
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 28 April 2023
Sec. Computational Physiology and Medicine
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1146910
 Pengyu Ye1
Pengyu Ye1 Sihe Li2
Sihe Li2 Zhongzheng Wang1
Zhongzheng Wang1 Siyu Tian1
Siyu Tian1 Yi Luo3
Yi Luo3 Zhanyong Wu4
Zhanyong Wu4 Yan Zhuang5
Yan Zhuang5 Yingze Zhang1
Yingze Zhang1 Marcin Grzegorzek2
Marcin Grzegorzek2 Zhiyong Hou1*
Zhiyong Hou1*Objective: To develop and test a deep learning (DL) model to distinguish acetabular fractures (AFs) on pelvic anteroposterior radiographs (PARs) and compare its performance to that of clinicians.
Materials and methods: A total of 1,120 patients from a big level-I trauma center were enrolled and allocated at a 3:1 ratio for the DL model’s development and internal test. Another 86 patients from two independent hospitals were collected for external validation. A DL model for identifying AFs was constructed based on DenseNet. AFs were classified into types A, B, and C according to the three-column classification theory. Ten clinicians were recruited for AF detection. A potential misdiagnosed case (PMC) was defined based on clinicians’ detection results. The detection performance of the clinicians and DL model were evaluated and compared. The detection performance of different subtypes using DL was assessed using the area under the receiver operating characteristic curve (AUC).
Results: The means of 10 clinicians’ sensitivity, specificity, and accuracy to identify AFs were 0.750/0.735, 0.909/0.909, and 0.829/0.822, in the internal test/external validation set, respectively. The sensitivity, specificity, and accuracy of the DL detection model were 0.926/0.872, 0.978/0.988, and 0.952/0.930, respectively. The DL model identified type A fractures with an AUC of 0.963 [95% confidence interval (CI): 0.927–0.985]/0.950 (95% CI: 0.867–0.989); type B fractures with an AUC of 0.991 (95% CI: 0.967–0.999)/0.989 (95% CI: 0.930–1.000); and type C fractures with an AUC of 1.000 (95% CI: 0.975–1.000)/1.000 (95% CI: 0.897–1.000) in the test/validation set. The DL model correctly recognized 56.5% (26/46) of PMCs.
Conclusion: A DL model for distinguishing AFs on PARs is feasible. In this study, the DL model achieved a diagnostic performance comparable to or even superior to that of clinicians.
Acetabular fractures (AFs) are one of the most complex traumas treated by orthopedic surgeons, which are mostly caused by high-energy trauma and have complicated and variable imaging features (Ziran et al., 2019). They are regularly accompanied by other concomitant injuries and occur in approximately 3–8 cases per 100,000 people (Laird and Keating, 2005; Melhem et al., 2020; Lundin et al., 2021). In addition, the hip joint is an essential weight-bearing joint with a wide range of motion, and its injury has a significant detrimental effect on an individual’s physical function, ability to work, and participation in social activities (FFA et al., 2021). Hence, AFs must be accurately and promptly detected, since diagnostic failure can lead to hip instability, post-traumatic osteoarthritis, and other poor outcomes (Scheinfeld et al., 2015).
A pelvic anteroposterior radiograph (PAR) provides visualization of the continuity and integrity of the bones in the pelvic and upper femur regions. It is the first choice for screening AFs (Trauma, 2018; Kitamura, 2020). Generally, traumas such as AFs are initially diagnosed and tended to by young trauma orthopedic surgeons because of their emergency nature (Oka et al., 2021). However, the diagnosis of AFs on X-ray images is difficult because AFs are uncommon, the femoral head and acetabulum overlap, and the potential existence of a single smaller fragment (Scheinfeld et al., 2015). Furthermore, radiologists are not always readily available, particularly in local hospitals or rural areas, which increases the uncertainty of diagnosis (Cheng et al., 2021; Zech et al., 2022). Thus, a new solution for the accurate and rapid detection of AFs is urgently required.
Deep learning (DL) is a group of artificial intelligence techniques that enable algorithms to learn from input data, identify features, and classify data after multiple iterations (Kuo et al., 2022; Zech et al., 2022). DL has evolved in leaps and bounds in the last few years and has been increasingly applied in radiology, orthopedics, and traumatology (Jones et al., 2020; Kuo et al., 2022). Previous studies have shown that fracture detection using DL can be on par with or exceed the diagnostic performance of physicians (Yamada et al., 2020; Cheng et al., 2021; Kuo et al., 2022). Although the detection of hip and pelvic ring fractures using DL on PAR has been reported (Kitamura, 2020; Yamada et al., 2020; Cheng et al., 2021; Liu et al., 2022), to our knowledge, studies focusing on identifying AFs are lacking. This study aimed to develop a DL model for detecting AFs on PAR and validate the strength of the model by comparing its diagnostic performance with that of orthopedic clinicians.
