- 1Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, China
- 2Department of Clinical Oncology, Queen Mary Hospital, Hong Kong, China
- 3Department of Clinical Oncology, The University of Hong Kong, Hong Kong, China
- 4Department of Nuclear Medicine, Queen Mary Hospital, Hong Kong, China
Purpose: This study aimed to develop and evaluate
Methods and Materials: The study used four-dimensional CT (4DCT) and single-photon emission computed tomography (SPECT) images and corresponding lung masks from 21 patients with lung cancer obtained from the Ventilation And Medical Pulmonary Image Registration Evaluation dataset. The lung volume of the exhale CT for each patient was segmented into hundreds of super-voxels using the Simple Linear Iterative Clustering (SLIC) method. These super-voxel segments were applied to the CT and SPECT images to calculate the mean density values (Dmean) and mean ventilation values (Ventmean), respectively. The final CT-derived ventilation images were generated by interpolation from the Dmean values to yield
Results: The correlation between the Dmean and Ventmean of the super-voxel was 0.59 ± 0.09, representing a moderate-to-high correlation at the super-voxel level. In the voxel-wise evaluation, the
Conclusion: The strong correlation between
1 Background
Lung cancer is the most common cause of cancer-related death in both men and women (Wild et al., 2020). Radiotherapy (RT) is an important treatment modality for lung cancer, especially in patients in whom surgical resection is contraindicated or those with mid- or late-stage lung cancers (Gadgeel et al., 2012). The functional lung volume that can be irradiated in such patients is limited, as irradiation of functioning tissue can lead to radiation pneumonitis (RP) and respiratory failure. Currently, the percentage of the lung volume receiving at least 20 Gy (V20) and the mean lung dose (MLD) are used to predict the risk of pulmonary injury (Lee et al., 2003) or the maximum acceptable dose to deliver to a lesion (Baisden et al., 2007). However, these parameters are evaluated across the whole lung volume and do not account for functional differences between lung regions. Recently, regional lung functionality assessment has been shown to enable highly functional lung areas to be spared from irradiation and thus can be used to design treatment plans that reduce the risk of injury (Hoover et al., 2014; Bucknell et al., 2018; Lee and Park, 2020; Vinogradskiy et al., 2022).
Lung ventilation images can provide regional functional information. Clinical-standard lung ventilation imaging techniques require radioactive gases or aerosols; for example, single-photon emission computed tomography (SPECT) uses Technetium-99 m (Tc-99 m) (Suga et al., 2004) and positron emission tomography (PET) uses Gallium-68 (Ga-68) (Ament et al., 2013). However, not all hospitals can perform PET or SPECT scans, and the radiopharmaceuticals used for imaging expose patients to additional radiation doses. Hyperpolarized noble gas magnetic resonance imaging (MRI) ventilation (Cai et al., 2007; Cai et al., 2009; Tustison et al., 2010; Roos et al., 2015) is another non-invasive imaging technique used to generate ionizing radiation-free ventilation images for lung function assessment. However, MRI ventilation requires a tracer gas and specialized equipment, which may limit the availability of this modality in clinical practice. CT-derived ventilation imaging (CTVI) is another method of generating ventilation images. Moreover, as CT scans of patients undergoing RT are routinely performed, CTVI methods could potentially help patients avoid unnecessary radiation doses and medical costs.
Current CTVI methods are mainly based on volume changes (Jacobian-based,
Current DIR-based CTVI methods are sensitive to both CT image quality and DIR algorithms, so the images they generate have a limited correlation with the gold-standard ventilation images generated using SPECT and PET (Vinogradskiy, 2019). Consequently, the results of CTVI are complicated and difficult to interpret, meaning they may be unsuitable for clinical application. The super-pixel concept was first proposed and developed as an image segmentation technology in 2003 (Ren and Malik, 2003). It uses pixel blocks that form specific patterns with adjacent pixels that have a similar texture, color, and other features. Images can be represented by a small number of super-pixels, which significantly reduces the complexity of image post-processing. A similar concept, the super-voxel, is used for three-dimensional (3D) image analysis. An air exchange unit is evaluated using a volume of approximately 2 cm3 (Levin et al., 2017) that contains a cluster of CT voxels with a resolution of approximately 1 mm × 1 mm × 3 mm. The CT image of a patient with lung cancer can be pre-processed by segmentation into a small number of super-voxels, where each super-voxel contains a cluster of voxels with similar features and forms perceptually meaningful anatomic features. Drawing on this principle, the current study devised a super-voxel-based method for generating robust lung ventilation images from the mean CT density value (Dmean) of super-voxels. The ventilation images generated are based on CT image features in the absence of DIR. The results are robust and expected to be directly interpretable and meaningful for predicting the outcomes of patients with lung cancer.
