- 1Department of Neonatology, University Children’s Hospital Basel (UKBB), Basel, Switzerland
- 2School of Human Sciences, The University of Western Australia, Crawley, WA, Australia
- 3Wal-Yan Respiratory Research Centre, Telethon Kids Institute, Nedlands, WA, Australia
- 4Neonatal Intensive Care Unit, King’s College Hospital NHS Foundation Trust Denmark Hill, London, United Kingdom
- 5Cambridge University Clinical School, Cambridge, United Kingdom
- 6Anaesthetic Department, Hammersmith Hospital, London, United Kingdom
Introduction: Instability of peripheral oxyhemoglobin saturation (SpO2) in preterm infants is correlated with late disability and is poorly understood. We hypothesised that a reduced ventilation to perfusion ratio (VA/Q) is the key predisposing factor for SpO2 instability.
Methods: We first used a mathematical model to compare the effects of reduced VA/Q or shunt on SaO2 stability (SaO2 and SpO2 are used for model and clinical studies respectively). Stability was inferred from the slope of the SaO2 vs. inspired oxygen pressure (PIO2) curve as it intersects the 21 kPa PIO2 line (breathing air). Then, in a tertiary neonatal intensive care unit, paired hourly readings of SpO2 and PIO2 were recorded over a 24 h period in week old extremely preterm infants. We noted SpO2 variability and used an algorithm to derive VA/Q and shunt from the paired SpO2 and PIO2 measurements.
Results: Our model predicted that when VA/Q < 0.4, a 1% change in PIO2 results in >8% fluctuation in SaO2 at 21 kPa PIO2. In contrast, when a 20% intrapulmonary shunt was included in the model, a 1% change in PIO2 results in <1% fluctuation in the SaO2. Moreover, further reducing the VA/Q from 0.4 to 0.3 at 21 kPa PIO2 resulted in a 24% fall in SaO2. All 31 preterm infants [mean gestation (±standard deviation) 26.2 (±1) week] had VA/Q < 0.74 (normal >0.85) but only two infants had increased shunt at 1.1 (±0.5) weeks’ postnatal age. Median (IQR) SpO2 fluctuation was 8 (7)%. The greatest SpO2 fluctuations were seen in infants with VA/Q < 0.52 (n = 10): SpO2 fluctuations ranged from 11%–17% at a constant PIO2 when VA/Q < 0.52. Two infants had reduced VA/Q and increased shunt (21% and 27%) which resolved into low VA/Q after 3–6 h.
Discussion: Routine monitoring of PIO2 and SpO2 can be used to derive a hitherto elusive measure of VA/Q. Predisposition to SpO2 instability results from reduced VA/Q rather than increased intrapulmonary shunt in preterm infants with cardiorespiratory disease. SpO2 instability can be prevented by a small increase in PIO2.
1 Introduction
Peripheral oxyhemoglobin saturation (SpO2) instability may result in more than 100 hypoxemic events per day within the first 8 weeks of life in preterm infants (Di Fiore et al., 2010). Moreover, SpO2 instability is poorly documented despite the correlation with an increased rate of late death or disability at 18 months of age (Poets et al., 2015). SpO2 instability increases when the infant is in supine compared to the prone position, independent of the mode of respiratory support (Miller-Barmak et al., 2020). A contributing factor for hypoxemic episodes in preterm infants includes sudden decrease in lung volume leading to small airway collapse and intrapulmonary shunt (Bolivar et al., 1995). We have previously shown in adults that SpO2 was unstable when ventilation to perfusion ratio (VA/Q) was reduced, but was stable with increased shunt (Jones and Jones, 2000).
The concept of VA/Q is well established but infrequently used in neonatal practice, as the technics for measuring VA/Q in infants are technically difficult e.g. A-a nitrogen difference, or impossible e.g. Multiple Inert Gas Elimination Technique (MIGET) (Corbet et al., 1974; Hand et al., 1990; Roca and Wagner, 1994). Our non-invasive method for measuring VA/Q in adults is based on the different effects on SpO2 of changing inspired oxygen pressure (PIO2) when either VA/Q is reduced or shunt increased (Jones and Jones, 2000). The method depends on the shape of the oxyhaemoglobin dissociation curve (ODC). We adapted this method for preterm infants by using the neonatal rather than the adult ODC as the reference and plotting SpO2 at different PIO2 (Lockwood et al., 2014). A computer algorithm derived a model of VA/Q and shunt from the paired SpO2 and PIO2 dataset. Decreasing VA/Q is reflected by a right shift of the SpO2 vs. PIO2 curve. The right shift leads to a steeper slope of the curve as it intersects the 21 kPa PIO2 line (PIO2 = FIO2 x (barometric pressure—saturated water vapour pressure), which means 21 kPa PIO2 is equal to room air at sea level (Figure 1). Any paired SpO2 vs. PIO2 measurement located on the steep section of the ODC may predispose the infant to SpO2 instability with small changes in alveolar oxygen.
