- 1College of Animal Science (College of Bee Science), Fujian Agriculture and Forestry University, Fuzhou, China
- 2Fujian Honey Bee Biology Observation Station, Ministry of Agriculture and Rural Affairs, Fuzhou, China
The strobilurin fungicide pyraclostrobin is widely used to prevent and control the fungal diseases of various nectar and pollen plants. Honeybees also directly or indirectly contact this fungicide with a long-term exposure period. However, the effects of pyraclostrobin on the development and physiology of Apis mellifera larvae and pupae during continuous exposure have been rarely known. To investigate the effects of field-realistic concentrations of pyraclostrobin on honeybee survival and development, the 2-day-old larvae were continuously fed with different pyraclostrobin solutions (100 mg/L and 83.3 mg/L), and the expression of development-, nutrient-, and immune-related genes in larvae and pupae were examined. The results showed that two field-realistic concentrations of pyraclostrobin (100 and 83.3 mg/L) significantly decreased the survival and capped rate of larvae, the weight of pupae and newly emerged adults, and such decrease was a positive correlation to the treatment concentrations. qPCR results showed that pyraclostrobin could induce the expression of Usp, ILP2, Vg, Defensin1, and Hymenoptaecin, decrease the expression of Hex100, Apidaecin, and Abaecin in larvae, could increase the expression of Ecr, Usp, Hex70b, Vg, Apidaecin, and Hymenoptaecin, and decreased the expression of ILP1, Hex100 and Defensin1in pupae. These results reflect pyraclostrobin could decrease nutrient metabolism, immune competence and seriously affect the development of honeybees. It should be used cautiously in agricultural practices, especially in the process of bee pollination.
1 Introduction
The honeybee is the most important economic insect. It is not only the ideal pollinator of crops and wild plants that subserve the agricultural production, plant diversity and ecological balance but also provides nutritious bee products (Gallai et al., 2009; Champetier et al., 2015; Zheng et al., 2019). In recent years, the honeybee colony losses were reported and the dramatic reductions caused significant economic losses all over the world (Van der Zee et al., 2012; van Dooremalen et al., 2018; Calatayud-Vernich et al., 2019; Kablau et al., 2020). The continued decline of honeybee colonies brings a serious crisis to plant pollination and food production, because nearly 75% of the major crop species rely on pollinators (Clermont et al., 2015; Shi et al., 2020). The main reasons were diverse agrochemicals, parasites, viruses, adjustment of crop planting structure and distribution, especially the noticeable toxic effects of pesticides on honeybees, and also these factors were complex and interacting (van Dooremalen et al., 2018; Duan et al., 2020a; Shi et al., 2020b). During the foraging process, honeybees can directly contact the pesticides remaining on the surface of plants (Legard et al., 2001; Xiong et al., 2022). At the same time, the systemic pesticide residues could be absorbed by plants and remain in nectar and pollen, then were taken back to the colony by the foraging bee and led to the contamination of honey, pollen and comb which were consumed by other members in the colony (Mullin et al., 2010). As a metamorphosis development insect, the life cycle of the honeybee consists of four developmental phases, including egg, larva, pupa, and adult. Furthermore, these pesticide residues could harm the health of honeybees at different stages of individuals and colonies by direct or indirect exposure (Krupke et al., 2017). Consequently, the primary problem in modern agricultural production is how to balance between protecting crops efficiently against pests and diseases and maintaining healthy pollinator populations (Godfray and Garnett, 2014; Xiong et al., 2022).
As an important class of plant protection products, fungicides have already accounted for more than 35% of the global pesticide market and are widely used in the disease control of nectar and pollen plants, such as maize, rape, sunflower, and alfalfa, which account for approximately 11% of total global pesticide use (Liao et al., 2018; Zhang 2018; Zubrod et al., 2019). According to the data of the Fungicide Resistance Action Committee (FRAC), the action mechanisms of fungicides to plant pathogenic microorganisms were the negative effects on the nucleic acid and protein synthesis, respiration, signal transduction, cell division, and membrane structure and function of microorganisms (Fungicide Resistance Action Committee, 2021). Therefore, the bioassay results showed that most fungicides were lower acutely toxic to honeybees and other non-target insects (Liao et al., 2018; Simondelso et al., 2018; Xiong et al., 2022). Normally, the field-realistic concentrations or residue in the colonies were considered to be low toxicity to cause illness or death of honeybees (Pettis et al., 2013). Furthermore, the fungicides were considered to be safe for honeybees and the chronic toxicity was ignored (Tadei et al., 2019; Duan et al., 2020a; Xiong et al., 2022). Thus bees are more likely to encounter fungicides than insecticides because fungicides can even be sprayed when insect-attractive crops are in bloom (Favaro et al., 2019; Gierer et al., 2019; Rondeau and Raine 2022).
However, a large number of scientific investigations have reported the chronic toxicity of fungicides on the development, detoxification, and immune function, foraging, and homing ability, olfactory memory of bees, which cause serious damage to the individual and colony of honeybees (Zhu et al., 2014; Carneiro et al., 2019; Duan et al., 2020a; Dai et al., 2021; Fisher et al., 2021; Traynor et al., 2021). After exposure to chlorothalonil for 3 days, the mortality of A. mellifera 4-day-old larvae was significantly increased over two-fold compared to untreated larvae, and also the pairing of chlorothalonil and coumaphos or fluvalinate produced synergistic interactions on the mortality of larvae (Zhu et al., 2014). The low field concentration of dimertachlone, prochloraz and iprodione could induce the activities of catalase (CAT), carboxylesterases (CarE) and glutathione S-transferase (GSTs), but the high concentration inhibits their activity (Duan et al., 2020a). The Iprodione (2 mg/kg) was not lethal to newly emerged bees, but it can inhibit the synthesis of glutathione, leading to the generation of reactive oxygen species and the cells of treated bees had signs of apoptosis (Carneiro et al., 2019). The benomyl stress (5 g/kg) led to a total of 5,759 DEGs being upregulated in the worker bees of A. mellifera, and most of the DEGs were involved in the functions of immunity, detoxification, biological metabolism, and regulation, such as light conduction, MAPK, calcium ion pathway and other 12 pathways (Dai et al., 2021). DesJardins et al. (2021) found the compound fungicides Pristine® showed significant sublethal effects on the learning performance of A. mellifera and lead the work type conversion of nurse bee to forage bee.