2 Materials and methods
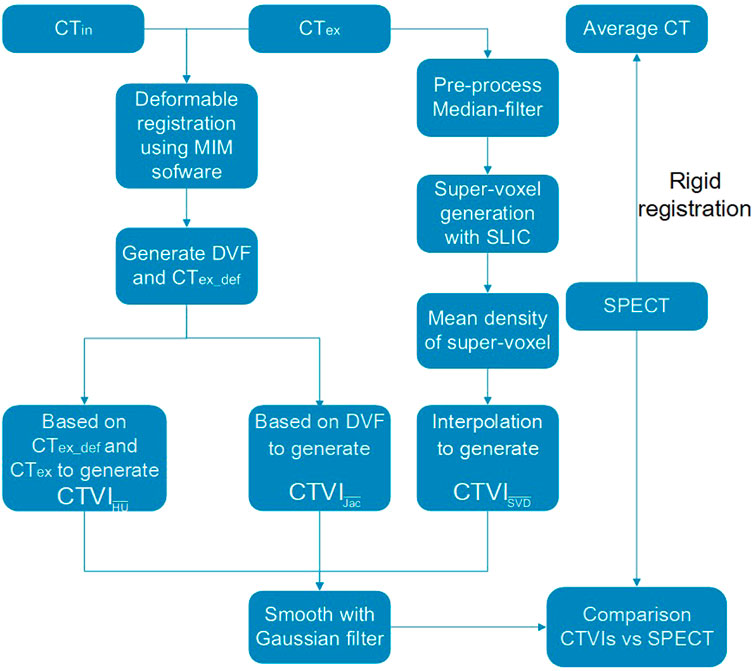
2.1 Workflow of the study
Figure 1 shows the main workflow of this study. The CTex and CTin were used to calculate the ventilation images. A clustering method was used to generate super-voxels, and the Dmean of each super-voxel was used to calculate the ventilation images
2.2 Image data
The data of 21 patients with lung cancer were acquired from the Ventilation And Medical Pulmonary Image Registration Evaluation (VAMPIRE) dataset (Kipritidis et al., 2019). All of the patients underwent 4DCT and diethylenetriamine pentaacetate (DTPA)-SPECT scans at Stanford University, United States (Yamamoto et al., 2014). All of the patients provided written informed consent to participate in a clinical trial of 4DCT ventilation imaging approved by the institutional review board for a study by Yamamoto (Yamamoto et al., 2014). Ten breathing phase CT images and a time-average CT with a slice thickness of 2.0, 2.5, or 3.0 mm were available for each patient. The average interval between the 4DCT and subsequent DTPA-SPECT (including low-dose attenuation correction CT) scans was 4 (±5) days. Rigid registration was performed between each SPECT image and the time-average CT image using Mattes mutual information rigid registration in Plastimatch. The DTPA-SPECT scans were linearly interpolated to match the dimensions of the time-average CT image (Kipritidis et al., 2019). The lung masks for all of the CT images (4DCT and attenuation correction CT) were also acquired from the VAMPIRE dataset, which used a region-growing method. The lung masks of the attenuation correction CT images were also used as the masks of the SPECT images. The CT values were converted to density values using Eq. 1, as follows:
2.3 DIR-based CTVI methods
The two main conventional DIR-based methods are
In
Both
2.4 Super-voxel segmentation
