- 1Center for Healthy Ageing and Wellness (H-CARE), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 2Center for Toxicology and Health Risk Studies (CORE), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 3Faculty of Medicine and Defence Health, National Defence University of Malaysia, Kuala Lumpur, Malaysia
Introduction: Studies have shown that exercise increases angiogenesis and perfusion in the hippocampus, activates neurogenesis in the dentate gyrus and increases synaptic plasticity, as well as increases the complexity and number of dendritic spines, all of which promote memory function and protect against cognitive decline. Flavonoids are gaining attention as antioxidants in health promotion due to their rich phenolic content, particularly for their modulating role in the treatment of neurodegenerative diseases. Despite this, there has been no comprehensive review of cognitive improvement supplemented with flavonoid and prescribed with exercise or a combination of the two interventions has been conducted. The purpose of this review is to determine whether a combined intervention produces better results when given together than when given separately.
Methods: Relevant articles assessing the effect of physical exercise, flavonoid or in combination on cognitive related biomarkers and neurobehavioral assessments within the timeline of January 2011 until June 2023 were searched using three databases; PubMed, PROQUEST and SCOPUS.
Results: A total of 705 articles were retrieved and screened, resulting in 108 studies which are in line with the objective of the current study were included in the analysis.
Discussion: The selected studies have shown significant desired effect on the chosen biomarkers and neurobehavioral assessments.
Systematic Review Registration: identifier: [CRD42021271001].
1 Introduction
Cognitive decline, which is initially slow yet progressive, can be exacerbated by various risk factors and events (Legdeur et al., 2018). Among the reported cellular and molecular events that underlie cognitive decline are oxidative stress, deposition of protein aggregates, neuroinflammation, impaired mitochondrial function, induction of apoptosis, and alteration of autophagy (Wang et al., 2018). These impairments negatively impact the quality of life and daily functions of affected individuals. Pharmacological treatments or drugs used in clinical practice are often directed toward alleviating disease symptoms rather than reversing or improving neurodegeneration or reducing cognitive and functional declines. Extensive studies have been conducted to prove that physical activities have a neuroprotective effect by slowing the progression of neurodegeneration and thus improving or enhancing cognitive function. The protective effect of physical exercise increases the level of brain-derived neurotrophic factor (BDNF) and catecholamines, such as dopamine and epinephrine (Meis et al., 2017). In addition to exercise, nutrition is an important means to reduce the incidence of cognitive decline, Alzheimer’s disease (AD), and Parkinson’s disease (PD). Therefore, polyphenols derived from plant food play an important role in supporting the development and maintenance of a healthy brain (Braga et al., 2018). Polyphenols principally comprise flavonoids, and previous research indicates that regular consumption of foods containing flavonoids may lower the risk of neurodegenerative diseases (Jung and Kim, 2018). Because of their ability to cross the blood–brain barrier (BBB), flavonoids are considered a potential agent for slowing down cognitive decline (Mansour et al., 2017). The current review aims to determine the effect of physical exercise, flavonoid supplementation, and/or a combination of both interventions on biomarkers and neurobehavioral outcomes in rodent models with cognitive impairment. Existing evidence has shown that physical exercise and flavonoids have individual benefits. However, little is known about the effectiveness of the intervention when administered concurrently in an animal model. Animal models would provide critical information and understanding of the synergistic effect of flavonoids and exercise on cognition enhancement. A positive outcome would help identify lifestyle alterations affecting cognition and prevent cognitive impairment in the aging society over time.
2 Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). We only included studies that examined cognitive decline with neurobehavioral and biomarker evaluation after administration of any amount of flavonoids, forced exercise, or both in male rodents. The meta-analysis included works that reported similar or related outcomes under similar or related experimental conditions. Other reports describing different measures or sets of experiments were also included in the review, but they were not included in the meta-analysis. The detailed protocol for this review has been registered in the PROSPERO database (Registration No: CRD42021271001).
2.1 Eligibility criteria
Studies using male rodents with the cognitive impairment model assessing the effects of exercise, flavonoids, or a combination of both were eligible for inclusion. The types of exercise searched were treadmill exercise, swimming training, wheel running, and rotarod exercise. Epigallocatechin-3-gallate (EGCG), grape seed proanthocyanidin extract (GSPE), 7,8-dihydroxyflavone (DHF), silibinin, quercetin, and spinosin were some of the commonly used flavonoids; they were studied and included. Comparators that were considered eligible were animals induced with aluminum chloride (AlCl3), scopolamine, amyloid beta (Aβ), streptozotocin (STZ), and young rats. Eligible studies were selected independent of the number of animals in the experiment, exercise speed, duration and time of training, and duration and dosage of flavonoids used. Only experimental studies were included in the review. These experimental studies must have used flavonoids, exercise, or a combination or both as interventions, and the studies must have conducted behavioral tests, biochemistry assessments, or both to determine cognitive improvement. However, studies on humans, female rodents, and non-rodent animals were excluded from this systematic review, as well as studies lacking original data (e.g., letters, review articles, or editorials), articles on unrelated topics, and non-specific exercise intervention. In this work, analysis and results were separated according to three types of interventions: aerobic exercise, flavonoids, and combined intervention. For the meta-analysis, results that allowed a pool of data were selected. When data were unavailable, the work was not included in the meta-analysis.
2.2 Data sources and search strategy
Comprehensive and systematic searches were conducted by an independent author using electronic databases such as PubMed, PROQUEST, and SCOPUS. The following MESH and search terms were used: [Flavonoids] AND [aerobic exercise] AND [Cognition] OR [Biomarkers]. Duplications were removed using Rayyan, a web application for systematic reviews (Ouzzani et al., 2016). Manual searches were also conducted and supplemented using reference lists from identified articles. Electronic databases were searched from January 2011 to June 2023.
2.3 Study selection and data extraction
The titles and abstracts of every citation in the literature search were independently screened by two (DK and AA) of the seven listed authors. Titles and abstracts clearly dealing with a different subject were excluded. All other data were extracted directly from the full-text articles, and those with potential relevance were examined for eligibility criteria. According to PRISMA recommendations, inclusion and exclusion criteria were based on relevant study characteristics (animals, intervention, comparator, outcome, and study design). Studies were included if 1) experiments involved male rodents; 2) the intervention was any form of supervised exercise; and 3) the comparator was exercise, flavonoids, or a combination of both in animal models of cognitive impairment. Any disagreement was resolved by consensus or a consultation with a third reviewer (NACR). After applying the exclusion and inclusion criteria in the selected articles, the following important data were extracted: author names, year of publication, study origin, animal model (species, age, and weight), duration of intervention, exercise protocol description, flavonoids, and dosage. The outcome data extracted comprised neurobehaviors and biomarkers assessed in the included studies. All authors involved were contacted to provide missing data or clarify if the data provided were ambiguous.
There were studies evaluating the effects of physical exercise in models of pregnancy, inflammation, ovariectomized animals, stress model, neuropathic pain, amyotrophic lateral sclerosis, intracerebral hemorrhage, smoking, encephalomyelitis, schizophrenia, and cerebral ischemia. All these findings were outside the scope of the current review and were thus excluded.
2.4 Risk of bias and calculations
All selected articles were evaluated according to the 10-item checklist for risk of bias of the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE). Possible biases affecting the selected articles were evaluated using SYRCLE’s Risk of Bias Tool, a screening instrument specifically developed for risk of bias assessment in animal studies (Hooijmans et al., 2014).
A “yes” score indicates a low risk of bias, a “no” score indicates a high risk of bias, and an “unclear” score indicates an unknown risk of bias. The quality of the included studies was independently evaluated by two reviewers (DK and AA). Any discrepancies were resolved by discussion or by consulting a third reviewer (NACR). A risk-of-bias summary table was created using Review Manager, version 5.3, and included in the Supplement (Figure 2).
2.5 Data synthesis and statistical analysis
The demographic information and quality of the included studies were described narratively and tabulated accordingly. Where appropriate, meta-analyses were conducted for data that were suitable to be pooled together using the RevMan 5.4 software.
The continuous data extracted from individual studies were pooled together using their reported mean and standard deviation (SD), with the mean difference (MD) and 95% confidence interval (CI) used as effect estimates. The pooled effect estimates were reported as MD together with its 95%CI. Heterogeneity across the included studies in a meta-analysis was assessed using the Chi-squared test and Higgin’s I2 test for heterogeneity. Heterogeneity is considered to be low, moderate, or high if the I2 test is 30%, 50%, or 75%, correspondingly. A random effects (RE) meta-analysis was performed considering the presence of heterogeneity across the included studies, with a p-value <0.05 indicating statistical significance. Sensitivity analysis was performed by conducting a fixed-effect (FE) model to test for result robustness. The limited number of studies available for meta-analysis did not permit a subgroup analysis. Publication bias was not evaluated because fewer than 10 studies were included in the meta-analyses conducted. The Cochrane Review (Page et al., 2019) recommended that publication bias is inappropriate when fewer than 10 studies are synthesized.
