- College of Plant Protection, Shandong Agricultural University, Taian, Shandong, China
Pheromone cues released from hosts or prey are of crucial importance to natural enemies for prey and habitat location. The use of herbivorous insect sex pheromones has long been considered as a potential pest control alternative that is non-toxic and harmless to beneficials. We hypothesized that Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), a major predatory coccinellid beetle of the devastating migratory pest Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae), could perceive and use the sex pheromone of S. frugiperda to locate its habitat. Here we tested the electrophysiological and behavioral responses of H. axyridis to the two components Z7-12:Ac and Z9-14:Ac of S. frugiperda sex pheromone by using electroantennography (EAG) and Y-tube bioassay. The 3D modeling of H. axyridis odorant-binding proteins (HaxyOBPs) and molecular docking were also performed. The results showed that both female and male H. axyridis exhibited significantly higher electrophysiological and behavioral responses to Z9-14:Ac at the concentrations of 0.001, 0.01, and 0.1 μg/μL, while no significant electrophysiological and behavioral responses of H. axyridis were observed to Z7-12:Ac. The blend of Z7-12:Ac and Z9-14:Ac at the ratio of 1:100 had a significant attraction to both male and female H. axyridis at the concentrations of 0.01 and 0.1 μg/μL based on electrophysiological and behavioral assays, but no significant behavioral responses were observed at the ratios of 1:9. According to the 3D modeling of HaxyOBPs and molecular docking, HaxyOBP12 has a good affinity with Z9-14:Ac. Z9-14:Ac is bound to the HaxyOBP12 by hydrogen bonding and hydrophobic interactions. However, there were no credible docking results between HaxyOBPs and Z7-12:Ac. Our findings revealed that H. axyridis can perceive Z9-14:Ac and could use it as a chemical cue to locate prey habitat. We speculated that Z7-12:Ac, which showed some antagonistic effect toward the response of H. axyridis to Z9-14:Ac, could improve the adaptability of S. frugiperda in the presence of predators. This study provides new insights into the application of pheromones to manipulate natural enemy behavior for pest control.
1 Introduction
Foraging behavior is a process in which insect natural enemies search for food resources or oviposition sites for survival, growth and reproduction (Kramer, 2001). Natural enemies of herbivores base their foraging decision on chemical cues from plant or herbivorous insects (Kaiser et al., 2017; Peñaflor, 2019). Pheromones released by hosts or prey may serve as kairomonal cues for parasitoids or predators, which can be used as kairomones for natural enemies to locate and control pests (Vaello et al., 2017). Common chemical signals released by the host or prey mainly include sex pheromones (Boo and Yang, 2000; Aukema and Raffa, 2005; Branco et al., 2006; Pekas et al., 2015; Shapira et al., 2018; Wang et al., 2018), alarm pheromones (Pickett et al., 2013; Wang et al., 2019; Liu et al., 2021; Qin et al., 2022), and aggregation pheromones (Kpongbe et al., 2019). To date, pheromone use is a promising way not only to monitor insect pests but to suppress their population growth by improving natural control function in agro-ecosystem.
Insects rely on sensitive olfactory systems to perceive chemical cues. Odorant-binding proteins (OBPs) are responsible for connecting the external environment and odorant receptors (ORs) (Brito et al., 2016). The interaction between OBPs and odor molecules is the first step in insect recognition of chemicals (Leal, 2012). The recognition mechanism of chemical signals of hosts or prey in natural enemies has been investigated within insect olfactory system. For example, aphid alarm pheromone E-β-Farnesene (EβF) was used as a foraging cue for many predators, such as hoverflies (Harmel et al., 2007; Wang et al., 2019), ground beetles (Kielty et al., 1996), green lacewings (Li et al., 2017; Li et al., 2018) and lady beetles (Liu et al., 2014).
Environmentally friendly pest management strategies including the use of natural enemies and pheromones are advocated. For example, slow release of aphid alarm pheromone EβF in wheat fields increases the abundance of mummified aphids by attracting parasitic wasps (Liu et al., 2021). However, the simultaneous use of these two methods may cause synergistic or antagonistic effects. The attraction of natural enemies to pheromones may enhance or interfere with biological control function when the natural enemies are able to generate kairomonal activity towards the pest pheromones (Shapira et al., 2018). Sex pheromone cues may arrest natural enemies to the source and enhance their foraging in the vicinity, thus contributing to the efficiency of pest control (Shapira et al., 2018). Alternatively, sex pheromone may attract natural enemies to the dispensers and reduce their densities in the field, thereby affecting the effectiveness of biological control (Pekas et al., 2015). Knowledge of the kairomonal effects of pheromones on enemies can help to improve lures that recruit and retain natural enemies and to improve the efficiency of biological control of crop pests (Ayelo et al., 2021; Ayelo et al., 2022).