Simple linear iterative clustering (SLIC) (Achanta et al., 2012) is a clustering method applied to lung CT 3D images to generate super-voxels with low computational power requirements. The SLIC algorithm first initializes the
where
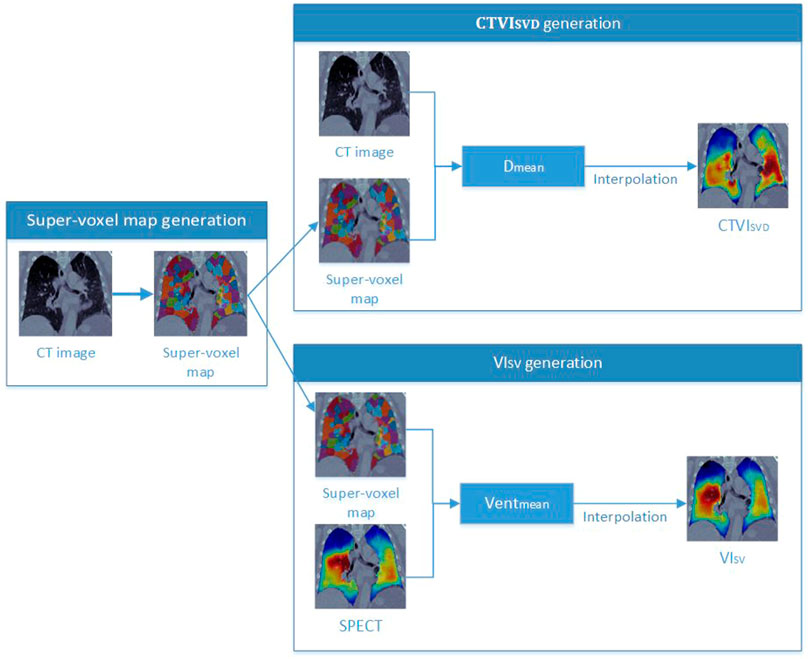
2.5 Super-voxel-based ventilation image
As shown in Figure 2, a super-voxel map was generated on CTex images (as described in Section 2.4), and the Dmean of each super-voxel was calculated. Other studies have used fixed threshold intervals of −1,024 to −400 HU to generate the lung parenchyma (Kemerink et al., 1998; Kuhnigk et al., 2005). In the current study, the same fixed threshold interval was applied to identify the non-lung region; a super-voxel with a Dmean greater than 0.6 according to Eq. 1 was assigned a value of 0 to remove clearly false results from consolidation of the tumor and abnormal tissues, which have a high density but should have a low ventilation value. The super-voxel segmentation results were then directly mapped on the SPECT images, as both the SPECT and time-average CT data were registered according to the VAMPIRE challenge, and the time-average CT and CTex images shared the same position. The mean ventilation value (Ventmean) was calculated using SPECT image data. The correlation between the Dmean and Ventmean of the super-voxels was determined using Spearman’s correlation analysis.
Figure 2 shows the workflow for generating the
where
2.6 Comparison of
The
2.7 Impact of the super-voxel number on
The size of the super-voxels may influence the results of
3 Results
3.1 Super-voxel segmentation
The SLIC method was used to divide the lung volumes of the 21 patients into 380–715 super-voxels at a
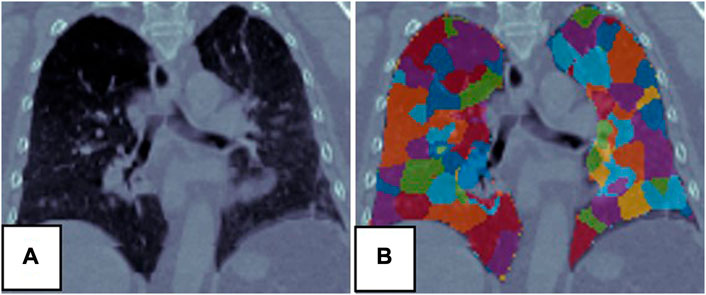
FIGURE 3. Super-voxel segmentation in the lungs of a patient. (A) Is the CT, (B) is the result of the super-voxel segmentation in the lung region.
