
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 30 March 2023
Sec. Invertebrate Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1155455
This article is part of the Research TopicThe Side Effects of Insecticides on Insects and the Adaptation Mechanisms of Insects to InsecticidesView all 15 articles
A correction has been applied to this article in:
Corrigendum: Effects of chlorantraniliprole on the life history traits of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae)
 Ali Hasnain1,2,3†
Ali Hasnain1,2,3† Shuirong Zhang1,2†
Shuirong Zhang1,2† Qinghua Chen4
Qinghua Chen4 Lijuan Xia5
Lijuan Xia5 Yutong Wu2
Yutong Wu2 Changwei Gong1,2
Changwei Gong1,2 Xuemei Liu1,2
Xuemei Liu1,2 Pu Jian1,2
Pu Jian1,2 Lei Zhang6
Lei Zhang6 Xuegui Wang1,2*
Xuegui Wang1,2*Introduction: Spodoptera frugiperda is an important nomadic agricultural pest with a diverse host range and resistance against several insecticides. The current study investigated the life history traits of two strains of the field-collected population against chlorantraniliprole using an age-stage two-sex life table.
Method: For this, we established the chlorantraniliprole-susceptible (Crp-SUS G12), and chlorantraniliprole-reduced susceptible (Crp-RES G12) strains derived from the sixth generation of the QJ-20 population having a resistance ratio (RR) of 10.39-fold, compared with the reported susceptible population.
Results: The results showed that the chlorantraniliprole-reduced susceptible strain attained a 4.0-fold RR, while the chlorantraniliprole-susceptible strain attained an RR of 0.85-fold, having overlapped fiducial limits (FLs) with the referred susceptible baseline. Meanwhile, the present study revealed that the development time of the susceptible strain was significantly longer than that of the reduced susceptible strain. Similarly, the mean longevity, adult pre-oviposition period (APOP), and total pre-oviposition period (TPOP) of the female chlorantraniliprole-susceptible strain were considerably longer than those of the female chlorantraniliprole-reduced susceptible strain. Contrarily, the population parameters, including the intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R), of the chlorantraniliprole-susceptible strain were considerably lower than those of the chlorantraniliprole-reduced susceptible strain, while the mean generation time (T) of the chlorantraniliprole-susceptible strain was substantially longer than the chlorantraniliprole-reduced susceptible strain. The age-stage characteristic survival rate (sxj) and age-stage characteristic life expectancy (exj) of the chlorantraniliprole-susceptible strain were longer than those of the chlorantraniliprole-reduced susceptible strain, but the age-stage-specific reproductive value (vxj) of the chlorantraniliprole-susceptible strain was shorter than that of the chlorantraniliprole-reduced susceptible strain. Moreover, the contents of vitellogenin (Vg) and VgR in the chlorantraniliprole-reduced susceptible strain were higher than those in the chlorantraniliprole-susceptible strain.
Discussion: These findings showed that reducing susceptibility to chlorantraniliprole promoted population growth in S. frugiperda. Therefore, this study could provide conceptual support for the integrated pest management (IPM) approach to control S. frugiperda in the field.
The fall armyworm (FAW), Spodoptera frugiperda (Lepidoptera, Noctuidae), a devastative agricultural pest in its innate range, North and South America, and during the last 10 years, has become a significant invasive pest on a global scale. Numerous variables, including a high reproductive capacity, long-distance migration, and multiple host plants, have been implicated in the widespread FAW (Barros et al., 2010; Westbrook et al., 2016). Additionally, it may move from overwintering areas to other suitable climate zones without entering diapause (Sparks, 1979; Hardke et al., 2015). FAW was first reported in China in January 2019, and there are several reports on severe damage caused by FAW to the fields of maize, rice, and wheat, threatening the food supply and causing yield loss in China (Xiao, 2021; Zhang et al., 2021). The continuous application of insecticides causes resistance in the pest population over time (Denholm and Devine, 2013). For FAW, the resistance development has been reported for about 29 different insecticides from America by 2017 (Gutierrez-Moreno et al., 2019) and is attributed to attaining exceptional deviations in physiology, behavior, reproduction, longevity, and biology (Haynes, 1988; Teke and Mutlu, 2021; Liu et al., 2022). The life history parameters, including insect fertility, mortality, and lifespan, can be better studied by using the age-stage two-sex life table because it computes the data by considering both sexes in the calculations, as compared to the conventional life table, which only focuses on the female population (Abdel-Khalek and Momen, 2022; Younas et al., 2022). Many studies have reported the usage of an age-stage two-sex life table in elaborating the life history traits of S. frugiperda that were reared on different host plants (Guo et al., 2021; Xie et al., 2021) and the effects of spinetoram on its growth and fecundity (Gao et al., 2021). Similarly, several other studies were conducted to elaborate the impact of different insecticides on the life history traits of insects, such as Spodoptera litura (Rehan et al., 2015), Oxycarenus hyalinipennis (Lygaeidae; Hemiptera) (Ullah et al., 2016), and Musca domestica (Shah et al., 2015). Vitellogenesis is vital for the reproduction of oviparous insects since it includes the synthesis and absorption of vitellogenin (Vg), a source of nutrients for embryonic development as it stimulates oocyte maturation (Roy et al., 2018; Wu et al., 2020), which is required for reproduction (Schneider, 1996; Ge et al., 2019). Therefore, studying the difference between the two strains was crucial because insecticidal stress also affects fecundity.
