- 1Educational and Scientific Institute of Neurosurgery, Peoples’ Friendship University of Russia (RUDN University), Moscow, Russian
- 2Academic Health System, Hamad Medical Corporation, Interim Translational Research Institute, Doha, Qatar
- 3Department of Urology, Harbin Medical University Cancer Hospital, Harbin, China
- 4Department of Obstetrics and Gynecology, Tyumen State Medical University, Tyumen, Russia
- 5Department of Internal Diseases, Bashkir State Medical University, Ufa, Russia
- 6Department of Neurosurgery, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
Gastric cancer (GC), being one of the most common malignant human tumors, occupies the second position in the structure of mortality in men and women. High rates of morbidity and mortality in this pathology determine its extremely high clinical and social significance. Diagnosis and timely treatment of precancerous pathology is the main way to reduce morbidity and mortality, and early detection of GC and its adequate treatment improve prognosis. The ability to accurately predict the development of GC and start treatment on time, as well as the ability to determine the stage of the disease if the diagnosis is confirmed - non-invasive biomarkers can become the key to solving these and many other problems of modern medicine. One of the promising biomarkers being studied are non-coding RNAs, namely, miсroRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). They are involved in a wide range of processes, including apoptosis, proliferation, differentiation, angiogenesis, which play a critical role in the development of GC oncogenesis. In addition, they are quite specific and stable due to their carriers (extracellular vesicles or Argonaute 2 protein) and can be detected in various human biological fluids, in particular gastric juice. Thus, miRNAs, lncRNAs, and circRNAs isolated from the gastric juice of GC patients are promising preventive, diagnostic and prognostic non-invasive biomarkers. This review article presents the characteristics of circulating or extracellular miRNAs, lncRNAs, and circRNAs in gastric juice, allowing their use in the GC preventive, diagnosis, prognosis and monitoring therapy.
1 Introduction
Gastric cancer (GC) ranks third in the world among malignant neoplasms and second in the structure of oncological mortality after lung cancer. The prognosis depends on the stage of the disease, where the 5-year survival rate of patients with GC is approximately 25%–30%, but this figure for patients with early GC after surgical treatment reaches 95% (Smyth et al., 2020). Unfortunately, more than 40% of all GC are still diagnosed at an advanced stage. Early GC is only 5%–10% of the total number of patients with GC (Petryszyn et al., 2020). In addition, metastases occur in 80%–90% of patients with GC (Petryszyn et al., 2020). As a rule, in the early stages of GC has unexpressed clinical manifestations and non-specific symptoms, which makes it difficult to accurately diagnose and prescribe appropriate therapy.
Diagnosis and timely treatment of precancerous pathology is the main way to reduce morbidity and mortality, and early detection of GC and its adequate treatment improve prognosis. This is facilitated by the timely implementation of such studies as, first of all, gastroscopy, which is the basis for screening and early detection of the disease, methods of ultrasound diagnostics, including endo-ultrasound during gastroscopy, various X-ray methods of research - computed tomography (CT), magnetic resonance imaging (MRI), and fluoroscopy (Yoon and Kim, 2015). But if gastroscopy reveals the very presence of a tumor, then all other studies determine the prevalence of the tumor process, which is a necessary condition for the correct staging of the disease and the choice of optimal tactics, as well as dynamic monitoring of the patient during and after treatment. However, the listed diagnostic methods do not always allow to accurately determine the prevalence of the process before the start of treatment, as, for example, in the case of initial peritoneal carcinomatosis, which is often an intraoperative finding, not to mention the dynamic observation after the treatment (Bleicher and Lambert, 2021). Currently, surgery or endoscopic resection of the gastric mucosa followed by histological analysis of the biomaterial are considered the most accurate diagnostic methods for GC (Yoon and Kim, 2015). One of the new and promising directions in improving the quality of diagnosis and prognosis of tumors is “liquid biopsy” - the determination of tumor biomarkers in human biological fluids using various modern methods of laboratory diagnostics. However, GC biomarkers such as cancer embryonic antigen (CEA), carbohydrate antigens cancer antigen 19–9 (CA19-9), cancer antigen 72-4 (CA72-4) and carbohydrate antigen 50 (CA50) for serum and CEA, CA19-9 and fetal sulfoglycoprotein antigen for gastric juice have rather low specificity and sensitivity and cannot be used for early diagnosis of GC (Wu et al., 2019). An increase in the level of these biomarkers occurs in only 20% of cases of GC and does not have a clear correlation with the stage of the disease at the time of its diagnosis (Matsuoka and Yashiro, 2018; Wu et al., 2019). Thus, there is a need to search for new, more specific and sensitive biomarkers for screening, early diagnosis, and prognosis of GC.
The characterization of the molecular mechanisms of GC development is the key not only to understanding the pathogenesis, but also to the creation of effective diagnostic and therapeutic approaches. Given the heterogeneity of GC, the complex nature of molecular, cellular and clinical information, it is now known that violations of epigenetic mechanisms have a significant impact on the development of GC (Guo and Yan, 2015). At the same time, non-coding RNAs (ncRNAs) are a type of epigenetic regulators of the expression of oncogenes and tumor suppressor genes (Guo and Yan, 2015). NcRNAs refer to RNAs that do not code for protein. They cover a huge number of RNA classes and perform a wide range of biological functions. One such class of ncRNAs that has been extensively studied are microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) (Guo and Yan, 2015). MiRNAs are endogenous RNAs of 18–22 nucleotides that inhibit gene expression by promoting degradation of their messenger RNA (mRNA) targets or inhibition of translation. It has been proven that miRNAs play an essential role in various biological processes, including the cell cycle, apoptosis, cell proliferation and differentiation, regulating the expression of about one-third of all human genes (Guo and Yan, 2015; Beylerli et al., 2022a).
LncRNAs are a group of ncRNAs longer than 200 nucleotides. Due to their length of nucleotide chains, lncRNAs have the unique ability to take on many complex secondary and tertiary structures, allowing them to perform specific functions such as catalysis, metabolism, and cell differentiation. LncRNAs cannot code for protein, but can modulate gene expression at epigenetic (e.g., DNA methylation and histone modification), transcriptional (e.g., recruitment of transcription factors), and post-transcriptional (e.g., regulation of miRNA and mRNA stability) levels (Guo and Yan, 2015; Ghafouri-Fard et al., 2020; Sufianova et al., 2022). CircRNAs are a class of regulatory ncRNAs with a closed structure of the ribose phosphate backbone. CircRNAs are neither polyadenylated nor capped. They are more stable than linear lncRNAs, making them more promising diagnostic markers and therapeutic agents. Like lncRNAs, circRNAs can interact with other RNAs, DNAs, and proteins and perform various functions in the cell. Many circRNAs contain binding sites for various miRNAs and act as a “sponge”, sorbing these molecules onto themselves (Guo and Yan, 2015; Sekar and Liang, 2019; Beilerli et al., 2022).
An increasing number of studies have demonstrated aberrant expression of a number of miRNAs, lncRNAs, and circRNAs in various types of tumors (Yan and Bu, 2021). The results of some studies have shown that these ncRNAs play a direct role in the development of GC (Xie et al., 2020). In addition, many miRNAs, lncRNAs, and circRNAs are found in the biological fluids of patients with GC: in whole blood, plasma/serum, and gastric juice. NcRNAs are present in the cell, in the cytoplasm, as well as in the nucleus, and are also secreted outside the cell via extracellular vesicles (EVs) (exosomes and microvesicles), which protects them from degradation. Thus, it is already known that the so-called circulating (cell-free or extracellular) miRNAs, lncRNAs and circRNAs are highly stable in biological fluids (including gastric juice), resistant to endogenous ribonucleases and adverse physical conditions, which allows them to be efficiently isolated from biological fluids, where their amount can be measured with high sensitivity and specificity using real-time PCR, DNA microarrays and RNA-sequencing (RNA-seq) methods (Gareev et al., 2020; Beylerli et al., 2022b). Thus, these ncRNAs have great potential for use in clinical practice as non-invasive biomarkers for screening, early diagnosis, prediction, and monitoring of the effectiveness of therapy for patients with GC. In this review paper, we will pay attention to cell-free miRNAs, lncRNAs and circRNAs circulating in the gastric juice, which can be used as biomarkers for minimally invasive diagnosis of GC, prognosis of the course of the disease, and evaluation of the effectiveness of therapy.
2 The choice of biological fluid
Since blood vessels permeate all tissues of the body, it is logical to assume that blood is the default source of various biomarkers, including extracellular ncRNAs, but the link between test samples and disease may be more important. So, in diseases of the central nervous system (CNS), you can use cerebrospinal fluid, blood is ideal for cardiovascular diseases, urine - for metabolic diseases, diseases of the liver or kidneys. In diseases of the gastrointestinal tract, including GC, gastric juice or saliva can be used as a source of biomarkers, in diseases of the lungs, saliva, sputum, or even exhaled air vapor (Aronson and Ferner, 2017). For diseases localized in a particular place, tissue or tissue fluid can be used. However, sometimes samples obtained from foci distant from the sources of the disease may also contain suitable biomarkers. There are a significant number of studies in which extracellular miRNAs, lncRNAs and circRNAs have been studied as biomarkers in GC. In all these studies, despite the difference in the choice of ncRNAs and in the search medium (whole blood, serum or plasma and gastric juice), the possibility of using ncRNAs in gastric juice as stable, sufficiently sensitive and specific biomarkers of the development of the tumor process has been shown.
