- 1Department of Basic Sciences, College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates
- 2Division of Medical Physiology, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa
Introduction: Rooibos (Aspalathin linearis), honeybush (Cyclopia intermedia), and sutherlandia (Sutherlandia frutescene) are three Southern Africa indigenous plants, of which the extracts have become house-hold items and are consumed on a large scale. Although, they are known for their antioxidant properties, studies have highlighted danger in the excessive intake. Therefore, the current study investigated whether treatment with rooibos, honeybush, and sutherlandia will impact sperm functional parameters positively or otherwise, in healthy rats.
Methods: Fourteen-week-old pathogen-free adult male Wistar rats (250–300 g) were randomly divided into four groups of ten, including a control, rooibos (RF), honeybush (HB) and a sutherlandia (SL) group. After 7 weeks of treatment, animals were sacrificed. Spermatozoa were retrieved from the cauda epididymis for motility, morphology and concentration analysis and the testis was used for all biochemical assays.
Results: The infusion treated animals (RF, HB, and SL) presented with a non-significant decrease of −14.3%, −18.2%, −17.2% and −24.8%, −20.7%, −27.3% in total motility and progressive motility when compared to the control group, respectively. There was a significant increase in number of spermatozoa with slow speed (p = 0.03), especially in SL treated group compared to the control (p = 0.03). Additionally, there was an increase of 28.8%, 31.7%, 23% in superoxide dismutase (SOD) activity of RF, HB and SL compared to control, respectively. This was accompanied with a percentage decrease of −21.1%, −23.7%, 45.9% in malondialdehyde (MDA) levels compared to the control group.
Conclusion: In summary, animals treated with the respective infusions presented with a percentage increase in SOD activity but have reduced sperm motility and decreased normal morphology. Paradoxically, they presented with increased sperm concentration. Hence, it is presumed that rooibos, honeybush and sutherlandia may enhance sperm quantity (concentration) but may impair sperm quality (motility morphology) when consumed by healthy animals.
1 Introduction
The use of medicinal plants in treating diseases have been used over many decades in traditional medicine. It is interesting to know that the world is becoming more intrigued in exploring the benefits of phytochemicals and to also investigate their importance in health.
Rooibos, honeybush and sutherlandia are indigenous Southern Africa plants, and they are caffeine-free beverages. Rooibos originate from the Cederberg Mountains of the Western Cape region of South Africa (McGaw et al., 2007; Joubert et al., 2008). The leaves and stems are used for the commercially available rooibos tea. Rooibos is generally available either as the unfermented (green rooibos) or the fermented (reddish-brown rooibos) form. The fermented tea is achieved by oxidation that result in the unique reddish-brown leaf colour with woody-fynbos-floral honey flavour (Koch et al., 2012). Whether unfermented or fermented rooibos, studies have shown the presence of numerous bioactive chemical compounds, which include nothofagin, aspalathin, C-5-hexosyl derivative of aspalathin, aspalalinin, phenypyruvic acid-2-0-glucoside, orientin, isoorientin, vitexin, isovitexin, luteolin, luteolin-7-0-glucoside, chyrysoerio, and many more (Beelders et al., 2012). Due to the presence of high aspalathin levels and other phytochemical contents, hot water extracts prepared from green and fermented rooibos are used as food and/or cosmetic ingredients. Although the aspalathin content of unfermented rooibos is greater (8%) than the fermented (2%), the latter is still more preferable in food because of the flavour, and it is considered more economical as there is a greater demand for it (Joubert and de Beer, 2012; de Beer et al., 2017). Studies have elucidated the role of rooibos in protecting against disease development (Lawal et al., 2019) and also the ameliorative effects on diverse disorders (Marnewick et al., 2005; Ajuwon et al., 2018). Akinrinmade et al. suggested that the long-term continuous consumption of fermented rooibos tea confer a mild neuroprotection against ischaemic brain injury in rats. This was evidenced by the decrease in brain oedema, neuronal apoptosis, decreased lipid peroxidation and increase total antioxidant capacity, which cumulatively improved neuro-behavioural outcomes in rats (Akinrinmade et al., 2017). Nash et al. reported that osteoblast (Saos2 cells) treated with luteolin and orientin (from rooibos) displayed elevated alkaline phosphatase and mitochondrial activities. This was associated with reduced toxicity and decreased expression of anti-inflammatory cytokines, which collectively suggest that these rooibos metabolites improved osteoblast mineral content (Nash et al., 2015). Other studies have reported its antioxidant (Hong et al., 2014), anti-diabetic (Son et al., 2013; Dludla et al., 2018; Layman et al., 2019; Orlando et al., 2019) and anti-inflammatory (Pyrzanowska et al., 2019) properties.
Honeybush is native to the Southeast and Southwest coastal areas of South Africa, and it forms a part of the fynbos biome with the family name Fabaceae. It has been shown to be used as a traditional tea since the 19th century (Joubert et al., 2011; Schloms and Swart, 2014). Honeybush tea does not only have a pleasant taste and scent, it also contains phytochemicals and volatile organic compounds that are beneficial to health (Joubert et al., 2008). The known health benefits of honeybush are ascribed to its rich bioactive compounds such as Eriodictyol, Hesperetin, Isokuranetin, Naringenin, Chrysoeriol, Luteolin, Kaempferol, Afrormosin, Calycosin, Formononetin, Fujikinetin, Pseudobaptigen, Wistin, Flemichapparin, Medicagol, Sophoracoumestan, Pinitol, Isomangiferin and Mangiferin (Kamara et al., 2003; Jang et al., 2008).
Choi et al. reported that honeybush improved skin wrinkles, elasticity and hydration in patients with crow’s feet wrinkles after daily supplementation during their randomized double-blinded controlled trial (Choi et al., 2018). The anti-wrinkle effect of honeybush has been reported in animals (Im et al., 2014) and in vitro studies (Magcwebeba et al., 2016). Furthermore, studies have also shown its antioxidant (Sánchez et al., 2000; Marnewick et al., 2003), anti-mutagenic (Kokotkiewicz and Luczkiewicz, 2009) and mucosa immune therapy (Murakami et al., 2018) effects in both in vivo and in vitro studies.
Sutherlandia has been used in traditional medicine since the 1800s. Sutherlandia has a variety of health benefits which are associated to its bioactive phytochemicals. The chemical compounds include, asparagine, proline and arginine (Moshe et al., 1998; van Wyk and Albrecht, 2008), ƴ-aminobutyric acid (GABA), L-carvanine, (Ortega, 2003), sutherlandin A, sutherlandin B, sutherlandin C, and sutherlandin D) (Avula et al., 2010), sutherlandioside A, sutherlandioside B, sutherlandioside C, sutherlandioside D (Avula et al., 2010) and cycloartane-type triterpene glycoside (van Wyk and Albrecht, 2008). Based on the array of pharmacological products present in sutherlandia, studies have highlighted its role in cancer (Tai et al., 2004; Grandi et al., 2005), Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) (Harnett et al., 2005; Mills et al., 2005; Wilson et al., 2015), diabetes (Sia, 2004; Chadwick et al., 2007; Mackenzie. et al., 2012), inflammation (Lei et al., 2015; Vasaikar et al., 2018) stress (Chuang et al., 2015; Sergeant et al., 2017) and oxidative stress (Fernandes et al., 2004; Tobwala et al., 2014).