This study was approved by the institutional review board. The requirement for informed consent was waived owing to the retrospective nature of the study.
Consecutive patients with AFs who underwent PAR from January 2013 to October 2021 at a big level I trauma center were retrospectively reviewed. Moreover, AF patients from two external hospitals from January 2020 to December 2020 were selected as validation data. Images were collected from a picture archiving and communication system (PACS). The inclusion criteria were as follows: 1) AFs with a clear history of trauma, 2) adults (>18 years old), and 3) the first PAR was taken after the patient arrived at our hospital. The exclusion criteria were as follows: 1) pathological fractures; 2) congenital hip malformations; 3) poor image quality or foreign bodies occluding the acetabulum. More advanced imaging examination evidence from PACS, such as CT, MRI (if any), and clinical information from patient files provide sufficient support for the diagnosis and classification of AFs.
Demographic characteristics, including age, sex, injured side, and mechanism of injury, were recorded from patient files. Two traumatic orthopedic surgeons evaluated the fracture type (one with 6 years of experience and the other with 10). When an inconsistency occurred, they discussed this and made a final decision with another orthopedic specialist with more than 25 years of experience. All AFs were classified into three main types: type A, type B, and type C, based on the “Three-column classification of AFs” (Zhang et al., 2019). According to the above classification, types A, B, and C represent fractures involving one, two, and three columns of the acetabulum, respectively, and indicate more severe and complicated fractures.
The original panoramic images of pelvic radiographs were obtained from the PACS with sizes of 1,024 × 1,024 pixels and 32-bit depth. The regions of interest (ROIs) containing the acetabulum were manually cropped using a 256 × 256 square bounding box with Snipaste software (v2.4.0-Beta) and converted to 8-bit depth grayscale images to match the predefined input image of the DL model. Both sides of the acetabulum were extracted individually and labelled as normal (negative) or not (positive). Moreover, because the left and right acetabula are symmetrical, the cropped left acetabular images were flipped horizontally and used as right acetabular developing images (Figure 1). DL often requires a lot of data to achieve satisfactory results. Data augmentation is a commonly used data preprocessing technique in DL that can increase the quantity of data for training. To improve the robustness of the model, the development dataset was augmented 15 times by brightness control, rotation, blurring, and noise addition. Details of the data augmentation are provided in the Supplementary Material.
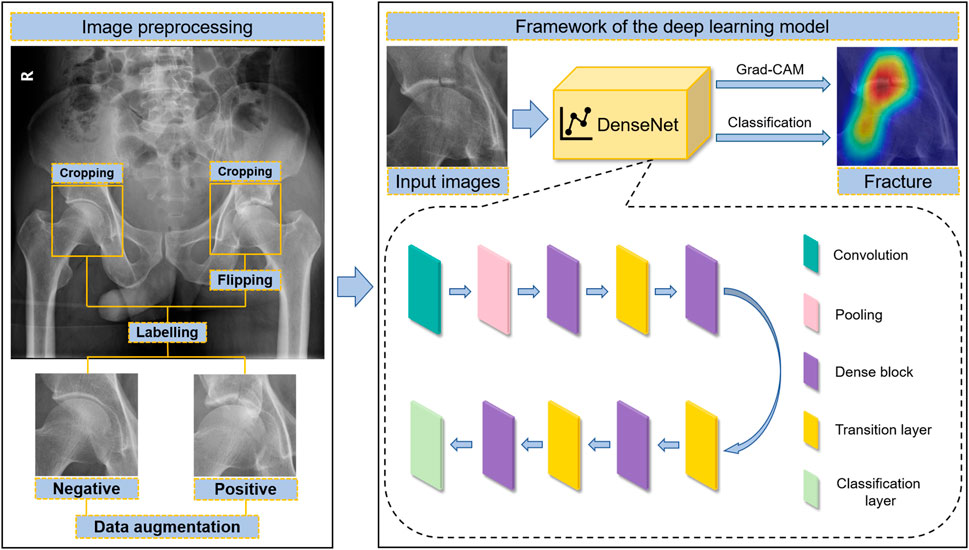
FIGURE 1. The workflow of acetabular fracture detection by artificial intelligence, including image preprocessing and the framework of the DL model.
