- 1Sport Science Department, Faculty of Literature and Humanities, Persian Gulf University, Boushehr, Iran
- 2Exercise Physiology Department, Sport Science Faculty, Shahid Rajaee Teacher Training University, Tehran, Iran
- 3Exercise Physiology Department, Sport Science Faculty, Tehran University, Tehran, Iran
- 4Cardiac Rehabilitation Specialist and Head of Rehabilitation Clinic of Tehran Heart Center Hospital, Tehran, Iran
Background: ProBNP1-108/BNP1-32, and NT-pro-BNP1-76/BNP1-32 ratios are significant indices for predicting complications after coronary artery bypass grafting (CABG) surgery. However, the effect of aerobic training types on these biomarkers has not been fully understood. So, the current study aimed to determine the impact of aerobic interval and continuous training programs on plasma ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 after coronary artery bypass grafting surgery.
Method: 36 patients were selected purposive (27 men and 9 women with mean of age 60.32 ± 5.81 years, height 164.64 ± 9.25 cm, weight 73.86 ± 14.23 kg, fat 32.30 ± 4.28, SBP 142.67 ± 6.49, DBP 84.5 ± 5.16 mmHg in seated position at rest situation and functional capacity of 7.08 ± 2.49 METs) and then divided randomly into three groups: control (C) group (without training program) moderate continuous training (MCT) and high intensity interval training (HIIT) (exercise training program was performed 3 days/week for 8 weeks) with intensities 65%–80% and 80%–95% of reserve heart rate in order. Blood samples were taken 48 h before the first session and 48 h after the last training session to measure the plasma levels of ProBNP1–108, corin enzyme, BNP1-32, and NT-pro-BNP1-76 using the enzyme-linked immunosorbent assay (ELISA) technique. Wilcoxin and kruskal wallis tests were used for analyzing data.
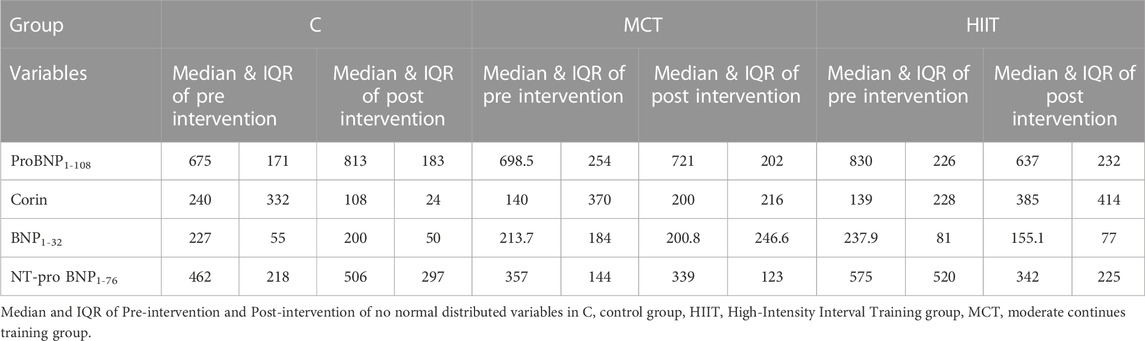
Results: The plasma corin enzyme was increased, and the ratios of proBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 were reduced in both training groups in compared with control group (p = 0.004, p = 0000, p = 0.016, p = 0.003, p = 0.009, and p = 0.016) when there was no significant difference was found between training groups (p = 0.074, p = 450, and p = 0.295).
Conclusion: Both high intensity interval training and moderate continuous training in compared with inactivity have positive effects on ratios of ProBNP1-108/BNP1-32, NT-pro-BNP1-76/BNP1-32 and could be effective to promote the health of coronary arteries and prevention of HF in post-CABG patients.
Introduction
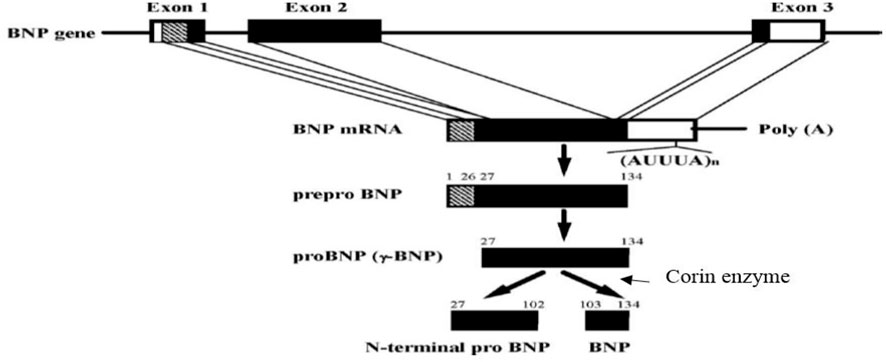
Coronary artery bypass grafting surgery (CABG) is an important therapeutic strategy for patients with coronary heart disease (Serruys et al., 2001). Although CABG can decrease the rates of morbidity and mortality in patients, by the improvement of blood circulation, in the heart, it is also accompanied by the emergence of some side effects. Postoperative heart failure (HF) is one of the significant outcomes that usually occurs after CABG (Siribaddana, 2012). Several lines of evidence indicate that the complications of CABG include inflammation and the production of free radicals due to reperfusion of the ischemic heart. These factors are the initiator of the fibrosis signaling pathway and negative remodeling of the heart. So, they can weaken the myocardial contractility and cause HF in the long term (Siribaddana, 2012; Cockburn et al., 2013). Brain natriuretic-related peptides (proBNP1-108, BNP1-32, and NT-pro-BNP1-76) are critical markers applied for the diagnosis of HF after surgery (Fox et al., 2011; Preeshagul et al., 2013; Fu et al., 2018a). For example, Fox et al. (2011) showed a direct and strong association between the increase of BNP1-32 after CABG and the development of HF in the next 5 years. Fu et al. (2018a) also reported that impairment in processing and degradation of BNP1-32 and NT-pro-BNP1-76 are effective in the occurrence of HF. Brain natriuretic peptide (BNP1-32) is a hormone released from the cardiac ventricles in response to ischemia and myocardial wall stress due to volume or pressure overload (Fu et al., 2018b). BNP1-32 is primarily synthesized from an inactive prohormone (proBNP1-108) that is cleaved into the active hormone (BNP1-32) and the inactive N-terminal fragment (NT-pro-BNP1-76) by the enzyme named corin. BNP1-32 is decomposed into the body after carrying out its biological function (Ichiki et al., 2011; Ichiki et al., 2013). BNP1-32 reduces blood pressure directly and indirectly by relaxing vascular smooth muscles, blocking the cardiac sympathetic nervous system, and increasing the diuretic and natriuretic effects. BNP1-32 also inhibits the renin-angiotensin-aldosterone system and has anti-proliferative and anti-fibrotic effects on the myocardium (Chopra et al., 2013). Although BNP1-32 is a cardiovascular protective peptide, it is measured in patients’ blood as a cardiovascular stress index. Recent studies indicated that in addition to BNP1-32 indices, namely, proBNP1-108, NT-pro-BNP1-76, like the ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 play a major role in predicting the development of HF (Jensen et al., 2010; Jensen et al., 2012; Miller et al., 2012; Barnet et al., 2015; Huntley et al., 2015; Fu