- 1School of Fisheries, Xinyang Agriculture and Forestry University, Xinyang, China
- 2School of Marine Sciences, Ningbo University, Ningbo, China
Introduction: Ammonia has been of concern for its high toxicity to animals. N-carbamylglutamate (NCG) can reduce blood ammonia levels in mammals, but studies on ammonia tolerance in fish are insufficient.
Methods: Juvenile yellow catfish were fed two levels of NCG (0.00% and 0.05%) for 84 days under three ammonia levels (0.00, 0.08, and 0.16 mg/L NH3).
Results and Discussion: The results showed that survival rate (SUR), final body weight (FBW), weight gain (WG), and serum total protein (TP), triglycerides (TG), glucose (Glu), ornithine (Orn), citrulline (Cit) contents, and liver superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), arginase (ARG), ornithine transcarbamylase (OTC) activities decreased with the increase of ammonia levels, on the contrary, feed conversion ratio (FCR), hepatosomatic index (HSI), and serum ammonia, urea, alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamine (Gln), arginine (Arg) contents, and liver malondialdehyde (MDA), tumor necrosis factor (TNF), interleukin (IL) 1, IL 8 contents, and mRNA expressions of cu/zn sod, cat, gpx, gr, tnf ɑ, il 1, and il 8 were significantly increased. Dietary 0.05% NCG supplementation had higher SUR, FBW, WG, feed intake (FI), whole-body protein, and serum TP, total cholesterol (TC), Glu, citrulline (Cit) contents, and liver SOD, GPx, argininosuccinate synthetase (ASS), argininosuccinate lyase (ASL), inducible nitric oxide synthase (iNOS) activities compared to 0.00% NCG group, but had lower serum ammonia, urea, ALT, AST, Gln, Arg contents, and liver MDA, TNF, IL 1, IL 8 contents, and neuronal nitric oxide synthase activity. At the end of bacterial challenge, cumulative mortality (CM) increased with ammonia levels increased, but serum antibody titer (AT), lysozyme (LYZ) activity, 50% hemolytic complement, immunoglobulin (Ig) contents, respiratory burst (RB), phagocytic indices decreased with ammonia levels increased. CM in 0.05% NCG group was lower than that in 0.00% NCG group, but serum AT, LYZ activity, Ig content, RB in 0.05% NCG group were significantly higher. The correlation analysis found that iNOS was positively correlated with ASS activity. This study indicates that dietary NCG supplementation can improve the ammonia tolerance of yellow catfish, and ASS may also be the target of NCG to activate the urea cycle.
1 Introduction
Ammonia is considered a toxic substance in aquaculture ecosystems, including unionized NH3 and NH4+ ions (Hegazi et al., 2010). The unionized NH3 causes poisoning in most fish, such as minnow Rhynchocypris lagowski (Yu et al., 2020), black sea bream Acanthopagrus schlegelii (Wang et al., 2020), common carp Cyprinus carpio (Xue et al., 2021), Nile tilapia Oreochromis niloticus (Esam et al., 2022), Japanese sea perch Lateolabrax japonicus (Zhang et al., 2022a) and hybrid snakehead Channa maculatus ♀ × Channa argus ♂ (Zuo et al., 2022). Acute or chronic ammonia stress can cause fish behavioral abnormalities, growth retardation, oxidative damage, and immunosuppression, making them more prone to disease outbreaks (Divya et al., 2020). It is well known that endogenous ammonia in fish is mostly excreted through gill tissues, but when ambient ammonia levels rise, they must convert it into less toxic substances, such as urea and glutamine (Ip and Chew, 2018). Recent studies have reported that improving the urea cycle efficiency of fish can effectively improve ammonia tolerance (Huang et al., 2019; Zhang et al., 2022b).
N-carbamylglutamate (NCG), an analogue of N-acetylglutamate (NAG), is a mandatory effector of the carbamyl phosphate synthase I (CPS I) reaction, an initial step in the urea cycle (Wu et al., 2004). In the feed industry, NCG has the advantages of being inexpensive and having a stable metabolism and high absorption rate compared with NAG (Chacher et al., 2013). NCG is clinically used to treat urea cycle disorder and restore ureagenesis and normalize blood ammonia levels in patients (Tuchman et al., 2008). In livestock and poultry breeding, NCG can significantly reduce their blood ammonia levels, increase plasma arginine contents, improve growth performance and antioxidant capacity, and inhibit inflammation, such in as chicken Gallus gallus (Huang et al., 2017), pig Susscrofa domestica (Wu et al., 2010; Yang et al., 2011), and sheep Ovis aries (Zhang et al., 2021). The vast majority of fish are known to excrete ammonia directly, so their urea cycle pathway may differ from that of mammals and birds (Anderson, 1995). In fact, many teleost species have enzymes involved in the urea cycle, such as mudskippers Periophthalmodon schlosseri, marble goby Oxyeleotris marmoratus, weather loach Misgurnus anguillicaudatus, small snakehead Channa asiatica, swamp eel Monopterus albus, mangrove killifish Rivulus marmoratus, central mud minnow Umbra limi (Ip and Chew, 2018), gulf toadfish Opsanus beta, oyster toadfish Opsanus tau, and plainfin midshipman Porichthys notatus (Wang and Walsh, 2000). So far, it has been reported that dietary NCG supplementation improves fish growth, but whether NCG can improve ammonia tolerance is unclear.
Yellow catfish is among the most economically valuable fish species in China, with a production of 587, 822 t in 2021 (MOAC, 2022). However, in recent years, ammonia stress has become the bottleneck in the development of yellow catfish aquaculture, and the yield and quality are being seriously degraded. The aim of this study was to investigate the changes in growth, blood health, antioxidant enzyme and ammonia metabolism enzyme activities, inflammation and disease resistance of yellow catfish fed a diet rich in NCG when ammonia stress occurs, so as to explore whether NCG can improve the ammonia tolerance of yellow catfish.
2 Materials and methods
2.1 Experimental diets
Two isonitrogenous (40% protein) and isolipidic (9% lipid) diets were prepared by adding 0.00% and 0.05% NCG to basal diets (Zhao et al., 2019). The NCG (97.5%) was supplied by Animore Sci. and Tech. Co., Ltd., Beijing, China. All the ingredients were ground into a powder through a 60-mesh, and the step-by-step expansion method was adopted to mix them evenly; then, they were processed into (2.00 × 2.00) mm pellets using a feed mill (F-26II, Science and Technology Industrial General Factory of South China University of Technology, China) and dried at room temperature to about 10% moisture. The diets were stored at −20°C until use. The diet formulation and proximate are presented in Table 1.
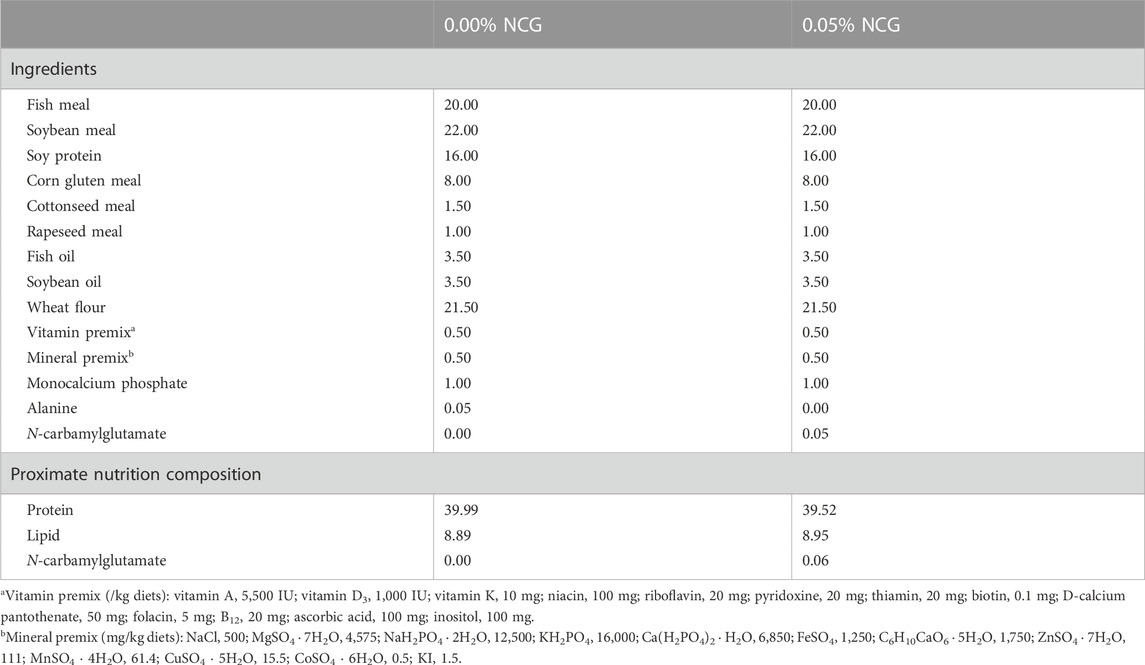
TABLE 1. The formula and approximate composition of the diets used in the experiment (% dry matter basis, DMB).
2.2 Animal and experimental design
Juvenile yellow catfish were obtained from the Ningbo Aquatic Products Market, China. All fish were fed on a control experimental diet for 2 weeks. The fish (1.01 ± 0.02 g) were selected and randomly placed in 18,500 L cylindrical buckets (diameter of 0.8 m), with 60 fish per bucket.