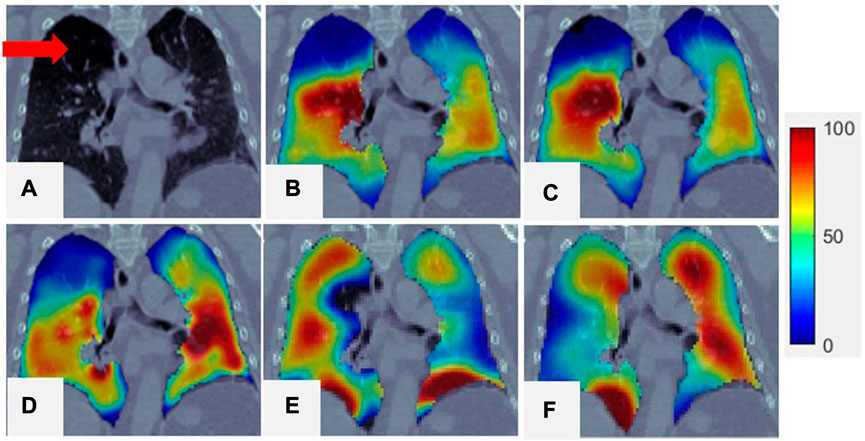
FIGURE 4. Comparison of SPECT image and
3.2 Comparison of
The correlation between the Dmean from CT and the Ventmean for the super-voxel volume from SPECT was 0.59 ± 0.09, indicating that super-voxels with a lower mean density tend to have a lower function value than super-voxels with a higher mean density. This moderate-to-strong correlation means that the Dmean of a super-voxel can be used as a surrogate for Ventmean when generating
The mean DSC values of the high-functioning (
For some patients,
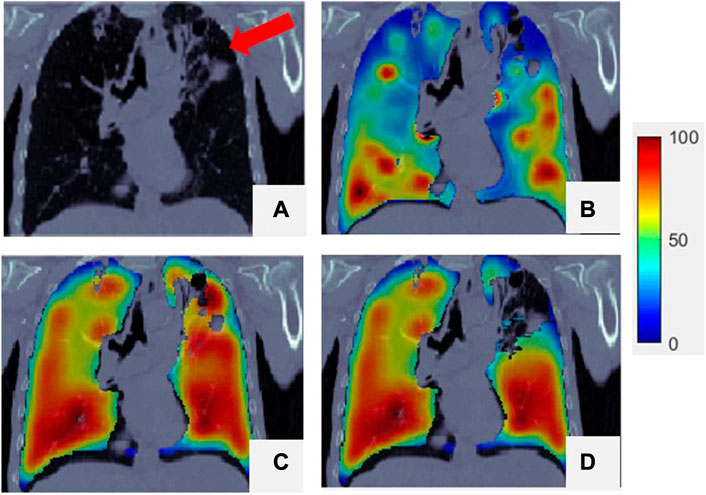
FIGURE 5. Comparison of SPECT image and
3.3 Evaluation of the impact of the super-voxel number on
Figure 6 shows super-voxel segmentation using two values of
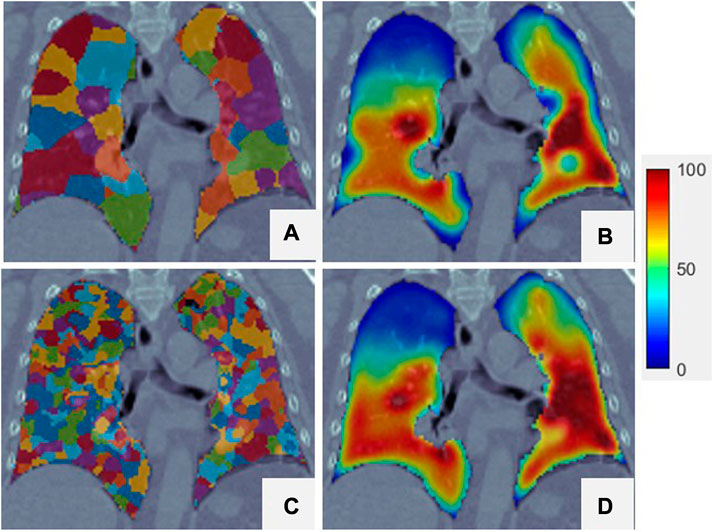
FIGURE 6. Two different super-voxel segmentations with different
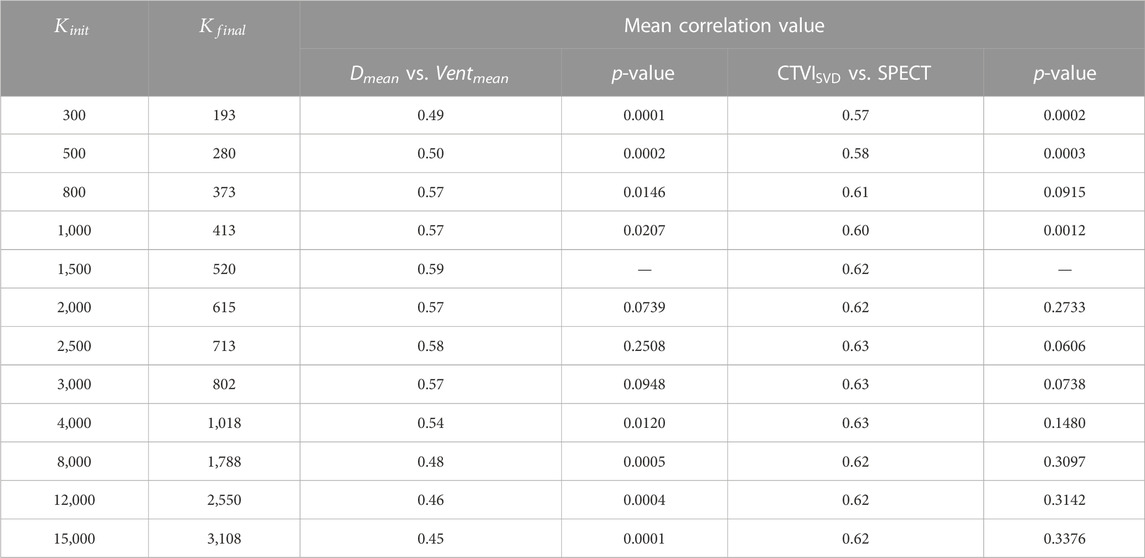
TABLE 1. The influence of the different numbers of the super-voxel.