With regards to the effects of these infusions on male reproduction, very few studies are available. This include a study that reported improvement in sperm motility, sperm concentration and sperm viability after treatment with unfermented rooibos in rats (Opuwari and Monsees, 2014). These authors investigated the effects of unfermented and fermented rooibos teas on male reproductive parameters when both forms are administered to rats at varying concentrations (2% or 5%) for 52 days. Their findings showed that although the sperm parameters of animals treated with unfermented rooibos significantly improved, that of those in the fermented groups either showed non-significant increasing trend or no change (Opuwari and Monsees, 2014). Ros-Santaella and Pintus showed that the in vitro treatment of boar semen with rooibos protected the sperm acrosome structure, which was accompanied by improved sperm velocity (Ros-Santaella and Pintus, 2017). Additionally, Awoniyi et al. suggested that rooibos may provide a measure of protection against induced oxidative damage by elevating antioxidant activities and thus improve sperm function (Awoniyi et al., 2012).
However, to the best of our knowledge, there are no studies reporting the effect of honeybush or sutherlandia on male reproduction. Despite the numerous health benefits of these infusions, there are concerns as to their safety in long-term use (Fantoukh et al., 2019). Opuwari and Monsees reported that lengthened exposure to rooibos induced acrosome reaction in rats, which can subsequently lead to impaired reproduction (Opuwari and Monsees, 2014). Additionally, the toxicity of canavanine (from sutherlandia) has been implicated in lupus erythematous syndrome. Long-term exposure to sutherlandia have likewise been reported to cause protein cross-links and it can as well result in autoimmunity and teratogenicity. Ngcobo et al. showed that the in vitro chronic treatment of normal T-lymphocyte cells with sutherlandia was toxic (Ngcobo et al., 2012), which means too much of sutherlandia may be hazardous. Therefore, the current study was designed to investigate whether treatment with rooibos, honeybush and sutherlandia will impact sperm functional parameters positively or otherwise, in healthy rats.
2 Materials and methods
2.1 Animal care
Fourteen-week-old healthy pathogen-free adult male Wistar rats (250–300 g) were housed at the Stellenbosch University’s Faculty of Medicine and Health Sciences’ Animal Unit at room temperature (18°C–23°C), under a normal 12:12 light/dark cycle. Animals were caged individually, had free access to food (standard raw chow) and water/infusions and were treated according to the recommendations of the Laboratory Animal Care of the National Society of Medical Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Maget, 1953). Ethics approval was obtained from the Stellenbosch University Animal Ethics Committee (SU-ACUD17-00016).
2.2 Infusion preparation
Fermented rooibos (2%), fermented honeybush (4%) and unfermented sutherlandia (0.2%) were prepared according to standard protocols. These concentrations were based on previously published studies. Preparation procedure conformed to the experimental established protocols for rooibos (Marnewick et al., 2011), honeybush (Du Toit and Joubert, 1999) and sutherlandia (Tobwala et al., 2014). In brief, 2% rooibos was prepared by adding 20 g of dried fermented rooibos in 1 L of boiling water and allowed to steep/rest for 30–45 min. The mixture was filtered three times using a cheesecloth, number 4 filter paper and number 1 filter paper (Whatman™, Buckinghamshire, United Kingdom) respectively. Filtered infusions were transferred to a dark bottle plastic container and stored at 4°C. Honeybush (4%; 40 g in 1 L) was prepared following the similar steps as used for making rooibos tea. However, due to the bitter taste of sutherlandia, the concentration was reduced to 0.2%. Briefly, 40 g of unfermented sutherlandia was prepared as described for rooibos, from the initial 4% solution, 2.5 mL was diluted in 50 mL of water to give the working concentration of 0.2%.
2.3 Experimental design
Forty animals were randomly divided into four groups of ten. The groups include a control group, which received tap water, rooibos group (RF) (2% fermented), honeybush (HB) (4% fermented) and a sutherlandia group (SL) (0.2% unfermented). Animals had access to food and fluid ad libitum. In the case of the treatment groups, the animals received the treatment through different and corresponding fluid infusions. Hence, as water was the only drinking source for the control animals, the respective infusions were the only drinking source for the animals in the treatment groups. During the first week of acclimatization, animals were fed with pellets (standard Epol™ rat chow) and water only. However, after the acclimatization period, rats were fed with standard rat chow, water, and the different infusions as appropriate. To keep up with the monitoring of the animals, food and fluid intake of the animals were measured three times weekly.
Animals were sacrificed after 7 weeks of treatment. Blood, testis, and epididymis were harvested. Blood was collected into a heparized tube, placed on ice for 30 min and afterwards centrifuged (3000 rpm at 4°C for 30 min). Plasma was stored at −80°C until further analysis. The testis was used for all biochemical analysis, while the caudal area of the epididymis was dissected for sperm retrieval. Relative testicular/epididymal weight were calculated by expressing the tissue weight as a percentage of body weight.
2.4 Sperm functional parameters
2.4.1 Motility
Sperm retrieval and motility analysis were carried out as described by (Omolaoye et al., 2018). Briefly, the left epididymis was rinsed in a Petri dish containing 2 mL Hams F-12 nutrient media (Sigma Chemicals, St Louis, MO, United States) at 37°C. The caudal area of the rinsed epididymis was dissected, and spermatozoa were retrieved into a separate 2 mL Hams F-12, at 37°C. After 30 s of retrieval, 2 µL of the sperm solution was infused into a 20 µL depth chamber slide (Leja, Netherlands) and placed on a Nikon Eclipse E200 microscope with an in-built heating stage (37°C). Sperm motility was measured via computer-aided sperm analysis (CASA) using the Sperm Class Analyser (SCA 6.3, Microptic, Barcelona, Spain). Total motility, progressive motility and the sperm kinematic parameters (curvilinear velocity (VCL), average path velocity (VAP), straight-line velocity (VSL), straight-line index (STR), linearity index (LIN), sperm oscillation index (WOB), amplitude lateral head (ALH) and beat frequency (BCF) were analysed. After analysing sperm motility at 30 s, sperm concentration was measured by dissecting the caudal epididymis into smaller pieces and was left for a further 5 min allowing a maximum number of spermatozoa to swim out. The pieces were removed after 5 min, and the sperm solution was mixed homogenously. Of the 2 mL solution, 10 µL was diluted in 50 µL of Hams F-12 and sperm concentration was analysed through CASA.