Blood (plasma and serum) is one of the available biological fluids for profiling the expression of circulating ncRNAs in patients with tumors. Blood plasma and serum turned out to be informative for profiling the expression of circulating ncRNAs for the purpose of diagnosing, predicting, assessing response to therapy and tumor recurrence, as well as identifying emerging resistance to therapy in various human tumors, including GC (Jelski and Mroczko, 2022; Mugoni et al., 2022; Powrózek and Ochieng Otieno, 2022). The detection of ncRNAs in plasma samples without measurable circulating tumor cells suggests that circulating miRNAs, lncRNAs and circRNAs can provide useful information about tumors, regardless of the presence of circulating tumor cells.
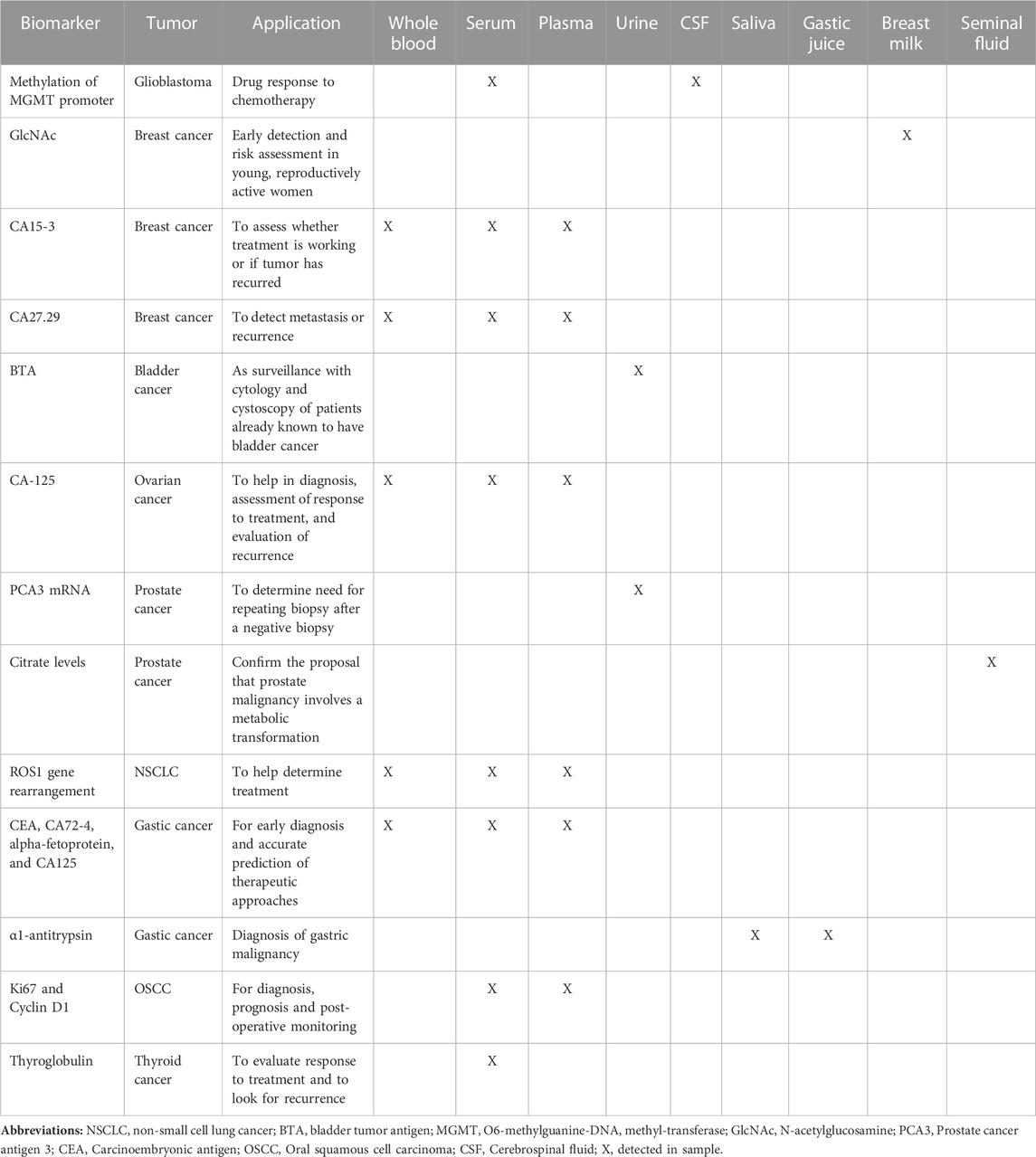
As described above, EVs are nanoparticles enclosed in membranes that are released from living tumor cells either as a result of fusion of the endosome with the plasma membrane (exosomes) or directly from the cell membrane-microvesicles (Abels and Breakefield, 2016). EVs are carriers of communication between different tumor compartments and its microenvironment, since other tumor cells and normal cells absorb them. It is important to note that EVs, which can be isolated from both blood and gastric juices, are a rich source of oncogenesis molecules such as ncRNA, since the structure of EVs protects them from nucleases, proteases, and the acidic environment of gastric juice (Kagota et al., 2019; Urabe et al., 2020). Unlike blood, gastric juice is in direct contact with the stomach and is a suitable source of biomarkers for gastric cancer. Gastric juice seems to be a very suitable source of EVs for solving the problem of finding non-invasive GC biomarkers, since the expected proportion of EVs originating from tumor cells and cells of the microenvironment should be significantly higher in this body fluid compared to the bloodstream (Skryabin et al., 2022). In addition, unlike blood, gastric juice should not contain ribonucleoproteins and lipoprotein complexes, which almost inevitably contaminate with EV (Skryabin et al., 2022). Surprisingly, gastric juice -derived EVs have barely been explored so far, except for a few studies (Kagota et al., 2019; Zeng et al., 2019; Tanaka et al., 2020; Skryabin et al., 2022). In addition, gastric juice is easier and safer to obtain than tumor tissue from a biopsy. Based on these arguments, this review paper will consider gastric juice miRNAs, lncRNAs and circRNAs in GC. For instance, the Table 1 provides some information on biomarkers and priority biological fluids for a range of tumors (Saijo, 2012; Crawford et al., 2014; Günther, 2015; Hayes, 2015; Sims et al., 2015; Wu and Qu, 2015; Zhou et al., 2015; Usman et al., 2023).
3 Extracellular miRNAs, lncRNAs and circRNAs as biomarkers and their advantages
The ability to accurately predict the development of GC and start treatment on time, the ability to choose therapy depending on the individual characteristics of the patient, the possibility of early diagnosis of the disease and determining the stage - biomarkers can become the key to solving these and many other problems of modern oncology. A biomarker is defined as a quantitatively and objectively measurable indicator of a biological, pathogenic process or pharmacological response to therapy and is used for a number of indicators (Figure 1) (Crowley et al., 2013; Wu and Qu, 2015; Aronson and Ferner, 2017).
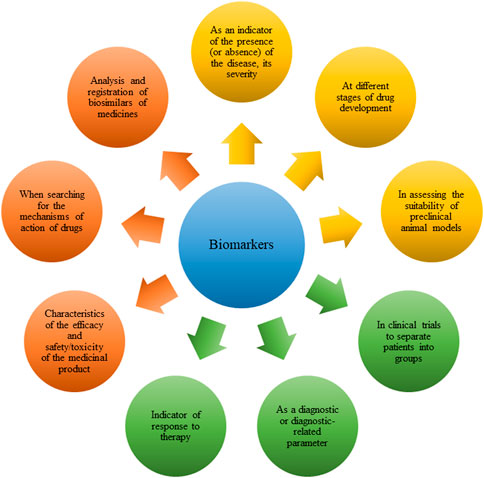
FIGURE 1. Indications for the use of biomarkers in various diseases, including in gastric cancer (GC).
Possessing most of the properties of ideal biomarkers, including resistance to non-nucleases, a unique nucleotide sequence, tissue specificity, minimally invasive and generally available sampling, relative stability at room temperature and repeated freeze/thaw cycles of blood samples, cell-free miRNAs, lncRNAs and circRNAs are deservedly considered in as promising biomarkers. However, to date, a consensus on the origin and biological functions of extracellular miRNAs, lncRNAs and circRNAs has not been formed. This may be due to the fact that the forms of extracellular existence of miRNAs, lncRNAs and circRNAs are inhomogeneous and differ in the way they are packaged. More information is needed on the mechanisms and causes of ncRNAs release from cells, which will be revealed first of all by analyzing the correlations between circulating and tissue concentrations of miRNAs, lncRNAs and circRNAs (Gareev et al., 2020; Szilágyi et al., 2020). However, three secretion pathways for cell-free miRNAs, lncRNAs and circRNAs are currently known: 1) passive secretion from damaged cells due to apoptosis or necrosis; 2) active secretion with the help of explosives, including exosomes and microvesicles, and as part of high density lipoproteins (HDL); and 3) active secretion of circulating miRNAs via an RNA-binding protein-dependent pathway (miRNA- Aurgonaute 2 (Ago2) complex) (Figure 2) (Turchinovich et al., 2011; Ding et al., 2018; Szilágyi et al., 2020; Zhao et al., 2020).