4 Discussion
In this study, a super-voxel-based method was developed to generate surrogate ventilation images directly from CT images. The SLIC method was employed to generate super-voxels inside the lung volume, and the Dmean of the super-voxels was used as a surrogate for the mean ventilation value to calculate a whole-lung ventilation image through interpolation. This novel
This study shows that the Dmean of a super-voxel is strongly correlated with the Ventmean of a super-voxel, which means that a lower super-voxel density is usually associated with less functional ventilation than a higher super-voxel density. Similar results have been shown in other studies (Lafata et al., 2019; Yang et al., 2021). As shown in Figure 4, the region with low ventilation function (indicated by arrows) is darker than the region with normal function. The low-functioning region may correspond to a defective lung region caused by emphysema, where healthy pulmonary tissue has been replaced increasingly by air due to alveolar damage and weakening and rupture of the inner walls of the air sacs. This was a preliminary study of the use of the Dmean of super-voxels to generate ventilation images, and only 21 patients were included. Other super-voxel features can be analyzed and combined with Dmean to build a more accurate and robust model for future CTVI studies involving more patient data.
According to Eq. 4, the total super-voxel number and compactness value affect segmentation of the super-voxels. The SLIC algorithm used in this study can refine the compactness value adaptively to reduce the influence of this variable without requiring pre-assignment. The only variable required for SLIC is the total number of super-voxels. The correlation between Dmean and Ventmean is strongest when approximately 520 super-voxels are extracted from the lung volume and decreases as the number of super-voxels increases. Meanwhile, as the number of super-voxel increases, the mean correlation between
This study has some limitations. Pulmonary ventilation refers to the air exchange between the atmosphere and the lungs. It involves the inflow of air through the airway to the alveoli, where the air exchange occurs, followed by outflow through the airway. Our results show that lung regions with lower density values exhibit lower ventilation values than those with higher density values. As previously mentioned, the damaged alveoli in a patient with emphysema lost their ability to expel air, leading to decreased intensity. However, in some cases, abnormal lung regions associated with pulmonary diseases can exhibit increased density, known as opacities, and fall into four patterns: consolidation, interstitial, nodules or masses, and atelectasis (L ung-disease, 2023). These diseases can also obstruct the airway or damage to the parenchyma, leading to a loss of air exchange capability. Consequently, some pulmonary diseases may affect the CTVI results in this study. However, the clinical presentation of pulmonary diseases on CT images can vary. Raju et al. categorized the signs of the lung disease into 22 groups (Raju et al., 2017). These signs can increase the difficulty of automatically recognizing defect regions. In this study, the super-voxel was the smallest unit of analysis and its features can be used directly to classify it as a defect or normal region. In future work, we will create a super-voxel-based model to automatically identify defect regions and correct the ventilation value to increase the accuracy of our method.
Moreover, some regions may have a low ventilation value due to pressure placed by the tumor on the central airway and blood vessels; this pressure can be recovered after radiotherapy (Yuan et al., 2012). Such regions need to be carefully protected during the treatment, and the dose should be as low as possible as normal lung regions. In cases with such regions, the patient’s dyspnea may be reduced and the lung function may increase if the tumor shrinks after treatment. From this perspective, CTVI can provide more information than SPECT. More investigation is needed to identify these regions and thus guide treatment planning.