The fall armyworm Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) is a devastating agricultural pest, spreading rapidly in China and causing substantial economic losses (Wang et al., 2020; Wu et al., 2021). Management of S. frugiperda relies on the use of chemical insecticides, extracts and metabolites from plants, entomopathogenic bacterium, natural enemies, and sex pheromone traps (Paredes-Sánchez et al., 2021). Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) is a dominant predator that contributes to the suppression of many pests, including various hemipterans, and the larvae and pupae of Coleoptera, Hymenoptera, Diptera, and Lepidoptera (Cheng et al., 2020; Di et al., 2021). H. axyridis is a highly voracious predator of the eggs and young larvae of S. frugiperda, and can be used as biocontrol agent of this pest (Di et al., 2021). The use of monitoring based on pheromone traps has been shown to be effective to predict the infestation of S. frugiperda (Paredes-Sánchez et al., 2021). To date, Z9-14:Ac and Z7-12:Ac are identified as the two principal sex pheromone components of the Yunnan population of S. frugiperda (Jiang et al., 2022). However, the functions of these two components on natural enemies remain elusive. So we hypothesized that H. axyridis could recognize and detect S. frugiperda sex pheromone cues and recruit these cues for prey habitat location and prey location.
Here, we tested the electrophysiological and behavioral responses of H. axyridis to the two components Z7-12:Ac and Z9-14:Ac of S. frugiperda sex pheromone by using electroantennography (EAG) and Y-tube bioassay. Further tests were performed on the blend of Z7-12:Ac and Z9-14:Ac at the ratios of 1:9 and 1:100 to determine the response of H. axyridis to the binary mixture. Subsequently, the binding mechanism between the two pheromone components and H. axyridis odorant-binding protein (HaxyOBPs) has been clarified. The potential use of sex pheromone to manipulate the foraging behavior of H. axyridis for S. frugiperda control in the crops was discussed.
2 Materials and methods
2.1 Insects
Colonies of H. axyridis were collected from corn fields at the experimental station of Shandong Agricultural University (Tai’an, Shandong Province, China). Sex determination of H. axyridis was based on the labrum pigmentation (female: dark; male: light) and the distal margin of the 5th visible abdominal sternite (female: convex; male: concave) (McCornack et al., 2007). The lady beetles were reared with the wheat aphid Sitobion miscanthi (Takahashi) in the laboratory and maintained at 25°C ± 2°C and 65% ± 5% r. h under 12:12 L:D photoperiod. All H. axyridis used in this study were naive, and used only once. Before each trial, H. axyridis adults were starved for 48 h to enhance their sensitivity to odors.
2.2 Chemicals
(Z)-9-tetradecenal acetate (Z9-14:Ac) and (Z)-7-dodecenyl acetate (Z7-12:Ac) with minimum purity >90% were purchased from Shanghai Yuanye Biotechnology Co., Ltd. n-hexane (≥98%) was purchased from Kaitong Chemical Reagents Co., Ltd., Tianjin, China.
2.3 Comparative EAG responses to the sex pheromone of S. frugiperda
The dose-dependent EAG responses of male and female antennae to synthetic standard chemical compounds, Z7-12:Ac, Z9-14:Ac were investigated. According to AndoLab-PheromoneDatabase, the ratios of the pheromone components of S. frugiperda population that broke out in mainland China and east Asia were identified and determined as 1:9 or 1:100, so the blends of Z7-12:Ac and Z9-14:Ac at the ratios of 1:9 and 1:100 were also tested. Five concentrations of each component and the blends (each compound/blend was diluted with n-hexane into solutions of different gradient concentrations at 0.00001 μg/μL, 0.0001 μg/μL, 0.001 μg/μL, 0.01 μg/μL, and 0.1 μg/μL) were tested. Six different antennae were tested as replications for each concentration. Each antenna was stimulated 3 times. The antennae of H. axyridis were removed from the base, and the distal tips were also removed. Each dissected antenna was immediately fastened with electrode gel (Spectra 360 Electrode Gel) onto two metal electrodes. Ten microliter of each chemical solution was applied to a piece of filter paper strip (0.8 × 1 cm). The solvent was allowed to evaporate for 1 min, then the paper strip was placed inside a glass Pasteur pipette (5 cm in length, 0.5 cm in internal diameter) in the EAG system directed at the antennal preparation.
The stimuli were provided as 0.5 s puffs of air into a humidified air flow at 0.4 mL/s generated by a stimulus controller. A 15 s interval between successive stimulations was allowed for antennal recovery. EAG response to 10 μL n-hexane was tested as control. Signals were stored and analyzed by using EAG ver. 2.5 software (Synthech, Hilversum, Netherlands).
2.4 Y-tube olfactometer bioassay
The attractiveness of the sex pheromone of S. frugiperda to H. axyridis was assessed in dual choice assays using a Y-tube olfactometer (common arm, 20.0 cm; arms, 15 cm at 75°angle; internal diameter, 1.5 cm). Air was pumped through an active charcoal filter and re-humidified by passing it through a bottle with distill water before being directed into the two arms of the olfactometer. Chemicals and the blends were prepared in different concentrations (0.00001, 0.0001, 0.001, 0.01 and 0.1 μg/μL) and n-hexane was used as control. An aliquot (10 μL) of each test solution was applied to a filter paper strip (2 × 1 cm2), and the solvent was allowed to evaporate for 1 min before inserting the strip into an odor-source glass bottle connected to one arm of the olfactometer. The control glass bottle connected to the other arm of the olfactometer contained a filter paper strip treated with 10 μL of hexane. Adult H. axyridis was recorded as having made a choice that crossed half of the arm within 5 min. Twenty-five females or males that made a choice were tested for each treatment, individuals making no response to either arm for 5 min were recorded but discarded. The olfactometer was rotated by 90° after each test to avoid any directional bias. The olfactometer was thoroughly washed and rinsed with acetone after five replicates.
