- 1Department of Reproductive Medicine, General Hospital of Northern Theater Command, Shenyang, China
- 2Department of Forensic Pathology, School of Forensic Medicine, China Medical University, Shenyang, China
Background: The success of embryo transfer cycle depends mainly on the quality of embryo and endometrial receptivity. Ultrasound examination is still the most widely used non-invasive evaluation method for its advantages of convenience, non-invasiveness and repeatability. Ultrasound-measured endometrial blood flow is one of the important evaluation indicators of morphology.
Aims: To investigate the effect of the number of endometrial blood flow branches on pregnancy outcome of frozen-thawed embryo transfer cycles which have undergoing hormone replacement therapy (HRT-FET).
Material and methods: A retrospective cohort study was performed looking at a total of 1390 HRT-FET cycles from our reproductive medicine center between January 2017 to December 2021, which transferred one blastocyst frozen on day 5 with good quality in morphology. Associations between endometrial blood flow branches and pregnancy outcomes were evaluated with multivariable linear regression analysis.
Results: The number of endometrial blood flow branches was independently associated with clinical pregnancy (OR 1.10; 95% CI 1.02–1.20). After adjusting for potential confounders, the effect size (odds ratio) was 1.09 (95% CI 1.00–1.19), and the results showed that the clinical pregnancy rate and live birth rate of T2 and T3 groups were significantly higher than those in group T1 (p < 0.05). Subgroup analysis showed that a consistent association between the endometrial blood flow branches and clinical pregnancy in all subgroups.
Conclusion: Our study provided evidence for the influence of endometrial blood flow on pregnancy outcomes. There may be an independent association between the number of endometrial blood flow branches and pregnancy outcomes in frozen-thawed single blastocyst transfer cycles.
Introduction
The prevalence of infertility patients has been increasing in recent years, and the global median prevalence rate is about 9% (Organization, 1987; Boivin et al., 2007). Assisted reproductive technology (ART) has become a way for most patients to address their fertility problems. In recent decades, improvements in laboratory techniques (Traub et al., 2009) and cryopreservation techniques have been rapidly developed (Pereira and Rosenwaks, 2016). Frozen embryo transfer (FET) reduces ART-related adverse consequences and risks, such as ovarian hyperstimulation syndrome (OHSS) (Aflatoonian et al., 2010; Evans et al., 2014), and can reduce the cost of multiple fresh cycles (Ghobara and Vandekerckhove, 2008; Roque et al., 2013), so FET cycles also increased substantially (Dyer et al., 2016).
The success of the embryo transfer cycle mainly depends on the quality of the embryo and endometrial receptivity (Wu et al., 2014). Either poor endometrial receptivity or poor embryo quality can affect the interaction between embryo and endometrium (Simon and Laufer, 2012). Therefore, the receptivity between endometrium and embryo during the in vitro fertilization process plays a very important role in achieving the improvement of pregnancy. More recently, methods for evaluating embryo receptivity in the endometrium are also increasing, including morphology, genomics, transcriptomics, proteomics, metabolomics, etc. (Craciunas et al., 2019). However, the morphological parameters evaluated by ultrasound are still commonly used in clinical evaluation.
Ultrasound-measured endometrial blood flow is one of the important evaluation indicators of endometrial morphology. There have been many studies about it (Son et al., 2014; Koo et al., 2018; Sadek et al., 2022), but ultrasound indicators which predicted of the outcomes of ART existed for endometrial blood flow are controversial. The relationship between endometrial blood flow and pregnancy outcome is even more uncertain. The number of endometrial blood flow branches is an ultrasonic parameter observed in the sensitive state when the power Doppler blood flow imaging mode is turned on, which can directly reflect the blood perfusion situation. The aim of this cohort study is to demonstrate a more accurate association between endometrial blood flow branches and pregnancy outcome in FET cycles.
Materials and methods
Research objects
This retrospective study analyzed infertility patients who undergoing FET cycles in the Department of Reproductive Medicine from January 2017 to December 2021. Inclusion criteria were women younger than 40 years who had one blastocyst frozen on day 5 with good quality in morphology and their endometrium are prepared with HRT protocol. Exclusion criteria: recurrent implantation failure (RIF); the thin endometrium, which the Endometrial thickness is <7.0 mm (Kupesic et al., 2001); the loss of endometrial blood flow in the diastolic period in the ultrasound examination; inappropriate endometrium for implantation, which included endometrial synechiae, endometrial polyp abnormal anatomy of uterine cavity and Untreated hydrosalpinx.