2.4.2 Morphology
From the sperm solution used for sperm concentration analysis, 10 µL was smeared on a glass slide and allowed to air dry. The dried slides were stained with Sperm Blue® fixative and stain (Microptic SL, Barcelona, Spain) for 3 min, rinsed for 3 s to remove excessive stain, air dried and then mounted (DPX, HiMedia Laboratories Pvt. Ltd., Mumbai, India). Sperm morphology was analysed through computer-aided sperm morphometry analysis (CASMA) using the SCA® (Maree et al., 2010).
2.5 Testosterone and estradiol
The plasma concentration of testosterone (E-EL-0072) and estradiol (E-EL-0065) were measured using a commercially available ELISA kit (Elabscience Biotechnology, Hubei) as per manufacturer’s instructions.
2.6 Biochemical analysis
2.6.1 Superoxide dismutase (SOD) activity
Testicular tissue samples were homogenized in lysis buffer (Na3PO4, 0.5% Triton X-100) and centrifuged at 15000 rpm for 20 min at 4°C. Tissue homogenates were diluted to 10x in deionized water. From the diluted standards and samples, 10 μL were dispensed into the microplate wells in triplicate, followed by adding 170 μL of diethylenetriaminepentaacetic acid (DETAPAC) and 5 µL of SOD assay buffer (50 mM Na/K Phosphate buffer at pH 7.4). SOD activity was measured on a plate reader (Multiskan spectrum) at 490 nm, 25°C for 5 min at 1 min interval using SkanIt RE for MSS 2.2 (ThermoScientific™ Inc. immediately after adding 15 μL of freshly prepared 6-hydroxydopamine (6-OHD) into the well.
2.6.2 Catalase
Tissue homogenates were obtained as described for SOD. From the diluted samples and standards, 5 μL were loaded in triplicate into UV microplate wells. Catalase assay buffer (170 μL) were added into each well and lastly, 50 μL of H2O2 was added into the wells and analysis was performed immediately on a plate reader (Multiskan spectrum) at 240 nm every 60 s over a 5 min period using SkanIt RE for MSS 2.2 (ThermoScientific™ Inc.) software.
2.6.3 Malonaldehyde (MDA) levels
Testicular tissue samples were homogenized in lysis buffer (0.1 M KPi, 1.15% KCl) by bullet blending at speed 9 for 3 min with a 1-min interval in-between. MDA levels in the testis was determined by pipetting 100 µL of standards and samples into corresponding 10 mL glass tubes, followed by adding 1 mL of SDS and 2 mL of 10%TCA-BHT buffer solution. Samples were vortexed, and after resting for 10 min, 2 mL of TBA was added and vortexed again. The standards and samples were covered with marbles and incubated in a water bath (1 h at 100°C), where after it was cooled on ice for 15 min. The standards and samples were centrifuged (3000 rpm, 15 min 4°C) and the supernatants retrieved. From the supernatants, 250 μL of each standard and sample were loaded in triplicate into microplate wells and analysed on a plate reader (Multiskan spectrum) at a 532 nm wavelength within 30 min after centrifugation.
2.7 Statistics
GraphPad Prism™ software (GraphPad™ Software, Version 8.2, CA, United States) was used. Normal data distribution was measured using the Anderson-Darling and Kolmogorov-Smirnov normality tests. When data passed all normality tests, a one-way ANOVA of variance with a Tukey’s Post-hoc Test were performed. Where data were not evenly distributed, a Kruskal–Wallis test and a Dunns Post-hoc Test were carried out. A probability level of p < 0.05 was considered statistically significant and results are expressed as mean ± SD.
3 Results
3.1 Anthropometric data
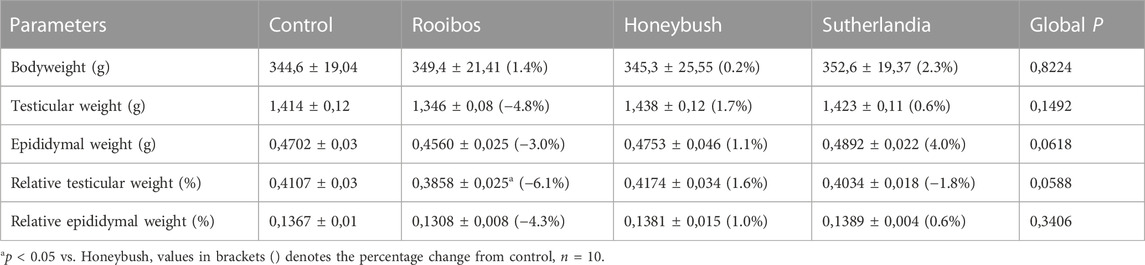
After 7 weeks of treatment, no significant differences were observed in the body, testicular and epididymal weights between any of the groups (Table 1). However, the SL group presented with a higher (7%) percentage epididymal weight mean compared to the RF group (p = 0.07). The RF treated animals displayed a significantly lower (−7.5%) relative testicular weight compared to the HB group (p = 0.04), while there was no significant difference in relative epididymal weight between the groups (p = 0.3) (Table 1).