Chlorantraniliprole, an anthranilic diamide, works by modulating ryanodine receptors (RyRs) in the sarcoplasmic reticulum membrane, causing permanent paralysis in insect bodies owing to acute muscular contraction caused by excessive Ca2+ release in the cytosol that causes insect feeding cessation and death (Nauen, 2006). It has been reported to eradicate various insect orders, including Coleoptera, Diptera, and Lepidoptera (Sattelle et al., 2008). As a new pesticide for managing lepidopterous pests, i.e., Spodoptera exigua and Tryporyza incertulas, it is considered a great alternative in integrated pest management (IPM) (Lai et al., 2011). However, prolonged use of chlorantraniliprole may impair the ecosystem; thus, its adverse effects on FAW must be evaluated to optimize the use and reduce environmental damage.
This study aimed to investigate the effects of chlorantraniliprole on the life history traits of susceptible and reduced susceptible strains of the field-collected population of S. frugiperda from Southwest China and the difference in vitellogenin content of the female strains. Moreover, the degree of chlorantraniliprole ferocity and its influence on all larval instars of S. frugiperda could appropriately highlight its efficacy. Therefore, the effects of chlorantraniliprole on the life stages (larva, pupa, and adult) of both male and female S. frugiperda could be used as a reference for its effective management under field conditions.
The field population of S. frugiperda was collected from Qianjiang (QJ-20), located in the southwestern part of China (N 29°32ˊ01, E 108°46ˊ15), during the summer of 2020. The bioassay result showed a moderate resistance with an RR of 10.39-fold against chlorantraniliprole. About 150–200 larvae were procured at the sample location. The larvae and adults were raised on synthetic food and 10% sugar syrup, respectively. Subsequently, we used sodium hypochlorite solution (0.2%–0.3%) to disinfect the fresh eggs and pupae. After the hatching of eggs, larvae at all growth stages were grown under a controlled environment at temperature, relative humidity (RH), and photoperiod of 26°C ± 1°C, 70%–80%, and 16:8 (light: dark), respectively. The target insecticide used for screening was 95% chlorantraniliprole (Corteva Agriscience, Indianapolis, United States).
The toxicity of chlorantraniliprole was determined in the third instar of S. frugiperda by topical application. At first, we determined the dose range for chlorantraniliprole in a preliminary experiment by using a series of its concentration (i.e., 200, 100, 50, and 10 mg a.i. L−1). Later, technical chlorantraniliprole was dissolved and diluted to a series of concentrations using acetone (i.e., ranged from 1.05 to 21.06 mg a.i. L−1) to determine the LD50 values of the subsequent generations of the two strains (established from the field population). A 50-μL micro-syringe, coupled with a micro-applicator (PB600-1 Repeating Dispenser, Hamilton Company), was used to apply 1 μL droplet of each prepared concentration on the dorsal side of the frontal thorax of the third-instar larva (average weight of 0.006 g) (Gutierrez-Moreno et al., 2019). Five different insecticidal concentrations and control (each in triplicate) were prepared, with 12 larvae in each replication. The LD50 values were expressed in µg g−1 (active ingredient/larval weight). A measure of 1 µL of acetone per larva was applied for the control treatment. The treated larvae were placed on a 12-compartment plate with enough food, with experiments performed in triplicate for each concentration. Mortality was observed at 48 h after treatment. The larvae that expressed severe inebriation signs (slow movement, twitching, feeding interruption, and severe growth inhibition) were considered dead.
Based on bioassay observations, the two strains derived from QJ-20 (RR = 10.39-fold) against chlorantraniliprole were prepared using the approach reported by Wang et al. (2006). About 200 F6-generation larvae of QJ-20 were divided equally into two groups: the susceptible strain (Crp-SUS G12) and the reduced susceptible strain (Crp-RES G12), using a single-pair mating method. The former generations were kept in the laboratory, without insecticidal contact, to free the field-collected population from biotic or abiotic stress.
For establishing the Crp-RES G12 strain, the screening doses, i.e., LD70 of each generation (6.31, 8.42, 9.47, 11.57, 12.62, and 13.68 mg a.i. L−1 from G7 to G12 generation, respectively) were prepared. About 250–300 third-instar larvae were treated topically with 1 μL droplet of each designed concentration on the dorsal side of the frontal thorax of the third-instar larva for each generation using a 50-μL micro-syringe (Hamilton Company, Reno, NV), coupled with a micro-applicator (PB600-1 Repeating Dispenser, Hamilton Company). After 48 h, the survived larvae were shifted into the glass tubes with a fresh artificial diet and raised for the next generation.
However, for the Crp-SUS G12 strain, male and female adults were coupled independently, and about 50 pairs were prepared during each generation. The progeny of about 20 pairs was considered for selection in every generation. A total of 20 third-instar larvae were given the same dosage of chlorantraniliprole (i.e., 5.26 mg a.i. L−1) in pair. Subsequently, after 48 h treatment, the mortality was checked, and the larvae with >80% mortality were raised for obtaining the next generation.
