
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 20 January 2023
Sec. Avian Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1110301
This article is part of the Research TopicNutritional Impacts the Health and Physiology of the Avian Gastro-intestinal TractView all 12 articles
 Ao-Chuan Yu1†
Ao-Chuan Yu1† Min-An Wang1†
Min-An Wang1† Li Chen2
Li Chen2 Cheng Long1
Cheng Long1 Yong Guo1
Yong Guo1 Xi-Hui Sheng1
Xi-Hui Sheng1 Xiang-Guo Wang1
Xiang-Guo Wang1 Kai Xing1
Kai Xing1 Long-Fei Xiao1
Long-Fei Xiao1 He-Min Ni1
He-Min Ni1 Jian-Tao Li3*
Jian-Tao Li3* Xiao-Long Qi1*
Xiao-Long Qi1*Aims: The study aimed to evaluate the effects of pretreated Chinese herbal medicine (PCHM) on egg quality, production performance, histopathological changes in the uterus, antiox idant capacity, and antioxidant gene expression in late-phase layers.
Methods: Jinghong No.1 layers (n = 360, 68 weeks old) were assigned randomly to one of f our dietary interventions. Each treatment was replicated six times. Repeat 15 chickens per g roup. All birds were fed a diet composed of a corn-soybean meal-based diet supplemented with 0, 0.2, 0.4, or 0.8% PCHM for 6 weeks.
Results: Dietary PCHM supplementation had no significant effects on laying rate, feed con sumption, yolk color, and shape index. With increasing PCHM level the Haugh unit linearly increased (P < 0.05). Supplementation of 0.8% PCHM increased egg weight, compared with the control (P < 0.05). PCHM can effectively alleviated the pathological changes caused by aging in the uterus including hemorrhage, and many inflammatory cell infiltrations. Supplementation of 0.4% PCHM increased glutathione peroxidase (GSHPx) in liver, magnum, and plasm considerably, compared with the control (P < 0.05). Supplementation of PCHM decr ease in the liver, magnum, and uterus on malondialdehyde (MDA) content, compared with the control (P < 0.05). Compared with the control group, mRNA expressions of glutathione peroxidase 1 (GPX1), peroxidase 4 (GPX4), catalase (CAT), and nuclear factor E2-related factor 2 (Nrf2) in the magnum, liver, and uterus were dramatically rose in the 0.4% PCHM supplementation group (P < 0.05). In summary, dietary supplementation after PCHM increased egg weight and quality in late-phase laying hens.
Conclusion: Dietary PCHM increased the antioxidative capacity of late-phase laying hens, which could be associated with increased mRNA expression of antioxidant enzymes and Nrf2. These findings provide potential for using PCHM to increase the production performance in late-phase laying hens.
The final stage of the laying cycle is characterized by a sharp drop in both egg performance (Attia et al., 2020) and egg quality (Park and Sohn, 2018). Yolk synthesis and accumulation are both reduced as egg output performance (Zakaria et al., 1983). Parallelly, egg size or weight increases, and Haugh unit (HU) and eggshell breaking strength (EBS) decrease as hens age. It has been observed that laying hens’ oviducal regression will directly affect egg production and quality (Gongruttananun et al., 2017). This may be related to the aging of oviducts. Age increases oxidant production from multiple sources, while antioxidant enzymes, the major lines of defense, decline (Zhang et al., 2015).
The activities of Chinese herbal medicines used as feed additives are extensive, including anti-apoptosis (Ibtisham et al., 2019), anti-inflammatory, and antioxidant ability (Xie et al., 2019). Angelica is a tonifying, nourishing, and edible herb with a long history of use, as well as for anti-inflammatory and antioxidant activity (Wei et al., 2016). Multiple live animal models of oxidative stress-related disease have shown that Astragalus extract offers substantial protection to the heart, kidneys, and intestines (Shahzad et al., 2016). Epimedium possesses multiple functions relevant in disease (Ma et al., 2011), including acting as an antioxidant (Yang et al., 2020). Houttuynia cordata also has medicinal properties (Wu et al., 2021), including acting as a free-radical scavenger and an anti-inflammatory (Ahn et al., 2017). Accumulating evidence has indicated that dietary herbs improve egg quality and production performance of laying hens. For example, Xie et al. (2019) found that the anti-inflammatory effect of Astragali can improve the albumen quality, and a blend of Lonicera confusa and Astragali Radix improved yolk color (Xie et al., 2019). Epimedium promotes follicular granulosa cell proliferation and differentiation, as well as hormone secretion and follicle development, which increases egg production rate (Guo et al., 2020a). Whole extracts or isolated compounds from Angelica, Astragalus, Epimedium, Houttuynia, and Leonurus can act as antioxidants. Currently, there are few studies on the five kinds of mixed Chinese herbal medicine in late-phase laying hens.
The objective of the current study was to investigate the effects of pretreated Chinese herbal medicine (PCHM) on production performance, egg quality, histopathological changes in the uterus, antioxidant capacity, and gene expression of antioxidant enzymes in late-phase laying hens.
The Beijing University of Agriculture’s Animal Care and Use Committee approved all experimental protocols (Approval ID: BUA-zc-20200073).
Epimedium, Astragalus, Angelica, Leonurus, and Houttuynia were all purchased from Yiren Pharmaceutical Co., Ltd. (Baoding, Hebei, China). The test bacteria and enzymolysis enzymes were purchased from Beijing challenge Biotechnology Co., Ltd. (Beijing, China). The main components of Angelica, Astragalus, Epimedium, Houttuynia, and Leonurus are Angelica polysaccharide, Astragalus polysaccharide, flavone, chlorogenic acid, and alkaloid, respectively (Table 1). In this experiment, Angelica and Astragalus were treated by fermentation, Leonurus was treated by enzymatic hydrolysis, and Houttuynia and Epimedium were not treated. According to the pre-experiment, the main components of Angelica and Astragalus reach their highest level through fermentation, the main components of Leonurus through enzymatic hydrolysis, and the main components of Houttuynia and Epimedium through no treatment at all. According to the Chinese veterinary medicine code, the mixing ratio of Epimedium, Astragalus, Angelica, Leonurus, and Houttuynia after pretreatment is 1:2:2:1:2. After processing, the mixed herbs were called PCHM. Fermentation conditions: Bacillus licheniformis, Bacillus coagulans, Aspergillus niger, Bacillus subtilis (1:1:1:1), conditions: time: 5 days, temperature: 37°C, water content: 45%. Enzymatic hydrolysis conditions: complex enzyme (pectinase, cellulase, laccase), conditions: time: 4 h, temperature: 55°C, water content: 45%.