Our DL network model was built based on DenseNet-169 architecture (Huang et al., 2017). DenseNet introduced dense connections to extract features from images. Compared with other convolutional networks, it can alleviate the vanishing-gradient problem, reduce the complexity of the model, and implement feature reuse (Huang et al., 2017). Our model was mainly composed of a 7 × 7 convolutional layer, followed by a max pooling layer, four dense blocks, three transition layers between dense blocks, and a classification layer at the end. Inside each dense block, 1 × 1 convolutional bottleneck layers and 3 × 3 convolutional layers were connected to each other in series. Pre-activation was introduced to each convolutional layer. The final classification layer was constructed using a fully connected layer, followed by sigmoid calculation. The output of the complete model was described as the probability of indicating the result of the prediction (Supplementary Table S1). The model was programmed using TensorFlow 2.5.0. PAR images were processed using OpenCV 4.5.4.60. The input images were grayscale images with a size of 256 × 256 pixels, and the value of each pixel was normalized from 0 to 1. The growth rate in the model was 12. Twelve feature maps were extracted in the bottleneck layers to decrease the model complexity and calculation computation.
The model was designed to classify whether the acetabular image included fractures. The training parameters were as follows: the batch size was set to 15 and the learning rate was initially set to 0.003 and was decreased every 10 epochs by 10%. An Adam optimizer was used, and a drop-box with a dropout rate of 0.2 was applied for training.
We recruited five residents and five attending orthopedic surgeons with 1–4 and 5–8 years of acute trauma-related experience, respectively, for the diagnostic tests. The aim was to compare the diagnostic performance of clinicians with that of the DL model. Before testing, all 10 clinicians were instructed to focus on only one question, to indicate whether there was an AF. Then, the ROI images of the test set were automatically presented on a screen one by one at 5-s intervals to each participating clinician. The entire experiment was divided into two parts, and 280 images were captured simultaneously. This process was adopted to ensure fairness between DL and clinicians and between clinicians, even though the test process was different from that in real clinical settings. The ROI images from the validation set were also performed at one time as described above. Based on the test results, a potential misdiagnosed case (PMC) was defined as a fracture missed by five or more of the 10 clinicians. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated to evaluate the clinicians’ diagnostic performance.
R 4.1.0 with “pROC” and “ggplot2” packages was used for statistical analyses and graphs. Continuous variables were compared using the Mann–Whitney U test, and categorical variables were compared using the chi-square and Fisher’s exact tests. The detection performance of the DL model was evaluated and compared using the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) with 95% corresponding confidence intervals (CIs), which were estimated by bootstrapping (2,000 times). The optimal threshold value was determined using Youden’s J-statistic. McNemar’s test was used to compare the sensitivity and specificity between the DL model and clinicians. The statistical significance level was set at p < 0.05.
A total of 1,120 patients in a single trauma center were enrolled and randomly divided into development and test sets at a ratio of 3:1. Among the 1,120 selected patients, 8 had bilateral AFs. Therefore, 840 patients with 846 injured acetabula and 834 normal acetabula in the development set and 280 patients with 282 broken acetabula and 278 normal acetabula in the test set were obtained. Additionally, 420 type A, 412 type B, and 296 type C AFs were identified and classified into 1,128 ROI fracture images. A total of 86 patients with 86 injured acetabula containing 33 type A, 36 type B, and 17 type C AFs from two hospitals were enrolled. The patients’ demographic data are shown in Table 1. Regarding age, sex, injured side, mechanism of injury, and fracture classification, there were no statistical differences (all p > 0.05) between the test and validation sets.
For the test/validation set comprising 560/172 ROI images, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the DL detection model were 0.926/0.872, 0.978/0.988, 0.978/0.987, 0.928/0.885, and 0.952/0.930, respectively. The ROC curve with visual AUC is shown in Figure 2. The optimal threshold value was 0.637/0.256, and the Youden index was 0.911 (sensitivity, 0.926; specificity, 0.985)/0.883 (sensitivity, 0.895; specificity, 0.988) for the test/validation sets.
