a significant correlation between CONUT score and the incidence of CVD events in diabetic kidney disease patients. The risk of CVD is high in patients with diabetic kidney disease, which is a major cause of mortality (Valmadrid et al., 2000; Gerstein et al., 2001). The potential mechanisms underlying the association between the CONUT score and CVD events are complex and multifaceted. but each component of the CONUT score is related to the CVD events. High cholesterol is a conventional risk factor for CVD in the general population. However, in dialysis patients, low levels of cholesterol are a high-risk factor for CVD events (Kalantar-Zadeh et al., 2003). Additionally, studies have shown that low levels of cholesterol are associated with CVD events in patients with chronic heart failure (Nakagomi et al., 2014). The reason for this lipid paradox may be that the inflammatory or malnutrition state of the organism leads to a disturbance in lipid metabolism, which increases the risk of adverse events (Liu et al., 2004). Also, lymphocytes, as immune cells, are involved in systemic inflammatory responses, and clinical and animal studies have shown that low lymphocyte counts promote atherosclerosis formation (Núñez et al., 2011). Finally, the synthesis of albumin is influenced by chronic inflammation and malnutrition, and lower levels of albumin may be a marker of continuous arterial injury, as well as the progression of atherosclerosis and thrombosis (Kuller et al., 1991). A clinical study has also shown that lower serum albumin levels are associated with a higher risk of CVD events in patients with diabetic kidney disease (Anavekar et al., 2004). Therefore, the CONUT score, as a combination of the above three indicators, may be a reliable biomarker for identifying high-risk CVD patients. Early assessment of the CONUT score can provide a preliminary understanding of the nutritional, immune, inflammatory, and lipid metabolism status of patients and provide a reference for clinical management.
In the present study, a higher CONUT score was related to higher all-cause mortality. Nutritional status has a strong correlation with mortality in patients diagnosed with diabetic kidney disease (Tauchi et al., 2020). A previous cohort study comprising 2,720 patients indicated that nutritional status assessed by prognostic nutritional index (PNI) was related to all-cause mortality in patients with diabetic kidney disease (Zhang et al., 2023). In line with these findings, our study revealed that higher CONUT scores implied poorer nutritional status and increased risk of all-cause mortality, thus further emphasizing the importance of nutritional status in the development of all-cause mortality in diabetic kidney disease patients. In addition, anemia and BMI are reliable indicators for assessing the nutritional status of patients, with previous studies highlighting that patients with anemia have a higher risk of mortality (Vlagopoulos et al., 2005), and low weight diabetic kidney disease patients, characterized by BMI (<18.5 kg/m2), also have a higher risk of mortality (Yokomichi et al., 2021). Consistent with previous studies, our univariate Cox analysis demonstrated that patients with lower hemoglobin and BMI had a higher risk of all-cause mortality. In general, the CONUT score, serving as a nutritional assessment index, effectively reflects the immune-nutritional status of patients. By identifying patients who are prone to malnutrition, early nutritional support can be administered, thereby reducing the incidence of mortality.
Some limitations in this study deserve our attention. First, we were a single-center study involving a small number of people and only included patients with renal biopsy-proven DKD, which may have had selective bias. Moreover, there was heterogeneity in the included patients with different types of treatment drugs, which may have an impact on the occurrence of endpoint events. Second, we only assessed the CONUT score at the time of patient enrollment without investigating the prognostic impact of CONUT score changes over time in patients with diabetic kidney disease. Third, the validity of nutritional status assessed by CONUT score remains uncertain because of the lack of comparison with comprehensive nutritional assessment tools such as the Subjective Global Assessment (SGA) and Mini Nutritional Assessment (MNA).
In conclusion, the CONUT score serves as a simple and easily accessible biomarker for predicting progression to ESRD, CVD events and all-cause mortality in patients with diabetic kidney disease. Early assessment of the CONUT score can identify people at high risk of diabetic kidney disease and is important for reducing adverse clinical outcomes.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of Xinqiao Hospital. The patients/participants provided their written informed consent to participate in this study.
QH draft the paper; JX analyzed the data; TH collected the data; JZ conceived the idea of the study and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by research grants from Joint Funds of the National Natural Science Foundation of China (U22A20279), Key program of the Natural Science Foundation of China (No. 82030023), the Natural Science Foundation of China (82270762), Chongqing Science and Technology Talent Program (cstc2021ycjh-bgzxm0145), Personal training Program for Clinical Medicine Research of Army Medical University (No. 2018XLC1007) and Frontier specific projects of Xinqiao Hospital (No. 2018YQYLY004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1231448/full#supplementary-material
Supplementary Figure S1 | Kaplan-Meier curves for (A) End-stage renal disease (ESRD), (B) Cardiovascular disease (CVD) events and (C) All-cause mortality in patients with biopsy-confirmed pure diabetic nephropathy (DN) across different controlling nutritional status score (CONUT) groups. Divide patients into two groups based on the median of CONUT. The low CONUT group (CONUT≤3), the high CONUT group.