3 Results
3.1 Study selection and data extraction
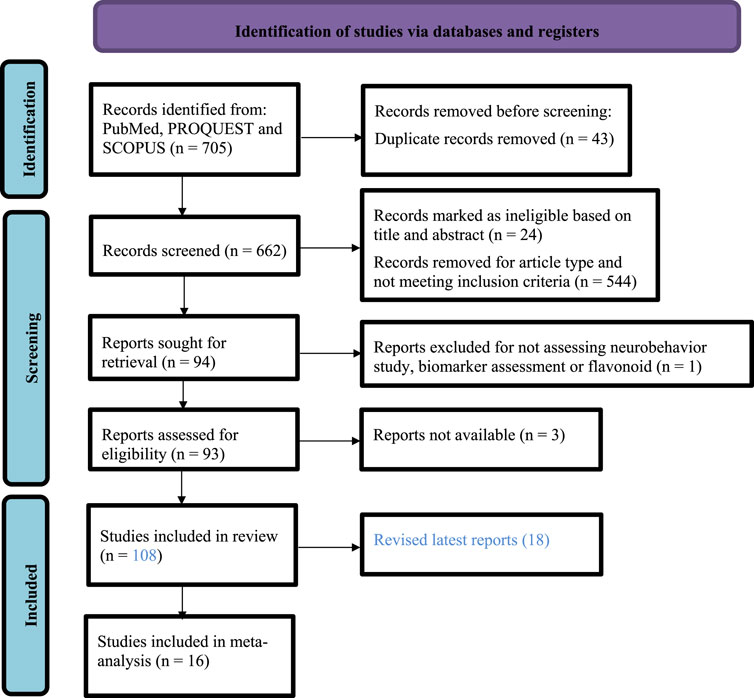
Initially, 705 articles were retrieved, of which, after thorough scrutinization, 63 were duplicated; 62 focused on other populations; 285 described other diseases; 203 were either reviews, short communication, conference abstracts, talks, or posters; 33 did not analyze the effects of exercise and flavonoids on brain alterations although cognitive impairment was reported, 44 focused on study design not related to flavonoids, exercise, or both on cognitive impairment; and 11 did not include neurobehavioral assessment or biomarkers. Finally, 108 studies that involved aerobic exercise and flavonoids and assessed neurophysiological effects on behavior and brain alterations were selected. For the meta-analysis, 16 studies with aggregate data for pooling were selected for meta-analysis on neurobehavioral tests and biomarkers. Figure 1 shows the flowchart of the data-gathering process. The included study characteristics are summarized in SupplementaryTable 1.
3.2 Study characteristics
The included animal species were male rodents aged between 4 weeks and 20 months. The number of animals in each group varied from 4 to 42.
3.2.1 Exercise
In the experimental models of exercise, exercise was in various forms, including aerobic exercise, running wheel, swim training, and rotarod device. The training time for voluntary and forced exercise ranged from 7 days to 16 weeks. The treadmill speed ranged from 8–13.2 m/min, and the duration was 30 min/m. The exercise was continued in one study until fatigue. The positive impact of exercise on cognition is through the molecular process of improving the redox state and enhancing inflammatory defenses (Benedetto et al., 2017). This would, in turn, trigger angiogenesis and neurogenesis and improve synapse formation (Chen et al., 2019). Exercise is considered as a safe and economical approach to neuroprotection and neurorestorative in cognitive impairment (Hsueh et al., 2018).
3.2.2 Flavonoids
Concerning flavonoids, the dosage ranged from 2 μg/kg to 1,650 mg/kg, and supplementation of flavonoids varied from 30 min to 9 months. The most common flavonoid supplementation protocol was 14 days (2 weeks). Among the common flavonoids found in the studies are catechin and epigallocatechin gallate (EGCG). EGCG is known to induce neurogenesis due to the ability of flavonoids and their metabolites to penetrate the blood–brain barrier and reach the brain (Unno et al., 2017). Moreover, EGCG eliminates ROS by inducing the production of proinflammatory cytokines (Wu et al., 2017). Conversely, catechin provides neuroprotection by reducing oxidative stress and decreasing lipid peroxidation, thus improving memory (Zamani et al., 2019).
3.3 Quality evaluation of the included studies
Studies included in this meta-analysis did not specifically describe sample-size calculation, allocation concealment, blinded assessment of outcomes, or reported animals excluded from the analysis, as is common in animal studies. Within each study, six domains were evaluated, and each was given a high, unclear, or low-risk rating. Across all the studies combined, high, unclear, or low risk was determined by the majority. All studies revealed a low risk of bias.
3.4 Results of individual studies
The Morris water maze (MWM) test (n = 71) was the most frequently used outcome measurement for neurobehavioral assessment, followed by the passive avoidance (n = 25), open-field (n = 17), Y-maze (n = 17), and novel object recognition (n = 15) test. Meanwhile, the two most commonly measured biomarkers of interest were acetylcholinesterase (AChE) (n = 24) and BDNF (n = 17), whereas malondialdehyde (MDA) (n = 25), SOD (n = 26), and CAT (n = 19) were the most commonly measured oxidative stress indicators in the selected studies.
Studies have reported that factors related to response to stress and neurogenesis were 24 on AChE. Reports on cerebral oxidative stress were 25 on MDA, whereas studies investigating spatial learning and memory in laboratory rats using the most common method (MWM) were 71. Regions frequently evaluated were the hippocampus, followed by the cerebral cortex. The included articles also reported the other means of assessing cognitive function, such as immunohistochemistry of vascular endothelial growth factor (VEGF)/platelet-derived growth factor (PDGF), neurological function of mice by Zea Longa scores, image analysis using light microscopy, electroencephalograph (EEG), circadian locomotor rhythm, and TUNEL assay for detecting apoptosis of hippocampal neurons. However, these methods are not within the scope of this review.
3.4.1 Morris water maze
Studies reported on various neurobehavioral tests, such as the radial maze test, Barnes hole-board maze, novel object recognition test, T-maze, open-field test (OFT), Y-maze test, and MWM test. The MWM test is employed to estimate memory and spatial learning in rodents, which assesses animals on time spent in the target quadrant, escape latency, and mean swimming speed. Fifty-nine studies reported on MWM, suggesting it is one of the most used neurobehavioral tests. Gibbons et al. (2014) administered a combined intervention of both flavonoids and exercise (epicatechin and β-alanine and voluntary wheel running). However, they did not observe any independent or additive/synergistic effects of the EGCG/β-ala diet on the performance in the MWM, whereas exercise improved age-related reductions in behavioral performance. This is because the dosages of EGCG and β-ala used in the study may have been too low. Conversely, Zhang et al. (2016) used combined intervention (epicatechin and treadmill exercise), indicating that treadmill exercise only improved spatial learning deficits rather than memory impairment. However, the combination therapy was able to improve both spatial learning and memory activity. Concerning flavonoid intervention, 75 studies reported its effect on learning and memory, of which only one study (Giacomini et al., 2019) showed that 40 days of 7,8-dihydroxyflavone had no sign of improvement in spatial learning and memory. It must be noted that the tests used to evaluate cognitive performance, such as contextual fear conditioning, novel object recognition, and MWM tests, may exhibit different sensitivity to treatment. Nevertheless, all other 74 studies using flavonoids reported a significant reduction in the MDA levels. These results indicated that flavonoids reduced the concentration of MDA to protect the antioxidant system.
3.4.2 Malondialdehyde
Thirty-eight studies reported several factors and variables related to oxidative stress; for example, superoxide dismutase (SOD) activity, catalase and glutathione (GSH) activity, nitric oxide synthase (iNOS), and lipid peroxidation (MDA) were evaluated. Nineteen studies reported on MDA. The combined study of flavonoids with treadmill exercise by Zhang et al. (2016) reported that impaired antioxidant enzymes were restored with epicatechin treatment alone. The possible explanation is that treadmill exercise may have elevated serum corticosterone levels similar to mild stress, which might offset the anti-oxidative effect of epicatechin in the combination group. However, the combined study of flavonoids with aerobic exercise by Abhijit et al. (2018) showed that adult rats benefited more from a combination of exercise and flavonoid supplementation than a single intervention. Similarly, all 24 studies with the intervention of flavonoids alone showed that the MDA level decreased in animals. Chronic stress can stimulate oxidative stress and the increased production of free radicals, which may contribute to cognition impairment. The outcomes of the studies show that flavonoids may inhibit neurological damage by suppression of oxidative stress and further protect against learning and memory impairments.