Pyraclostrobin is a high-efficiency, low-toxic, and broad-spectrum systemic strobilurin fungicide which was registered and widely used to prevent and control diseases caused by fungi on various nectar and pollen plants (Bartlett et al., 2002). The bactericidal mechanism of pyraclostrobin was to inhibit cell respiration in fungi and the acute oral and contact toxicity of pyraclostrobin to the worker bee of A. mellifera was low toxicity (LC50 > 100 μg (a.i.)/bee) (Earley et al., 2012; Tan et al., 2021). Pyraclostrobin was chronic toxicity to honeybees and could directly inhibit the mitochondrial function in vitro (Campbell et al., 2016; Nicodemo et al., 2020). The field-relevant doses of pyraclostrobin decreased the height of secretory cells and volume of mandibular glands with 6 days continuous exposure and influence the behavior of newly emerged workers and young workers (Zaluski et al., 2017; Tadei et al., 2019). Meanwhile, pyraclostrobin was widely residual in the pollen of treated crops and in honeybee colonies which may influence the health of honeybees (Yoder et al., 2013; David et al., 2015).
However, the effect of pyraclostrobin on the development and physiology of larvae and pupae of A. mellifera is rarely known. To investigate the influence of field-realistic concentrations of pyraclostrobin on larvae and pupae, the survival and developmental state of A. mellifera worker bees from larvae to adult stage in each treatment were documented daily. Further, the effect of pyraclostrobin on the development-related genes ecdysone receptor (Ecr) and ultraspiracle protein (Usp), nutrient metabolism-related genes insulin-like peptides 1 (ILP 1), insulin-like peptides 2 (ILP 2), Hexamerin 70b (Hex 70b), Hexamerin 110 (Hex 110), Vitellogenin (Vg) and immune-related genes Apidaecin, Abaecin, Hymenoptaecin, Defensin1 in larvae and pupae were examined, respectively. This study will provide new evidence of pyraclostrobin exposure on honeybee larvae and pupae development, and also provide the theoretical basis for the pollination safety and the management of pesticides.
2 Materials and methods
2.1 The fungicide and treated concentrations
The 25% pyraclostrobin suspension concentrate was purchased from Hebei Chengyue Chemical Co., Ltd. According to the fungicide instruction manual, the recommended dilution multiple of pyraclostrobin for disease control was 2500–3000. Considering the actual use of the field, two field-realistic concentrations of pyraclostrobin 100 mg/L (2500 fold) and 83.3 mg/L (3000 fold) were designed and diluted by the artificial diet of larvae, which were stored at −4°C and used up within 7 days. Different day-old larvae have different artificial diets which should be prepared when using. Diet A for 1 and 2 day-old larvae (royal jelly 50%, glucose 6%, fructose 6%, yeast extract 1%, and water 37%), Diet B for 3 day-old larvae (royal jelly 50%, glucose 7.5%, fructose 7.5%, yeast extract 1.5%, and water 33.5%) and Diet C for 4, 5 and 6 day-old larvae (royal jelly 50%, glucose 9%, fructose 9%, yeast extract 2%, and water 30%) (Ministry of Agriculture of the People’s Republic of China, 2017).
2.2 The honeybee
Ten healthy honeybee colonies were reared in the experimental apiary of the College of Animal Science (College of Bee Science), Fujian Agriculture and Forestry University (Fuzhou, China, and 26.08°N 119.23°E). Before the experiment, these colonies were not exposed to pesticides and the test larvae and pupae were obtained by the following method: Five healthy egg-laying queens were confined, respectively in empty combs for laying eggs within 8 h, and then these combs with new-laid eggs were moved to a separated place in the same colony. 3 days later, the 1-day-old larvae were swiftly transferred from the combs to the 96-well tissue culture plates by a Chinese grafting tool in the laboratory and kept in a dark incubator (Ningbo Jiangnan Instrument Factory) at 34°C ± 1°C, 95% ± 2% RH (Duan et al., 2021).
2.3 Fungicide treatment of A. mellifera larvae
Larvae in the plates were reared according to the method by Jensen et al. (2009) with a few modifications. Three tissue culture plates were taken as the control group, and the other six plates were taken as the two different concentration treatment groups. Three replicates per group and forty-eight larvae were treated per replicate. In the fungicide treatment groups, each larva was fed a contaminated diet containing different concentrations of pyraclostrobin, 1 day-old larvae were fed 20 μL Diet A containing fungicide, 3 day-old larvae were fed 20 μL Diet B including fungicide, and also 4, 5, and 6 day-old larvae were fed the Diet C with fungicide for 30, 40, and 50 μL, respectively. Meanwhile, the larvae in the control group were fed a normal diet with the same quantity as fungicide treatments (Ministry of Agriculture of the People’s Republic of China 2017; Dai et al., 2018). The artificial diet with fungicide was changed daily. The larvae normally pupate on the 7th day, so they were checked every 6 h before pupation, and the dead larva was removed and recorded. When they begin to emerge on the 19th day, the number of pupation and eclosion of larvae in each group was also recorded. The calculation method of capped rate and emergence rate was referred to Shi et al. (2020).
Fifteen white-eye pupae and fifteen newly emerged bees were randomly selected from each treatment and individually weighed to calculate the pupa weight and newly emerged bee birth weight. The newly emerged bee’s weight must be measured within 2 h after emergence. Moreover, ten 6-day-old larvae and ten pupae were randomly sampled from each treatment and immediately frozen with liquid nitrogen for RNA extraction.