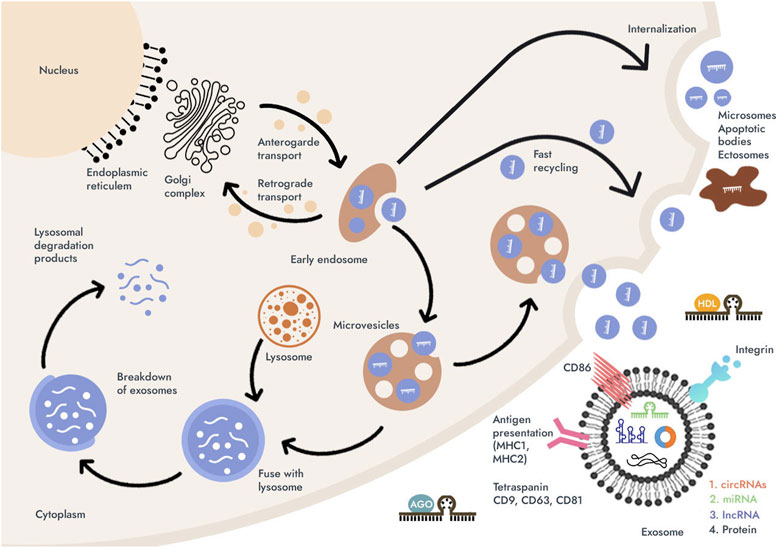
FIGURE 2. Possible pathways for the secretion of microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) into the biological fluids (blood or gastric juice) and their vehicles. MiRNAs, lncRNAs, and circRNAs can be secreted by the cell as microscopic extracellular vesicles (EVs) (exosomes and microvesicles) or released as part of apoptotic bodies; miRNAs can be found associated with high-density lipoproteins (HDL) and mostly in the form of complexes with Argonauts proteins (with Ago2). Then, regardless of the forms of miRNAs, lncRNAs and circRNAs pass from the extracellular space into the biological fluid. About 90% of circulating miRNAs are in a non-vesicular form, namely, they are associated with Ago2. In addition to miRNAs, lncRNAs and circRNAs, exosomes contain a diverse array of molecules such as translation factors, metabolic enzymes, apoptotic proteins (Alix), proteolytic proteins chaperones, and contain other types of nucleic acids such as mRNAs. MiRNAs, lncRNAs, and circRNAs account for almost 50% of the total vesicular RNA.
Exosomes are small membrane-bound vesicles of endosomal origin with a diameter of 30–100 nm. They are secreted by different types of cells: normal or pathological. Previously, exosomes for the cell were considered as a way to get rid of unnecessary “garbage”, for example, “obsolete” proteins. However, it has now been proven that exosomes are more than just “garbage bins” and take a significant part in intercellular communication (Kalluri and LeBleu, 2020). Exosomes, like microvesicles, are used by cells to transfer information (mRNA, viruses, proteins, miRNAs, lncRNAs and circRNAs) from 1 cell to another. Tumor cells play a particularly important role in the production of exosomes. Exosomes of tumor cells are able to remotely participate in the formation of “niches” (Zhang and Yu, 2019). In addition to vesicular forms of extracellular miRNAs, long noncoding RNAs, and circular RNAs, apoptotic bodies or cell masses 1–4 nm in size after necrotic processes, which also contain ncRNA data, can be found in biological fluids cell-free miRNAs, lncRNAs and circRNAs can also be secreted outside the vesicles. About 90% of circulating miRNAs are in a non-vesicular form, namely, they are associated with Ago2 proteins. It is reported that HDL are involved in the mechanism of intercellular communication and are involved in the transport and delivery of ncRNA (Turchinovich et al., 2011; Sims et al., 2015; Xu et al., 2022). However, the origin of the lipoprotein fraction of circulating miRNAs, lncRNAs and circRNAs is still in question.
3.1 Preventive (screening) diagnostic
There is no evidence for the efficacy of screening for GC based on randomized controlled trials. The only country that screens for stomach cancer is Japan, due to the high incidence of this form of cancer. The survey increases the early diagnosis of GC by 30%–40% (Mabe et al., 2022). In European countries, screening is not practiced because of the high cost, in the United States - because of the low incidence. In the United Kingdom, only patients with chronic stomach conditions are screened (Crew and Neugut, 2006). Early diagnosis of gastric cancer leads to more effective therapy, resulting in an increase in the duration and quality of life of patients. Therefore, the search for reliable and accurate ways to predict the malignancy of normal stomach cells into cancer cells is an acute problem to this day.
As already mentioned, the use of liquid biopsy based on the detection of specific molecules in human biological fluids for malignancy processes is an important direction in the field of preventive diagnosis of GC. Protein molecules as biomarkers are sensitive indicators of normal biological, pathogenic processes, including oncogenesis, or response to a specific intervention or therapy. Although circulating CEA, CA19.9, and CA72.4 have notable predictive value for predicting GC recurrence and metastases after surgery and chemotherapy, as of 2017, none of these tumor antigens had significant value for gastric screening (Wu et al., 2019; de Mello et al., 2021). In particular, circulating ncRNAs in plasma or serum have been found to be extremely stable after long-term storage or repeated freeze-thaw cycles and can be reliably studied in both fresh and stored samples (Vogiatzi et al., 2018). The main reasons for this stability are their associations with protein complexes or small membrane vesicles known as exosomes or microvesicles (Gareev et al., 2020).
In addition, circulating miRNAs, lncRNAs and circRNAs have advantages over protein-based biomarkers because they are less complex, are found in different tissues or biological media, and can be easily identified using common laboratory methods. Circulating miRNAs, lncRNAs and circRNAs can be detected at the early stages of oncogenesis, when protein tumor markers are already active at the active stage of tumor development and progression. That is, aberrant expression of extracellular ncRNAs indicates the disease even before the manifestation of the clinical picture (in the latent period), and the profile may vary depending on the degree and severity of the disease, which is especially important for determining the stage of oncological disease (Schwarzenbach et al., 2011; Sims et al., 2015; Diez-Fraile et al., 2020; Gareev et al., 2020). All this makes them potentially useful as non-invasive biomarkers for use in screening studies for GC.
3.2 Diagnostic value
One of the main factors determining the tactics of managing patients with primary or metastatic GC is the determination of the type of tumor. The type of GC is usually identified on the basis of studies of the tumor tissue (biopsy) performed during gastroscopy, and the subsequent decision on the type of treatment depends on the results of the biopsy (Yoon and Kim, 2015). Even for those patients who are suspected to have benign gastric tumors, tissue biopsy is currently required to establish an accurate diagnosis and plan further care. The ability to distinguish benign gastric tumors from GC in real time by analyzing biomarkers based on gastric juice will help plan the course of treatment (e.g., use neoadjuvant therapy). In addition, in the modern age of using molecular parameters in the classification of GC, certain circulating ncRNAs as biomarkers with predictive value can help in planning operations, decision making during surgery and enrollment in clinical trials (Choi et al., 2019).
3.3 Detection of recurrence
Monitoring the risk of recurrence in the immediate or long term after surgical, radio- and chemotherapeutic treatment of GC is a difficult task (Foller et al., 2018). Due to the specialist’s inability to distinguish pseudoprogression from recurrence using imaging studies, a number of patients without true GC recurrence undergo potentially unnecessary surgical procedures, such as repeat tissue biopsy, to confirm tumor recurrence, which (surgical manipulation) may have a high sampling error rate. It is assumed that changes in the expression level of circulating ncRNAs can correlate with tumor burden. In this scenario, it is reasonable to assume that the progression of GC will lead to an increase in the level of expression of certain circulating miRNAs, lncRNAs and circRNAs in GC patients, and this will help to identify the progression already at the molecular level (Zhang et al., 2019a).
3.4 Monitoring response to therapy
Of the instrumental methods for examining GC, X-ray and endoscopic methods are mainly used, where these methods often do not provide convincing evidence that allows one to observe the effectiveness of the response to tumor treatment (Yoon and Kim, 2015). As mentioned earlier, CEA, CA19.9, and CA72.4 are biomarkers for several tumors, including GC, which are found in plasma or serum; however, none of them was effective in monitoring the quality of therapy (de Mello et al., 2021). The lack of sensitive and specific biomarkers to assess response to treatment in gastric cancer patients makes it difficult to develop new therapeutic agents (Wu and Qu, 2015). Future studies should aim to fill this knowledge gap by systematically evaluating gastric juice samples for circulating ncRNAs and correlating the expression level of specific circulating ncRNAs with tumor volume as determined by radiological and endoscopic techniques.