3.4.3 Acetylcholinesterase
Of 24 studies, 23 evaluated the content of AChE in rodents treated with flavonoids and one evaluated that content with a combination of flavonoids and exercise. The study using the combination of flavonoids and exercise (n = 44) reported that single interventions failed to show any significant decreases in AChE activity, unlike the combined interventions that resulted in a significant decrease. Among the 23 studies involving flavonoids, Mundugaru et al. (2017) reported no statistically significant changes (n = 24). However, they revealed a reduction, although the changes were not statistically significant compared to the AlCl3-alone group. All other studies showed a significant reduction in the level of AChE with the duration of flavonoid administration ranging from 30 min before sacrifice for AChE activity assay to 60 days. AChE is essential in maintaining the normal function of the nervous system, and an increased AChE level causes cognitive dysfunction. In the studies examined, it can be concluded that both administration of flavonoids alone and in combination with exercise could ameliorate learning and memory impairments by inhibiting AChE activity and elevating the level of neurotransmitter ACh in the cortex and hippocampus.
3.5 Meta-analysis
3.5.1 Morris water maze
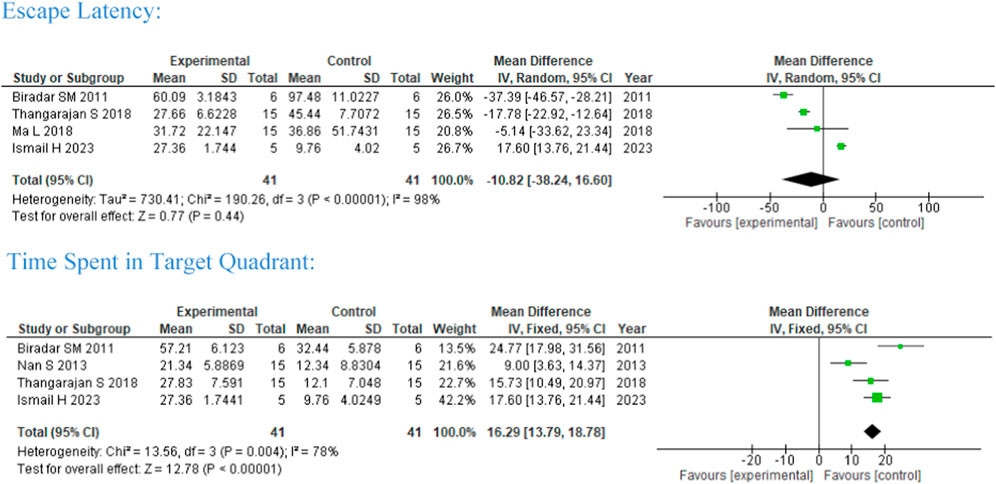
Five studies were included in the meta-analysis on the effect of flavonoid treatment on the neurobehavioral study of MWM (rodents, n = 41) (Figure 2). One subgroup analysis was performed with flavonoid intervention between 2 and 8 weeks. Hippocampal spatial perception and learning are commonly assessed using MWM through escape latency and time spent in the target quadrant. Among the studies, a 2-week intervention of Morin and Vitis vinifera (Ma et al., 2018; Thangarajan et al., 2018) and a 15-day intervention of Ageratum conyzoides (Biradar and Joshi, 2011) favors experimental/intervention group shortened escape latency and stronger learning ability, but they did not reach significance.
While 8-week intervention of EGCG (Nan et al., 2021) shows that studies conducted by Nan et al. (2013), Biradar and Joshi (2011), Thangarajan et al. (2018), and Ismail et al. (2023) favor control significantly for time spent at the target quadrant, implying memory was not improved (four studies, MD = 16.29, 95%CI 13.79, 18.78) with a heterogenicity of 78% between studies.
3.5.2 Malondialdehyde
The meta-analysis focused on assessing the effect of flavonoid treatment on the oxidative stress marker of MDA (rodents, n = 80) (Figure 3). The nine studies included in this meta-analysis examined the effect of flavonoids on the MDA level. Brain cells are most susceptible to lipid peroxidation, which is indicated by the presence of MDA in tissues. A lower level of MDA indicates reduced oxidative stress and an improved antioxidant system in the brain tissue. This reflects on better cognitive function. The statistical results demonstrated that flavonoid administration [nine studies; mean difference (MD), −1.98 nmol/mg protein; 95%CI (−3.20, −0.77)] has a significant effect in reducing MDA level among the animals with cognitive impairment as the effect favors experimental/intervention group, with a heterogenicity of 87% between studies. The nine studies included are as follows: Wu et al. (2012) administered green tea extract (GTex), epigallocatechin gallate (EGCG), for 7 days; Jia et al. (2016) administered liquiritin for 2 weeks; Mansour et al. (2017) administered 5, 7-dihydroxyflavone for 3 weeks; Peter AN and Rehab AA 2021 administered hesperidin for 8 weeks; Kwon et al. (2022) administered Rhodiola sachalinensis for 3 weeks; Hawash et al. (2023) administered jambolan fruit ethanolic extract for 28 days; Jadhav and Kulkarni (2023) administered baicalein for 21 days and quercetin for 42 days, respectively; and finally, Kim et al. (2023) administered Sesamum indicum L. for 4 weeks.
3.5.3 Acetylcholinesterase
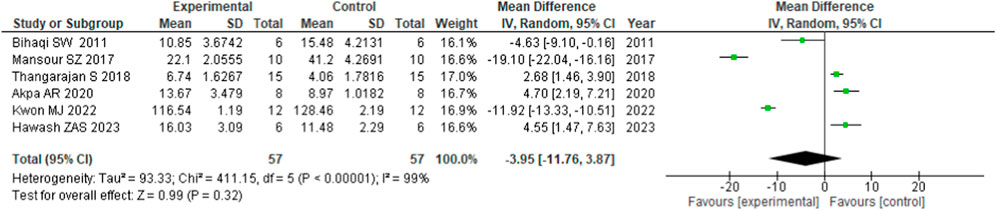
Finally, the meta-analysis studied the effect of flavonoids on AChE secretion (rodents, n = 57) (Figure 4). The forest plot of the pooled level of AChE for each study comparison is presented in Figure 4. Compared with the control group, Convolvulus pluricaulis was administered for 7 days (Bihaqi et al., 2011), 5, 7-dihydroxyflavone and Rhodiola sachalinensis were administered for 3 weeks, respectively (Mansour et al., 2017; Kwon et al., 2022) favors experimental/intervention group, which had positive effects on cognitive-enhancing activities from the inhibition of the AChE activity. Administration of flavonoids resulted in the improvement of the AchE activity, which invariably enhanced the neuromuscular activity. This showed that flavonoids have AChE restoration properties. However, other studies [i.e., Thangarajan et al. (2018) administering Morin for 2 weeks; Akpa et al. (2020) and Hawash et al. (2023) administering fisetin for 7 weeks and Convolvulus pluricaulis 1 week, respectively] showed a negative effect, favoring control. In addition, six studies [mean difference (MD), −3.95 U/mg protein; 95%CI (−11.76, 3.87)] found a trend in flavonoids improving cognition through AChE but did not reach significance, with a heterogenicity of 99% between studies.
95%CI values are presented for individual studies as squares and lines and meta-analysis results as diamonds.
4 Discussion
Physical exercise and flavonoids as a non-conventional approach/intervention for medical disease have proven beneficial in reducing the risk for many diseases, including stroke, high blood pressure, and mental disorders, such as chronic stress and depression (da Silva et al., 2012; Singh et al., 2014). Although the impact of physical exercise and flavonoid supplementation on cognition is well understood, the combined effect of the two interventions is still in its infancy (Wang et al., 2022). Neurodegeneration in the brain is progressive in nature and is an irreversible process. There is no effective treatment for age-related memory impairment, emphasizing the importance of developing preventive strategies before or during aging (Souza et al., 2015). Exercise has been shown to decrease the levels of circulating inflammatory cytokines, whereas flavonoids have been shown to exert beneficial effects in a variety of bodily functions and organs, including the brain (El-Kader and Al-Shreef, 2018; Nkpaa and Onyeso, 2018). The mechanisms by which flavonoids exert their effects depend largely on their antioxidant properties. Flavonoids may also interact with neuronal receptors and kinase signaling pathways, thereby modulating certain cellular processes.