Based on the generational screening result of the two strains, a prominent resistance difference (i.e., 4.0-fold) was progressively developed. Then, about 100 adults of each strain were kept in a clean cage covered with a clean muslin cloth. At the time of high fecundity, we randomly selected at least five egg masses and allowed them to air-dry at room temperature. Later, 100 larvae from the hatched eggs were raised separately in an artificial diet in a pre-labeled glass tube with 1.8 cm diameter and 10 cm height. Larvae at all growth stages were monitored daily and transferred to a six-compartment dish/plate. All the eggs and pupae were disinfected using sodium hypochlorite solution.
The fresh adults (developed from artificially raised larvae) were paired, and each pair was grown separately in plastic cups (500 mL; 9.5 cm diameter and 13.7 cm height) to establish a family. Each cup contained a wet cotton ball soaked daily with 10% sugar solution. Eventually, 30 families of each strain were established, and the population characteristics, including longevity, fecundity, and developmental time, were recorded every day until the couple’s death. The eggs were precisely counted and recorded. The life parameters, including time and duration of each growth stage, the emergence, life span, mating, and fecundity intensity of the adults, were recorded. The dataset of the values was analyzed to establish the life table.
The ovaries from five adult female adults of each strain were collected 2 days after their emergence (Zhen et al., 2018), weighed. A measure of 1 mL of PBS (pH 7.3) was added and then manually homogenized. The supernatant was then collected by centrifuging the mixture at 2,500 g for 20 min. The protein contents of Vg and VgR were determined according to the instructions of the ELISA kit (Shanghai Enzyme Biotechnology Co., Ltd., Product Code: mlbio104703 for insect vitellogenin and mlbio104704 for insect vitellogenin receptor): Insect Vg (or VgR) was added to a microtiter plate well that had been coated with a particular insect Vg (or VgR) antibody and then combined with an antibody that had been labeled with horseradish peroxidase (HRP) to form an antibody–antigen–enzyme–antibody complex. After thoroughly cleaning the plate, 3, 3′5, 5′-tetramethyl benzidine (TMB) substrate solution was added until the substrate turned blue, signifying HRP enzyme catalysis. The reaction was then stopped by adding sulfuric acid solution. The concentrations of Vg (or VgR) were calculated by comparing the absorbance (OD) of the samples to the standard curve using a microplate reader (Model 680 Microplate Reader, Bio-Rad) that measured the absorbance (OD) at 450 nm.
The POLO 2.0 program (LeOra Software, www.leorasoftware.com) was used to calculate the slope, LD50, 95% fiducial limits (FLs), and chi-square (X2) value of the insecticide after 48 h of treatment (Wang et al., 2018). The susceptible baseline value was referred from Wang et al. (2021). The raw data of the life table were examined using age-stage two-sex life table computer software. The basic parameters, including age-specific survival rate (lx), age-stage survival rate (sxj), finite rate of increase (λ), reproductive value (vxj), intrinsic rate of increase (r), net reproductive rate (R0), and mean generation time (T), were analyzed. Based on the confidence interval of the differences, the adult longevity, fecundity, adult pre-oviposition period (APOP), total pre-oviposition period (TPOP), developmental growth, and other population parameters (r, λ, R0, and T) were compared using the paired bootstrap test with 10,000 random resamplings. The intrinsic rate of increase (r) and the finite rate of increase (λ) are the two critical metrics for estimating the capacity for population expansion, used to indicate the population’s fitness. A sigma plot (SigmaPlot 12.0) was used for the graphical representation of survival rate and reproductive value curves.
The third-instar larvae were screened for toxicity of the two strains: Crp-SUS G12 and Crp-RES G12. The increased toxicity to chlorantraniliprole was observed for Crp-SUS G12 (LD50: 0.349 μg·g−1), having almost similar susceptibility to the referred susceptible strain as observed by the overlapping FL (fiducial limit) of its LD50 values (Table 1). In contrast, the Crp-RES G12 strain showed reduced susceptibility to chlorantraniliprole (LD50: 1.63 μg·g−1), and the resistance ratio increased by four-fold compared to the susceptible strain. Moreover, no overlapping of CI for LD50 was observed between Crp-RES G12 and susceptible strains (Table 2).
The life history traits of both strains were significantly different from each other. The developmental times of varying life stages of Crp-SUS G12 were considerably longer than those of Crp-RES G12 (p < 0.05) (Table 3). The mean longevity of male and female adults, APOP (adult pre-oviposition period), and TPOP (total pre-oviposition period) for Crp-SUS G12 were significantly longer than those for the Crp-RES G12 (p < 0.05), while the fecundity of Crp-SUS G12 was considerably lower than that of Crp-RES G12 (p < 0.05) (Table 4). However, the mean generation time (T) of the Crp-SUS G12 strain was much longer than that of the Crp-RES G12 strain, but the intrinsic rate of increase (r), finite rate of increase (λ), and net reproduction rate (R0) of the Crp-SUS G12 strain were all significantly lower than those of the Crp-RES G12 strain (Table 5).
