- 1Department of Neurology, People’s Hospital of Deyang City, Deyang, China
- 2Department of Neurology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 3Department of Neurology, The Suining Central Hospital, Suining, China
Objectives: We aimed to explore sex-specific association between genes involved in inflammation and endothelial function and vulnerable carotid plaque, a subclinical precursor of ischemic stroke.
Methods: Carotid plaque and plaque phenotype were assessed by carotid ultrasound in high-risk participants for stroke drawn from a multicenter, cross-sectional survey in southwestern China. We examined 18 single nucleotide polymorphisms (SNPs) in 10 genes related to inflammation and endothelial function. Sex differences in the genotype of the candidate SNPs and risk of vulnerable carotid plaques were assessed. Interaction tests were performed to identify the SNPs that might modify the association between the sex and vulnerable plaques. For SNPs with suggestive evidence for interaction with sex (p for interaction<0.05), stratification analysis by sex was performed to evaluate the sex-specific association between the SNP and vulnerable plaques.
Results: 2,644 high-risk individuals were enrolled, comprising 1,202 (45.5%) men and 1,442 (54.5%) women. Vulnerable carotid plaques were detected in 425 (16.1%) participants. Among candidate SNPs, the genotype frequencies of 5 SNPs (TNFSF4 rs11811788, TNFSF4 rs1234313, IL6R rs4845625, VCAM1 rs2392221, and ITGA2 rs1991013) were significantly different between sex (all p < 0.05). Univariable and multivariable analyses suggested that male individuals had a significantly higher prevalence of vulnerable carotid plaques (20.0% vs. 12.8%, adjusted OR 1.72, 95% CI 1.12–2.66, p = 0.014), while none of the candidate SNPs was significantly associated with vulnerable plaques (all p > 0.05). Interaction tests found the association between sex and vulnerable plaques is affected by the genotype of IL6R rs4845625 (p for interaction = 0.031). Stratification analysis revealed a strong association between IL6R rs4845625 and vulnerable carotid plaque in man (dominant model TT vs. CT + CC: adjusted OR 1.52, 95% CI 1.12–2.07, p = 0.007; codominant model TT vs. CC: adjusted OR 1.50, 95% CI 1.00–2.25, p = 0.048) but not in women (p > 0.05 in all genetic models).
Conclusion: The rs4845625 polymorphism in IL6R has sex-specific effects on vulnerable carotid plaque in Chinese Han high-risk individuals for stroke. Our findings provide a plausible genetic basis underlying the sex difference in carotid plaque vulnerability.
Introduction
With rapidly aging population and an ongoing high prevalence of risk factors, the burden of stroke is expected to increase significantly worldwide (GBD 2016 Causes of Death Collaborators, 2017). Atherosclerosis is responsible for at least 20% of ischemic strokes, as a result of both cerebral embolism/thrombosis from an atherothrombotic plaque rupture and luminal stenosis (Petty et al., 1999; Hollander et al., 2002; Prasad, 2015). Atherosclerosis in the carotid artery can lead to plaque vulnerability, which is an important subclinical precursor of ischemic stroke and other vascular diseases (Rundek et al., 2008; Puig et al., 2020). Atherosclerosis is a diffuse, chronic inflammatory disease (Mangge and Almer, 2019; Wolf and Ley, 2019). Several different mechanisms play important roles in the pathogenesis of atherosclerosis, including endothelial injury, recruitment and activation of immune inflammatory cells, lipid accumulation, extensive degradation of extracellular matrix components, and smooth muscle cell proliferation (Berliner et al., 1995; Mangge and Almer, 2019; Wolf and Ley, 2019). Meanwhile, genetic factors play an important role in the determination of subclinical carotid atherosclerosis (Moskau et al., 2005; Zhao et al., 2008; Sacco et al., 2009). It has been reported that only 19.5% of the carotid plaque burden could be explained by the contribution of traditional vascular risk factors (Kuo et al., 2012). Therefore, genetic variants involved in endothelial function and inflammation may affect carotid atherogenesis and plaque vulnerability.
Although the incidence of ischemic stroke in young women is higher than men (especially for young adults ≤ 35 years), and several risk factors such as heart disease, heavy alcohol consumption, previous venous thromboembolism, diabetes mellitus, hypertension, migraine and use of combined oral contraceptives have been identified to contributing to these (Nightingale and Farmer, 2004; Leppert et al., 2022), the total ischemic stroke incidence rates are higher in man than in women according to the most up-to-date statistics (Tsao et al., 2022). Sex differences have also been recognized in the risk of carotid atherosclerotic plaque (Iemolo et al., 2004). Observational studies conducted in patients with moderate/severe carotid stenosis, or undergoing carotid endarterectomy reported that men had more high-risk vulnerable plaques compared with women, after controlling for cardiovascular risk factors (Hellings et al., 2007; Ota et al., 2010; Vrijenhoek et al., 2013) Although traditional cardiovascular risk factors such as age, hypertension, diabetes, and current smoking are associated with the prevalence of carotid plaque (Sturlaugsdottir et al., 2016; Bian et al., 2018; Santos-Neto et al., 2021), the variation in traditional cardiovascular risk factors between the sex could not fully explain these differences (Silander et al., 2008). Our previous study conducted in high-risk individuals for stroke also demonstrated that male individuals had a higher risk of vulnerable carotid plaque independent of classical vascular risk factors, suggesting sex-dependent genetic risk factors may play an important role in the progression of atherosclerosis (Li et al., 2021). Numerous studies have explored associations between polymorphisms in inflammation and endothelial function relevant genes and carotid atherosclerosis with few exploring the sex-specific genetic effects (Gardener et al., 2011a; Wang et al., 2011; Yi et al., 2020).
Therefore, in this study, we aimed to explore sex-dependent associations between genes involved in inflammation and endothelial function and vulnerable carotid plaque, a subclinical precursor of ischemic stroke.
Materials and methods
Study population
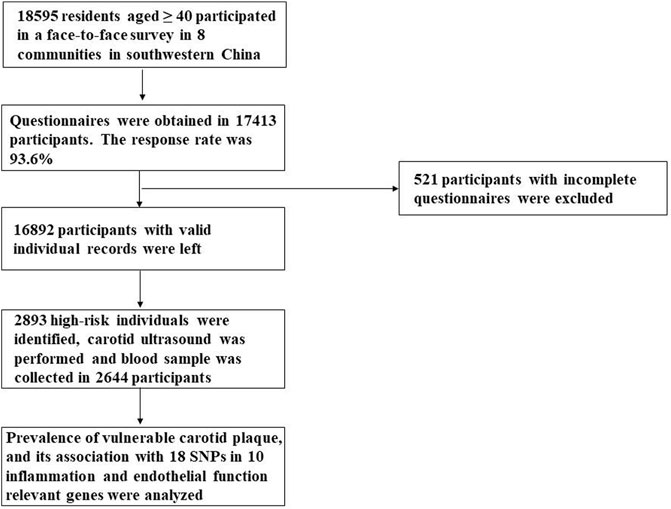
18,595 residents aged ≥40 participated in a face-to-face survey in eight communities in Sichuan province in the year 2015. This multicenter, cross-sectional survey was a branch of the China National Stroke Screening Survey (CNSSS) program of the National Health and Family Planning Commission of China (grant No. 2011BAI08B01) (Li et al., 2015; Stroke Prevention Project Committee, 2022), which have been elaborated in our previous studies (Yi et al., 2020; Li et al., 2021). The eight stroke-related risk factors were evaluated, including hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, current smoking, physical inactivity, overweight/obesity and a family history of stroke, which has been described in our previous study (Yi et al., 2020; Li et al., 2021). The participants with at least three of the eight aforementioned risk factors or a history of stroke were identified as high-risk individuals for stroke (Wang et al., 2017; Stroke Prevention Project Committee, 2022). 2,644 high-risk participants for stroke who had a carotid ultrasound performed and a blood sample collected were enrolled in the present study. A flow diagram of the data preparing and cleaning process in our study is provided in Figure 1. Study protocol was approved by the Ethics Committee of the People’s Hospital of Deyang City (Reference No. 2015-024). Informed consent was obtained from each participant during recruitment.