A total of three hundred and sixty 68-week-old Jinghong No. 1 laying hens (initial body weights 1.65 ± 0.10 kg) with a similar weight and genetic background were used. A completely randomized design was used to divide the birds into the four treatment groups. Each treatment had six replicates with 15 birds each. All birds were fed a basal diet for 1 week before being assigned a diet containing maize-soybean meal containing 0, 0.2, 0.4, or 0.8% PCHM for 6 weeks. Table 2 displays the composition and nutritional levels of the diet based on maize-soybean meal. The experimental diet feeding period lasts for 6 weeks and the birds in the experiment were exposed to 16-h light cycles and had free access to water and the experimental diets at all times.
Every day, data on egg production and weight were logged. The number of eggs produced was expressed as an average hen-day production, which was derived by dividing the total number of hen-days by the number of eggs. Average egg weight was calculated as total egg weight divided by the number of eggs. Feed consumption was recorded on a replicate basis at weekly intervals. The feed conversion ratio was measured as the amount of feed consumed relative to the amount of eggs produced in kilograms. Egg quality was measured on three eggs collected randomly from each replicate on the 14th, 28th, and 42nd days. One healthy bird was chosen at random from each replicate at the end of the feeding period (one bird per replicate, and 24 birds in total). Exsanguination of the left jugular vein with scalpels was used to collect blood samples, which were then centrifuged at 4°C at 4,000 g for 10 min to separate plasma. Following collection, plasma samples were flash-frozen at −80°C and stored in the freezer until analysis. Birds were euthanized by exsanguination and necropsied, and the liver, magnum, and uterus were separated immediately and quickly frozen at −80°C for further analysis. Approximately 2 cm medial portion sections of the uterus were removed and cleaned thoroughly with 0.9% saline, then placed in 4% formaldehyde solution for tissue fixation and histological measurement.
The rate of egg production was determined as follows: egg production rate = eggs laid per day/(birds counted per day). Feed efficiency was calculated weekly. Haugh units, yolk color, and albumen height were measured using an egg analyzer (Orka Food Technology Ltd., Ramat Hasharon, Israel). Yolk color was defined according to the Roche yolk color fan, where 1 represents bright yellow and 15 represents dark yellow. An egg force reader (Herzliya, Tel Aviv, Israel) was used to measure eggshell strength. The thickness of the eggshell (ST) was calculated as follows: [ST, mm = SW/(ES d)], where SW is the weight of the eggshell, ES is the surface area of the egg, and d is the density of the material (2.3 g/cm3 for calcium carbonate).
Uterine tissues were fixed in 4% paraformaldehyde and embedded in paraffin, and the 5 µm-thick sections were stained with hematoxylin-eosin (H&E). Samples were trimmed, dehydrated, embedded, sliced, dyed, and sealed in strict accordance with the standard operating procedures for pathological experiment detection of the service-bio unit (Liu et al., 2022). Histological samples were evaluated for degree of injury, using variables such as the integrity of tissue structure and the numbers of infiltrated inflammatory cells (service-bio, Wuhan, China).
The plasma, liver, magnum, and uterus tissue concentrations of glutathione peroxidases (GSH-Px), catalase (CAT), superoxide dismutase (SOD), and the ability to scavenge superoxide anion radicals and the hydroxyl radical malondialdehyde (MDA) were measured by enzyme-linked immunosorbent assay (ELISA) using a commercial ELISA kit for chicken (Nanjing Jiancheng Bioengineering Institute, Nanjing, China; catalog no. A003-1-2, A007-1-1, A001-1-2, A005-1-2, A052-1-1, A018-1-1) according to the manufacturer’s instructions. T-SOD activity was determined by using the xanthine/xanthine oxidase method, which is based on the inhibition of nitroblue tetrazolium formazan. The activity of GSH-Px was measured using H2O2 as a substrate in the presence of reduced glutathione. The GSH-Px activity was reported in micromoles of oxidized NADPH per minute.
Total RNA was extracted from 0.1 g of liver, magnum, and uterus tissue sample using TRIzol reagent (Thermo Fisher Scientific, Shanghai, China), as per the manufacturer’s instructions, and then reverse-transcribed into single-stranded cDNA using the Thermo First cDNA Synthesis Kit (Promega, Beijing, China). The expressions of nuclear factor E2 related factor 2 (Nrf2), Cu-Zn superoxide dismutase (SOD) 1, Mn superoxide dismutase (SOD) 2, catalase (CAT), glutathione peroxidase 1 (GPX1), and peroxidase 4 (PX4) were determined using qRT-PCR with specific primers (Table 3). An AriaMx Real-Time PCR system (Agilent Technologies, Santa Clara, California, United States) was used for quantitative PCR analysis. After initial denaturation at 95°C for 10 min, 40 cycles of amplification were carried out (95°C for 10 s and 58.2°C for 30 s), followed by the generation of melt curves that could be used to verify the specificity of amplification. The samples were tested in triplicate. The 2−ΔΔCT (Livak and Schmittgen, 2001) method was used to calculate the relative gene expression levels. Refer to the operation of (Madkour et al., 2021) for specific process.
All statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, United States). Data are presented as mean ± standard deviation of the mean (SD). Additionally, polynomial regression analysis was used to test the linear and quadratic nature of the response to the additive PCHM dosage, and Tukey’s multiple comparison tests were used to analyze the differences among various treatments. p < 0.05 was considered significant.
Comparison of the main components of five Chinese herbal medicines is shown in Table 4. Angelica, Astragalus fermentation treatment, Leonurus enzymolysis treatment, Houttuynia, and Epimedium did not have treatment.
The effects of dietary supplementation of PCHM on performance and egg quality are shown in Table 5 and Table 6. PCHM supplementation had no effect on laying rate, feed consumption, yolk color, shell thickness, shape index; PCHM supplementation had a significant effect on feed efficiency, egg weight, albumen height, and eggshell strength (p < 0.05). With increasing dietary supplementation levels of PCHM, the Haugh unit increased linearly (p < 0.05).