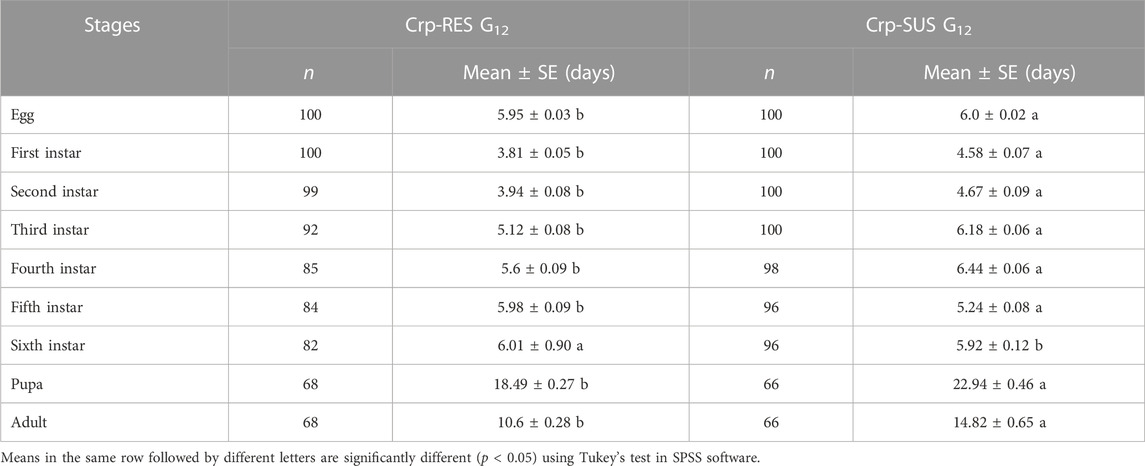
TABLE 3. Developmental time durations of various life stages of the Crp-RES G12 and Crp-SUS G12 strains.
The Crp-SUS G12 and Crp-RES G12 strains overlapped at various developmental phases and showed earlier terminating curves for female larvae than male larvae in the displayed peaks for each developmental stage. These peaks were more significant in the Crp-SUS G12 strain than in the Crp-RES G12 strain, indicating that male adults of the former had better survival rates. However, Crp-RES G12 female adults showed identical survival rates to male adults, but Crp-SUS G12 female larvae showed a lower peak and consequently poorer survival rates (Figure 1). During the pupal stage, the vxj value of the Crp-SUS G12 individuals was lower than that of the Crp-RES G12 individuals. When the female adults emerged, the Crp-RES G12 strain’s plotted curve increased somewhat higher than the Crp-SUS G12 strain before significantly declining. In the Crp-SUS G12 strain, the maximum vxj value was 241.1 d−1 on the 57th day, but in the Crp-RES G12 strain, it was 301.74 d−1 on the 56th day (Figure 2).
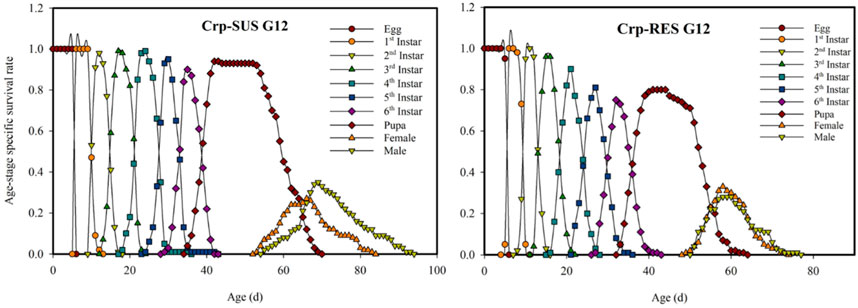
FIGURE 1. Age-stage-specific survival rate (sxj) for the Crp-RES G12 and Crp-SUS G12 strains of Spodoptera frugiperda.
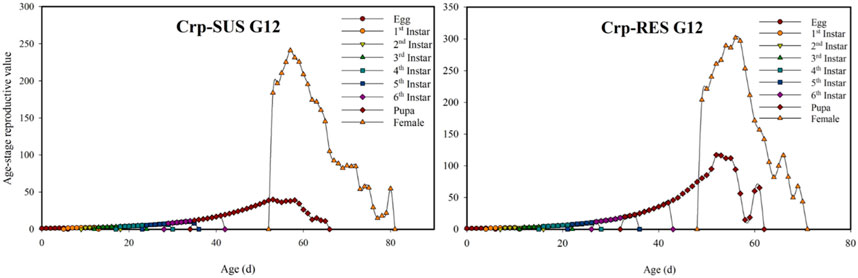
FIGURE 2. Age-stage reproductive value (vxj) for the Crp-RES G12 and Crp-SUS G12 strains of S. frugiperda.
In this research, the Crp-SUS G12 strain had a higher age-stage-specific life expectancy (exj) than the Crp-RES G12 strain (Figure 3). The age-specific survival rates (lx) for these strains indicated that the lx value decreased more quickly for the Crp-RES G12 strain at 15 d than the Crp-SUS G12 strain. In addition, the highest mean fecundity (mx) for Crp-RES G12 was 140.85 eggs compared to the Crp-SUS G12 that showed a mean fecundity of 85 eggs (Figure 4).