Carotid ultrasonography measurements
Diagnostic ultrasound was performed in 2,644 high-risk participants to assessed bilateral common and internal carotid arteries, as well as bifurcations according to a standard scanning and reading protocol (Rundek et al., 2008). Detailed procedures for evaluating the characteristics of carotid plaque have been described in our previous study (Yi et al., 2020; Li et al., 2021). Atherosclerotic plaque was defined as an endoluminal protrusion of at least 1.5 mm or a focal wall thickening>50% than the surrounding vessel wall (Rundek et al., 2008). Based on the plaque echogenicity and surface characteristics, a carotid plaque was further graded from class I to class IV as echolucent, predominantly echolucent, predominantly echogenic, and echogenic, respectively (Mathiesen et al., 2001). Carotid plaque characteristics were evaluated independently by one sonologist blinded to clinical information of participants. Plaque of class I or class II was defined as vulnerable plaque, and plaque of class III or class IV was defined as stable plaque (Yi et al., 2020). According to the results of carotid ultrasound, the enrolled participants were divided into two groups: vulnerable plaque group (with at least one vulnerable plaque) or non-vulnerable plaque group (without carotid plaque or with stable plaque).
Gene and single nucleotide polymorphism selection
Based on a literature review (Gardener et al., 2011a; Yi et al., 2020), we selected 10 inflammation and endothelial function relevant gene that have been implicated in atherosclerosis from the NCBI database (http://www.ncbi.nlm.nih.gov/SNP). These genes included tumor necrosis factor superfamily member 4 (TNFSF4), interleukin-6 receptor (IL6R), interleukin-1α (IL1A), Toll-like receptor 4 (TLR4), tumor necrosis factor (TNF), nitric oxide synthase 2A (NOS2A), peroxisome proliferator-activated receptor-α (PPARA), vascular cell adhesion molecule-1 (VCAM-1), integrin-α2 (ITGA2), hyaluronic acid binding protein 2 (HABP2). 18 tagging or functional SNPs in these genes were evaluated.
DNA extraction and single nucleotide polymorphism genotyping
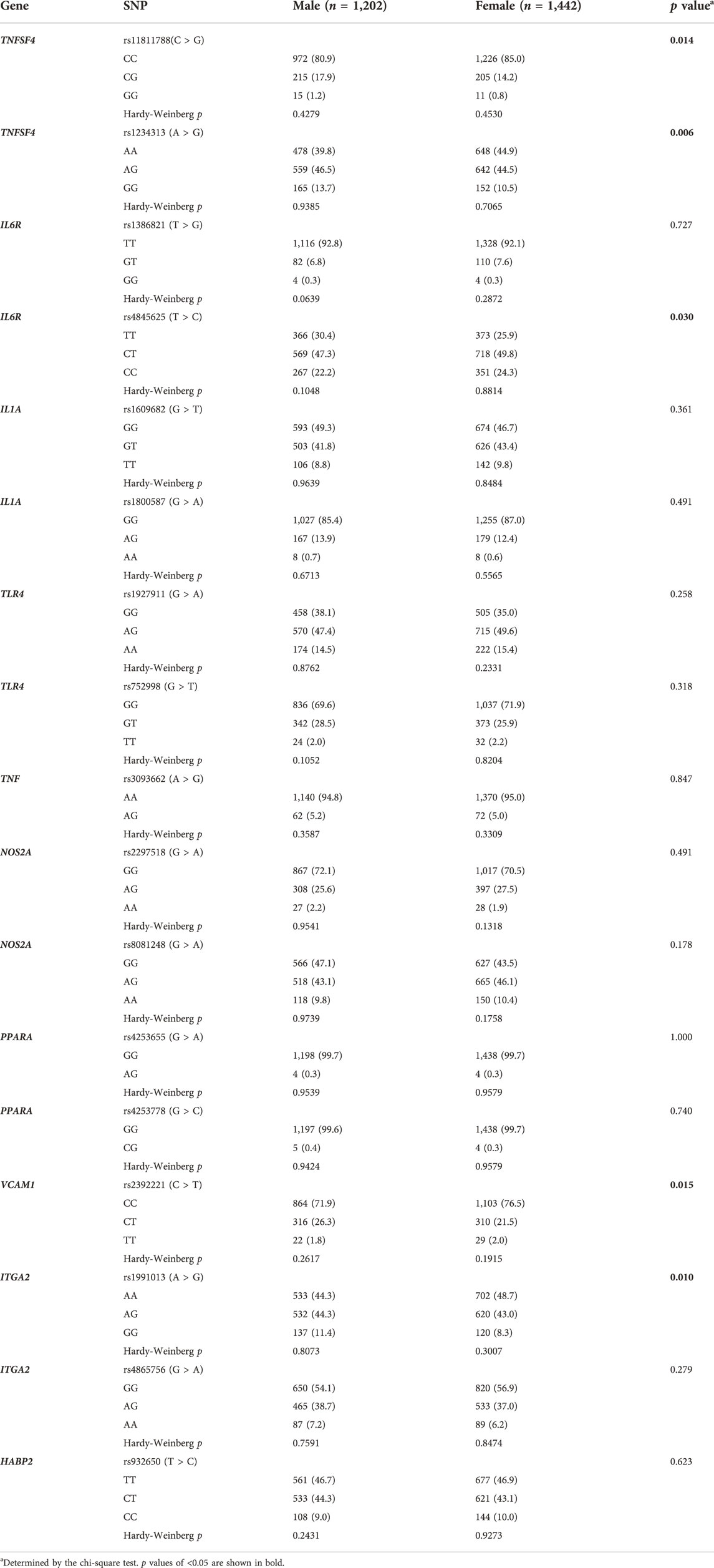
Whole blood samples (3 ml, elbow vein) from the 2,644 participants were drawn into sterile tubes containing ethylene diamine tetraacetic acid and were stored at −80°C until genotype analysis was performed. Genomic deoxyribonucleic acid (DNA) was extracted from peripheral blood using a modified phenol/chloroform method and purified using the UNIQ-10 kit (Sangon Biotech Co., Ltd. Shanghai, China). The genotyping of the 18 SNPs was performed by investigators blinded to the basic characteristics of the participants using the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) method, which has been elaborated upon in our previous study (Yi et al., 2016). The genotype frequencies for Hardy-Weinberg equilibrium were assessed using Pearson’s chi-squared test. One SNP (HABP2 rs7923349) which did not pass the Hardy-Weinberg equilibrium test was excluded from analyses (p = 6.591E-13). The remaining 17 SNPs showed no significant deviation from Hardy-Weinberg equilibrium (all p > 0.05).