A total of 1390 FET cycles were analyzed (Supplementary Figure S1). This was a retrospective study and we collected the clinical data from our electronic medical record system for all patients who underwent conventional FET.
Treatment protocol
For the HRT cycles, patients begin oral administration of estradiol [1 mg (Progynova); Bayer, Leverkusen, Germany] with 4 mg/day from cycle day 3 of the cycle. Transvaginal ultrasound examination was conducted to evaluate the endometrial thickness and ovulation and the dose of estradiol was adjusted according to the endometrial thickness every 4 days. When the endometrial thickness reached 7 mm or more, 40 mg intramuscular administration of progesterone and oral progesterone were given and included in the study. A single frozen-thawed blastocyst was transferred on the 5th day after progesterone initiation. If pregnancy was achieved intramuscular administration and oral progesterone was continued until 10 weeks’ gestation.
Blastocyst quality assessment
According to the prescribed procedure, at least two experienced embryologists independently evaluate the blastocysts according to the Gardner and Schoolcraft grading system (Gardner et al., 2000). The blastocyst score is mainly determined by three morphological parameters: blastocyst expansion, ICM, and TE. Blastocysts with an expansion stage >3, ICM grade, and TE grade higher than C (≥4BB) are considered to be good quality (Hu et al., 2021).
Ultrasonic measurement
All the ultrasound measurement assessments were carried out by specialist sonographers using the same standardized protocols on the same ultrasound machines in our department (GE Voluson E8, the United States).
On the transfer day, the patient was evacuated, the bladder lithotomy position was taken, the breathing was calmed. Endometrial thickness (Dickey et al., 1993) and endometrial patterns (Gonen and Casper, 1990) was measured as described in our previous paper (Zhang et al., 2021). Endometrial blood flow detects blood flow signals under and around the endometrium. The power Doppler blood flow imaging function was activated, and the sensitive state was adjusted to observe endometrial blood flow branches. Detection of blood flow signals in the endometrium: the median sagittal section of the uterus, the region of interest surrounding the intima and 1/3 of the myometrium at the endometrium, and the activation of Doppler flow imaging. The pulse repetition frequency PRF was set at 0.6 MHz. The number of endometrial blood flow branches was observed and recorded. After at least 5 consecutive waveforms were obtained, the resistive index (RI) and pulsatility index (PI) were checked.
Pregnancy determination
Serum human chorionic gonadotropin (hCG) was measured 14 days after embryo transfer. Clinical pregnancy was confirmed by ultrasound observation 2–3 weeks after a positive hCG test was recorded. The identification of a gestational sac with fetal heart activity on ultrasound examination is defined as a clinical pregnancy. Live birth was defined as one or more live babies delivered beyond 28 weeks of gestation.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (normal distribution) and Categorical variables were expressed in frequency or as a percentage. The χ 2 test (categorical variables), one-way ANOVA (normal distribution), or Kruskal–Wallis H test (skewed distribution) were used to calculate for differences among endometrial blood flow branches. Univariate and multivariable linear regression analysis was used to estimate the effect values (OR) and 95% confidence intervals (CIs) for the association between endometrial blood flow branches and clinical pregnancy outcomes. Results adjusted for age and BMI in model I. To investigate the independent association, we further adjusted for some covariables. Whether the covariables were adjusted was determined according to the recommendations of the article (Kernan et al., 2000) published in the New England Journal of Medicine. If, when the variable was added to this model, the matched odds ratio was changed by at least 10% then an adjustment was made. At the same time, conforming to the recommendations of the STROBE statement (von Elm et al., 2014) the results were analyzed from unadjusted or minimally adjusted and fully adjusted in parallel. The endometrial blood flow branches were converted into a categorical variable by tertiles. Tests for trends were computed by modeling three groups as continuous variables. Interaction and stratified analyses were performed to evaluate whether covariates influenced the associations between endometrial blood flow branches and clinical pregnancy outcomes. Statistical significance was indicated by a two-sided p-value < 0.05. All analyses were performed using EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc.) and R 3.6.3 (http://www.r-project.org).
Results
Baseline characteristics
Totally, 1390 FET cycles were finally included in this data analysis. Table 1 present the baseline characteristics by tertiles of the endometrial blood flow branches and the average of it was 8.47 ± 1.32. The average age of the study population was 32.45 ± 3.76 years. There were significant differences between age, BMI, Diagnoses of infertility, endometrial pattern and pregnancy outcomes among groups of the endometrial blood flow branches (p < 0.05).