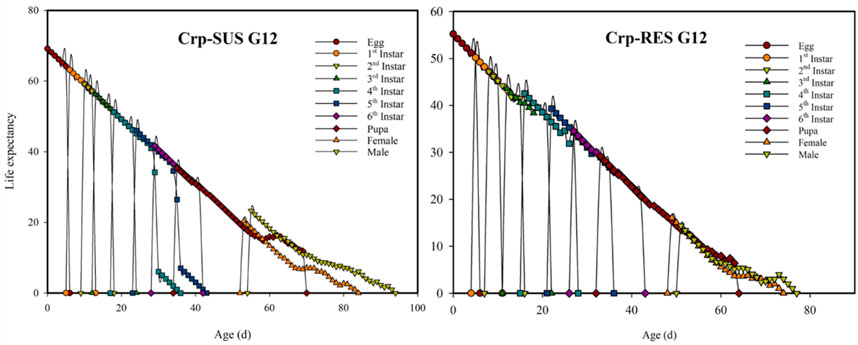
FIGURE 3. Age-stage-specific life expectancies (exj) for the Crp-RES G12 and Crp-SUS G12 strains of S. frugiperda.
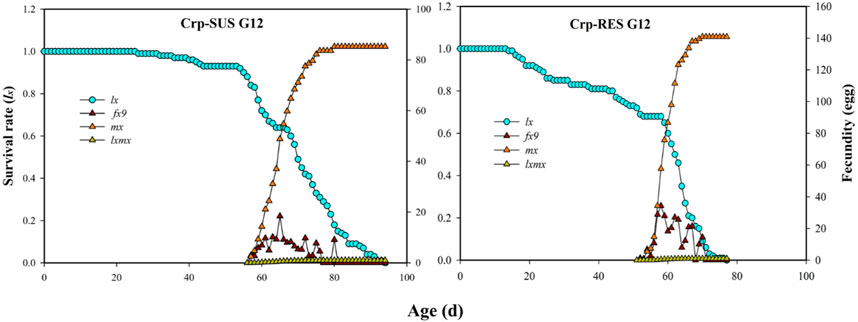
FIGURE 4. Age-specific survival rate (lx), female age-specific fecundity (fx9), age-specific fecundity of the total population (mx), and age-specific net maternity (lxmx) of the Crp-RES G12 and Crp-SUS G12 strains of S. frugiperda.
The results indicated that the Vg content in the Crp-RES G12 strain (8,702.9 ng/g) was significantly more than that in the Crp-SUS G12 strain (7,581.7 ng/g) (p < 0.01). Similarly, the VgR content in Crp-RES G12 (414.6 ng/g) was significantly more than that in Crp-SUS G12 (318.8 ng/g) (p < 0.01) (Figure 5).
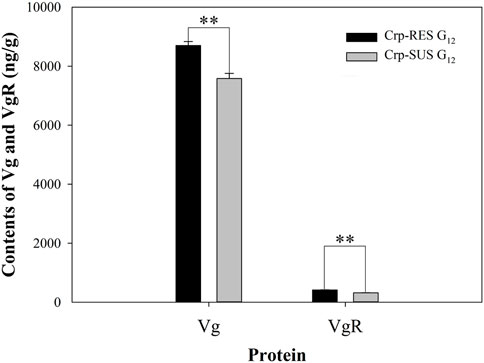
FIGURE 5. Contents of Vg and VgR for Crp-RES G12 and Crp-SUS G12 strains were presented as the mean of three replications ± SE. The asterisks above the bars indicate statistical differences between Vg and VgR contents of both strains (Student’s t-test, **p < 0.01).
The development of insect life tables is crucial for pest control methods when evaluating insect population dynamics (Gabre et al., 2005). Numerous biological and non-biological elements, including temperature, light, and the host plants, cause changes in insect populations (Tuan et al., 2014). Chemicals are currently considered one of the most effective integrated pest control strategies. However, the pervasive use of pesticides has led to substantial resistance, which is now a significant factor altering the parameters of insects’ life tables. Adequate knowledge about the life cycle, survival rate, and reproduction can be helpful in developing efficient management strategies against insect pests (Harcourt, 1969). In this study, we established the susceptible and reduced susceptible strains of S. frugiperda against chlorantraniliprole, and resistance ratios were gradually decreased (RR = 0.85 -fold) and increased (RR = 4.0 -fold) in both strains, respectively. Based on the results, all life stages of the Crp-SUS G12 strain had significantly longer time duration than those of the Crp-RES G12 strain, which is consistent with the findings of Abbas et al. (2012) in which imidacloprid application enhanced the emergence rate of healthy adults, developmental time, and fecundity of S. litura. In contrast, it was also reported that the fecundity of S. litura was reduced under the influence of emamectin benzoate, along with an increase in the larval duration and developmental time (Zaka et al., 2014), which was similar in the case of the Crp-SUS G12 strain in this study. Similarly, the thiamethoxam-selected individuals of Mythimna separata had prolonged larval and developmental times (Yasoob et al., 2018). This tendency suggests that different pesticides can alter the insect life history characteristics, which are also affected by many other variables, including the temperature (Chi et al., 2016), host plants (Nematikalkhoran et al., 2018), and insecticides (Steinbach et al., 2017).