Statistical analyses
Sex differences in the baseline characteristics of high-risk individuals for stroke, the prevalence of carotid plaque, and genotype distributions of the 18 SNPs were assessed for significance using the χ2 tests (categorical variables) or the student’s t-tests (continuous variables). Univariable and multivariable analyses were performed to identify the risk factors associated with the prevalence of vulnerable carotid plaques. In this stage, multivariate logistic regression was performed in 2 different models. Model 1 was adjusted for variables which had a potential association with vulnerable carotid plaque in univariate analysis excluding genotype distribution which had a potentially higher risk of vulnerable plaque (p < 0.1), while Model 2 was adjusted for variables including genotype distribution with a potentially higher risk of vulnerable plaque (p < 0.1).
Then, interaction tests were performed via using multiple logistic regression adjusting for confounders (p < 0.1 in univariate models), to identify the SNPs (assuming a dominant genetic model) that might modify the association between sex and vulnerable carotid plaques. The significance of interaction was tested by the log-likelihood ratio test. A significant probability value for the interaction (p for interaction<0.05) would suggest that there was a sex-dependent difference in the association between the SNP and vulnerable carotid plaques.
Furthermore, stratification analysis was performed for SNPs with suggestive evidence for interaction with sex (p for interaction <0.05) in different genetic models (dominant, recessive and codominant). Separately in each sex stratum, multiple logistic regression was done to explore the association between each SNP and vulnerable plaques controlling for potential confounders. Sex-specific odds ratio (OR) and 95% confidence interval (CI) for SNP association were estimated in each sex stratum.
All statistical analysis was performed using SPSS v21.0 (IBM, Chicago, IL, United States), the statistical software packages R (http://www.R-project.org, The R Foundation, version 3.4.3) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, United States), which have been described in our previous studies (Li et al., 2021). Two-sided p < 0.05 was considered to be statistically significant.
Results
Sex differences in the baseline characteristics of participants and prevalence of vulnerable carotid plaque
A total of 2,644 subjects at high risk of stroke were enrolled, comprising 1,202 (45.5%) men and 1,442 (54.5%) women. Carotid plaques were detected in 904 (34.2%) participants, and 479 (18.1%) subjects had stable plaques, whereas 425 (16.1%) had vulnerable plaques. Sex differences in the baseline characteristics of high-risk individuals and the prevalence of carotid plaque have been detailly described in our previous published study (Li et al., 2021). Compared with women, men were younger (62.7 ± 10.3 vs. 63.7 ± 9.4 years, p < 0.01), had higher levels of education (p < 0.01), more history of former and current smoking (13.9%, 54.2% vs. 1.7%, 4.4%, respectively, p < 0.01) and regular alcohol consumption (18.7% vs. 1.6%, p < 0.01). Meanwhile, men had larger waist circumference than women in the current survey (88.9 ± 10.0 vs. 86.4 ± 11.6 cm, p < 0.01). However, men had less history of ischemic stroke or transient ischemic stroke (TIA) (14.4% vs. 20.5%, p < 0.01), hypertension (78.6% vs. 81.6%, p = 0.05), diabetes (28.4% vs. 39.3%, p < 0.01), dyslipidemia (67.8% vs. 76.5%, p < 0.01) and atrial fibrillation (7.8% vs. 11.0%, p < 0.01) than women. The total prevalence of vulnerable carotid plaque was higher in men than in women (20.0% vs. 12.8%, p < 0.01).
Sex differences in genotype distributions of the candidate single nucleotide polymorphisms
One SNP (HABP2 rs7923349) which did not pass the Hardy-Weinberg equilibrium test was excluded from analyses (p = 6.591E-13). Genotype distributions of the remaining 17 SNPs were compared between sex. As shown in Table 1, the genotype frequencies of 5 SNPs (TNFSF4 rs11811788, TNFSF4 rs1234313, IL6R rs4845625, VCAM1 rs2392221, and ITGA2 rs1991013) were significantly different between sex (all p < 0.05).
Univariable and multivariable analyses for risk factors associated with vulnerable carotid plaque
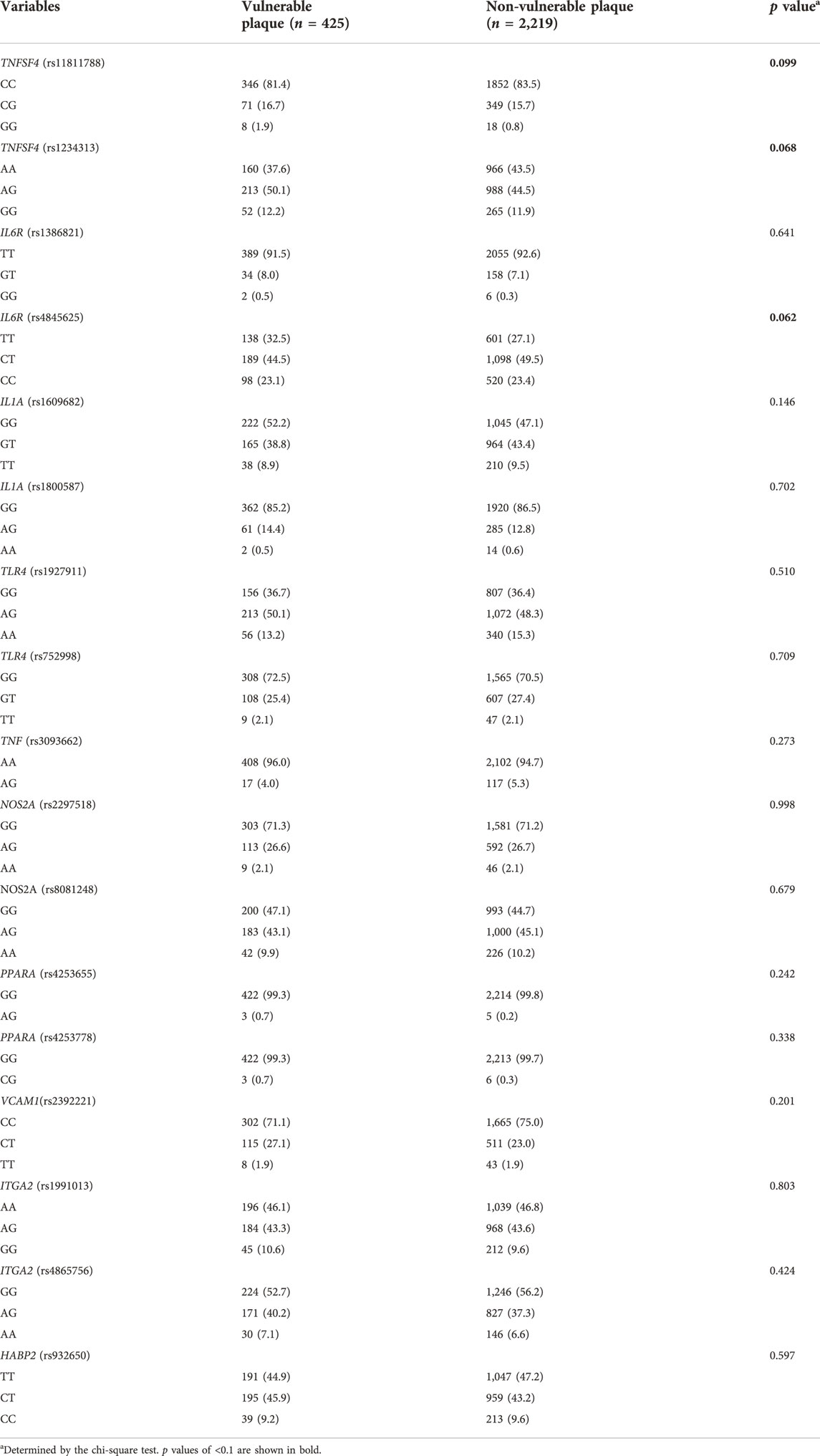
Genotype distribution of 18 SNPs between high-risk individuals with vulnerable plaque or not were presented in Table 2. Univariable analyses showed that there was a potential for differences in the genotype distributions of 3 SNPs (TNFSF4 rs11811788, TNFSF4 rs1234313, and IL6R rs4845625) between high-risk participants with vulnerable plaque or not (all p < 0.1). Meanwhile, as shown in our previous published study (Li et al., 2021), age, sex, family history of stroke, hypertension, smoking status, and body mass index (BMI) had a potential association with vulnerable carotid plaque (all p < 0.1) in univariable analyses.