The histological analysis was performed to assess the pathological changes in uterine tissues. The results demonstrated that aging resulted in severe injury, including hyperemia, hemorrhage, and many inflammatory cell infiltrations (Figure 1A). However, the aging-induced pathological changes were dramatically improved by PCHM at doses of 0.2 (Figure 1B), 0.4 (Figure 1C), and 0.8% (Figure 1D). These results indicate that PCHM could effectively attenuate aging-induced pathological changes.
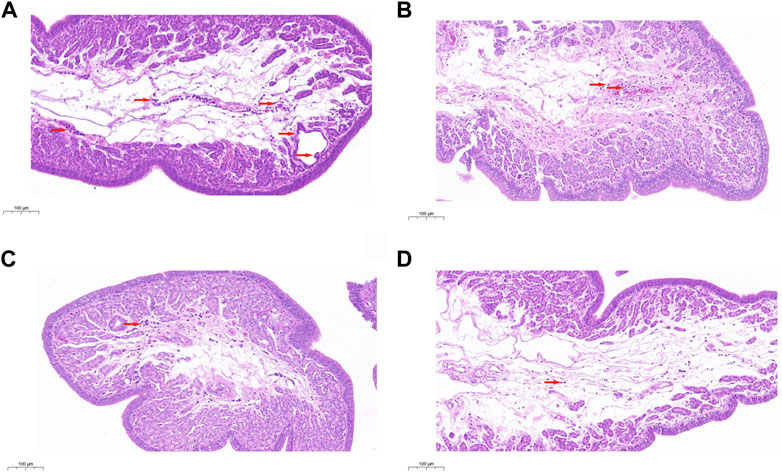
FIGURE 1. Tissues from the uterus were removed and stained with H&E. Scale bar: 100 μm. (A) Uterine histopathology in the control group; (B–D) Uterine histopathology with PCHM treatment groups at 0%, 0.2%, 0.4%, and 0.8%, respectively. The locations of tissue damage and inflammatory cell infiltration are denoted by the directional arrows in red.
The effect of dietary PCHM supplementation on the antioxidant enzyme activity and free radical scavenging capabilities in plasma, liver, magnum, and uterus are depicted in Figures 2–5, respectively. The PCHM supplementation significantly influenced the antioxidant enzyme activity and free radical scavenging capabilities in plasma, liver, magnum, and uterus. In the plasma, a significant (p < 0.05) increased in the protein level of CAT and GSH-Px was noticed in the 0.2% and 0.4% PCHM groups, and CAT and SOD in 0.8% PCHM group supplemented were observed as compared to the other experimental groups. In liver, a significant (p < 0.05) increased in the protein level of GSH-Px and free radical scavenging capabilities was noticed in the PCHM supplementation as compared to the control. Further, in liver a significant (p < 0.05) decreased the content of MDA in the PCHM supplementation as compared to the control. In magnum, a significant (p < 0.05) increased of GSH-Px protein level in the 0.4% PCHM groups, SOD protein level in 0.8% PCHM group, the ability to scavenge superoxide anion in 0.4% and 0.8% PCHM groups were observed as compared to the other experimental groups. Further, in magnum a significant (p < 0.05) decreased the content of MDA in the PCHM supplementation as compared to the control. Similarly, in uterus, a significant (p < 0.05) increased of CAT protein level in the 0.4% and 0.8% PCHM groups, the ability to scavenge hydroxyl radical in 0.2% and 0.4% PCHM group were observed as compared to the other experimental groups. Further, in uterus, significantly (p < 0.05) decreased the content of MDA in the 0.4% PCHM groups were evident as compared to the other experimental groups.
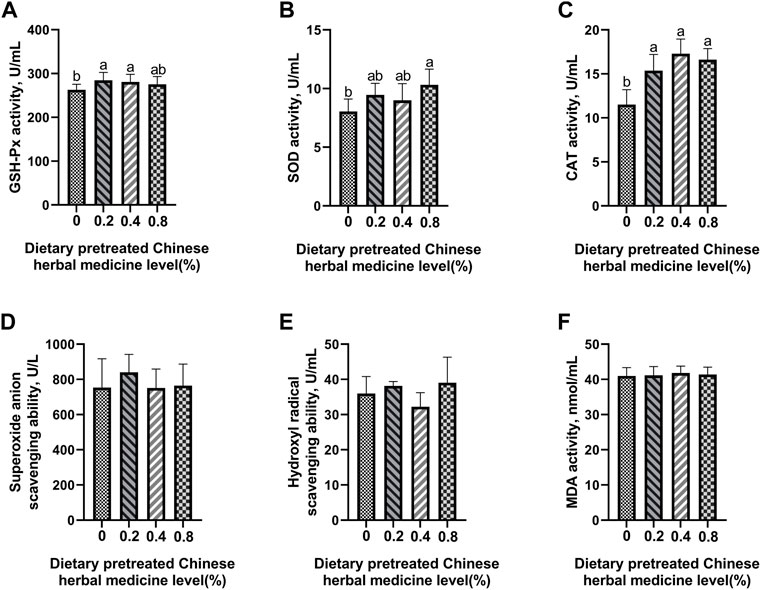