Furthermore, chlorantraniliprole significantly affected the total pre-oviposition period (TPOP), mean longevities of male (M) and female (F) adults, and developmental times (T) of Crp-RES G12. These were positively related to the variations in intrinsic rate of increase (r), net reproductive rate (R), and finite rate of increase (λ). The fecundity and reproduction of both strains were greatly affected by chlorantraniliprole in this study. The plotted curves of the age-specific survival rate (lx) showed that the resistant strain showed the fitness cost of its normal survival rate and adapted to increase its eggs for better survival while shortening its life span. However, the Crp-SUS G12 strain modified itself toward only better survival and tried to maintain an average life duration. The age-stage reproductive value (vxj) also revealed the same trend with increased life span and decreased fecundity in the Crp-SUS G12 strain and vice versa. Similar results were also reported by Huang et al. (2019) that the bistrifluron-resistant strain of S. litura had higher values of population metrics along with an increase in fecundity than the bistrifluron-susceptible strain. However, in contrast, Han et al. (2012) showed that the fecundity decreased dramatically, along with the decrease in the values of R, r, and λ, when the sublethal doses of chlorantraniliprole were applied to the larvae of Plutella xylostella. Still, as in our study, we established Crp-RES G12 by screening LD70 of each generation (which is more than the median lethal dosage), and thus its effects are different from the sublethal doses. Moreover, the varied amounts of pesticides may contribute to differences between past and present results, based on the diverse species of insects evaluated and the pesticide application method.
Moreover, the fecundity in female adults is primarily governed by vitellogenin (Vg), and its receptor (VgR), as a precursor to Vg, is involved in the sequestration of Vg into the oocytes through endocytosis during the development and subsequently crucial for Vg uptake and oocyte maturation (Lu et al., 2015). The results of our study showed that the protein contents of Vg and VgR for Crp-RES G12 were higher than those for Crp-SUS G12, which supported the findings of Chen et al. (2020) that under the influence of triflumezopyrim, Vg and VgR contents increased in the subsequent F4 generation of Sogatella furcifera. In addition, Ge et al. (2011) reported that Vg content in the female larvae of Nilaparvata lugens significantly increased under the influence of triazophos. Similarly, Liu et al. (2016) also reported that the resistant strain of Tetranychus cinnabarinus treated with fenpropathrin had more protein content than the sensitive strain.
These results from life table data proved that the reduction in susceptibility against chlorantraniliprole positively affected the life history traits of S. frugiperda. However, to comprehend the impact of chlorantraniliprole on the life parameters of the individuals of Crp-SUS G12 and Crp-RES G12, further screening and follow-up research can be carried out to get more resistant individuals. In addition, as an alternate plan to integrated pest management, to prevent chlorantraniliprole from promoting the population dynamics of S. frugiperda, alternative no cross-resistance chemical insecticides or natural control agents should be used.
This study about the life history traits revealed that female adults of the reduced susceptible strain increased their fertility while lowering their life span to withstand chlorantraniliprole. In contrast, female adults of the susceptible strain decreased their fecundity while increasing their life span. These findings may serve as a foundation for subsequent research on chlorantraniliprole resistance in S. frugiperda and can contribute to developing the integrated pest management (IPM) program for fall armyworms in the future.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
AH and XW: Conceptualization and methodology. AH, SZ, and XW: Writing the original draft. AH, QC, LX, YW, CG, LZ, and XL: Investigation and formal analysis. LZ, PJ, and XW: Supervision, review, and editing. XW: Project administration and funding acquisition.