2.5 3D modeling and molecular docking
The OBPs sequences of H. axyridis were identified by Qu et al. (2021). Two strategies were employed to predict the 3D structure of OBPs. The online program SWISS MODEL (Waterhouse et al., 2018) was used for predicting the OBPs that have >30% homology with the templates in the Protein Data Bank (http://www.rcsb.org/pdb). While the OBPs that have less than 30% homology were generated using a deep residual neural network trRosetta (https://yanglab.nankai.edu.cn/trRosetta; Yang et al., 2020). The final 3D models were assessed by Procheck, Verify_3D and ERRAT (http://services.mbi.ucla.edu/SAVES/).
The binding mode between HaxyOBPs and S. frugiperda sex pheromone was performed using Surflex-Dock suit SYBYL X 2.1.1. The results of binding mode were evaluated according to the total score. The PyMOL and LigPlot+ (Laskowski and Swindells, 2011) were used to visualize conformations and interactions.
2.6 Data analysis
Data on EAG responses to each concentration of the sex pheromone components were analyzed using analysis of variance (ANOVA followed by LSD test). Preference numbers of Y-tube olfactory bioassay were evaluated by χ2 test. Individuals that did not make a choice were excluded from the statistical analysis.
3 Results
3.1 EAG response of H. axyridis to S. frugiperda pheromone
The dose-EAG responses of male and female antennae of H. axyridis to Z7-12:Ac and Z9-14:Ac were measured. Z9-14:Ac elicited higher EAG responses in both females and males than those of Z7-12:Ac (Figure 1). The concentration of 0.0001–0.01 μg/μL Z9-14:Ac caused significantly higher EAG responses in the males than those of Z7-12:Ac and n-hexane (0.0001 μg/μL: F = 7.072, df = 2, p = 0.007; 0.001 μg/μL: F = 9.884, df = 2, p = 0.002; 0.01 μg/μL: F = 4.971, df = 2, p = 0.022; 0.1 μg/μL: F = 14.782, df = 2, p < 0.001) (Figure 1A). Females showed significantly higher EAG responses to Z9-14:Ac at the concentrations of 0.0001 and 0.1 μg/μL than those of Z7-12:Ac and n-hexane (0.0001 μg/μL: F = 6.956, df = 2, p = 0.007; 0.1 μg/μL: F = 4.682, df = 2, p = 0.026) (Figure 1B).
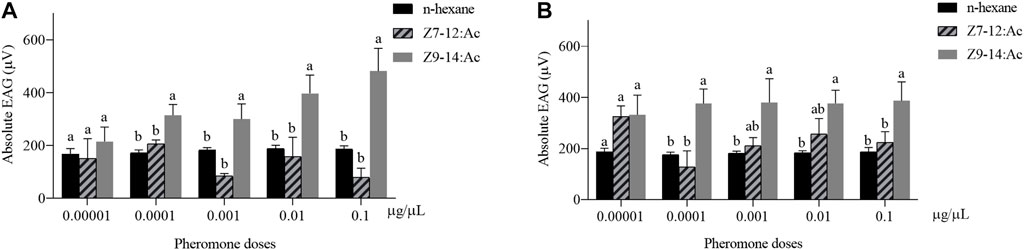
FIGURE 1. Electroantennogram (EAG) responses of male (A) and female (B) Harmonia axyridis to Z7-12:Ac and Z9-14:Ac with various doses (0.00001–0.1 μg/μL). Six different antennae were tested as replications for each concentration. Different letters indicate significant differences between different compounds (p < 0.05) at each concentration.
The EAG response of H. axyridis to the blend of Z7-12:Ac and Z9-14:Ac at the ratio of 1:100 was higher than that to 1:9 (Figure 2). The responses of male to the ratio of 1:100 were significantly higher than those to 1:9 at the concentration of 0.0001–0.1 μg/μL (0.0001 μg/μL: F = 5.793, df = 2, p = 0.012; 0.001 μg/μL: F = 6.975, df = 2, p = 0.007; 0.01 μg/μL: F = 7.426, df = 2, p = 0.006; 0.1 μg/μL: F = 9.564, df = 2, p = 0.002) (Figure 2A). The female responses to 1:100 were significantly higher than those to 1:9 at the concentrations of 0.001 and 0.1 μg/μL (0.001 μg/μL: F = 12.157, df = 2, p = 0.022; 0.1 μg/μL: F = 8.472, df = 2, p = 0.002) (Figure 2B).