3.2 Sperm functional parameters
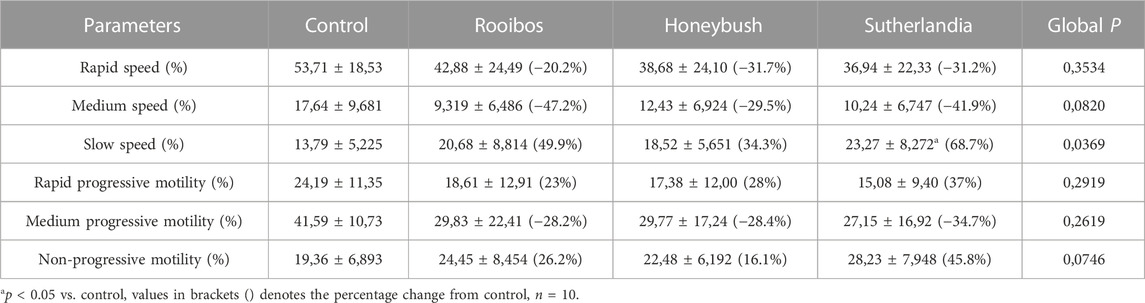
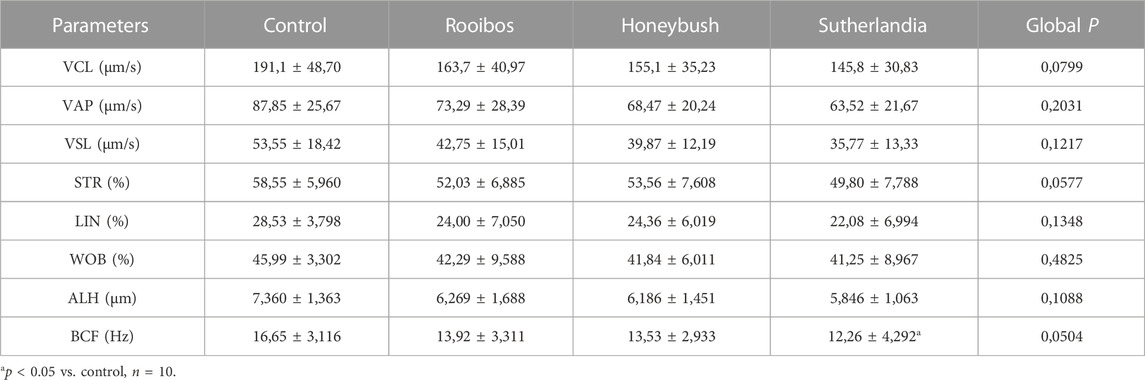
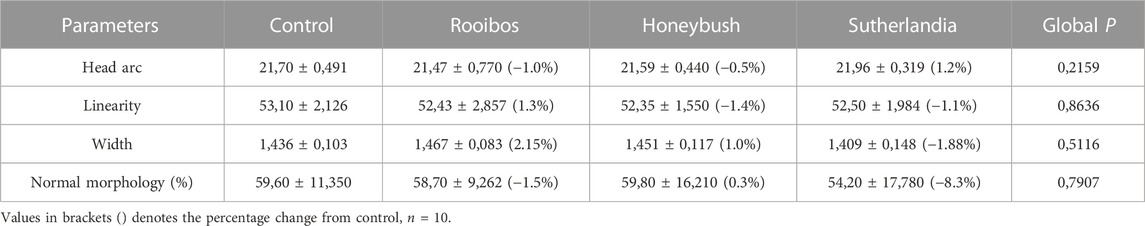
The infusion treated animals (RF, HB, and SL) presented with a non-significant decrease (−14.3%, −18.2%, −17.2%) (−24.8%, −20.7%, −27.3%) in total motility and progressive motility when compared to the control group respectively (Figures 1A, B). Although not significant, the rapid progressive motility of RF, HB and SL animals reduced by 23%, 28% and 37% respectively (Table 2), and there was a significant increase in the number of spermatozoa with slow speed (p = 0.03), especially the SL treated group compared to the control (p = 0.03) (Table 2). All sperm kinematic parameters of the infusion treated animals were non-significantly reduced compared to the control (Table 3). RF, HB and SL treated animals displayed a decrease in VCL (−14.3%, −18.8%, −23.7%); VAP (−16.5%, −22%, −27.6%); VSL (−20%, −25.5%, −33.2%); STR (−11.1%, −8.5%, −14.9%); LIN (−15.8%, −14.6%, −22.6%) WOB (−8%, −9%, −10%); ALH (−14.8%, −15.9%, −20.5%) and BCF (−16.3%, −18.7%, −26.3%), compared to the control group respectively (Table 3). Interestingly, infusion treated groups displayed a non-significant increase in sperm concentration compared to the control group (Figure 2). However, there was no significant differences in the percentage of morphologically normal spermatozoa between the groups, but RF and SL are on the decrease compared to the control (Table 4). Taken together, findings showed that the percentage of rapidly progressive motile spermatozoa follows the order of control > RF > HB > SL.
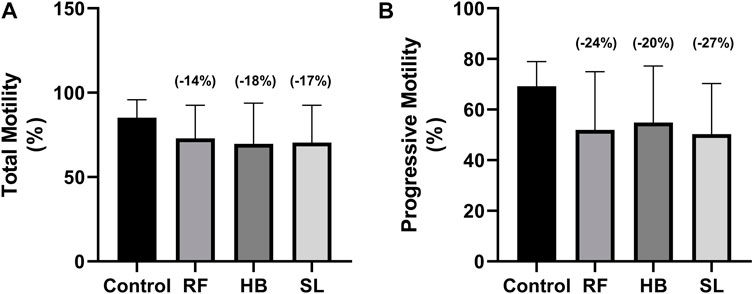
FIGURE 1. Sperm total and progressive motilities at 30 s. (A) total motility, (B) progressive motility, RF = rooibos, HB = honeybush, SL = sutherlandia, values in brackets () denotes the percentage change from control, n = 10.
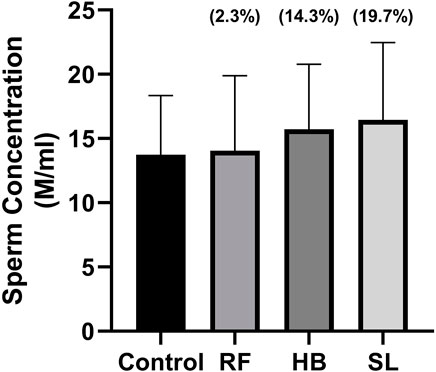
FIGURE 2. Sperm concentration measured after 5 min of retrieval. RF = rooibos, HB = honeybush, SL = sutherlandia, values in brackets () denotes the percentage change from control, n = 10.
3.3 Hormones and biochemical assays
There were no statistical difference in the concentration of testosterone and estradiol between the groups (Figures 3A, B). However, there was an increase of 28.8%, 31.7%, and 23% in SOD activity, decrease of −11.3%, −16.6%, 23.7% in catalase activity and a decrease of −21.1%, −23.7%, 45.9% in MDA levels of RF, HB and SL groups when compared to the control group respectively (Figures 4A–C).
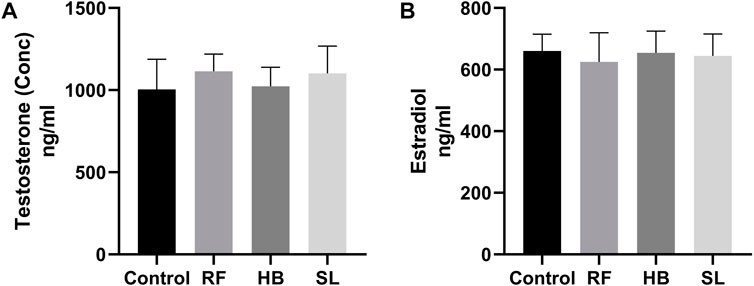
FIGURE 3. Testosterone and estradiol concentration. (A) testosterone, (B) estradiol, RF = rooibos, HB = honeybush, SL = sutherlandia, n = 9.