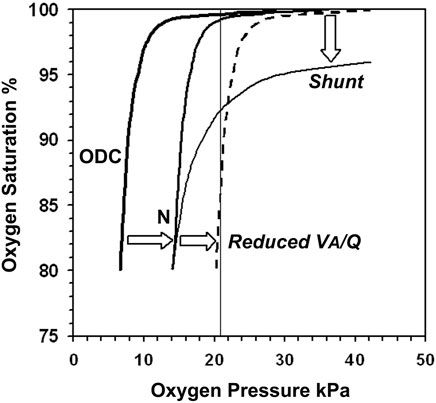
FIGURE 1. The neonatal oxyhaemoglobin dissociation curve is derived by plotting oxygen saturation (SaO2) against arterial oxygen pressure (PaO2) and determines the shape of the normal infant SpO2 vs. PIO2 curve (N), which is displaced to the right proportional to PCO2. Reducing Ventilation to Perfusion ratio (VA/Q) shifts the curve further to the right (dashed line). Its slope is now steep as it intersects the 21 kPa PIO2 line where a 1% change in PIO2 results in large SpO2 instability. Shunt displaces the plateau downwards with trivial effect on stability.
We hypothesised that SpO2 instability in preterm infants with cardiorespiratory disease is not an epiphenomenon but an important clinical sign of a reduced VA/Q rather than right to left intrapulmonary shunt. We used a new algorithm and routine SpO2 monitoring to derive VA/Q and shunt.
2 Methods
We conducted a prospective observational study in two phases. Firstly, we used a mathematical model of pulmonary gas exchange to examine changes in VA/Q or increasing shunt on the slope of the SpO2 vs. PIO2 curve.
Secondly, we recorded 24 hourly SpO2 vs. PIO2 measurements in extremely preterm infants requiring continuous SpO2 monitoring. From these measurements, we derived VA/Q and shunt using a pulmonary gas exchange algorithm (Lockwood et al., 2014).
2.1 Gas exchange model
We explored the effects of reducing VA/Q or increasing shunt on the arterial oxygen saturation (SaO2) vs. PIO2 curve using a mathematical model of pulmonary gas exchange described by Olszowka and Wagner (Olszowka et al., 1980). Datasets were generated using the equations implemented on a spreadsheet supplied by Dr AJ Olszowka. The model allowed calculation of exact values of SaO2 for a given PIO2. The model lung is subdivided into three compartments: a shunt and two ventilated regions with different alveolar ventilation-perfusion ratios (VA/Q). The values of cardiac output, oxygen consumption, hemoglobin concentration, shunt fraction, and the distribution of blood flow and alveolar ventilation to the ventilated compartments can be set. The perfusion of one compartment was set at 90% of non-shunt flow while VA/Q was reduced stepwise from 0.85 to 0.3. Shunt was fixed at 2%, PIO2 was varied between 15 kPa and 30 kPa (FIO2 = 0.15–0.3) and the SaO2 was derived at the corresponding PIO2. In the next step, the VA/Q was kept constant at 0.85 and the shunt was increased stepwise from 2%–25%. The PIO2 was again varied between 15 and 30 kPa (FIO2 = 0.15–0.3) and the corresponding SpO2 recorded. The slope of the SpO2 vs. PIO2 curve was calculated as it intersected the 21 kPa PIO2 line (FIO2 = 0.21).
2.2 Clinical study
2.2.1 Study design
We conducted a prospective observational study at King Edward Memorial Hospital in Perth in Western Australia. The study was approved by the Women and Newborn Health Service Human Research Ethics Committee (HREC:1883EW and 20130193EW) in Perth.
2.2.2 Recruitment period, inclusion and exclusion criteria
Preterm infants born ≤28 weeks’ gestation without major congenital malformations were recruited from the Neonatal Clinical Care Unit at King Edward Memorial Hospital for Women in Perth, Western Australia (KEMH) between 21st August 2017 and the 1st February 2018. All included infants were part of the Preterm Infant Functional and Clinical Outcomes (PIFCO) study (ACTRN12613001062718). We started recruitment for this substudy based on the findings from the main PIFCO cohort, hence the shorter recruitment period and the much smaller number of infants included in the study (Svedenkrans et al., 2019). Informed consent was obtained from parents before the first measurement.