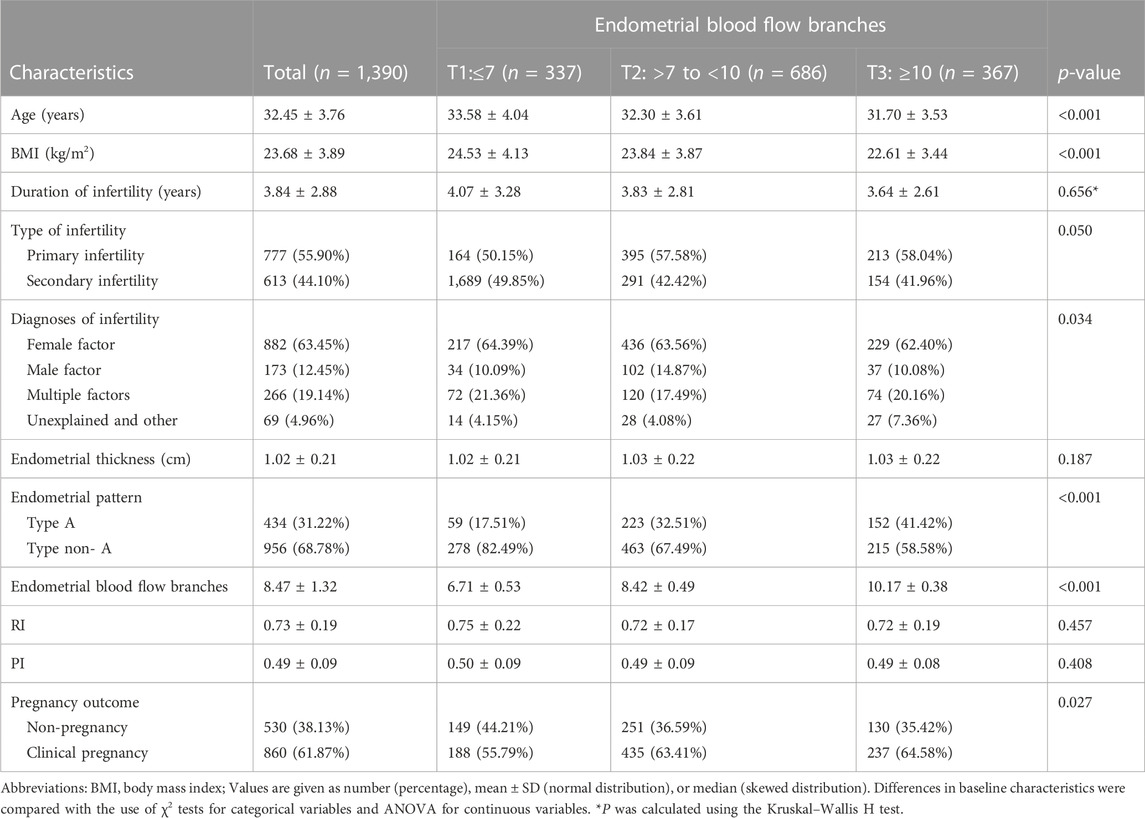
TABLE 1. Baseline characteristics of the study population by tertiles of the endometrial blood flow branches.
Factors correlated with clinical pregnancy
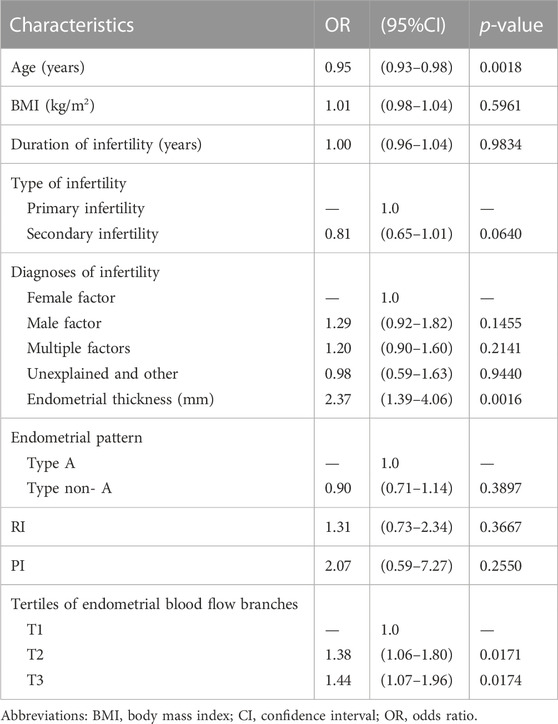
As shown in Table 2, we evaluated the association of clinical and ultrasound parameters with early clinical pregnancy using univariate linear regression analysis. In non-adjusted model, there was a significant negative correlation between age and pregnancy outcomes (p = 0.0018). Endometrial thickness and different groups of endometrial blood flow branches had a significant positive correlation with clinical outcomes (p < 0.05). Other variable included BMI, duration of infertility, type of infertility, diagnosis of infertility, endometrial pattern, RI and PI did not remain significantly associated with pregnancy outcomes (p > 0.05).
