- 1School of Agriculture, Ludong University, Yantai, China
- 2College of Animal Science and Technology, Northwest A&F University, Xianyang, China
Gonadotropin-releasing hormone (GnRH) controls synthesis of sex steroid hormones through hypothalamic-pituitary-gonadal (HPG) axis in vertebrates. But in mollusks, research on neuroendocrine control of gonadal function, such as the function of GnRH during gonadal development is limited. In this study, we investigated the morphology and structure of the nerve ganglia of Zhikong scallop Chlamys farreri by physiological and histological observations. We also cloned the ORF and studied the expression patterns of GnRH in the scallop. Tissue expression analysis showed that GnRH was highly expressed in parietovisceral ganglion (PVG). The in situ hybridization result further confirmed that GnRH mRNA only distributed in some good-sized neurons in the posterior lobe (PL) and some pint-sized neurons in the lateral lobe (LL). In addition, by examining the expression of GnRH during gonadal development in ganglia, we found GnRH displayed higher expression in the female scallops, and showed significant high expression at the growing stage of female scallops in PVG. This study would contribute to gaining insight into the mechanism underlying reproduction regulation by GnRH in the scallop and help to provide a better understanding of reproductive neuroendocrine in mollusks.
1 Introduction
Gonadotropin-releasing hormone (GnRH) is the major neurohormone in hypothalamic–pituitary–gonadal (HPG) axis, which plays a key role in sexual development and reproduction regulation in vertebrates (Bliss et al., 2010). GnRH is released from hypothalamus and received by the receptor for releasing gonadotropins including luteinizing hormone and follicle-stimulating hormone in the pituitary (Jin and Yang, 2014). These hormones are then transported to the gonads to stimulate the synthesis of sex steroid hormones, and then modulate reproductive process (Huggard-Nelson et al., 2002; Schulster et al., 2016). In invertebrates, with an absence of complex brain tissues such as hypothalamus and pituitary, GnRH also be found in nervous system and may play an important role of reproductive function (Tinikul et al., 2014; Sukhan et al., 2021; Ojima et al., 2022).
Mollusks are one of the most abundant and biologically diverse groups in the animal kingdom. Although they do not possess the complex brain structure as vertebrates, they have various kinds of nerve ganglia as central nervous system (CNS). The neurohormones are synthesized in nerve ganglia and released into the haemolymph via a network of neurohemal organs to regulate growth, immune, reproduction and other physiological functions (Zhang et al., 2018; Li et al., 2019; Kotsyuba and Dyachuk, 2022). Although there is no “HPG axis” in mollusks, GnRH has been identified in various mollusks (Zhang et al., 2008; Bigot et al., 2012; Nagasawa et al., 2015; Kim et al., 2017; Rosa-Casillas et al., 2021). Molluscan GnRH was suggested to be associated with reproduction (Morishita et al., 2010; Osada and Treen, 2013; Ding et al., 2021; Murata et al., 2021).
The Zhikong scallop, Chlamys farreri is an important maricultural bivalve in China and Japan. Due to its commercial value, the reproduction of C. farreri has received a lot of research focus. Previously, vertebrate-type steroids and steroidogenesis genes have been reported in C. farreri and seem to regulate reproductive events (Qin et al., 2012; Liu et al., 2014a; Zheng et al., 2014). But the study on reproductive regulation of neuroendocrine in C. farreri, especially the potential reproductive function of GnRH, is still blank until now. In order to illustrate how the GnRH regulate gonadal development, we investigated the CNS and gonads of Zhikong scallop and studied its tissue and spatiotemporal expression. We suggested that GnRH may display an important role at the growing stage of female Zhikong scallops. This study will contribute to a better understanding of reproductive neuroendocrine in mollusks.
2 Materials and methods
2.1 Sample collection and histology
One-year-old scallops were obtained from Yantai, Shandong Province, China. The scallops were acclimated at 16°C in aerated seawater (30‰) for 1 week, and were fed with Spirulina platensis powder. After acclimation, nerve ganglia, gonadal tissues, peripheral tissues (mantle, gill, kidney, digestive gland and adductor muscle) were dissected, immediately frozen in liquid nitrogen and stored at −80°C before use. The gonadal development stages were distinguished by examining gonadal morphology and by histological observations. The structure of neural ganglion system was also investigated by histological observation.
2.2 RNA extraction and first-strand cDNA synthesis
Total RNA was extracted using Tiangen RNA Extraction Kit (Tiangen Biotech, China). RNA concentration and purity were determined using a Nanovue Plus spectrophotometer (GE Healthcare, NJ, United States), and RNA integrity was verified by agarose gel electrophoresis. First-strand cDNA synthesis was performed using the Evo MMLV Plus cDNA Synthesis Kit (Accurate Biology, China). The reverse transcription reaction conditions were as follows: 30 min at 42°C and termination by heating at 95°C for 5 min.