2.2.3 Conduct of study
Infants were assessed at 1 week of age. SpO2 measurements were recorded at hourly intervals for 24 h (Masimo Infant Pulse Oximeter Adhesive Sensor RD SET® Inf; Philips Monitor. IntelliVue MP50 or MP70 Neonatal). Measurements were postponed until the following day in infants with changing respiratory support on the day of measurement. FIO2 was adjusted by the bedside nurses to achieve a SpO2 within the target range SpO2 90%–94%. FIO2 was later converted into PIO2 (PIO2 = FIO2 x (barometric pressure – saturated water vapour pressure).
2.2.4 Analysis of results
The slope of the SpO2 vs. PIO2 curve in preterm infants was analysed using the pulmonary gas exchange algorithm with three lung compartments (Lockwood et al., 2014). Reference normative data of VA/Q, right shift of the oxyhemoglobin dissociation curve and right to left shunt in healthy term infants studied in the first week of life were used to quantify the magnitude of the abnormalities in our population of extremely preterm infants (Dassios et al., 2017; Dassios et al., 2019).
2.2.5 Background data
Patient data information including duration of respiratory support and oxygen therapy were collected from the medical charts or the discharge summaries.
2.2.6 Statistical analyses
Study data were collected and managed using Research Electronic Data Capture (REDCap) software hosted at The University of Western Australia. REDCap is a secure, web-based application designed to support data capture for research studies (Harris et al., 2009).
Parametric data are reported as mean and standard deviation (SD) and non-parametric data as median and variance. Statistical analyses included Student’s t-test for the comparison of parametric and Mann-Whitney-U test for the comparison of non-parametric data. Data were analysed within SPSS (v25·0·0·1; IBM Corp, United States).
3 Results
3.1 Gas exchange model
Reducing VA/Q shifted the SaO2 vs. PIO2 curve to the right so that its slope increased as it intercepted the 21 kPa PIO2 line (Figure 2A). SaO2 at the intercept fell in increasingly large increments particularly with VA/Q below 0.5: e.g., a 24% SaO2 fall when VA/Q dropped from 0.4 to 0.3. The effect of VA/Q on the slope of the curve (Figure 2B) suggested an unstable SaO2 once VA/Q fell below 0.5: a 1% change in PIO2 at 0.3 VA/Q resulted in a >8% change in SaO2.
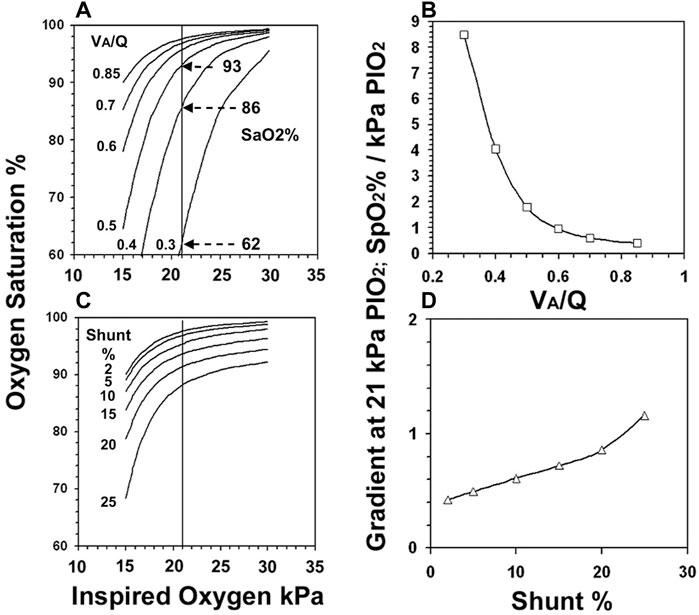
FIGURE 2. The gas exchange model derived PIO2 vs. SaO2 curves when VA/Q was reduced from 0.85 to 0.3. (Figure 2A). The slope of the curve as it intercepted the 21 kPa PIO2 line is shown for VA/Q (Figure 2B). The PIO2 vs. SaO2 curves when shunt increased from 2%–25% (Figure 2C) and their slopes (Figure 2D).
In contrast, increasing shunt (Figure 2C) displaced the curve downwards but even large changes in PIO2 caused only small changes in both SaO2 and slope (Figure 2D): for example in the setting of a 20% intrapulmonary shunt, a 1% change in PIO2 resulted in <1% change in SaO2.