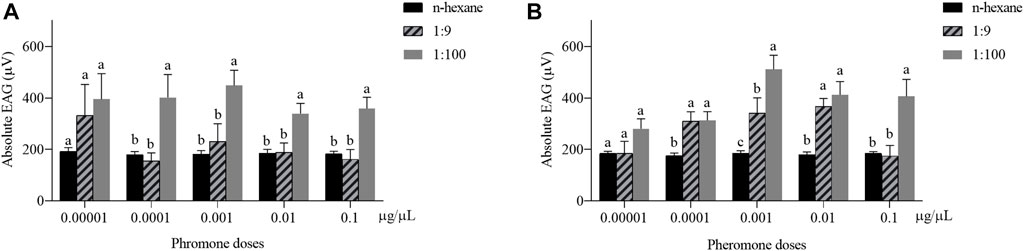
FIGURE 2. Electroantennogram (EAG) responses of male (A) and female (B) Harmonia axyridis to the blend of Z7-12:Ac and Z9-14:Ac at the ratios of 1:100 and 1:9. Six different antennae were tested as replications for each concentration. Different letters indicate significant differences at each concentration (p < 0.05).
3.2 Olfactory response of H. axyridis to S. frugiperda pheromone
Behavioral response tests showed that Z7-12:Ac elicited no significant responses of the male and female H. axyridis (Figure 3), while H. axyridis exhibited strongly attractive response to Z9-14:Ac (Figure 4). At the concentrations ranging from 0.0001 to 0.1 μg/μL, Z9-14:Ac attracted significantly more males than that of n-hexane (Figure 4A), while females showed a significant tendency to Z9-14:Ac at the concentrations ranging from 0.001 to 0.1 μg/μL (Figure 4B).

FIGURE 3. Olfactory responses of male (A) and female (B) Harmonia axyridis to Z7-12:Ac in a Y-tube olfactometer. Twenty-five females or males that made a choice were tested for each treatment. N: the number of non-responses.
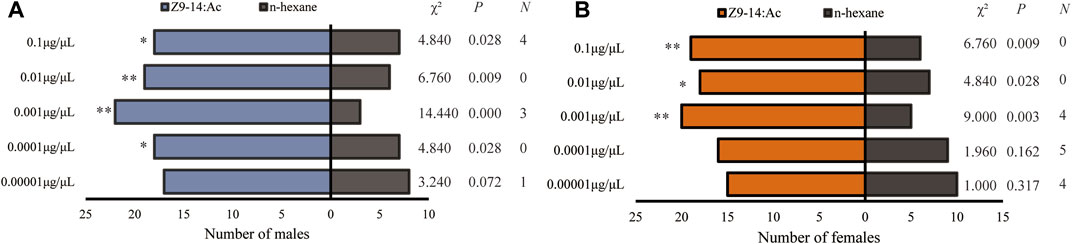
FIGURE 4. Olfactory responses of male (A) and female (B) Harmonia axyridis to Z9-14:Ac in a Y-tube olfactometer. Twenty-five females or males that made a choice were tested for each treatment. N: the number of non-responses. The asterisks indicate significance differences at p < 0.05 (*) and p < 0.01 (**).
H. axyridis showed completely different olfactory responses to the blends of Z7-12:Ac and Z9-14:Ac at the ratios of 1:9 and 1:100. Neither male nor female showed a preference for a ratio of 1:9 (Figures 5A, B). Males displayed significant preferences for 0.01 and 0.1 μg/μL at the ratio of 1:100 (Figure 5C), and females did for 0.001, 0.01 and 0.1 μg/μL (Figure 5D).
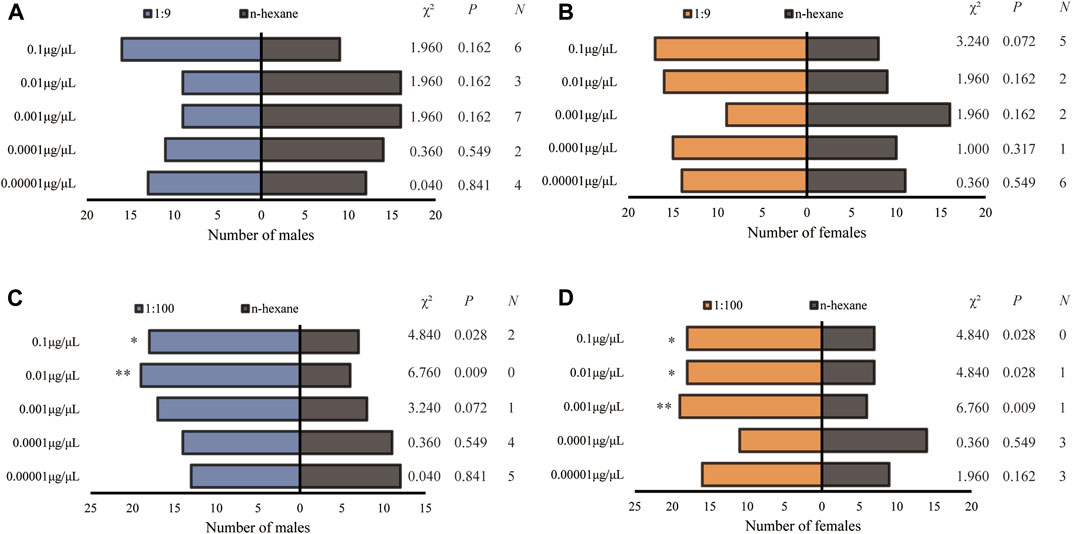
FIGURE 5. Olfactory responses of male and female Harmonia axyridis to the blend of Z7-12: Ac and Z9-14: Ac at the ratios of 1:9 ((A): male; (B) female) and 1:100 ((C): male; (D) female) in a Y-tube olfactometer. Twenty-five females or males that made a choice were tested for each treatment. N: the number of non-responses. The asterisks indicate significance differences at p < 0.05 (*) and p < 0.01 (**).