The authors gratefully acknowledge the financial support of the Science and Technology Program of Sichuan, China (2022YFSY0034).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PL declared a shared affiliation with the author LZ to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, N., Shad, S. A., and Razaq, M. (2012). Fitness cost, cross resistance and realized heritability of resistance to imidacloprid in Spodoptera litura, (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 103, 181–188. doi:10.1016/j.pestbp.2012.05.001
Abdel-Khalek, A. A., and Momen, F. M. (2022). Biology and life table parameters of Proprioseiopsis lindquisti on three eriophyid mites (Acari: Phytoseiidae: Eriophyidae). Persian J. Acarol. 11, 59–69. doi:10.22073/pja.v11i1.68574
Barros, E. M., Torres, J. B., Ruberson, J. R., and Oliveira, M. D. (2010). Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 137, 237–245. doi:10.1111/j.1570-7458.2010.01058.x
Chen, L., Wang, X., Zhang, Y., Yang, R., Zhang, S., Xu, X., et al. (2020). The population growth, development and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) under the sublethal dose of triflumezopyrim. Chemosphere 247, 125865. doi:10.1016/j.chemosphere.2020.125865
Chi, B., Zheng, X., Liang, X., and Liu, Y. (2016). Temperature-dependent demography of Agriphila aeneociliella (Lepidoptera: Crambidae), a new insect pest of wheat in China. Agric. For. Entomol. 18, 189–197. doi:10.1111/afe.12151
Denholm, I., and Devine, G. (2013). Insecticide resistance. Encycl. Biodivers. 3, 298–307. doi:10.1016/B978-0-12-384719-5.00104-0
Gabre, R. M., Adham, F. K., and Chi, H. (2005). Life table of Chrysomya megacephala (fabricius) (Diptera: Calliphoridae). Acta Oecol 27, 179–183. doi:10.1016/j.actao.2004.12.002
Gao, Z., Chen, Y., He, K., Guo, J., and Wang, Z. (2021). Sublethal effects of the microbial-derived insecticide spinetoram on the growth and fecundity of the fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 114, 1582–1587. doi:10.1093/jee/toab123
Ge, L. Q., Cheng, Y., Wu, J. C., and Jahn, G. C. (2011). Proteomic analysis of insecticide triazophos-induced mating-responsive proteins of Nilaparvata lugens stål (Hemiptera: Delphacidae). J. Proteome Res. 10, 4597–4612. doi:10.1021/pr200414g
Ge, L. Q., Zheng, S., Gu, H. T., Zhou, Y. K., Zhou, Z., Song, Q. S., et al. (2019). Jinggangmycin-induced UDP-glycosyltransferase 1-2-like is a positive modulator of fecundity and population growth in Nilaparvata lugens (stål) (Hemiptera: Delphacidae). Front. Physiol. 10, 747. doi:10.3389/fphys.2019.00747
Guo, J., Zhang, M., Gao, Z., Wang, D., He, K., and Wang, Z. (2021). Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Sci. 28, 602–610. doi:10.1111/1744-7917.12830
Gutierrez-Moreno, R., Mota-Sanchez, D., Blanco, C. A., Whalon, M. E., Teran-Santofimio, H., Rodriguez-Maciel, J. C., et al. (2019). Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 112, 792–802. doi:10.1093/jee/toy372
Han, W., Zhang, S., Shen, F., Liu, M., Ren, C., and Gao, X. (2012). Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 68, 1184–1190. doi:10.1002/ps.3282
Harcourt, D. G. (1969). The development and use of life tables in the study of natural insect populations. Annu. Rev. Entomol. 14, 175–196. doi:10.1146/annurev.en.14.010169.001135
Hardke, J. T., Lorenz, G. M., and Leonard, B. R. (2015). Fall armyworm (Lepidoptera: Noctuidae) ecology in southeastern cotton. J. Integr. Pest Manag. 6, 10. doi:10.1093/jipm/pmv009
Haynes, K. F. (1988). Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 33, 149–168. doi:10.1146/annurev.en.33.010188.001053
Huang, Q., Wang, X., G., Yao, X., Gong, C., and Shen, L. (2019). Effects of bistrifluron resistance on the biological traits of Spodoptera litura (fab.) (Noctuidae: Lepidoptera). Ecotoxicology 28, 323–332. doi:10.1007/s10646-019-02024-2
Lai, T. C., Li, J., and Su, J. Y. (2011). Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 101, 198–205. doi:10.1016/j.pestbp.2011.09.006
Liu, X., Shen, G. M., Xu, H. R., and He, L. (2016). The fenpropathrin resistant Tetranychus cinnabarinus showed increased fecundity with high content of vitellogenin and vitellogenin receptor. Pestic. Biochem. Physiol. 134, 31–38. doi:10.1016/j.pestbp.2016.04.010
Liu, Z. K., Li, X. L., Tan, X. F., Yang, M. F., Idrees, A., Liu, J. F., et al. (2022). Sublethal effects of emamectin benzoate on fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Agriculture 12, 959. doi:10.3390/agriculture12070959
Lu, K., Shu, Y. H., Zhou, J. L., Zhang, X. Y., Zhang, X. Y., Chen, M. G., et al. (2015). Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stål). J. Insect Physiol. 73, 20–29. doi:10.1016/j.jinsphys.2015.01.007
Nauen, R. (2006). Insecticide mode of action: Return of the ryanodine receptor. Pest Manag. Sci. 62, 690–692. doi:10.1002/ps.1254
Nematikalkhoran, M., Razmjou, J., Borzoui, E., and Naseri, B. (2018). Comparison of life table parameters and digestive physiology of Rhyzopertha dominica (Coleoptera: Bostrichidae) fed on various barley cultivars. J. Insect Sci. 18 (2), 31. doi:10.1093/jisesa/iey022