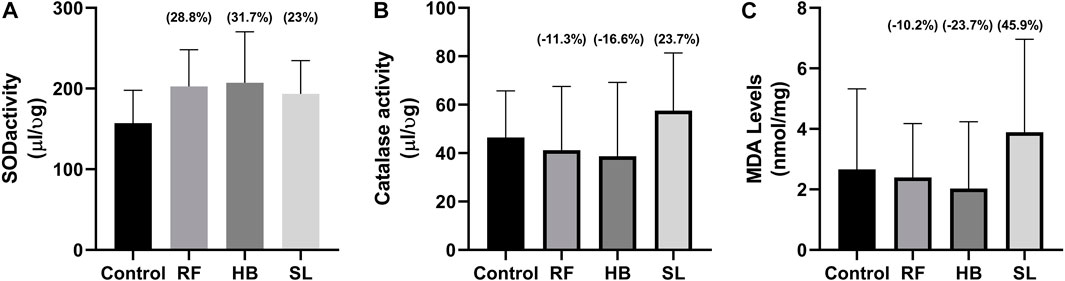
FIGURE 4. Oxidative stress parameters. (A) SOD activity, (B) catalase activity, (C) MDA levels, RF = rooibos, HB = honeybush, SL = sutherlandia, values in brackets () denotes the percentage change from control, n = 10.
4 Discussion
The use of natural products in the prevention and treatment of diverse diseases is increasing. This is due to the availability of phytochemical bioactive compounds in these plants, which are able to mimic the pharmacokinetics of synthetic drugs with fewer resultant adverse effects (Mills et al., 2005; Jang et al., 2008; van Wyk and Albrecht, 2008). Rooibos, honeybush and sutherladia are plants with bioactive compounds and they have been shown to have numerous health benefits (van Wyk and Albrecht, 2008). However, only a few studies have been performed regarding their effects on male reproductive function. From these few studies, concerns regarding long term use have already been highlighted. Opuwari and Monsees reported that long-term consumption of rooibos resulted in spontaneous acrosome reaction, which may impair male reproduction (Opuwari and Monsees, 2014). The current study investigated the effects of rooibos, honeybush and sutherlandia on male reproductive functional parameters in rats.
After 7 weeks of treatment, there was no significant difference in body and organ (testes and epididymides) weights. This is similar to the report of Opuwari and Monsees, who also showed no significant difference in the body and tissue weight gain after 52 days of rooibos consumption in rat (Opuwari and Monsees, 2014). Many natural products of plant origin contains flavonoids, and flavonoids are considered endocrine disruptors (Patisaul and Jefferson, 2010). Chandra et al. reported that green tea administered in relatively high dose inhibited the activities of testicular 3β and 17β-hydoxy-steriod dehydrogenase, decreased serum testosterone and reduced testicular weight, which cumulatively resulted in inhibition of spermatogenesis. This suggest that high dose of this tea may impair the morphological and normal function of the testis (Das and Karmakar, 2015). Regarding rooibos, an in vitro study reported decrease in the production of testosterone by the Leydig cells after treatment with rooibos (Opuwari and Monsees, 2015). However, In the current, although RF animals presented with a decrease in relative testicular weight, there was no significant difference in the serum concentration of testosterone, but the estradiol levels decreased by 5%. The observed result of testosterone in the current study is supported by a study that reported no significant difference in serum testosterone after administration of both fermented and unfermented forms of rooibos in rats (Opuwari and Monsees, 2014). The role of rooibos as endocrine disruptor still remains inconclusive, because, recently, Noh reported that MR-10, a novel complex of dandelion and rooibos increases testosterone levels and also improved sperm production in older (>45) men (Noh, 2018). Hence, more studies are required to ascertain the role of rooibos on the endocrine regulation of male reproduction. Honeybush has been shown to have mild oestrogenic activity as it was reported to induce proliferation of oestrogen-insentive MDA-MD-231 cells (Verhoog et al., 2007). As previously stated, studies are lacking on its role in male reproduction, thus more studies are required to investigate the role on male reproduction.
In the current study, the effects of rooibos, honeybush and sutherlandia on the sperm motility, concentration and morphology represent an ilicit/confusing situation. Animals treated with the respective infusion (RF, HB, SL) presented with a decrease in all sperm kinematic parameters. This is in contrast to the report of Ros-Santaella and Pintus. They showed that after treating boar semen with rooibos, there was an increase in sperm kinematic parameters (Ros-Santaella and Pintus, 2017). The contrasting result may be due to the difference in models. Additionally, there was a decrease in the total motility (−14%, −18%, −17%), progressive motility (−24%, −20%, −27%) and normal sperm morphology (−1.5%, 0.3% and −9%) of animals treated with RF, HB and SL, with SL animals showing a significant increase in the percentage of spermatozoa with slow speed. The results of the total and progressive motilities of the current study are in contrast with the reports of Awoniyi et al. and Ayeleso et al. (Awoniyi et al., 2012; Ayeleso et al., 2014b). The difference may be due to the health status of animals analysed. Awoniyi et al. reported an increase in the total and progressive motilities of diabetic animals treated with rooibos and Ayelso et al. showed an improvement in the total and progressive motilities of animals that were induced with OS. Additionally, from data obtained in our laboratory (SURRG Stellenbosch University Reproductive Research Group), we observed that diabetic animals treated with fermented rooibos displayed an increase that was up to 5% in total and progressive motilities when compared to non-diabetic animals (Omolaoye et al., 2021). This suggest that rooibos consumption may be beneficial in a diseased state, especially, DM-related male reproductive function impairment in rats. However, when consumed by healthy rats, it may reduce sperm motility. Since studies on honeybush and sutherlandia are lacking, little can be said about them. We can speculate that when administered to healthy animals, there is a possibility of sperm function impairment since they follow the same trend as rooibos. Nevertheless, if the three infusions are compared, findings showed that the percentage of rapidly progressive motile spermatozoa follows the order of control > RF > HB > SL.
Interestingly, animals treated with these infusions displayed a non-significant increase in sperm concentration. This is partly supported by Opuwari and Monsees. They reported that unfermented rooibos supplementation significantly enhanced sperm concentration in rats (Opuwari and Monsees, 2014). Several studies have shown the antioxidant potential of rooibos, honeybush and sutherlandia on diverse diseases (Fernandes et al., 2004; Ayeleso et al., 2014a). In like manner, the RF, HB and SL animals of the current study presented with an increase in SOD activity accompanied with reduced MDA levels in RF and HB groups, with SL group showing higher MDA levels. The former is supported by several studies that have shown increased testicular antioxidant enzyme activity after treatment with rooibos either in disease or health (Awoniyi et al., 2012; Ayeleso et al., 2014b; Opuwari and Monsees, 2014). Although, SOD activity was increased in the infusion treated groups of the current study, the sperm motility and morphology were adversely affected. The mechanisms behind these outcomes were not investigated, but it can be speculated that, the increased SOD and reduced catalase activities observed are compensatory responses. As SL that displayed higher SOD and catalase activities, resultantly showed a non-significant 45% increase in MDA levels.