2.3 Cloning of GnRH ORF and sequences analyses
Using the GnRH gene sequences of other scallops Mizuhopecten (Patinopecten) yessoensis (XM_021491876.1), Pecten maximus (XM_033891046.1) and Argopecten irradians (Li et al., 2020) through alignment, we designed primers (Supplementary Table S1) to amplify GnRH gene. After purification with the SanPrep Column DNA Gel Extraction Kit (Sangon Biotech, China), the PCR product was ligated into the pEASY-T1 vector (TransGen Biotech, China). Then, the T-vector was transformed into competent E. coli cells. The positive clones were selected and sent to Personal BioTechnology (Shanghai, China) for sequencing.
2.4 Gene identification and phylogenetic analyses
After sequencing, the amino acid sequence was deduced. The function domains were predicted using the Simple Modular Architecture Research Tool (SMART) (http://smart.emblheidelberg.de). The GnRH family protein sequences of different species were obtained from the NCBI (http://www.ncbi.nlm.nih.gov/guide/proteins/) and MolluscDB (http://mgbase.qnlm.ac/page/download/download) (Liu et al., 2021) database. Multiple sequence alignments were performed with ClustalW (Thompson et al., 1994), and the results were annotated with GeneDoc. A phylogenetic tree was constructed using the neighbor-joining method with 1,000 bootstrap replicates using the MEGA7 (Kumar et al., 2016).
2.5 Reverse transcription quantitative PCR (RT-qPCR)
Gene-specific primers were designed using Primer Premier 5.0 (Supplementary Table S1). RT-qPCR was conducted using SYBR Premix Ex Taq II (TaKaRa, Japan) and a Bio-Rad CFX Connect PCR instrument with the following program: 94°C for 10°min, followed by 40 cycles of 94°C for 15°s and 60°C for 1 min EF1A (elongation factor 1-alpha) was used as an endogenous control for the normalization of gene expression. The relative expression level of each gene was calculated using the 2−ΔΔCt method. All data were analyzed using SPSS 21.0 (IBM Corp, Armonk, United States). p-values lower than 0.05 were considered statistically significant.
2.6 Tissue in situ hybridization
The cDNA fragments were amplified with the GnRH_ISH primers (Supplementary Table S1). The template for in vitro transcription was conducted using the primers containing T7/SP6 promoter sequence. The anti-sense and sense probes were in vitro transcribed with a DIG RNA Labeling Kit (SP6/T7) (Roche, Mannheim, Germany). Sections of the PVG were serially rehydrated in PBST and digested with 2 mg/mL proteinase K at 37°C for 13 min. After pre-hybridization at 60°C for 4 h, hybridization was performed with 1 mg/mL denatured RNA probe in hybridization buffer at 60°C for 16 h. After hybridization, the probes were washed away, and antibody incubation was performed in a fresh solution of anti-digoxigenin-AP Fab fragments (Roche, Mannheim, Germany) coupled with blocking buffer (diluted 1:2000) at 4°C for 14 h. After extensive washing with maleic acid buffer, sections were incubated with NBT/BCIP substrate solution in the darkness and counterstained with 1% neutral red solution.
3 Results
3.1 Physiological and histological observation of nerve ganglion system
The neural ganglion system of C. farreri consisted of one pair of separated cerebral ganglia (CG), one pair of connected pedal ganglia (PG) and one parietovisceral ganglion (PVG). By the physiological observation, the CG and PG were located next to each other between the gonad and the hepatopancreas (Figure 1A). By HE staining and tissue section observation, ganglia consisted of a region of neuron-cell bodies (RNB) and a region of central neuropile (RCN). The nerve fibers were distributed in the central part of ganglia or along nerve cords, where were colored lighter. Neuronal cell bodies in ganglia section were mainly distributed in the outboard surface where were colored deeply. The majority of neurons in the CG were the good-sized (>15 μm), and the number of neurons at pint size (4–5 μm) were more than that of those in good size in PG (Figure 1B). The PVG was above the middle region of adduct muscle, where it connected with the gonad (Figure 1C). The structure of PVG was comparatively complex (Figure 1D), including two anterior lobes (AL), one posterior lobe (PL), two lateral lobes (LL) and two osphradium lobes (OL). There were more good-sized neuron cells in AL and PL, and only pint-sized cells in LL and OL. Different lobes could be distinguished by the tissue morphology and the neuron size.