2.4 Gene expression analysis
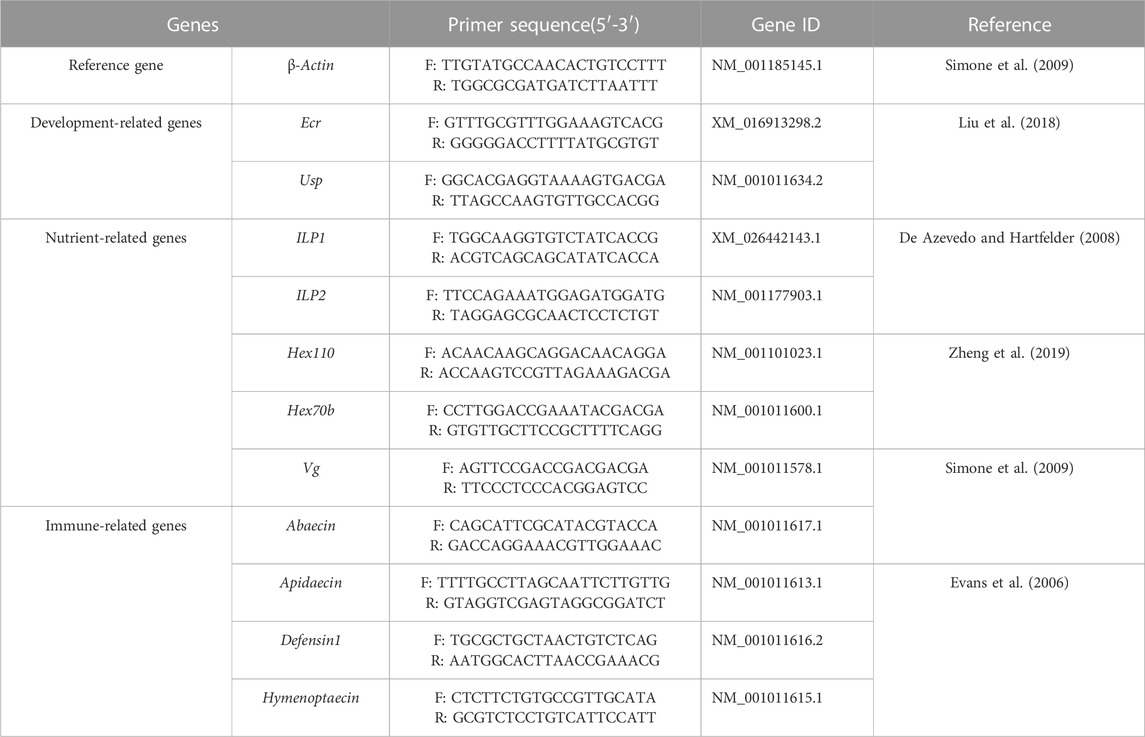
The total RNA of each individual was exacted by TRIzol® Reagent (TransGen Biotech Co., Ltd. Beijing, China). After quality and concentration detection, the qualified RNA was used for cDNA synthesized by PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Japan) and the cDNA samples were stored at −20°C. The qPCR assay was performed to examine the relative expression of development-related genes Ecr and Usp, nutrient metabolism-related genes ILP1, ILP2, Hex70b, Hex110, and Vg, and immune-related genes Apidaecin, Abaecin, Defensin1 and Hymenoptaecin in larvae and pupae, respectively (Table 1). The β-actin was used as the reference gene (Duan et al., 2021) and the gene-specific primers were shown in Table 1. The qPCR was performed in ABI QuantStudio six Flex System (Thermo Fisher Scientific, Waltham, MA, and United States) with a 10 µL reaction volume containing TB Green Premix Ex Taq Ⅱ (2×) 5, cDNA 1 μL, each gene-specific primers (10 μM) 0.4 μL, ROX Reference Dye Ⅱ (50×) 0.2 μL and H2O 3 μL. The thermal procedure include 95°C for 30 s, followed by quantification for 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 30 s, and a final melt-curve step was rung from 60°C–95°C for 10 s at 1°C increment to check for non-specific amplification. Both technical and biological triplicates were performed at least three in all experiments.
2.5 Statistical analysis
The Ct values of development-, nutrient-, and immune-related genes were normalized by the corresponding Ct value of reference gene β-actin, and then the relative expression levels of genes were calculated using the 2−△△CT method (Livak and Schmittgen, 2001). All data are presented as mean ± standard error (S.E.). The one-way ANOVA was used to determine the significance of the differences in gene expression. With homogeneity of variance, the One-way analysis was followed by Tukey’s test. The significance level was set at a value of p < 0.05. All data analyses and figures were carried out using Graphpad Prism 8 (GraphPad Software, Inc., San Diego, CA, and United States).
3 Results
3.1 Pyraclostrobin exposure decreased the survival rate of larvae and pupae
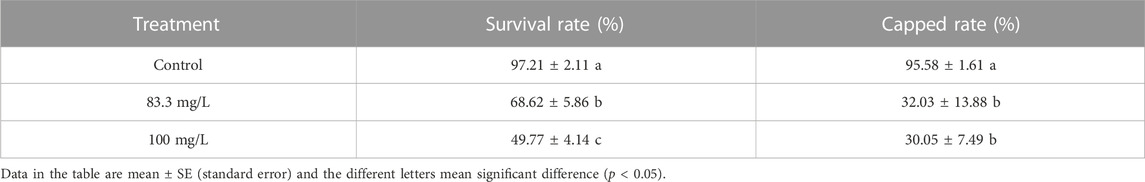
The field concentrations of pyraclostrobin could repress the survival and development of A. mellifera larvae and pupae with noticeable toxic effects indicated by the significantly different survival and development index of A. mellifera larvae and pupae among fungicide treatments. The survival rate and capped rate of A. mellifera larvae from two pyraclostrobin treatments (83.3 and 100 mg/L) were significantly lower than that of the control groups (68.62%, 49.77% and 32.03%, 30.05%, p < 0.05, Table 2), and also the weight, emergence rate of pupae and newly emerged bee weight were also significantly decreased (p < 0.05, Table 3). Remarkably, there was a concentration-effect between treatment concentration and these development indexes, and a significant difference in the survival rate of larvae and birth weight of newly emerged bees between 83.3 and 100 mg/L treatment (p < 0.05).
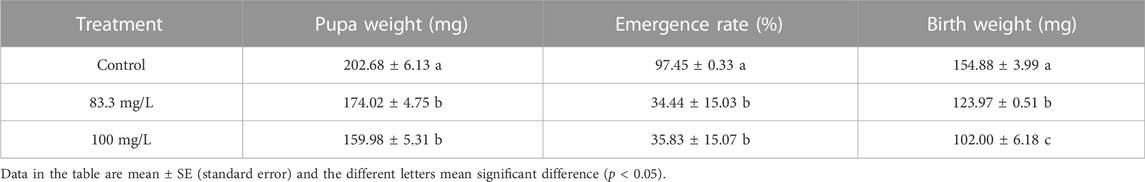
TABLE 3. Effects of pyraclostrobin on the pupa weight, emergence rate and newly emerged bee birth weight of Apis mellifera.
3.2 Pyraclostrobin exposure interrupted the expression of development-related genes
The relative expression of development-related genes (Ecr and Usp) in A. mellifera larvae and pupae was affected after exposure to field concentrations of pyraclostrobin (83.3 and 100 mg/L, Figure 1). The field concentrations of pyraclostrobin inhibited the expression level of Ecr in larvae and gradually downregulated with the increasing treatment concentrations (0.6-fold for 100 mg/L; 0.73-fold for 83.3 mg/L, p < 0.05). Meanwhile, the relative expression of Usp in larvae with 100 mg/L treatment was significantly upregulated than both the 83.3 mg/L treatment and control. However, the pyraclostrobin could induce the expression level of Ecr and Usp in pupae. The higher the treatment concentration, the stronger the induction effect. The expression level of Usp was significantly higher than in control (1.52-fold for 100 mg/L; 1.63-fold for 83.3 mg/L, p < 0.05), and the 100 mg/L treatment could significantly induce the expression level of Usp of pupae.