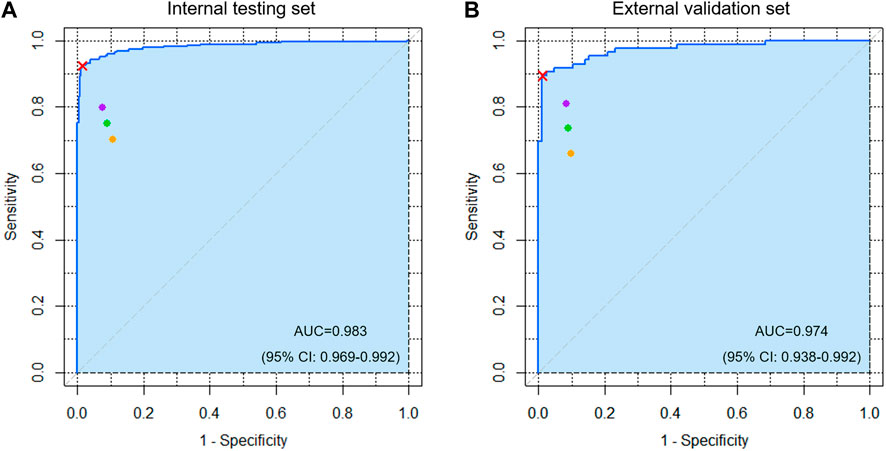
FIGURE 2. The receiver operating characteristic (ROC) curve of the detection algorithm for acetabular fractures in the (A) internal testing and (B) external validation sets. The cyan area indicates AUC. The red cross mark represents the performance on the probability cutoff value calculated by Youden’s J statistic. The purple, orange, and green spots represent the performance of five attending orthopedic surgeons, five residents, and ten clinicians, respectively.
Table 2 shows the diagnostic performance of the DL model, clinicians, and their comparisons. The mean sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the 10 clinicians were 0.750/0.735, 0.909/0.909, 0.894/0.890, 0.786/0.774, and 0.829/0.822 for the test/validation sets, respectively. Moreover, the mean results of the five attending orthopedic surgeons are higher than that of the residents. However, the DL model performed better (p < 0.05) with respect to sensitivity and specificity, except for the sensitivity of one clinician and specificity of three clinicians in the test set and the sensitivity and specificity of four clinicians in the validation set.
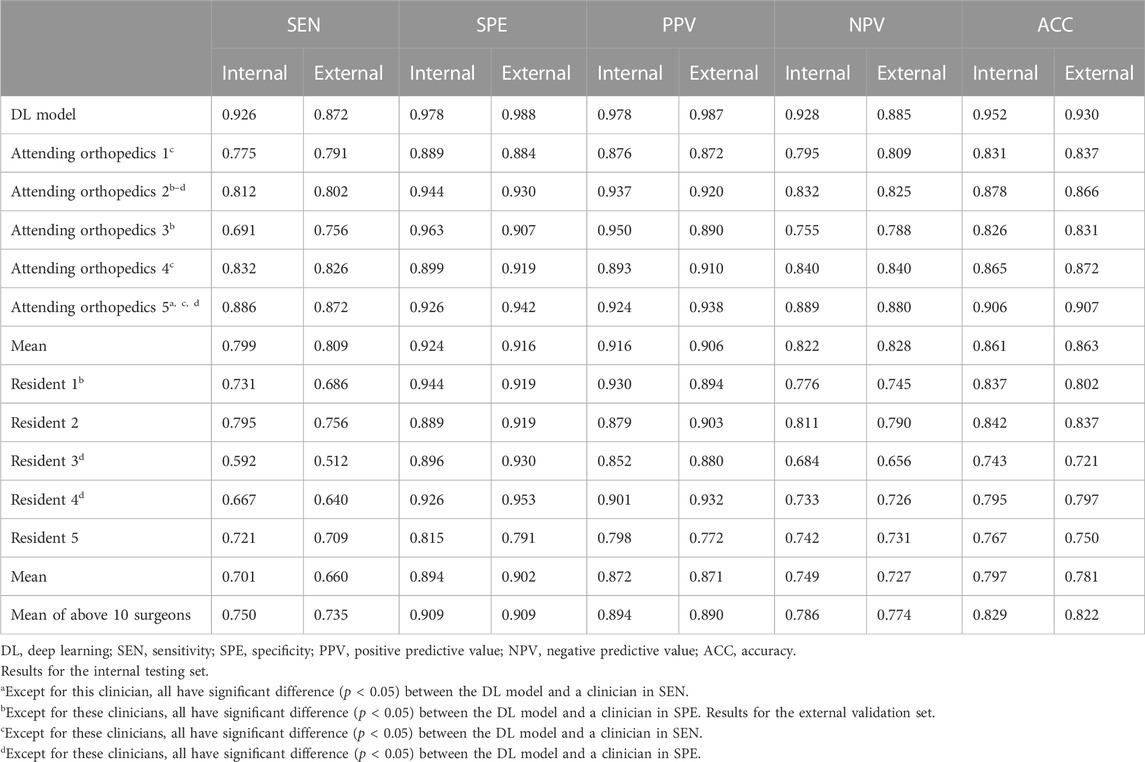
TABLE 2. Performance comparison between the DL model and clinicians in detecting acetabular fractures.