et al., 2018a). The generation and degradation of BNP1-32 is a significant problem in cardiac patients. For example, Barnet et al. (2015) have shown that the amount of corin enzyme involved in the conversion of proBNP1-108 to BNP1-32 decreases after CABG. Whereas proBNP1-108 is increased simultaneously, indicating a gap in the production process of BNP1-32, particularly after CABG (Barnet et al., 2015). In other words, the enzymatic activity is impaired in the myocardium of patients, especially after CABG. Hence, the ProBNP1-108/BNP1-32 ratio could be increased in these patients and may result in an increased risk of HF (Barnet et al., 2015). On the other hand, proper breakdown of BNP1-32 and NT-pro-BNP1-76 is essential for heart functions; the accumulation of BNP1-32 leads to the saturation of its receptor and inefficiency of BNP1-32 in patients with cardiovascular diseases (Huntley et al., 2015). Some studies indicated that the impairment of BNP1-32 processing and its degradation system could reduce the levels of BNP1-32 and increase the levels of inactive ProBNP1-108 and NT-pro-BNP1-76. This condition, a paradox of BNP1-32, is medically considered a dilemma in patients with cardiovascular diseases (Huntley et al., 2015). Numerous BNP-related peptides are circulated in the bloodstream; however, they have no beneficial effect on the health status of patients with cardiovascular disorders, as most of these peptides are found inactive and have no functionality (Huntley et al., 2015; Zarekarizak et al., 2017). Hence, the ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 could be regarded as two important indices for BNP1-32 processing and degradation, which are defective in patients with cardiovascular diseases. The dysfunction of BNP1-32 processing and its degradation retain a fundamental role in the development of HF in the long-term period (Jensen et al., 2010; Jensen et al., 2012; Barnet et al., 2015; Huntley et al., 2015). For example, Suzuki and Sugiyama, (2018) showed a significant risk of HF in patients with a high ratio of NT-proBNP1-76/BNP1-32 according to Kaplan-Meier analysis (Suzuki and Sugiyama, 2018). In other words, the positive effects of exercise on cardiac rehabilitation, especially ACT, have been addressed and can act as a therapeutic strategy to prevent secondary problems and mortality after surgery (Dendale et al., 2005; Conraads and Beckers, 2010; Valkeinen et al., 2010; Ghashghaei et al., 2012; Meyer et al., 2013; Nishitani et al., 2013). High Intensity Interval Training (HIIT) is also an exercise training employed for cardiovascular adaptations and improvement of functional capacity (Wisloff et al., 2007; Gibala et al., 2012; Guiraud et al., 2012). HIIT is characterized by repeated bouts of high-intensity exercise interspersed by rest periods or low-intensity exercise for recovery (Gibala et al., 2012). HIIT is recommended for patients with coronary heart disease because of the higher tolerability of this type of exercise than continuous exercise programs (Guiraud et al., 2012). In this regard, studies have been performed exercise training on BNP1-32 and NT-proBNP1-76 levels in cardiac patients. For example, Pearson et al. (2018) reported a positive effect of exercise on BNP1-32 and NT-proBNP1-76 scores among patients with HF (Pearson et al., 2018). Wisløff et al. (2007) also examined the impacts of eight weeks of high-intensity interval training upon moderate-intensity continuous training. They showed a greater reduction of BNP1-32 in high-intensity interval training than in continuous training in HF patients (Wisloff et al., 2007). Additionally, many studies have reported the beneficial impacts of HIIT, but there are conflicts about the optimal training program compared to MCT in patients with coronary heart disease. Besides, the effects of these two training programs remained unclear on the processing and degradation of BNP1-32, as shown by the ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32. Therefore, the purpose of this study was to determine the impact of HIIT and MCT on plasma ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 in patients who underwent CABG. The chemical structure and production processing of BNP1-32 and NT-pro-BNP1-76 is depicted in Figure 1.
Material and methods
Subjects
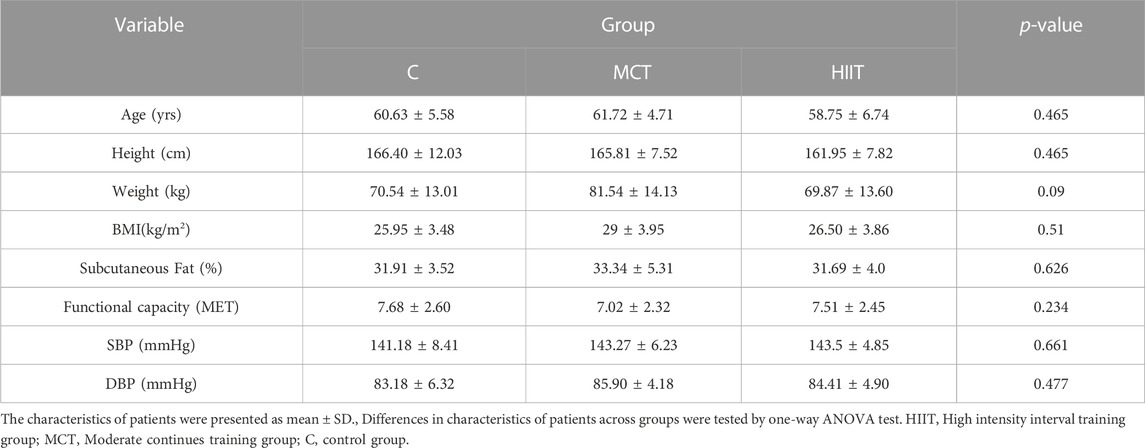
The statistical population were all post CABG patients referred to Tehran Heart Center Hospital. Sample size were 36 patients because according to studies there must be at least chosen 30 samples to semi-experimental studies (Dietz and Kalof, 2009). so first of all we selected 36 patients purposive (27 men’s and 9 women) according our criteria and then allocated them to three groups according to homogeneity and gender randomly (9 men and 3 women in each group). The mean of their age were 60.32 ± 5.81 years old, height 164.64 ± 9.25 cm, weight 73.86 ± 14.23 kg, body mass index (BMI) 27.24 ± 3.90 kg/m2, systolic blood pressure (SBP) 142.67 ± 6.49, diastolic blood pressure 9 (DBP) 84.5 ± 5.16 mmHg, and functional capacity 7.08 ± 2.49 (METs). Two patients withdrew from the study during the experiment, and 34 patients were remained in our research. Table 3 illustrates patients’ characteristics for each group.