4 Gastric juice miRNAs and GC
Analysis of the expression of extracellular miRNAs in gastric juice can face a number of problems. Gastric juice is a mixture consisting mainly of proteins and hydrochloride (HCl). Chen et al. demonstrated in their work that microRNAs (e.g., miR-25, miR-223, and let-7a) of serum and plasma treated for 3 h with exogenous HCl solution remain stable. In doing so, they examined the stability of circulating miRNAs under conditions of boiling, low/high pH, and freeze-thaw cycles; this indicates that miRNAs in gastric juice are stable and can be used as new biomarkers for GC (Chen et al., 2008). In another study, Cui et al. observed that the real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) method for detecting extracellular miRNAs in gastric juice was satisfactorily reproducible, and sequencing results confirmed the stability of miRNAs in gastric juice (Cui et al., 2013). In addition, they observed significantly lower expression levels of circulating miR-21 and miR-106a in the gastric juice of patients with GC compared to the gastric juice of patients with benign gastric diseases. However, expression levels of circulating miR-21 and miR-106a have been reported to be elevated in the plasma and tissues of GC patients, as confirmed by gastric mucosal biopsy. The results of this study are indeed noteworthy. Therefore, in the continuation of this study, the authors found that circulating miR-21 and miR-106a based on gastric juice have a more reliable diagnostic value compared to circulating miR-21 and miR-106a based on blood, where the receiver operating characteristic (AUC) reached 0.96 and 0.87, respectively. Shao et al. also demonstrated that cell-free miR-133a expression levels in gastric juice were significantly lower in GC patients than in healthy individuals and those with benign gastric disease, in particular demonstrating superior diagnostic value, namely, with an AUC of 0.90 and a sensitivity of 85 .9% and a specificity of 84.8%, respectively (Shao et al., 2016).
Several other studies have also shown aberrant expression of a number of other circulating miRNAs, such as miR-129-1-3p, miR-129-2-3p and miR-421, in gastric juice, which may also be good candidates as non-invasive biomarkers in GC (Zhang et al., 2012; Yu et al., 2013). After all, there is evidence that some of these circulating miRNAs, like miR-421, are involved in oncogenic activity, and the authors found them in low amounts in GC patients (Ge et al., 2016; Yang et al., 2017; Jingyue et al., 2019). There are suggestions that these miRNAs may be involved in the process of crosstalk in the tumor microenvironment by being secreted into the gastric juice. All authors who have studied gastric juice miRNAs have come to the conclusion that clinicians can easily obtain samples of gastric juice using a string test that is both economical and acceptable to patients. Thus, for the diagnosis of GC, the use of circulating miRNAs in gastric juice has obvious advantages, since gastric juice is present in direct contact with GC. Table 2 summarizes the results of studies demonstrating the potential of gastric juice miRNAs as non-invasive biomarkers in GC (Zhang et al., 2012; Cui et al., 2013; Yu et al., 2013; Shao et al., 2016; Skryabin et al., 2022)
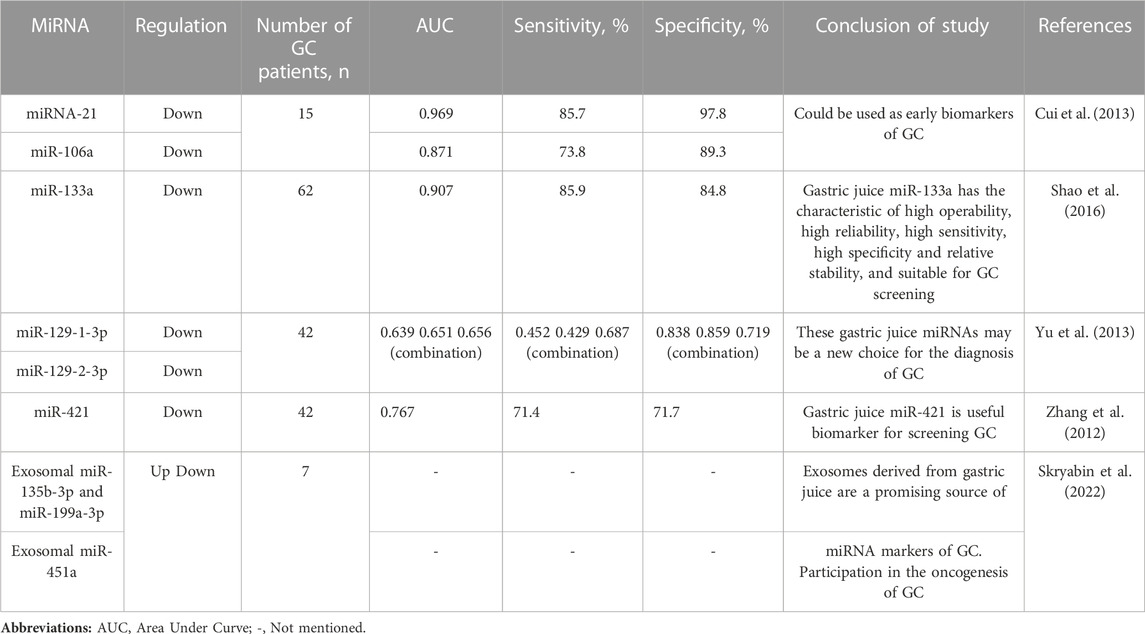
TABLE 2. Summarized several microRNAs (miRNAs) presenting modified circulating expression in gastric juice of gastric cancer (GC) patients compared to healthy controls or benign gastric diseases.
5 Gastric juice lncRNAs and GC
LncRNAs are an important group of ncRNAs ranging in length from several hundred to several thousand nucleotides. Genetic changes and aberrant lncRNA expression may be a factor in the development of cancer. Several lncRNAs, such as H19 and LINC00152, correlate with progression and metastases of GC (Li et al., 2014; Wang et al., 2021). Li et al. demonstrated that H19 was significantly increased in GC tissues than in noncancerous tissues, and H19 expression was positively correlated with lymph node metastases (LNM) and clinical stage (Li et al., 2014). In addition, the oncogenic effect of H19 in GC is mediated by direct activation of ISM1 and indirect downregulation of CALN1 expression via miP-675. In other study, Wang et al., demonstrated that LINC00152 expression is upregulated in GC and positively correlated with GC progression (Wang et al., 2021). Moreover, LINC00152 knockdown plays an anti-tumor role in GC by targeting miR-138/SIRT2 axis.
Cell-free lncRNAs have great potential to be considered as non-invasive biomarkers. Because they are more stable, have high tissue/cell specificity, and are readily detectable in body fluids (e.g., gastric juice) (Szilágyi et al., 2020). In addition, there is increasing evidence that aberrant expression of circulating lncRNAs is of clinical importance in the diagnosis and prognosis of GC. As a new diagnostic and prognostic non-invasive biomarker in gastric juice of GC patients, circulating H19, LINC00152 and others are already being studied. For instance, Chen et al. showed that H19 expression was significantly higher in GC tissues compared with adjacent normal tissues (Chen et al., 2016). Moreover, the authors demonstrated that the high H19 group GC patients showed higher invasion depth, advanced TNM (tumor, nodus and metastases) stage and regional lymph nodes metastases than the lower H19 expression group GC patients. In this study, GC patients with a high expression of H19 seemed to have shorter overall survival (OS) and disease-free survival (DFS) than GC patients with lower levels. In addition, the authors found that H19 levels in gastric juice from patients with GC were significantly higher than those from normal subjects. In this study, the authors suggested that H19 might be useful as a diagnostic and prognostic biomarker for GC and might be a possible will be as non-invasive biomarker based gastric juice. However, further studies will be needed to confirm these preliminary results. Pang et al., showed that the expression level of LINC00152 in GC tissues was significantly higher than that in matched paracancerous tissues (Pang et al., 2014). Increased expression levels of LINC00152 was significantly associated with the depth of GC invasion. In addition, the results showed that cell-free LINC00152 levels in gastric juice from GC patients were significantly higher than those from normal controls. Circulating lncRNAs that have been linked to gastric juice in GC are listed in Table 3 (Pang et al., 2014; Shao et al., 2014; Chen et al., 2016; Fei et al., 2016; Yang et al., 2016; Yuan et al., 2016).