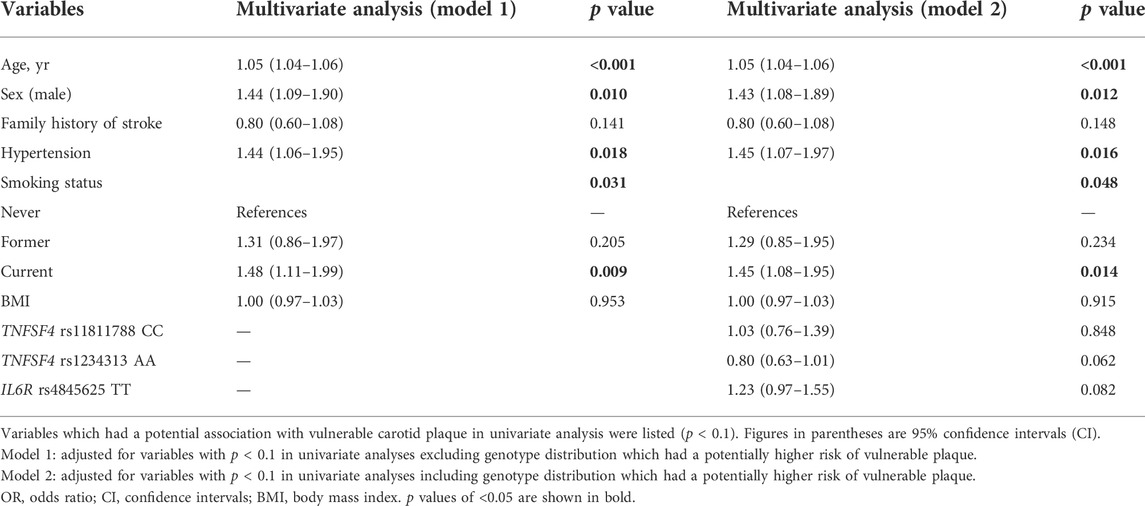
Multivariate logistic regression was conducted to identify the independent factors associated with the prevalence of vulnerable carotid plaques (Table 3). After adjusting for age, family history of stroke, hypertension, smoking status, and BMI (model 1), male sex was significantly associated with vulnerable carotid plaque (adjusted OR 1.11, 95% CI 1.09–1.90, p = 0.01). When genotype distribution of 3 SNPs (TNFSF4 rs11811788, TNFSF4 rs1234313, and IL6R rs4845625) which had a potentially higher risk of vulnerable plaque (p < 0.1) were included in the multivariate logistic regression (model 2), male sex was still an independent risk factor for vulnerable plaque (adjusted OR 1.43, 95% CI 1.08–1.89, p = 0.012). However, none of the 3 SNPs was significantly associated with vulnerable plaques (all p > 0.05).
Interaction tests to identify the SNP that might modify the association between sex and vulnerable carotid plaques.
We looked for evidence for interactions between the sex and SNPs in association with vulnerable carotid plaques. Among 18 SNPs in 10 genes, interaction tests revealed that only one SNP (IL6R rs4845625) had suggestive evidence for interaction with sex (p for interaction<0.05), and might modify the association between sex and vulnerable carotid plaques, as shown in Table 4.
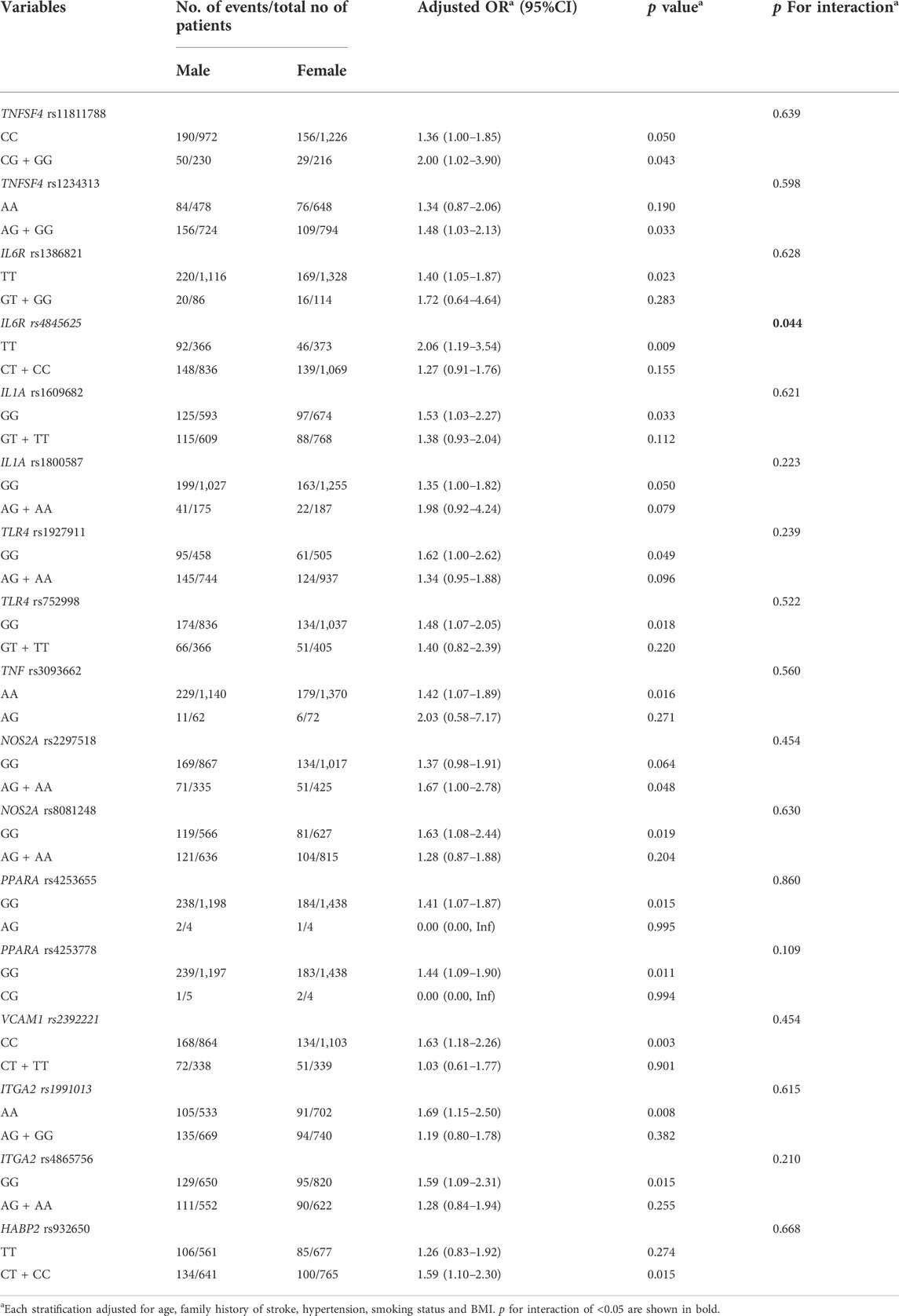
TABLE 4. Interaction tests to identify the SNP that might modify the association between sex and vulnerable carotid plaque.