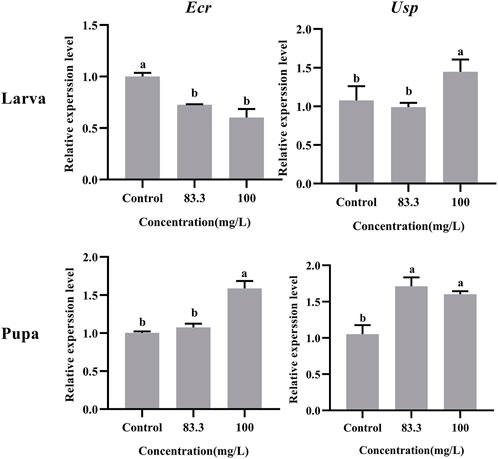
FIGURE 1. Effects of pyraclostrobin on the relative expression of development-related genes (Ecr and Usp) in Apis mellifera larva and pupa. The data in the figures are mean ± SE (standard error) and different letters above bars mean significant difference (p < 0.05, Fisher’s LSD test).
3.3 Pyraclostrobin exposure interfered with the nutrition metabolism of larvae and pupae
These five nutrient-related genes were all expressed in A. mellifera larvae and pupae but at varying levels under different pyraclostrobin concentrations (Figure 2). For larvae, the pyraclostrobin can significantly upregulate the expression of ILP2 and Vg, but the expression of Hex110 was downregulated in both treated concentrations (p < 0.05). And also the high concentration pyraclostrobin (100 mg/L) can significantly downregulate the expression of Hex70b in larvae. For pupae, the expression levels of Hex70b and Vg were significantly upregulated in two fungicide treatments (p < 0.05). Meanwhile, the expression levels of ILP1 and Hex110 were significantly downregulated with both pyraclostrobin treatments (p < 0.05). Despite the expression of both ILP1 in larvae and ILP2 in pupae being induced by low pyraclostrobin concentration (83.3 mg/L), which was also inhibited by high concentration treatment (100 mg/L), there were no statistical differences between the treatments and control.
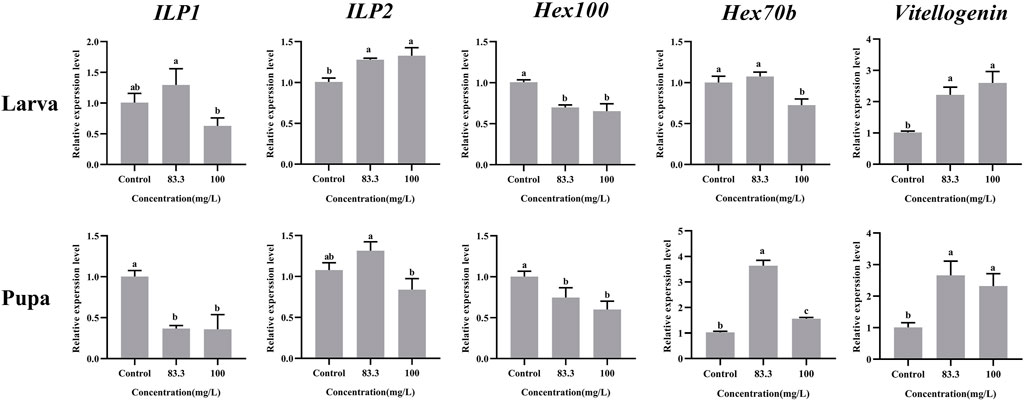
FIGURE 2. Effects of pyraclostrobin on the relative expression of nutrient-related genes (ILP1, ILP2, Hex110, Hex70b, and, Vg) in Apis mellifera larvae and pupae. The data in the figures are mean ± SE (standard error) and different letters above bars mean significant difference (p < 0.05, Fisher’s LSD test).
3.4 Pyraclostrobin exposure disturbed the immunity of larvae and pupae
The mRNA levels of these four immune-related genes in both larvae and pupae were influenced by pyraclostrobin exposure (Figure 3). For larvae, the expression of Abaecin and Apidaecin was significantly decreased in fungicide treatments (p < 0.05). However, the pyraclostrobin can induce the expression of Defensin1 and Hymenoptaecin. The expression of Defensin1 was significantly increased after exposure to 83.3 mg/L pyraclostrobin treatment, but the expression of Hymenoptaecin was significantly increased after exposure to high pyraclostrobin treatment (100 mg/L). For pupae, the expression level of Apidaecin and Hymenoptaecin was increased after exposure, and the expression of Apidaecin in 100 mg/L treatment was significantly higher than control. In addition, the expression of Hymenoptaecin was also significantly affected by pyraclostrobin. The expression of Defensin1 was significantly decreased in fungicide treatments (p < 0.05). Although the expression of Apidaecin was lower than control, there was no significant difference (p > 0.05).
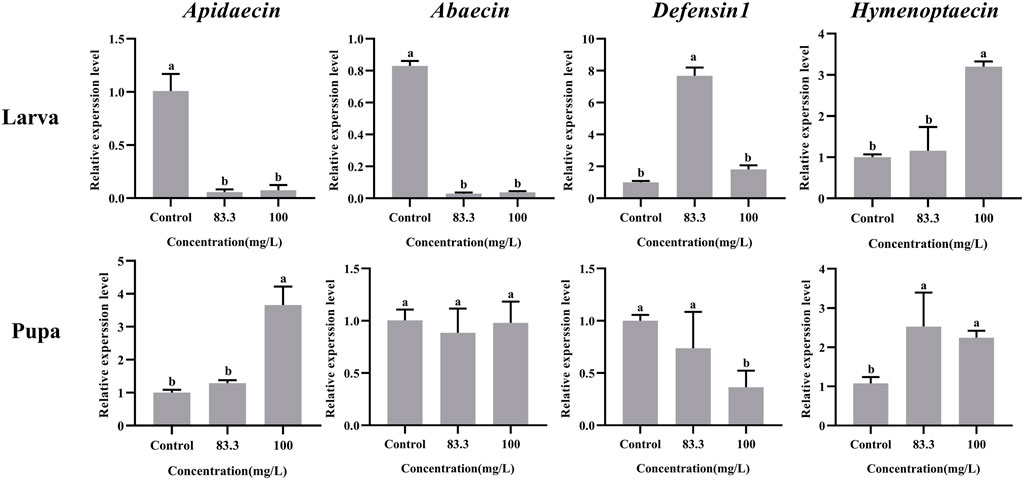
FIGURE 3. Effects of pyraclostrobin on the relative expression of immune-related genes (Apidaecin, Abaecin, Defensin1, and Hymenoptaecin) in Apis mellifera larvae and pupae. The data in the figures are mean ± SE (standard error) and different letters above bars mean significant difference (p < 0.05, Fisher’s LSD test).