For types A, B, and C AFs, the DL model had the best diagnostic performance for type C fractures, with an AUC value of 1.000 (95% CI: 0.975–1.000)/1.000 (95% CI: 0.897–1.000); type A fracture was the worst, with an AUC value of 0.963 (95% CI: 0.927–0.985)/0.950 (95% CI: 0.867–0.989); and type B had an AUC value of 0.991 (95% CI: 0.967–0.999)/0.989 (95% CI: 0.930–1.000) in the test/validation sets (Figure 3). The gradient-weighted class activation mapping (Grad-CAM) method was applied to visualize the probable AF regions of different fracture types determined by the DL model (Supplementary Figure S1) (Selvaraju et al., 2017).
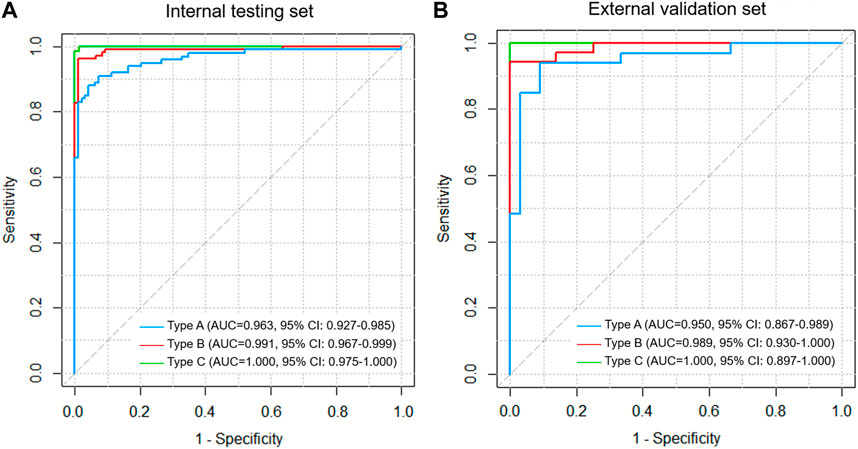
FIGURE 3. ROC curve analysis for types A, B, and C fractures based on the three-column classification of acetabular fractures in the (A) internal testing and (B) external validation set.
A total of 46 PMCs were misdiagnosed by at least five clinicians in the internal test and external validation sets, including five PMCs by five clinicians, eight PMCs by six clinicians, eight PMCs by seven clinicians, six PMCs by eight clinicians, ten PMCs by nine clinicians, and nine PMCs by ten clinicians. Of the 46 PMCs, 40 were type A fractures, 6 were type B, and none were type C. The DL model correctly recognized 26 of the 46 PMCs (Supplementary Figure S2).
In this study, we first developed a specific DL model to detect AFs on PARs with high sensitivity, specificity, and accuracy. According to our results, the diagnostic performance of the DL model was equal to or even better than that of the clinicians. Our model showed the highest performance in detecting the most complex type C fractures among all AF types. Furthermore, the model successfully identified 26 of the 46 cases in which the clinicians had a high probability of misjudgment. This shows that the model has a promising ability to detect PMCs.
The overall miss rate of fractures identified by clinicians on PARs was estimated to be 10% (Hakkarinen et al., 2012). However, unlike fractures in other areas, clinicians cannot easily identify Afs on PARs. In a recent study, only 42.4% (61/144) of AFs were detected by an attending trauma radiologist on PARs (Benjamin et al., 2022). Another study involving 129 patients showed that AFs were the most commonly missed trauma on PARs (Kessel et al., 2007). Similarly, our study found that clinicians missed up to 20% of the AFs cases. As mentioned above, diagnosis by humans is not very accurate. Nevertheless, we did not find previous specific studies on DL for detecting AFs on PARs, and insufficient image data on AFs could be one of the barriers to the development of an appropriate DL model. A 2019 study reported that AFs could be detected using traditional machine learning methods, achieving an accuracy of 80% in a test set containing as few as five cases (Castro-Gutierrez et al., 2019). Therefore, we conducted this study to prove that our DL model has great potential for diagnosing AFs.
Kuo et al. (2022) analyzed 37 studies of DL on X-rays for fracture detection from January 2018 to July 2020 and demonstrated that the median number of participants, the median size of development sets, the pooled sensitivity of test sets, and the pooled specificity of test sets were 1,169 (interquartile range, 425–2,417), 1,898 (interquartile range, 784–7,646), 92% (95% CI: 88–94), and 91% (95% CI: 88–93), respectively. Although both the number of participants and development set in this study were comparatively low, similar sensitivities and specificities were obtained. This indicates that the DL model is efficient.