Stratification analysis for single nucleotide polymorphisms with suggestive evidence for interaction with sex
Stratification analysis was performed to explore sex-specific genotypic association of IL6R rs4845625 with vulnerable carotid plaque, in dominant, recessive and codominant model, respectively. We found that there is a strong association between IL6R rs4845625 and vulnerable carotid plaque in man (dominant model TT vs. CT + CC: adjusted OR 1.52, 95% CI 1.12–2.07, p = 0.007; codominant model TT vs. CC: adjusted OR 1.50, 95% CI 1.00–2.25, p = 0.048) but not in women (all p > 0.05), after adjusting for age, family history of stroke, hypertension, smoking status, and BMI (Table 5).
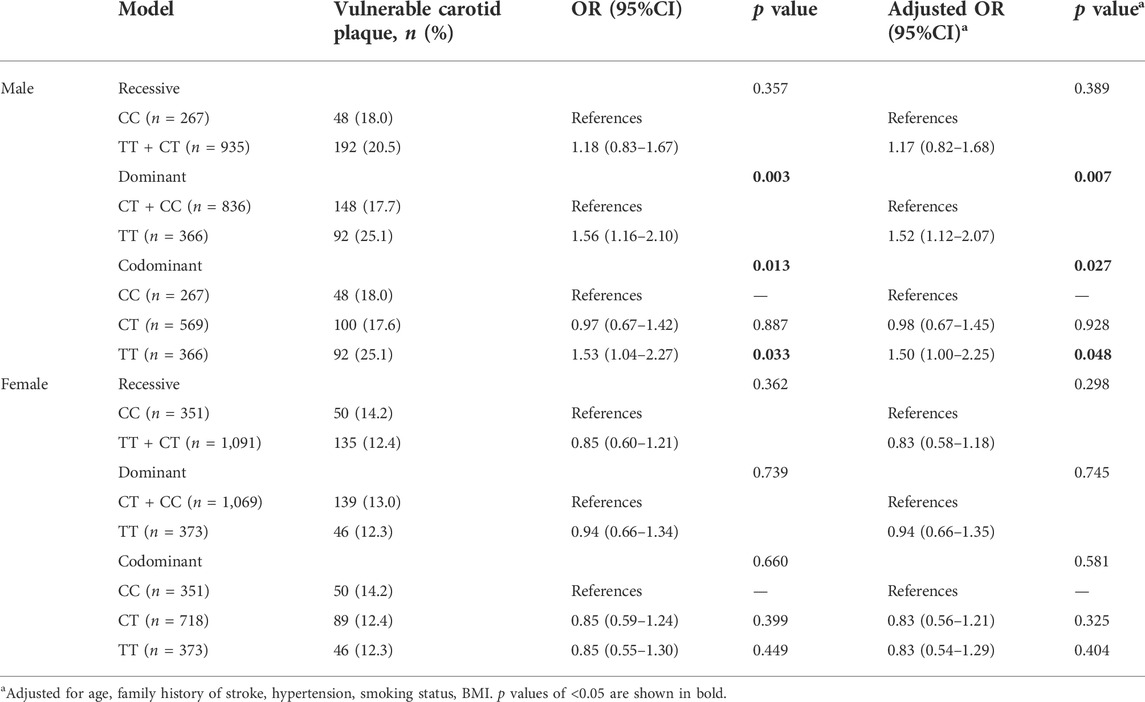
TABLE 5. Sex-specific genotypic association of IL6R rs4845625 with vulnerable carotid plaque in different genetic models.
Discussion
Although male individuals tend to have a higher risk of vulnerable carotid plaque independent of traditional vascular risk factors (Iemolo et al., 2004; Hellings et al., 2007; Ota et al., 2010; Vrijenhoek et al., 2013; Li et al., 2021), the sex-dependent genetic contribution to vulnerable plaque is still unclear. In the present study we analyzed the sex-specific genotype distribution of 10 genes involved in inflammation and endothelial function and their association with the prevalence of vulnerable carotid plaques in 2,644 high-risk individuals for stroke. Although significant difference was observed in the genotype frequencies of 5 SNPs (TNFSF4 rs11811788, TNFSF4 rs1234313, IL6R rs4845625, VCAM1 rs2392221, and ITGA2 rs1991013) between sex, none of the candidate SNPs was significantly associated with vulnerable carotid plaque in univariable and multivariable analyses. The sex differences in the genotype distribution of the 5 SNPs have not ever been reported in literature. However, experimental studies have shown that sex steroids might play an important role in vascular disease, via regulation the sex-specific expression of VCAM-1 in endothelial cells (McGrath et al., 2010; Cutini et al., 2012). The most compelling finding is that the association between sex and vulnerable plaques was affected by the genotype of IL6R rs4845625 in interaction tests. Further stratification analysis revealed men carrying the TT genotype of IL6R rs4845625 had significantly higher risk of vulnerable carotid plaque (TT vs. CT + CC: adjusted OR 1.52, 95% CI 1.12–2.07; TT vs. CC: adjusted OR 1.50, 95% CI 1.00–2.25), which was not noted in women who had a lower frequency of TT genotype than men. We have provided statistical evidence that the rs4845625 polymorphism in IL6R has sex-specific effects on vulnerable carotid plaque in Chinese Han high-risk individuals for stroke.