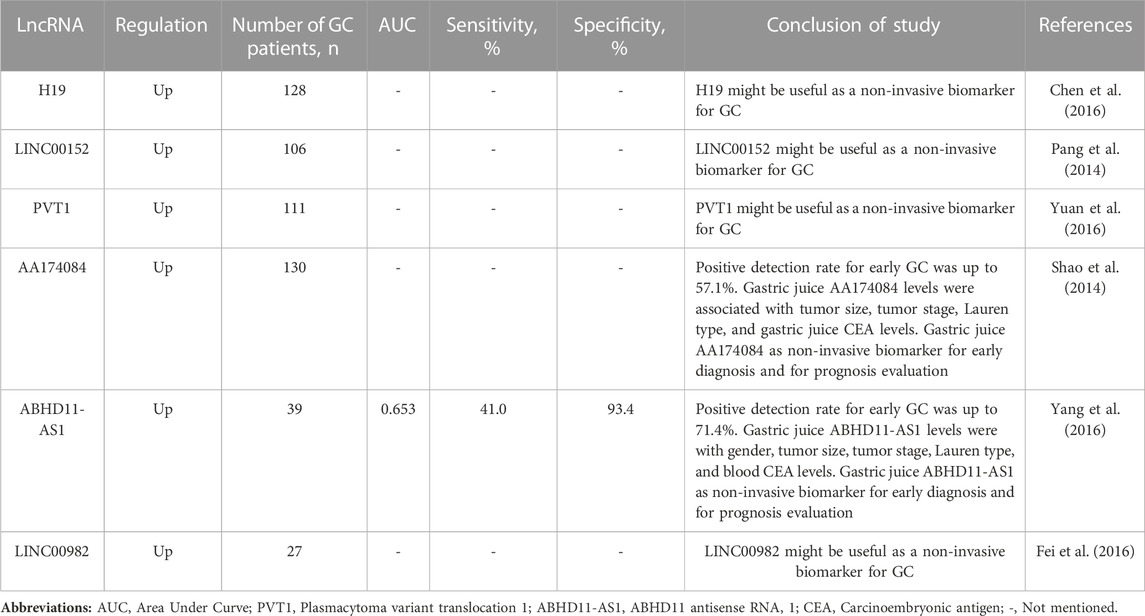
TABLE 3. Summarized several long non-coding RNAs (lncRNAs) presenting modified circulating expression in gastric juice of gastric cancer (GC) patients compared to healthy controls or benign gastric diseases.
6 Gastric juice circRNAs and GC
Thanks to the achievements of bioinformatics and RNA-seq methods, it can be argued that the circRNAs deserve recognition of the “new players” in the regulation of genes expression. However, the functional significance of the circRNAs is still far from understanding compared to progress achieved in the field of other ncRNAs such as miRNAs and lncRNAs. The vast majority of the circRNAs has not yet been studied, and their functions remain unknown. In addition, in a number of works it is reported that the same circRNAs are involved in various processes of GC progression (Shan et al., 2019). Secondly, the strategies that can be used to deeply study the gastric juice circRNAs for GC are not sufficiently developed. For instance, Shao et al., used microarray method to investigate the differential expression profiles of circRNAs between GC tissues and paired noncancerous tissues (Shao et al., 2017). They identified 20 top differentially expressed circRNAs in GC tissue, where ten upregulated were hsa_circ_0035445, hsa_circ_0003789, hsa_circ_0063809, hsa_circ_0074362, hsa_circ_0006282, hsa_circ_0011107, hsa_circ_0084606, hsa_circ_0005556, hsa_circ_0050547, and hsa_circ_0006470, while the top ten downregulated ones were hsa_circ_0007099, hsa_circ_0001897, hsa_circ_0007707, hsa_circ_0008832, hsa_circ_0001546, hsa_circ_0002089, hsa_circ_0004680, hsa_circ_0000154, hsa_circ_0004458, and hsa_circ_0008394. Further, using the qRT-PCR, the authors identified the specific gastric juice hsa_circ_0014717could be used as a biomarker for screening GC patients. In results, they showed that cell-free hsa_circ 0014717 can stably exist in human gastric juice, and cell-free hsa_circ 0014717 has the potential to be used as biomarkers for the screening of people with high-risk of GC. In other study, almost the same team led by Shao Y. detected and compared gastric juice hsa_circ_0065149 levels in healthy volunteers, gastric ulcer patients, chronic atrophic gastritis patients, and GC patients (Shao et al., 2020). Unexpectedly, maybe due to small number of samples tested, hsa_circ_0065149 levels have no significant difference among four groups. However, the expression levels of exosomal hsa_circ_0065149 in plasma from patents with early GC were significantly decreased than those from healthy control. Moreover, it should be noted that the levels of exosomal hsa_circ_0065149 in gastric juice do not correspond to its levels in GC tissues and levels of exosomal hsa_circ_0065149 in plasma. The authors conclude that this opposite trend may be related to the function of exosomes; and the mechanism of selective change hsa_circ_0065149 in various body fluids needs further study. Song et al., showed that the hsa_circ_000780 levels were significantly decreased in the gastric juice of the GC group patients. However, no significant difference in gastric juice hsa_circ_000780 levels was found between the advanced GC and early GC groups or between the chronic non-atrophic gastritis and chronic atrophic gastritis groups (Song et al., 2021). This finding indicates that cell-free hsa_circ_000780 could be detected in the gastric juice, and has the potential for use as a non-invasive biomarker for early GC screening.
Regarding the use of extracellular circRNAs based on gastric juice as non-invasive biomarkers GC, we come to the conclusion that this area of study is still in its infancy. The studies are represented by a small sample size, the absence of independent comparison groups and a limited analysis of the correlation with the characteristics of the disease.
7 Potential non-invasive biomarkers
Gastric juice can be a good source of circulating ncRNAs as biomarkers for GC, since it is directly secreted by cells without elimination by the liver. Nevertheless, today there are not so many papers that have studied circulating ncRNAs as non-invasive biomarkers based on gastric juice with GC. Therefore, there is a reason to consider the most studied/studied miRNAs, lncRNAs, and circRNAs which directly interpret in the oncogenesis of GC, and which can be directly considered in future studies on the study of non-invasive biomarkers based on gastric juice for GC screening, diagnosis and prognosis (Figure 3).
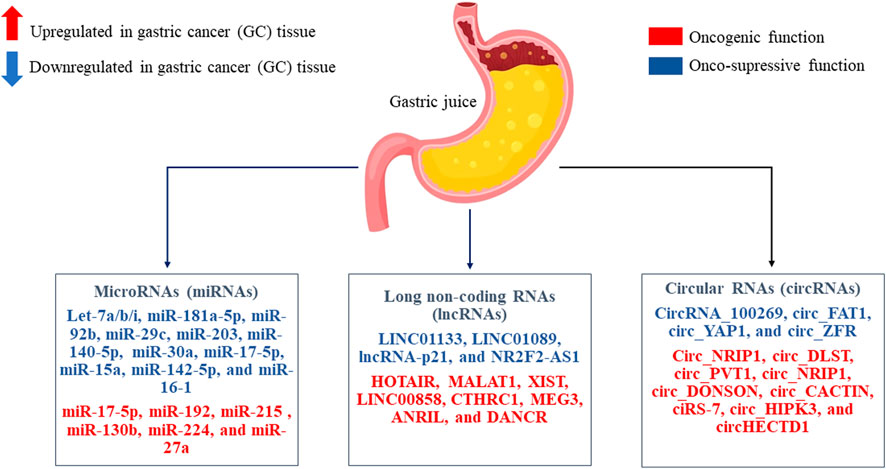
FIGURE 3. The most studied/studied microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) in the oncogenesis of gastric cancer (GC) tissue are presented. These non-coding RNAs (ncRNAs) that play a role as onco-suppressors or oncogenic role. Perhaps it will be possible to consider them as non-invasive biomarkers based on gastric juice in GC with further study.
Tumor-suppressive let-7 family is generally downregulated in GC tissues and inhibit GC formation or metastases by attenuating some oncogenes. For instance, let-7a expression is significantly reduced in GC tissues, and its onco-suppressive function is associated with inhibition of GC cell differentiation. In addition, upregulation of let-7a leads to inhibition of GC cell proliferation and GC growth due to a decrease in the expression level of RAB40C (Yang et al., 2011). In addition, let-7a is able to induce cellular autophagy by inhibiting rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) expression in GC cells (Fan et al., 2018). Another member of the let-7 family, let-7b is also downregulated in GC tissues, and its suppression is negatively correlated with poor survival and LNM in GC patients (Motoyama et al., 2008). Overexpression of let-7b suppresses the invasion and migration of GC cells by reducing the expression of inhibitor of growth protein 1 (ING1) (Han et al., 2015). Let-7b also attenuates GC growth and resistance of GC cells to cisplatin by inhibiting Aurora kinase B (AURKB) (Han et al., 2018). In addition, let-7i acts as a tumor suppressor, preventing invasion and metastases of GC cells, promoting the inactivation of collagen, type I, alpha 1 (COL1A1) (Shi et al., 2019).
HOX antisense intergenic RNA (HOTAIR) is known to be an oncogenic lncRNA in GC. Increased HOTAIR expression is highly correlated with LNM, TNM staging, and poor overall survival in patients with GC (Liu et al., 2016). HOTAIR enhances the invasiveness and process of epithelial-mesenchymal transition (EMT) of GC cells, as well as the expression of matrix metallopeptidase-1 (MMP-1) and matrix metallopeptidase-3 (MMP-3) (Xu et al., 2013). Or metastases-associated lung adenocarcinoma transcript 1 (MALAT1) is also oncogenic lncRNAs. It is known that its overexpression correlates with a poor prognosis in patients with GC (Xu et al., 2021). MALAT1 is involved in the direct progression and metastases of GC by activating E-cadherin/β-catenin, extracellular signal-regulated kinases (ERK)/MMP and focal adhesion kinase (FAK)/paxillin signaling pathways (Li et al., 2017).