Our previous study reported that the lethal concentration of 50% (LC50) of yellow catfish exposed to ammonia at 96 h was 57.00 mg/L total ammonia nitrogen (TA-N) (0.80 mg/L NH3; pH 6.6; 28°C) (Li et al., 2020). For the ammonia challenge, the fish were divided into a 0.00 mg/L NH3 group (<0.001 mg/L NH3; control group), 0.08 mg/L NH3 group (1/10 LC50), and 0.16 mg/L NH3 group (1/5 LC50). Ammonium chloride was added to the aquaculture system through a metering pump (Iwaki, Japan) to achieve the required ammonia levels. The actual ammonia levels were measured twice daily (YSI ProPlus Multi-Parameter Water Quality Instrument, YSI, United States). The fish were fed twice daily (07:00–07:30 a.m. and 18:00–18:30 p.m.) until apparent satiation for 84 days. The amounts of diet consumed were recorded daily. During the trail, the water temperature was 26°C–28°C, the pH was maintained within the range of 6.6–6.7, and the dissolved oxygen remained >7.00 mg/L and nitrate <0.1 mg/L, with a natural photoperiod.
2.3 Sample collections
We stopped feeding the experiment fish for 24 h, and then they were anesthetized (20 mg/L eugenol). Three fish from each bucket were randomly selected, minced, pooled, and stored at −20°C for a whole-body proximate composition analysis. We picked the other three fish from each bucket, and blood was collected from the tail vein, then centrifuged at 836 g to obtain serum and stored at −20°C for analysis of the serum biochemical index, free amino acid content, antioxidant enzyme activity, and inflammation response. Then, the liver samples were quickly removed and weighed for the hepatosomatic index, one part of which was stored at −20°C for ammonia metabolism enzyme activity analysis and another part of which was stored at −80°C for qRT-PCR analysis. All analyses were completed within 2 weeks of sampling.
2.4 Bacterial challenge
The frozen Aeromonas hydrophila was resuscitated in nutrient agar at 30°C under light for 48 h. The bacterial solution was concentrated to 1 × 108 colony-forming units (CFU)/mL before the bacterial challenge.
After feeding for 84 days, 20 fish from each bucket were randomly selected and intraperitoneally injected with 0.1 mL of 1 × 106 CFU/mL of A. hydrophila (Zhang et al., 2022b). Mortality was recorded daily during the 7 days of experiment, and dead fish were removed. We continued to feed all the fish experiment diets once a day, and the experimental conditions were maintained. At the end of the bacterial challenge, blood (three fish per bucket) was drawn from the caudal vasculature, and serum was stored at −20°C for analysis of the immune indexes; then, the head kidney was quickly removed for macrophage isolation.
2.5 Biochemical analysis
The diets and whole body of the fish were analyzed for proximate composition following the AOAC (2000) standard method. Protein was measured by the combustion method using the FP-528 Nitrogen Analyzer (Leco, United); lipid was measured by the ether extraction method using HT6 Soxtec System (FOSS, Sweden); ash was determined by incineration in the muffle furnace at 550°C for 8 h; and moisture was determined by oven drying at 105°C to a constant weight. The concentration of NCG was measured by high-performance liquid chromatography (HPLC; Agilent, California, United States).
A serum biochemical reagent (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was added to each well of the microtiter plate at 250 µL/well. Then, 5 µL of serum was added to each well. After 30-min incubation at room temperature, the analysis was carried out using the Hitachi 7600-110 automatic chemistry analyzer (Hitachi Ltd., Tokyo, Japan), including on the urea, total protein (TP), total cholesterol (TC), triglyceride (TG), glucose (Glu), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Serum ammonia content was detected by a kit (Nanjing Jiancheng, China) using a PT-3502C full-wavelength microplate reader (Beijing Potenov, China).
Serum was thoroughly mixed with 10 mmol/L D-nor-leucine and acetonitrile, and the supernatant was obtained by centrifugation. Serum free amino acid (glutamine, Gln; arginine, Arg; ornithine, Orn; citrulline, Cit) content was analyzed using a LC-20AD liquid chromatograph (Shumadzu, Japan).
Serum superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities, and malondialdehyde (MDA) content were analyzed with commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). One unit of SOD activity was calculated using the amount of superoxide dismutase required to inhibit the reduction of nitroblue tetrazolium by 50%; one unit of CAT activity was defined as the amount of CAT required to transform 1 μmol of H2O2 per min; and one unit of GPX activity was defined as the amount of GPX required to oxidize 1 μmol of NADPH per min.
Serum inflammation response (tumor necrosis factor, TNF; interleukin 1, IL 1; interleukin 8, IL 8), liver ammonia metabolism enzymes (argininosuccinate synthetase, ASS; argininosuccinate lyase, ASL; arginase, ARG; ornithine transcarbamylase, OTC), and nitric oxide synthase (neuronal nitric oxide synthase, nNOS; inducible nitric oxide synthase, iNOS) were determined by the ELISA method with kits (Nanjing Jiancheng, China). In short, TMB was converted to blue under the catalysis of peroxidase and finally to yellow under the action of acid. There was a positive correlation between the color and the cytokines in the samples. The absorbance was measured at 450 nm with a microplate reader, and the sample concentration was calculated.
Serum lysozyme (LYZ) activity was analyzed with a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The assay was based on the lysis of lysozyme-sensitive Gram-positive bacterium via the lysozyme present in the serum. Serum antibody titer (AT), 50% hemolytic complement (CH50), and total immunoglobulin (Ig) contents were analyzed using commercially available assay kits (Zhejiang Elikan Biological Technology Co., Ltd., Wenzhou, China). After 3 µL of serum was added to the 300 μL reagent, the sample was incubated for 10 min at 37°C. The absorbance of the samples was read at 340 nm. The head kidney was transferred to a L-15 culture medium (100 IU/mL penicillin, 100 μg/mL streptomycin, 10 IU/mL heparin, 2% fetal bovine serum), then filtered through a 100 μm metal mesh. The cell suspension was enriched by centrifugation at 600 g for 5 min at 4°C on the 34%/51% Percoll density gradient. The cells were collected at the 34%–51% interface and washed twice (cell concentration 1 × 107/mL; cell viability >95%). The respiratory burst (RB) and phagocytic index (PI) were measured following the method of Zhang et al. (2018).
2.6 qRT-PCR analysis
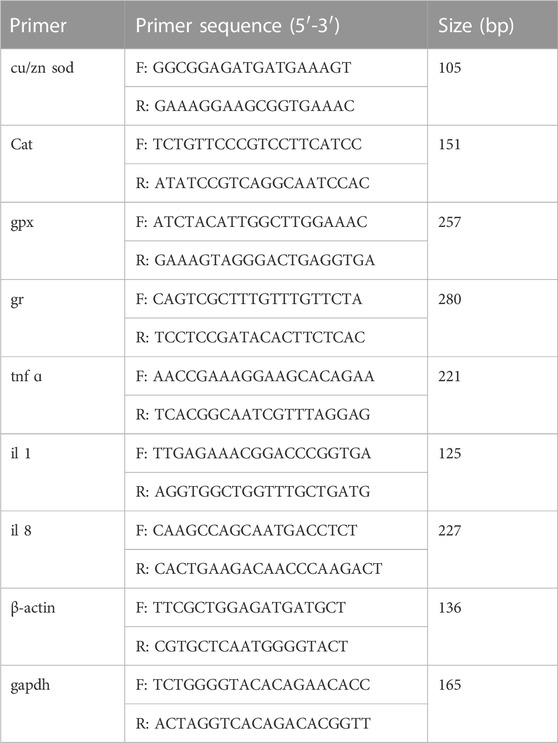
Total RNA extraction was performed using the RNAiso Reagent kit (Takara, China), and cDNA was synthesized using the Prime Script PT reagent Kit (Takara, China). Table 2 lists the forward and reverse primers of genes. qRT-PCR was performed using a LightCycler® 480 II Real-Time PCR system (Roche, Switzerland). The PCR temperature conditions were 95°C for 5 min, followed by 40 cycles of 95°C for 20 s, 57°C for 25 s, and 72°C for 25 s. Each sample was analyzed in triplicate, and the internal control genes included β-actin and GAPDH. The expression levels were calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
2.7 Statistical analysis
All analyses were performed using SPSS 18.0.0 (SPSS, United States). Data were tested for normal distribution using the Kolmogorov–Smirnov test. The results were subjected to a two-way analysis of variance (ANOVA) followed by Tukey’s multiple range test. The correlations between the measured nitric oxide synthase activity and ammonia metabolism enzyme activity were conducted using the Pearson correlation. The level of significance was set at p < 0.05.
3 Results
3.1 Growth performance and body composition
The survival rate (SUR), final body weight (FBW), and weight gain (WG) decreased with the increase of ammonia levels (p < 0.05) (Table 3). On the contrary, the feed conversion ratio (FCR) and hepatosomatic index (HSI) were significantly increased (p < 0.05). SUR, FBW, WG, and feed intake (FI) in the 0.05% NCG group were significantly higher than those in the 0.00% NCG group (p < 0.05). The interactions of the dietary NCG supplementation and ammonia level were observed in SUR (p = 0.003), FBW (p = 0.048), and WG (p = 0.045).