AFs are classified based on radiographic imaging features. The Letournel-Judet criterion (Letournel, 1980) is the most widely applied classification of AFs, but it only covers 80% of them (Herman et al., 2018). The choice of an appropriate classification theory capable of covering and classifying all AFs is essential for presenting and discussing the test results in this study. Therefore, a more comprehensive three-column classification theory was adopted. All AFs were classified into three types, with higher grades indicating more areas involved and more severe fractures. The results showed that the DL model had excellent recognition ability for type C fractures, where injuries were severe and likely to be combined with other injuries, and low recognition ability for type A fractures, where injuries were relatively mild. This helps to quickly diagnose and save critically injured patients with AFs.
Clinician experience is negatively associated with fracture misdiagnosis rates; however, timely specialist consultations are usually unavailable (Williams et al., 2000). In this study, we defined the concept of PMC. PMC was neither summarized by the results of X-ray reports nor conferred by experienced specialists, but was based on the test results of 10 clinicians, which is closer to the real situation. DL algorithms are liable to misdiagnosis when abnormal features are subtle, even for experienced radiologists (Li et al., 2021). Although our model identified only 56.5% (26/46) of PMCs, it could still be a valuable solution compared with clinicians’ performance. Furthermore, a previous study demonstrated the utilization of the Grad-CAM algorithm in medical education and practice (Cheng et al., 2020b). Grad-CAM was used in this study to provide a reference for clinicians to assist in fracture diagnosis. The heat maps generated by Grad-CAM enable improved diagnostic accuracy with limited learning cases (Cheng et al., 2020b).
DenseNet was selected in this study because it has the main advantage of balancing the performance on the development, test and validation sets compared to some other common models (Cheng et al., 2020a). In addition, it has the following advantages: 1) a smaller number of parameters, 2) encouraging feature reuse, 3) easier network training, and 4) alleviating the problems of gradient vanishing and model degradation (Huang et al., 2017). Many successful presentations of the DenseNet model have been reported in the field of orthopedics, such as vertebral compression fractures (Monchka et al., 2021), proximal femoral fractures (Oakden-Rayner et al., 2022), distal radio-ulnar fractures (Kim et al., 2021), hip fractures (Krogue et al., 2020; Cheng et al., 2021), and hip osteoarthritis (von Schacky et al., 2020).
This study has some limitations. First, ROI images were generated by manual cropping, which resulted in subtle variations. Second, the diagnostic performance of clinicians may be underestimated because the procedure is not a real clinical scenario; for example, no information was available regarding patients’ clinical backgrounds. Lastly, intra-observer variability could be affected by the factors such as the surroundings and the clinician’s self-efficacy, which may affect the study findings. The algorithm should be externally validated by large numbers of cases in a multicenter prospective clinical setting in the future.
In conclusion, we developed a DL algorithm for detecting AFs, which achieved a diagnostic performance comparable to or even superior to that of clinicians. The algorithm has an excellent detection rate for severely injured type C fractures and is useful for detecting inconspicuous AFs. Future studies should conduct prospective controlled trials in real-world clinical settings to further demonstrate that DL can be used as a tool to aid clinicians in AF diagnosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study was approved by the ethics committee of the Third Hospital of Hebei Medical University, Orthopedic Hospital of Xingtai and Xi’an Honghui Hospital. The ethics committee waived the requirement of written informed consent for participation.
All authors contributed to the study conception and design. Data collection and analysis were performed by ZWa, ST, ZWu, and YZ (7th author). The first draft of the manuscript was written by PY and SL. PY, SL, and YL involved in methodology and software. YZ (8th author), MG, and ZH were involved in review and editing. All authors read and approved the final manuscript.
This study has received funding by the National Natural Science Foundation of China (Grant No. 82072523).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1146910/full#supplementary-material
Benjamin, E. R., Jakob, D. A., Myers, L., Liasidis, P., Lewis, M., Fu, Y., et al. (2022). The trauma pelvic X-ray: Not all pelvic fractures are created equally. Am. J. Surg. 224, 489–493. doi:10.1016/j.amjsurg.2022.01.009
Castro-Gutierrez, E., Estacio-Cerquin, L., Gallegos-Guillen, J., Obando, J. D., and Year), (2019). “Detection of acetabulum fractures using X-Ray imaging and processing methods focused on noisy images,” in Amity International Conference on Artificial Intelligence (AICAI), United Arab Emirates, 04-06 February 2019 (IEEE), 296–302.