CircNRIP1 and circDLST, whose expression is elevated in GC tissues, are termed as oncogenic circRNAs, which promote GC progression and metastases. A decrease in circDLST expression inhibits cell viability, invasion, and liver metastases of GC (Chen et al., 2020). CircDLST has been shown to activate the neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS)/MEK1/Ras-dependent extracellular signal-regulated kinase 1/2 (ERK 1/2) signaling pathway, acting as a miR-502–3p sponge in GC cells (Zhang et al., 2019b). Although the function of the circDLST/miR-502–3p axis in the progression of GC has been determined, evidence for the presence of the NRAS/MEK1/ERK1/2 signaling pathway in this role is still insufficient. In a recent study, it was found that circNRIP1 is significantly upregulated in human GC tissues and can successfully inactivate the onco-suppressive miR-149–5p, promoting proliferation, migration, and invasion of GC cells (Zhang et al., 2019a). In addition, inhibition of circNRIP1 expression can block the malignant behavior of GC cells through a RAC-alpha serine/threonine-protein kinase 1 (AKT1)/mammalian target of rapamycin (mTOR) signaling pathway.
8 Author opinions
As can be seen from this work of the currently available literature, liquid biopsy is a promising direction in the diagnosis and dynamic monitoring of cancer patients. In the case of GC, when histological material sampling can be performed during routine studies (gastroscopy), it is most likely that liquid biopsy for primary diagnosis is unlikely to find its distribution. Although, if a highly sensitive and specific biomarker common to a particular nosology is detected, as in the case of ncRNAs, it can be used for screening in the early stages of GC, which may be more convenient for the patient than other diagnostic methods.
However, the main application of liquid biopsy is seen in predicting the aggressiveness of the tumor process. For example, when aberrant miRNAs, lncRNAs or circRNAs expression in tissue or biofluids is detected, it may indicate high GC aggression, even in the absence of distant metastases. Thus, dynamic monitoring of patients with gastric cancer becomes more effective if a relapse of the disease is suspected much earlier than it can be detected by modern instrumental research methods, which will allow timely prescribing or changing the necessary therapy. It is possible to control the effectiveness of chemotherapy treatment, both conventional regimens and targeted therapy, which would allow changing the drug or treatment regimen at the right time.
Applicable to GC, the detection of extracellular ncRNAs could play an important role in the dynamic monitoring of patients undergoing radical surgery, due to the rather aggressive course of this disease even at its early stages, the high frequency of relapses and progression, and the low survival rate of these patients. For example, it is known that the “gold standard” for screening and diagnosing prostate cancer (PC) is the determination of the level of prostate-specific antigen (PSA). However, PSA is not a tumor-specific marker, but an organ-specific one: an increase in PSA is caused not only by PC, but also by benign prostatic hyperplasia and chronic prostatitis. Prostate cancer specific antigen 3 (PCA3) is a lncRNA that is specific to the prostate. Increased expression of PCA3 is noted in prostate cancer (in 95% of all cases of PC) (Gareev et al., 2021). PCA3 expression is superior to total PSA and percent free PSA in determining PC in patients with elevated PSA, as PCA3 has a significantly higher AUC (Gareev et al., 2021). PCA3 testing is most useful in determining the need for repeat biopsy in patients with negative histological findings from primary biopsy specimens. In other words, the PSA-3 test helps to detect PC already in routine clinical practice at different stages - from early to progressive. This confirms the future potential of using ncRNA in clinical practice for GC patients.
One of the key issues in the field of research on extracellular ncRNAs as non-invasive biomarkers is the choice of priority biological fluid (whole blood, plasma or serum, and gastric juice), and it must be addressed in order to maximize the potential of circulating miRNAs, lncRNAs and circRNAs for prevention (screening), real-time diagnostics, prognosis and tracking the effectiveness of therapy in GC patients. Venipuncture is a minimally invasive, simple and affordable method for obtaining biomaterial, and therefore the study and detection of biomarkers in the blood are of great interest. However, gastric juice obtained by gastroscopy is in direct contact with tumor tissue, and we suggest that circulating ncRNAs obtained from it can serve as more reliable biomarkers of GC. In addition, the change in the expression profile of circulating miRNAs, lncRNAs and circRNAs in gastric juice and blood in the same GC patient is unique, since gastric juice probably reflects local events of damaged gastric mucosa compared to blood flow. In addition, a specific panel of circulating ncRNAs in gastric juice can help distinguish GC from precancerous diseases of the gastric, which can be indispensable during screening examinations.
Nevertheless, despite the availability of various modern and sufficiently accurate methods of liquid biopsy, only a few are used in clinical practice and for a limited number of pathologies. Perhaps this is due to a large number of techniques, the lack of common markers for a specific pathology, the complexity of performing research and the economic component of the issue. Therefore, new methods for determining circulating miRNAs, lncRNAs and circRNAs in gastric juice should be developed, and the targets for their search should be standardized for more sensitive and specific diagnostics, which will undoubtedly be carried out in the near future due to the great promise in GC.
9 Conclusion
It is no longer a secret that miRNAs, lncRNAs and circRNAs are involved not only in physiological processes in the human body, but also interacting with each other, participate in the proliferation of tumor cells, in the control of the cell cycle and apoptosis, migration, invasion and angiogenesis, affecting numerous target genes. These ncRNAs are also involved in the regulation of malignancy and differentiation of normal gastric cells into cancer cells, thus showing that the aberrant expression of some miRNAs, lncRNAs and circRNAs correlates with the clinical prognosis of GC. The use of gastric juice miRNAs, lncRNAs and circRNAs as biomarkers for GC will be ideal when methods for profiling the expression of circulating ncRNAs become sufficiently sensitive and specific for quantification with small sample volumes. Despite advances in expression profiling technology for gastric juice miRNAs, lncRNAs and circRNAs, their use as biomarkers has some limitations. One of them is the lack of proper protocols for handling and storing specimens in the clinical setting. Limited knowledge of environmental factors that may influence the expression of circulating miRNAs, lncRNAs and circRNAs in GC patients may also limit clinical use.
Author contributions
IG: conceptualization, writing—original draft, and supervision. OB: writing—review and editing, investigation, project administration, and resources. AA: formal analysis and methodology. JW, AB, and TI: data curation. AS: validation, visualization, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bashkir State Medical University Strategic Academic Leadership Program (PRIORITY-2030).
Conflict of interest
Author AA was employed by the Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abels, E. R., and Breakefield, X. O. (2016). Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol. Neurobiol. 36 (3), 301–312. doi:10.1007/s10571-016-0366-z
Aronson, J. K., and Ferner, R. E. (2017). Biomarkers-A general review. Curr. Protoc. Pharmacol. 76, 9.23.1–9.23.17. doi:10.1002/cpph.19
Beilerli, A., Gareev, I., Beylerli, O., Yang, G., Pavlov, V., Aliev, G., et al. (2022). Circular RNAs as biomarkers and therapeutic targets in cancer. Semin. Cancer Biol. 83, 242–252. doi:10.1016/j.semcancer.2020.12.026
Beylerli, O., Gareev, I., Sufianov, A., Ilyasova, T., and Guang, Y. (2022). Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res. 7 (2), 66–70. doi:10.1016/j.ncrna.2022.02.004
Beylerli, O., Sufianova, G., Shumadalova, A., Zhang, D., and Gareev, I. (2022). MicroRNAs-mediated regulation of glucose transporter (GLUT) expression in glioblastoma. Noncoding RNA Res. 7 (4), 205–211. doi:10.1016/j.ncrna.2022.09.001
Bleicher, J., and Lambert, L. A. (2021). A palliative approach to management of peritoneal carcinomatosis and malignant ascites. Surg. Oncol. Clin. N. Am. 30 (3), 475–490. doi:10.1016/j.soc.2021.02.004
Chen, H., Liang, C., Wang, X., Liu, Y., Yang, Z., Shen, M., et al. (2020). The prognostic value of circRNAs for gastric cancer: A systematic review and meta-analysis. Cancer Med. 9 (23), 9096–9106. doi:10.1002/cam4.3497
Chen, J. S., Wang, Y. F., Zhang, X. Q., Lv, J. M., Li, Y., Liu, X. X., et al. (2016). H19 serves as a diagnostic biomarker and up-regulation of H19 expression contributes to poor prognosis in patients with gastric cancer. Neoplasma 63 (2), 223–230. doi:10.4149/207_150821N454
Chen, X., Ba, Y., Ma, L., Cai, X., Yin, Y., Wang, K., et al. (2008). Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 18 (10), 997–1006. doi:10.1038/cr.2008.282
Choi, R. S., Lai, W. Y. X., Lee, L. T. C., Wong, W. L. C., Pei, X. M., Tsang, H. F., et al. (2019). Current and future molecular diagnostics of gastric cancer. Expert Rev. Mol. Diagn 19 (10), 863–874. doi:10.1080/14737159.2019.1660645
Crawford, E. D., Ventii, K., and Shore, N. D. (2014). New biomarkers in prostate cancer. Oncol. Willist. Park) 28 (2), 135–142.