Great attention has been attracted to inflammatory molecules and their genetic variant in the pathogenesis of atherosclerosis. The human IL6R gene is localized on chromosome 1 band q21 (Kim et al., 2003), encoding the receptor for interleukin-6 (IL-6), which is a member of the pro-inflammatory cytokine family (Uciechowski and Dempke, 2020). IL-6 is a multifunction cytokine mainly secreted by T lymphocytes, macrophages, endothelial cells, smooth muscle cells, and adipocytes, eliciting pro-inflammatory signals in target tissues through the binding to the membrane-bound (IL6R and gp130) or circulating soluble interleukin-6 receptor (sIL6R and sgp130) on monocytes, hepatocytes, and endothelial cells (Naka et al., 2002). As we known, persistent local and systemic inflammation has been implicated in all stages of atherogenesis, from endothelial dysfunction to onset of atherosclerotic plaque rupture and their thrombotic complications (Ross, 1999), while IL-6 signaling pathway is a master player closely associated with the pathogenesis of atherosclerotic disease (Scheller and Rose-John, 2012). It has been reported that high circulating concentration of IL-6 is associated with increased risk of coronary heart disease in prospective observational studies (Ridker et al., 2000; Danesh et al., 2008). Several studies have suggested different SNPs in the IL-6/IL-6R were associated with several inflammatory cytokines and in relation to the susceptibility to coronary atherosclerosis (Deloukas et al., 2013; Mitrokhin et al., 2017). Meta-analyses including individual participant data from Mendelian randomization studies suggested a specific functional genetic variant Asp358Ala (rs2228145) in the IL6R had effects on biomarkers of inflammation and related pathways (soluble IL-6 and IL6R, C-reactive protein, fibrinogen, and others), and was associated with a reduced risk of coronary heart disease (Sarwar et al., 2012; Swerdlow et al., 2012). On the basis of genetic evidence, IL6R-related pro-inflammatory pathway seems to have a causal role in the pathogenesis of coronary atherosclerosis, and IL6R blockade could be a novel therapeutic approach for prevention of coronary atherosclerotic disease (Boekholdt and Stroes, 2012).
Compared with coronary artery disease, there is less evidence supporting IL6R signaling pathway contributing to carotid atherosclerotic diseases. Elevated IL-6 levels appear to be associated with lower echogenicity of carotid plaques, unstable plaques and internal carotid artery stenosis in several observational studies, suggesting a link between IL-6 and the pathogenesis and progression of carotid atherosclerosis (Yamagami et al., 2004; Puz et al., 2013; Hassan et al., 2020). Meanwhile, a cohort study conducted in patients undergoing carotid endarterectomy found that all components of the IL-6 signaling pathways are expressed in carotid plaques, and IL6R expression are higher in patients who had a history of cerebrovascular event (Ziegler et al., 2021). Thus, it is logical that the IL-6/IL6R gene polymorphisms could affect carotid atherosclerosis. Previous studies could not demonstrate the association between IL-6 gene polymorphisms (which had been associated with coronary artery disease) and carotid atherosclerosis (Cunnington et al., 2009; Hulkkonen et al., 2009; Riikola et al., 2009). Until recently, there is limited information regarding the association between IL6R gene polymorphisms and carotid atherosclerosis. A candidate gene study examined the association between genes involved in inflammation and endothelial function carotid plaque phenotypes in the single SNP analysis, and found that IL6R SNP (rs1386821) was strongly associated with thick plaque phenotype (Gardener et al., 2011b). Genetic studies have indicated that the presence of the T allele of rs4845625 in the intron of the IL6R gene was associated with an increased risk of cardiovascular disease such as coronary artery disease and atrial fibrillation (Schnabel et al., 2011; Deloukas et al., 2013; Zhang et al., 2022). However, there is still a lack of evidence on the association between IL6R gene (rs4845625) polymorphism and carotid atherosclerosis. To the best of our knowledge, this is the first time we revealed that carriers of the IL6R rs4845625 TT genotype was associated with an increased risk of vulnerable carotid plaque in Chinese Han high-risk individuals for stroke in a sex-specific manner. Our results suggested that IL6R SNPs might participate in the pathogenesis of carotid atherosclerosis and plaque vulnerability in male individual. The Cardiovascular Risk in Young Finns Study also reported that the IL-6 promoter gene polymorphism (IL6-174 G>C) was associated with markers of subclinical carotid atherosclerosis in men, but not significant in women (Hulkkonen et al., 2009). IL6R signaling pathway could be an important therapeutic target for the prevention of carotid atherosclerosis and ischemic cerebrovascular events in male high-risk individuals. Further studies are needed to explain the molecular mechanisms in future.
Sex has long been recognized as a strong modifier of cerebrovascular disease risk. It is also worth noting that in our study population, men had more carriers of the IL6R rs4845625 TT genotype than women (30.4% vs. 25.9%), which had been associated with an increased risk of vulnerable carotid plaque. Sex differences have been recognized in the risk of carotid atherosclerotic plaque (Iemolo et al., 2004; Hellings et al., 2007; Ota et al., 2010; Vrijenhoek et al., 2013; Li et al., 2021). A recent prospective cohort study conducted in patients with recent ischemic cerebrovascular events and mild-to-moderate carotid stenosis also demonstrated that men are more likely to have a high-risk vulnerable carotid plaque with intraplaque hemorrhage and lipid-rich necrotic core than women, no matter the total plaque burden (van Dam-Nolen et al., 2022). Our findings provide a plausible genetic basis for the sex difference in carotid plaque vulnerability. It is not clear that how the variant rs4845625 confers sex-dependent effects on vulnerable carotid plaque. Sex-differences in other vascular risk factors could interact with the genetic variation and contribute to the sex-specific genetic effect. Future studies exploring the gene-environment interactions can help to illustrate the biological basis for the sex-specific effects.
Limitations
The present study has several limitations. First, we only enrolled residents who were aged ≥40 years and identified as the high-risk individuals for stroke, therefore, our results can not represent the whole population. Second, the main objective of this study was to explore the association between genes involved in inflammation and endothelial function with vulnerable carotid plaque, a subclinical precursor of ischemic stroke. Thus, carotid intima thickness and carotid stenosis were not involved in our analyses. Third, this is a cross-sectional study, so the prospective prediction of the IL6R rs4845625 genotypes effects on the development of vulnerable carotid plaque is impossible at this stage. Besides, carotid plaque vulnerability was evaluated by ultrasound but not high-resolution magnetic resonance imaging. Fourth, the mean age of female individuals at high-risk of stroke in the present study are 63.7 years old. As we know, sex hormone levels in postmenopausal women might have an effect on atherosclerosis, however, we did not collect information about the time of menopause of women, and whether women received a hormonal treatment such as estrogen/progesterone. In addition, we conducted a candidate gene study and only a total of 18 SNPs in 10 gene were examined, further study is needed to test other inflammation and endothelial function related genes to validate the findings in our study. Furthermore, we did not explore the effect of antihypertensive drugs, statins, and antiplatelet drugs on the carotid plaque vulnerability due to a lack of data. Finally, limited to the study protocol of the CNSSS program, we could not provide information on biomarkers of inflammation such as soluble IL-6 and IL6R, C-reactive protein, fibrinogen, and others. It has been reported that sex differences exist in monocyte expression of IL-6 (O’Connor et al., 2007), the measurement of IL-6 in the participants’ serum would be an experimental technique to explore the correlation that the sex-difference in the SNP polymorphism of the ILR6 gene contributes to the level plasma IL-6. Further studies are needed to explore this issue.