The objective of the current study was to conduct a systematic review and meta-analysis to identify and evaluate the scientific literature published on the effect of exercise and flavonoid intervention on cognitive impairment, either alone or in combination. A total of 83 studies (n = 3,658–3,823) investigated the effect of flavonoid treatment on cognitive impairment. Five studies (Mundugaru et al., 2017; da Silveira et al., 2016; Peter and Rehab, 2021; Giacomini et al., 2019; Mallien et al., 2019) that administered flavonoids to rodents with cognitive impairment found no significant effect. A non-significant effect was observed in behavioral studies, which is the hallmark of learning, memory, and motor function. This could result from tests used to evaluate cognitive performance with different sensitivity to treatment in terms of dosage and duration. One study showed no difference in oxidative stress markers of SOD and CAT activities. This is most likely because the antioxidant activities of flavonoids may involve enzymatic and non-enzymatic pathways, such as GSH. However, the results of the remaining studies predominantly showed cognitive improvement. Elevated MDA means elevated lipid peroxidation and increased oxidative stress. Increase in lipid peroxidation of nueronal membrane leads to neuronal damage and apoptosis (Park et al., 2018). Dietary antioxidants enhance antioxidative systems and administration of phenolic-rich compounds, inhibiting MDA production (You et al., 2020). Thus, biomarker selection is critical to accurately reflect the actual scenario. Acetylcholine is one of the most important neurotransmitters involved in cognitive function regulation, and flavonoids appear to improve learning/acquisition and memory retention by lowering AChE levels. Acetylcholine modulates synaptic plasticity via BDNF. BDNF is crucial in supporting the survival and function of existing nerve cells while promoting the growth and differentiation of new neurons and synapses. As a result, BDNF is critical in learning, memory, and motor function.
Although improved behavioral performance is interpreted as improved cognition in terms of learning and memory, Chen et al. (2019) investigated the effect of treadmill exercise on cognitive impairment. The neurobehavioral radial maze test revealed that treadmill exercise for 7 or 14 days improves motor and cognitive functions. This demonstrates that exercise can improve cerebrovascular and neuronal plasticity, thereby protecting the brain from cognitive dysfunction and neurodegenerative diseases. Enhanced BDNF levels were also observed from treadmill training because BDNF is involved in the brain plasticity processes associated with cognitive recovery. Finally, six studies reported combined interventions of flavonoids and exercise. However, Bhattacharya et al. (2015) found no significant influence on learning and memory measures when the interventions were combined. Two studies (Abhijit et al., 2017; Abhijit et al., 2018) examining the combined effect showed that flavonoids alone improved the neurobehavioral test or biomarker. However, the combined intervention did not show further improvement. Two other studies (Gibbons et al., 2014; Zhang et al., 2016) found that exercise alone improved the results. Nevertheless, Ramis et al. (2021) reported that the combined action of exercise and a polyphenol-enriched diet could be very useful as a therapy to delay or ameliorate the cognitive and motor decline associated with aging by improving monoaminergic neurotransmitters. In summary, the outcomes of the studies that combine physical exercise and dietary flavonoids are varied. Additionally, the number of studies with combined intervention is limited in this review. Thus, further studies are required to properly deduce whether a physically active lifestyle in combination with the intake of antioxidants can be the most effective management strategy to alleviate the cognitive and motor deterioration associated with aging.
5 Strength and limitations
Although our search was comprehensive, we may have overlooked potentially relevant studies published in a language other than English. Moreover, the studies available for the meta-analysis were few. However, the strength of this study lies in its comprehensive assessment of this topic.
6 Conclusion
Considering the findings of this study, as well as the limitations, it can be concluded that combined intervention of exercise and flavonoids suggests a positive effect on cognitive function compared to flavonoids and exercise alone. Nevertheless, the results yielded from this review should be interpreted with caution due to the high heterogeneity observed across the included studies. Therefore, further research is necessary to define more specific recommendations on these interventions, in terms of quantity and type of polyphenol, as well as exercise strategies, to recommend these interventions as part of a healthy lifestyle regime in humans.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
Writing—original draft preparation, DJ; writing—review and editing, DJ, AL, and NR. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge Universiti Kebangsaan Malaysia DIP-2021-023 for their contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1216948/full#supplementary-material
Abbreviations
BDNF, brain-derived neurotrophic factor; MWM, Morris water maze; AChE, acetylcholinesterase; MDA, malondialdehyde; EGCG, epigallocatechin-3-gallate; PAT, passive avoidance test.
References
Abhijit S., Subramanyam M. V. V., Devi S. A. (2017). Grape seed proanthocyanidin and swimming exercise protects against cognitive decline: A study on M1 acetylcholine receptors in aging male rat brain. Neurochem. Res. 42 (12), 3573–3586. doi:10.1007/s11064-017-2406-6
Abhijit S., Tripathi S. J., Bhagya V., Shankaranarayana Rao B. S., Subramanyam M. V., Asha Devi S. (2018). Antioxidant action of grape seed polyphenols and aerobic exercise in improving neuronal number in the hippocampus is associated with decrease in lipid peroxidation and hydrogen peroxide in adult and middle-aged rats. Exp. Gerontol. 101, 101–112. doi:10.1016/j.exger.2017.11.012
Akpa A. R., Ayo J. O., Mika'il H. G., Zakari F. O. (2020). Protective effect of fisetin against subchronic chlorpyrifos-induced toxicity on oxidative stress biomarkers and neurobehavioral parameters in adult male albino mice. Toxicol. Res. 37 (2), 163–171. doi:10.1007/s43188-020-00049-y
Alaqeel N. K., AlSheikh M. H., Al-Hariri M. T. (2022). Quercetin nanoemulsion ameliorates neuronal dysfunction in experimental Alzheimer's disease model. Antioxidants (Basel) 11 (10), 1986. doi:10.3390/antiox11101986
Ali D. E., Bassam S. M., Elatrebi S., Habiba E. S., Allam E. A., Omar E. M., et al. (2023). HR LC-MS/MS metabolomic profiling of Yucca aloifolia fruit and the potential neuroprotective effect on rotenone-induced Parkinson's disease in rats. PLoS One 18 (2), e0282246. doi:10.1371/journal.pone.0282246
Ay M., Luo J., Langley M., Jin H., Anantharam V., Kanthasamy A., et al. (2017). Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson's Disease. J. Neurochem. 141 (5), 766–782. doi:10.1111/jnc.14033
Baitharu I., Jain V., Deep S. N., Shroff S., Sahu J. K., Naik P. K., et al. (2014). Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia. PLoS One 9 (10), e105311. doi:10.1371/journal.pone.0105311