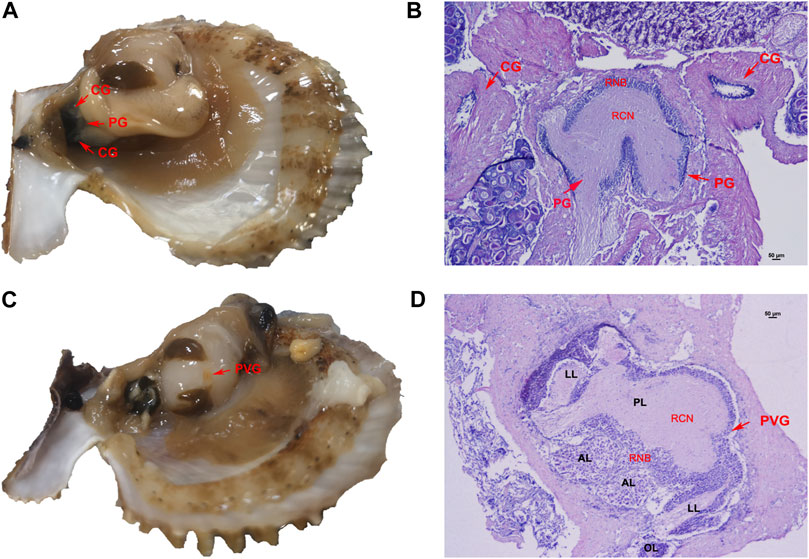
FIGURE 1. Nerve ganglion system of C. farreri. (A) morphological and (B) histological structure of CG and PG. (C) morphological and (D) histological observation of PVG. Abbreviations: RCN, region of central neuropile; RNB, region of neuron-cell bodies; AL, anterior lobe; LL, lateral lobe; PL, posterior lobe; OL, osphradium lobe.
3.2 Sequence characterization and phylogenetic analysis
The cDNA sequence of GnRH was obtained from nerve ganglia, and deposited in GenBank with an accession number of OQ200481. The partial-length cDNA consisted of 525-bp with a 5′-UTR of 19-bp, a 3′-UTR of 194-bp, and an open reading frame (ORF) of 312-bp (Figure 2A). The ORF encoded a precursor of 103 residues, including a 24-residue signal peptide, a conserved 11 residue GnRH peptide and a GKR sequence serving as a combined amidation and cleavage site. The deduced mature GnRH peptide was aligned with representative vertebrate GnRH1, GnRH2, GnRH3 and GnRH4 sequences along with a variety of echinodermal and molluscan GnRH members. Mature Cf-GnRH exhibits a high degree of identity with other molluscan GnRHs as well as some relatedness with vertebrate and echinodermal GnRHs (Figure 2B). The phylogenetic tree of GnRH precursor sequences showed that vertebrate GnRHs can be classified into four groups: GnRH-I (hypothalamic/preoptic forms of GnRH), GnRH-II (mesencephalic form of GnRH), fish-specific GnRH-III and lamprey-specific GnRH-IV, as previously reported (Roch et al., 2014). The sequence of Cf-GnRH clustered mainly with invertebrates, especially classified as a member of molluscan GnRH-V (Figure 2C).
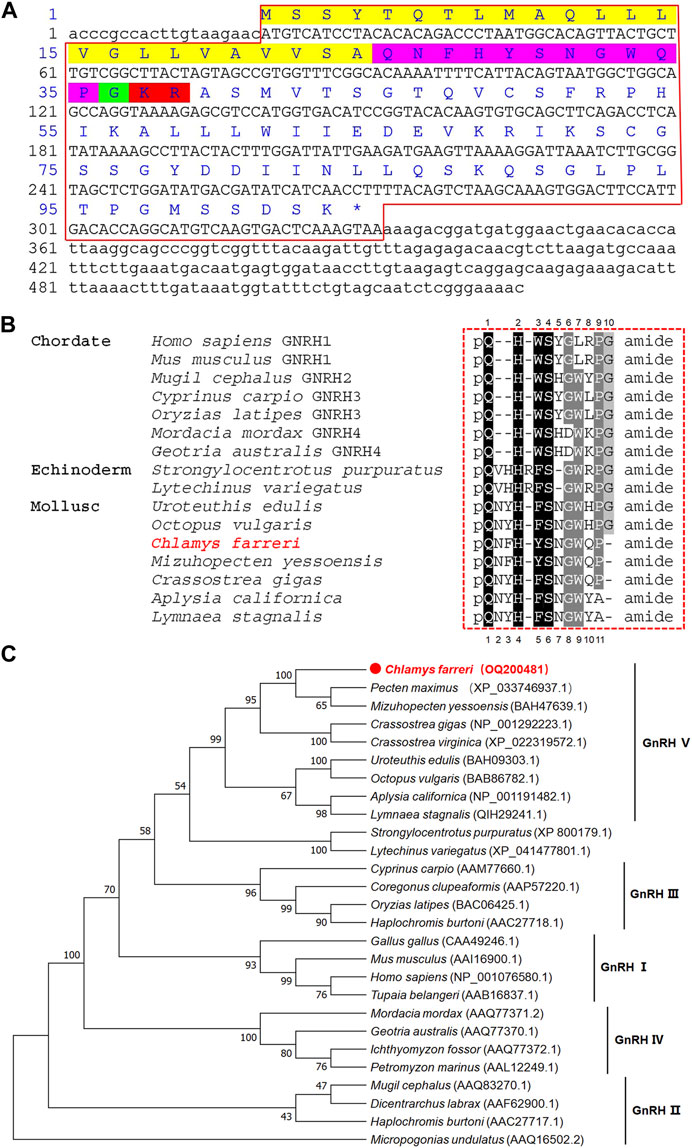
FIGURE 2. Sequence and phylogenetic analysis of GnRH. (A) Sequence of Zhikong scallop GnRH. Yellow, signal peptide; purple pink, GnRH peptide; green, C-terminal glycine; red, cleavage site. (B) Alignment of GnRH peptides among vertebrates, echinoderms and mollusks. (C) Neighbor-joining phylogenetic tree of GnRH.