Conclusion
Despite the above limitations, the present study provides clear evidence that the rs4845625 polymorphism in IL6R has sex-specific effects on vulnerable carotid plaque in Chinese Han high-risk individuals for stroke. This variant might be a genetic risk factor for vulnerable carotid plaque in Chinese male individuals. Our findings provide a plausible genetic basis underlying the sex difference in carotid plaque vulnerability. A better understanding of these sex-specific genetic effects will help identify high-risk individuals for carotid atherosclerosis and new pharmaceutical targets, as well as help to design novel strategies for the prevention and treatment of ischemic stroke.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the People’s Hospital of Deyang City. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Dr. Li, Zhang- conceived and designed the study, acquired the funding, collected, analyzed, and interpreted the data, as well as drafted the manuscript. Dr. Li, Zhang, Yi, Chen - participated in study administration, investigation, and data collection. Dr. Li, Zhang, Yi - contributed to study design, funding acquisition, and study administration. Dr. Luo, Yu, Wang, Chen- contributed to study design, administration, and supervision. All authors critically revised the manuscript for important intellectual content and approved the final manuscript.
Funding
This research was funded by the Scientific Research Foundation, Health and Family Planning Commission of Sichuan Province in China (Nos. 17PJ084 and 16ZD046), and the Science and Technology Research Foundation of Deyang City (Nos. 2020SZZ069 and 2021SZZ065).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/10.3389/fphys.2022.977578/full#supplementary-material
References
Berliner J. A., Navab M., Fogelman A. M., Frank J. S., Demer L. L., Edwards P. A., et al. (1995). Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation 91, 2488–2496. doi:10.1161/01.cir.91.9.2488
Bian L., Xia L., Wang Y., Jiang J., Zhang Y., Li D., et al. (2018). Risk factors of subclinical atherosclerosis and plaque burden in high risk individuals: Results from a community-based study. Front. Physiol. 9, 739. doi:10.3389/fphys.2018.00739
Boekholdt S. M., Stroes E. S. (2012). The interleukin-6 pathway and atherosclerosis. Lancet 379 (9822), 1176–1178. doi:10.1016/S0140-6736(12)60361-4
Cunnington M. S., Mayosi B. M., Hall D. H., Avery P. J., FarrallM. , Vickers M. A., et al. (2009). Novel genetic variants linked to coronary artery disease by genome-wide association are not associated with carotid artery intima-media thickness or intermediate risk phenotypes. Atherosclerosis 203 (1), 41–44. doi:10.1016/j.atherosclerosis.2008.06.025
Cutini P. H., Campelo A. E., Agriello E., Sandoval M. J., Rauschemberger M. B., Massheimer V. L. (2012). The role of sex steroids on cellular events involved in vascular disease. J. Steroid Biochem. Mol. Biol. 132 (3-5), 322–330. doi:10.1016/j.jsbmb.2012.08.001
Danesh J., Kaptoge S., Mann A. G., Sarwar N., Wood A., Angleman S. B., et al. (2008). Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 5, e78. doi:10.1371/journal.pmed.0050078
Deloukas P., Kanoni S., Willenborg C., Farrall M., Assimes T. L., Thompson J. R., et al. (2013). Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45 (1), 25–33. doi:10.1038/ng.2480
Gardener H., Beecham A., Cabral D., Yanuck D., Slifer S., Wang L., et al. (2011). Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanics from northern Manhattan. Stroke 42, 889–896. doi:10.1161/STROKEAHA.110.591065
Gardener H., Beecham A., Cabral D., Yanuck D., Slifer S., Wang L., et al. (2011). Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanics from northern Manhattan. Stroke 42 (4), 889–896. doi:10.1161/STROKEAHA.110.591065
GBD 2016 Causes of Death Collaborators (2017). Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the global burden of disease study 2016. Lancet 390, 1151–1210. doi:10.1016/S0140-6736(17)32152-9
Hassan M. O., Duarte R., Dickens C., Dix-Peek T., Naidoo S., Vachiat A., et al. (2020). Interleukin-6 gene polymorhisms and interleukin-6 levels are associated with atherosclerosis in CKD patients. Clin. Nephrol. 93 (1), 82–86. doi:10.5414/CNP92S114
Hellings W. E., Pasterkamp G., Verhoeven B. A. N., De Kleijn D. P. V., De Vries J. P. P. M., Seldenrijk K. A., et al. (2007). Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J. Vasc. Surg. 45 (2), 289–296. doi:10.1016/j.jvs.2006.09.051
Hollander M., Bots M. L., Del Sol A. I., Koudstaal P. J., Witteman J. C. M., Grobbee D. E., et al. (2002). Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: The rotterdam study. Circulation 105 (24), 2872–2877. doi:10.1161/01.cir.0000018650.58984.75
Hulkkonen J., Lehtimäki T., Mononen N., Juonala M., Hutri-Kahonen N., Taittonen L., et al. (2009). Polymorphism in the IL6 promoter region is associated with the risk factors and markers of subclinical atherosclerosis in men: The Cardiovascular Risk in Young Finns Study. Atherosclerosis 203 (2), 454–458. doi:10.1016/j.atherosclerosis.2008.07.014
Iemolo F., Martiniuk A., Steinman D. A., Spence J. D. (2004). Sex differences in carotid plaque and stenosis. Stroke 35, 477–481. doi:10.1161/01.STR.0000110981.96204.64
Kim L. H., Lee H. S., Kim Y. J., Jung J. H., Kim J. Y., Park B. L., et al. (2003). Identification of novel SNPs in the interleukin 6 receptor gene (IL6R). Hum. Mutat. 21 (4), 450–451. doi:10.1002/humu.9130
Kuo F., Gardener H., Dong C., Cabral D., Della-Morte D., Blanton S. H., et al. (2012). Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke 43 (7), 1755–1760. doi:10.1161/STROKEAHA.112.651059
Leppert M. H., Burke J. F., Lisabeth L. D., Madsen T. E., Kleindorfer D. O., Sillau S., et al. (2022). Systematic review of sex differences in ischemic strokes among young adults: Are young women disproportionately at risk? Stroke 53 (2), 319–327. doi:10.1161/STROKEAHA.121.037117
Li J., Gao L., Zhang P., Liu Y., Zhou J., Yi X., et al. (2021). Vulnerable plaque is more prevalent in male individuals at high risk of stroke: A propensity score-matched study. Front. Physiol. 12, 642192. doi:10.3389/fphys.2021.642192
Li J., Wang L., Chao B., Liu Y. (2015). Prevalence of stroke in China: An epidemiological study based on the national stroke screening survey. Lancet 386, S49. doi:10.1016/s0140-6736(15)00630-3
Mangge H., Almer G. (2019). Immune-mediated inflammation in vulnerable atherosclerotic plaques. Molecules 24 (17), 3072. doi:10.3390/molecules24173072
Mathiesen E. B., Bønaa K. H., Joakimsen O. (2001). Low levels of high-density lipoprotein cholesterol are associated with echolucent carotid artery plaques: The tromsø study. Stroke 32 (9), 1960–1965. doi:10.1161/hs0901.095639