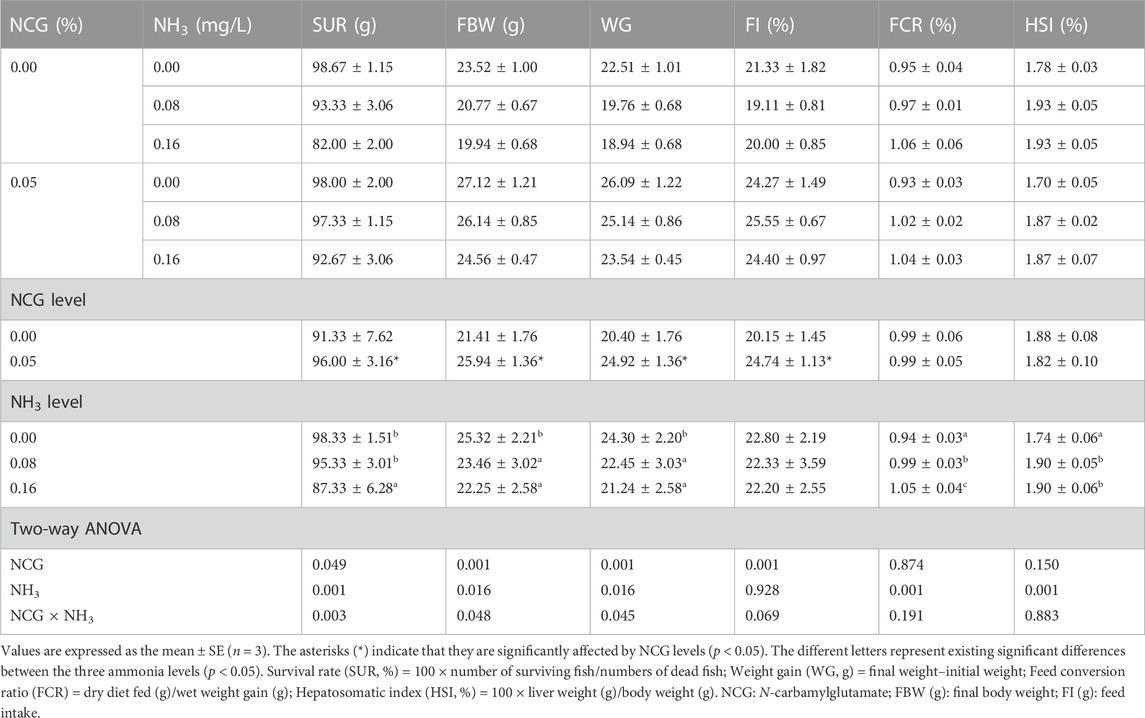
TABLE 3. Effects of dietary N-carbamylglutamate supplementation on growth performance of yellow catfish exposed to different ammonia levels for 84 days.
Whole-body protein content in the 0.05% NCG group was significantly higher than in the 0.00% NCG group (p < 0.05) (Table 4). Dietary NCG supplementation and ammonia stress had no effect on lipid, ash, and moisture contents (p > 0.05).

TABLE 4. Effects of dietary N-carbamylglutamate supplementation on whole-body proximate composition of yellow catfish exposed to different ammonia levels for 84 days.
3.2 Serum biochemical index and free amino acid content
The serum ammonia, urea, ALT, and AST contents increased as ammonia levels increased, while TP, TG, and Glu contents decreased (p < 0.05) (Table 5). Serum ammonia, urea, ALT, and AST in the 0.05% NCG group were significantly lower than those in the 0.00% NCG group, but TP, TC, and Glu contents were significantly higher than those in the 0.00% NCG group (p < 0.05). The interactions of the dietary NCG supplementation and ammonia level were observed in serum ammonia (p = 0.001), urea (p = 0.001), TP (p = 0.014), Glu (p = 0.001), ALT (p = 0.001), and AST (p = 0.001).
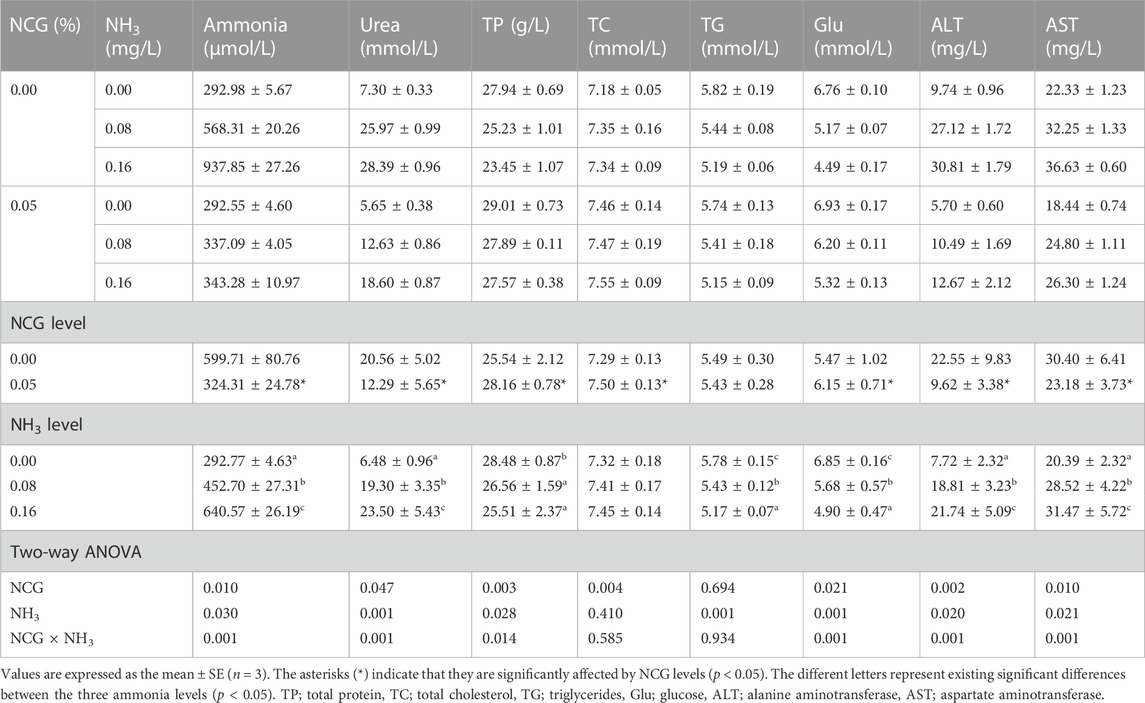
TABLE 5. Effects of dietary N-carbamylglutamate supplementation on serum biochemical index of yellow catfish exposed to different ammonia levels for 84 days.
The serum Gln and Arg contents increased as ammonia levels increased, while the Orn and Cit contents decreased (p < 0.05) (Table 6). The serum Gln and Arg contents in the 0.05% NCG group were significantly lower than those in the 0.00% NCG group, but the Cit content was higher (p < 0.05). The interactions of the dietary NCG supplementation and ammonia level were observed in Gln (p = 0.001), Arg (p = 0.001), and Cit (p = 0.009).
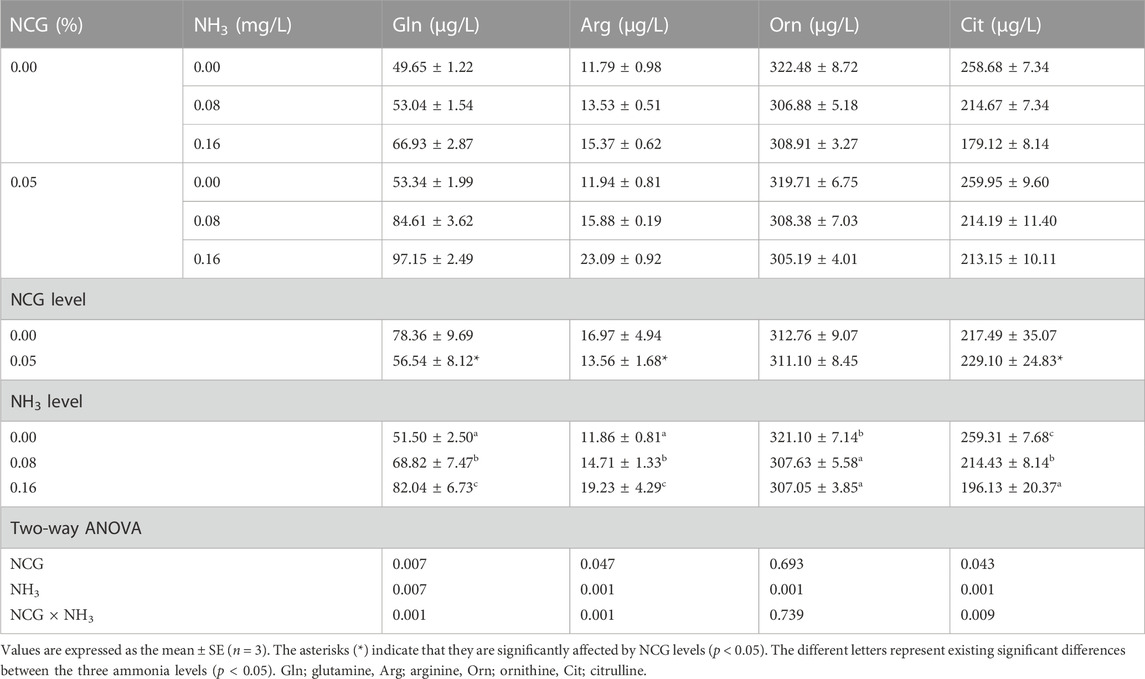
TABLE 6. Effects of dietary N-carbamylglutamate supplementation on serum free amino acid content of yellow catfish exposed to different ammonia levels for 84 days.