3.3 Tissue distribution and spatial expression of GnRH
Relative expression analysis revealed that the mRNA expression of GnRH was mainly detected in the nerve ganglia. It was significantly (p < 0.05) higher in the PVG than in any other examined tissues (Figure 3A). We further investigated the spatial expression in PVG using in situ hybridization (Figure 3B). Strong hybridization signals were found in the cytoplasm of some good-sized neurons (15–20 μm) in posterior lobe (PL) (Figures 3C, E, F) and some pint-sized neurons (<15 μm) in lateral lobe (LL) (Figures 3D, F). The mRNA was only detected in a few dispersed neurons, but most neurons did not have any signal distribution.
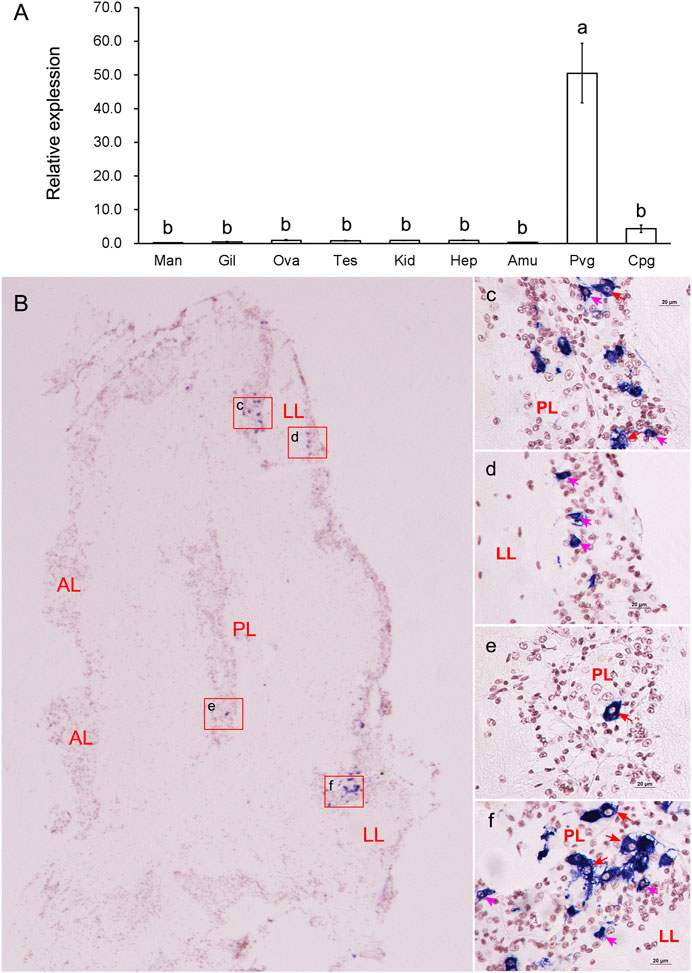
FIGURE 3. Tissue expressions and spatial localization of GnRH. (A) Relative expressions in different tissues. Abbreviations: Man, mantle; Gil, gill; Ova, ovary; Tes, testis; Kid, kidney, Hep, hepatopancreas; Amu, adduct muscle; Pvg, parietovisceral ganglion; Cpg, cerebral and pedal ganglia. (B) Localization of GnRH mRNA in PVG. (C-F): The enlarged areas in the red boxes corresponding to panel (B). Positive signals with an antisense probe are indicated in blue, and the cell nuclei are dyed red. Red arrows indicate good-sized neurons; pink arrows indicate pint-sized neurons.