FIGURE 2. Effect of dietary PCHM supplementation on the antioxidant enzyme activity and free radicals in the plasma of late-phase laying hens. (A–C) Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) activity in the plasma. (D,E) Scavenging free radical abilities in the plasma. (F) Malondialdehyde (MDA) level in the plasma. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
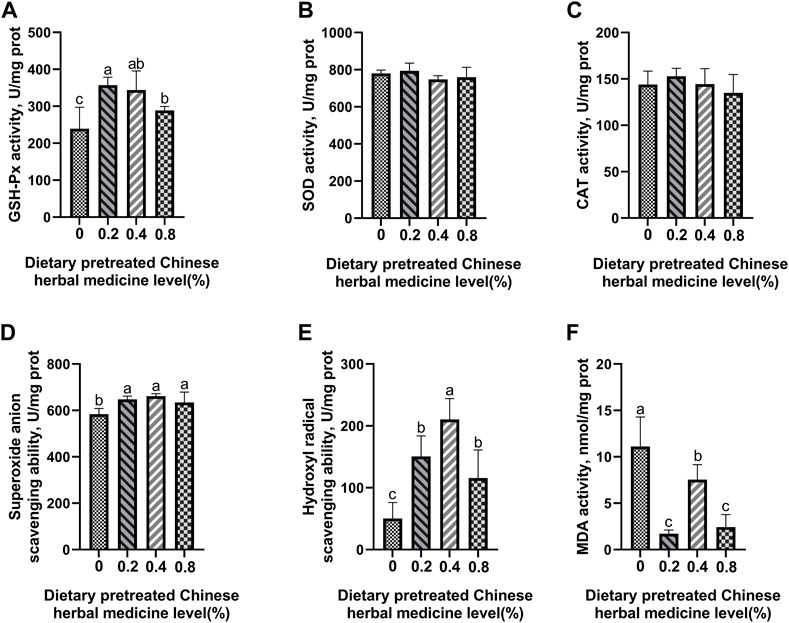
FIGURE 3. Effect of dietary PCHM supplementation on the antioxidant enzyme activity and free radicals in the liver of late-phase laying hens. (A–C) Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) activity in the liver. (D,E) Scavenging free radical abilities in the liver. (F) Malondialdehyde (MDA) level in the liver. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
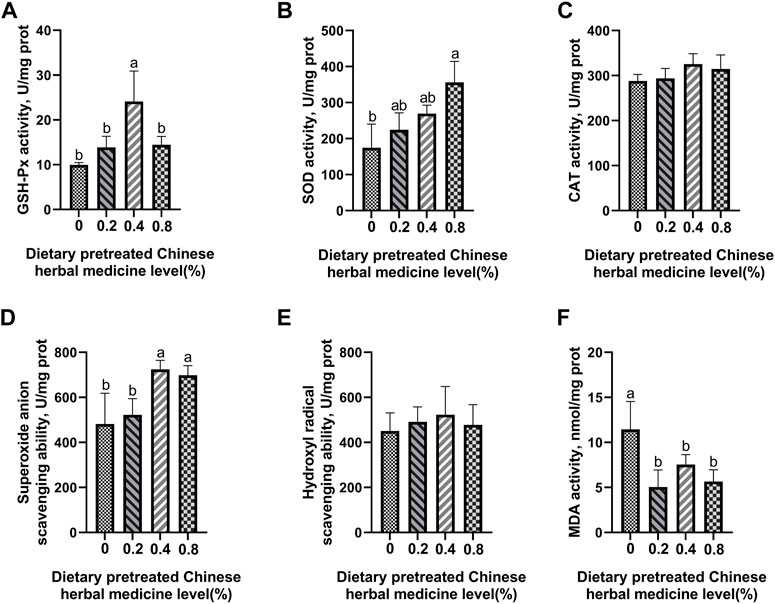
FIGURE 4. Effect of dietary PCHM supplementation on the antioxidant enzyme activity and free radicals in the magnum of late-phase laying hens. (A–C) Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) activity in the magnum. (D,E) Scavenging free radical abilities in the magnum. (F) Malondialdehyde (MDA) level in the magnum. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
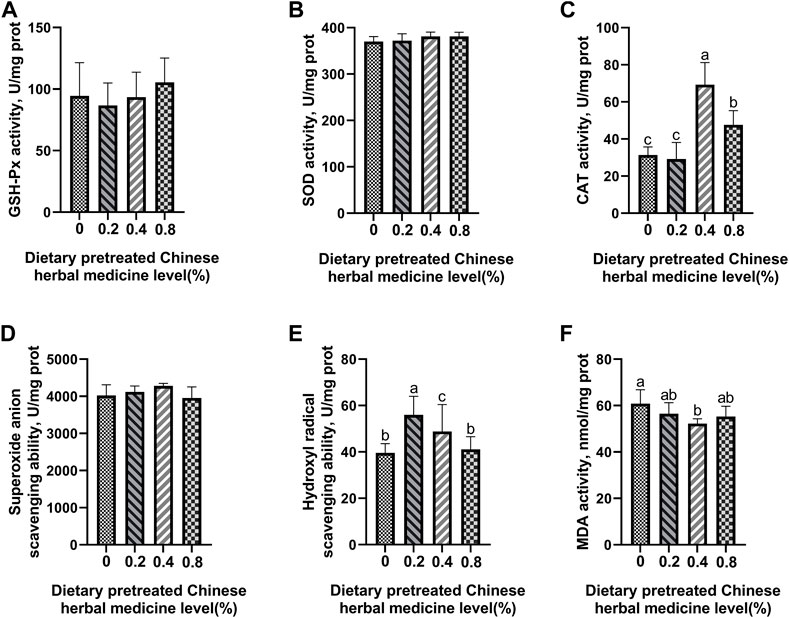
FIGURE 5. Effect of dietary PCHM supplementation on the antioxidant enzyme activity and free radicals in the uterus of late-phase laying hens. (A–C) Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) activity in the uterus. (D,E) Scavenging free radical abilities in the uterus. (F) Malondialdehyde (MDA) level in the uterus. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
The effect of dietary PCHM supplementation on the expressions of CAT, SOD1, SOD2, GPX1, GPX4, and Nrf2 genes in liver, magnum and uterus are depicted in Figures 6–8, respectively. The PCHM supplementation significantly influenced the expression of these genes in liver, magnum, and uterus. Expression of all the genes was significantly (p < 0.05) upregulated in liver with 0.4% PCHM supplementation as compared to the control. In magnum, a significant (p < 0.05) increase in the expression of CAT and Nrf2 was noticed in the 0.2% and 0.8% PCHM supplemented as compared to the other groups. Further, in magnum, significantly (p < 0.05) greater expression of SOD1 and GPX4 in the 0.2 PCHM groups, and SOD2 and GPX1 in the 0.8% PCHM group were evident as compared to the other experimental groups. Similarly, in uterus, a significant (p < 0.05) upregulated expression of CAT, SOD1 and GPX4 in the 0.2% and 0.4% PCHM groups, SOD2 in 0.2% PCHM group, GPX1 in 0.2%, 0.4%, and 0.8% PCHM groups, and Nrf2 in the 0.4% and 0.8% PCHM groups were observed as compared to the other experimental groups.