3.3 Antioxidant enzyme activity and inflammation
Serum SOD, CAT, and GPx activities decreased as ammonia levels increased, while MDA, TNF, IL 1, and IL 8 contents decreased (p < 0.05) (Table 7). Serum SOD and GPx activities in the 0.05% NCG group were significantly higher than those in the 0.00% NCG group, but MDA, TNF, IL 1, and IL 8 contents were lower (p < 0.05). The interactions of the dietary NCG supplementation and ammonia level were observed in SOD (p = 0.020), CAT (p = 0.016), GPx (p = 0.001), MDA (p = 0.025), TNF (p = 0.001), IL 1(p = 0.001), and IL 8 (p = 0.017).
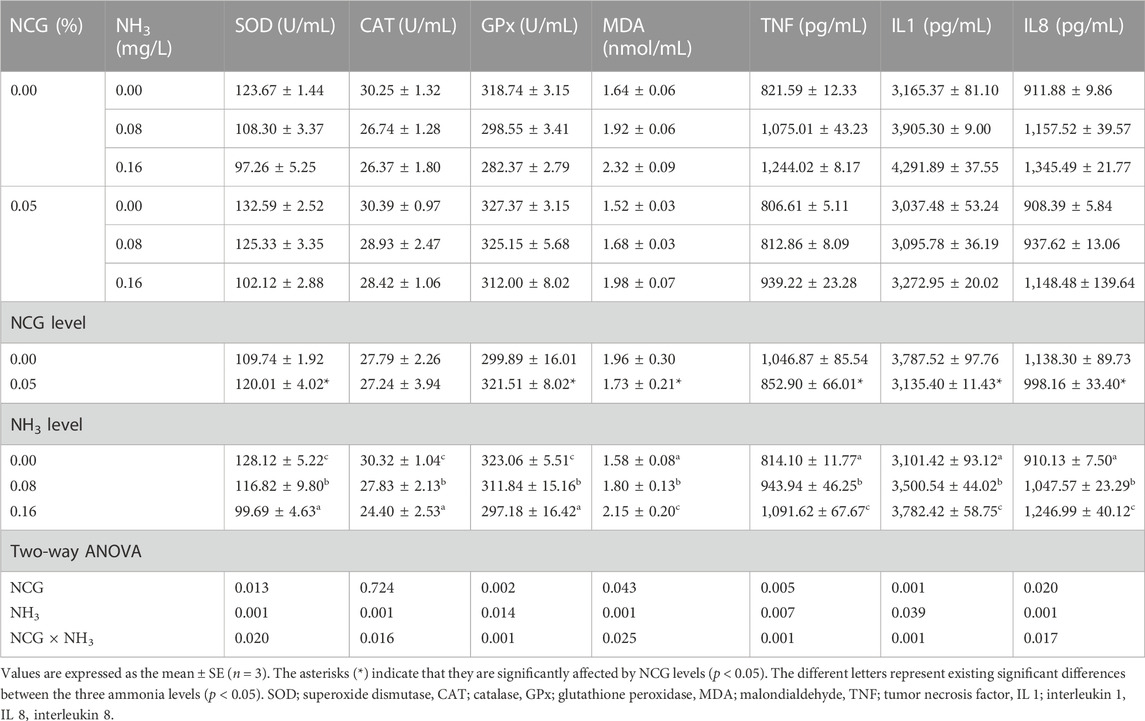
TABLE 7. Effects of dietary N-carbamylglutamate supplementation on serum antioxidant enzyme activity and inflammation of yellow catfish exposed to different ammonia levels for 84 days.
The cu/zn sod, cat, gpx, and gr expressions in the liver increased as ammonia levels increased (p < 0.05) (Figure 1). The interactions of the dietary NCG supplementation and ammonia level were observed in cu/zn sod (p = 0.001), cat (p = 0.001), gpx (p = 0.004), and gr (p = 0.018) expressions.
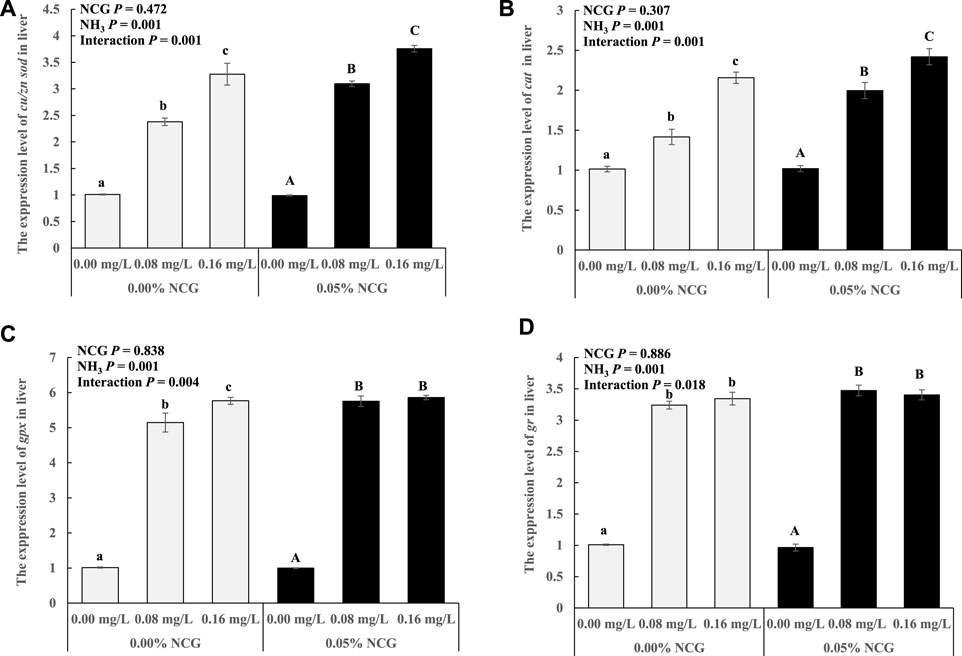
FIGURE 1. Relative expression levels of antioxidant-related genes cu/zn sod (A), cat (B), gpx (C), and gr (D) in liver. The relative expression of the transcript from qRT-PCR was calculated based on the standard curve and normalized to the β-actin and gapdh mRNA level (n = 3). The different letters represent existing significant differences between the three ammonia levels (p < 0.05).
The tnf ɑ, il 1, and il 8 expression in the liver increased as ammonia levels increased (p < 0.05) (Figure 2). The interactions of the dietary NCG supplementation and ammonia level were observed in tnf ɑ (p = 0.001), il 1 (p = 0.003), and il 8 (p = 0.001) expression.
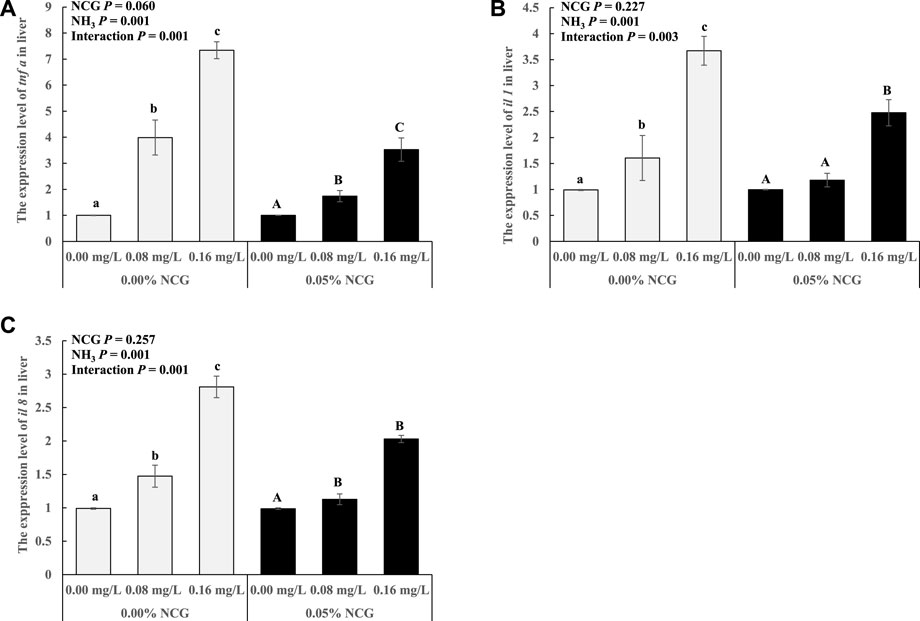
FIGURE 2. Relative expression levels of antioxidant-related genes tnf ɑ (A), il 1 (B), and il 8 (C) in liver. The relative expression of the transcript from qRT-PCR was calculated based on the standard curve and normalized to the β-actin and gapdh mRNA level (n = 3). The different letters represent existing significant differences between the three ammonia levels (p < 0.05).