Inclusion and exclusion criteria
All inclusion and exclusion criteria were considered according to patients’ medical records.
Inclusion criteria were as follows passing one month after following CABG surgery, low cardiovascular risk stratification (Thow, 2006), level 1 hypertension (SBP 140–159 mmHg or DBP 90–99 mmHg or both (Giles et al., 2005), and simultaneous consumption of aspirin, beta-blockers, anti-hypertensive drugs, and statins. Since all subjects were selected with low cardiovascular risk stratification and level 1 hypertension, the dosage of the drugs used by patients was almost the same. Furthermore, all patients had concentric pathologic hypertrophy (LVMI1 more than116 g/m2 and RWT2 was more than 0.42) (Lang et al., 2006).
Exclusion criteria were myocardial infarction, heart valve surgery history, ejection fraction <30%, and movement limitation.
Procedures
All procedures in this study have been endorsed by the Ethics Committee of Shahid Rajaee Teacher Training University of Tehran (IRSRTTU.SSF.2020.104). Informed consent was obtained from all participants. All measurements were performed as a pre-test after one week of familiarization and 48 h before the first exercise training session. After 2 months and 48 h after the last session of exercise training, all measurements were conducted as a post-test in similar conditions in which the pre-test was carried out. Exercise training was performed along with other routine rehabilitation programs such as psychological rehabilitation (counseling sessions to decrease anxiety and depression of patients), pharmacological treatments (aspirin, beta-blockers, anti-hypertensive drugs, and statins); lifestyle correction counseling (encouragement to increase the physical activity in daily life); diet modification and cessation of cigarette smoking performed in the cardiac rehabilitation clinic.
Exercise training protocol
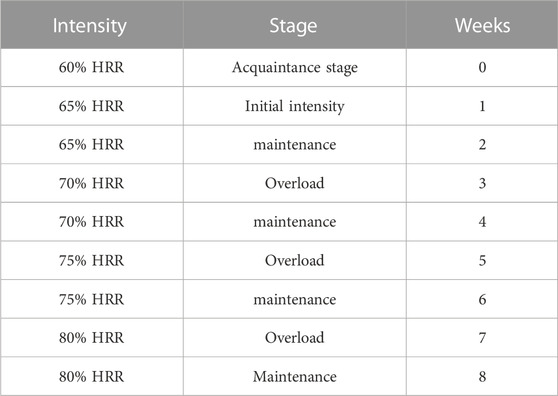
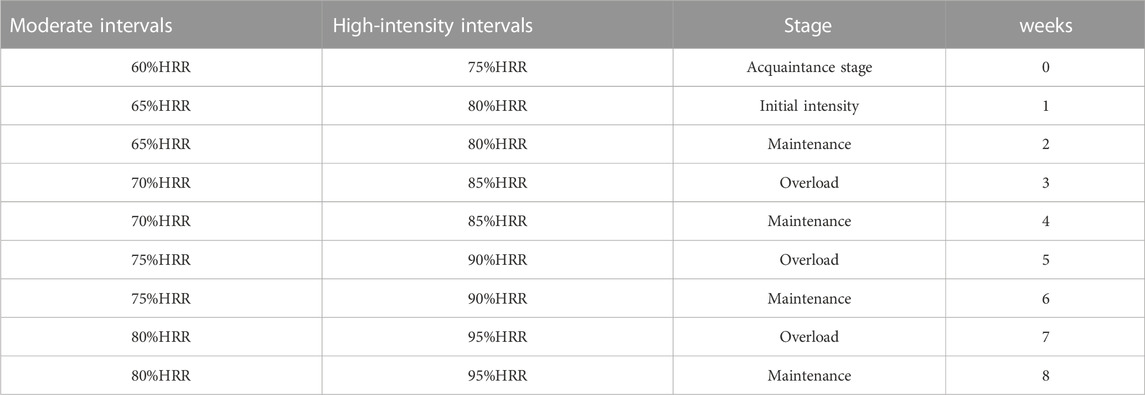
A comprehensive cardiac rehabilitation program (CR) includes six main aspects: 1) initial patient assessment, 2) nutritional counseling and weight management, 3) ongoing management of coronary risk factors, 4) psychological management, 5) physical activity counseling, and 6) exercise training (de Waard et al., 2021). In the cardiac rehabilitation clinic, exercise training programs were conducted three days a week over 8 weeks training groups performed exercise training on the treadmill (HP Cosmos, Germany) under the supervision of a physician, nurses, and the exercise physiologist. The rate of perceived exertion, rhythm, and arrhythmia was measured by Borg scale, ECG (United States; MHC 1,200), and remote control system (Iran, Avicenna Company; Telemetry), respectively, during each exercise training session. HIIT included 30 min exercises that consisted of 7 min warm-up (walking at 50%–55% of heart rate reserve (HRR), 4 intense intervals for 2.30 min at 80%–95% of HRR, 4 light intervals for 2.30 min at 65%–80% HRR between intense intervals, and finally 3 min cool-down (walking at 50%–55% of HRR). MCT included 33 min exercise on a treadmill that consisted of 7 min warm-up (walking at 50%–55% of HRR), followed by 23 min exercise on a treadmill at 65%–80% HRR, and, finally, 3 min cool-down (walking at 50%–55% of HRR). These protocols were similar to those used in a study by Wisloff et al. (2007) with slight modifications. We considered a similar training load (volume × intensity) to redesign the protocols for HIIT and MCT groups (Figure 2). Furthermore, the mode of intensity overload in the continuous and interval training group have been shown in Tables 1, 2. The maximum heart rate was defined according to the modified Bruce test result. The training heart rate was calculated by the Karvonen equation, as mentioned below (Sagiv, 2012).