Bax E. N., Cochran K. E., Mao J., Wiedmeyer C. E., Rosenfeld C. S. (2019). Opposing effects of S-equol supplementation on metabolic and behavioral parameters in mice fed a high-fat diet. Nutr. Res. 64, 39–48. doi:10.1016/j.nutres.2018.12.008
Ben-Azu B., Aderibigbe A. O., Ajay A. M., Omogbiya I. A., Uruaka C. I., Umukoro S., et al. (2021). Evaluation of the role of monoaminergic and nonmonoaminergic systems in the psychotropic effects of morin in mice: An interaction study with receptor blockers. Nutrire 46, 8. doi:10.1186/s41110-021-00137-5
Bhatnagar M., Goel I., Roy T., Shukla S. D., Khurana S. (2017). Complete Comparison Display (CCD) evaluation of ethanol extracts of Centella asiatica and Withania somnifera shows that they can non-synergistically ameliorate biochemical and behavioural damages in MPTP induced Parkinson's model of mice. PLoS One 12 (5), e0177254. doi:10.1371/journal.pone.0177254
Bhattacharya T. K., Pence B. D., Ossyra J. M., Gibbons T. E., Perez S., McCusker R. H., et al. (2015). Exercise but not (-)-epigallocatechin-3-gallate or β-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol. Behav. 145, 29–37. doi:10.1016/j.physbeh.2015.03.023
Bihaqi S. W., Singh A. P., Tiwari M. (2011). In vivo investigation of the neuroprotective property of Convolvulus pluricaulis in scopolamine-induced cognitive impairments in Wistar rats. Indian J. Pharmacol. 43 (5), 520–525. doi:10.4103/0253-7613.84958
Biradar S. M., Joshi H. K. (2011). Psychopharmacological investigations on the benefits of Ageratum conyzoides in the modulation of neurodegenerative disorder of Alzheimer′s type. Int. J. Green Pharm. 5 (3), 205. doi:10.4103/0973-8258.91229
Braga R., Teles D. A., Diniz T. C., Coimbra T., Pinto C., Gonçalves R., et al. (2018). Flavonoids as therapeutic agents in alzheimer’s and Parkinson’s diseases: A systematic review of preclinical evidences. Oxid. Med. Cell. Longev. 2018, 1–21. doi:10.1155/2018/7043213
Chen Q., Hu P. (2017). Proanthocyanidins prevent ethanol-induced cognitive impairment by suppressing oxidative and inflammatory stress in adult rat brain. Neuroreport 28 (15), 980–986. doi:10.1097/WNR.0000000000000867
Chen X., Wu H., Chen H., Wang Q., Xie X. J., Shen J. (2019). Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK signaling cascades. Mol. Neurobiol. 56 (4), 3053–3067. doi:10.1007/s12035-018-1294-3
Chen Z., Hu Q., Xie Q., Wu S., Pang Q., Liu M., et al. (2019). Effects of treadmill exercise on motor and cognitive function recovery of MCAO mice through the caveolin-1/VEGF signaling pathway in ischemic penumbra. Neurochem. Res. 44 (4), 930–946. doi:10.1007/s11064-019-02728-1
Chen Z. J., Yang Y. F., Zhang Y. T., Yang D. H., 2018, Dietary total prenylflavonoids from the fruits of Psoralea corylifolia L. Prevents age-related cognitive deficits and down-regulates Alzheimer's markers in SAMP8 mice. Molecules. 18;23(1):196, doi:10.3390/molecules23010196
Choi S. Y., Lee J., Lee D. G., Lee S., Cho E. J. (2017). Acer okamotoanum improves cognition and memory function in Aβ25–35-induced Alzheimer’s mice model. Appl. Biol. Chem. 60 (1), 1–9. doi:10.1007/s13765-016-0244-x
Cong W. H., Yang B., Xu L., Dong X. X., Sheng L. S., Hou J. C., et al. (2012). Herbal extracts combination (WNK) prevents decline in spatial learning and memory in APP/PS1 mice through improvement of hippocampal aβ plaque formation, histopathology, and ultrastructure. Evid. Based Complement. Altern. Med. 2012, 478190. doi:10.1155/2012/478190
da Silva G. S., Unsain N., Mascó D. H., Toscano-Silva M., de Amorim H. A., Silva Araújo B. H., et al. (2012). Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus 22 (2), 347–358. doi:10.1002/hipo.20903
da Silveira C. C. S. M., Fernandes L. M., Silva M. L., Luz D. A., Gomes A. R., Monteiro M. C., et al. (2016). Neurobehavioral and antioxidant effects of ethanolic extract of yellow propolis. Oxid. Med. Cell. Longev. 2016, 2906953. doi:10.1155/2016/2906953
Das J., Singh R., Ladol S., Nayak S. K., Sharma D. (2020). Fisetin prevents the aging-associated decline in relative spectral power of α, β and linked MUA in the cortex and behavioral alterations. Exp. Gerontol. 138, 111006. doi:10.1016/j.exger.2020.111006
Das J., Singh R., Sharma D. (2017). Antiepileptic effect of fisetin in iron-induced experimental model of traumatic epilepsy in rats in the light of electrophysiological, biochemical, and behavioral observations. Nutr. Neurosci. 20 (4), 255–264. doi:10.1080/1028415X.2016.1183342
Doungue H. T., Kengne A. P. N., Kuate D. (2018). Neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract, and juice in aluminum chloride-induced Alzheimer’s disease rats. Nutrire 43, 23. doi:10.1186/s41110-018-0082-1
El-Gazar A. A., Soubh A. A., Mohamed E. A., Awad A. S., El-Abhar H. S. (2019). Morin post-treatment confers neuroprotection in a novel rat model of mild repetitive traumatic brain injury by targeting dementia markers, APOE, autophagy and Wnt/β-catenin signaling pathway. Brain Res. 1717, 104–116. doi:10.1016/j.brainres.2019.04.003
El-Kader S. M. A., Al-Shreef F. M. (2018). Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 18 (1), 120–131. doi:10.4314/ahs.v18i1.16
Gao W. L., Li X. H., Dun X. P., Jing X. K., Yang K., Li Y. K. (2020). Grape seed proanthocyanidin extract ameliorates streptozotocin-induced cognitive and synaptic plasticity deficits by inhibiting oxidative stress and preserving AKT and ERK activities. Curr. Med. Sci. 40 (3), 434–443. doi:10.1007/s11596-020-2197-x
George A., Ng C. P., O'Callaghan M., Jensen G. S., Wong H. J. (2014). In vitro and ex-vivo cellular antioxidant protection and cognitive enhancing effects of an extract of Polygonum minus Huds (Lineminus™) demonstrated in a Barnes Maze animal model for memory and learning. BMC Complement. Altern. Med. 14, 161. doi:10.1186/1472-6882-14-161
Giacomini A., Stagni F., Emili M., Uguagliati B., Rimondini R., Bartesaghi R., et al. (2019). Timing of treatment with the flavonoid 7,8-DHF critically impacts on its effects on learning and memory in the Ts65Dn mouse. Antioxidants (Basel) 8 (6), 163. doi:10.3390/antiox8060163
Gibbons T. E., Pence B. D., Petr G., Ossyra J. M., Mach H. C., Bhattacharya T. K., et al. (2014). Voluntary wheel running, but not a diet containing (-)-epigallocatechin-3-gallate and β-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav. Brain Res. 272, 131–140. doi:10.1016/j.bbr.2014.05.049
Gong Y., Yang Y., Chen X., Yang M., Huang D., Yang R., et al. (2017). Hyperoside protects against chronic mild stress-induced learning and memory deficits. Biomed. Pharmacother. 91, 831–840. doi:10.1016/j.biopha.2017.05.019
Halawany A. M. E., Sayed N. S. E., Abdallah H. M., Dine R. S. E. (2017). Protective effects of gingerol on streptozotocin-induced sporadic Alzheimer's disease: Emphasis on inhibition of β-amyloid, COX-2, alpha-beta - secretases and APH1a. Sci. Rep. 7 (1), 2902. doi:10.1038/s41598-017-02961-0
Hawash Z. A. S., Yassien E. M., Alotaibi B. S., El-Moslemany A. M., Shukry M., 2023, Assessment of anti-alzheimer pursuit of jambolan fruit extract and/or choline against AlCl3 toxicity in rats. Toxics. 6;11(6):509, doi:10.3390/toxics11060509
He L., Deng Y., Gao J., Zeng L., Gong Q. (2018). Icariside II ameliorates ibotenic acid-induced cognitive impairment and apoptotic response via modulation of MAPK pathway in rats. Phytomedicine 41, 74–81. doi:10.1016/j.phymed.2018.01.025