3.2 Clinical study
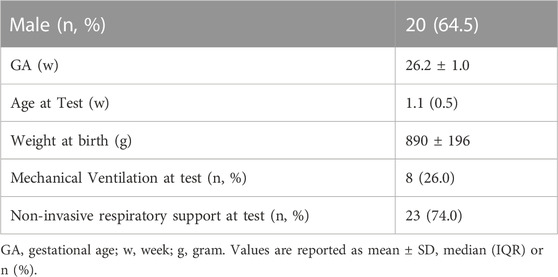
We studied 31 extremely preterm infants (mean (SD) 26.2 (1.0) weeks’ gestation) at median (IQR) 1.1 (0.5) weeks’ postnatal age. Eight infants were mechanically ventilated and 23 infants were on non-invasive respiratory support during the measurements. Demographics for this infant cohort are shown in Table 1.
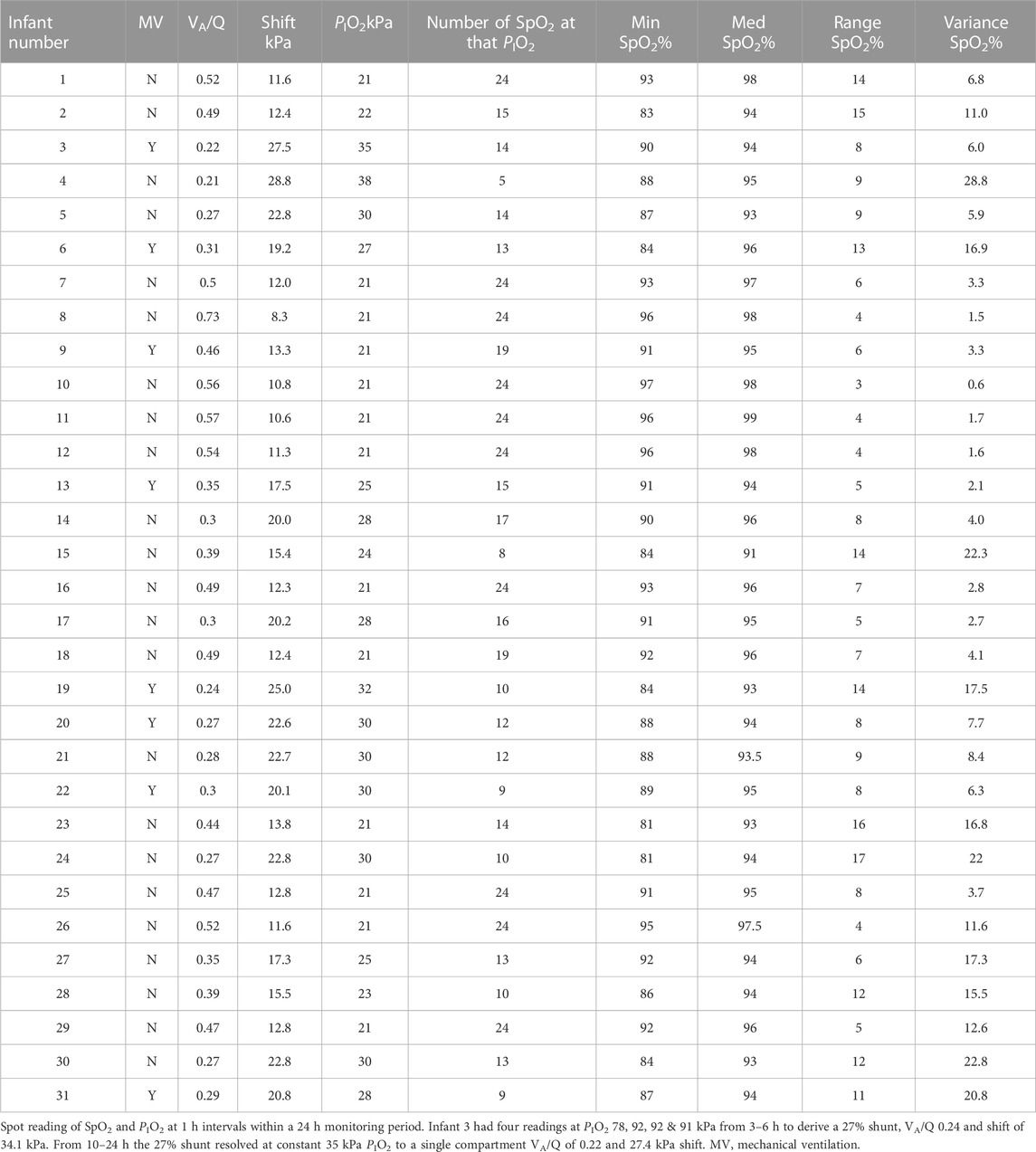
Ten infants received 21 kPa PIO2 throughout the 24 h monitoring period. Median (IQR) PIO2 in the remainder was 28 (Jones and Jones, 2000) % to maintain target SpO2 90%–94%. The number of consecutive hourly readings at the same PIO2 in each infant are shown in Table 2. There were sufficient data to describe the steep part of the SpO2 vs. PIO2 curve in all infants in terms of VA/Q and shift, but complete characterization of the top end of the curve was constrained by the upper limit of the SpO2 target range and therefore restricted PIO2. The VA/Q was less than 0.74 in every infant and only two infants (Nr. 3 and 6) had a large shunt resolving after a few hours into predominantly low VA/Q compartments. The median (IQR) SpO2 of all preterm infants was 8.0 (7.0) % at a constant PIO2 over at least 5 h. At a VA/Q < 0.52, in 10 out of 31 infants the variability of the SpO2 ranged from 11%–17%, whereas in the four infants with VA/Q > 0.52, the range of SpO2 recorded was ≤4% (VA/Q 0.73, 0.56, 0.57, 0.54). Seven infants had a profound desaturation with a SpO2 ≤ 84%. All seven infants had a VA/Q ≤ 0.49 and four of them VA/Q < 0.31. Examples of the pattern of SpO2 in three infants are shown in Figure 3 using the gas exchange algorithm (Lockwood et al., 2014) and a format similar to Figure 1.