The relationship between endometrial blood flow branches and pregnancy outcomes
To reveal the association between endometrial blood flow branches and early clinical pregnancy, we utilized Multivariate linear regression in Table 3. In the crude model, an increase in endometrial blood flow branches results in an 10% elevation in clinical pregnancy. Compared with the crude model (OR 1.10; 95% CI 1.02–1.20), the model I which only adjusted for age and BMI had showed a similar trend that endometrial blood flow branches were still positively correlated with early clinical pregnancy (OR 1.09; 95% CI 1.00–1.10). After adjusting for age, BMI, duration of infertility, diagnosis of infertility, endometrial thickness, RI and PI, the effect size of model II was 1.09 (95% CI 1.00–1.19), and the result showed the clinical pregnancy and live birth of T2 and T3 groups were significantly higher than group T1 (p < 0.05). For the purpose of sensitivity analysis, we converted the endometrial blood flow branches into categorical variable by tertiles and found the same trend (showed in Table 3).
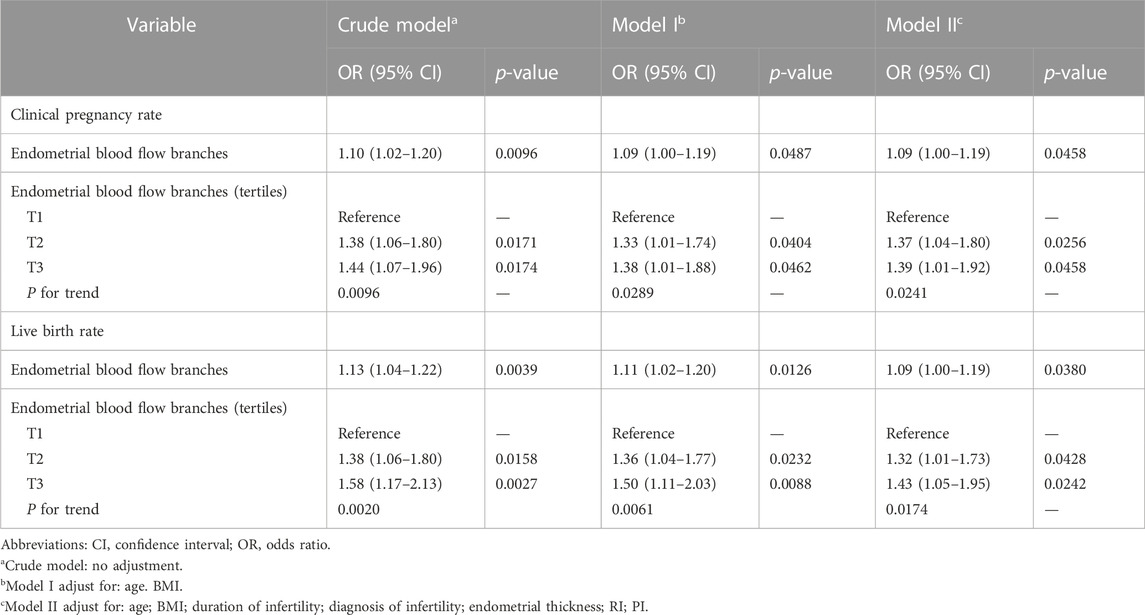
TABLE 3. Multivariable analysis to assess the independent impact of endometrial blood flow branches on clinical pregnancy outcomes.
Stratified analysis of clinical pregnancy
We further performed exploratory subgroup analysis to assess the association between endometrial blood flow branches and clinical pregnancy outcomes. As shown in Figure 1, all covariates including age, BMI, duration of infertility, type of infertility, diagnosis of infertility, endometrial thickness, endometrial pattern, RI and PI, did not significantly modify the association between elevated endometrial blood flow branches and clinical pregnancy outcomes. Similarly, after stratified analysis of the above variables, the relationship was consistent in the subgroups (all P for interaction >0.05).