3.3 3D modeling and molecular docking
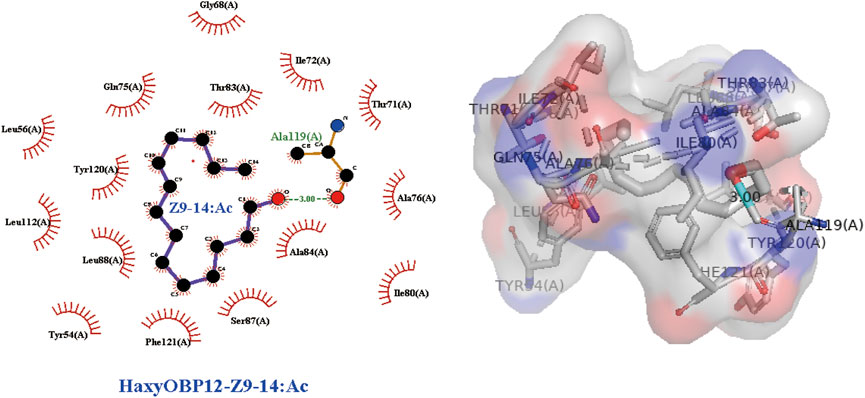
In order to take insight into the mechanism of the responses of H. axyridis to the two components of S. frugiperda sex pheromone, 3D modeling of H. axyridis odorant-binding protein (HaxyOBPs) and molecular docking were performed. Sequence alignments showed that HaxyOBP12, HaxyOBP13 and HaxyOBP14 shared more than 30% similarity with template proteins (Supplementary Table S1). In addition to these three OBPs, the other 16 OBPs used trRosetta for 3D structure prediction. The predicted results all matched very high (Supplementary Table S2). In order to provide evidence on whether H. axyridis could sense and detect S. frugiperda sex pheromone Z7-12: Ac and Z9-14: Ac, SYBYL was used to analyze the molecular interactions between identified HaxyOBPs and Z7-12:Ac or Z9-14:Ac respectively. The results revealed that HaxyOBP12 has a good affinity with Z9-14:Ac (Total score = 8.261, C-score = 4). However, there were no credible docking results between HaxyOBPs and Z7-12:Ac (Table 1). Z9-14:Ac bound to HaxyOBP12 by hydrogen bonding interactions and hydrophobic interactions (Figure 6). The Z9-14:Ac was stabilized by a hydrogen bond with Ala119 of HaxyOBP12 (3.00 Å). Additionally, Z9-14:Ac was also buried in tight hydrophobic cavities formed by residues including Thr83, Tyr120, Leu88, Phe121, Ser87, Ala84, Ile72, Gln75, Thr71, Gly68, Leu56, Leu112, Tyr54, Ile80, and Gly68.
4 Discussion
Chemical cues play a key role in mediating the interactions between natural enemies and their hosts or prey. The host or prey can release kairomones that differs from the plant background odors, thus providing the most reliable source of chemical information for natural enemies to detect host and prey in the natural environment (Rodriguez-Saona and Stelinski, 2009; Ayelo et al., 2022). In this study, we examined and determined the kairomonal effect of S. frugiperda sex pheromone on the coccinellid predator H. axyridis, and the binding properties of H. axyridis OBPs to the components of the sex pheromone. Our findings could provide new strategies for the combined application of sex pheromone and natural enemies for S. frugiperda control.
The pheromone composition of S. frugiperda was inconsistent among different geographic populations (Jiang et al., 2022). To sum up, Z9-14:Ac, Z7-12:Ac, Z9-12:Ac, Z11-16:Ac and E7-12:Ac were employed by different geographic populations (Sekul and Sparks, 1967; Sekul and Sparks, 1976; Tumlinson et al., 1986; Groot et al., 2008; Jiang et al., 2022). The sex pheromone components of the Yunnan population C-strain of S. frugiperda are confirmed as Z7-12:Ac and Z9-14:Ac (Jiang et al., 2022). Based on the pre-test of the EAG responses of S. frugiperda to the five identified sex pheromone components, we found that Z9-14:Ac and Z7-12:Ac elicited strong electrophysiological responses (Supplementary Figure S1). Therefore, these two components were as the putative candidates for the experiments.
Electrophysiological and olfactory response assays of H. axyridis demonstrated that both female and male H. axyridis were strongly attracted to Z9-14:Ac. Molecular docking showed that OBP12 and Z9-14:Ac could be closely bonded through hydrogen bonding and hydrophobic interaction, while there was no good docking effect between OBP and Z7-12:Ac. The transcript of HaxyOBP12 is mainly restricted to adult antennae, implying a potential role in olfactory chemoreception (Qu et al., 2021). These results revealed that H. axyridis can sense the stimulation of Z9-14:Ac and transfer the stimulus to its central nervous system. It was suggested that the sex pheromone component Z9-14:Ac of S. frugiperda could be recognized by H. axyridis and was acted as an important kairomonal signal when H. axyridis prey on the eggs and larvae of S. frugiperda. Moreover, HaxyOBP12 is abundant in the larval stage (Qu et al., 2022), indicating that it is also involved in the recognition of Z9-14:Ac by H. axyridis in the larval stage to locate prey.