HRR, heart rate reserve; MHR, maximum heart rate attained at a peak stress test; and RHR, resting heart rate; THR, training heart rate.
Laboratory measurements
All participants’ exact height (cm) and weight (kg) were measured for their BMI. Lange Skinfold caliper, 3-site formula, and Siri equation were used to estimate body fat percentage. Resting blood pressure was also measured by the digital blood pressure system (medical space labs made in the United States) from the brachial artery at a seating position. Blood samples (10 mL) were collected into the standard EDTA-containing tubes and immediately transferred to the laboratory to measure values at baseline and after the experiment. The sample plasma was separated by centrifugation at 3,000 rpm for about 10 min, then stored at −80°C until analysis. Corin, BNP1-32, proBNP1-108, and NT-pro-BNP1-76 were measured using the ELISA technique (kits: BOSTER Company made in United States Cat. No: EK1283, BIOMEDICA Company made in Austria Cat. No: BI-20852W and EASTBIOPHARM Company made in china Cat. No: CK-E90422, CK-E10219).
Statistical analysis
The data were presented as mean and standard deviation (mean ± SD). The Kolmogorov-Simonov test was used to specify the normality of data distribution. We assessed normal distributed data (witness variables: blood pressure and subcutaneous fat, the ratios of proBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32) with the paired sample test and one-way ANOVA to determine within-group variations and between-group differences in the delta of means from pre-test to post-test, respectively. Kruskal-Wallis and Mann-Whitney U tests were used for non-normal distributed data (proBNP1-108, Corin, BNP1-32, and NT-pro-BNP1-76) to analyze within-group variations and between-group differences in the delta of means, from pre-test to post-test, respectively. A p-value of less than 0.05 was statistically considered significant for tests.
Results
The plasma corin enzyme was increased, and the ratios of proBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 were reduced in both training groups in compared with control group (p = 0.004, p = 0000, p = 0.016, p = 0.003, p = 0.009, and p = 0.016) when there was no significant difference was found between training groups (p = 0.074, p = 450, and p = 0.295).
The statistical results are depicted in Tables 3–5. Within and between groups, changes in PROBNP1-108, CORIN, BNP1-32, NT-PROBNP1-76, ProBNP/BNP ratio and NTProBNP/BNP ratio are shown in Figures 3A–F.
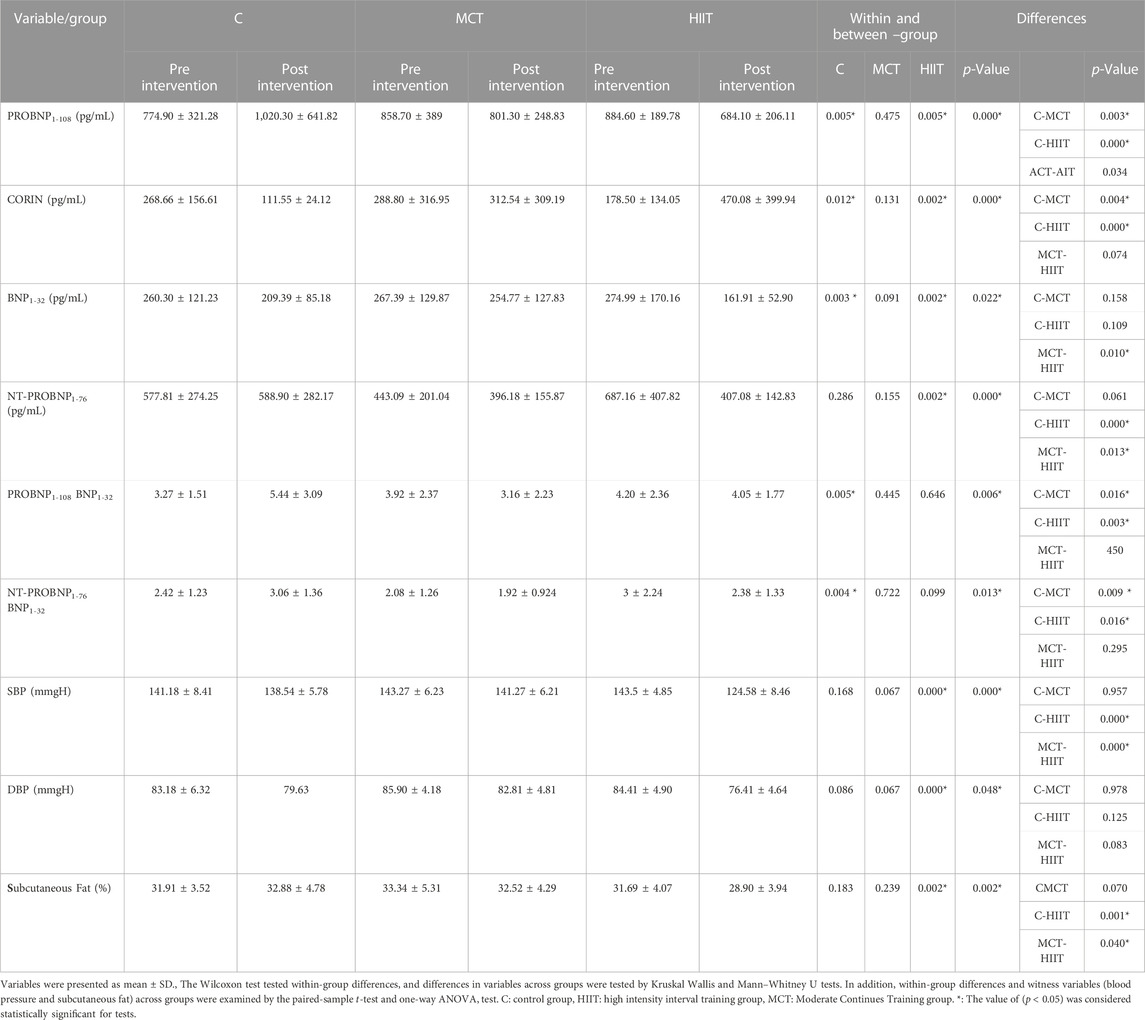
TABLE 4. Within and between-group differences, analyzed by Wilcoxon, Kruskal-Wallis, and Mann-Whitney U tests.