4 Discussion
So far, pyraclostrobin has been on the market for over 20 years and was registered and used for fungi diseases control of various plants in different countries (Luo et al., 2022). With widely and irrational use, the accumulation and pollution of pyraclostrobin in the soil, water and other ecosystems represent high potential risks to the environment and organisms. As an indicator of environmental pollution, honeybees also directly or indirectly contact with this pollutant which led to harmful effects on individuals and colonies (Yoder et al., 2013; David et al., 2015; Zaluski et al., 2017; Tadei et al., 2019; Xiong et al., 2022).
Despite the low acute oral and contact toxicity of pyraclostrobin to honeybees (Tan et al., 2021), long-time exposure to pyraclostrobin could lead to irreversible adverse effects on honeybees. The results showed pyraclostrobin (100 and 83.3 mg/L) were chronic toxicity effects on the survival and development of A. mellifera. da Costa Domingues et al. (2020) found the forager workers of Melipona scutellaris exposed to pyraclostrobin showed a reduced survival rate. Compared with control, both two pyraclostrobin concentrations exposure significantly reduced the survival rate of A. mellifera larvae with a significant concentration effect (p < 0.05; Table 2). Meanwhile, the significantly low capped rate of larvae and emergence rate of pupae after pyraclostrobin exposure causes unsuccessful metamorphic development from larvae to pupae with a high mortality rate. During the process of pupation and emergence, honeybees consume the energy which they have previously stored to synthesize new substances. Owing to the low concentration of pyraclostrobin could inhibit the mitochondrial respiratory of A. mellifera (Nicodemo et al., 2020), which means the fungicide exposure-treated A. mellifera larvae and pupae need to consume more material to complete metamorphosis. Thus significantly decreased the weight of pupae and newly emerged bees (Table 3), suggesting pyraclostrobin could affect the normal growth and metabolism of A. mellifera larvae and pupae, especially for pupae with a low emergence rate and weight, though they did not feed during pupal stage. The quantity and quality of brood (larvae and pupae) are critical to the population size of the colony (Duan et al., 2020b; Xiong et al., 2022) and these findings indicate pyraclostrobin can cause serious damage to bee colonies by suppressing the survival and development of individuals.
During the larval-pupal transition of A. mellifera, the development rhythm of metamorphosis was regulated by juvenile hormones (JH) and molting hormone (20-hydroxyecdysone, 20E) (Liu et al., 2014). The 20E, ecdysteroid receptor (Ecr) and ultraspiracle protein (Usp) constitute the ligand-receptor complex (20E-Ecr-Usp) and then activate the metamorphosis process (Riddiford et al., 2000). Therefore, the Ecr and Usp were considered to be key genes responsible for the transduction of the JH/20E signals during metamorphosis development (Barchuk et al., 2008; Duan et al., 2021). In the present study, the RT-PCR results showed the expression levels of Ecr and Usp in larvae and pupae were altered with pyraclostrobin exposure. The expression level of Ecr and Usp in pupae were significantly upregulated after pyraclostrobin exposure (100 and 83.3 mg/L). Furthermore, the low emergence rate and weight of pupae also confirmed that pyraclostrobin could disturb the normal development process leading to a high mortality rate of A. mellifera pupae.
Pyraclostrobin could cause the energy deficiency of A. mellifera, and more nutrition materials need to be metabolized for normal development (Nicodemo et al., 2020). Moreover, extra nutrient consumption may cause the weight loss of pupae and newly emerged bees. The present results suggested that the weight loss might come from either some nutrient metabolic pathway disturbance or the decreased hexamerins for building the pupae tissues, which may already be disrupted by pyraclostrobin. There are two insulin-like peptides (ILPs) in honeybees that have profound effects on invertebrate metabolism, nutrient storage and fertility (de Azeved and Hartfelder, 2008; Duan et al., 2021). The ILP1 gene potentially functions in lipid and protein metabolism while ILP2 is a more general indicator of nutritional status (Ihle et al., 2014). Compared with control, the high concentration of pyraclostrobin (100 m/L) inhibits ILP1 expression in both larvae and pupae. However, the expression of ILP2 in 83.3 mg/L treatment exhibited upregulation. The abnormal expression phenomenon of ILP1 and ILP2 in honeybees would lead to nutritional and metabolic disorders (Wang et al., 2013). The hexamerins were synthesized in the fat body during the larval growth phase and used for pupal development and adult differentiation (Burmester and Scheller, 1999). The subunits of Hex 110 were highly abundant in A. mellifera larval hemolymph and the gene activity obeys a nutritional control (Bitondi et al., 2006). The expression of Hex100 was significantly downregulated indicating malnutrition and developmental abnormalities of larvae and pupae after pyraclostrobin exposure. Meanwhile, the larvae could use Hex 70b to compensate for the lack of proteins (Cunha et al., 2005). And the expression of Hex 70b was significantly induced in pupae by pyraclostrobin (Figure 2). Vitellogenin (Vg) is an egg-yolk precursor in insect reproduction and multiple roles of Vgs, such as immunity, life span, and antioxidation in non-reproduction were also uncovered (Havukainen et al., 2013; Salmela et al., 2015; Salmela and Sundström, 2017). Pyraclostrobin exposure could result in oxidative stress in zebrafish embryos (Li et al., 2018; Kumar et al., 2020). In the present study, Vg has significantly upregulated expression in larvae and pupae which acts as a ‘defender’ against infection and reactive oxygen species for a prolonged life span (p < 0.05) (Havukainen et al., 2013; Salmela et al., 2015).
Honey bee innate immunity provides immediate responses against invading pathogens, especially antimicrobial peptides (AMPs) in cell-free humoral immunity (Danihlík et al., 2015; Duan et al., 2021; Xiong et al., 2022). Four families of AMPs (i.e., apidaecins, abaecin, hymenoptaecin and defensins) with a variety of antimicrobial activities have been described in the honey bee and their expressions were regulated by two intracellular signaling pathways Toll and Imd/JNK (Evans et al., 2006; Danihlík et al., 2015). It should be noted that that pyraclostrobin had negative effects on the immunity of bee larvae and pupae, leading to a low survival rate (Figure 3). At the larvae stage, the expression of Apidaecin and Abaecin were significantly downregulated (p < 0.05) indicating that exposure to pyraclostrobin makes the honeybee would be more sensitive to pathogens, which may be the main reason for the increased Nosema ceranae infection rates in adult bees (Pettis et al., 2013). While the Defensin1 and Hymenoptaecin genes exhibited upregulation in two pyraclostrobin treatments suggesting that larvae can coordinate different immune genes in response to the effects of fungicides on their immunity (Shi et al., 2020). However, at the pupae stage, the pyraclostrobin exposure could induce the expression of Apidaecin and Hymenoptaecin, and inhibit the expression of Defensin1 which means pupae had different defense strategies for stress to immunity than larvae. Furthermore, combined with the results of immune-genes expression with different exposure concentrations, these four immune genes have different response mechanisms to two pyraclostrobin treatment concentrations. Considering the regulation of four immune genes by Toll and Imd/JNK metabolic pathways (Evans et al., 2006), the influence of pyraclostrobin on two Toll and Imd/JNK metabolic pathways also needs to be further evaluated and attention.