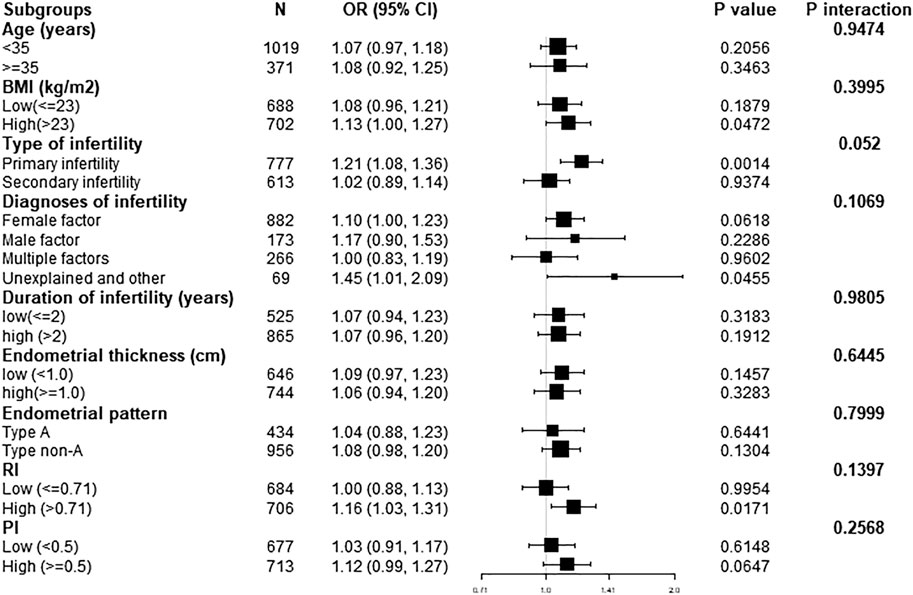
FIGURE 1. Effect size of endometrial blood flow branches on clinical pregnancy outcomes in each subgroup. Adjusted, if not stratified, for age; BMI; duration of infertility; diagnosis of infertility; type of infertility; endometrial thickness; endometrial patten; RI; PI. BMI, body mass index; Cl, confidence interval; FET, frozen embryo transfer; OR, odds ratio.
Discussion
How to improve the success rate of embryo transfer has always been the focus of reproductive attention. A better maternal condition and good embryo quality are necessary for successful embryo implantation. The endometrium with normal tissue function has the ability to allow a competent embryo to attach to and invade into it (Hou et al., 2019). How to assess the ability of the endometrium to receive embryos is one of the current research directions. Ultrasound checking is still the most widely used non-invasive evaluation method because of its convenience, non-invasiveness and repeatability. In the present study, we found that a positive association between the number of endometrial blood flow branches and clinical pregnancy in FET cycles. And it also proved the importance of endometrial blood supply in embryo transfer from a new perspective.
Angiogenesis plays an important role in the reproductive process of implantation (Karizbodagh et al., 2017). A good blood supply to the endometrium is generally considered to be a basic requirement for embryo implantation. In the mid-to-late proliferative and early-mid secretory phases, the mean vessel length density was significantly greatest in the subepithelial capillary plexus (Gambino et al., 2002), creating a suitable environment for implanting embryos. The blood flow of endometrial micro vessels could be determined by ultrasound Doppler technique prior to the transfer day of FET cycles. However, the existing ultrasound indicators for uterine blood flow, whether in 2D or 3D, were controversial.
In some studies, 2D Doppler flow indices of spiral arteries such as RI, PI and peak systolic velocity (PSV) are not predictive of pregnancy. One study (Son et al., 2014) assessed 70 women on the day of embryo transfer and reported similar uterine artery Pls and Rls and subendometrial Rls and Pls between clinically pregnant and not pregnant women in FET cycles. But there are also dissenters. Sadek et al. (2022) found pregnancy and live births were associated with a lower mean percentage drop in blood flow from day 15 to the day of transfer and elevated RI and S/D ratio on transfer day. Sardana said (Sardana et al., 2014) that the presence of endometrial blood flow was highly correlated with the cycle outcome in HRT-FET cycles, and the embryo implantation rate and clinical pregnancy rate of the endometrial blood flow signal group were significantly higher than those with the absented blood flow group in the subendometrial-endometrial region.