Ahn, S. S., Yoo, J., Jung, S. M., Song, J. J., Park, Y. B., and Lee, S. W. (2019). Comparison of the clinical implications among five different nutritional indices in patients with lupus nephritis. Nutrients 11 (7), 1456. doi:10.3390/nu11071456
Anavekar, N. S., Gans, D. J., Berl, T., Rohde, R. D., Cooper, W., Bhaumik, A., et al. (2004). Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: A case for albuminuria. Kidney Int. 92, S50–S55. doi:10.1111/j.1523-1755.2004.09213.x
Arero, G., Arero, A. G., Mohammed, S. H., and Vasheghani-Farahani, A. (2022). Prognostic potential of the controlling nutritional status (CONUT) score in predicting all-cause mortality and major adverse cardiovascular events in patients with coronary artery disease: A meta-analysis. Front. Nutr. 9, 850641. doi:10.3389/fnut.2022.850641
Duan, S., Sun, L., Zhang, C., Wu, L., Nie, G., Huang, Z., et al. (2021). Association of platelet-to-lymphocyte ratio with kidney clinicopathologic features and renal outcomes in patients with diabetic kidney disease. Int. Immunopharmacol. 93, 107413. doi:10.1016/j.intimp.2021.107413
Filippatos, G., Anker, S. D., August, P., Coats, A. J. S., Januzzi, J. L., Mankovsky, B., et al. (2023). Finerenone and effects on mortality in chronic kidney disease and type 2 diabetes: A FIDELITY analysis. Eur. Heart J. Cardiovasc Pharmacother. 9 (2), 183–191. doi:10.1093/ehjcvp/pvad001
Gerstein, H. C., Mann, J. F., Yi, Q., Zinman, B., Dinneen, S. F., Hoogwerf, B., et al. (2001). Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama 286 (4), 421–426. doi:10.1001/jama.286.4.421
Giglio, R. V., Patti, A. M., Rizvi, A. A., Stoian, A. P., Ciaccio, M., Papanas, N., et al. (2023). Advances in the pharmacological management of diabetic nephropathy: A 2022 international update. Biomedicines 11 (2), 291. doi:10.3390/biomedicines11020291
Giunti, S., Barit, D., and Cooper, M. E. (2006). Diabetic nephropathy: From mechanisms to rational therapies. Minerva Med. 97 (3), 241–262.
Ignacio de Ulíbarri, J., González-Madroño, A., de Villar, N. G., González, P., González, B., Mancha, A., et al. (2005). CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 20 (1), 38–45.
Kalantar-Zadeh, K., Block, G., Humphreys, M. H., and Kopple, J. D. (2003). Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 63 (3), 793–808. doi:10.1046/j.1523-1755.2003.00803.x
Kuller, L. H., Eichner, J. E., Orchard, T. J., Grandits, G. A., McCallum, L., and Tracy, R. P. (1991). The relation between serum albumin levels and risk of coronary heart disease in the Multiple Risk Factor Intervention Trial. Am. J. Epidemiol. 134 (11), 1266–1277. doi:10.1093/oxfordjournals.aje.a116030
Liu, X. Y., Zhang, X., Zhang, Q., Xie, H. L., Ruan, G. T., Liu, T., et al. (2022). Value of the controlling nutritional status score in predicting the prognosis of patients with lung cancer: A multicenter, retrospective study. JPEN J. Parenter. Enter. Nutr. 46 (6), 1343–1352. doi:10.1002/jpen.2321
Liu, Y., Coresh, J., Eustace, J. A., Longenecker, J. C., Jaar, B., Fink, N. E., et al. (2004). Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. Jama 291 (4), 451–459. doi:10.1001/jama.291.4.451
Liu, Y., Lv, Y., Zhang, T., Huang, T., Lang, Y., Sheng, Q., et al. (2023). T cells and their products in diabetic kidney disease. Front. Immunol. 14, 1084448. doi:10.3389/fimmu.2023.1084448
Nakagomi, A., Seino, Y., Noma, S., Kohashi, K., Kosugi, M., Kato, K., et al. (2014). Relationships between the serum cholesterol levels, production of monocyte proinflammatory cytokines and long-term prognosis in patients with chronic heart failure. Intern Med. 53 (21), 2415–2424. doi:10.2169/internalmedicine.53.2672
Núñez, J., Miñana, G., Bodí, V., Núñez, E., Sanchis, J., Husser, O., et al. (2011). Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 18 (21), 3226–3233. doi:10.2174/092986711796391633
Selby, N. M., and Taal, M. W. (2020). An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 22 (1), 3–15. doi:10.1111/dom.14007
Takagi, K., Takahashi, H., Miura, T., Yamagiwa, K., Kawase, K., Muramatsu-Maekawa, Y., et al. (2022). Prognostic value of the controlling nutritional status (CONUT) score in patients at dialysis initiation. Nutrients 14 (11), 2317. doi:10.3390/nu14112317
