
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Physiol. , 05 June 2023
Sec. Membrane Physiology and Membrane Biophysics
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1227565
This article is part of the Research Topic Chloride Homeostasis in Animal Cell Physiology View all 6 articles
Editorial on the Research Topic
Chloride homeostasis in animal cell physiology
Chloride (Cl−) homeostasis is a critical aspect of animal cell physiology that is often overlooked, but it plays a vital role in maintaining the balance of charge within cells. Chloride ions, along with sodium (Na+) and potassium (K+), are responsible for osmotic pressure and acid-base balance and determine fundamental biological functions in all tissues. Cl− is not in electrochemical equilibrium in most cell types, and its regulation involves the coordination of several processes. Cotransporters and exchangers utilize electric potential and/or chemical gradients to move two or more protons and ions in the same or opposite directions across the cell membrane. Impaired Cl− transport affects diverse processes ranging from neuron excitability to water secretion, which underlie pathological conditions such as epilepsy, deafness, imbalance, brain edema and ischemia, pain and neurogenic inflammation, hypertrophy, or heart failure-induced remodelling, chronic kidney disease and cystic fibrosis, etc. Therefore, further investigation to explore the molecular mechanisms of nociception occurring via membrane-bound Cl− ion channels or co-transporters is vital for understanding cellular physiology and pathophysiology, as well as for developing a new class of therapeutics.
The Research Topic includes two original research papers and three reviews from prominent researchers in the field and provides readers of the journal with recent results or summaries in the area of mechanisms of chloride ion channels and co-transporters in cellular Cl− volume regulation, Cl− homeostasis, creatine balance, exocytosis, mucus secretion, and formation of extracellular vesicles (Figure 1), as well as new strategies for drug development.
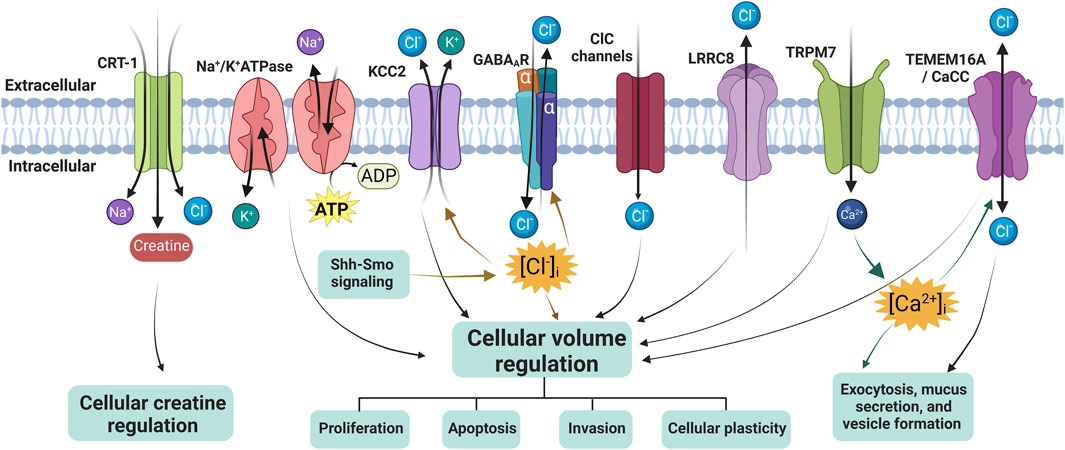
FIGURE 1. Overview of chloride ion channels, ion cotransporters, and creatine transporter in regulating cellular volume or creatine. CRT-1, creatine transporter 1; KCC2, K+-Cl− co-transporter 2; ClC channels, Cl− channels; LRRC8, leucine-rich repeat-containing 8; TMEM16A, transmembrane protein 16A; TRPM7, transient receptor potential cation channel subfamily M member 7; CaCC, Ca2+-activated Cl− channel, and GABAAR, γ-aminobutyric acid (GABA) A receptor. The diagram was created using BioRender.com.
The volume-regulated anion channel (VRAC) is present in various cell types and plays a crucial role in facilitating swelling-induced Cl− currents (ICl, swell) for cell volume regulation. To date, VRAC primarily consists of three main components: 1) a family of leucine-rich repeat containing 8 (LRRC8) proteins, which includes five members (LRRC8A-E); 2) the solute carrier organic anion transporter family member 2a1 gene (SLCO2A1), responsible for encoding organic anion transporting polypeptide (OATP) 2A1, also known as prostaglandin (PG) transporter, is a high-affinity prostanoid carrier; and 3) transmembrane protein 206 (TMEM206), an essential subunit of the acid-sensitive outwardly rectifying (ASOR) anion channel. Most of these channels have been identified as crucial core or pore-forming elements in various anion channels. Dysregulation of them can lead to cellular swelling or shrinkage, which can have significant consequences for cell function and survival. In their comprehensive review, Okada et al. discuss the current understanding of these channels with respect to their molecular identities, functional properties, structural features, and physiological roles. The review summarizes that these anion channels play a crucial role not only in essential physiological cell functions but also in pathological conditions by regulating/dysregulating cell volume and releasing organic signals.
KCC2, a K+-Cl− co-transporter mainly found in neurons, is a vital molecule that regulates Cl− extrusion and establishes the resting level of (Cl−)i in both developing and mature mammalian neurons, thereby tuning the strength and polarity of GABAA receptor-mediated transmission (Tillman and Zhang, 2019). One of the factors influencing the functioning of KCC2 and the development of inhibitory circuits is Smoothened (Smo), which acts as a transducer in the receptor complex of the developmental protein Sonic Hedgehog (Shh) (Delmotte et al., 2020). In this timely review, Hamze et al. delve into the latest research on how the Shh-Smo signaling pathways affect Cl− homeostasis by regulating KCC2 membrane trafficking, which, in turn, impacts inhibitory neurotransmission and network activity during postnatal development. Indeed, this new discovery opens up new avenues of research on Shh and GABA for physiological research, as well as for identifying potential pharmacological targets against neuropsychiatric and neurological diseases.