3.4 Temporal expression of GnRH in neural ganglia during gonadal development
Three different stages (proliferative stage, growing stage and mature stage) of testis and ovary were distinguished by histological observation (Figure 4A). The GnRH in nerve ganglia displayed higher expression in the female scallops than in the male individuals. During the gonadal development, GnRH displayed significant high expression at the growing stage of female scallops in PVG (Figure 4B). But the expression of GnRH in CPG didn’t show any significant change during the three stages in female and male scallops (Figure 4C).
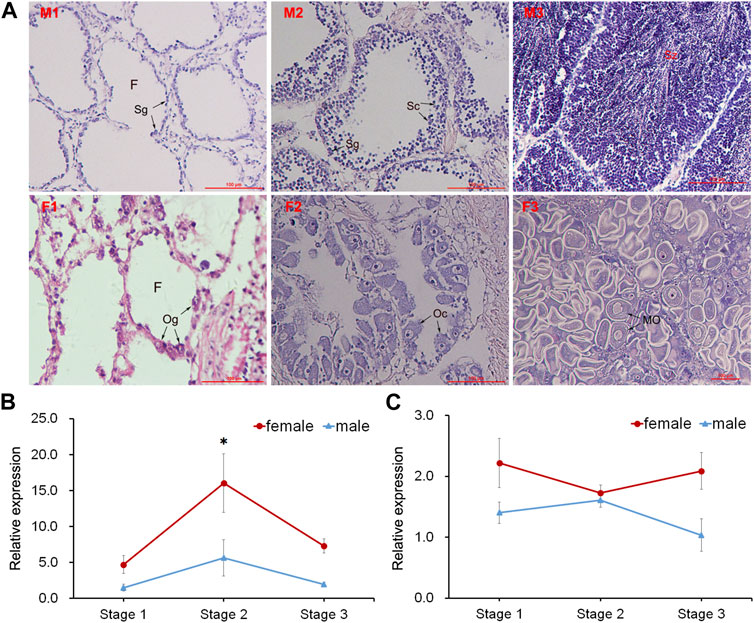
FIGURE 4. Histological observation of gonads and temporal expression of GnRH in ganglia. (A) Sections of testis and ovary during different gonadal development. Abbreviations: M, testis; F, ovary; 1, proliferative stage; 2, growing stage; 3, mature stage. F, follicle; Sg, spermatogonium; Sc, spermatocyte; Sz, spermatozoon; Og, oogonium; Oc, oocyte; MO, mature oocyte. Relative expressions of GnRH in (B) PVG and (C) CPG.
4 Discussion
In the present study, we characterized the prepro-GnRH from the Zhikong scallop. Similar to the known GnRH precursors, the scallop GnRH precursor exhibited a conserved structure consisting of a signal peptide, a mature GnRH peptide and a cleavage site. In the comparison of all the examined species, the site of prepro-GnRH was found to be strictly conserved at the pyroglutamic N-terminal and the amidated C-terminal. In contrast to vertebrate GnRHs with 10 amino acids, invertebrate GnRHs are more variable in their length. The molluscan GnRHs exhibit the typical extra dipeptide insertion between the N-terminal pyro-Glu and His (Minakata and Tsutsui, 2016; Kim et al., 2017; Li et al., 2020). Our results clearly demonstrate that the scallop GnRH is a member of the molluscan GnRH group, and its characterization helps to clarify the structural significance of invertebrate GnRHs. By comparing the GnRHs in four species of scallops (Zhikong scallop, Yesso scallop, King scallop and Bay scallop, we found these GnRHs showed high similarity and shared a same mature GnRH peptide (Supplementary Figure S2). However, whether these scallop GnRHs has the same function in gonadal development in other species of scallops needs studying further.
In previous study, GnRH was more strongly expressed in ganglia than other tissues in Yesso scallop (Zhang et al., 2020), and PVG is the major producing organ rather than CPG (Nagasawa et al., 2015). Similarly, our study suggested that GnRH was mainly expressed in PVG rather than CPG in Zhikong scallop. We also found that the female scallops had higher expression of GnRH than the male scallops, it was also similar to the expression characteristics of Yesso scallop (Zhang et al., 2020). According to the ISH result, we found the GnRH neurons were mainly distributed in posterior and lateral lobes. The tissue distribution and spatial expression both showed similar characterization with those of Yesso scallop (Nagasawa et al., 2015; Zhang et al., 2020). Previous neuro-endocrine studies in snail suggested that clusters of neurons in different lobes may be involved in different reproduction process (Koene, 2010). Combined with our study, we suppose PL and LL of PVG may be the regions project into reproduction in Zhikong scallop.