3D ultrasound and power Doppler angiography with the aid of the VOCAL can be used to provide a fast means of measuring endometrial parameters. In FET cycles, results varied as well. Nandi et al. (2014) found no statistically significant differences in endometrial the vascularization index (VI), flow index (FI), and vascularization-flow index (VFI) between the pregnant and non-pregnant groups. However, Mercé et al. (2008) found that the endometrial 3D power Doppler flow indices were statistically significantly higher in the pregnant group yet two other studies showed no significant difference in Doppler blood flow in pregnant and non-pregnant women. A meta-analysis in 2018 (Wang et al., 2018) suggested that VI, FI and VFI of the endometrial/subendometrial vasculature were helpful in identify the appropriate timing for FET.
Furthermore, Researchers are also trying to choose different ways to assess endometrial blood flow. But few of them investigated the number of endometrial blood flow branches. Wei et al. (2018) studied endometrial blood flow in patients with repeated transplant failure. They only studied a higher number of endometrial blood flow branches and clinical pregnancy rate in the HCG group compared with the control group. But they did not explore correlation between them.
Subgroup analysis and interaction analysis are extremely important for a scientific study. It could exclude potential confounding factors. In the present study, factors related to early clinical pregnancy were used as stratified variables. After careful adjustments, this positive effect was evident in most of subgroups. As we know, age and embryo quality and quantity affect pregnancy outcome. In order to exclude the influence, the present study only examined those aged under 40 years and who transferred one blastocyst of good quality in HRT cycles. From the known factors affecting the treatment outcome of assisted reproductive technology, these analyses would help us to better understand the independent association between the number of endometrial blood flow branches and pregnancy outcomes. As the continuous improvement and maturity of IVF-ET technology, and the deepening recognition of the huge risk and cost of multiple pregnancy, single embryo transfer is advocated to reduce the risk of multiple pregnancy, so as to improve perinatal outcomes. Déniz et al. (2018) also believed that elective single embryo transfer (eSET) could significantly reduce the incidence of multiple pregnancies. According to our study in this paper, there was a significant correlation between the number of endometrial blood flow branches and the outcome of early pregnancy in single embryo transfer. Therefore, in the trend of single blastocyst transplantation (Gazdaru et al., 2017), the number of endometrial blood flow branches plays a very important role in improving the pregnancy rate.
There are limitations to this study. Firstly, the present study was a retrospective cohort study of single-center. Secondly, our control of important epidemiologic and clinical covariables in the analyses, we could not exclude the possibility of residual confounding. Furthermore, the number of endometrial blood flow branches used in this study is likely to be more prone to error, as this index is artificially measured and recorded. It is noteworthy that the potential exposure misclassification resulting from such errors would bias toward to the null and thus result in an underestimation of the association between endometrial blood flow branches and early pregnancy outcomes. Finally, the data of each patient for each cycle was not reviewed, and the findings might not be generalized to the patient level. Caution is needed in interpreting the results.
Conclusion
In summary, this study suggests that there may be an independent association between the number of endometrial blood flow branches and pregnancy outcomes, including early clinical pregnancy and live birth. The number of endometrial blood flow branches was positively correlated with clinical pregnancy in patients under 40 years of age who transferred one blastocyst of good morphological quality during HRT cycles.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the medical ethics committee of the General Hospital of Northern Theater Command. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
QZ, YY, and HL conceived and designed the experiments. ZL and YW collected the data. QZ and XW analyzed the data. QZ drafted the manuscript. HL, YX, and YY provided critical revision. All authors reviewed and approved the final manuscript.