FIGURE 6. Effects of dietary PCHM supplementation on antioxidation-related mRNA expression in liver. The effect of adding PCHM (0, 0.2%, 0.4%, 0.8%) in diets in the liver, measured by quantitative PCR: (A) CAT, (B) SOD1, (C) SOD2, (D) GPX1, (E) GPX4, and (F) Nrf2. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
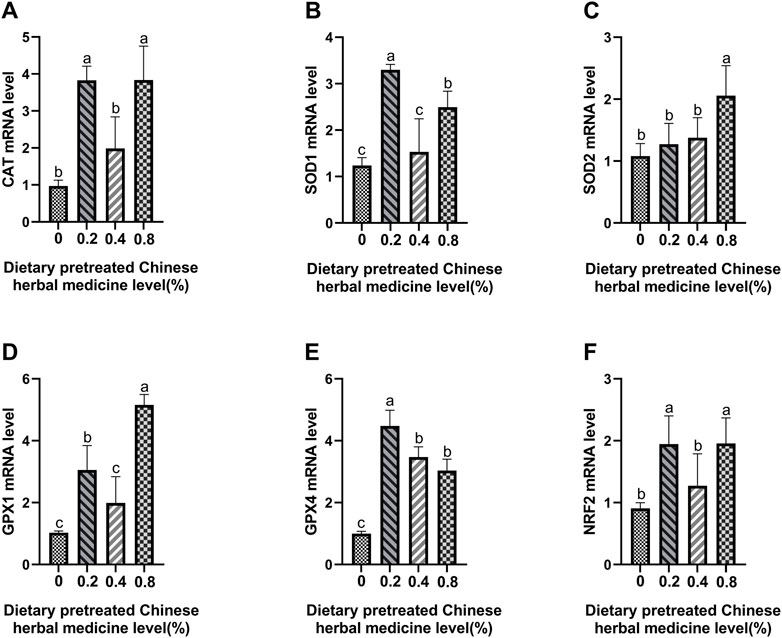
FIGURE 7. Effects of dietary PCHM supplementation on antioxidation-related mRNA expression in magnum. The effect of adding natural herbs (0, 0.2%, 0.4%, 0.8%) in diets in the magnum, measured by quantitative PCR: (A) CAT, (B) SOD1, (C) SOD2, (D) GPX1, (E) GPX4, and (F) Nrf2. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
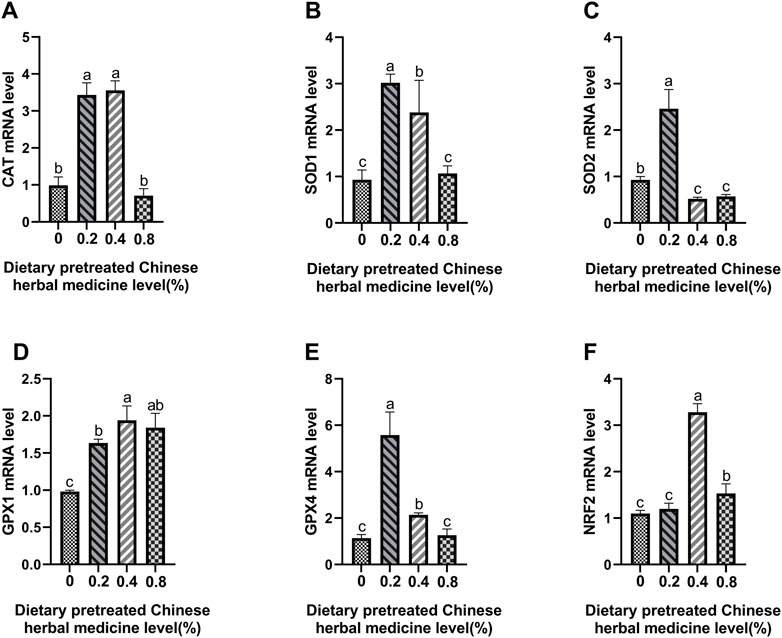
FIGURE 8. Effects of dietary PCHM supplementation on antioxidation-related mRNA expression in uterus. The effect of adding PCHM (0, 0.2%, 0.4%, 0.8%) in diets in the uterus, measured by quantitative PCR: (A) CAT, (B) SOD1, (C) SOD2, (D) GPX1, (E) GPX4, and (F) Nrf2. Values are expressed as means ± SEM of six birds per treatment. Means without a common letter differ (p < 0.05).
The purpose of this study was to investigate how dietary supplementation of PCHM affected production performance, egg quality, histopathological changes in the uterus, antioxidant capacity, and antioxidant enzyme gene expression in late-phase laying hens. Egg quality is important in commercial layers (Odabasi et al., 2007). The quality of eggs, notably their shells, gradually decreases as hens age past their prime laying years (Bain et al., 2016). It has been reported that supplementation of laying hen diets with herbs can improve their egg-laying rate (Li et al., 2017), Haugh unit (Moon et al., 2021), and shell strength (Xiao et al., 2019). The present study revealed that dietary supplementation of PCHM increased average egg weight, shell thickness, eggshell strength, Haugh unit and albumen height, but did not affect the laying rate, feed consumption, yolk color, or shape index. With increasing dietary levels of PCHM, the Haugh unit increased linearly. The improvement of Haugh unit may be caused by the polysaccharide contained in PCHM (Guo et al., 2020b). Similar findings were reported by Xie et al. (2019), who observed that diets including herbal medicine (Lonicera confusa and Astragali Radix) improved the albumen quality in laying hens. The antioxidant ability of PCHM’s bioactive components is responsible for these changes in Haugh unit. In particular, prior investigations (Costa et al., 2019) have revealed the antioxidant capabilities of PCHM, which likely prevent protein oxidation in eggs.
To explore a possible mechanism for the improvement in egg qualities, and feed efficiency in late-phase laying hens with PCHM supplementation, histopathological changes in the uterus and antioxidant status were determined. The ability to ovulate was closely related to the uterus (Kong et al., 2012). Aging causes certain changes in the tissue morphology of uterus such as swelling of collagen fibres, elastic tissue fragments and oedema (Mulholland and Jones, 1993). Bitter ginseng bases extracted from the Chinese herb bitter ginseng were reported to significantly reduce uterine damage in a mouse model induced by Staphylococcus aureus lipophosphatidic acid (Jiang et al., 2019). In the current study, PCHM supplementation attenuated the uterine injury in late-phase laying hens, which the improvement of uterine injury was probably related to the ability of PCHM to inhibit lipid peroxidation (Sikiru et al., 2019). Recent research too demonstrates that ginsenoside ameliorates pathological uterine damage by boosting the anti-oxidant enzymes SOD and GSH (He et al., 2021).