In vertebrates, GnRH plays a key role in HPG axis. But research on GnRH signaling pathway is scarce in mollusks. Although vertebrate sex steroids have been detected in molluscan tissues and the sex steroid levels have seasonal changes during the reproductive cycle and seem to regulate reproductive events in mollusks (Siah et al., 2002; Avila-Poveda et al., 2015), a controversy exists regarding whether mollusks can synthesize vertebrate sex steroids (Fernandes et al., 2011; Nuurai et al., 2020; Fodor and Pirger, 2022). In Yesso scallop, a putative pathway for sex steroid synthesis has been proposed (Thitiphuree et al., 2019), and GnRH may participate in the regulation of steroidogenesis (Zhang et al., 2020). These studies make it more convincible that scallops can produce sex steroids endogenously by a potential GnRH signaling pathway. In previous study, sex steroids have been also found in Zhikong scallop and correlated with gametogenesis (Liu et al., 2014b; Zheng et al., 2014). In our study, we found GnRH displayed higher expression in the female than the male. GnRH showed significant high expression at the growing stage in PVG during the gonadal development. We suggested that the GnRH may regulate gonadal development in the growing stage of the female scallops. However, our knowledge on the GnRH function of Zhikong scallop is still fragmentary, and whether the GnRH could regulate gonadal development and/or sex steroids synthesis remains to be answered.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Institutional Review Board of Ludong University (LDU-IRB20230322NXY-6).
Author contributions
JT: Investigation, Formal analysis. MY, JW, and QL: Investigation. BH and LW: Data curation. YL and YH: Formal analysis. XZ: Experimental materials. XnW: Project administration. MZ: Conceptualization, Methodology, Investigation, Writing. XtW: Conceptualization, Supervision, Writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 42076088); Key Research and Development Program of Shandong Province (2022LZGC015); Special Funds for Taishan Scholar Project of Shandong Province, China (No. tsqn201812094); Special Supporting Funding for Leading Talents above provincial level in Yantai City (No. 202203); Modern Agricultural Industry Technology System of Shandong Province, China (SDAIT-14-03) and Plan of Excellent Youth Innovation Team of Colleges and Universities in Shandong Province, China (2019KJF004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1180725/full#supplementary-material
References
Avila-Poveda, O. H., Montes-Pérez, R. C., Koueta, N., Benítez-Villalobos, F., Ramírez-Pérez, J. S., Jimenez-Gutierrez, L., et al. (2015). Seasonal changes of progesterone and testosterone concentrations throughout gonad maturation stages of the Mexican octopus, Octopus maya (octopodidae: Octopus). J. Malacol. Soc. Aust. 35 (3), 161–172. doi:10.1080/13235818.2015.1045055
Bigot, L., Zatylny-Gaudin, C., Rodet, F., Bernay, B., Boudry, P., and Favrel, P. (2012). Characterization of GnRH-related peptides from the pacific oyster Crassostrea gigas. Peptides 34 (2), 303–310. doi:10.1016/j.peptides.2012.01.017
Bliss, S. P., Navratil, A. M., Xie, J., and Roberson, M. S. (2010). GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocrinol. 31 (3), 322–340. doi:10.1016/j.yfrne.2010.04.002
Ding, M. J. S., Miao, J., and Pan, L. (2021). Possible roles of gonadotropin-releasing hormone (GnRH) and melatonin in the control of gonadal development of clam Ruditapes philippinarum. Comp. Biochem. Physiology Part A Mol. Integr. Physiology 262, 111059. doi:10.1016/j.cbpa.2021.111059
Fernandes, D., Loi, B., and Porte, C. (2011). Biosynthesis and metabolism of steroids in molluscs. J. steroid Biochem. Mol. Biol. 127 (3-5), 189–195. doi:10.1016/j.jsbmb.2010.12.009
Fodor, I., and Pirger, Z. (2022). From dark to light - an overview of over 70 Years of endocrine disruption research on marine mollusks. Front. Endocrinol. 13, 903575. doi:10.3389/fendo.2022.903575
Huggard-Nelson, D., Nathwani, P., Kermouni, A., and Habibi, H. R. (2002). Molecular characterization of LH-β and FSH-β subunits and their regulation by estrogen in the goldfish pituitary. Mol. Cell. Endocrinol. 188 (1-2), 171–193. doi:10.1016/s0303-7207(01)00716-x