Crew, K. D., and Neugut, A. I. (2006). Epidemiology of gastric cancer. World J. Gastroenterol. 12 (3), 354–362. doi:10.3748/wjg.v12.i3.354
Crowley, E., Di Nicolantonio, F., Loupakis, F., and Bardelli, A. (2013). Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 10 (8), 472–484. doi:10.1038/nrclinonc.2013.110
Cui, L., Zhang, X., Ye, G., Zheng, T., Song, H., Deng, H., et al. (2013). Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer. Cancer 119 (9), 1618–1626. doi:10.1002/cncr.27903
de Mello, R. A., Amaral, G. A., Neves, N. M., Lippo, E. G., Parini, F., Xu, S., et al. (2021). Current and potential biomarkers in gastric cancer: A critical review of the literature. Future Oncol. 17 (25), 3383–3396. doi:10.2217/fon-2021-0084
Diez-Fraile, A., Ceulaer, J., Derpoorter, C., Spaas, C., Backer, T., Lamoral, P., et al. (2020). Circulating non-coding RNAs in head and neck cancer: Roles in diagnosis, prognosis, and therapy monitoring. Cells 10 (1), 48. doi:10.3390/cells10010048
Ding, M., Wang, C., Lu, X., Zhang, C., Zhou, Z., Chen, X., et al. (2018). Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal. Bioanal. Chem. 410 (16), 3805–3814. doi:10.1007/s00216-018-1052-4
Fan, H., Jiang, M., Li, B., He, Y., Huang, C., Luo, D., et al. (2018). MicroRNA-let-7a regulates cell autophagy by targeting Rictor in gastric cancer cell lines MGC-803 and SGC-7901. Oncol. Rep. 39 (3), 1207–1214. doi:10.3892/or.2018.6194
Fei, Z. H., Yu, X. J., Zhou, M., Su, H. F., Zheng, Z., and Xie, C. Y. (2016). Upregulated expression of long non-coding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer. Tumour Biol. 37 (2), 1983–1993. doi:10.1007/s13277-015-3979-9
Foller, S., Oppel-Heuchel, H., and Grimm, M. O. (2018). Tumor assessment in immune checkpoint inhibitor therapy: Tumor response, progression and pseudoprogression. Urol. A 57 (11), 1316–1325. German. doi:10.1007/s00120-018-0788-y
Gareev, I., Beylerli, O., Yang, G., Sun, J., Pavlov, V., Izmailov, A., et al. (2020). The current state of MiRNAs as biomarkers and therapeutic tools. Clin. Exp. Med. 20 (3), 349–359. doi:10.1007/s10238-020-00627-2
Gareev, I., Gileva, Y., Dzidzaria, A., Beylerli, O., Pavlov, V., Agaverdiev, M., et al. (2021). Long non-coding RNAs in oncourology. Noncoding RNA Res. 6 (3), 139–145. doi:10.1016/j.ncrna.2021.08.001
Ge, X., Liu, X., Lin, F., Li, P., Liu, K., Geng, R., et al. (2016). MicroRNA-421 regulated by HIF-1α promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget 7 (17), 24466–24482. doi:10.18632/oncotarget.8228
Ghafouri-Fard, S., Dashti, S., Taheri, M., and Omrani, M. D. (2020). Tincr: An lncRNA with dual functions in the carcinogenesis process. Noncoding RNA Res. 5 (3), 109–115. doi:10.1016/j.ncrna.2020.06.003
Günther, U. L. (2015). Metabolomics biomarkers for breast cancer. Pathobiology 82 (3-4), 153–165. doi:10.1159/000430844
Guo, M., and Yan, W. (2015). Epigenetics of gastric cancer. Methods Mol. Biol. 1238, 783–799. doi:10.1007/978-1-4939-1804-1_41
Han, X., Chen, Y., Yao, N., Liu, H., and Wang, Z. (2015). MicroRNA let-7b suppresses human gastric cancer malignancy by targeting ING1. Cancer Gene Ther. 22 (3), 122–129. doi:10.1038/cgt.2014.75
Han, X., Zhang, J. J., Han, Z. Q., Zhang, H. B., and Wang, Z. A. (2018). Let-7b attenuates cisplatin resistance and tumor growth in gastric cancer by targeting AURKB. Cancer Gene Ther. 25 (11-12), 300–308. doi:10.1038/s41417-018-0048-8
Hayes, D. F. (2015). Biomarker validation and testing. Mol. Oncol. 9 (5), 960–966. doi:10.1016/j.molonc.2014.10.004
Jelski, W., and Mroczko, B. (2022). Molecular and circulating biomarkers of gastric cancer. Int. J. Mol. Sci. 23 (14), 7588. doi:10.3390/ijms23147588
Jingyue, S., Xiao, W., Juanmin, Z., Wei, L., Daoming, L., and Hong, X. (2019). TFAP2E methylation promotes 5-fluorouracil resistance via exosomal miR-106a-5p and miR-421 in gastric cancer MGC-803 cells. Mol. Med. Rep. 20 (1), 323–331. doi:10.3892/mmr.2019.10237
Kagota, S., Taniguchi, K., Lee, S. W., Ito, Y., Kuranaga, Y., Hashiguchi, Y., et al. (2019). Analysis of extracellular vesicles in gastric juice from gastric cancer patients. Int. J. Mol. Sci. 20 (4), 953. doi:10.3390/ijms20040953
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Li, H., Yu, B., Li, J., Su, L., Yan, M., Zhu, Z., et al. (2014). Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget 5 (8), 2318–2329. doi:10.18632/oncotarget.1913
Li, Y., Wu, Z., Yuan, J., Sun, L., Lin, L., Huang, N., et al. (2017). Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 395, 31–44. doi:10.1016/j.canlet.2017.02.035
Liu, F. T., Qiu, C., Luo, H. L., Zhang, Y., Xia, G. F., Hao, T. F., et al. (2016). The association of HOTAIR expression with clinicopathological features and prognosis in gastric cancer patients. Panminerva Med. 58 (2), 167–174.
Mabe, K., Inoue, K., Kamada, T., Kato, K., Kato, M., and Haruma, K. (2022). Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig. Endosc. 34 (3), 412–419. doi:10.1111/den.14063
Matsuoka, T., and Yashiro, M. (2018). Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 24 (26), 2818–2832. doi:10.3748/wjg.v24.i26.2818
Motoyama, K., Inoue, H., Nakamura, Y., Uetake, H., Sugihara, K., and Mori, M. (2008). Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin. Cancer Res. 14 (8), 2334–2340. doi:10.1158/1078-0432.CCR-07-4667
Mugoni, V., Ciani, Y., Nardella, C., and Demichelis, F. (2022). Circulating RNAs in prostate cancer patients. Cancer Lett. 524, 57–69. doi:10.1016/j.canlet.2021.10.011
Pang, Q., Ge, J., Shao, Y., Sun, W., Song, H., Xia, T., et al. (2014). Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 35 (6), 5441–5447. doi:10.1007/s13277-014-1709-3
Petryszyn, P., Chapelle, N., and Matysiak-Budnik, T. (2020). Gastric cancer: Where are we heading? Dig. Dis. 38 (4), 280–285. doi:10.1159/000506509
Powrózek, T., and Ochieng Otieno, M. (2022). Blood circulating non-coding RNAs for the clinical management of triple-negative breast cancer. Cancers (Basel) 14 (3), 803. doi:10.3390/cancers14030803
Saijo, N. (2012). Critical comments for roles of biomarkers in the diagnosis and treatment of cancer. Cancer Treat. Rev. 38 (1), 63–67. doi:10.1016/j.ctrv.2011.02.004
Schwarzenbach, H., Hoon, D. S., and Pantel, K. (2011). Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 11 (6), 426–437. doi:10.1038/nrc3066
Sekar, S., and Liang, W. S. (2019). Circular RNA expression and function in the brain. Noncoding RNA Res. 4 (1), 23–29. doi:10.1016/j.ncrna.2019.01.001
Shan, C., Zhang, Y., Hao, X., Gao, J., Chen, X., and Wang, K. (2019). Biogenesis, functions and clinical significance of circRNAs in gastric cancer. Mol. Cancer 18 (1), 136. doi:10.1186/s12943-019-1069-0
Shao, J., Fang, P. H., He, B., Guo, L. L., Shi, M. Y., Zhu, Y., et al. (2016). Downregulated MicroRNA-133a in gastric juice as a clinicopathological biomarker for gastric cancer screening. Asian Pac J. Cancer Prev. 17 (5), 2719–2722.