5 Conclusion
In this study, we developed a super-voxel-based method to generate surrogate ventilation images from CT data. The observed correlation between
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Departmental Research Committee Department of Health Technology and Informatics. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZC: data analysis, methodology, writing—original draft. Y-HH: data acquisition and analysis, writing—editing. F-MK and WH: result checking, writing—review. GR and JC: supervision, writing—review and editing.
Funding
This work was partly supported by the General Research Fund (GRF 15103520) from The University Grants Committee, and Health and Medical Research Fund (HMRF 07183266, HMRF 09200576) from The Health Bureau, The Government of the Hong Kong Special Administrative Regions.
Acknowledgments
We would like to acknowledge the data provided by the VAMPIRE challenge.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achanta, R., Shaji, A., Smith, K., Lucchi, A., Fua, P., and Süsstrunk, S. (2012). SLIC superpixels compared to state-of-the-art superpixel methods. IEEE Trans. pattern analysis Mach. Intell. 34 (11), 2274–2282. doi:10.1109/TPAMI.2012.120
Ament, S. J., Maus, S., Reber, H., Buchholz, H. G., Bausbacher, N., Brochhausen, C., et al. (2013). PET lung ventilation/perfusion imaging using 68 Ga aerosol (Galligas) and 68 Ga-labeled macroaggregated albumin. Recent Results Cancer Res. 2013, 395–423. doi:10.1007/978-3-642-27994-2_22
Baisden, J. M., Romney, D. A., Reish, A. G., Cai, J., Sheng, K., Jones, D. R., et al. (2007). Dose as a function of lung volume and planned treatment volume in helical tomotherapy intensity-modulated radiation therapy-based stereotactic body radiation therapy for small lung tumors. Int. J. Radiat. Oncology* Biology* Phys. 68 (4), 1229–1237. doi:10.1016/j.ijrobp.2007.03.024
Bucknell, N. W., Hardcastle, N., Bressel, M., Hofman, M. S., Kron, T., Ball, D., et al. (2018). Functional lung imaging in radiation therapy for lung cancer: A systematic review and meta-analysis. Radiotherapy Oncol. 129 (2), 196–208. doi:10.1016/j.radonc.2018.07.014
Cai, J., Altes, T. A., Miller, G. W., Sheng, K., Read, P. W., Mata, J. F., et al. (2007). MR grid-tagging using hyperpolarized helium-3 for regional quantitative assessment of pulmonary biomechanics and ventilation. Magnetic Reson. Med. 58 (2), 373–380. doi:10.1002/mrm.21288
Cai, J., Sheng, K., Benedict, S. H., Read, P. W., Larner, J. M., Mugler, J. P., et al. (2009). Dynamic MRI of grid-tagged hyperpolarized helium-3 for the assessment of lung motion during breathing. Int. J. Radiat. Oncology* Biology* Phys. 75 (1), 276–284. doi:10.1016/j.ijrobp.2009.03.051
Castillo, E., Castillo, R., Vinogradskiy, Y., Nair, G., Grills, I., Guerrero, T., et al. (2020). Technical Note: On the spatial correlation between robust CT-ventilation methods and SPECT ventilation. Med. Phys. 47 (11), 5731–5738. doi:10.1002/mp.14511
Gadgeel, S. M., Ramalingam, S. S., and Kalemkerian, G. P. (2012). Treatment of lung cancer. Radiol. Clin. 50 (5), 961–974. doi:10.1016/j.rcl.2012.06.003
Hoover, D. A., Reid, R. H., Wong, E., Stitt, L., Sabondjian, E., Rodrigues, G. B., et al. (2014). SPECT-Based functional lung imaging for the prediction of radiation pneumonitis: A clinical and dosimetric correlation. J. Med. Imaging Radiat. Oncol. 58 (2), 214–222. doi:10.1111/1754-9485.12145
Kemerink, G. J., Lamers, R. J., Pellis, B. J., Kruize, H. H., and van Engelshoven, J. M. (1998). On segmentation of lung parenchyma in quantitative computed tomography of the lung. Med. Phys. 25 (12), 2432–2439. doi:10.1118/1.598454
Kipritidis, J., Hofman, M. S., Siva, S., Callahan, J., Le Roux, P. Y., Woodruff, H. C., et al. (2016). Estimating lung ventilation directly from 4D CT Hounsfield unit values. Med. Phys. 43 (1), 33–43. doi:10.1118/1.4937599
Kipritidis, J., Tahir, B. A., Cazoulat, G., Hofman, M. S., Siva, S., Callahan, J., et al. (2019). The VAMPIRE challenge: A multi-institutional validation study of CT ventilation imaging. Med. Phys. 46 (3), 1198–1217. doi:10.1002/mp.13346
Kuhnigk, J.-M., Dicken, V., Zidowitz, S., Bornemann, L., Kuemmerlen, B., Krass, S., et al. (2005). Informatics in radiology (infoRAD): New tools for computer assistance in thoracic CT. Part 1. Functional analysis of lungs, lung lobes, and bronchopulmonary segments. Radiographics 25 (2), 525–536. doi:10.1148/rg.252045070
Lung-disease (2023). Lung diseases four-pattern approach. Available from: https://radiologyassistant.nl/chest/chest-x-ray/lung-disease.