Funding
This work was supported by Key Research and Development Program of Liaoning Province (2020JH2/10300118) and Military Family Planning Professional Scientific Research Project (21JSZ11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1113853/full#supplementary-material
References
Aflatoonian A., Mansoori Moghaddam F., Mashayekhy M., Mohamadian F. (2010). Comparison of early pregnancy and neonatal outcomes after frozen and fresh embryo transfer in ART cycles. J. Assist. Reprod. Genet. 27 (12), 695–700. doi:10.1007/s10815-010-9470-z
Boivin J., Bunting L., Collins J. A., Nygren K. G. (2007). International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 22 (6), 1506–1512. doi:10.1093/humrep/dem046
Craciunas L., Gallos I., Chu J., Bourne T., Quenby S., Brosens J. J., et al. (2019). Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Update 25 (2), 202–223. doi:10.1093/humupd/dmy044
Déniz F. P., Encinas C., Fuente J. (2018). Morphological embryo selection: An elective single embryo transfer proposal. JBRA Assist. Reprod. 22 (1), 20–25. doi:10.5935/1518-0557.20180015
Dickey R. P., Olar T. T., Taylor S. N., Curole D. N., Matulich E. M. (1993). Relationship of endometrial thickness and pattern to fecundity in ovulation induction cycles: Effect of clomiphene citrate alone and with human menopausal gonadotropin. Fertil. Steril. 59 (4), 756–760. doi:10.1016/s0015-0282(16)55855-5
Dyer S., Chambers G. M., de Mouzon J., Nygren K. G., Zegers-Hochschild F., Mansour R., et al. (2016). International committee for monitoring assisted reproductive technologies world report: Assisted reproductive technology 2008, 2009 and 2010. Hum. Reprod. 31 (7), 1588–1609. doi:10.1093/humrep/dew082
Evans J., Hannan N. J., Edgell T. A., Vollenhoven B. J., Lutjen P. J., Osianlis T., et al. (2014). Fresh versus frozen embryo transfer: Backing clinical decisions with scientific and clinical evidence. Hum. Reprod. Update 20 (6), 808–821. doi:10.1093/humupd/dmu027
Gambino L. S., Wreford N. G., Bertram J. F., Dockery P., Lederman F., Rogers P. A. (2002). Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum. Reprod. 17 (5), 1199–1206. doi:10.1093/humrep/17.5.1199
Gardner D. K., Lane M., Stevens J., Schlenker T., Schoolcraft W. B. (2000). Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 73 (6), 1155–1158. doi:10.1016/s0015-0282(00)00518-5
Gazdaru S., Surbone A., Mathevet P., Leyvraz-Recrosio C., Primi M. P., Vulliemoz N. (2017). Assisted reproductive technology: Strategies of elective single embryo transfer. Rev. Med. Suisse 13 (580), 1832–1837.
Ghobara T., Vandekerckhove P. (2008). Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst. Rev. 1, CD003414. doi:10.1002/14651858.CD003414.pub2
Gonen Y., Casper R. F. (1990). Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). J Vitro Fert Embryo Transf 7 (3), 146–152. doi:10.1007/bf01135678
Hou Z., Zhang Q., Zhao J., Xu A., He A., Huang X., et al. (2019). Value of endometrial echo pattern transformation after hCG trigger in predicting IVF pregnancy outcome: A prospective cohort study. Reprod. Biol. Endocrinol. 17 (1), 74. doi:10.1186/s12958-019-0516-5
Hu K. L., Zheng X., Hunt S., Li X., Li R., Mol B. W. (2021). Blastocyst quality and perinatal outcomes in women undergoing single blastocyst transfer in frozen cycles. Hum. Reprod. Open 2021 (4), hoab036. doi:10.1093/hropen/hoab036
Karizbodagh M. P., Rashidi B., Sahebkar A., Masoudifar A., Mirzaei H. (2017). Implantation window and angiogenesis. J. Cell Biochem 118 (12), 4141–4151. doi:10.1002/jcb.26088
Kernan W. N., Viscoli C. M., Brass L. M., Broderick J. P., Brott T., Feldmann E., et al. (2000). Phenylpropanolamine and the risk of hemorrhagic stroke. N. Engl. J. Med. 343 (25), 1826–1832. doi:10.1056/nejm200012213432501
Koo H. S., Park C. W., Cha S. H., Yang K. M. (2018). Serial evaluation of endometrial blood flow for prediction of pregnancy outcomes in patients who underwent controlled ovarian hyperstimulation and in vitro fertilization and embryo transfer. J. Ultrasound Med. 37 (4), 851–857. doi:10.1002/jum.14418
Kupesic S., Bekavac I., Bjelos D., Kurjak A. (2001). Assessment of endometrial receptivity by transvaginal color Doppler and three-dimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J. Ultrasound Med. 20 (2), 125–134. doi:10.7863/jum.2001.20.2.125
Mercé L. T., Barco M. J., Bau S., Troyano J. (2008). Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome? Fertil. Steril. 89 (1), 111–117. doi:10.1016/j.fertnstert.2007.02.029
Nandi A., Martins W. P., Jayaprakasan K., Clewes J. S., Campbell B. K., Raine-Fenning N. J. (2014). Assessment of endometrial and subendometrial blood flow in women undergoing frozen embryo transfer cycles. Reprod. Biomed. Online 28 (3), 343–351. doi:10.1016/j.rbmo.2013.11.004
Organization W. H. (1987). Infections, pregnancies, and infertility: Perspectives on prevention. World health organization. Fertil. Steril. 47 (6), 964–968.