Maintaining appropriate levels of Cl− through Cl− channels is crucial for the normal functioning of the central nervous system (CNS). These channels consist of voltage-gated Cl− channels (ClC family), such as ClC-1 and ClC-2; Ca2+-activated Cl− channels (CaCCs), including Anoctamins 1 and 2 and Bestrophin1 (Best1); Cystic fibrosis transmembrane conductance regulator (CFTR); VRAC; GABAA-gated Cl− channels (GABAA receptor); and maxi anion channels (MAC). Wang and Choi have reviewed five families of Cl− channels expressed by neurons and glia and their role in the pathogenesis of CNS disorders. They have also summarized the small molecule Cl− channel modulators currently used as potential therapeutics in CNS disorders. Additionally, a possible strategy for optimizing drug permeation through the blood-brain barrier (BBB) has been suggested.
The creatine transporter (CRT) is a Na+- and Cl− -dependent transporter that is primarily responsible for transporting creatine across the cell membrane. CRT-1, a member of the SLC6 family (SLC6A8), is believed to play a crucial role in supplying creatine to skeletal and cardiac muscle, as well as the nervous system, where it acts as a precursor to phosphocreatine, a substance that replenishes spent ATP. Mutations in CRT-1 are implicated in several neurological disorders (Bizzi et al., 2002; Hahn et al., 2002), underscoring the importance of gaining insight into its mechanism and the effects of mutations on its function. However, our understanding of its molecular mechanisms lags far behind that of other well-studied SLC transporters and remains a critical question to be answered. Farr et al. used whole-cell patch clamp electrophysiological recordings on CRT-1-expressing HEK293 cells in combination with mathematical modelling to develop a kinetic model of CRT-1-mediated transport. They concluded that to maintain cytosolic creatine concentrations as observed, CRT-1 must destabilize binary complexes rather than ternary ones. Additionally, they provide a plausible explanation as to why neurons, heart, and skeletal muscle cells require a transporter that releases creatine, facilitating the quick balancing of creatine levels within these cells.
The Ca2+-activated Cl− channel TMEM16A and the Cl− permeable phospholipid scramblase TMEM16F are examples of Cl− channels that could have an impact on the intracellular Cl− concentration ([Cl−]i), potentially serving as an intracellular signal. If TMEM16A expression is lost in the airway, it can lead to a significant increase in the population of secretory cells such as goblet and club cells, resulting in the differentiation of the airway epithelium into a secretory one (He et al., 2020). Centeio et al. evaluated the role of TMEM16A and TMEM16F, as well as the Notch pathway, in airway epithelial cell differentiation using epithelial cultures and conditional knockout models. Their data found that TMEM16A/F are important for exocytosis, mucus secretion, and formation of extracellular vesicles (exosomes or ectosomes). However, their present data do not support a functional role of TMEM16A/F in Notch-mediated differentiation of BCi-NS1.1 cells towards a secretory epithelium.
Overall, this Research Topic summarizes important findings and recent research progress related to chloride ion channels and co-transporters, their mechanisms of regulation, and new strategies of drug development.
JZ conceptualized the Research Topic and was responsible for writing the whole passage. JZ, A-MH, and JG were responsible for checking and revision. All authors contributed to the article and approved the submitted version.
We are very grateful for the financial support from the National Natural Science Foundation of China (Grant Nos: 82170406, and 81970238), and The Royal Society United Kingdom (Grant No: IEC\NSFC\201094).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bizzi, A., Bugiani, M., Salomons, G. S., Hunneman, D. H., Moroni, I., Estienne, M., et al. (2002). X-Linked creatine deficiency syndrome: A novel mutation in creatine transporter gene SLC6A8. Ann. Neurol. 52, 227–231. doi:10.1002/ana.10246
Delmotte, Q., Hamze, M., Medina, I., Buhler, E., Zhang, J., Belgacem, Y. H., et al. (2020). Smoothened receptor signaling regulates the developmental shift of GABA polarity in rat somatosensory cortex. J. Cell. Sci. 133, jcs247700. doi:10.1242/jcs.247700
Hahn, K. A., Salomons, G. S., Tackels-Horne, D., Wood, T. C., Taylor, H. A., Schroer, R. J., et al. (2002). X-linked mental retardation with seizures and carrier manifestations is caused by a mutation in the creatine-transporter gene (SLC6A8) located in Xq28. Am. J. Hum. Genet. 70, 1349–1356. doi:10.1086/340092
He, M., Wu, B., Ye, W., Le, D. D., Sinclair, A. W., Padovano, V., et al. (2020). Chloride channels regulate differentiation and barrier functions of the mammalian airway. Elife 9, e53085. doi:10.7554/eLife.53085
Keywords: chloride ion channels and cotransporters, cellular Cl-volume regulation, Cl-homeostasis, creatine balance, exocytosis, mucus secretion, drug development
Citation: Zhang J, Hartmann A-M and Guo J (2023) Editorial: Chloride homeostasis in animal cell physiology. Front. Physiol. 14:1227565. doi: 10.3389/fphys.2023.1227565
Received: 23 May 2023; Accepted: 30 May 2023;
Published: 05 June 2023.
Edited and reviewed by:
Christoph Fahlke, Helmholtz Association of German Research Centres (HZ), GermanyCopyright © 2023 Zhang, Hartmann and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinwei Zhang, ai56aGFuZzVAZXhldGVyLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.