5 Conclusion
The present study evaluated the role of rooibos, honeybush and sutherlandia on sperm functional parameters in healthy rats. Animals treated with the respective infusions presented with percentage increase in antioxidant enzyme activity but have reduced sperm motility and decreased normal morphology. Paradoxically, they presented with increased sperm concentration. Hence, it is concluded that rooibos, honeybush and sutherlandia may enhance sperm concentration, which represent sperm quantity, but they may impair sperm motility and morphology (sperm quality) when consumed by healthy animals. Thus, caution should be taken regarding the quantity of teas consumed when healthy, as it can affect the antioxidant shift (antioxidant paradox). That is, too much of antioxidants can push into the opposite state of oxidative stress and rather lead to reductive stress.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Stellenbosch University Animal Ethics Committee (SU-ACUD17-00016).
Author contributions
TSO-conceptualized, animal monitoring, data analysis, original draft; BTS-review and editing; SSDP-conceptualized, review, editing, supervised. All authors contributed to the article and approved the submitted version.
Funding
The authors would like to thank Harry Crossley Foundation for the research grant (2018/2019) provided.
Acknowledgments
The authors would like to thank Dr Michelle Smit-van Schalkwyk and Dr Shantal Windvogel for providing the animals used. This study was partly supported by Al Jalila Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajuwon, O. R., Ayeleso, A. O., and Adefolaju, G. A. (2018). The potential of South African herbal tisanes, rooibos and honeybush in the management of type 2 diabetes mellitus. Molecules 23, 3207–3225. doi:10.3390/molecules23123207
Akinrinmade, O., Omoruyi, S., Dietrich, D., and Ekpo, O. (2017). Long-term consumption of fermented rooibos herbal tea offers neuroprotection against ischemic brain injury in rats. Acta Neurobiol. Exp. (Wars). 77, 94–105. doi:10.21307/ane-2017-040
Avula, B., Wang, Y. H., Smillie, T. J., Fu, X., Li, X. C., Mabusela, W., et al. (2010). Quantitative determination of flavonoids and cycloartanol glycosides from aerial parts of Sutherlandia frutescens (L) R. BR. by using LC-UV/ELSD methods and confirmation by using LC-MS method. J. Pharm. Biomed. Anal. 52, 173–180. doi:10.1016/j.jpba.2010.01.010
Awoniyi, D. O., Aboua, Y. G., Marnewick, J., and Brooks, N. (2012). The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress-induced rats. Phyther. Res. 26, 1231–1239. doi:10.1002/ptr.3717
Ayeleso, A., Brooks, N., and Oguntibeju, O. (2014a). Modulation of antioxidant status in streptozotocin-induced diabetic male wistar rats following intake of red palm oil and/or rooibos. Asian pac. J. Trop. Med. 7, 536–544. doi:10.1016/S1995-7645(14)60090-0
Ayeleso, A. O., Oguntibeju, O. O., Aboua, Y. G., and Brooks, N. L. (2014b). Effects of red palm oil and rooibos on sperm motility parameters in streptozotocin-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 11, 8–15. doi:10.4314/ajtcam.v11i5.2
Beelders, T., Kalili, K. M., Joubert, E., De Beer, D., and De Villiers, A. (2012). Comprehensive two-dimensional liquid chromatographic analysis of rooibos (Aspalathus linearis) phenolics. J. Sep. Sci. 35, 1808–1820. doi:10.1002/jssc.201200060
Chadwick, W. A., Roux, S., van de Venter, M., Louw, J., and Oelofsen, W. (2007). Anti-diabetic effects of Sutherlandia frutescens in Wistar rats fed a diabetogenic diet. J. Ethnopharmacol. 109, 121–127. doi:10.1016/j.jep.2006.07.012
Choi, S. Y., Hong, J. Y., Ko, E. J., Kim, B. J., Hong, S. W., Lim, M. H., et al. (2018). Protective effects of fermented honeybush (cyclopia intermedia) extract (HU-018) against skin aging: A randomized, double-blinded, placebo-controlled study. J. Cosmet. Laser Ther. 20, 313–318. doi:10.1080/14764172.2017.1418512
Chuang, D. Y., Cui, J., Simonyi, A., Engel, V. A., Chen, S., Fritsche, K. L., et al. (2015). Dietary sutherlandia and elderberry mitigate cerebral ischemia-induced neuronal damage and attenuate p47phox and phospho-ERK1/2 expression in microglial cells. ASN Neuro 6, 1759091414554946–1759091414554949. doi:10.1177/1759091414554946
Das, S. K., and Karmakar, S. N. (2015). Effect of green tea (Camellia sinensis l) leaf extract on reproductive system of adult male albino rats. Int. J. Physiol. Pathophysiol. Pharmacol. 7, 178–184.
de Beer, D., Miller, N., and Joubert, E. (2017). Production of dihydrochalcone-rich green rooibos (Aspalathus linearis) extract taking into account seasonal and batch-to-batch variation in phenolic composition of plant material. South Afr. J. Bot. 110, 138–143. doi:10.1016/j.sajb.2016.02.198
Dludla, P. V., Gabuza, K. B., Muller, C. J. F., Joubert, E., Louw, J., and Johnson, R. (2018). Aspalathin, a C-glucosyl dihydrochalcone from rooibos improves the hypoglycemic potential of metformin in type 2 diabetic (db/db) mice. Physiol. Res. 67, 813–818. doi:10.33549/physiolres.933891
Du Toit, J., and Joubert, E. (1999). Optimization of the fermentation parameters of honeybush tea (Cyclopia). J. Food Qual. 22, 241–256. doi:10.1111/j.1745-4557.1999.tb00555.x
Fantoukh, O. I., Dale, O. R., Parveen, A., Hawwal, M. F., Ali, Z., Manda, V. K., et al. (2019). Safety assessment of phytochemicals derived from the globalized South African rooibos tea (aspalathus linearis) through interaction with CYP, PXR, and P-gp. J. Agric. Food Chem. 67, 4967–4975. doi:10.1021/acs.jafc.9b00846
Fernandes, A. C., Cromarty, A. D., Albrecht, C., and Jansen Van Rensburg, C. E. (2004). The antioxidant potential of Sutherlandia frutescens. J. Ethnopharmacol. 95, 1–5. doi:10.1016/j.jep.2004.05.024
Grandi, M., Roselli, L., and Vernay, M. (2005). Lessertia (Sutherlandia frutescens) et la fatigue en cancérologie*Lessertia (Sutherlandia frutescens) and fatigue during cancer treatment. Phytotherapie 3, 110–113. doi:10.1007/s10298-005-0083-0