Hong S. W., Heo H., Yang J. H., Han M., Kim D. H., Kwon Y. K. (2013). Soyasaponin I improved neuroprotection and regeneration in memory deficient model rats. PLoS One 8 (12), e81556. doi:10.1371/journal.pone.0081556
Huang P., Fang R., Li B. Y., Chen S. D. (2016). Exercise-related changes of networks in aging and mild cognitive impairment brain. Front. Aging Neurosci. 8, 47. doi:10.3389/fnagi.2016.00047
Imran I., Javaid S., Waheed A., Rasool M. F., Majeed A., Samad N., et al. (2021). Grewia asiatica berry juice diminishes anxiety, depression, and scopolamine-induced learning and memory impairment in behavioral experimental animal models. Front. Nutr. 7, 587367. doi:10.3389/fnut.2020.587367
Ismail H., Khalid D., Ayub S. B., Ijaz M. U., Akram S., Bhatti M. Z., et al. (2023). Effects of phoenix dactylifera against streptozotocin-aluminium chloride induced alzheimer’s rats and their in Silico study. BioMed Res. Int. 1725638, 1725638. doi:10.1155/2023/1725638
Jadhav R., Kulkarni Y. A. (2023). Neuroprotective effect of quercetin and memantine against AlCl3-induced neurotoxicity in albino wistar rats. Molecules 28 (1), 417. doi:10.3390/molecules28010417
Jadhav R., Kulkarni Y. A. (2023). The combination of Baicalein and memantine reduces oxidative stress and protects against β-amyloid-Induced Alzheimer's disease in rat model. Antioxidants (Basel). 12 (3), 707. doi:10.3390/antiox12030707
Jajin A. E., Esmaeili A., Rahgozar S., Noorbakhshnia M., 2021, Quercetin-conjugated superparamagnetic iron oxide nanoparticles protect AlCl3-induced neurotoxicity in a rat model of alzheimer’s disease via antioxidant genes, APP gene, and miRNA-101. Front. Neurosci. 14:598617.doi:10.3389/fnins.2020.598617
Jia S. L., Wu X. L., Li X. X., Dai X. L., Gao Z. L., Lu Z., et al. (2016). Neuroprotective effects of liquiritin on cognitive deficits induced by soluble amyloid-β1-42 oligomers injected into the hippocampus. J. Asian Nat. Prod. Res. 18 (12), 1186–1199. doi:10.1080/10286020.2016.1201811
Jung I. H., Lee H. E., Park S. J., Ahn Y. J., Kwon G., Woo H., et al. (2014). Ameliorating effect of spinosin, a C-glycoside flavonoid, on scopolamine-induced memory impairment in mice. Pharmacol. Biochem. Behav. 120, 88–94. doi:10.1016/j.pbb.2014.02.015
Jung U. J., Kim S. R. (2018). Beneficial effects of flavonoids against Parkinson’s disease. J. Med. Food 21, 421–432. doi:10.1089/jmf.2017.4078
Kang H., Zhou H., Ye Y., Yang J., Liu Z., He P., et al. (2021). Tieguanyin oolong tea extracts alleviate behavioral abnormalities by modulating neuroinflammation in APP/PS1 mouse model of Alzheimer's disease. Foods 11 (1), 81. doi:10.3390/foods11010081
Keser H., Doğramacı Ş., Şahin E., Sağlam N., Erdem M., Alver A., et al. (2020). The TrkB agonist 7,8-dihydroxyflavone improves sensory-motor performance and reduces lipid peroxidation in old mice. Gen. Physiol. Biophys. 39 (5), 471–479. doi:10.4149/gpb_2020022
Khan A., Ali T., Rehman S. U., Khan M. S., Alam S. I., Ikram M., et al. (2018). Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol. 9, 1383. doi:10.3389/fphar.2018.01383
Kim C. J., Ryu H. Y., Lee S., Lee H. J., Chun Y. S., Kim J. K., et al. (2021). Neuroprotective effect and antioxidant potency of fermented cultured wild ginseng root extracts of panax ginseng C.A. Meyer in mice. Molecules 26, 3001. doi:10.3390/molecules26103001
Kim H. J., Baek S. Y., Sok D. E., Lee K. J., Kim Y. J., Kim M. R. (2020). Neuroprotective activity of polyphenol-rich ribes diacanthum pall against oxidative stress in glutamate-stimulated HT-22 cells and a scopolamine-induced amnesia animal model. Antioxidants (Basel) 9 (9), 895. doi:10.3390/antiox9090895
Kim J., Seo Y. H., Kim J., Goo N., Jeong Y., Bae H. J., et al. (2020). Casticin ameliorates scopolamine-induced cognitive dysfunction in mice. J. Ethnopharmacol. 259, 112843. doi:10.1016/j.jep.2020.112843
Kim J. M., Park S. K., Kang J. Y., Park S. B., Yoo S. K., Han H. J., et al. (2019). Green tea seed oil suppressed aβ1⁻42-induced behavioral and cognitive deficit via the aβ-related akt pathway. Int. J. Mol. Sci. 20 (8), 1865. doi:10.3390/ijms20081865
Kim M. S., Jeon W. K., Lee K. W., Park Y. H., Han J. S., et al. (2015). Evaluation of low-dose aspirin for primary prevention of ischemic stroke among patients with diabetes: A retrospective cohort study. Altern. Med. 102734, 8. doi:10.1186/s13098-015-0002-y
Kim M. Y., Kim S., Lee J., Kim J. I., Oh E., Kim S. W., et al. (2023). Lignan-rich sesame (Sesamum indicum L) cultivar exhibits in vitro anti-cholinesterase activity, anti-neurotoxicity in amyloid-β induced SH-SY5Y cells, and produces an in vivo nootropic effect in scopolamine-induced memory impaired mice. Antioxidants (Basel) 12 (5), 1110. doi:10.3390/antiox12051110
Koh E. J., Seo Y. J., Choi J., Lee H. Y., Kang D. H., Kim K. J., et al. (2017). Spirulina maxima extract prevents neurotoxicity via promoting activation of BDNF/CREB signaling pathways in neuronal cells and mice. Molecules 22 (8), 1363. doi:10.3390/molecules22081363
Kwon M. J., Lee J. W., Kim K. S., Chen H., Cui C. B., Lee G. W., et al. (2022). The influence of tyrosol-enriched Rhodiola sachalinensis extracts bioconverted by the mycelium of bovista plumbe on scopolamine-induced cognitive, behavioral, and physiological responses in mice. Molecules 27 (14), 4455. doi:10.3390/molecules27144455
Lee H. E., Jeon S. J., Ryu B., Park S. J., Ko S. Y., Lee Y., et al. (2016). Swertisin, a C-glucosylflavone, ameliorates scopolamine-induced memory impairment in mice with its adenosine A1 receptor antagonistic property. Behav. Brain Res. 306, 137–145. doi:10.1016/j.bbr.2016.03.030
Lee H. Y., Weon J. B., Jung Y., Kim N., Kim M., Ma C. J. (2016). Genet variation of ectomycorrhizal suillus granulatus fruiting bodies in pinus strobus stands. Altern. Med. 6145926, 7–13. doi:10.5941/MYCO.2016.44.1.7
Lee J. H., Kim S. W., Lee S. H., Cho J. Y., Hwang S. H., Lee W. W., et al. (2023). The mixture of gastrodia elata and Glycyrrhiza uralensis attenuates scopolamine-induced cognitive disorder. Appl. Sci. 13, 3690. doi:10.3390/app13063690
Lee S., Jang G., Jung J., Park S., Lee J., Lee Y., et al. (2023). Memory-improving activity of the flower extract from Chrysanthemum boreale (makino) maskino in scopolamine-treated rodents. Processes 11, 159. doi:10.3390/pr11010159
Lee Y., Jeon S. J., Lee H. E., Jung I. H., Jo Y. W., Lee S., et al. (2016). Spinosin, a C-glycoside flavonoid, enhances cognitive performance and adult hippocampal neurogenesis in mice. Pharmacol. Biochem. Behav. 145, 9–16. doi:10.1016/j.pbb.2016.03.007
Legdeur N., Heymans M. W., Comijs H. C., Huisman M., Maier A. B., Visser P. J. (2018). Age dependency of risk factors for cognitive decline. BMC Geriatr. 18, 187. doi:10.1186/s12877-018-0876-2
Li E., Deng H., Wang B., Fu W., You Y., Tian S. (2016). Apelin-13 exerts antidepressant-like and recognition memory improving activities in stressed rats. Eur. Neuropsychopharmacol. 26 (3), 420–430. doi:10.1016/j.euroneuro.2016.01.007
Li R., Zang A., Zhang L., Zhang H., Zhao L., Qi Z., et al. (2014). Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol. Sci. 35 (10), 1527–1532. doi:10.1007/s10072-014-1784-7
Li S., Zhang Q., Ding Y., Wang X., Liu P. (2020). Flavonoids ameliorate aluminum chloride-induced learning and memory impairments via suppression of apoptosis and oxidative stress in rats. J. Inorg. Biochem. 212, 111252. doi:10.1016/j.jinorgbio.2020.111252