Oxidative stress contributes to aging and age-related disorders (Miyazawa et al., 2009). MDA is a lipid peroxidation marker used to evaluate lipid peroxidation as a result of elevated oxidative stress (Ottolenghi et al., 2019). Oxidative stress may come from either an excess of free radical generation or a breakdown of antioxidant defense systems (Torun et al., 2009). It has been reported that some herbs possess strong radical-scavenging ability (Jarco et al., 2021). In the current study, PCHM was added to the diet to effectively improve the free-radical scavenging capacity and reduce the content of MDA in liver, magnum, and uterus. A previous report indicates that supplementation of 0.8 g/kg herbs lowers MDA content in egg yolk by increasing antioxidant enzyme activity (Chen et al., 2018). Recent studies have shown that improvement of free-radical scavenging ability may be related in part to antioxidant enzyme activity (Heng et al., 2021). It is reported that GSH-Px is an important antioxidant enzyme that can convert hydrogen peroxide into water (Zhou et al., 2021). Similarly, dietary supplementation of ginger powder increases the antioxidant enzymatic activity of laying hens (Lee et al., 2012). These results of the current study indicate that dietary supplementation with PCHM at 0.4% was most effective in improving the antioxidant capacity of late-phase laying hens.
Superoxide dismutases (SODs) are an important class of metallo-antioxidant enzymes in the metabolism of ROS in living organisms (Zhao et al., 2021). Selenium (Se) is of great importance in the treatment of diseases caused by oxidative stress (Rashid and Coombs, 2019). Previous studies have shown that selenomethionine promotes the expression of Nrf2 transcription factor-related genes in cells during lipopolysaccharide stimulation (Adeniran et al., 2022). The change in antioxidant enzyme activity is related to gene expression (Guo et al., 2014). Previous research has demonstrated that the addition of tanshinone can boost GPX1 mRNA expression in macrophages (Li et al., 2008). Dietary supplementation with PCHM elevated the mRNA expression of CAT, SOD1, SOD2, GPX1, and GPX4 in the liver and uterine in the current study. In particular, dietary supplementation with 0.4% PCHM is most effective in the liver.
Nrf2 is regarded as an important regulator of the cellular antioxidant response (Motohashi and Yamamoto, 2004). Studies have shown that with the overexpression of Nrf2, its downstream gene expression levels were significantly upregulated (Xiao et al., 2020; Xue et al., 2021). Nrf2-null affects mRNA expression of SOD1, SOD2, and CAT (Reisman et al., 2009). A previous study showed that theaflavin promotes resistance to oxidative stress-induced cell damage by activating Nrf2 (Li et al., 2021). In this research, adding PCHM to the diet dramatically increased Nrf2 mRNA levels in the liver and the uterine. The reason for improvement is related to the polysaccharide contained in PCHM (Liu et al., 2022).
In conclusion, PCHM supplementation boosted egg weight and quality in late-stage laying hens. This result may be attributable to the increase in antioxidant enzyme activity, which may be associated with increased antioxidant enzyme mRNA expression and Nrf2 expression (Figure 9). However, the underlying mechanism through which dietary supplementation of PCHM enhances the mRNA expression of antioxidant enzymes requires additional investigation.
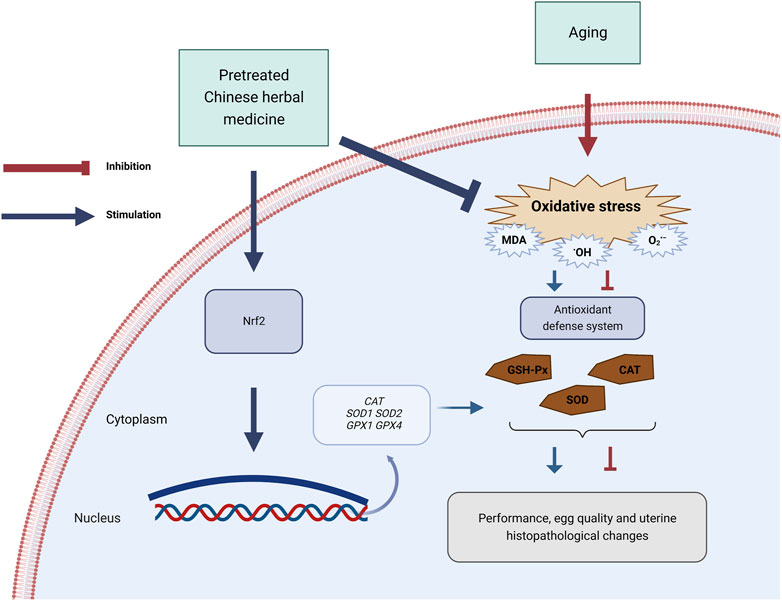
FIGURE 9. Schematic diagram summarizing the mechanisms by which pretreated Chinese herbal medicine (PCHM) promote the antioxidant defense system in late laying hens. The antioxidant defense system is downregulated in the natural aging process in laying hens. PCHM attenuated the oxidative stress in the uterus via the activation of the nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway to increase the age-related up-produce in commercial laying hens. CAT, catalase; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde; SOD1, Cu-Zn superoxide dismutase; SOD2, Mn superoxide dismutase; CAT, catalase; GPX1, glutathione peroxidase 1; GPX4, peroxidase 4. Figures generated with BioRender (https://biorender.com/).
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Beijing University of Agriculture’s Animal Care and Use Committee approved all experimental protocols. Written informed consent was obtained from the owners for the participation of their animals in this study.
A-CY: Prepared and wrote the manuscript; M-AW: Designed the study and contributed to the experiment; CL: Edited the English language and wrote the manuscript; YG, H-MN, KX, and LC: Supervised and directed the experiment; X-GW, L-FX, and X-HS: Supervised and directed molecular experiments; J-TL and X-LQ: Designed the study and reviewed the manuscript.
This study was supported by Special Fund for Beijing Innovation Team Construction of Modern Agricultural Industrial Technology System (BJJQ-G11), Technology Innovation “spark action” support program of Beijing University of Agriculture (BUA-HHXD2022008) and the Project of Tianjin Jiuzhou Dadi Feed Co., Ltd. “Oviduct Health Technical Services for Laying Hens” (20210802).