3.4 Ammonia metabolism enzyme activity
The liver ARG and OTC activities decreased as ammonia levels increased (p < 0.05) (Table 8). The liver ASS, ASL, and iNOS activities in the 0.05% NCG group were significantly higher than those in the 0.00% NCG group, but nNOS activity was lower (p < 0.05). The interactions of the dietary NCG supplementation and ammonia level were observed in ASS (p = 0.001), ASL (p = 0.001), nNOS (p = 0.001), and iNOS (p = 0.001).
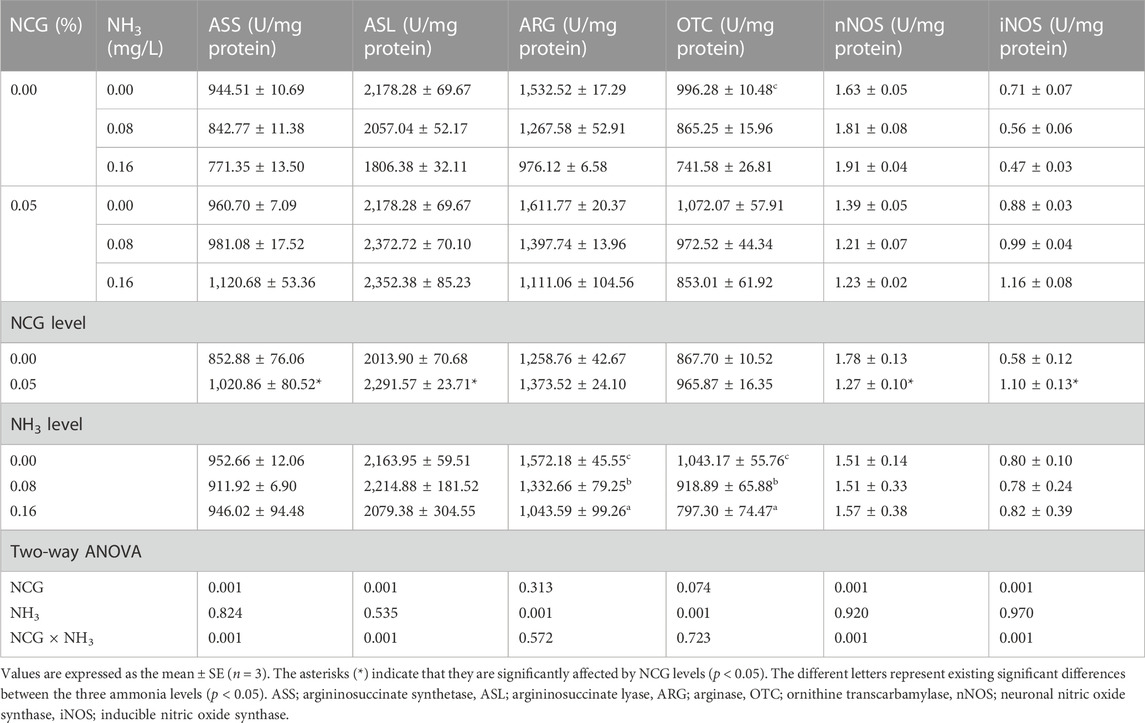
TABLE 8. Effects of dietary N-carbamylglutamate supplementation on activities of ammonia metabolism enzyme and nitric oxide synthase in liver of yellow catfish exposed to different ammonia levels for 84 days.
3.5 Bacterial challenge
At the end of the bacterial challenge, CM increased as ammonia levels increased (p < 0.05) (Table 9). The serum AT and LYZ activity, the CH50 and Ig content, and RB and PI decreased as ammonia levels increased (p < 0.05). CM in the 0.05% NCG group was lower than in the 0.00% NCG group, but serum AT and LYZ activity, Ig content, and RB in the 0.05% NCG group were significantly higher than in the 0.00% NCG group (p < 0.05). The interactions of the dietary NCG supplementation and ammonia level were observed in CM (p = 0.021), LYZ (p = 0.001), Ig (p = 0.001), RB (p = 0.001), and PI (p = 0.007).
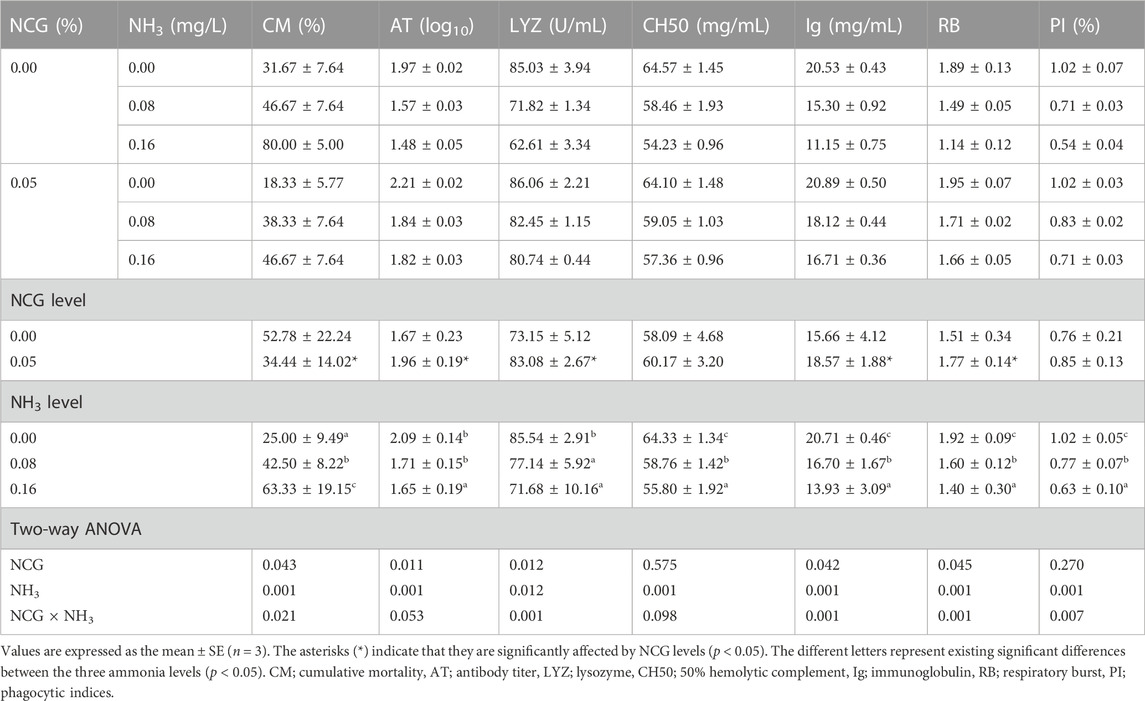
TABLE 9. Effects of dietary N-carbamylglutamate supplementation and ammonia stress on cumulative mortality and serum immune response of yellow catfish 7 days post-challenge with A. hydrophila.
3.6 Correlation analysis
As shown in Figure 3, nNOS was significantly negatively correlated with ARG in 0.00 mg/L NH3 level (p < 0.05). iNOS was significantly positively correlated with ASS, ASL, and OTC in 0.16 mg/L NH3 level, but nNOS was significantly negatively correlated with ASS, ASL, and OTC (p < 0.05). iNOS was significantly positively correlated with ASL, ASS, and OTC in 0.16 mg/L NH3 level, but nNOS was significantly negatively correlated with ASL and ASS (p < 0.05). Dietary 0.00% NCG supplementation and iNOS were significantly positively correlated with ASL, ARG, ASS, and OTC, but those trends were reversed in nNOS (p < 0.05). Dietary 0.05% NCG supplementation and iNOS were significantly positively correlated with ASS but significantly negatively correlated with ARG and OTC, and nNOS was positively correlated with ARG (p < 0.05).
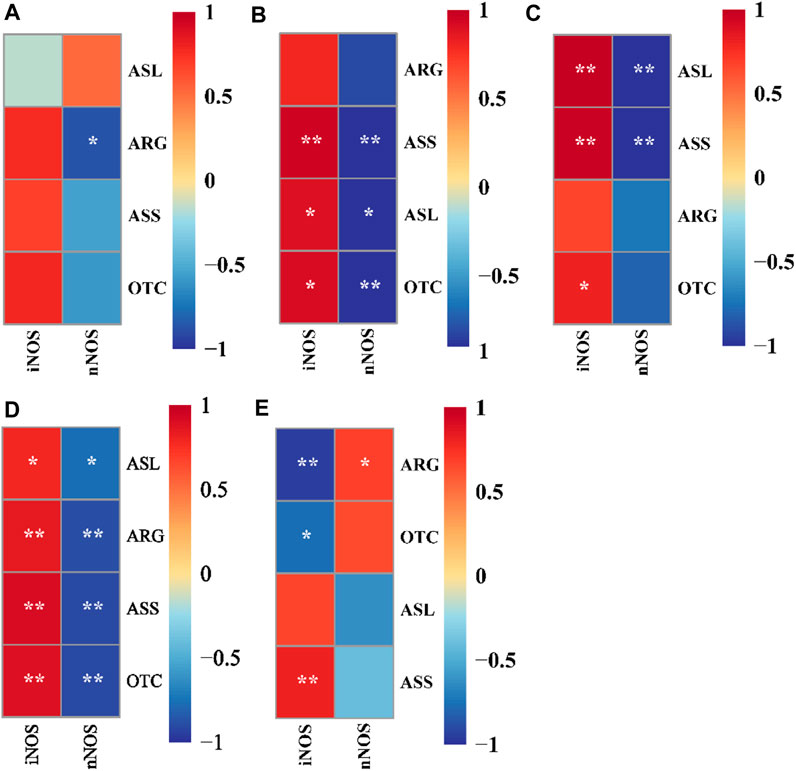
FIGURE 3. Correlation analysis between nitric oxide synthase activity and ammonia metabolism enzyme activity in yellow catfish fed with different N-carbamylglutamate under 0.00 mg/L NH3 (A), 0.08 mg/L NH3 (B), and 0.16 mg/L NH3 (C) ammonia levels, respectively. Correlation analysis between nitric oxide synthase activity and ammonia metabolism enzyme activity in yellow catfish fed diets with 0.00% (D) and 0.05% (E) N-carbamylglutamate under different ammonia levels. A significant difference was marked as ∗ at p < 0.05 and ∗∗ at p < 0.001. nNOS; neuronal nitric oxide synthase, iNOS; inducible nitric oxide synthase, ASS; argininosuccinate synthetase, ASL; argininosuccinate lyase, ARG; arginase, OTC; ornithine transcarbamylase.