Rehan, A., Freed, S., and Abbas, N. (2015). Fitness cost of methoxyfenozide and the effects of its sublethal doses on development, reproduction, and survival of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Neotrop. Entomol. 44, 513–520. doi:10.1007/s13744-015-0306-5
Roy, S., Saha, T. T., Zou, Z., and Raikhel, A. S. (2018). Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 63, 489–511. doi:10.1146/annurev-ento-020117-043258
Sattelle, D. B., Cordova, D., and Cheek, T. R. (2008). Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invert. Neurosci. 8 (3), 107–119. doi:10.1007/s10158-008-0076-4
Schneider, W. J. (1996). Vitellogenin receptors: Oocyte-specific members of the low-density lipoprotein receptor supergene family. Int. Rev. Cytol. 166, 103–137. doi:10.1016/s0074-7696(08)62507-3
Shah, R. M., Shad, S. A., and Abbas, N. (2015). Mechanism, stability and fitness cost of resistance to pyriproxyfen in the house fly, Musca domestica L. (Diptera: Muscidae). Pestic. Biochem. Physiol. 119, 67–73. doi:10.1016/j.pestbp.2015.02.003
Sparks, A. N. (1979). A review of the biology of the fall armyworm. Fla. Entomol. 62, 82–87. doi:10.2307/3494083
Steinbach, D., Moritz, G., and Nauen, R. (2017). Fitness costs and life table parameters of highly insecticide-resistant strains of Plutella xylostella (L.) (Lepidoptera: Plutellidae) at different temperatures. Pest Manag. Sci. 73 (9), 1789–1797. doi:10.1002/ps.4597
Teke, M. A., and Mutlu, Ç. (2021). Insecticidal and behavioral effects of some plant essential oils against Sitophilus granarius L. and Tribolium castaneum (Herbst). J. Plant Dis. Prot. 128, 109–119. doi:10.1007/s41348-020-00377-z
Tuan, S. J., Lee, C. C., and Chi, H. (2014). Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 70, 805–813. doi:10.1002/ps.3618
Ullah, S., Shad, S. A., and Abbas, N. (2016). Resistance of dusky cotton bug, Oxycarenus hyalinipennis costa (lygaidae: Hemiptera), to conventional and novel chemistry insecticides. J. Econ. Entomol. 109 (1), 345–351. doi:10.1093/jee/tov324
Wang, H. H., Lv, S. L., Zhao, R., Liang, P., Zhang, S., Gao, X. W., et al. (2021). Establishment of the relative susceptible baselines of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae to commonly used insecticides. Acta. Entomol. sini. 64, 1427–1432. doi:10.16380/j.kcxb.2021.12.008
Wang, W., Mo, J., Cheng, J., Zhuang, P., and Tang, Z. (2006). Selection and characterization of spinosad resistance in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 84, 180–187. doi:10.1016/j.pestbp.2005.07.002
Wang, X. G., Xiang, X., Yu, H. L., Liu, S., Yin, Y., Cui, P., et al. (2018). Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan province, China. Pestic. Biochem. Physiol. 146, 71–79. doi:10.1016/j.pestbp.2018.02.008
Westbrook, J. K., Nagoshi, R. N., Meagher, R. L., Fleischer, S. J., and Jairam, S. (2016). Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 60, 255–267. doi:10.1007/s00484-015-1022-x
Wu, Z., Yang, L., He, Q., and Zhou, S. (2020). Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 8, 593613. doi:10.3389/fcell.2020.593613
Xiao, Y. T. (2021). Research on the invasive pest of fall armyworm (Spodoptera frugiperda) in China. J. Integr. Agric. 20, 633–636. doi:10.1016/s2095-3119(21)63623-7
Xie, W., Zhi, J., Ye, J., Zhou, Y., Li, C., Liang, Y., et al. (2021). Age-stage, two-sex life table analysis of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) reared on maize and kidney bean. Chem. Biol. Technol. Agric. 8, 44. doi:10.1186/s40538-021-00241-8
Yasoob, H., Abbas, N., Li, Y., and Zhang, Y. (2018). Selection for resistance, life history traits and the biochemical mechanism of resistance to thiamethoxam in the maize armyworm, Mythimna separata (Lepidoptera: Noctuidae). Phytoparasitica 46, 627–634. doi:10.1007/s12600-018-0692-4
Younas, H., Razaq, M., Farooq, M. O., and Saeed, R. (2022). Host plants of Phenacoccus solenopsis (Tinsley) affect parasitism of Aenasius bambawalei (Hayat). Phytoparasitica 50, 669–681. doi:10.1007/s12600-022-00980-w
Zaka, S. M., Abbas, N., Shad, S. A., and Shah, R. M. (2014). Effect of emamectin benzoate on life history traits and relative fitness of Spodoptera litura, (Lepidoptera: Noctuidae). Phytoparasitica 42, 493–501. doi:10.1007/s12600-014-0386-5
Zhang, D. D., Xiao, Y. T., Xu, P. J., Yang, X. M., Wu, Q. L., and Wu, K. M. (2021). Insecticide resistance monitoring for the invasive populations of fall armyworm, Spodoptera frugiperda in China. J. Integr. Agric. 19 (0), 783–791. doi:10.1016/s2095-3119(20)63392-5
Keywords: Spodoptera frugiperda, resistance, life history traits, chlorantraniliprole, vitellogenin
Citation: Hasnain A, Zhang S, Chen Q, Xia L, Wu Y, Gong C, Liu X, Jian P, Zhang L and Wang X (2023) Effects of chlorantraniliprole on the life history traits of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Front. Physiol. 14:1155455. doi: 10.3389/fphys.2023.1155455
Received: 31 January 2023; Accepted: 14 March 2023;
Published: 30 March 2023.
Edited by:
Xiaoming Xia, Shandong Agricultural University, ChinaReviewed by:
Pei Liang, China Agricultural University, ChinaCopyright © 2023 Hasnain, Zhang, Chen, Xia, Wu, Gong, Liu, Jian, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuegui Wang, d2FuZ3h1ZWd1aUBzaWNhdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.