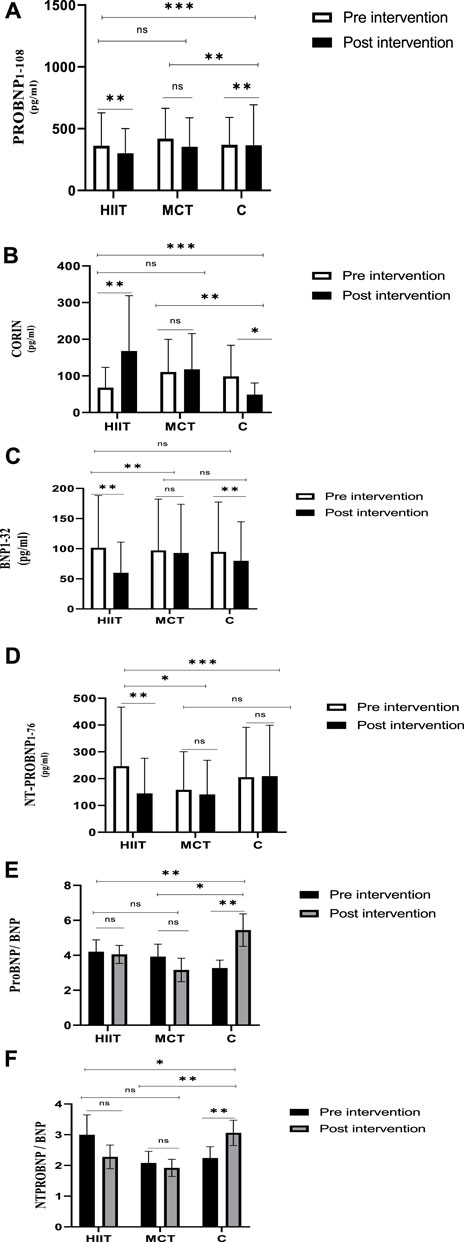
FIGURE 3. The differences in variables from Pre-Intervention to Post-intervention and between-group differences of variables (A) Within and between groups, changes in PROBNP1-108. (B) Within and between groups, changes in CORIN. (C) Within and between groups, changes in BNP1-32, (D) Within and between groups, changes in NT-PROBNP1-76. (E) Within and between groups, changes in ProBNP/BNP ratio. (F) Within and between groups, changes in NTProBNP/BNP ratio.
Discussion
The results of the present study indicated that 8-week HITT, in comparison with MCT, significantly decreased the plasma levels of ProBNP1-108, BNP1-32, and NT-pro-BNP1-76, on the other hand, increased plasma corin enzyme. Our findings mirror those of similar studies. For instance, Smart and Still (2010) showed the role of resistance and aerobic exercise in reducing BNP1-32 and NT-pro-BNP1-76 in HF patients (Smart and Steele, 2010). Santoso et al., 2020 have also demonstrated that aerobic exercise can reduce NT-pro-BNP1-76 and increase the left ventricular function in HF patients (Santoso et al., 2020). With this line, some studies have shown no effect of exercise on BNP1-32 dependent peptides, such as NT-pro-BNP1-76; for example, Zdrenghea et al. (2014) have investigated the effect of two methods, including exercise on the Ergometer and isometric handgrip test on NT-pro-BNP1-76 level of HF patients and reported any change. Perhaps the reason for the inconsistency of the mentioned study with the present study was the difference in the kind of patients HF patients versus post-CABG patients (in fact, HF is a secondary adverse consequence of CABG in long term. Although HF is a multifactorial problem, it seems hypertension, due to the pressure and volume overload on the heart, and with the direct effect on the structure and function of cardiomyocytes and fibroblasts, causes the gradual development of the fibrotic process, and pathologic hypertrophy. Finally, these structural changes lead to a rise in diastolic heart failure (impairment in optimal ventricular filling), systolic heart failure (impairment in Ejection fraction), and possibly patient mortality (Zile et al., 2011; Zare Karizak et al., 2017). So the improvement of the BNP processing system is important as an effective factor in blood pressure and consequently pathologic hypertrophy and Hf.), type, and duration of exercise (Isotonic and isometric exercise in single sessions versus eight weeks of aerobic interval and continuous training). As previously mentioned, BNP1-32 is primarily synthesized by the converting enzyme (corin) from an inactive prohormone (proBNP1-108) that is cleaved into the active hormone (BNP1-32) and inactive N-terminal fragment (NT-pro-BNP1-76). So in the present study probably, the reduction of prohormone (proBNP1-108) and increase of corin enzyme indicates an improvement in the production system of BNP1-32 due to an increase of converting enzyme (corin) in training groups. These results indicated an enhancement in the production system of BNP1-32; the amount of BNP1-32 and NT-pro-BNP1-76, as the final products, decreased in both training groups, especially in the HIIT group. This event may be owing to the regulatory role of BNP1-32, which could be increased as a compensatory mechanism in response to pressure overload, volume overload, and ischemic conditions (Fu et al., 2018b). Hence, its concentration is initially elevated in patients with cardiovascular disorders, while it could be decreased after 8-week HIIT. This phenomenon stems from the reduction of stress conditions. Consequently, studies indicated that body adaptation with HIIT could diminish the pressure overload by decreasing vasoconstriction factors, such as angiotensin II, endothelin, and increment in vasodilator agents, such as nitric oxide and prostaglandins (Passino et al., 2006) and decrease of adrenomedullin as a stress index on heart (Zarekarizak et al., 2021). HIIT also decreases volume overload by inhibiting the renin-angiotensin-aldosterone system (Gademan et al., 2007). Other studies have also indicated that HIIT can attenuate the ischemic condition by incrementing the quantity and quality of micro-vessels and improving oxygen delivery to the heart (Macheret et al., 2011). Besides, our findings confirmed that HIIT could dramatically decrease blood pressure compared to other groups. Furthermore, while body fat reduction is associated with an increased ratio of the biological natriuretic peptide receptors (NPR-A to NPR-C), it has been suggested that the body fat percentage influences the sensitivity of the human body to BNP1-32 (Dessì-Fulgheri et al., 1997; Marney et al., 2014; Arora et al., 2015). The reduction in body fat percentage after exercise training appears to correlate with the reduction of BNP1-32 and an increase in the body’s sensitivity to BNP1-32. Our results showed that the rate of the reduction of the body fat percentage is significantly higher at HIIT in comparison with MCT. On the other hand, the ratio of ProBNP1-108/BNP1-32 was increased in the control group compared with other groups. As previously mentioned, the ratio of ProBNP1-108/BNP1-32 is an indicator of the production system of BNP1-32. The increment ratio in the control group is associated with the impaired production system of BNP1-32, which may result from the reduction of the corin enzyme of the control group in the present study. Furthermore, the reduction of degradation enzymes of proBNP1-108 has disrupted the BNP1-32 production system