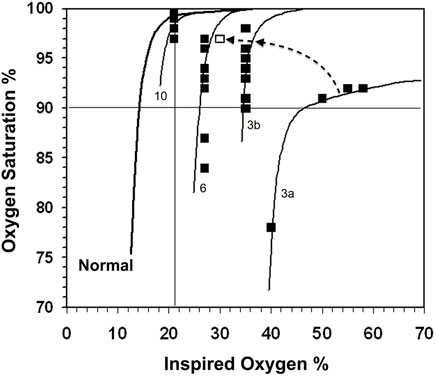
FIGURE 3. The normal PIO2 vs. SpO2 curve is shown on the left. SpO2 readings (closed boxes) with the calculated gas exchange model curves for infants 3, 6 and 10. Infant 3 had 27% shunt and VA/Q of 0.24 (3a), which resolved during the day to a single VA/Q compartment of 0.22 and a SpO2 range of 8% (3b) and subsequently into a final point (open box) at the end of the day to almost overlying the VA/Q 0.31 line of infant 6 (the changes are indicated by the dashed arrow). Infant 6 initially had a 21% shunt and VA/Q of 0.43 (not shown in Figure), which resolved into a VA/Q of 0.31 in the 9–24 h study period. Infant 10 had a VA/Q of 0.56 and a SpO2 range of 3% from 24 SpO2 values.
One infant (Nr. 3) required a PIO2 > 50 kPa to maintain SpO2 within the target range. This infant had an initial large shunt and greatly reduced VA/Q, which later resolved into a single low VA/Q compartment. Infant Nr. 3, 6 and 10 are described in detail in the legend to Figure 3.
The 10 infants exhibiting the most variable SpO2 (range >10% in SpO2) were equally distributed between those breathing spontaneously (n = 5) and those requiring mechanical ventilation (n = 5) (Table 1). The increased variance in SpO2 as VA/Q falls below 0.5 is illustrated in Figure 4A shift increases >11 kPa in Figure 4B.