4 Discussion
To protect fishery resources, the Chinese government has recommended a threshold of 0.020 mg/L NH3 for fisheries (Fan et al., 2021). However, the NH3 levels in the intensive aquaculture systems are usually maintained at 0.052–0.064 mg/L (data not published), which is more than upper limit of ammonia tolerance in many cultured fishes, such as burbot Lota lota, Atlantic salmon Salmo salar, Pacific cod Gadus macrocephalus, rainbow trout, channel catfish Ictalurus punctatus (Vaage and Myrick, 2021), golden pompano Trachinotus ovatus (Liu et al., 2021), Japanese sea perch (Zhang et al., 2022a), and Nile tilapia (Esam et al., 2022). Similarly, this study also found that ammonia stress (>0.16 mg/L NH3) can lead to a lower survival rate of yellow catfish. It is worth noting that the dietary 0.05% NCG supplementation significantly increased the survival rate of yellow catfish at this ammonia level, which may be related to the reduction of stress, energy consumption, and internal ammonia load. A previous study confirmed that dietary NCG contents at 0.03%–0.05% can improve the growth, digestive enzyme activity, oxidation resistance status, and immunity of yellow catfish (Zhao et al., 2019). As was expected, we found that the dietary 0.05% NCG supplementation improves the growth (FBW and WG) of yellow catfish under ammonia stress.
Fish mainly rely on gill tissue to remove toxic ammonia from their bodies, but when the ambient ammonia is too high, some fish also can convert ammonia to non-toxic urea by the urea cycle pathway, such as marble goby (Jow et al., 1999), mudskippers (Lim et al., 2001), weather loach (Chew et al., 2001), swamp eel (Tay et al., 2003), walking catfish Clarias batrachus (Banerjee et al., 2017), magur catfish Clarias magur (Banerjee et al., 2020), common carp (Xue et al., 2021), and rainbow trout (Clark et al., 2019). The urea cycle consists of five key enzymes, namely, CPS I, ornithine transcarbamylase (OTC), argininosuccinate synthetase (ASS), argininosuccinate lyase (ASL), and arginase (ARG). Recent studies have suggested that ARG deficiency may be a major cause of ammonia poisoning in some fish, such as rainbow trout (Clark et al., 2019), Dolly Varden char Salvelinus malma (Zhu et al., 2020), and yellow catfish (Zhang et al., 2022b). NCG is mainly used to treat urea cycle disorders caused by CPS I deficiency in clinical practice (Ucar et al., 2009). Although this study found that dietary NCG could not alleviate the inhibition of ARG activity caused by ammonia stress, serum Arg and urea accumulations were alleviated, which indicates that dietary NCG can alleviate urea cycle disorder in fish to a certain extent. In addition, this study found that dietary NCG increased the activities of ASS and ASL, which are key enzymes linking the urea and nitric oxide synthesis (Husson et al., 2003). Nitric oxide is considered to be a very important immune signaling molecule (Colasanti and Suzuki, 2000). Unlike mammals, nitric oxide in fish is produced by two nitric oxide synthase isoforms: inducible nitric oxide synthase (iNOS) and neuronal nitric oxide synthase (nNOS) (Vemuganti and Raghavendra, 2002). nNOS exists in the central nervous system (CNS), and its production of nitric oxide can affect the neuron function in the brain, which is considered the main mediator of neuronal death (Kiss, 2000). A previous study reported that nNOS knockout mice were more resistant to neuro-excitotoxic injury (Ayata et al., 1997). Fish ammonia poisoning typically results in abnormal behavior, including polypnea, hyperexcitability, mania, convulsions, and syncope (Zhang et al., 2022b). In this study, an important finding was that dietary 0.05% NCG supplementation decreased nNOS activity, which proves that NCG could alleviate the neurotoxicity caused by ammonia poisoning in yellow catfish. In addition, 0.05% NCG was added to the diet, which significantly increased iNOS activity in the liver of yellow catfish. iNOS was isolated from macrophages and involved in mitochondrial superoxide anion scavenging (Moncada and Higgs, 1993), and it mediated immune functions (Iadecola, 1997). Based on this finding, we hypothesized that NCG may have a positive effect on the health status of yellow catfish under ammonia stress, including immunity and disease resistance.
In this study, ammonia stress was found to induce the deterioration of blood health (TP, TG, and Glu decreased), which is consistent with other ammonia poisoning fish, such as blunt snout bream Megalobrama amblycephala (Zhang et al., 2019), Dolly Varden char (Zhu et al., 2020), and common carp (Xue et al., 2022). A recent study reported that dietary NCG supplements effectively improved the blood health of Japanese seabass, decreasing plasma low-density lipoproteins and ammonia contents, increasing antioxidant enzyme activity, and decreasing inflammation and apoptosis (Huang et al., 2019). This is consistent with the findings of this study, in which dietary 0.05% NCG supplementation decreased serum ammonia and urea contents and increased TP, TC, and Glu contents. In addition, NCG has also been reported to improve liver health in animals (Huang et al., 2019). The AST and ALT are indicators that can be released into the bloodstream following the occurrence of liver damage (Lin et al., 2010). This study found that dietary 0.05% NCG supplementation decreased the AST and ALT contents in the serum of yellow catfish. This may be related to the antioxidant capacity of NCG, which reduces oxidative damage of the liver induced by ammonia toxicity.
For a long time, fish ammonia poisoning has been thought to be related to oxidative damage, such as in hybrid grouper Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀ (Kim et al., 2020), flounder Paralichthys olivaceus (Cui et al., 2020), and golden pompano (Liu et al., 2021). It has been confirmed in mammals that stress causes overactivation of N-methyl-d-aspartate glutamate receptors in neurons, resulting in the production of a large number of reactive oxygen species (ROS), which is the main cause of oxidative damage (Hermenegildo et al., 2000). In general, the scavenging of ROS in fish depends on the activation of antioxidant enzymes (including SOD, CAT, and GPx), which are regulated by related genes expression (Trenzado et al., 2009). In this study, the cu/zn sod, cat, gpx, and gr expression were gradually up-regulating as ammonia levels increased, but the serum SOD, CAT, and GPx activities did not increase as expected. We speculated that this might be related to the excessive accumulation of MDA, because serum MDA contents continued to increase throughout the experiment. MDA can cross-link with the nucleophilic groups of proteins, nucleic acids, and amino phospholipids, leading to cytotoxicity and protein denaturation (Trenzado et al., 2009).
Previous studies have confirmed that the overproduction of ROS will further promote the release of proinflammatory cytokines (TNF, IL, and TGF) and induce apoptosis and even necrosis (Koca et al., 2008; Malaguarnera et al., 2009). TNFα is classified as a proinflammatory mediator that induces cell death by playing an initiating role in hepatocyte apoptosis; IL 1 stimulates T cell activation and promotes B cell proliferation and antibody secretion; IL 8 can recruit and activate macrophages and neutrophils, remove cell debris, and invade microorganisms (Budhu and Wang, 2006; Dhanasekaran and Reddy, 2008; Yin et al., 2014). In this study, the contents of serum proinflammatory cytokines (TNF, IL 1, and IL 8) were analyzed by the ELISA kit, and the tnf ɑ, il 1, and il 8 expression levels were also analyzed. The results showed that they all increased with increasing ammonia levels, but we observed a decrease in inflammation with the dietary 0.05% NCG intake, which means that NCG can reduce the negative effects of inflammation.