Pereira N., Rosenwaks Z. (2016). A fresh(er) perspective on frozen embryo transfers. Fertil. Steril. 106 (2), 257–258. doi:10.1016/j.fertnstert.2016.06.028
Roque M., Lattes K., Serra S., Sola I., Geber S., Carreras R., et al. (2013). Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 99 (1), 156–162. doi:10.1016/j.fertnstert.2012.09.003
Sadek S., Matitashvili T., Kovac A., Ramadan H., Stadtmauer L. (2022). Assessment of uterine receptivity by endometrial and sub-endometrial blood flow using SlowflowHD in hormone prepared frozen embryo transfer cycles: A pilot study. J. Assist. Reprod. Genet. 39 (5), 1069–1079. doi:10.1007/s10815-022-02454-8
Sardana D., Upadhyay A. J., Deepika K., Pranesh G. T., Rao K. A. (2014). Correlation of subendometrial-endometrial blood flow assessment by two-dimensional power Doppler with pregnancy outcome in frozen-thawed embryo transfer cycles. J. Hum. Reprod. Sci. 7 (2), 130–135. doi:10.4103/0974-1208.138872
Simon A., Laufer N. (2012). Repeated implantation failure: Clinical approach. Fertil. Steril. 97 (5), 1039–1043. doi:10.1016/j.fertnstert.2012.03.010
Son J. B., Jeong J. E., Joo J. K., Na Y. J., Kim C. W., Lee K. S. (2014). Measurement of endometrial and uterine vascularity by transvaginal ultrasonography in predicting pregnancy outcome during frozen-thawed embryo transfer cycles. J. Obstet. Gynaecol. Res. 40 (6), 1661–1667. doi:10.1111/jog.12406
Traub M. L., Van Arsdale A., Pal L., Jindal S., Santoro N. (2009). Endometrial thickness, caucasian ethnicity, and age predict clinical pregnancy following fresh blastocyst embryo transfer: A retrospective cohort. Reprod. Biol. Endocrinol. 7, 33. doi:10.1186/1477-7827-7-33
von Elm E., Altman D. G., Egger M., Pocock S. J., Gøtzsche P. C., Vandenbroucke J. P., et al. (2014). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 12 (12), 1495–1499. doi:10.1016/j.ijsu.2014.07.013
Wang J., Xia F., Zhou Y., Wei X., Zhuang Y., Huang Y. (2018). Association between endometrial/subendometrial vasculature and embryo transfer outcome: A meta-analysis and subgroup analysis. J. Ultrasound Med. 37 (1), 149–163. doi:10.1002/jum.14319
Wei W., Yue Y., Ting Y., Xueling J., Lihui Z., Junping H., et al. (2018). Study of human chorionic gonadotrophin in improving endometrial thickness and blood flow in recurrent implantation failure patients during frozen-thawed embryo transfer cycles. Chin. J. Reproduction Contracept. 38 (10), 837–841. doi:10.3760/cma.j.issn.2096-2916.2018.10.009
Wu Y., Gao X., Lu X., Xi J., Jiang S., Sun Y., et al. (2014). Endometrial thickness affects the outcome of in vitro fertilization and embryo transfer in normal responders after GnRH antagonist administration. Reprod. Biol. Endocrinol. 12, 96. doi:10.1186/1477-7827-12-96
Keywords: embryo transfer, pregnancy outcome, assisted reproductive technique, blood supply, frozen transfer cycles
Citation: Zhang Q, Wang X, Li Z, Wang Y, Lu H, Xiao Y and Yu Y (2023) Association between endometrial blood and clinical outcome in frozen single blastocyst transfer cycles. Front. Physiol. 14:1113853. doi: 10.3389/fphys.2023.1113853
Received: 01 December 2022; Accepted: 02 March 2023;
Published: 13 March 2023.
Edited by:
Sebastian Peter Galuska, Leibniz Institute for Farm Animal Biology (FBN), GermanyCopyright © 2023 Zhang, Wang, Li, Wang, Lu, Xiao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuexin Yu, eXV5dWV4aW5waW5nYW5AMTYzLmNvbQ==; Yuhong Xiao, eGlhb3NodWdlZ2UyQDE2My5jb20=
 Qian Zhang1
Qian Zhang1 Xiaolong Wang
Xiaolong Wang Yuexin Yu
Yuexin Yu