Liu B., Liu W., Liu P., Liu X., Song X., Hayashi T., et al. (2019). Silibinin alleviates the learning and memory defects in overtrained rats accompanying reduced neuronal apoptosis and senescence. Neurochem. Res. 44 (8), 1818–1829. doi:10.1007/s11064-019-02816-2
Liu P., Cui L., Liu B., Liu W., Hayashi T., Mizuno K., et al. (2020). Silibinin ameliorates STZ-induced impairment of memory and learning by up-regulating insulin signaling pathway and attenuating apoptosis. Physiol. Behav. 213, 112689. doi:10.1016/j.physbeh.2019.112689
Liu W., Zhu Y., Wang Y., Qi S., Wang Y., Ma C., et al. (2017). Anti-amnesic effect of extract and alkaloid fraction from aerial parts of Peganum harmala on scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 204, 95–106. doi:10.1016/j.jep.2017.04.019
Long J. Y., Chen J. M., Liao Y. J., Zhou Y. J., Liang B. Y., Zhou Y. (2020). Naringin provides neuroprotection in CCL2-induced cognition impairment by attenuating neuronal apoptosis in the hippocampus. Behav. Brain Funct. 16, 4. doi:10.1186/s12993-020-00166-6
Ma L., Xiao H., Wen J., Liu Z., He Y., Yuan F. (2018). Possible mechanism of Vitis vinifera L. flavones on neurotransmitters, synaptic transmission and related learning and memory in Alzheimer model rats. Lipids Health Dis. 17, 152. doi:10.1186/s12944-018-0708-6
Mallien A. S., Soukup S. T., Pfeiffer N., Brandwein C., Kulling S. E., Chourbaji S., et al. (2019). Effects of soy in laboratory rodent diets on the basal, affective, and cognitive behavior of C57bl/6 mice. J. Am. Assoc. Lab. Anim. Sci. 58 (5), 532–541. doi:10.30802/AALAS-JAALAS-18-000129
Mani V., Arfeen M., Dhaked D. K., Mohammed H. A., Amirthalingam P., Elsisi H. A. (2023). Neuroprotective effect of methanolic ajwa seed extract on lipopolysaccharide-induced memory dysfunction and neuroinflammation: In vivo, molecular docking and dynamics studies. Plants (Basel). 12 (4), 934. doi:10.3390/plants12040934
Mansour S. Z., Moawed F. S. M., Elmarkaby S. M. (2017). Protective effect of 5, 7-dihydroxyflavone on brain of rats exposed to acrylamide or γ-radiation. J. Photochem Photobiol. B 175, 149–155. doi:10.1016/j.jphotobiol.2017.08.034
Massaquoi M. S., Liguore W. A., Churchill M. J., Moore C., Melrose H. L., Meshul C. K. (2020). Gait deficits and loss of striatal tyrosine hydroxlase/trk-B are restored following 7,8-dihydroxyflavone treatment in a progressive MPTP mouse model of Parkinson's disease. Neuroscience 433, 53–71. doi:10.1016/j.neuroscience.2020.02.046
Matias I., Diniz L. P., Buosi A., Neves G., Stipursky J., Gomes F. C. A. (2017). Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-β1. Front. Aging Neurosci. 9, 184. doi:10.3389/fnagi.2017.00184
Meis S., Endres T., Munsch T., Lessmann V. (2017). The relation between long-term synaptic plasticity at glutamatergic synapses in the amygdala and fear learning in adult heterozygous BDNF knockout mice. Cereb. Cortex 28, 1195–1208. doi:10.1093/cercor/bhx032
Moreno-Ulloa A., Nogueira L., Rodriguez A., Barboza J., Hogan M. C., Ceballos G., et al. (2015). Recovery of indicators of mitochondrial biogenesis, oxidative stress, and aging with (-)-Epicatechin in senile mice. J. Gerontol. A Biol. Sci. Med. Sci. 70 (11), 1370–1378. doi:10.1093/gerona/glu131
Mostafa N. (2018). β-Amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. Rec. Nat. Prod. 12 (5), 480–492. doi:10.25135/rnp.47.17.10.062
Mundugaru R., Sivanesan S., Udaykumar P., Rao N., Chandra N. (2017). Protective effect of pluchea lanceolata against aluminum chloride-induced neurotoxicity in Swiss albino mice. Pharmacogn. Mag. 3, S567–S572. doi:10.4103/pm.pm_124_17
Nan S., Wang P., Zhang Y., Fan J. (2021). Epigallocatechin-3-Gallate provides protection against Alzheimer's disease-induced learning and memory impairments in rats. Drug Des. Devel Ther. 15, 2013–2024. doi:10.2147/DDDT.S289473
Nassiri-Asl M., Moghbelinejad S., Abbasi E., Yonesi F., Haghighi M. R., Lotfizadeh M., et al. (2013). Effects of quercetin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav. 28 (2), 151–155. doi:10.1016/j.yebeh.2013.04.019
Nkpaa K. W., Onyeso G. I. (2018). Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neurosci. Lett. 682, 92–99. doi:10.1016/j.neulet.2018.06.023
Noshy P. A., Azouz R. A. (2021). Neuroprotective effect of hesperidin against emamectin benzoate-induced neurobehavioral toxicity in rats. Neurotoxicol Teratol. 86, 106981. doi:10.1016/j.ntt.2021.106981
Ortiz-López L., Márquez-Valadez B., Gómez-Sánchez A., Silva-Lucero M. D., Torres-Pérez M., Téllez-Ballesteros R. I., et al. (2016). Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience 322, 208–220. doi:10.1016/j.neuroscience.2016.02.040
Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. (2016). Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5, 210. doi:10.1186/s13643-016-0384-4
Oyovwi M. O., Ben-Azu B., Tesi E. P., Oyeleke A. A., Uruaka C. I., Rotu R. A., et al. (2021). Repeated endosulfan exposure induces changes in neurochemicals, decreases ATPase transmembrane ionic-pumps, and increased oxidative/nitrosative stress in the brains of rats: Reversal by quercetin. Pestic. Biochem. Physiol. 175, 104833. doi:10.1016/j.pestbp.2021.104833
Page M. J., Higgins J. P. T., Sterne J. A. C. (2019). Assessing risk of bias due to missing results in a synthesis. Cochrane Handb. Syst. Rev. Interv. 2019, 349–374.
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, 790–799. doi:10.1016/j.rec.2021.07.010
Pang Q. Q., Kim J. H., Choi J. M., Song J. L., Lee S., Cho E. J. (2022). Cirsium japonicum var. Maackii improves cognitive impairment under amyloid beta25-35-induced Alzheimer's disease model. Biomed. Res. Int. 4513998, 1–11. doi:10.1155/2022/4513998
Parashar A., Mehta V., Udayabanu M. (2017). Rutin alleviates chronic unpredictable stress-induced behavioral alterations and hippocampal damage in mice. Neurosci. Lett. 656, 65–71. doi:10.1016/j.neulet.2017.04.058
Prakash R. S., Voss M. W., Erickson K. I., Kramer A. F. (2015). Physical activity and cognitive vitality. Ann. Rev. Psychol. 66, 769–797. doi:10.1146/annurev-psych-010814-015249
Prema A., Thenmozhi A. J., Manivasagam T., Essa M. M., Akbar M. D., Akbar M. (2016). Fenugreek seed powder nullified aluminium chloride induced memory loss, biochemical changes, aβ burden and apoptosis via regulating akt/gsk3β signaling pathway. PLoS One 11 (11), e0165955. doi:10.1371/journal.pone.0165955
Pyrzanowska J., Fecka I., Mirowska-Guzel D., Joniec-Maciejak I., Blecharz-Klin K., Piechal A., et al. (2019). Long-term administration of Aspalathus linearis infusion affects spatial memory of adult Sprague-Dawley male rats as well as increases their striatal dopamine content. J. Ethnopharmacol. 238, 111881. doi:10.1016/j.jep.2019.111881
Ramis M. R., Sarubbo F., Moranta D., Tejada S., Lladó J., Miralles A., et al. (2021). Neurochemical and cognitive beneficial effects of moderate physical activity and catechin in aged rats. Antioxidants 10, 621. doi:10.3390/antiox10040621
Sethiya N. K., Nahata A., Singh P. K., Mishra S. H. (2019). Neuropharmacological evaluation on four traditional herbs used as nervine tonic and commonly available as Shankhpushpi in India. J. Ayurveda Integr. Med. 10 (1), 25–31. doi:10.1016/j.jaim.2017.08.012
Singh R. P., Sharad S., Kapur S. (2014). Free radicals and oxidative stress in neurodegenerative diseases: Relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 5, 218–225.