in the control group likely and increased the ProBNP1-108/BNP1-32 ratio in this group. In contrast, the ratio of ProBNP1-108/BNP1-32 was remarkably decreased in plasma levels of both training groups compared with the control group. However, since ProBNP1-108/BNP1-32 decreased in both training groups, the rate of change in the ratio ProBNP1-108/BNP1-32 would remain unchanged. Therefore, no significant difference was found in that ratio between training groups. The statistical analysis demonstrated a significant difference in the ratio of ProBNP1-108/BNP1-32 between the HIIT and control groups and between the MCT and control groups. The ratio of NT-pro-BNP1-76/BNP1-32 was also increased in the group of control but decreased in the training groups. The ratio of NT-pro-BNP1-76/BNP1-32 is an indicator of the degradation system of BNP1-32. Like the production system of BNP1-32, its degradation system was impaired in patients and exacerbated, resulting from the inactivity of the control group. BNP1-32 is cleaved in several ways, such as guanylyl cyclase receptor type C and glomerular filtration (Barnet et al., 2015; Huntley et al., 2015), while the breakdown of NT-pro-BNP1-76 is exclusively performed by glomerular filtration (Holm, 2013). Also, inflammation is associated with a decreased breakdown of NT-pro-BNP1-76 compared to BNP1-32 and an increased ratio of NT-pro-BNP1-76/BNP1-32 (Jensen et al., 2010). Several lines of evidence indicated that exercise training decreases inflammation in post-CABG patients (Goto, 2010), so it could be an influential factor in the degradation of BNP1-32 and NT-pro-BNP1-76. However, this possible mechanism was not examined in our study and was considered a limitation of the experiment. There was no significant difference between the training groups between ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 ratios. Therefore, both HIIT and MCT may be useful to regulate production and degradation systems for BNP1-32 in underwent CABG patients. However, due to the multiplicity of cardiac protection pathways and HF prevention, the lack of more mechanisms was the limitation of this study. For example, BNP1-32 is cleaved by several enzymes, including dipeptidyl peptidase-4 (DPPIV), neprilysin (NEP), and insulin-degrading enzyme (IDE) so further studies are required to survey the effects of MCT and HIIT on these mechanisms. Furthermore, the number of patients was small and this limits the reliability of the results and was limitation of our study. it should be consider more patients for future studies.
Conclusion
The inactivity of post-CABG patients harms the corin enzyme; ProBNP1-108/BNP1-32 ratios and NT-pro-BNP1-76/BNP1-32, while both HIIT and MCT have a positive effect on ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 and could be effective to promote the health of coronary arteries and prevention of HF in post-CABG patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shahid Rajaee Teacher Training University of Tehran (IRSRTTU.SSF.2020.104). Informed consent was obtained from all participants. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors have been involved in gathering information, writing, and reviewing the article.
Acknowledgments
We would like to appreciate the staff of cardiac rehabilitation clinic of the Tehran heart center hospital and all patients that participated in this study as subjects.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Left ventricular mass index (LVMI).
2Relative wall thickness (RWT).
References
Arora, P., Reingold, J., Baggish, A., Guanaga, D. P., Wu, C., Ghorbani, A., et al. (2015). Weight loss, saline loading, and the natriuretic peptide system. J. Am. Heart Assoc. 4, e001265. doi:10.1161/JAHA.114.001265
Barnet, C. S., Liu, X., Body, S. C., Collard, C. D., Shernan, S. K., Muehlschlegel, J. D., et al. (2015). Plasma corin decreases after coronary artery bypass graft surgery and is associated with postoperative heart failure: A pilot study. J. Cardiothorac. Vasc. Anesth. 29 (2), 374–381. doi:10.1053/j.jvca.2014.11.001
Chopra, S., Cherian, D., Verghese, P. P., and Jacob, J. J. (2013). Physiology and clinical significance of natriuretic hormones. Indian J. Endocrinol. metabolism 17 (1), 83–90. doi:10.4103/2230-8210.107869
Cockburn, J., Blows, L., Cohen, A., Holmberg, S., Hyde, J., Lewis, M., et al. (2013). Acute ischemic complications of PCI and CABG: Who should cover whom for coronary revascularization? J. Interventional Cardiol. 26 (4), 372–377. doi:10.1111/joic.12045
Conraads, V. M., and Beckers, P. J. (2010). Exercise training in heart failure: Practical guidance. Heart 96 (24), 2025–2031. doi:10.1136/hrt.2009.183889
de Waard, D., Fagan, A., Minnaar, C., and Horne, D. (2021). Management of patients after coronary artery bypass grafting surgery: A guide for primary care practitioners. Can. Med. Assoc. J. 193 (19), E689–E694. doi:10.1503/cmaj.191108
Dendale, P., Berger, J., Hansen, D., Vaes, J., Benit, E., and Weymans, M. (2005). Cardiac rehabilitation reduces the rate of major adverse cardiac events after percutaneous coronary intervention. Eur. J. Cardiovasc. Nurs. 4 (2), 113–116. doi:10.1016/j.ejcnurse.2004.11.003
Dessì-Fulgheri, P., Sarzani, R., Tamburrini, P., Moraca, A., Espinosa, E., Cola, G., et al. (1997). Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J. Hypertens. 15 (12), 1695–1699. doi:10.1097/00004872-199715120-00074
Dietz, T., and Kalof, L. (2009). Introduction to social statistics: The logic of statistical reasoning. New York, United States: John Wiley and Sons.