Although H. axyridis showed a higher kairomonal response to Z9-14:Ac, further research is needed to determine whether it could be efficient to be used as a pheromone bait to attract and assemble local population of H. axyridis in the fields. Several enemies, such as H. axyridis (Verheggen et al., 2007), Coccinella septempunctata (Ninkovic et al., 2001), the hoverfly Episyrphus balteatus (Verheggen et al., 2008), and aphid parasitoids (Liu et al., 2021), are able to perceive the aphid alarm pheromone EβF and show attractant behavior. But in the field, EβF had no effect on the number of predator (Araneae, Opilionidae, Carabidae, Coccinellidae, Staphilinidae, Forficulidae, Syrphidae, Formicidae, Polistinae, Parasitiod, and Chrysopidae) visits to aphid colony or on predator patch residence times (Joachim & Weisser, 2015). Thus, while sex pheromones may have potential for application in agricultural pest management strategies, their ecological role as kairomone in natural settings is worth pondering. The dose-dependent response tests showed that H. axyridis seemed to respond to higher concentrations of Z9-14:Ac, suggesting that the potential effect of concentration on natural enemy populations must be taken into consideration when determining the most effective dose to use (Branco et al., 2006).
In addition, H. axyridis had a significantly higher response to the mixture of Z7-12:Ac and Z9-14:Ac at the ratio of 1:100 than to 1:9. It suggested that Z7-12:Ac might increase the avoidance effect of Z9-14:Ac on H. axyridis. We speculated that insect pest populations have probably evolved in producing a variety of components of sex pheromones with various ratios, thus not only forming reproductive isolation, but also avoiding natural enemy predation or parasite. Therefore, the ratio effect of the two components of S. frugiperda sex pheromone on natural enemies should be considered when it is applied for monitoring and control of S. frugiperda.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YL and JW contributed to the concept and design. JW, YZ, and XK performed the experiments. JW and YZ were involved in data analysis. YL, YZ, and JW drafted and revised the manuscript. All authors reviewed the manuscript and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2021YFE0115600).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1167174/full#supplementary-material
References
Aukema, B. H., and Raffa, K. F. (2005). Selective manipulation of predators using pheromones: Responses to frontalin and ipsdienol pheromone components of bark beetles in the great lakes region. Agric. For. Entomol. 7, 193–200. doi:10.1111/j.1461-9555.2005.00250.x
Ayelo, P. M., Pirk, C. W. W., Yusuf, A. A., Chailleux, A., Mohamed, S. A., and Deletre, E. (2021). Exploring the kairomone-based foraging behaviour of natural enemies to enhance biological control: A review. Front. Ecol. Evol. 9, 641974. doi:10.3389/fevo.2021.641974
Ayelo, P. M., Yusuf, A. A., Chailleux, A., Mohamed, S. A., Pirk, C., and Deletre, E. (2022). Chemical cues from honeydew and cuticular extracts of Trialeurodes vaporariorum serve as kairomones for the parasitoid Encarsia formosa. J. Chem. Ecol. 48, 370–383. doi:10.1007/s10886-022-01354-6
Boo, K. S., and Yang, J. P. (2000). Kairomones used by Trichogramma chilonis to find Helicoverpa assulta eggs. J. Chem. Ecol. 26, 359–375. doi:10.1023/A:1005453220792
Branco, M., Lettere, M., Franco, J. C., Binazzi, A., and Jactel, H. (2006). Kairomonal response of predators to three pine bast scale sex pheromones. J. Chem. Ecol. 32, 1577–1586. doi:10.1007/s10886-006-9071-6
Brito, N. F., Moreira, M. F., and Melo, A. (2016). A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 95, 51–65. doi:10.1016/j.jinsphys.2016.09.008
Cheng, J., Li, P., Zhang, Y., Zhan, Y., and Liu, Y. (2020). Quantitative assessment of the contribution of environmental factors to divergent population trends in two lady beetles. Biol. Control 145, 104259. doi:10.1016/j.biocontrol.2020.104259
Di, N., Zhang, K., Xu, Q., Zhang, F., Harwood, J. D., Wang, S., et al. (2021). Predatory ability of Harmonia axyridis (Coleoptera: Coccinellidae) and Orius sauteri (Hemiptera: Anthocoridae) for suppression of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 12, 1063. doi:10.3390/insects12121063
Groot, A. T., Marr, M., Schöfl, G., Lorenz, S., Svatos, A., and Heckel, D. G. (2008). Host strain specific sex pheromone variation in Spodoptera frugiperda. Front. Zool. 5, 20. doi:10.1186/1742-9994-5-20
Harmel, N., Almohamad, R., Fauconnier, M. L., Jardin, P. D., Verheggen, F., Marlier, M., et al. (2007). Role of terpenes from aphid-infested potato on searching and oviposition behavior of Episyrphus balteatus. Insect Sci. 14, 57–63. doi:10.1111/j.1744-7917.2007.00126.x
Jiang, N. J., Mo, B. T., Guo, H., Yang, J., Tang, R., and Wang, C. Z. (2022). Revisiting the sex pheromone of the fall armyworm Spodoptera frugiperda, a new invasive pest in South China. Insect Sci. 29 (3), 865–878. doi:10.1111/1744-7917.12956