Lafata, K. J., Zhou, Z., Liu, J. G., Hong, J., and Kelsey, C. R. (2019). An exploratory radiomics approach to quantifying pulmonary function in CT images. Sci. Rep. 9 (1), 11509–9. doi:10.1038/s41598-019-48023-5
Lee, H. K., Vaporciyan, A. A., Cox, J. D., Tucker, S. L., Putnam, J. B., Ajani, J. A., et al. (2003). Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: Correlation with pulmonary dose-volume histogram parameters. Int. J. Radiat. Oncol. Biol. Phys. 57 (5), 1317–1322. doi:10.1016/s0360-3016(03)01373-7
Lee, S. J., and Park, H. J. (2020). Single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging for radiotherapy planning in patients with lung cancer: A meta-analysis. Sci. Rep. 10 (1), 14864–14911. doi:10.1038/s41598-020-71445-5
Levin, D. L., Schiebler, M. L., and Hopkins, S. R. (2017). Physiology for the pulmonary functional imager. Eur. J. radiology 86, 308–312. doi:10.1016/j.ejrad.2016.09.027
Liu, Z., Miao, J., Huang, P., Wang, W., Wang, X., Zhai, Y., et al. (2020). A deep learning method for producing ventilation images from 4DCT: First comparison with technegas SPECT ventilation. Med. Phys. 47 (3), 1249–1257. doi:10.1002/mp.14004
Raju, S., Ghosh, S., and Mehta, A. C. (2017). Chest CT signs in pulmonary disease: A pictorial review. Chest 151 (6), 1356–1374. doi:10.1016/j.chest.2016.12.033
Reinhardt, J. M., Ding, K., Cao, K., Christensen, G. E., Hoffman, E. A., and Bodas, S. V. (2008). Registration-based estimates of local lung tissue expansion compared to xenon CT measures of specific ventilation. Med. image Anal. 12 (6), 752–763. doi:10.1016/j.media.2008.03.007
Ren, G., Lam, S. K., Zhang, J., Xiao, H., Cheung, A. L. Y., Ho, W. Y., et al. (2021). Investigation of a novel deep Learning-Based computed tomography perfusion mapping framework for functional lung avoidance radiotherapy. Front. Oncol. 11, 644703. doi:10.3389/fonc.2021.644703
Ren, G., Zhang, J., Li, T., Xiao, H., Cheung, L. Y., Ho, W. Y., et al. (2021). Deep learning-based computed tomography perfusion mapping (DL-CTPM) for pulmonary CT-to-perfusion translation. Int. J. Radiat. Oncology* Biology* Phys. 110 (5), 1508–1518. doi:10.1016/j.ijrobp.2021.02.032
Ren, X., and Malik, J. (2003). “Learning a classification model for segmentation,” in IEEE International Conference on Computer Vision, Nice, France, 13-16 October 2003.