Jin, J.-M., and Yang, W.-X. (2014). Molecular regulation of hypothalamus–pituitary–gonads axis in males. Gene 551 (1), 15–25. doi:10.1016/j.gene.2014.08.048
Kim, T. H., Kim, M. A., Kim, K. S., Kim, J. W., Lim, H. K., Lee, J. S., et al. (2017). Characterization and spatiotemporal expression of gonadotropin-releasing hormone in the Pacific abalone, Haliotis discus hannai. Comp. Biochem. Physiology Part A Mol. Integr. Physiology 209, 1–9. doi:10.1016/j.cbpa.2017.04.001
Koene, J. (2010). Neuro-endocrine control of reproduction in hermaphroditic freshwater snails: Mechanisms and evolution. Front. Behav. Neurosci. 4, 167. doi:10.3389/fnbeh.2010.00167
Kotsyuba, E., and Dyachuk, V. (2022). Effect of air exposure-induced hypoxia on neurotransmitters and neurotransmission enzymes in ganglia of the scallop Azumapecten farreri. Int. J. Mol. Sci. 23 (4), 2027. doi:10.3390/ijms23042027
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33 (7), 1870–1874. doi:10.1093/molbev/msw054
Li, M., Wang, M., Wang, W., Wang, L., Liu, Z., Sun, J., et al. (2019). The immunomodulatory function of invertebrate specific neuropeptide FMRFamide in oyster Crassostrea gigas. Fish Shellfish Immunol. 88, 480–488. doi:10.1016/j.fsi.2019.03.023
Li, W., Zhang, L., and Zhang, M. (2020). Cloning and expression analysis of gonadotropin-releasing hormone gene in the bay scallop. Periodical Ocean Univ. China 50 (2), 68–74. (in Chinese). doi:10.16441/j.cnki.hdxb.20180430
Liu, F., Li, Y., Yu, H., Zhang, L., Hu, J., Bao, Z., et al. (2021). MolluscDB: An integrated functional and evolutionary genomics database for the hyper-diverse animal phylum Mollusca. Nucleic Acids Res. 49 (D1), D988–D997. doi:10.1093/nar/gkaa918
Liu, J., Zhang, Z., Ma, X., Liang, S., and Yang, D. (2014a). Characteristics of 17β-hydroxysteroid dehydrogenase 8 and its potential role in gonad of Zhikong scallop Chlamys farreri. J. steroid Biochem. Mol. Biol. 141, 77–86. doi:10.1016/j.jsbmb.2014.01.008
Liu, J., Zhang, Z., Zhang, L., Liu, X., Yang, D., and Ma, X. (2014b). Variations of estradiol-17β and testosterone levels correlated with gametogenesis in the gonad of Zhikong scallop (Chlamys farreri) during annual reproductive cycle. Can. J. Zoology 92 (3), 195–204. doi:10.1139/cjz-2013-0202
Minakata, H., and Tsutsui, K. (2016). Oct-GnRH, the first protostomian gonadotropin-releasing hormone-like peptide and a critical mini-review of the presence of vertebrate sex steroids in molluscs. General Comp. Endocrinol. 227, 109–114. doi:10.1016/j.ygcen.2015.07.011
Morishita, F., Furukawa, Y., Matsushima, O., and Minakata, H. (2010). Regulatory actions of neuropeptides and peptide hormones on the reproduction of molluscsThe present review is one of a series of occasional review articles that have been invited by the Editors and will feature the broad range of disciplines and expertise represented in our Editorial Advisory Board. Can. J. Zoology 88 (9), 825–845. doi:10.1139/z10-041
Murata, R., Mushirobira, Y., Tanaka, Y., and Soyano, K. (2021). Expression profile of GnRH-like peptide during gonadal sex differentiation in the cephalopod kisslip cuttlefish, Sepia lycidas. General Comp. Endocrinol. 304, 113718. doi:10.1016/j.ygcen.2021.113718
Nagasawa, K., Osugi, T., Suzuki, I., Itoh, N., Takahashi, K. G., Satake, H., et al. (2015). Characterization of GnRH-like peptides from the nerve ganglia of Yesso scallop, Patinopecten yessoensis. Peptides 71, 202–210. doi:10.1016/j.peptides.2015.07.022
Nuurai, P., Wanichanon, C., and Wanichanon, R. (2020). Effect of gonadotropin releasing hormone on the expression of luteinizing hormone and estrogen in the nerve ganglia and ovary of a tropical abalone, Haliotis asinina Linnaeus. Acta Histochem. 122 (1), 151454. doi:10.1016/j.acthis.2019.151454
Ojima, D., Hirano-Maeda, Y., Matsubara, A., and Amano, M. (2022). Changes in gonadotropin-releasing hormone in the cerebral ganglion of the Manila clam Ruditapes philippinarum during gonadal development. Comp. Biochem. Physiology Part A Mol. Integr. Physiology 270, 111228. doi:10.1016/j.cbpa.2022.111228
Osada, M., and Treen, N. (2013). Molluscan GnRH associated with reproduction. General Comp. Endocrinol. 181, 254–258. doi:10.1016/j.ygcen.2012.09.002