Harnett, S. M., Oosthuizen, V., and Van De Venter, M. (2005). Anti-HIV activities of organic and aqueous extracts of Sutherlandia frutescens and Lobostemon trigonus. J. Ethnopharmacol. 96, 113–119. doi:10.1016/j.jep.2004.08.038
Hong, I. S., Lee, H. Y., and Kim, H. P. (2014). Anti-oxidative effects of Rooibos tea (Aspalathus linearis) on immobilization-induced oxidative stress in rat brain. PLoS One 9, e87061–e87069. doi:10.1371/journal.pone.0087061
Im, A. R., Song, J. H. youn., Lee, M. Y. oun., Yeon, S. H. u., Um, K. A. n., and Chae, S. (2014). Anti-wrinkle effects of fermented and non-fermented Cyclopia intermedia in hairless mice. BMC Complement. Altern. Med. 14, 424–426. doi:10.1186/1472-6882-14-424
MacKenzie, J., Roux, S., van de Venter, M., and Dealtry, G. B. (2012). Effect of sutherlandia frutescens on the lipid metabolism in an insulin resistant rat model and 3T3-L1 adipocytes. Phyther. Res. 26, 1830, 1837. doi:10.1002/ptr.4653
Jang, M. H., Piao, X. L., Kim, J. M., Kwon, S. W., and Park, J. H. (2008). Inhibition of cholinesterase and amyloid-β aggregation by resveratrol oligomers from Vitis amurensis. Phyther. Res. 22, 544–549. doi:10.1002/ptr.2406
Joubert, E., and de Beer, D. (2012). Phenolic content and antioxidant activity of rooibos food ingredient extracts. J. Food Compos. Anal. 27, 45–51. doi:10.1016/j.jfca.2012.03.011
Joubert, E., Gelderblom, W. C. A., Louw, A., and de Beer, D. (2008). South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides-A review. J. Ethnopharmacol. 119, 376–412. doi:10.1016/j.jep.2008.06.014
Joubert, E., Joubert, M. E., Bester, C., de Beer, D., and De Lange, J. H. (2011). Honeybush (Cyclopia spp): From local cottage industry to global markets - the catalytic and supporting role of research. South Afr. J. Bot. 77, 887–907. doi:10.1016/j.sajb.2011.05.014
Kamara, B. I., Brandt, E. V., Ferreira, D., and Joubert, E. (2003). Polyphenols from honeybush tea (Cyclopia intermedia). J. Agric. Food Chem. 51, 3874–3879. doi:10.1021/jf0210730
Koch, I. S., Muller, M., Joubert, E., van der Rijst, M., and Næs, T. (2012). Sensory characterization of rooibos tea and the development of a rooibos sensory wheel and lexicon. Food Res. Int. 46, 217–228. doi:10.1016/j.foodres.2011.11.028
Kokotkiewicz, A., and Luczkiewicz, M. (2009). Honeybush (Cyclopia sp) - a rich source of compounds with high antimutagenic properties. Fitoterapia 80, 3–11. doi:10.1016/j.fitote.2008.11.001
Lawal, A. O., Oluyede, D. M., Adebimpe, M. O., Olumegbon, L. T., Awolaja, O. O., Elekofehinti, O. O., et al. (2019). The cardiovascular protective effects of rooibos (Aspalathus linearis) extract on diesel exhaust particles induced inflammation and oxidative stress involve NF-κB- and Nrf2-dependent pathways modulation. Heliyon 5, 014266–e1522. doi:10.1016/j.heliyon.2019.e01426
Layman, J. I., Pereira, D. L., Chellan, N., Huisamen, B., and Kotzé, S. H. (2019). A histomorphometric study on the hepatoprotective effects of a green rooibos extract in a diet-induced obese rat model. Acta histochem. 121, 646–656. doi:10.1016/j.acthis.2019.05.008
Lei, W., Browning, J. D., Eichen, P. A., Brownstein, K. J., Folk, W. R., Sun, G. Y., et al. (2015). Unveiling the anti-inflammatory activity of Sutherlandia frutescens using murine macrophages. Int. Immunopharmacol. 29, 254–262. doi:10.1016/j.intimp.2015.11.012
Magcwebeba, T., Swart, P., Swanevelder, S., Joubert, E., and Gelderblom, W. (2016). Anti-inflammatory effects of aspalathus linearis and cyclopia spp. extracts in a UVB/keratinocyte (HaCaT) model utilising interleukin-1α accumulation as biomarker. Molecules 21, 1323–1421. doi:10.3390/molecules21101323
Maget, M. (1953). Guide d’étude directe des comportements culturelsGuide d’etude directe des comportements culturels. Paris: CNRS.
Maree, L., Du Plessis, S. S., Menkveld, R., and Van Der Horst, G. (2010). Morphometric dimensions of the human sperm head depend on the staining method used. Hum. Reprod. 25, 1369–1382. doi:10.1093/humrep/deq075
Marnewick, J., Joubert, E., Joseph, S., Swanevelder, S., Swart, P., and Gelderblom, W. (2005). Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 224, 193–202. doi:10.1016/j.canlet.2004.11.014
Marnewick, J. L., Joubert, E., Swart, P., Van Der Westhuizen, F., and Gelderblom, W. C. (2003). Modulation of hepatic drug metabolizing enzymes and oxidative status by rooibos (aspalathus linearis) and honeybush (cyclopia intermedia), green and black (camellia sinensis) teas in rats. J. Agric. Food Chem. 51, 8113–8119. doi:10.1021/jf0344643
Marnewick, J. L., Rautenbach, F., Venter, I., Neethling, H., Blackhurst, D. M., Wolmarans, P., et al. (2011). Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 133, 46–52. doi:10.1016/j.jep.2010.08.061
McGaw, L. J., Steenkamp, V., and Eloff, J. N. (2007). Evaluation of Athrixia bush tea for cytotoxicity, antioxidant activity, caffeine content and presence of pyrrolizidine alkaloids. J. Ethnopharmacol. 110, 16–22. doi:10.1016/j.jep.2006.08.029
Mills, E., Cooper, C., Seely, D., and Kanfer, I. (2005). African herbal medicines in the treatment of HIV: Hypoxis and sutherlandia. An overview of evidence and pharmacology. Nutr. J. 4, 19–26. doi:10.1186/1475-2891-4-19
Moshe, D., Van Der Bank, H., Van Der Bank, M., and Van Wyk, B. E. (1998). Lack of genetic differentiation between 19 populations from seven taxa of Sutherlandia Tribe: Galegeae, Fabaceae. Biochem. Syst. Ecol. 26, 595–609. doi:10.1016/S0305-1978(98)00002-7
Murakami, S., Miura, Y., Hattori, M., Matsuda, H., Malherbe, C. J., Muller, C. J. F., et al. (2018). Cyclopia extracts enhance Th1-Th2-and Th17-type T cell responses and induce Foxp3 + cells in murine cell culture. Planta Med. 84, 311–319. doi:10.1055/s-0043-121270
Nash, L. A., Sullivan, P. J., Peters, S. J., and Ward, W. E. (2015). Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 59, 443–453. doi:10.1002/mnfr.201400592