Roos, J. E., McAdams, H. P., Kaushik, S. S., and Driehuys, B. (2015). Hyperpolarized gas MR imaging: Technique and applications. Magn. Reson. Imaging Clin. 23 (2), 217–229. doi:10.1016/j.mric.2015.01.003
Simon, B. A. (2000). Non-invasive imaging of regional lung function using x-ray computed tomography. J. Clin. Monit. Comput. 16 (5), 433–442. doi:10.1023/a:1011444826908
Suga, K., Kawakami, Y., Zaki, M., Yamashita, T., Shimizu, K., and Matsunaga, N. (2004). Clinical utility of co-registered respiratory-gated 99mTc-Technegas/MAA SPECT-CT images in the assessment of regional lung functional impairment in patients with lung cancer. Eur. J. Nucl. Med. Mol. imaging 31 (9), 1280–1290. doi:10.1007/s00259-004-1558-1
Szmul, A., Matin, T., Gleeson, F. V., Schnabel, J. A., Grau, V., and Papież, B. W. (2019). Patch-based lung ventilation estimation using multi-layer supervoxels. Comput. Med. Imaging Graph. 74, 49–60. doi:10.1016/j.compmedimag.2019.04.002
Tustison, N. J., Awate, S. P., Cai, J., Altes, T. A., Miller, G. W., de Lange, E. E., et al. (2010). Pulmonary kinematics from tagged hyperpolarized helium-3 MRI. J. Magnetic Reson. Imaging 31 (5), 1236–1241. doi:10.1002/jmri.22137
Vinogradskiy, Y., Castillo, R., Castillo, E., Schubert, L., Jones, B. L., Faught, A., et al. (2022). Results of a multi-institutional phase 2 clinical trial for 4DCT-ventilation functional avoidance thoracic radiation therapy. Int. J. Radiat. Oncology* Biology* Phys. 112 (4), 986–995. doi:10.1016/j.ijrobp.2021.10.147
Vinogradskiy, Y. (2019). CT-based ventilation imaging in radiation oncology. BJR| Open 1, 20180035. doi:10.1259/bjro.20180035
Wild, C., Weiderpass, E., and Stewart, B. W. (2020). World cancer report: Cancer research for cancer prevention. France: IARC Press.
Yamamoto, T., Kabus, S., Lorenz, C., Mittra, E., Hong, J. C., Chung, M., et al. (2014). Pulmonary ventilation imaging based on 4-dimensional computed tomography: Comparison with pulmonary function tests and SPECT ventilation images. Int. J. Radiat. Oncology* Biology* Phys. 90 (2), 414–422. doi:10.1016/j.ijrobp.2014.06.006
Yamamoto, T., Kabus, S., von Berg, J., Lorenz, C., and Keall, P. J. (2011). Impact of four-dimensional computed tomography pulmonary ventilation imaging-based functional avoidance for lung cancer radiotherapy. Int. J. Radiat. Oncology* Biology* Phys. 79 (1), 279–288. doi:10.1016/j.ijrobp.2010.02.008
Yang, Z., Lafata, K. J., Chen, X., Bowsher, J., Chang, Y., Wang, C., et al. (2021). Quantification of lung function on CT images based on pulmonary radiomic filtering. Med. Phys. 49, 7278. doi:10.1002/mp.15837
Yuan, S. T., Frey, K. A., Gross, M. D., Hayman, J. A., Arenberg, D., Cai, X. W., et al. (2012). Changes in global function and regional ventilation and perfusion on SPECT during the course of radiotherapy in patients with non-small-cell lung cancer. Int. J. Radiat. Oncology* Biology* Phys. 82 (4), e631–e638. doi:10.1016/j.ijrobp.2011.07.044
Keywords: ventilation, 4DCT, super-voxel, radiotherapy, lung cancer
Citation: Chen Z, Huang Y-H, Kong F-M, Ho WY, Ren G and Cai J (2023) A super-voxel-based method for generating surrogate lung ventilation images from CT. Front. Physiol. 14:1085158. doi: 10.3389/fphys.2023.1085158
Received: 31 October 2022; Accepted: 06 April 2023;
Published: 26 April 2023.
Edited by:
Sam Bayat, Université Grenoble Alpes, FranceReviewed by:
Yi Xin, University of Pennsylvania, United StatesMaciej Orkisz, Université Claude Bernard Lyon 1, France
Copyright © 2023 Chen, Huang, Kong, Ho, Ren and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Ren, Z2FyeS1nZS5yZW5AcG9seXUuZWR1Lmhr; Jing Cai, amluZy5jYWlAcG9seXUuZWR1Lmhr
 Zhi Chen
Zhi Chen Yu-Hua Huang
Yu-Hua Huang Feng-Ming Kong
Feng-Ming Kong Wai Yin Ho4
Wai Yin Ho4 Ge Ren
Ge Ren Jing Cai
Jing Cai