5 Conclusion
In the current study, two field-recommended concentrations pyraclostrobin (100 and 83.3 mg/L) showed significant adverse effects on the development of honey bee, resulting in a significantly lower survival rate, capped rate, emergence rate and body weight. Meanwhile, with long-term pyraclostrobin exposure, the expression levels of development-, nutrient- and immune-related genes in both larvae and pupae were also abnormally altered, indicating that pyraclostrobin could impair the development, nutrient metabolism and immunity of larvae and pupae. These findings demonstrate that the low acute toxic fungicide pyraclostrobin has deleterious effects on A. mellifera larvae and pupae with continuous exposure. Thus, it is necessary to re-evaluate the safety and potential risks of the fungicides to honey bee, bumble bee and solitary bee in the future. And the health welfare of pollinators should be emphasized in integrated pest management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author contributions
Conceived and designed the experiments: MX, JL, and XD; Performed the experiments: MX, GQ, RZ, and LW; Analyzed the data: MX, RW, XL, QL, SH, and XD; Wrote the paper: MX and XD. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Fujian Province of China (2022J01585), the Special Foundation of Investigation on Basic Resources of Ministry of Science and Technology of China (2018FY100402). Training Program for College Students Innovation and Entrepreneurship Project of Fujian Province (202110389159, X202210389074).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barchuk A. R., Figueiredo V. L., Simões Z. L. (2008). Downregulation of ultraspiracle gene expression delays pupal development in honeybees. J. Insect. Physiol. 54 (6), 1035–1040. doi:10.1016/j.jinsphys.2008.04.006
Bartlett D. W., Clough J. M., Godwin J. R., Hall A. A., Hamer M., Parr-Dobrzanski B. (2002). The strobilurin fungicides. Pest Manag. Sci. 58, 649–662. doi:10.1002/ps.520
Bitondi M. M., Nascimento A. M., Cunha A. D., Guidugli K. R., Nunes F. M., Simões Z. L. (2006). Characterization and expression of the Hex 110 gene encoding a glutamine-rich hexamerin in the honey bee, Apis mellifera. Arch. Insect Biochem. Physiol. 63 (2), 57–72. doi:10.1002/arch.20142
Burmester T., Scheller K. (1999). Ligands and receptors: Common theme in insect storage protein transport. Naturwissenschaften 86 (10), 468–474. doi:10.1007/s001140050656
Calatayud-Vernich P., Calatayud F., Simó E., Aguilar J. A. P., Picó Y. (2019). A two-year monitoring of pesticide hazard in-hive: High honey bee mortality rates during insecticide poisoning episodes in apiaries located near agricultural settings. Chemosphere 232, 471–480. doi:10.1016/j.chemosphere.2019.05.170
Campbell J. B., Nath R., Gadau J., Fox T., DeGrandi-Hoffman G., Harrison J. F. (2016). The fungicide Pristine® inhibits mitochondrial function in vitro but not flight metabolic rates in honey bees. J. Insect Physiol. 86, 11–16. doi:10.1016/j.jinsphys.2015.12.003
Carneiro L. S., Martínez L. C., Gonçalves W. G., Santana L. M., Serrão J. E. (2019). The fungicide iprodione affects midgut cells of non-target honey bee Apis mellifera workers. Ecotox. Environ. Safe. 189, 109991. doi:10.1016/j.ecoenv.2019.109991
Champetier A., Sumner D. A., Wilen J. E. (2015). The bio-economics of honey bees and pollination. Environ. Resour. Econ. 60 (1), 143–164. doi:10.1007/s10640-014-9761-4
Clermont A., Eickermann M., Kraus F., Hoffmann L., Beyer M. (2015). Correlations between land covers and honey bee colony losses in a country with industrialized and rural regions. Sci. Total Environ. 532, 1–13. doi:10.1016/j.scitotenv.2015.05.128
Cunha A. D., Nascimento A. M., Guidugli K. R., Simões Z. L., Bitondi M. M. (2005). Molecular cloning and expression of a hexamerin cDNA from the honey bee, Apis mellifera. Apis Mellifera. J. Insect Physiol. 51 (10), 1135–1147. doi:10.1016/j.jinsphys.2005.06.004
da Costa Domingues C. E., Bello Inoue L. V., da Silva-Zacarin E. C. M., Malaspina O. (2020). Fungicide pyraclostrobin affects midgut morphophysiology and reduces survival of Brazilian native stingless bee Melipona scutellaris. Ecotoxicol. Environ. Saf. 206, 111395. doi:10.1016/j.ecoenv.2020.111395
Dai J. J., Shu R., Liu J., Xia J. F., Jiang X. S., Zhao P. (2021). Transcriptome analysis of Apis mellifera under benomyl stress to discriminate the gene expression in response to development and immune systems. J. Environ. Sci. Heal. B 56 (6), 594–605. doi:10.1080/03601234.2021.1930795
Dai P. L., Yan Z. X., Ma S. L., Yang Y., Wang Q., Hou C. S., et al. (2018). The herbicide glyphosate negatively affects midgut bacterial communities and survival of honey bee during larvae reared in vitro. J. Agr. Food Chem. 66 (29), 7786–7793. doi:10.1021/acs.jafc.8b02212
Danihlík J., Aronstein K., Petřivalský M. (2015). Antimicrobial peptides: A key component of honey bee innate immunity. J. Apic. Res. 54 (2), 123–136. doi:10.1080/00218839.2015.1109919
David A., Botías C., Abdul-Sada A., Goulson D., Hill E. M. (2015). Sensitive determination of mixtures of neonicotinoid and fungicide residues in pollen and single bumblebees using a scaled down QuEChERS method for exposure assessment. Anal. Bioanal. Chem. 407, 8151–8162. doi:10.1007/s00216-015-8986-6
de Azevedo S. V., Hartfelder K. (2008). The insulin signaling pathway in honey bee (Apis mellifera) caste development - differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol. 54 (6), 1064–1071. doi:10.1016/j.jinsphys.2008.04.009
DesJardins N. S., Fisher A., Ozturk C., Fewell J. H., DeGrandi-Hoffman G., Harrison J. F., et al. (2021). A common fungicide, Pristine®, impairs olfactory associative learning performance in honey bees (Apis mellifera). Enviro. Pollu. 288, 117720. doi:10.1016/j.envpol.2021.117720
Duan X. L., Xiong M. Q., Liu W. B., Zhao B. A., Huang S. K., Li J. H. (2020a). Effects of three fungicides on the activities of protective enzymes and detoxifying enzymes in Apis mellifera. Acta pratac. Sin. 29 (11), 74–82. doi:10.11686/cyxb2020148
Duan X. L., Zhao B. A., Jin X., Cheng X., Huang S. K., Li J. H. (2021). Antibiotic treatment decrease the fitness of honeybee (Apis mellifera) larvae. Insects 12, 301. doi:10.3390/insects12040301
Duan X. L., Zhao B. A., Liu Y., Xiong M. Q., Huang S. K., Huang W. F., et al. (2020b). Development and characterization of six novel microsatellite markers for honey bee parasitic mite Varroa destructor (Mesostigmata: Varroidae). Syst. Appl. Acarol. 25 (10), 1733–1744. doi:10.11158/saa.25.10.2
Earley F., Sauter H., Rheinheimer J., Rieck H., Coqueron P. Y., Whittingham W. G., et al. (2012). “Fungicides acting on oxidative phosphorylation,” in Modern crop protection compounds (Hoboken: John Wiley & Sons), 559–691.