Soubh A. A., El-Gazar A. A., Mohamed E. A., Awad A. S., El-Abhar H. S. (2021). Further insights for the role of Morin in mRTBI: Implication of non-canonical Wnt/PKC-α and JAK-2/STAT-3 signaling pathways. Int. Immunopharmacol. 100, 108123. doi:10.1016/j.intimp.2021.108123
Souza L. C., Antunes M. S., Filho C. B., Del Fabbro L., de Gomes M. G., Goes A. T., et al. (2015). Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 134, 22–30. doi:10.1016/j.pbb.2015.04.010
Subramanian P., Jayakumar M., Singaravel M., Kumar D., Basu P., Jayapalan J. J., et al. (2015). Fisetin, a dietary flavonoid, attenuates hyperammonemia and improves circadian locomotor deficits, redox balance, and astrocytic markers in rats. J. Funct. Foods 12, 409–419. doi:10.1016/j.jff.2014.11.025
Sun K., Yang P., Zhao R., Bai Y., Guo Z. (2018). Matrine attenuates D-galactose-induced aging-related behavior in mice via inhibition of cellular senescence and oxidative stress. Oxidative Med. Cell. Longev. 7108604, 7108604. doi:10.1155/2018/7108604
Sun P., Yin J. B., Liu L. H., Guo J., Wang S. H., Qu C. H., et al. (2019). Protective role of Dihydromyricetin in Alzheimer's disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci. Rep. 39 (1), BSR20180902. doi:10.1042/BSR20180902
Thangarajan S., Vedagiri A., Somasundaram S., Sakthimanogaran R., Murugesan M. (2018). Neuroprotective effect of morin on lead acetate-induced apoptosis by preventing cytochrome c translocation via regulation of Bax/Bcl-2 ratio. Neurotoxicol Teratol. 66, 35–45. doi:10.1016/j.ntt.2018.01.006
Thangthaeng N., Poulose S. M., Gomes S. M., Miller M. G., Bielinski D. F., Shukitt-Hale B. (2016). Tart cherry supplementation improves working memory, hippocampal inflammation, and autophagy in aged rats. Age (Dordr). 38 (5-6), 393–404. doi:10.1007/s11357-016-9945-7
Unno K., Pervin M., Nakagawa A., Iguchi K., Hara A., Takagaki A., et al. (2017). Blood-brain barrier permeability of green tea catechin metabolites and their neuritogenic activity in human neuroblastoma SH-SY5Y cells. Mol. Nutr. Food Res. 61, 1700294. doi:10.1002/mnfr.201700294
Vivar C., Potter M. C., van Praag H. (2012). All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Neurogenes. Neural Plast. 15, 189–210. doi:10.1007/7854_2012_220
Wang G. W., Cao J., Wang X. Q. (2019). Effects of ethanol extract from Bidens pilosa L. on spontaneous activity, learning and memory in aged rats. Exp. Gerontol. 125, 110651. doi:10.1016/j.exger.2019.110651
Wang J., Li L., Wang Z., Cui Y., Tan X., Yuan T., et al. (2018). Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. Jun 56, 16–25. doi:10.1016/j.jnutbio.2018.01.009
Wang R. S., Wang B. L., Huang Y. N., Thomas T. H. W. (2022). The combined effect of physical activity and fruit and vegetable intake on decreasing cognitive decline in older Taiwanese adults. Sci. Rep. 12, 9825. doi:10.1038/s41598-022-14219-5
Wang S., Yu Y., Feng Y., Zou F., Zhang X., Huang J., et al. (2016). Protective effect of the orientin on noise-induced cognitive impairments in mice. Behav. Brain Res. 296, 290–300. doi:10.1016/j.bbr.2015.09.024
Wang Y., Li M., Xu X., Song M., Tao H., Bai Y. (2012). Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 56 (8), 1292–1303. doi:10.1002/mnfr.201200035
Wang Y. J., Wang X. Y., Hao X. Y., Yan Y. M., Hong M., Wei S. F., et al. (2019). Ethanol extract of centipeda minima exerts antioxidant and neuroprotective effects via activation of the Nrf2 signaling pathway. Oxid. Med. Cell. Long. 9421037, 9421037. doi:10.1155/2019/9421037
Wei P., Li X., Wang S., Dong Y., Yin H., Gu Z., et al. (2022). Silibinin ameliorates formaldehyde-induced cognitive impairment by inhibiting oxidative stress. Oxid. Med. Cell. Longev. 2022, 5981353. doi:10.1155/2022/5981353
Wen H., Cui H., Tian H., Zhang X., Ma L., Ramassamy C., et al. (2021). Isolation of neuroprotective anthocyanins from black chokeberry (aronia melanocarpa) against amyloid-β-induced cognitive impairment anthocyanins from black chokeberry (aronia melanocarpa) against amyloid-induced cognitive. Impair. Foods 10, 63. doi:10.3390/foods10010063
Weon J. B., Lee J., Eom M. R., Jung Y. S., Ma C. J. (2014). The effects of loranthus parasiticus on scopolamine-induced memory impairment in mice. Evid. Based Complement. Altern. Med. 2014, 860180. doi:10.1155/2014/860180
Weon J. B., Yun B. R., Lee J., Eom M. R., Kim J. S., Lee H. Y., et al. (2013). The ameliorating effect of steamed and fermented codonopsis lanceolata on scopolamine-induced memory impairment in mice. Evid. Based Complement. Altern. Med. 2013, 464576. doi:10.1155/2013/464576
Wu K. J., Hsieh M. T., Wu C. R., Wood W. G., Chen Y. F. (2012). Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evid. Based Complement. Altern. Med. 2012, 163106. doi:10.1155/2012/163106
Wu Y. R., Choi H. J., Kang Y. G., Kim J. K., Shin J. W. (2017). In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano- and microscale particles. Int. J. Nanomed. 12, 7007–7013. doi:10.2147/IJN.S146296
Xu L., Li M., Wei A., Yang M., Li C., Liu R., et al. (2022). Treadmill exercise promotes E3 ubiquitin ligase to remove amyloid β and P-tau and improve cognitive ability in APP/PS1 transgenic mice. J. Neuroinflammation 19 (1), 243. doi:10.1186/s12974-022-02607-7
Yeh T. M., Chang C. D., Liu S. S., Chang C. I., Shih W. L. (2022). Tea seed kaempferol triglycoside attenuates LPS-induced systemic inflammation and ameliorates cognitive impairments in a mouse model. Molecules 27 (7), 2055. doi:10.3390/molecules27072055
Zamani M., Rohampour K., Rashtiani S., Dolati M., Fallahian F., Kalhor N. (2019). Effect of epigallocatechin gallate and catechin on overexpression of GSK-3β and IR genes induced by streptozotocin in rat brain. J. title 5 (4), 161–167. doi:10.32598/cjns.5.18.161
Zhang L., Liu C., Yuan M. (2020). Eriodictyol produces antidepressant-like effects and ameliorates cognitive impairments induced by chronic stress. Neuroreport 31 (15), 1111–1120. doi:10.1097/WNR.0000000000001525
Zhang Y., Liu J., Yao M., Song W., Zheng Y., Xu L., et al. (2019). Sailuotong capsule prevents the cerebral ischaemia-induced neuroinflammation and impairment of recognition memory through inhibition of LCN2 expression. Oxid. Med. Cell. Longev. 2019, 8416105. doi:10.1155/2019/8416105
Zhang Y., Qiao L., Song M., Wang L., Xie J., Feng H. (2014). Hplc-ESI-MS/MS analysis of the water-soluble extract from Ziziphi spinosae semen and its ameliorating effect of learning and memory performance in mice. Pharmacogn. Mag. 10 (40), 509–516. doi:10.4103/0973-1296.141777
Zhang Z., Wu H., Huang H. (2016). Epicatechin plus treadmill exercise are neuroprotective against moderate-stage amyloid precursor protein/presenilin 1 mice. Phcog Mag. 12, S139–S146. doi:10.4103/0973-1296.182174
Zhao L., Wang J. L., Liu R., Li X. X., Li J. F., Zhang L. (2013). Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer's disease mouse model. Molecules 18 (8), 9949–9965. doi:10.3390/molecules18089949
Zhao Q., Yokozawa T., Tsuneyama K., Tanaka K., Miyata T., Shibahara N., et al. (2011). Chotosan (Diaoteng San)-induced improvement of cognitive deficits in senescence-accelerated mouse (SAMP8) involves the amelioration of angiogenic/neurotrophic factors and neuroplasticity systems in the brain. Chin. Med. 6, 33. doi:10.1186/1749-8546-6-33
Zhong S., Ye J., Deng Y., Zhang M., Zou M., Yao X., et al. (2023). Quercetagitrin inhibits tau accumulation and reverses neuroinflammation and cognitive deficits in P301S-tau transgenic mice. Molecules 28 (9), 3964. doi:10.3390/molecules28093964
Keywords: flavonoids, aerobic exercise, cognition, biomarkers, neurobehavioral assessment abbreviations
Citation: Joseph DK, Mat Ludin AF, Ibrahim FW, Ahmadazam A, Che Roos NA, Shahar S and Rajab NF (2023) Effects of aerobic exercise and dietary flavonoids on cognition: a systematic review and meta-analysis. Front. Physiol. 14:1216948. doi: 10.3389/fphys.2023.1216948
Received: 04 May 2023; Accepted: 12 July 2023;
Published: 16 August 2023.
Edited by:
Muaz Belviranli, Selçuk University, TürkiyeReviewed by:
Sandeep Kumar Singh, Indian Scientific Education and Technology Foundation, IndiaSusana González Manzano, University of Salamanca, Spain
Copyright © 2023 Joseph, Mat Ludin, Ibrahim, Ahmadazam, Che Roos, Shahar and Rajab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nor Fadilah Rajab, bmZhZGlsYWhAdWttLmVkdS5teQ==
 Daren Kumar Joseph
Daren Kumar Joseph Arimi Fitri Mat Ludin
Arimi Fitri Mat Ludin Farah Wahida Ibrahim
Farah Wahida Ibrahim Amalina Ahmadazam1
Amalina Ahmadazam1 Nur Aishah Che Roos
Nur Aishah Che Roos Nor Fadilah Rajab
Nor Fadilah Rajab