Tauchi, E., Hanai, K., and Babazono, T. (2020). Effects of dietary protein intake on renal outcome and mortality in patients with advanced diabetic nephropathy. Clin. Exp. Nephrol. 24 (2), 119–125. doi:10.1007/s10157-019-01796-5
Tervaert, T. W., Mooyaart, A. L., Amann, K., Cohen, A. H., Cook, H. T., Drachenberg, C. B., et al. (2010). Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 21 (4), 556–563. doi:10.1681/asn.2010010010
Tsuda, S., Nakayama, M., Tanaka, S., Haruyama, N., Yoshitomi, R., Fukui, A., et al. (2023). The association of controlling nutritional status score and prognostic nutritional index with cardiovascular diseases: The fukuoka kidney disease registry study. J. Atheroscler. Thromb. 30 (4), 390–407. doi:10.5551/jat.63501
Valmadrid, C. T., Klein, R., Moss, S. E., and Klein, B. E. (2000). The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch. Intern Med. 160 (8), 1093–1100. doi:10.1001/archinte.160.8.1093
Vlagopoulos, P. T., Tighiouart, H., Weiner, D. E., Griffith, J., Pettitt, D., Salem, D. N., et al. (2005). Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: The impact of chronic kidney disease. J. Am. Soc. Nephrol. 16 (11), 3403–3410. doi:10.1681/asn.2005030226
Xue, W., Zhang, Y., Wang, H., Zhang, Y., and Hu, X. (2021). Multicenter study of controlling nutritional status (CONUT) score as a prognostic factor in patients with HIV-related renal cell carcinoma. Front. Immunol. 12, 778746. doi:10.3389/fimmu.2021.778746
Yang, S., Zhao, L., Mi, Y., and He, W. (2022). Effects of sodium-glucose cotransporter-2 inhibitors and aldosterone antagonists, in addition to renin-angiotensin system antagonists, on major adverse kidney outcomes in patients with type 2 diabetes and chronic kidney disease: A systematic review and network meta-analysis. Diabetes Obes. Metab. 24 (11), 2159–2168. doi:10.1111/dom.14801
Yang, X., and Mou, S. (2017). Role of immune cells in diabetic kidney disease. Curr. Gene Ther. 17 (6), 424–433. doi:10.2174/1566523218666180214100351
Yang, Y., Zhou, H., Zhang, P., Chao, W., Zou, Y., and Yang, M. (2020). Evaluation of objective nutritional indexes as predictors of worse outcomes in peritoneal dialysis patients. Nutrition 79-80, 110963. doi:10.1016/j.nut.2020.110963
Yokomichi, H., Mochizuki, M., Hirata, M., Nagai, A., Kojima, R., Horiuchi, S., et al. (2021). All-cause and cardiovascular disease mortality in underweight patients with diabetic nephropathy: BioBank Japan cohort. J. Diabetes Investig. 12 (8), 1425–1429. doi:10.1111/jdi.13483
Zhang, J., Chen, Y., Zou, L., and Gong, R. (2023). Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. 60 (2), 235–245. doi:10.1007/s00592-022-01985-x
Zhang, J., Xiao, X., Wu, Y., Yang, J., Zou, Y., Zhao, Y., et al. (2022). Prognostic nutritional index as a predictor of diabetic nephropathy progression. Nutrients 14 (17), 3634. doi:10.3390/nu14173634
Zhang, J., Zhang, R., Wang, Y., Li, H., Han, Q., Wu, Y., et al. (2019). The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J. Diabetes Res. 2019, 7825804. doi:10.1155/2019/7825804
Zhang, J., Zhang, R., Wang, Y., Wu, Y., Li, H., Han, Q., et al. (2019a). Effects of neutrophil-lymphocyte ratio on renal function and histologic lesions in patients with diabetic nephropathy. Nephrol. Carlt. 24 (11), 1115–1121. doi:10.1111/nep.13517
Zhou, H., Chao, W., Cui, L., Li, M., Zou, Y., and Yang, M. (2020). Controlling Nutritional Status (CONUT) score as immune-nutritional predictor of outcomes in patients undergoing peritoneal dialysis. Clin. Nutr. 39 (8), 2564–2570. doi:10.1016/j.clnu.2019.11.018
Keywords: diabetic kidney disease, controlling nutritional status (CONUT) score, end-stage renal disease, cardiovascular events, mortality
Citation: Huo Q, He T, Xiong J and Zhao J (2023) Controlling nutritional status score is associated with renal progression, cardiovascular events, and all-cause mortality in biopsy-proved diabetic kidney disease. Front. Physiol. 14:1231448. doi: 10.3389/fphys.2023.1231448
Received: 30 May 2023; Accepted: 26 July 2023;
Published: 07 August 2023.
Edited by:
Shiren Sun, Air Force Medical University, ChinaReviewed by:
Guisen Li, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaCopyright © 2023 Huo, He, Xiong and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiachuan Xiong, eGlvbmdqY0B0bW11LmVkdS5jbg==; Jinghong Zhao, emhhb2poQHRtbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.