Fox, A. A., Marcantonio, E. R., Collard, C. D., Thoma, M., Perry, T. E., Shernan, S. K., et al. (2011). Increased peak postoperative B-type natriuretic peptide predicts decreased longer-term physical function after primary coronary artery bypass graft surgery. Anesthesiol. J. Am. Soc. Anesthesiol. 114 (4), 807–816. doi:10.1097/ALN.0b013e31820ef9c1
Fu, S., Ping, P., Wang, F., and Luo, L. (2018). Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 12 (1), 2. doi:10.1186/s13036-017-0093-0
Fu, S., Ping, P., Zhu, Q., Ye, P., and Luo, L. (2018). Brain natriuretic peptide and its biochemical, analytical, and clinical issues in heart failure: A narrative review. Front. physiology 9, 692. doi:10.3389/fphys.2018.00692
Gademan, M. G., Swenne, C. A., Verwey, H. F., Van Der Laarse, A., Maan, A. C., Van De Vooren, H., et al. (2007). Effect of exercise training on autonomic derangement and neurohumoral activation in chronic heart failure. J. cardiac Fail. 13 (4), 294–303. doi:10.1016/j.cardfail.2006.12.006
Ghashghaei, F. E., Sadeghi, M., Marandi, S. M., and Ghashghaei, S. E. (2012). Exercise-based cardiac rehabilitation improves hemodynamic responses after coronary artery bypass graft surgery. ARYA Atheroscler. 7 (4), 151–156.
Gibala, M. J., Little, J. P., MacDonald, M. J., and Hawley, J. A. (2012). Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. physiology 590 (5), 1077–1084. doi:10.1113/jphysiol.2011.224725
Giles, T. D., Berk, B. C., Black, H. R., Cohn, J. N., Kostis, J. B., Izzo, J. L., et al. (2005). Expanding the definition and classification of hypertension. J. Clin. Hypertens. 7 (9), 505–512. doi:10.1111/j.1524-6175.2005.04769.x
Goto, Y. (2010). Exercise training in post-CABG patients at low prognostic risk–beyond recovery from surgery–. Circulation J. 74 (12), 2548–2549. doi:10.1253/circj.cj-10-1061
Guiraud, T., Nigam, A., Gremeaux, V., Meyer, P., Juneau, M., and Bosquet, L. (2012). High-intensity interval training in cardiac rehabilitation. Sports Med. 42 (7), 587–605. doi:10.2165/11631910-000000000-00000
Holm, J. (2013). Markers of hemodynamic state and heart failure as predictors for outcome in cardiac surgery: With special reference to mixed venous oxygen saturation and natriuretic peptides. Linköping: Linköping University Electronic Press.
Huntley, B. K., Sandberg, S. M., Heublein, D. M., Sangaralingham, S. J., Burnett, J. C., and Ichiki, T. (2015). Pro–B-Type natriuretic peptide-1-108 processing and degradation in human heart failure. Circ. Heart Fail. 8 (1), 89–97. doi:10.1161/CIRCHEARTFAILURE.114.001174
Ichiki, T., Boerrigter, G., Huntley, B. K., Sangaralingham, S. J., McKie, P. M., Harty, G. J., et al. (2013). Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. American Journal of Physiology-Regulatory. Integr. Comp. Physiology 304 (2), R102–R9.
Ichiki, T., Huntley, B. K., Heublein, D. M., Sandberg, S. M., McKie, P. M., Martin, F. L., et al. (2011). Corin is present in the normal human heart, kidney, and blood, with pro–B-type natriuretic peptide processing in the circulation. Clin. Chem. 57 (1), 40–47. doi:10.1373/clinchem.2010.153908
Jensen, J., Ma, L-P., Bjurman, C., Hammarsten, O., and Fu, M. L. (2012). Prognostic values of NTpro BNP/BNP ratio in comparison with NTpro BNP or BNP alone in elderly patients with chronic heart failure in a 2-year follow up. Int. J. Cardiol. 155 (1), 1–5. doi:10.1016/j.ijcard.2011.01.083
Jensen, J., Ma, L-P., Fu, M. L., Svaninger, D., Lundberg, P-A., and Hammarsten, O. (2010). Inflammation increases NT-proBNP and the NT-proBNP/BNP ratio. Clin. Res. Cardiol. 99 (7), 445–452. doi:10.1007/s00392-010-0140-z
Lang, R. M., Bierig, M., Devereux, R. B., Flachskampf, F. A., Foster, E., Pellikka, P. A., et al. (2006). Recommendations for chamber quantification. Eur. J. Echocardiogr. 7 (2), 79–108. doi:10.1016/j.euje.2005.12.014
Macheret, F., Boerrigter, G., McKie, P., Costello-Boerrigter, L., Lahr, B., Heublein, D., et al. (2011). Pro–B-Type natriuretic peptide1–108 circulates in the general community: Plasma determinants and detection of left ventricular dysfunction. J. Am. Coll. Cardiol. 57 (12), 1386–1395. doi:10.1016/j.jacc.2011.01.005
Marney, A. M., Brown, N. J., Tamboli, R., and Abumrad, N. (2014). Changes in B-type natriuretic peptide and BMI following Roux-en-Y gastric bypass surgery. Diabetes care 37 (4), e70–e71. doi:10.2337/dc13-2449
Meyer, P., Gayda, M., Juneau, M., and Nigam, A. (2013). High-intensity aerobic interval exercise in chronic heart failure. Curr. heart Fail. Rep. 10 (2), 130–138. doi:10.1007/s11897-013-0130-3
Miller, W. L., Grill, D. E., and Jaffe, A. S. (2012). Comparison of novel pro-BNP1–108 and standard BNP assays in heart failure patients. Clin. Chim. acta 413 (9-10), 920–926. doi:10.1016/j.cca.2012.02.007
Nishitani, M., Shimada, K., Masaki, M., Sunayama, S., Kume, A., Fukao, K., et al. (2013). Effect of cardiac rehabilitation on muscle mass, muscle strength, and exercise tolerance in diabetic patients after coronary artery bypass grafting. J. Cardiol. 61 (3), 216–221. doi:10.1016/j.jjcc.2012.11.004
Passino, C., Severino, S., Poletti, R., Piepoli, M. F., Mammini, C., Clerico, A., et al. (2006). Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J. Am. Coll. Cardiol. 47 (9), 1835–1839. doi:10.1016/j.jacc.2005.12.050
Pearson, M. J., King, N., and Smart, N. A. (2018). Effect of exercise therapy on established and emerging circulating biomarkers in patients with heart failure: A systematic review and meta-analysis. Open heart 5 (2), e000819. doi:10.1136/openhrt-2018-000819
Preeshagul, I., Gharbaran, R., Jeong, K. H., Abdel-Razek, A., Lee, L. Y., Elman, E., et al. (2013). Potential biomarkers for predicting outcomes in CABG cardiothoracic surgeries. J. Cardiothorac. Surg. 8 (1), 176. doi:10.1186/1749-8090-8-176
Sagiv, M. S. (2012). Exercise cardiopulmonary function in cardiac patients: Springer science and business media. Berlin, Germany: Springer.