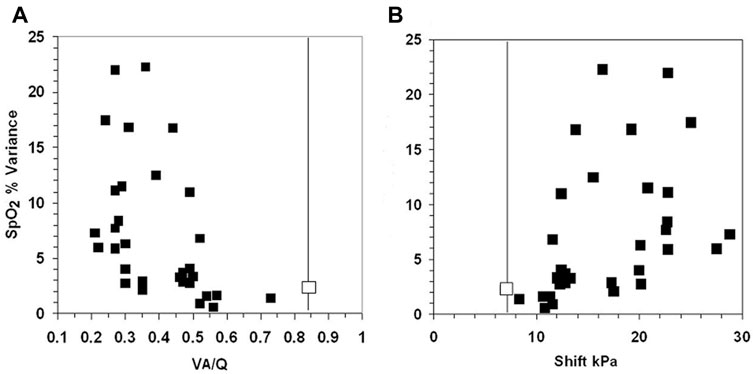
FIGURE 4. The fall in VA/Q below 0.52 (Figure 4A) and an increase in shift >11 kPa (Figure 4B), were associated with an abrupt widening of the variance of SpO2. The two uppermost points have a range of 16% and 17% SpO2 in infants 23 and 24. The normal values for VA/Q and shift are shown by the vertical line. The normal variance for SpO2 is 2.2 shown by the contrasting boxes on the vertical line (Dassios et al., 2017; Dassios et al., 2019).
4 Discussion
We suggest that a reduced VA/Q predisposes preterm infants to SpO2 instability. The pulmonary gas exchange model showed that reducing VA/Q shifted the PIO2 vs. SaO2 curve to the right, whereas shunt displaced the curve downwards. Right shift increased the effective curve slope up to 16 times where it intercepted the 21 kPa PIO2 line e.g., breathing room air. The right shift predicted large fluctuations in SaO2 with small changes in alveolar oxygen. Reducing VA/Q from 0.4 to 0.3 predicted a 24% fall in SaO2 breathing air. In contrast a large shunt had little effect on curve slope and a small fall in SaO2.
These predictions were confirmed in extremely preterm infants by routine monitoring of SpO2 and PIO2. The paired SpO2 vs. PIO2 measurements were used to derive VA/Q, right shift and shunt using Lockwood’s algorithm (Lockwood et al., 2014). All infants had a reduced VA/Q. The SpO2 was unstable with a median (IQR) 8.0 (7.0) % at a constant PIO2 compared to a mean (±SD) of 3% (±1.5%) in healthy newborn infants breathing room air (Dassios et al., 2017; Dassios et al., 2019). Infants with VA/Q < 0.52 had considerable increase in SpO2 variability, seven infants had episodes with SpO2 ≤ 84%, whilst four of these infants were dependent on supplemental oxygen ≥27%. Two infants had transient shunt lasting for a few hours, which was not associated with SpO2 instability. In the remaining infants, the PIO2 was insufficiently high to fully characterize shunt due to the SpO2 target range (90%–94%).
The non-invasive method for deriving VA/Q and shunt normally depends on varying PIO2 (Rowe et al., 2010; Bamat et al., 2015; Dassios et al., 2017; Dassios et al., 2019; Svedenkrans et al., 2019; Bamat et al., 2022). More recently, we showed that VA/Q and/or shift can be derived from serial SpO2 measurements at a fixed PIO2 (Svedenkrans et al., 2019; Stoecklin et al., 2021). A reduced VA/Q lowers SpO2 at any given PIO2 and increases the effective slope of the SpO2 vs. PIO2 curve, but does not by itself make SpO2 unstable unless VA/Q itself is unstable (Jones, 2021). The reduced VA/Q amplifies the destabilizing effects on SpO2 during changes in cardiac output, ventilation, central or obstructive apnea and posture. Interestingly, the 10 infants with the most unstable SpO2 were equally distributed between those breathing spontaneously and those on mechanical ventilation. Reduced VA/Q can lead to SpO2 instability independent of the mode of respiratory support used in an infant.
In agreement with our results, a reduced VA/Q in adults leads to SpO2 instability breathing room air. By adding a time dimension, the different effects of VA/Q or shunt in individual adult patients breathing air is more clearly illustrated by dynamic waterfall plots of continuously monitored SpO2 (Entwistle et al., 1991; Jones and Jones, 2000; Jones, 2021) than do “static” histograms (Borenstein-Levin et al., 2020). A typical example of unstable SpO2 in a patient with reduced VA/Q breathing air is shown in Figure 3 in Ref (Jones, 2021). This patient had blunt curves with SpO2 fluctuating from 77% to 95%. In contrast, a patient with increased shunt had superimposed stable SpO2 peaks within a much narrower SpO2 range.
We note that the right to left shunt in our study might include some element of cardiac shunting occurring via an open ductus arteriosus. Our method does not allow differentiation between intrapulmonary or cardiac shunt. However, in the majority of preterm infants the ductus arteriosus would have functionally closed on day seven of life. In the few infants with a persistent ductus arteriosus, at day seven of life a predominantly left to right shunt would be expected which would not affect our calculations (de Klerk et al., 2020).