Shao, Y., Li, J., Lu, R., Li, T., Yang, Y., Xiao, B., et al. (2017). Global circular RNA expression profile of human gastric cancer and its clinical significance. Cancer Med. 6 (6), 1173–1180. doi:10.1002/cam4.1055
Shao, Y., Tao, X., Lu, R., Zhang, H., Ge, J., Xiao, B., et al. (2020). Hsa_circ_0065149 is an indicator for early gastric cancer screening and prognosis prediction. Pathol. Oncol. Res. 26 (3), 1475–1482. doi:10.1007/s12253-019-00716-y
Shao, Y., Ye, M., Jiang, X., Sun, W., Ding, X., Liu, Z., et al. (2014). Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer 120 (21), 3320–3328. doi:10.1002/cncr.28882
Shi, Y., Duan, Z., Zhang, X., Zhang, X., Wang, G., and Li, F. (2019). Down-regulation of the let-7i facilitates gastric cancer invasion and metastasis by targeting COL1A1. Protein Cell 10 (2), 143–148. doi:10.1007/s13238-018-0550-7
Sims, J. S., Ung, T. H., Neira, J. A., Canoll, P., and Bruce, J. N. (2015). Biomarkers for glioma immunotherapy: The next generation. J. Neurooncol 123 (3), 359–372. doi:10.1007/s11060-015-1746-9
Skryabin, G. O., Vinokurova, S. V., Galetsky, S. A., Elkin, D. S., Senkovenko, A. M., Denisova, D. A., et al. (2022). Isolation and characterization of extracellular vesicles from gastric juice. Cancers (Basel) 14 (14), 3314. doi:10.3390/cancers14143314
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric cancer. Lancet 396 (10251), 635–648. doi:10.1016/S0140-6736(20)31288-5
Song, J., Yu, S., Zhong, D., Yang, W., Jia, Z., Yuan, G., et al. (2021). The circular RNA hsa_circ_000780 as a potential molecular diagnostic target for gastric cancer. BMC Med. Genomics 14 (1), 282. doi:10.1186/s12920-021-01096-6
Sufianova, G., Shumadalova, A., Wenhao, Y., and Gareev, I. (2022). Long non-coding RNAs as biomarkers and therapeutic targets for ischemic stroke. Noncoding RNA Res. 7 (4), 226–232. doi:10.1016/j.ncrna.2022.09.004
Szilágyi, M., Pös, O., Márton, É., Buglyó, G., Soltész, B., Keserű, J., et al. (2020). Circulating cell-free nucleic acids: Main characteristics and clinical application. Int. J. Mol. Sci. 21 (18), 6827. doi:10.3390/ijms21186827
Tanaka, F., Takashima, S., Nadatani, Y., Otani, K., Hosomi, S., Kamata, N., et al. (2020). Exosomal hsa-miR-933 in gastric juice as a potential biomarker for functional dyspepsia. Dig. Dis. Sci. 65 (12), 3493–3501. doi:10.1007/s10620-020-06096-7
Turchinovich, A., Weiz, L., Langheinz, A., and Burwinkel, B. (2011). Characterization of extracellular circulating microRNA. Nucleic Acids Res. 39 (16), 7223–7233. doi:10.1093/nar/gkr254
Urabe, F., Kosaka, N., Ito, K., Kimura, T., Egawa, S., and Ochiya, T. (2020). Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 318 (1), C29–C39. doi:10.1152/ajpcell.00280.2019
Usman, M., Beilerli, A., Sufianov, A., Kudryashov, V., Ilyasova, T., Balaev, P., et al. (2023). Investigations into the impact of non-coding RNA on the sensitivity of gastric cancer to radiotherapy. Front. Physiol. 14, 1149821. doi:10.3389/fphys.2023.1149821
Vogiatzi, G., Oikonomou, E., Deftereos, S., Siasos, G., and Tousoulis, D. (2018). Peripheral artery disease: A micro-RNA-related condition? Curr. Opin. Pharmacol. 39, 105–112. doi:10.1016/j.coph.2018.04.001
Wang, J., Wu, J., Wang, L., Min, X., and Chen, Z. (2021). The linc00152/miR-138 Axis facilitates gastric cancer progression by mediating SIRT2. J. Oncol. 2021, 1173869. doi:10.1155/2021/1173869
Wu, D., Zhang, P., Ma, J., Xu, J., Yang, L., Xu, W., et al. (2019). Serum biomarker panels for the diagnosis of gastric cancer. Cancer Med. 8 (4), 1576–1583. doi:10.1002/cam4.2055
Wu, L., and Qu, X. (2015). Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 44 (10), 2963–2997. doi:10.1039/c4cs00370e
Xie, S., Chang, Y., Jin, H., Yang, F., Xu, Y., Yan, X., et al. (2020). Non-coding RNAs in gastric cancer. Cancer Lett. 493, 55–70. doi:10.1016/j.canlet.2020.06.022
Xu, W., Ding, M., Wang, B., Cai, Y., Guo, C., and Yuan, C. (2021). Molecular mechanism of the canonical oncogenic lncRNA MALAT1 in gastric cancer. Curr. Med. Chem. 28 (42), 8800–8809. doi:10.2174/0929867328666210521213352
Xu, Y. X., Pu, S. D., Li, X., Yu, Z. W., Zhang, Y. T., Tong, X. W., et al. (2022). Exosomal ncRNAs: Novel therapeutic target and biomarker for diabetic complications. Pharmacol. Res. 178, 106135. doi:10.1016/j.phrs.2022.106135
Xu, Z. Y., Yu, Q. M., Du, Y. A., Yang, L. T., Dong, R. Z., Huang, L., et al. (2013). Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int. J. Biol. Sci. 9 (6), 587–597. doi:10.7150/ijbs.6339
Yan, H., and Bu, P. (2021). Non-coding RNA in cancer. Essays Biochem. 65 (4), 625–639. doi:10.1042/EBC20200032
Yang, P., Zhang, M., Liu, X., and Pu, H. (2017). MicroRNA-421 promotes the proliferation and metastasis of gastric cancer cells by targeting claudin-11. Exp. Ther. Med. 14 (3), 2625–2632. doi:10.3892/etm.2017.4798
Yang, Q., Jie, Z., Cao, H., Greenlee, A. R., Yang, C., Zou, F., et al. (2011). Low-level expression of let-7a in gastric cancer and its involvement in tumorigenesis by targeting RAB40C. Carcinogenesis 32 (5), 713–722. doi:10.1093/carcin/bgr035
Yang, Y., Shao, Y., Zhu, M., Li, Q., Yang, F., Lu, X., et al. (2016). Using gastric juice lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of gastric cancer. Tumour Biol. 37 (1), 1183–1188. doi:10.1007/s13277-015-3903-3
Yoon, H., and Kim, N. (2015). Diagnosis and management of high risk group for gastric cancer. Gut Liver 9 (1), 5–17. doi:10.5009/gnl14118
Yu, X., Luo, L., Wu, Y., Yu, X., Liu, Y., Yu, X., et al. (2013). Gastric juice miR-129 as a potential biomarker for screening gastric cancer. Med. Oncol. 30 (1), 365. doi:10.1007/s12032-012-0365-y
Yuan, C. L., Li, H., Zhu, L., Liu, Z., Zhou, J., and Shu, Y. (2016). Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma 63 (3), 442–449. doi:10.4149/314_150825N45
Zeng, B., Chen, T., Xie, M. Y., Luo, J. Y., He, J. J., Xi, Q. Y., et al. (2019). Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. J. Dairy Sci. 102 (8), 6726–6737. doi:10.3168/jds.2019-16257
Zhang, J., Hou, L., Liang, R., Chen, X., Zhang, R., Chen, W., et al. (2019). CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling. Mol. Cancer 18 (1), 80. doi:10.1186/s12943-019-1015-1
Zhang, L., and Yu, D. (2019). Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 1871 (2), 455–468. doi:10.1016/j.bbcan.2019.04.004
Zhang, X., Cui, L., Ye, G., Zheng, T., Song, H., Xia, T., et al. (2012). Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 33 (6), 2349–2355. doi:10.1007/s13277-012-0497-x
Zhang, X., Wang, S., Wang, H., Cao, J., Huang, X., Chen, Z., et al. (2019). Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer 18 (1), 20. doi:10.1186/s12943-018-0935-5
Zhao, C., Lv, Y., Duan, Y., Li, G., and Zhang, Z. (2020). Circulating non-coding RNAs and cardiovascular diseases. Adv. Exp. Med. Biol. 1229, 357–367. doi:10.1007/978-981-15-1671-9_22
Keywords: gastric juice ncRNAs and gastric cancer non-coding RNAs, gastric cancer, gastric juice, liquid biopsy, non-invasive biomarkers, extracellular vesicles, theories, clinical perspectives
Citation: Gareev I, Ahmad A, Wang J, Beilerli A, Ilyasova T, Sufianov A and Beylerli O (2023) Gastric juice non-coding RNAs as potential biomarkers for gastric cancer. Front. Physiol. 14:1179582. doi: 10.3389/fphys.2023.1179582
Received: 04 March 2023; Accepted: 18 April 2023;
Published: 26 April 2023.
Edited by:
Cuncong Zhong, University of Kansas, United StatesReviewed by:
Syed Musthapa Meeran, Central Food Technological Research Institute (CSIR), IndiaTao Tan, The Ohio State University, United States
Copyright © 2023 Gareev, Ahmad, Wang, Beilerli, Ilyasova, Sufianov and Beylerli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ozal Beylerli, b2JleWxlcmxpQG1haWwucnU=; Albert Sufianov, c3VmaWFub3ZAZ21haWwuY29t
 Ilgiz Gareev
Ilgiz Gareev Aamir Ahmad
Aamir Ahmad Jiaqi Wang
Jiaqi Wang Aferin Beilerli
Aferin Beilerli Tatiana Ilyasova5
Tatiana Ilyasova5 Albert Sufianov
Albert Sufianov Ozal Beylerli
Ozal Beylerli