Ngcobo, M., Gqaleni, N., Chelule, P. K., Serumula, M., and Assounga, A. (2012). Effects of Sutherlandia frutescens extracts on normal t-lymphocytes in vitro. Afr. J. Tradit. Complement. Altern. Med. 9, 73–80. doi:10.4314/ajtcam.v9i1.11
Noh, Y. H. (2018). MR-10 enhances men’s health by improving endogenous male sex hormone generation. J. Med. Food 21, 1288–1294. doi:10.1089/jmf.2018.4201
Omolaoye, T. S., Skosana, B. T., and du Plessis, S. S. (2018). Diabetes mellitus-induction: Effect of different streptozotocin doses on male reproductive parameters. Acta histochem. 120, 103–109. doi:10.1016/j.acthis.2017.12.005
Omolaoye, T., Windvogel, S., and du Plessis, S. (2021). Testicular oxidative stress and apoptosis status in streptozotocin-induced diabetic rats after treatment with rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia), and sutherlandia (Lessertia frutescens) infusions. Asian Pac. J. Reprod. 10, 11–20. doi:10.4103/2305-0500.306432
Opuwari, C. S., and Monsees, T. K. (2014). In vivo effects of Aspalathus linearis (rooibos) on male rat reproductive functions. Andrologia 46, 867–877. doi:10.1111/and.12158
Opuwari, C. S., and Monsees, T. K. (2015). Reduced testosterone production in TM3 Leydig cells treated with Aspalathus linearis (Rooibos) or Camellia sinensis (tea). Andrologia 47, 52–58. doi:10.1111/and.12221
Orlando, P., Chellan, N., Louw, J., Tiano, L., Cirilli, I., Dludla, P., et al. (2019). Aspalathin-rich green rooibos extract lowers LDL-cholesterol and oxidative status in high-fat diet-induced diabetic vervet monkeys. Molecules 1713, 1713–1719. doi:10.3390/molecules24091713
Ortega, A. (2003). A new role for GABA: Inhibition of tumor cell migration. Trends Pharmacol. Sci. 24, 151–154. doi:10.1016/S0165-6147(03)00052-X
Patisaul, H. B., and Jefferson, W. (2010). The pros and cons of phytoestrogens. Front. Neuroendocrinol. 31, 400–419. doi:10.1016/j.yfrne.2010.03.003
Pyrzanowska, J., Fecka, I., Mirowska-Guzel, D., Joniec-Maciejak, I., Blecharz-Klin, K., Piechal, A., et al. (2019). Long-term administration of Aspalathus linearis infusion affects spatial memory of adult Sprague-Dawley male rats as well as increases their striatal dopamine content. J. Ethnopharmacol. 238, 111881–111912. doi:10.1016/j.jep.2019.111881
Ros-Santaella, J. L., and Pintus, E. (2017). Rooibos (Aspalathus linearis) extract enhances boar sperm velocity up to 96 hours of semen storage. PLoS One 12, 01836822–e183713. doi:10.1371/journal.pone.0183682
Sánchez, G. M., Re, L., Giuliani, A., Núñez-Sellés, A. J., Davison, G. P., and León-Fernández, O. S. (2000). Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol. Res. 42, 565–573. doi:10.1006/phrs.2000.0727
Schloms, L., and Swart, A. C. (2014). Rooibos flavonoids inhibit the activity of key adrenal steroidogenic enzymes, modulating steroid hormone levels in H295R cells. Molecules 19, 3681–3695. doi:10.3390/molecules19033681
Sergeant, C. A., Africander, D., Swart, P., and Swart, A. C. (2017). Sutherlandia frutescens modulates adrenal hormone biosynthesis, acts as a selective glucocorticoid receptor agonist (SEGRA) and displays anti-mineralocorticoid properties. J. Ethnopharmacol. 202, 290–301. doi:10.1016/j.jep.2017.03.019
Sia, C. (2004). Spotlight on ethnomedicine: Usability of sutherlandia frutescens in the treatment of diabetes. Rev. Diabet. Stud. 1, 145–149. doi:10.1900/rds.2004.1.145
Son, M. J., Minakawa, M., Miura, Y., and Yagasaki, K. (2013). Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 52, 1607–1619. doi:10.1007/s00394-012-0466-6
Tai, J., Cheung, S., Chan, E., and Hasman, D. (2004). In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J. Ethnopharmacol. 93, 9–19. doi:10.1016/j.jep.2004.02.028
Tobwala, S., Fan, W., Hines, C. J., Folk, W. R., and Ercal, N. (2014). Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement. Altern. Med. 14, 271. doi:10.1186/1472-6882-14-271
van Wyk, B. E., and Albrecht, C. (2008). A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J. Ethnopharmacol. 119, 620–629. doi:10.1016/j.jep.2008.08.003
Vasaikar, N., Mahajan, U., Patil, K. R., Suchal, K., Patil, C. R., Ojha, S., et al. (2018). D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on pro-inflammatory cytokines. Chem. Biol. Interact. 290, 6–11. doi:10.1016/j.cbi.2018.05.003
Verhoog, N. J. D., Joubert, E., and Louw, A. (2007). Evaluation of the phytoestrogenic activity of Cyclopia genistoides (honeybush) methanol extracts and relevant polyphenols. J. Agric. Food Chem. 55, 4371–4381. doi:10.1021/jf063588n
Wilson, D., Goggin, K., Williams, K., Gerkovich, M. M., Gqaleni, N., Syce, J., et al. (2015). Consumption of Sutherlandia frutescens by HIV-seropositive South African adults: An adaptive double-blind randomized placebo controlled trial. PLoS One 10, 01285222–e128614. doi:10.1371/journal.pone.0128522
Keywords: sperm, motility, sperm concentration, rooibos (Aspalathin linearis), honeybush (Cyclopia intermedia), sutherlandia (Sutherlandia frutescene)
Citation: Omolaoye TS, Skosana BT and du Plessis SS (2023) The effect of Aspalathin linearis, Cyclopia intermedia and Sutherlandia frutescene on sperm functional parameters of healthy male wistar rats. Front. Physiol. 14:1211227. doi: 10.3389/fphys.2023.1211227
Received: 24 April 2023; Accepted: 30 May 2023;
Published: 07 June 2023.
Edited by:
Alexandre Rodrigues Silva, Federal University Rural Semi-Arid, BrazilReviewed by:
Wael A. Khalil, Mansoura University, EgyptAlexsandra Fernandes Pereira, Federal University Rural Semi-Arid, Brazil
Copyright © 2023 Omolaoye, Skosana and du Plessis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Temidayo S. Omolaoye, dGVtaWRheW8ub21vbGFveWVAbWJydS5hYy5hZQ==
 Temidayo S. Omolaoye
Temidayo S. Omolaoye Bongekile T. Skosana
Bongekile T. Skosana Stefan S. du Plessis
Stefan S. du Plessis