The clinical applicability of our findings is that routine measurements of SpO2 and PIO2 can be analysed with our algorithm to derive VA/Q and shunt, thereby characterizing respiratory disease in preterm infants. VA/Q and shunt incorporate information on the mechanisms of hypoxemia and explain SpO2 instability. Consequently, SpO2 instability can be prevented by a small increase in PIO2.
5 Conclusion
In conclusion, we reported that predisposition to oxygen saturation instability in preterm infants results from a reduced ventilation to perfusion ratio rather than from increased intrapulmonary shunt. We have highlighted how routine monitoring can be used to derive non-invasive measurements of oxygenation impairment in preterm infants. Future research should aim at strategies to not only detect SpO2 instability, but to also prevent such instability.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the Women and Newborn Health Service Human Research Ethics Committee (HREC:1883EW and 20130193EW) in Perth. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
BS collected and analysed the data, interpreted the data, assisted with the REDCap database design and development, drafted the initial manuscript together with JGJ, performed literature search, and approved the final manuscript as submitted. YC collected the data, reviewed and revised the manuscript, and approved the final manuscript as submitted. JGJ developed the algorithms used for the calculation of shunt, shift and VA/Q, drafted reviewed and revised the manuscript, drafted the Figures and approved the final manuscript as submitted. GL developed the algorithms used for the calculation of shunt, shift and VA/Q, reviewed and revised the manuscript. TD drafted the initial manuscript, revised and approved the final manuscript. JP was the principal investigator obtaining funding, leading study design including development of the REDCap database, verified all statistical calculations, interpreted the data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Funding
All phases of this study were supported by the University of Western Australia and the Women and Newborn Health Service of Western Australia. Funded by National Health and Medical Research Council (NHMRC) of Australia (GNT1047689, GNT1057514) and the Metropolitan Health Research Infrastructure Fund (MHRIF). BS was supported by the Swiss National Science Foundation (P2BSP3_158837) and a Research Training Program scholarship from The University of Western Australia. JP was supported by a NHMRC Fellowships (RF1077691, GNT1196188).
Acknowledgments
The authors thank Dr A. Olszowka, Department of Physiology, University of Buffalo, New York, United States for providing his pulmonary gas exchange program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bamat N., Ghavam S., Liu Y., DeMauro S. B., Jensen E. A., Roberts R., et al. (2015). Reliability of a noninvasive measure of V./Q. Mismatch for bronchopulmonary dysplasia. Ann. Am. Thorac. Soc. 12 (5), 727–733. doi:10.1513/AnnalsATS.201410-462OC
Bamat N. A., Orians C. M., Abbasi S., Morley C. J., Ross Russell R., Panitch H. B., et al. (2022). Use of ventilation/perfusion mismatch to guide individualised CPAP level selection in preterm infants: A feasibility trial. Arch Dis Child Fetal Neonatal Ed. doi:10.1136/archdischild-2022-324474
Bolivar J. M., Gerhardt T., Gonzalez A., Hummler H., Claure N., Everett R., et al. (1995). Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. J. Pediatr. 127 (5), 767–773. doi:10.1016/s0022-3476(95)70171-0
Borenstein-Levin L., Konikoff L., Solimano A. (2020). Clinical quantification of SpO2 instability using a new histogram classification system: A clinical study. Pediatr. Res. 87 (4), 716–720. doi:10.1038/s41390-019-0566-6
Corbet A. J., Ross J. A., Beaudry P. H., Stern L. (1974). Ventilation-perfusion relationships as assessed by a ADN2 in hyaline membrane disease. J. Appl. Physiol. 36 (1), 74–81. doi:10.1152/jappl.1974.36.1.74
Dassios T., Ali K., Rossor T., Greenough A. (2019). Using the fetal oxyhaemoglobin dissociation curve to calculate the ventilation/perfusion ratio and right to left shunt in healthy newborn infants. J. Clin. Monit. Comput. 33 (3), 545–546. doi:10.1007/s10877-018-0168-6
Dassios T., Ali K., Rossor T., Greenough A. (2017). Ventilation/perfusion ratio and right to left shunt in healthy newborn infants. J. Clin. Monit. Comput. 31 (6), 1229–1234. doi:10.1007/s10877-016-9969-7
de Klerk J. C. A., Engbers A. G. J., van Beek F., Flint R. B., Reiss I. K. M., Voller S., et al. (2020). Spontaneous closure of the ductus arteriosus in preterm infants: A systematic review. Front. Pediatr. 8, 541. doi:10.3389/fped.2020.00541
Di Fiore J. M., Bloom J. N., Orge F., Schutt A., Schluchter M., Cheruvu V. K., et al. (2010). A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157 (1), 69–73. doi:10.1016/j.jpeds.2010.01.046
Entwistle M. D., Roe P. G., Sapsford D. J., Berrisford R. G., Jones J. G. (1991). Patterns of oxygenation after thoracotomy. Br. J. Anaesth. 67 (6), 704–711. doi:10.1093/bja/67.6.704
Hand I. L., Shepard E. K., Krauss A. N., Auld P. A. (1990). Ventilation-perfusion abnormalities in the preterm infant with hyaline membrane disease: A two-compartment model of the neonatal lung. Pediatr. Pulmonol. 9 (4), 206–213. doi:10.1002/ppul.1950090404
Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42 (2), 377–381. doi:10.1016/j.jbi.2008.08.010
Jones J. G., Jones S. E. (2000). Discriminating between the effect of shunt and reduced VA/Q on arterial oxygen saturation is particularly useful in clinical practice. J. Clin. Monit. Comput. 16 (5-6), 337–350. doi:10.1023/a:1011495416005
Jones J. G. (2021). Oxygen saturation instability in suspected covid-19 patients; contrasting effects of reduced VA/Q and shunt. medRxiv.