Cheng, C. T., Chen, C. C., Cheng, F. J., Chen, H. W., Su, Y. S., Yeh, C. N., et al. (2020a). A human-algorithm integration system for hip fracture detection on plain radiography: System development and validation study. JMIR Med. Inf. 8 (11), e19416. doi:10.2196/19416
Cheng, C. T., Chen, C. C., Fu, C. Y., Chaou, C. H., Wu, Y. T., Hsu, C. P., et al. (2020b). Artificial intelligence-based education assists medical students' interpretation of hip fracture. Insights Imaging 11 (1), 119. doi:10.1186/s13244-020-00932-0
Cheng, C. T., Wang, Y., Chen, H. W., Hsiao, P. M., Yeh, C. N., Hsieh, C. H., et al. (2021). A scalable physician-level deep learning algorithm detects universal trauma on pelvic radiographs. Nat. Commun. 12 (1), 1066. doi:10.1038/s41467-021-21311-3
Ffa, I. J., Meesters, A. M. L., Merema, B. B. J., Ten Duis, K., de Vries, J. P. M., Banierink, H., et al. (2021). Feasibility of imaging-based 3-dimensional models to design patient-specific osteosynthesis plates and drilling guides. JAMA Netw. Open 4 (2), e2037519. doi:10.1001/jamanetworkopen.2020.37519
Hakkarinen, D. K., Banh, K. V., and Hendey, G. W. (2012). Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J. Emerg. Med. 43 (2), 303–307. doi:10.1016/j.jemermed.2012.01.037
Herman, A., Tenenbaum, S., Ougortsin, V., and Shazar, N. (2018). There is No column: A new classification for acetabular fractures. J. Bone Jt. Surg. Am. 100 (2), e8. doi:10.2106/JBJS.17.00600
Huang, G., Liu, Z., Maaten, L. V. D., and Weinberger, K. Q. (2017). Densely connected convolutional networks. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR) (p), 2261–2269. doi:10.1109/CVPR.2017.243
Jones, R. M., Sharma, A., Hotchkiss, R., Sperling, J. W., Hamburger, J., Ledig, C., et al. (2020). Assessment of a deep-learning system for fracture detection in musculoskeletal radiographs. NPJ Digit. Med. 3, 144. doi:10.1038/s41746-020-00352-w
Kessel, B., Sevi, R., Jeroukhimov, I., Kalganov, A., Khashan, T., Ashkenazi, I., et al. (2007). Is routine portable pelvic X-ray in stable multiple trauma patients always justified in a high technology era? Injury 38 (5), 559–563. doi:10.1016/j.injury.2006.12.020
Kim, M. W., Jung, J., Park, S. J., Park, Y. S., Yi, J. H., Yang, W. S., et al. (2021). Application of convolutional neural networks for distal radio-ulnar fracture detection on plain radiographs in the emergency room. Clin. Exp. Emerg. Med. 8 (2), 120–127. doi:10.15441/ceem.20.091
Kitamura, G. (2020). Deep learning evaluation of pelvic radiographs for position, hardware presence, and fracture detection. Eur. J. Radiol. 130, 109139. doi:10.1016/j.ejrad.2020.109139
Krogue, J. D., Cheng, K. V., Hwang, K. M., Toogood, P., Meinberg, E. G., Geiger, E. J., et al. (2020). Automatic hip fracture identification and functional subclassification with deep learning. Radiol. Artif. Intell. 2 (2), e190023. doi:10.1148/ryai.2020190023
Kuo, R. Y. L., Harrison, C., Curran, T. A., Jones, B., Freethy, A., Cussons, D., et al. (2022). Artificial intelligence in fracture detection: A systematic review and meta-analysis. Radiology 1, 211785. doi:10.1148/radiol.211785
Laird, A., and Keating, J. F. (2005). Acetabular fractures: A 16-year prospective epidemiological study. J. Bone Jt. Surg. Br. 87 (7), 969–973. doi:10.1302/0301-620x.87b7.16017
Letournel, E. (1980). Acetabulum fractures: Classification and management. Clin. Orthop. Relat. Res. 151, 81–S2. doi:10.1097/BOT.0000000000001424
Li, Y., Zhang, Y., Zhang, E., Chen, Y., Wang, Q., Liu, K., et al. (2021). Differential diagnosis of benign and malignant vertebral fracture on CT using deep learning. Eur. Radiol. 31 (12), 9612–9619. doi:10.1007/s00330-021-08014-5
Liu, P., Lu, L., Chen, Y., Huo, T., Xue, M., Wang, H., et al. (2022). Artificial intelligence to detect the femoral intertrochanteric fracture: The arrival of the intelligent-medicine era. Front. Bioeng. Biotechnol. 10, 927926. doi:10.3389/fbioe.2022.927926