Joachim, C., and Weisser, W. W. (2015). Does the aphid alarm pheromone (E)-β-farnesene act as a kairomone under field conditions? J. Chem. Ecol. 41 (3), 267–275. doi:10.1007/s10886-015-0555-0
Kaiser, L., Ode, P., van Nouhuys, S., Calatayud, P. A., Colazza, S., Cortesero, A. M., et al. (2017). “The plant as a habitat for entomophagous insects,” in insect-plant interactions in a crop protection perspective,” in Advances in botanical research. Editors N. Sauvion, P-A. Calatayud, and D. Thiéry (Londres: Elsevier Ltd.), 179–223. doi:10.1016/bs.abr.2016.09.006
Kielty, J. P., Allen-Williams, L. J., Underwood, N., and Eastwood, E. A. (1996). Behavioral responses of three species of ground beetle (Coleoptera: Carabidae) to olfactory cues associated with prey and habitat. J. Insect Behav. 9, 237–250. doi:10.1007/BF02213868
Kpongbe, H., van den Berg, J., Khamis, F., Tamò, M., and Torto, B. (2019). Isopentyl butanoate: Aggregation pheromone of the Brown spiny bug, Clavigralla tomentosicollis (Hemiptera: Coreidae), and kairomone for the egg parasitoid gryon sp. (Hymenoptera: Scelionidae). J. Chem. Ecol. 45, 570–578. doi:10.1007/s10886-019-01081-5
Kramer, D. L. (2001). “Foraging behavior,” in Evolutionary ecology, concepts and cases studies. Editors C. W. Fox, D. A. Roff, and D. J. Fairbairn (Oxford: Oxford University Press), 232–246.
Laskowski, R. A., and Swindells, M. B. (2011). LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786. doi:10.1021/ci200227u
Leal, W. S. (2012). Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391. doi:10.1146/annurev-ento-120811-153635
Li, Z. Q., Zhang, S., Cai, X. M., Luo, J. Y., Dong, S. L., and Cui, J. J. (2017). Three odorant binding proteins may regulate the behavioural response of Chrysopa pallens to plant volatiles and the aphid alarm pheromone (E)-β-farnesene. Insect Mol. Biol. 26, 255–265. doi:10.1111/imb.12295
Li, Z. Q., Zhang, S., Cai, X. M., Luo, J. Y., Dong, S. L., and Cui, J. J. (2018). Distinct binding affinities of odorant-binding proteins from the natural predator Chrysoperla sinica suggest different strategies to hunt prey. J. insect physiol. 111, 25–31. doi:10.1016/j.jinsphys.2018.10.004
Liu, J. H., Zhao, X. J., Zhan, Y. D., Wang, K., Francis, F., and Liu, Y. (2021). New slow release mixture of E-β-farnesene with methyl salicylate to enhance aphid biocontrol efficacy in wheat ecosystem. Pest Manag. Sci. 77, 3341–3348. doi:10.1002/ps.6378
Liu, Y. J., Chi, B. J., Yang, B., Zhu, Y. F., and Yong, L. (2014). Effects of E-β-farnesene release on the spatial distribution patterns of cabbage aphids and lady beetles. Acta phytophy. Sin. 41, 754–760.
McCornack, B. P., Koch, R. L., and Ragsdale, D. W. (2007). A simple method for in-field sex determination of the multicolored Asian lady beetle Harmonia axyridis. J. Insect Sci. 7, 1–12. doi:10.1673/031.007.1001
Ninkovic, V., Al Abassi, S., and Pettersson, J. (2001). The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol. Control 21, 191–195. doi:10.1006/bcon.2001.0935
Paredes-Sánchez, F. A., Rivera, G., Bocanegra-García, V., Martínez-Padrón, H. Y., Berrones-Morales, M., Niño-García, N., et al. (2021). Advances in control strategies against Spodoptera frugiperda. A Rev. Mol. 26, 5587. doi:10.3390/molecules26185587
Pekas, A., Navarro-Llopis, V., Garcia-Marí, F., Primo, J., and Vacas, S. (2015). Effect of the California red scale Aonidiella aurantii sex pheromone on the natural parasitism by Aphytis spp. in Mediterranean citrus. Biol. Control 90, 61–66. doi:10.1016/j.biocontrol.2015.05.016
Peñaflor, M. F. G. V. (2019). “Use of semiochemical-based strategies to enhance biological control,” in Natural enemies of insect pests in neotropical agroecosystems: Biological control and functional biodiversity. Editors B. Souza, L. L. Vazquez, and R. C. Marucci (Cham: Springer Nature Switzerland), 479–488. doi:10.1007/978-3-030-24733-1_41
Pickett, J. A., Allemann, R. K., and Birkett, M. A. (2013). The semiochemistry of aphids. Nat. Prod. Rep. 30, 1277–1283. doi:10.1039/C3NP70036D
Qin, Y. G., Zhang, S. Y., and Li, Z. X. (2022). Kairomonal effect of aphid alarm pheromones and analogues on the parasitoid Diaeretiella rapae. PREPRINT (Version 1) available at Research Square. doi:10.21203/rs.3.rs-1706467/v1
Qu, C., Wang, R., Che, W. N., Li, F. Q., Zhao, H. P., Wei, Y. Y., et al. (2021). Identification and tissue distribution of odorant binding protein genes in Harmonia axyridis (Coleoptera: Coccinellidae). J. Integr. Agr. 20, 2204–2213. doi:10.1016/S2095-3119(20)63297-X
Qu, C., Yang, Z. K., Wang, S., Zhao, H. P., Li, F. Q., Yang, X. L., et al. (2022). Binding affinity characterization of four antennae-enriched odorant-binding proteins from Harmonia axyridis (Coleoptera: Coccinellidae). Front. Physiol. 13, 829766. doi:10.3389/fphys.2022.829766
Rodriguez-Saona, C., and Stelinski, L. L. (2009). “Behavior-modifying strategies in IPM: Theory and practice,” in Integrated pest management: Innovation and development. Editor A. K. D. R. Peshin (New York: Springer), 261–312.