Thanks to Yifan Bao, (Department of Physical Chemistry, University of Vienna) for exporting Figure 9 from Borender.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adeniran S. O., Zheng P., Feng R., Adegoke E. O., Huang F., Ma M., et al. (2022). The antioxidant role of selenium via GPx1 and GPx4 in LPS-Induced oxidative stress in bovine endometrial cells. Biol. Trace Elem. Res. 200 (3), 1140–1155. doi:10.1007/s12011-021-02731-0
Ahn J., Chae H., Chin Y., Kim J. (2017). Alkaloids from aerial parts of Houttuynia cordata and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 27 (12), 2807–2811. doi:10.1016/j.bmcl.2017.04.072
Attia Y. A., Al-Harthi M. A., Abo El-Maaty H. M. (2020). Calcium and cholecalciferol levels in Late-Phase laying hens: Effects on productive traits, egg quality, blood biochemistry, and immune responses. Front. veterinary Sci. 7, 389. doi:10.3389/fvets.2020.00389
Bain M. M., Nys Y., Dunn I. C. (2016). Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Brit. Poult. Sci. 57 (3), 330–338. doi:10.1080/00071668.2016.1161727
Chen Y., Chen H., Li W., Miao J., Chen N., Shao X., et al. (2018). Polyphenols in Eucalyptus leaves improved the egg and meat qualities and protected against ethanol-induced oxidative damage in laying hens. J. Anim. Physiol. Anim. Nutr. Berl. 102 (1), 214–223. doi:10.1111/jpn.12680
Costa I. M., Lima F. O. V., Fernandes L. C. B., Norrara B., Neta F. I., Alves R. D., et al. (2019). Astragaloside IV supplementation promotes a neuroprotective effect in experimental models of neurological disorders: A systematic review. Curr. Neuropharmacol. 17 (7), 648–665. doi:10.2174/1570159X16666180911123341
Gongruttananun N., Kochagate P., Poonpan K., Yu-Nun N., Aungsakul J., Sopa N. (2017). Effects of an induced molt using cassava meal on body weight loss, blood physiology, ovarian regression, and postmolt egg production in late-phase laying hens. Poult. Sci. 96 (6), 1925–1933. doi:10.3382/ps/pew457
Guo H., Wu B., Cui H., Peng X., Fang J., Zuo Z., et al. (2014). NiCl2-down-regulated antioxidant enzyme mRNA expression causes oxidative damage in the broiler(')s kidney. Biol. Trace Elem. Res. 162 (1-3), 288–295. doi:10.1007/s12011-014-0132-3
Guo Y., Li Y., Zhang S., Wu X., Jiang L., Zhao Q., et al. (2020a). The effect of total flavonoids of Epimedium on granulosa cell development in laying hens. Poult. Sci. 99 (9), 4598–4606. doi:10.1016/j.psj.2020.05.032
Guo Y., Zhao Z., Pan Z., An L., Balasubramanian B., Liu W. (2020b). New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 99 (4), 2100–2107. doi:10.1016/j.psj.2019.12.032
He L., Wang X., Cheng D., Xiong Z., Liu X. (2021). Ginsenoside Rg1 improves pathological damages by activating the p21‑p53‑STK pathway in ovary and Bax‑Bcl2 in the uterus in premature ovarian insufficiency mouse models. Mol. Med. Rep. 23 (1), 37. doi:10.3892/mmr.2020.11675
Heng N., Gao S., Chen Y., Wang L., Li Z., Guo Y., et al. (2021). Dietary supplementation with natural astaxanthin from Haematococcus pluvialis improves antioxidant enzyme activity, free radical scavenging ability, and gene expression of antioxidant enzymes in laying hens. Poult. Sci. 100 (5), 101045. doi:10.1016/j.psj.2021.101045
Ibtisham F., Nawab A., Niu Y., Wang Z., Wu J., Xiao M., et al. (2019). The effect of ginger powder and Chinese herbal medicine on production performance, serum metabolites and antioxidant status of laying hens under heat-stress condition. J. Therm. Biol. 81, 20–24. doi:10.1016/j.jtherbio.2019.02.002
Jarco S., Pilawa B., Ramos P. (2021). Free radical scavenging activity of infusions of different medicinal plants for use in obstetrics. Plants (Basel) 10 (10), 2016. doi:10.3390/plants10102016
Jiang K., Guo S., Yang J., Liu J., Shaukat A., Zhao G., et al. (2019). Matrine alleviates Staphylococcus aureus lipoteichoic acid-induced endometritis via suppression of TLR2-mediated NF-κB activation. Int. Immunopharmacol. 70, 201–207. doi:10.1016/j.intimp.2019.02.033
Kong S., Zhang S., Chen Y., Wang W., Wang B., Chen Q., et al. (2012). Determinants of uterine aging: Lessons from rodent models. Sci. China Life Sci. 55 (8), 687–693. doi:10.1007/s11427-012-4356-1
Lee M. Y., Shin I. S., Jeon W. Y., Seo C. S., Ha H., Huh J. I., et al. (2012). Protective effect of Bojungikki-tang, a traditional herbal formula, against alcohol-induced gastric injury in rats. J. Ethnopharmacol. 142 (2), 346–353. doi:10.1016/j.jep.2012.04.043
Li X. L., He W. L., Yang M. L., Yan Y. M., Xue Y. H., Zhao S. T. (2017). Effect of dietary supplementation of Ligustrum lucidum on performance, egg quality and blood biochemical parameters of Hy-Line Brown hens during the late laying period. Anim. Camb. Engl. 11 (11), 1899–1904. doi:10.1017/S1751731117000532
Li Y., Elmer G., LeBoeuf R. C. (2008). Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase. Life Sci. 83 (15), 557–562. doi:10.1016/j.lfs.2008.08.003
Li Z., Zhu J., Wan Z., Li G., Chen L., Guo Y. (2021). Theaflavin ameliorates renal ischemia/reperfusion injury by activating the Nrf2 signalling pathway in vivo and in vitro. Biomed. Pharmacother. 134, 111097. doi:10.1016/j.biopha.2020.111097
Liu W., Zhuang D., Zhao Y., Balasubramanian B., Zhao Z. (2022). Seaweed-Derived polysaccharides attenuate heat Stress-Induced splenic oxidative stress and inflammatory response via regulating nrf2 and NF-κB signaling pathways. Mar. Drugs 20 (6), 358. doi:10.3390/md20060358
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25 (4), 402–408. doi:10.1006/meth.2001.1262
Ma H., He X., Yang Y., Li M., Hao D., Jia Z. (2011). The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 134 (3), 519–541. doi:10.1016/j.jep.2011.01.001