During the bacterial challenge, ammonia stress resulted in increased CM while decreasing serum AT, LYZ, CH50, Ig, RB, and PI values. In higher animals, dietary NCG has a regulatory effect on their immune function. A dietary supplementation of 50 mg/kg NCG can improve the intestinal mucosal immune response of Escherichia coli-challenged piglets (Zhang et al., 2013). Moreover, dietary NCG supplementation has been found to enhance the immunity of PRRSV-infected sows (Yang et al., 2011). This study found that a dietary 0.05% NCG supplementation significantly reduced the CM of yellow catfish and significantly increased the serum immune response (AT, LYZ, Ig, and RB). So far, there have been few studies on the effects of dietary NCG on fish immunity and disease resistance; we speculate that nitric oxide may be involved. As an immunomodulatory molecule, nitric oxide is involved in regulation of T-lymphocyte proliferation, antibody immune response, and natural killer cells activity (Ye and Tian, 2010). As discussed earlier in this study, nitric oxide produced by nNOS can affect the brain function, but when produced by iNOS, it is involved in immune regulation. Based on the results of the correlation analysis between the nitric oxide synthase activity and ammonia metabolism enzyme activity, we propose: 1) when ammonia stress occurred, iNOS was positively correlated with ASS, ASL, and OTC activities (Figures 3B, C), which suggests that improving the urea cycle efficiency may effectively alleviate the negative effects of ammonia stress on the immunity of yellow catfish; 2) compared with the 0.00% NCG group (Figure 3D), iNOS in the 0.05% NCG group was only positively correlated with ASS activity (Figure 3E), which suggests that ASS may be another target of NCG to activate the urea cycle, though more evidence is needed.
5 Conclusion
When ammonia stress occurs, dietary NCG supplementation can improve the growth, hematological index, oxidation resistance status, immune response, and disease resistance of yellow catfish, and it can thus improve their ammonia tolerance. This study hypothesized that ASS may be another target of NCG to activate the urea cycle.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Professional Committee of Xinyang Agriculture and Forestry University.
Author contributions
DF and ML designed the experiments; DF carried out the experimental work; DF wrote the manuscript under the direction of ZY.
Funding
This work was supported by the National Natural Science Foundation of China (32072948) and the Fundamental Research Funds for the Provincial Universities of Zhejiang (SJLY2020009). The Special Fund for Henan Agriculture Research System (HARS-22-16-G3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson, P. M. (1995). “3 urea cycle in fish: Molecular and mitochondrial studies,” in Fish Physiology. Editors C. M. Wood, and T. J. Shuttleworth (Utah: Academic Press), 57–83.
Ayata, C., Ayata, G., Hara, H., Matthews, R. T., Beal, M. F., Ferrante, R. J., et al. (1997). Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knockout mice. J. Neurosci. 17, 6908–6917. doi:10.1523/JNEUROSCI.17-18-06908.1997
Banerjee, B., Koner, D., Hasan, R., and Saha, N. (2020). Molecular characterization and ornithine-urea cycle genes expression in air-breathing magur catfish (Clarias magur) during exposure to high external ammonia. Genomics 112, 2247–2260. doi:10.1016/j.ygeno.2019.12.021
Banerjee, B., Koner, D., Saha, P. N., and Saha, N. (2017). Unique mitochondrial localization of arginase 1 and 2 in hepatocytes of air-breathing walking catfish, Clarias batrachus and their differential expression patterns under hyper-ammonia stress. Gene 622, 13–22. doi:10.1016/j.gene.2017.04.025
Budhu, A., and Wang, X. W. (2006). The role of cytokines in hepatocellular carcinoma. J. Leukoc. Biol. 80, 1197–1213. doi:10.1189/jlb.0506297
Chacher, B., Liu, H. Y., Wang, D. M., and Liu, J. X. (2013). Potential role of N-carbamoyl glutamate in biosynthesis of arginine and its significance in production of ruminant animals. J. Anim. Sci. Biotechnol. 4, 16. doi:10.1186/2049-1891-4-16
Chew, S. F., Jin, Y., and Ip, Y. K. (2001). The loach Misgurnus anguillicaudatus reduces amino acid catabolism and accumulates alanine and glutamine during aerial exposure. Physiol. Biochem. Zool. 74, 226–237. doi:10.1086/319663
Clark, T. C., Tinsley, J., Macqueen, D. J., and Martin, S. A. M. (2019). Rainbow trout (Oncorhynchus mykiss) urea cycle and polyamine synthesis gene families show dynamic expression responses to inflammation. Fish. Shellfish Immunol. 89, 290–300. doi:10.1016/j.fsi.2019.03.075
Colasanti, M., and Suzuki, H. (2000). The dual personality of NO. Trends Pharmacol. Sci. 21, 249–252. doi:10.1016/S0165-6147(00)01499-1
Cui, W., Cao, L., Liu, J., Ren, Z., Zhao, B., and Dou, S. (2020). Effects of seawater acidification and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total Environ. 718, 137234. doi:10.1016/j.scitotenv.2020.137234
Dhanasekaran, D. N., and Reddy, E. P. (2008). JNK signaling in apoptosis. Oncogene 27, 6245–6251. doi:10.1038/onc.2008.301
Divya, M., Gopi, N., Iswarya, A., Govindarajan, M., Alharbi, N. S., Kadaikunnan, S., et al. (2020). β-Glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 139, 103917. doi:10.1016/j.micpath.2019.103917
Esam, F., Khalafalla, M. M., Gewaily, M. S., Abdo, S., Hassan, A. M., and Dawood, M. A. O. (2022). Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 231, 113187. doi:10.1016/j.ecoenv.2022.113187
Fan, B., Li, J., Wang, X., Chen, J., Gao, X., Li, W., et al. (2021). Ammonia spatiotemporal distribution and risk assessment for freshwater species in aquatic ecosystem in China. Ecotoxicol. Environ. Saf. 207, 111541. doi:10.1016/j.ecoenv.2020.111541
Hegazi, M. M., Attia, Z. I., and Ashour, O. A. (2010). Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 99, 118–125. doi:10.1016/j.aquatox.2010.04.007
Hermenegildo, C., Monfort, P., and Felipo, V. (2000). Activation of N-methyl-d-aspartate receptors in rat brain in vivo following acute ammonia intoxication: Characterization by in vivo brain microdialysis. Hepatology 31, 709–715. doi:10.1002/hep.510310322
Huang, H. Y., Chen, P., Liang, X. F., Wu, X. F., Wang, C. P., Gu, X., et al. (2019). Dietary N-Carbamylglutamate (NCG) alleviates liver metabolic disease and hepatocyte apoptosis by suppressing ERK1/2-mTOR-S6K1 signal pathway via promoting endogenous arginine synthesis in Japanese seabass (Lateolabrax japonicus). Fish. Shellfish Immunol. 90, 338–348. doi:10.1016/j.fsi.2019.04.294
Huang, Y. L., Zhou, Z. B., Huang, W. M., Peng, T., and Hou, X. L. (2017). Effects of N-carbamylglutamate on growth performance, blood parameters and serum free amino acid content of three yellow chicken. Feed Ind. 4, 10.
Husson, A., Brasse-Lagnel, C., Fairand, A., Renouf, S., and Lavoinne, A. (2003). Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 270, 1887–1899. doi:10.1046/j.1432-1033.2003.03559.x
Iadecola, C. (1997). Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 20, 132–139. doi:10.1016/S0166-2236(96)10074-6
Ip, Y. K., and Chew, S. F. (2018). Air-breathing and excretory nitrogen metabolism in fishes. Acta histochem. 120, 680–690. doi:10.1016/j.acthis.2018.08.013
Jow, L. Y., Chew, S. F., Lim, C. B., Anderson, P. M., and Ip, Y. K. (1999). The marble goby Oxyeleotris marmoratus activates hepatic glutamine synthetase and detoxifies ammonia to glutamine during air exposure. J. Exp. Biol. 202, 237–245. doi:10.1242/jeb.202.3.237
Kim, J. H., Cho, J. H., Kim, S. R., and Hur, Y. B. (2020). Toxic effects of waterborne ammonia exposure on hematological parameters, oxidative stress and stress indicators of juvenile hybrid grouper, Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀. Environ. Toxicol. Pharmacol. 80, 103453. doi:10.1016/j.etap.2020.103453
Kiss, P. J. (2000). Role of nitric oxide in the regulation of monoaminergic neurotransmission. Brain Res. Bull. 52, 459–466. doi:10.1016/S0361-9230(00)00282-3
Koca, S. S., Bahcecioglu, I. H., Poyrazoglu, O. K., Ozercan, I. H., Sahin, K., and Ustundag, B. J. I. (2008). The treatment with antibody of TNF-alpha reduces the inflammation, necrosis and fibrosis in the non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Inflammation 31, 91–98. doi:10.1007/s10753-007-9053-z
Li, M., Zhang, M. Z., Qian, Y. X., Shi, G., and Wang, R. X. (2020). Ammonia toxicity in the yellow catfish (Pelteobagrus fulvidraco): The mechanistic insight from physiological detoxification to poisoning. Fish. Shellfish Immunol. 102, 195–202. doi:10.1016/j.fsi.2020.04.042
Lim, C. B., Anderson, P. M., Chew, S. F., and Ip, Y. K. (2001). Reduction in the rates of protein and amino acid catabolism to slow down the accumulation of endogenous ammonia: A strategy potentially adopted by mudskippers (Periophthalmodon schlosseri and Boleophthalmus boddaerti) during aerial exposure in constant darkness. J. Exp. Biol. 204, 1605–1614. doi:10.1242/jeb.204.9.1605
Lin, J. D., Lin, P. Y., Chen, L. M., Fang, W. H., Lin, L. P., and Loh, C. H. (2010). Serum glutamicoxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res. Dev. Disabil. 31, 172–177. doi:10.1016/j.ridd.2009.08.005
Liu, M. J., Guo, H. Y., Zhu, K. C., Liu, B. S., Liu, B., Guo, L., et al. (2021). Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat. Toxicol. 240, 105969. doi:10.1016/j.aquatox.2021.105969
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using realtime quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods 5, 402–408. doi:10.1006/meth.2001.1262
Malaguarnera, M., Di Rosa, M., Nicoletti, F., and Malaguarnera, L. J. (2009). Molecular mechanisms involved in NAFLD progression. J. Mol. Med. 87, 679–695. doi:10.1007/s00109-009-0464-1
MOAC (Ministry of Agriculture, China) (2022). China fisheries yearbook. Beijing, China: China Agriculture Publisher.