Sekul, A. A., and Sparks, A. N. (1976). Sex attractant of the fall armyworm moth. USDA Tech. Bull. 1542, 1–6.
Sekul, A. A., and Sparks, A. N. (1967). Sex pheromone of the fall armyworm Moth1: Isolation, identification, and Synthesis23. J. Econ. Entomol. 60, 1270–1272. doi:10.1093/jee/60.5.1270
Shapira, I., Keasar, T., Harari, A. R., Gavish-Regev, E., Kishinevsky, M., Steinitz, H., et al. (2018). Does mating disruption of Planococcus ficus and Lobesia botrana affect the diversity, abundance and composition of natural enemies in Israeli vineyards? Pest Manag. Sci. 74, 1837–1844. doi:10.1002/ps.4883
Tumlinson, J. H., Mitchell, E. R., Teal, P. E. A., Heath, R. R., and Mengelkoch, L. J. (1986). Sex pheromone of fall armyworm, Spodoptera frugiperda (J.E. Smith). J. Chem. Ecol. 12, 1909–1926. doi:10.1007/BF01041855
Vaello, T., Casas, J. L., Pineda, A., de Alfonso, I., and Marcos-García, M. Á. (2017). Olfactory response of the predatory bug Orius laevigatus (Hemiptera: Anthocoridae) to the aggregation pheromone of its prey, Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol. 46, 1115–1119. doi:10.1093/ee/nvx141
Verheggen, F. J., Arnaud, L., Bartram, S., Gohy, M., and Haubruge, E. (2008). Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol. 34, 301–307. doi:10.1007/s10886-008-9434-2
Verheggen, F. J., Fagel, Q., Heuskin, S., Lognay, G., Francis, F., and Haubruge, E. (2007). Electrophysiological and behavioral responses of the multicolored Asian lady beetle, Harmonia axyridis Pallas, to sesquiterpene semiochemicals. J. Chem. Ecol. 33, 2148–2155. doi:10.1007/s10886-007-9370-6
Wang, K., Liu, J., Zhan, Y., and Liu, Y. (2019). A new slow-release formulation of methyl salicylate optimizes the alternative control of Sitobion avenae (Fabricius) (Hemiptera: Aphididae) in wheat fields. Pest Manag. Sci. 75, 676–682. doi:10.1002/ps.5164
Wang, L., Wang, K., Dong, J., and Liu, Y. (2018). Behavioral responses of Aphidius avenae Haliday and A. gifuensis Ashmead to the aphid pheromones (E)-β-farnesene and nepetalactone. Chin. J. Appl. Entomol. 55, 223–229. doi:10.7679/j.issn.2095-1353.2018.031
Wang, R., Jiang, C., Guo, X., Chen, D., You, C., Zhang, Y., et al. (2020). Potential distribution of Spodoptera frugiperda (J.E. Smith) in China and the major factors influencing distribution. Glob. Ecol. Conserv. 21, e00865. doi:10.1016/j.gecco.2019.e00865
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., et al. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46 (W1), W296–W303. doi:10.1093/nar/gky427
Wu, Q. L., Jiang, Y. Y., Liu, J., Hu, G., and Wu, K. M. (2021). Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 28, 649–661. doi:10.1111/1744-7917.12852
Keywords: chemical cue, predatory beetle, Spodoptera frugiperda, odorant-binding protein, population adaptability
Citation: Zhan Y, Wang J, Kong X and Liu Y (2023) Perception and kairomonal response of the coccinellid predator (Harmonia axyridis) to the fall armyworm (Spodoptera frugiperda) sex pheromone. Front. Physiol. 14:1167174. doi: 10.3389/fphys.2023.1167174
Received: 16 February 2023; Accepted: 27 March 2023;
Published: 10 April 2023.
Edited by:
Ettore Tiraboschi, University of Trento, ItalyReviewed by:
Hamadttu Abdel Farag El-Shafie, University of Khartoum, SudanZhi-Wei Kang, Hebei University, China
Copyright © 2023 Zhan, Wang, Kong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Liu, bGl1eW9uZ0BzZGF1LmVkdS5jbg==
†These authors share first authorship
 Yidi Zhan
Yidi Zhan Jiaojiao Wang†
Jiaojiao Wang† Yong Liu
Yong Liu