Lockwood G. G., Fung N. L., Jones J. G. (2014). Evaluation of a computer program for non-invasive determination of pulmonary shunt and ventilation-perfusion mismatch. J. Clin. Monit. Comput. 28 (6), 581–590. doi:10.1007/s10877-014-9554-x
Miller-Barmak A., Riskin A., Hochwald O., Haddad J., Dinur G., Vortman R., et al. (2020). Oxygenation instability assessed by oxygen saturation histograms during supine vs prone position in very low birthweight infants receiving noninvasive respiratory support. J. Pediatr. 226, 123–128. doi:10.1016/j.jpeds.2020.06.066
Olszowka A. J., Wagner P. D. (1980). “8 - numerical analysis of gas exchange,” in Ventilation, blood flow, and diffusion. Editor J. B. West (Academic Press), 263–306.
Poets C. F., Roberts R. S., Schmidt B., Whyte R. K., Asztalos E. V., Bader D., et al. (2015). Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. Jama 314 (6), 595–603. doi:10.1001/jama.2015.8841
Roca J., Wagner P. D. (1994). Contribution of multiple inert gas elimination technique to pulmonary medicine. 1. Principles and information content of the multiple inert gas elimination technique. Thorax 49 (8), 815–824. doi:10.1136/thx.49.8.815
Rowe L., Jones J. G., Quine D., Bhushan S. S., Stenson B. J. (2010). A simplified method for deriving shunt and reduced VA/Q in infants. Archives Dis. Child. Fetal neonatal Ed. 95 (1), F47–F52. doi:10.1136/adc.2009.160010
Stoecklin B., Choi J., Rakshasbhuvankar A., Svedenkrans J., Jones J. G., Pillow J. (2021). Simplified bedside assessment of pulmonary gas exchange in very preterm infants at 36 weeks’ postmenstrual age. Thorax 76, 689–695. doi:10.1136/thoraxjnl-2020-214659
Keywords: infant, premature, neonatal intensive care unit, pulmonary gas exchange, oxygen inhalation therapy
Citation: Stoecklin B, Choi YJ, Dassios T, Jones JG, Lockwood GG and Pillow JJ (2023) Unstable SpO2 in preterm infants: The key role of reduced ventilation to perfusion ratio. Front. Physiol. 14:1112115. doi: 10.3389/fphys.2023.1112115
Received: 30 November 2022; Accepted: 25 January 2023;
Published: 07 February 2023.
Edited by:
Rajasvaran Logeswaran, Asia Pacific University of Technology & Innovation, MalaysiaReviewed by:
Kevin Dysart, Alfred I. duPont Hospital for Children, United StatesJuliann Di Fiore, Case Western Reserve University, United States
Copyright © 2023 Stoecklin, Choi, Dassios, Jones, Lockwood and Pillow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Stoecklin, YmVuamFtaW4uc3RvZWNrbGluQHVrYmIuY2g=
 Benjamin Stoecklin
Benjamin Stoecklin Y. Jane Choi
Y. Jane Choi Theodore Dassios
Theodore Dassios J. Gareth Jones
J. Gareth Jones Geoffrey G. Lockwood6
Geoffrey G. Lockwood6 J. Jane Pillow
J. Jane Pillow