Lundin, N., Huttunen, T. T., Berg, H. E., Marcano, A., Fellander-Tsai, L., and Enocson, A. (2021). Increasing incidence of pelvic and acetabular fractures. A nationwide study of fractures over a 16-year period in Sweden. Injury 308, 87. doi:10.1016/j.injury.2021.03.013
Melhem, E., Riouallon, G., Habboubi, K., Gabbas, M., and Jouffroy, P. (2020). Epidemiology of pelvic and acetabular fractures in France. Orthop. Traumatol. Surg. Res. 106 (5), 831–839. doi:10.1016/j.otsr.2019.11.019
Monchka, B. A., Kimelman, D., Lix, L. M., and Leslie, W. D. (2021). Feasibility of a generalized convolutional neural network for automated identification of vertebral compression fractures: The Manitoba Bone Mineral Density Registry. Bone 150, 116017. doi:10.1016/j.bone.2021.116017
Oakden-Rayner, L., Gale, W., Bonham, T. A., Lungren, M. P., Carneiro, G., Bradley, A. P., et al. (2022). Validation and algorithmic audit of a deep learning system for the detection of proximal femoral fractures in patients in the emergency department: A diagnostic accuracy study. Lancet Digital Health 4, e351–e358. doi:10.1016/s2589-7500(22)00004-8
Oka, K., Shiode, R., Yoshii, Y., Tanaka, H., Iwahashi, T., and Murase, T. (2021). Artificial intelligence to diagnosis distal radius fracture using biplane plain X-rays. J. Orthop. Surg. Res. 16 (1), 694. doi:10.1186/s13018-021-02845-0
Scheinfeld, M. H., Dym, A. A., Spektor, M., Avery, L. L., Dym, R. J., and Amanatullah, D. F. (2015). Acetabular fractures: What radiologists should know and how 3D CT can aid classification. Radiographics 35 (2), 555–577. doi:10.1148/rg.352140098
Selvaraju, R. R., Cogswell, M., Das, A., Vedantam, R., Parikh, D., and Batra, D. (2017). "Grad-CAM: Visual explanations from deep networks via gradient-based localization", in IEEE International Conference on Computer Vision 22-29 October 2017, Venice, Italy, ICCV.
von Schacky, C. E., Sohn, J. H., Liu, F., Ozhinsky, E., Jungmann, P. M., Nardo, L., et al. (2020). Development and validation of a multitask deep learning model for severity grading of hip osteoarthritis features on radiographs. Radiology 295 (1), 136–145. doi:10.1148/radiol.2020190925
Williams, S. M., Connelly, D. J., Wadsworth, S., and Wilson, D. J. (2000). Radiological review of accident and emergency radiographs: A 1-year audit. Clin. Radiol. 55 (11), 861–865. doi:10.1053/crad.2000.0548
Yamada, Y., Maki, S., Kishida, S., Nagai, H., Arima, J., Yamakawa, N., et al. (2020). Automated classification of hip fractures using deep convolutional neural networks with orthopedic surgeon-level accuracy: Ensemble decision-making with antero-posterior and lateral radiographs. Acta Orthop. 91 (6), 699–704. doi:10.1080/17453674.2020.1803664
Zech, J. R., Santomartino, S. M., and Yi, P. H. (2022). Artificial intelligence (AI) for fracture diagnosis: An overview of current products and considerations for clinical adoption, from the AJR special series on AI applications. AJR Am. J. Roentgenol. 219 (6), 869–878. doi:10.2214/AJR.22.27873
Zhang, R., Yin, Y., Li, A., Wang, Z., Hou, Z., Zhuang, Y., et al. (2019). Three-column classification for acetabular fractures: Introduction and reproducibility assessment. J. Bone Jt. Surg. Am. 101 (22), 2015–2025. doi:10.2106/JBJS.19.00284
Keywords: deep learning, acetabular fracture, pelvic anteroposterior radiograph, DenseNet, diagnosis
Citation: Ye P, Li S, Wang Z, Tian S, Luo Y, Wu Z, Zhuang Y, Zhang Y, Grzegorzek M and Hou Z (2023) Development and validation of a deep learning-based model to distinguish acetabular fractures on pelvic anteroposterior radiographs. Front. Physiol. 14:1146910. doi: 10.3389/fphys.2023.1146910
Received: 31 January 2023; Accepted: 12 April 2023;
Published: 28 April 2023.
Edited by:
Yi Jiang, Georgia State University, United StatesReviewed by:
Jan Kubicek, VSB-Technical University of Ostrava, CzechiaCopyright © 2023 Ye, Li, Wang, Tian, Luo, Wu, Zhuang, Zhang, Grzegorzek and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Hou, ZHJ6eWhvdUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.