Evans J. D., Aronstein K., Chen Y. P., Hetru C., Imler J. L., Jiang H., et al. (2006). Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 15 (5), 645–656. doi:10.1111/j.1365-2583.2006.00682.x
Favaro R., Bauer L. M., Rossi M., D’Ambrosio L., Bucher E., Angeli S. (2019). Botanical origin of pesticide residues in pollen loads collected by honeybees during and after apple bloom. Front. Physiol. 10, 1069. doi:10.3389/fphys.2019.01069
Fisher A., DeGrandi-Hoffman G., Smith B. H., Johnson M., Harrison J. F., Cogley T., et al. (2021). Colony field test reveals dramatically higher toxicity of a widely-used mito-toxic fungicide on honey bees (Apis mellifera). Environ. Pollut. 269, 115964. doi:10.1016/j.envpol.2020.115964
Fungicide Resistance Action Committee (FRAC) (2021). Fungal control agents sorted by cross resistance pattern and mode of action. Available at: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-(2022)--final.pdf?sfvrsn=b6024e9a_2 (Accessed March 15, 2022).
Gallai N., Salles J. M., Settele J., Vaissiere B. E. (2009). Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821. doi:10.1016/j.ecolecon.2008.06.014
Gierer F., Vaughan S., Slater M., Thompson H. M., Elmore J. S., Girling R. D. (2019). A review of the factors that influence pesticide residues in pollen and nectar: Future research requirements for optimising the estimation of pollinator exposure. Environ. Pollut. 249, 236–247. doi:10.1016/j.envpol.2019.03.025
Godfray H. C. J., Garnett T. (2014). Food security and sustainable intensification. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369 (1639), 20120273. doi:10.1098/rstb.2012.0273
Havukainen H., Münch D., Baumann A., Zhong S., Halskau Ø., Krogsgaard M., et al. (2013). Vitellogenin recognizes cell damage through membrane binding and shields living cells from reactive oxygen species. J. Biol. Chem. 288, 28369–28381. doi:10.1074/jbc.M113.465021
Ihle K. E., Baker N. A., Amdam G. V. (2014). Insulin-like peptide response to nutritional input in honey bee workers. J. Insect Physiol. 69, 49–55. doi:10.1016/j.jinsphys.2014.05.026
Jensen A. B., Pedersen B. V., Eilenberg J. (2009). Differential susceptibility across honey bee colonies in larval chalkbrood resistance. Apidologie 40 (5), 524–534. doi:10.1051/apido/2009029
Kablaua A., Eckertb J. H., Pistoriusb J., Sharbatia S., Einspanier R. (2020). Effects of selected insecticidal substances on mRNA transcriptome in larvae of Apis melliferaffects of selected insecticidal substances on mRNA transcriptome in larvae of Apis mellifera. Pestic. Biochem. Phys. 170, 104703. doi:10.1016/j.pestbp.2020.104703
Krupke C. H., Hunt G. J., Eitzer B. D., Andino G., Given K. (2017). Multiple routes of pesticide exposure for honey bees living near agricultural fields. PloS ONE 7 (1), e29268. doi:10.1371/journal.pone.0029268
Kumar N., Willis A., Satbhai K., Ramalingam L., Schmitt C., Moustaid-Moussa N., et al. (2020). Developmental toxicity in embryo-larval zebrafish (Danio rerio) exposed to strobilurin fungicides (azoxystrobin and pyraclostrobin). Chemosphere 241, 124980. doi:10.1016/j.chemosphere.2019.124980
Legard D. E., Xiao C. L., Mertely J. C., Chandler C. K. (2001). Management of Botrytis fruit rot in annual winter strawberry using captan, thiram, and iprodione. Plant Dis. 85 (1), 31–39. doi:10.1094/PDIS.2001.85.1.31
Li H., Cao F., Zhao F., Yang Y., Teng M., Wang C., et al. (2018). Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere 207, 781–790. doi:10.1016/j.chemosphere.2018.05.146
Liao J. H., Cheng Y., Bu Y. Q., Tan L. C., Zhou J. Y., Shan Z. J. (2018). Review of the pesticides registered on major nectar crops in China and the primary risk assessment of the insecticides to honey bees. Chin. J. Pestic. Sci. 20 (01), 100–109. doi:10.16801/j.issn.1008-7303.2018.0007
Liu C. D., Wang Q., Yu H., Zhang Z. B., Zheng B. Y., Li J. H. (2018). Expression and functional analysis of early-response factors ecr and usp of ecdysteroid in different castes of. Apis mellifera. genom. Appl. Biol. 37, 686–692. doi:10.13417/j.gab.037.000686
Liu H., Wang J., Li S. (2014). E93 predominantly transduces 20-hydroxyecdysone signaling to induce autophagy and caspase activity in Drosophila fat body. Insect biochem. Mol. Biol. 45, 30–39. doi:10.1016/j.ibmb.2013.11.005
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
Luo Y., Wu X. M., Hu X. F., Yao X. L., Liu X. D., Han L. (2022). Research progress on pyraclostrobin degradation, metabolism, and toxicology. J. Agric. Resour. Environ. 13, 651–663. doi:10.13254/j.jare.2021.0169
Ministry of Agriculture of the People's Republic of China (2017).Chemical pesticide-Guideline on honeybee (Apis mellifera L.) larval toxicity test (NY/T 3085-2017). Beijing: Ministry of Agriculture of the People's Republic of China.