Qin, Z., Li, Y., Sun, D., Shao, M., and Zhang, Z. (2012). Cloning and expression analysis of the vitellogenin gene in the scallop Chlamys farreri and the effects of estradiol-17β on its synthesis. Invertebr. Biol. 131 (4), 312–321. doi:10.1111/ivb.12006
Roch, G., Busby, E., and Sherwood, N. (2014). GnRH receptors and peptides: Skating backward. General Comp. Endocrinol. 209, 118–134. doi:10.1016/j.ygcen.2014.07.025
Rosa-Casillas, M., de Jesús, P., Vicente Rodríguez, L., Habib, M. R., Croll, R. P., and Miller, M. W. (2021). Identification and localization of a gonadotropin-releasing hormone-related neuropeptide in Biomphalaria, an intermediate host for schistosomiasis. J. Comp. Neurology 529 (9), 2347–2361. doi:10.1002/cne.25099
Schulster, M., Bernie, A. M., and Ramasamy, R. (2016). The role of estradiol in male reproductive function. Asian J. Androl. 18 (3), 435–440. doi:10.4103/1008-682X.173932
Siah, A., Pellerin, J., Benosman, A., Gagné, J. P., and Amiard, J. C. (2002). Seasonal gonad progesterone pattern in the soft-shell clam Mya arenaria. Comp. Biochem. Physiology Part A Mol. Integr. Physiology 132 (2), 499–511. doi:10.1016/s1095-6433(02)00095-8
Sukhan, Z. P., Cho, Y., Sharker, M. R., Hossen, S., Rha, S. J., and Kho, K. H. (2021). Effective accumulative temperature affects gonadal maturation by controlling expression of GnRH, GnRH receptor, serotonin receptor and APGWamide gene in Pacific abalone, Haliotis discus hannai during broodstock conditioning in hatcheries. J. Therm. Biol. 100, 103037. doi:10.1016/j.jtherbio.2021.103037
Thitiphuree, T., Nagasawa, K., and Osada, M. (2019). Molecular identification of steroidogenesis-related genes in scallops and their potential roles in gametogenesis. J. steroid Biochem. Mol. Biol. 186, 22–33. doi:10.1016/j.jsbmb.2018.09.004
Thompson, J. D., Higgins, D. G., and GibsonCLUSTAL, T. J. .W. (1994). Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids Res. 22 (22), 4673–4680. doi:10.1093/nar/22.22.4673
Tinikul, Y., Poljaroen, J., Tinikul, R., Anuracpreeda, P., Chotwiwatthanakun, C., Senin, N., et al. (2014). Effects of gonadotropin-releasing hormones and dopamine on ovarian maturation in the Pacific white shrimp, Litopenaeus vannamei, and their presence in the ovary during ovarian development. Aquaculture 420-421, 79–88. doi:10.1016/j.aquaculture.2013.10.036
Zhang, L., Tello, J. A., Zhang, W., and Tsai, P. S. (2008). Molecular cloning, expression pattern, and immunocytochemical localization of a gonadotropin-releasing hormone-like molecule in the gastropod mollusk, Aplysia californica. General & Comp. Endocrinol. 156 (2), 201–209. doi:10.1016/j.ygcen.2007.11.015
Zhang, M., Wang, Y., Li, Y., Li, W., Li, R., Xie, X., et al. (2018). Identification and characterization of neuropeptides by transcriptome and proteome analyses in a bivalve mollusc Patinopecten yessoensis. Front. Genet. 9, 197. doi:10.3389/fgene.2018.00197
Zhang, M., Wei, H., Liu, T., Li, W., Li, Y., Wang, S., et al. (2020). Potential GnRH and steroidogenesis pathways in the scallop Patinopecten yessoensis. J. Steroid Biochem. Mol. Biol. 204, 105756. doi:10.1016/j.jsbmb.2020.105756
Keywords: Chlamys farreri, GnRH, nerve ganglia, gonadal development, in situ hybridization
Citation: Tang J, Yuan M, Wang J, Li Q, Huang B, Wei L, Liu Y, Han Y, Zhang X, Wang X, Zhang M and Wang X (2023) Identification and characterization of gonadotropin-releasing hormone (GnRH) in Zhikong scallop Chlamys farreri during gonadal development. Front. Physiol. 14:1180725. doi: 10.3389/fphys.2023.1180725
Received: 06 March 2023; Accepted: 16 May 2023;
Published: 31 May 2023.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaReviewed by:
Yang Zhang, Chinese Academy of Sciences (CAS), ChinaQingheng Wang, Guangdong Ocean University, China
Copyright © 2023 Tang, Yuan, Wang, Li, Huang, Wei, Liu, Han, Zhang, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meiwei Zhang, bWVpd3poQDE2My5jb20=; Xiaotong Wang, d2FuZ3hpYW90b25nOTk5QDE2My5jb20=
 Juyan Tang
Juyan Tang Mengqiang Yuan
Mengqiang Yuan Jia Wang2
Jia Wang2 Yaqiong Liu
Yaqiong Liu Meiwei Zhang
Meiwei Zhang Xiaotong Wang
Xiaotong Wang