Madkour M., Aboelenin M. M., Aboelazab O., Elolimy A. A., El-Azeem N. A., El-Kholy M. S., et al. (2021). Hepatic expression responses of DNA methyltransferases, heat shock proteins, antioxidant enzymes, and NADPH 4 to early life thermal conditioning in broiler chickens. Ital. J. Anim. Sci. 20 (1), 433–446. doi:10.1080/1828051X.2021.1890645
Miyazawa M., Ishii T., Yasuda K., Noda S., Onouchi H., Hartman P. S., et al. (2009). The role of mitochondrial superoxide Anion(O2-)on physiological aging in C57BL/6J mice. J. Radiat. Res. 50 (1), 73–83. doi:10.1269/jrr.08097
Moon S. G., Lee S. K., Lee W. D., Niu K. M., Hwang W. U., Oh J. S., et al. (2021). Effect of dietary supplementation of a phytogenic blend containing Schisandra chinensis, Pinus densiflora, and Allium tuberosum on productivity, egg quality, and health parameters in laying hens. Anim. Biosci. 34 (2), 285–294. doi:10.5713/ajas.20.0552
Motohashi H., Yamamoto M. (2004). Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10 (11), 549–557. doi:10.1016/j.molmed.2004.09.003
Mulholland J., Jones C. J. (1993). Characteristics of uterine aging. Microsc. Res. Tech. 25 (2), 148–168. doi:10.1002/jemt.1070250207
Odabasi A. Z., Miles R. D., Balaban M. O., Portier K. M. (2007). Changes in Brown eggshell color as the hen ages. Poult. Sci. 86 (2), 356–363. doi:10.1093/ps/86.2.356
Ottolenghi S., Rubino F. M., Sabbatini G., Coppola S., Veronese A., Chiumello D., et al. (2019). Oxidative stress markers to investigate the effects of hyperoxia in anesthesia. Int. J. Mol. Sci. 20 (21), 5492. doi:10.3390/ijms20215492
Park J. A., Sohn S. H. (2018). The influence of hen aging on eggshell ultrastructure and shell mineral components. Korean J. Food Sci. Anim. Resour. 38 (5), 1080–1091. doi:10.5851/kosfa.2018.e41
Rashid M. U., Coombs K. M. (2019). Serum-reduced media impacts on cell viability and protein expression in human lung epithelial cells. J. Cell. Physiol. 234 (6), 7718–7724. doi:10.1002/jcp.27890
Reisman S. A., Yeager R. L., Yamamoto M., Klaassen C. D. (2009). Increased nrf2 activation in livers from Keap1-Knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol. Sci. 108 (1), 35–47. doi:10.1093/toxsci/kfn267
Shahzad M., Shabbir A., Wojcikowski K., Wohlmuth H., Gobe G. C. (2016). The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets 17 (12), 1331–1340. doi:10.2174/1389450116666150907104742
Sikiru A. B., Arangasamy A., Alemede I. C., Guvvala P. R., Egena S. S. A., Ippala J. R., et al. (2019). Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon 5 (9), e02470. doi:10.1016/j.heliyon.2019.e02470
Torun A. N., Kulaksizoglu S., Kulaksizoglu M., Pamuk B. O., Isbilen E., Tutuncu N. B. (2009). Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. Oxf. 70 (3), 469–474. doi:10.1111/j.1365-2265.2008.03348.x
Wei W. L., Zeng R., Gu C. M., Qu Y., Huang L. F. (2016). Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 190, 116–141. doi:10.1016/j.jep.2016.05.023
Wu Z., Deng X., Hu Q., Xiao X., Jiang J., Ma X., et al. (2021). Houttuynia cordata thunb: An ethnopharmacological review. Front. Pharmacol. 12, 714694. doi:10.3389/fphar.2021.714694
Xiao C., Xu C., He N., Liu Y., Wang Y., Zhang M., et al. (2020). Atractylenolide II prevents radiation damage via MAPKp38/Nrf2 signaling pathway. Biochem. Pharmacol. 177, 114007. doi:10.1016/j.bcp.2020.114007
Xiao Y. Q., Shao D., Sheng Z. W., Wang Q., Shi S. R. (2019). A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, Brown laying hens. Poult. Sci. 98 (8), 3298–3303. doi:10.3382/ps/pez178
Xie T., Bai S. P., Zhang K. Y., Ding X. M., Wang J. P., Zeng Q. F., et al. (2019). Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 98 (10), 4838–4847. doi:10.3382/ps/pez219
Xue J., Yu C., Tang Y., Mo W., Tang Z., Sheng W., et al. (2021). NF-E2-Related factor 2 (Nrf2) ameliorates radiation-induced skin injury. Front. Oncol. 11, 680058. doi:10.3389/fonc.2021.680058
Yang X., Li L., Xue Y., Zhou X., Tang J. (2020). Flavonoids from Epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-Px of Drosophila melanogaster, .PeerJ (San Francisco, CA) 8.e8361 doi:10.7717/peerj.8361
Zakaria A. H., Miyaki T., Imai K. (1983). The effect of aging on the ovarian follicular growth in laying hens. Poult. Sci. 62 (4), 670–674. doi:10.3382/ps.0620670
Zhang H., Davies K. J. A., Forman H. J. (2015). Oxidative stress response and Nrf2 signaling in aging. Free Radic. Bio. Med. 88, 314–336. doi:10.1016/j.freeradbiomed.2015.05.036
Zhao H., Zhang R., Yan X., Fan K. (2021). Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mat. Chem. B 9 (35), 6939–6957. doi:10.1039/d1tb00720c
Keywords: antioxidant capacity, egg quality, late-phase laying hens, pretreated Chinese herbal medicine, performance
Citation: Yu A-C, Wang M-A, Chen L, Long C, Guo Y, Sheng X-H, Wang X-G, Xing K, Xiao L-F, Ni H-M, Li J-T and Qi X-L (2023) Effects of dietary pretreated Chinese herbal medicine supplementation on production performance, egg quality, uterine histopathological changes, and antioxidant capacity in late-phase laying hens. Front. Physiol. 14:1110301. doi: 10.3389/fphys.2023.1110301
Received: 28 November 2022; Accepted: 11 January 2023;
Published: 20 January 2023.
Edited by:
Krystyna Pierzchala-Koziec, University of Agriculture in Krakow, PolandReviewed by:
Mahmoud Madkour, National Research Centre, EgyptCopyright © 2023 Yu, Wang, Chen, Long, Guo, Sheng, Wang, Xing, Xiao, Ni, Li and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Tao Li, bGlqdDEwMjRAdmlwLnNpbmEuY29t; Xiao-Long Qi, YnVhcXhsQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.