Mullin C. A., Frazier M., Frazier J. L., Ashcraft S., Simonds R., Vanengelsdorp D., et al. (2010). High levels of miticides and agrochemicals in north American apiaries: Implications for honey bee health. PloS ONE 5 (3), e9754. doi:10.1371/journal.pone.0009754
Nicodemo D., Mingatto F. E., De Jong D., Bizerra P. F. V., Tavares M. A., Bellini W. C., et al. (2020). Mitochondrial respiratory inhibition promoted by pyraclostrobin in fungi is also observed in honey bees. Environ. Toxicol. Chem. 39 (6), 1267–1272. doi:10.1002/etc.4719
Pettis J. S., Lichtenberg E. M., Andree M., Stitzinger J., Rose R., van Engelsdorp D. (2013). Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8 (7), e70182. doi:10.1371/journal.pone.0070182
Riddiford L. M., Cherbas P., Truman J. W. (2000). Ecdysone receptors and their biological actions. Vitam. Horm. 60, 1–73. doi:10.1016/s0083-6729(00)60016-x
Rondeau S., Raine N. E. (2022). Fungicides and bees: A review of exposure and risk. Environ. Inter. 165, 107311. doi:10.1016/j.envint.2022.107311
Salmela H., Amdam G. V., Freitak D. (2015). Transfer of immunity from mother to offspring is mediated via egg-yolk protein vitellogenin. PLoS Pathog. 11, e1005015. doi:10.1371/journal.ppat.1005015
Salmela H., Sundström L. (2017). Vitellogenin in inflammation and immunity in social insects. Inflamm. Cell Signal 5, e1506. doi:10.14800/ics.1506
Shi J. L., Yang H. Y., Yu L. T., Liao C. H., Liu Y., Jin M. J., et al. (2020). Sublethal acetamiprid doses negatively affect the lifespans and foraging behaviors of honey bee (Apis mellifera L.) workers. Sci. Total Environ. 738, 139924. doi:10.1016/j.scitotenv.2020.139924
Shi J. L., Zhang R. N., Pei Y. L., Liao C. H., Wu X. B. (2020). Exposure to acetamiprid influences the development and survival ability of worker bees (Apis mellifera L.) from larvae to adults. Environ. Pollut. 266, 115345. doi:10.1016/j.envpol.2020.115345
Simondelso N., Martin G. S., Bruneau E., Hautier L. (2018). Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci. Rep. 8 (1), 7241. doi:10.1038/s41598-018-24746-9
Simone M., Evans J. D., Spivak M. (2009). Resin collection and social immunity in honey bees. Evolution 63, 3016–3022. doi:10.1111/j.1558-5646.2009.00772.x
Tadei R., Domingues C. E. C., Malaquias J. B., Camilo E. V., Malaspina O., Silva-Zacarin E. C. M. (2019). Late effect of larval co-exposure to the insecticide clothianidin and fungicide pyraclostrobin in Africanized Apis mellifera. Sci. Rep. 9 (1), 3277. doi:10.1038/s41598-019-39383-z
Tan L. C., Ge F., Cheng Y., Shan Z. J. (2021). Acute toxicity of four commonly used strobilurin fungicides. Pestic. Sci. Admin. 42 (1), 33–38. doi:10.7524/AJE.1673-5897.20170512001
Traynor K. S., Van Engelsdorp D., Lamas Z. S. (2021). Social disruption: Sublethal pesticides in pollen lead to Apis mellifera queen events and brood loss. Ecotox. Environ. Safe. 214, 112105. doi:10.1016/j.ecoenv.2021.112105
Van der Zee R., Pisa L., Andonov S., Brodschneider R., Charrière J. D., Chlebo R., et al. (2012). Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of (2008)-9 and (2009)-10. J. Apic. Res. 51 (1), 100–114. doi:10.3896/IBRA.1.51.1.12
van Dooremalen C., Cornelissen B., Poleij-Hok-Ahin C., Blacquière T. (2018). Single and interactive effects of Varroa destructor, Nosema spp., and imidacloprid on honey bee colonies (Apis mellifera)ffects of Varroa destructor, Nosema spp., and imidacloprid on honey bee colonies (Apis mellifera). Ecosphere 9, 02378. doi:10.1002/ecs2.2378
Wang Y., Azevedo S. V., Hartfelder K., Amdam G. V. (2013). Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L.). J. Exp. Biol. 216, 4347–4357. doi:10.1242/jeb.085779
Xiong M. Q., Qin G., Wang L. Z., Huang S. K., Li J. H., Duan X. L. (2022). Toxic effects of fungicides on physiology and behavior of honeybee. Asian J. ecotox. 2022 (6), 111–120. doi:10.7524/AJE.1673-5897.(2021)1019002
Yoder J. A., Jajack A. J., Rosselot A. E., Smith T. J., Yerke M. C., Sammataro D. (2013). Fungicide contamination reduces benefcial fungi in bee bread based on an area-wide feld study in honey bee, Apis mellifera, colonies. J. Toxicol. Environ. Health. A 76, 587–600. doi:10.1080/15287394.2013.798846
Zaluski R., Justulin L. A., Orsi R. (2017). Field-relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Sci. Rep. 7, 15217. doi:10.1038/s41598-017-15581-5
Zhang W. J. (2018). Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8, 1–27. doi:10.13140/RG.2.2.28978.30405
Zheng B. Y., Zhao B. A., Jin X., Duan X. L., Huang S. K., Li J. H. (2019). Effect of Sacbrood virus infection on nutritional and immune responses of Apis cerana cerana (Hymenoptera: Apidae). Acta Entomol. Sin. 62 (09), 1054–1064. doi:10.16380/j.kcxb.2019.09.006
Zhu W., Schmehl D. R., Mullin C. A., Frazier J. L. (2014). Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 9 (1), e77547. doi:10.1371/journal.pone.0077547
Keywords: Apis mellifera, pyraclostrobin, larvae, pupae, gene expression
Citation: Xiong M, Qin G, Wang L, Wang R, Zhou R, Luo X, Lou Q, Huang S, Li J and Duan X (2023) Field recommended concentrations of pyraclostrobin exposure disturb the development and immune response of worker bees (Apis mellifera L.) larvae and pupae. Front. Physiol. 14:1137264. doi: 10.3389/fphys.2023.1137264
Received: 04 January 2023; Accepted: 26 January 2023;
Published: 09 February 2023.
Edited by:
Chunsheng Hou, Institute of Bast Fiber Crops (CAAS),, ChinaReviewed by:
Xinzheng Huang, China Agricultural University, ChinaSu-Qin Shang, Gansu Agricultural University, China
Hongxia Zhao, Guangdong Institute of Applied Biological Resources, China
Copyright © 2023 Xiong, Qin, Wang, Wang, Zhou, Luo, Lou, Huang, Li and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinle Duan, eGlubGVkdWFuQGZhZnUuZWR1LmNu
 Manqiong Xiong1
Manqiong Xiong1 Xinle Duan
Xinle Duan