Moncada, S., and Higgs, A. (1993). The L-arginine-nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012. doi:10.1056/NEJM199312303292706
Tay, S. L. A., Chew, S. F., and Ip, Y. K. (2003). The swamp eel Monopterus albus reduces endogenous ammonia production and detoxifies ammonia to glutamine during aerial exposure. J. Exp. Biol. 206, 2473–2486. doi:10.1242/jeb.00464
Trenzado, C. E., Morales, A. E., Palma, J. M., and Higuer, M. (2009). Blood antioxidant defenses and hematological adjustments in crowded/uncrowded rainbowtrout (Oncorhynchus mykiss) fed on diets with different levels of antioxidant vitamins and HUFA. Aquaculture 149, 440–447. doi:10.1016/j.cbpc.2008.10.105
Tuchman, M., Caldovic, L., Daikhin, Y., Horyn, O., Nissim, I., Nissim, I., et al. (2008). N-Carbamylglutamate markedly enhances ureagenesis in N-acetylglutamate deficiency and propionic acidemia as measured by isotopic incorporation and blood biomarkers. Pediatr. Res. 64, 213–217. doi:10.1203/PDR.0b013e318179454b
Ucar, S. K., Coker, M., Habif, S., Saz, E. U., Karapinar, B., Ucar, H., et al. (2009). The first use of N-carbamylglutamate in a patient with decompensated maple syrup urine disease. Metab. Brain Dis. 24, 409–414. doi:10.1007/s11011-009-9155-4
Vaage, B., and Myrick, C. (2021). The effects of acute and chronic exposure of ammonia on juvenile burbot (Lota lota) growth and survival. Aquaculture 542, 736891. doi:10.1016/j.aquaculture.2021.736891
Vemuganti, L., and Raghavendra, R. (2002). Nitric oxide in hepatic encephalopathy and hyperammonemia. Neurochem. Int. 41, 161–170. doi:10.1016/S0197-0186(02)00038-4
Wang, L., Sun, Y. X., Xu, B. Y., Sagada, G., Chen, K., Xiao, J. X., et al. (2020). Effects of berberine supplementation in high starch diet on growth performance, antioxidative status, immune parameters and ammonia stress esponse of fingerling black sea bream (Acanthopagrus schlegelii). Aquaculture 527, 735473. doi:10.1016/j.aquaculture.2020.735473
Wang, Y. X., and Walsh, P. J. (2000). High ammonia tolerance in fishes of the family Batrachoididae (Toadfish and Midshipmen). Aquat. Toxicol. 50, 205–219. doi:10.1016/S0166-445X(99)00101-0
Wu, G. Y., Knabe, D. A., and Kim, S. W. (2004). Arginine nutrition in neonatal pigs. J. Nutr. 134, 2783S–2790S. doi:10.1093/jn/134.10.2783S
Wu, X., Ruan, Z., Gao, Y., Yin, Y., Zhou, X., Wang, M., et al. (2010). Dietary supplementation with l-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a cornand soybean meal-based diet. Amino Acids 39, 831–839. doi:10.1007/s00726-010-0538-y
Xue, S. Q., Chen, S. M., Ge, Y. X., Guan, T., and Han, Y. (2022). Regulation of glutathione on growth performance, biochemical parameters, non-specific immunity, and related genes of common carp (Cyprinus carpio) exposed to ammonia. Aquaculture 546, 737241. doi:10.1016/j.aquaculture.2021.737241
Xue, S. Q., Lin, J. W., Zhou, Q., Wang, H. T., and Han, Y. (2021). Effect of ammonia stress on transcriptome and endoplasmic reticulum stress pathway for common carp (Cyprinus carpio) hepatopancreas. Aquacul. Rep. 20, 100694. doi:10.1016/j.aqrep.2021.100694
Yang, P., Wu, D., Che, L., Fang, Z., Lin, Y., Qiao, S., et al. (2011). Effects of dietary L-arginine or N-carbamylglutamate supplementation on reproductive performance and immune function of PRRSV-infected pregnant sows. Chin. J. Anim. Nutr. 23, 1351–1360.
Ye, T., and Tian, K. X. (2010). Effect of arginine on the immune system and related mechanisms. Chin. J. Feed Rev. 7, 11–13.
Yin, G., Li, W., Lin, Q., Lin, X., Lin, J., Zhu, Q., et al. (2014). Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish. Shellfish Immunol. 41, 402–406. doi:10.1016/j.fsi.2014.09.027
Yu, Z., Wu, X. Q., Zheng, L. J., Dai, Z. Y., and Wu, L. F. (2020). Effect of acute exposure to ammonia and BFT alterations on Rhynchocypris lagowski: Digestive enzyme, inflammation response, oxidative stress and immunological parameters. Environ. Toxicol. Pharmacol. 78, 103380. doi:10.1016/j.etap.2020.103380
Zhang, F., Zeng, X., Yang, F., Huang, Z., Liu, H., Ma, X., et al. (2013). Dietary N-carbamylglutamate supplementation boosts intestinal mucosal immunity in Escherichia coli challenged piglets. PLoS ONE 8, e66280. doi:10.1371/journal.pone.0066280
Zhang, H., Liu, X., Ren, S., Elsabagh, M., Wang, M., and Wang, H. (2021). Dietary N-carbamylglutamate or l-arginine supplementation improves hepatic energy status and mitochondrial function and inhibits the AMP-activated protein kinase-peroxisome proliferator-activated receptor γ coactivator-1α-transcription factor A pathway in intrauterine-growth-retarded suckling lambs. Anim. Nutr. 7, 859–867. doi:10.1016/j.aninu.2021.02.005
Zhang, M. Z., Li, M., Wang, R. X., and Qian, Y. X. (2018). Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish. Shellfish Immunol. 79, 313–320. doi:10.1016/j.fsi.2018.05.036
Zhang, M. Z., Song, P. W., Jiang, H. B., and Li, M. (2022b). The argininosuccinate synthetase can differentially regulate nitric oxide synthase in yellow catfish Pelteobagrus fulvidraco. Fish. Shellfish Immunol. 127, 991–1000. doi:10.1016/j.fsi.2022.07.044
Zhang, M. Z., Wang, S. D., Sun, Z., Jiang, H. B., Qian, Y. X., Wang, R. X., et al. (2022a). The effects of acute and chronic ammonia exposure on growth, survival, and free amino acid abundance in juvenile Japanese sea perch Lateolabrax japonicus. Aquaculture 560, 738512. doi:10.1016/j.aquaculture.2022.738512
Zhang, W. X., Xia, S. L., Zhu, J., Miao, L. H., Ren, M. C., Lin, Y., et al. (2019). Growth performance, physiological response and histology changes of juvenile blunt snout bream, Megalobrama amblycephala exposed to chronic ammonia. Aquaculture 506, 424–436. doi:10.1016/j.aquaculture.2019.03.072
Zhao, H., Qiao, G., Cao, J., Huang, Y., Wang, G., Chen, B., et al. (2019). Dietary supplementation of N-carbamylglutamate and effects on growth, intestinal enzyme activities, immunological and antioxidant abilities of juvenile yellow catfish (Pelteobagrus fulvidraco). Aquacult. Nutr. 25, 1250–1260. doi:10.1111/anu.12939
Zhu, X. Z., Li, M., and Liu, B. (2020). Acute ammonia poisoning in dolly varden char (Salvelinus malma) and effect of methionine sulfoximine. Fish. Shellfish Immunol. 101, 198–204. doi:10.1016/j.fsi.2020.03.068
Zuo, Z. H., Wang, S. D., Wang, Q. J., Wang, D. J., Wu, Q. P., Xie, S. L., et al. (2022). Effects of partial replacement of dietary flour meal with seaweed polysaccharides on the resistance to ammonia stress in the intestine of hybrid snakehead (Channa maculatus ♀ × Channa argus ♂). Fish. Shellfish Immunol. 127, 271–279. doi:10.1016/j.fsi.2022.06.035
Keywords: N-carbamylglutamate, ammonia, urea cycle, nitric oxide synthase, Pelteobagrus fulvidraco
Citation: Feng D, Yang Z and Li M (2023) Dietary N-carbamylglutamate supplementation improves ammonia tolerance of juvenile yellow catfish Pelteobagrus fulvidraco. Front. Physiol. 14:1191468. doi: 10.3389/fphys.2023.1191468
Received: 22 March 2023; Accepted: 13 April 2023;
Published: 24 April 2023.
Edited by:
Shengming Sun, Shanghai Ocean University, ChinaReviewed by:
Fenglu Han, Hainan University, ChinaYafei Duan, South China Sea Fisheries Research Institute, China
Copyright © 2023 Feng, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Li, bGltaW5nMUBuYnUuZWR1LmNu
 Dexiang Feng
Dexiang Feng Zhiguo Yang1
Zhiguo Yang1 Ming Li
Ming Li