Santoso, A., Maulana, R., Alzahra, F., Prameswari, H. S., Ambari, A. M., Hartopo, A. B., et al. (2020). The effects of aerobic exercise on N-terminal pro-B-type natriuretic peptide and cardiopulmonary function in patients with heart failure: A meta-analysis of randomised clinical trials. Lung Circulation 29 (12), 1790–1798. doi:10.1016/j.hlc.2020.05.098
Serruys, P. W., Unger, F., Sousa, J. E., Jatene, A., Bonnier, H. J., Schönberger, J. P., et al. (2001). Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N. Engl. J. Med. 344 (15), 1117–1124. doi:10.1056/NEJM200104123441502
Siribaddana, S. (2012). Cardiac dysfunction in the CABG patient. Curr. Opin. Pharmacol. 12 (2), 166–171. doi:10.1016/j.coph.2012.01.010
Smart, N. A., and Steele, M. (2010). Systematic review of the effect of aerobic and resistance exercise training on systemic brain natriuretic peptide (BNP) and N-terminal BNP expression in heart failure patients. Int. J. Cardiol. 140 (3), 260–265. doi:10.1016/j.ijcard.2009.07.004
Suzuki, S., and Sugiyama, S. (2018). The molar ratio of N-terminal pro-B-type natriuretic peptide/B-type natriuretic peptide for heart failure-related events in stable outpatients with cardiovascular risk factors. Intern. Med. 57 (18), 2621–2630. doi:10.2169/internalmedicine.0471-17
Thow, M. (2006). Exercise leadership in cardiac rehabilitation: An evidence-based approach. New York, United States: John Wiley and Sons.
Valkeinen, H., Aaltonen, S., and Kujala, U. (2010). Effects of exercise training on oxygen uptake in coronary heart disease: A systematic review and meta-analysis. Scand. J. Med. Sci. sports 20 (4), 545–555. doi:10.1111/j.1600-0838.2010.01133.x
Wisloff, U., Stoylen, A., Loennechen, J. P., Bruvold, M., Haram, P. M., Tjonna, A. E., et al. (2007). Superior cardiovascular effect of aerobic interval-training versus moderate continuous training in elderly heart failure patients: 651May 31 8: 15 AM-8: 30 am. Med. Sci. Sports Exerc. 39 (5), S32. doi:10.1249/01.mss.0000273010.06226.99
Zare Karizak, S., Kashef, M., Gaeini, A. A., and Nejatian, M. (2017). The comparison of two protocol of interval and continues aerobic training on level of concentric pathologic hypertrophy and cardiac function in patients after coronary artery bypass grafting surgery. J. Pract. Stud. Biosci. Sport 5 (9), 9–20.
Zarekarizak, S., Kashef, M., and Gaeini, A. A. (2017). The comparison of eight weeks interval and continuous training on PRO-BNP/CORIN system in coronary artery disease patients after CABG surgery. Sport Physiol. 9 (33), 131–152.
Zarekarizak, S., Kashef, M., Nejatian, M., and Hamid, R. (2021). Variations of plasma adrenomedullin, ventricular ejection fraction and resting rate pressure product, following rehabilitation programs of interval and continuous training after coronary artery bypass grafting surgery. Journal for Research in Sport Rehabilitation. 8 (16), 15–23.
Zdrenghea, D. T., Ilea, M., Bodizs, G., Sitar-Tăut, A., Zdrenghea, M., and Pop, D. (2014). NT-pro-BNP during isotonic and isometric exercise in heart failure patients with preserved LV ejection fraction. Clin. Lab. 60 (12), 2055–2061. doi:10.7754/clin.lab.2014.140417
Zile, M. R., DeSantis, S. M., Baicu, C. F., Stroud, R. E., Thompson, S. B., McClure, C. D., et al. (2011). Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ. Heart Fail. 4 (3), 246–256. doi:10.1161/CIRCHEARTFAILURE.110.958199
Keywords: high intensity interval training (HIIT), moderate continuous training (MCT), ProBNP1-108/BNP1-32, NT-pro-BNP1-76/BNP1-32, CABG surgery
Citation: Zare Karizak S, Kashef M, Gaeini AA and Nejatian M (2023) Impact of high intensity interval and moderate continuous training on plasma ratios of ProBNP1-108/BNP1-32 and NT-pro-BNP1-76/BNP1-32 after coronary artery bypass grafting surgery. Front. Physiol. 14:1114813. doi: 10.3389/fphys.2023.1114813
Received: 02 December 2022; Accepted: 22 February 2023;
Published: 07 March 2023.
Edited by:
Jinlei Nie, Macao Polytechnic University, Macao SAR, ChinaReviewed by:
Mousa Khalafi, University of Kashan, IranAleksey M. Chaulin, Samara State Medical University, Russia
Einat Kodesh, University of Haifa, Israel
Copyright © 2023 Zare Karizak, Kashef, Gaeini and Nejatian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Zare Karizak, c2FyYXphcmVrYXJpemFrQHlhaG9vLmNvbQ==
 Sara Zare Karizak
Sara Zare Karizak Majid Kashef2
Majid Kashef2