McGrath K. C., Hill M. D., McRobb L. S., Heather A. K. (2010). The androgen receptor drives the sex-specific expression of vascular cell adhesion molecule-1 in endothelial cells but not lipid metabolism genes in monocyte-derived macrophages. Horm. Mol. Biol. Clin. Investig. 2 (1), 203–209. doi:10.1515/HMBCI.2010.022
Mitrokhin V., Nikitin A., Brovkina O., Khodyrev D., Zotov A., Vachrushev N., et al. (2017). Association between interleukin-6/6R gene polymorphisms and coronary artery disease in Russian population: Influence of interleukin-6/6R gene polymorphisms on inflammatory markers. J. Inflamm. Res. 10, 151–160. doi:10.2147/JIR.S141682
Moskau S., Golla A., Grothe C., Boes M., Pohl C., Klockgether T. (2005). Heritability of carotid artery atherosclerotic lesions: An ultrasound study in 154 families. Stroke 36 (1), 5–8. doi:10.1161/01.STR.0000149936.33498.83
Naka T., Nishimoto N., Kishimoto T. (2002). The paradigm of IL-6: From basic science to medicine. Arthritis Res. 4 (3), S233–S242. doi:10.1186/ar565
Nightingale A. L., Farmer R. D. (2004). Ischemic stroke in young women: A nested case-control study using the UK general practice research database. Stroke 35 (7), 1574–1578. doi:10.1161/01.STR.0000129789.58837.e4
O'Connor M. F., Motivala S. J., Valladares E. M., Olmstead R., Irwin M. R. (2007). Sex differences in monocyte expression of IL-6: Role of autonomic mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293 (1), R145–R151. doi:10.1152/ajpregu.00752.2006
Ota H., Reeves M. J., Zhu D. C., Majid A., Collar A., Yuan C., et al. (2010). Sex differences in patients with asymptomatic carotid atherosclerotic plaque: In vivo 3.0-T magnetic resonance study. Stroke 41 (8), 1630–1635. doi:10.1161/STROKEAHA.110.581306
Petty G. W., Brown R. D., Whisnant J. P., Sicks J. D., O'Fallon W. M., Wiebers D. O. (1999). Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke 30 (12), 2513–2516. doi:10.1161/01.str.30.12.2513
Prasad K. (2015). Pathophysiology and medical treatment of carotid artery stenosis[J]. Int. J. angiology 24 (3), 158–172. doi:10.1055/s-0035-1554911
Puig N., Jiménez-Xarrié E., Camps-Renom P., Benitez S. (2020). Search for reliable circulating biomarkers to predict carotid plaque vulnerability. Int. J. Mol. Sci. 21 (21), 8236. doi:10.3390/ijms21218236
Puz P., Lasek-Bal A., Ziaja D., Kazibutowska Z., Ziaja K. (2013). Inflammatory markers in patients with internal carotid artery stenosis. Arch. Med. Sci. 9 (2), 254–260. doi:10.5114/aoms.2013.34533
Ridker P. M., Rifai N., Stampfer M. J., Hennekens C. H. (2000). Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101, 1767–1772. doi:10.1161/01.cir.101.15.1767
Riikola A., Sipilä K., Kähönen M., Jula A., Nieminen M. S., Moilanen L., et al. (2009). Interleukin-6 promoter polymorphism and cardiovascular risk factors: The Health 2000 survey. Atherosclerosis 207 (2), 466–470. doi:10.1016/j.atherosclerosis.2009.06.004
Ross R. (1999). Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 14340 (2), 115–126. doi:10.1056/NEJM199901143400207
Rundek T., Arif H., Boden-Albala B., Elkind M. S., Paik M. C., Sacco R. L. (2008). Carotid plaque, a subclinical precursor of vascular events: The northern manhattan study. Neurology 70 (14), 1200–1207. doi:10.1212/01.wnl.0000303969.63165.34
Sacco R. L., Blanton S. H., Slifer S., Beecham A., Glover K., Gardener H., et al. (2009). Heritability and linkage analysis for carotid intima-media thickness: The family study of stroke risk and carotid atherosclerosis. Stroke 40 (7), 2307–2312. doi:10.1161/STROKEAHA.109.554121
Santos-Neto P. J., Sena-Santos E. H., Meireles D. P., Bittencourt M. S., Santos I. S., Bensenor I. M., et al. (2021). Association of carotid plaques and common carotid intima-media thickness with modifiable cardiovascular risk factors. J. Stroke Cerebrovasc. Dis. 30 (5), 105671. doi:10.1016/j.jstrokecerebrovasdis.2021.105671
Sarwar N., Butterworth A. S., Freitag D. F., Gregson J., Willeit P., Gorman D. N., et al. (2012). Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 379 (9822), 1205–1213. doi:10.1016/S0140-6736(11)61931-4
Scheller J., Rose-John S. (2012). The interleukin 6 pathway and atherosclerosis. Lancet 380 (9839), 338. doi:10.1016/S0140-6736(12)61246-X
Schnabel R. B., Kerr K. F., Lubitz S. A., Alkylbekova E. L., Marcus G. M., Sinner M. F., et al. (2011). Large-scale candidate gene analysis in whites and african Americans identifies IL6R polymorphism in relation to atrial fibrillation: The national heart, lung, and blood institute's candidate gene association resource (CARe) project. Circ. Cardiovasc. Genet. 4 (5), 557–564. doi:10.1161/CIRCGENETICS.110.959197
Silander K., Alanne M., Kristiansson K., Saarela O., Ripatti S., Auro K., et al. (2008). Gender differences in genetic risk profiles for cardiovascular disease. PLoS One 3, e3615. doi:10.1371/journal.pone.0003615
Stroke Prevention Project Committee (2022). Program of stroke screening and intervention for high-risk population.Http://cnstroke.com/WebManage/InterveneProject/Index
Sturlaugsdottir R., Aspelund T., Bjornsdottir G., Sigurdsson S., Thorsson B., Eiriksdottir G., et al. (2016). Prevalence and determinants of carotid plaque in the cross-sectional REFINE-Reykjavik study. BMJ open 6 (11), e012457. doi:10.1136/bmjopen-2016-012457
Swerdlow D. I., Holmes M. V., Kuchenbaecker K. B., Engmann J. E., Shah T., Sofat R., et al. (2012). The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 379 (9822), 1214–1224. doi:10.1016/S0140-6736(12)60110-X
Tsao C. W., Aday A. W., Almarzooq Z. I., Alonso A., Beaton A. Z., Bittencourt M. S., et al. (2022). Heart disease and stroke statistics-2022 update: A report from the American heart association. Circulation 145 (8), e153–e639. doi:10.1161/CIR.0000000000001052
Uciechowski P., Dempke W. C. M. (2020). Interleukin-6: A master player in the cytokine network. Oncology 98 (3), 131–137. doi:10.1159/000505099
van Dam-Nolen D. H. K., van Egmond N. C. M., Dilba K., Nies K., van der Kolk A. G., Liem M. I., et al. (2022). Sex differences in plaque composition and morphology among symptomatic patients with mild-to-moderate carotid artery stenosis. Stroke 53 (2), 370–378. doi:10.1161/STROKEAHA.121.036564
Vrijenhoek J. E. P., Den Ruijter H. M., De Borst G. J., de Kleijn D. P. V., De Vries J. P. P. M., Bots M. L., et al. (2013). Sex is associated with the presence of atherosclerotic plaque hemorrhage and modifies the relation between plaque hemorrhage and cardiovascular outcome. Stroke 44 (12), 3318–3323. doi:10.1161/STROKEAHA.113.002633
Wang L., Yanuck D., Beecham A., Gardener H., Slifer S., Blanton S. H., et al. (2011). A candidate gene study revealed sex-specific association between the OLR1 gene and carotid plaque. Stroke 42 (3), 588–592. doi:10.1161/STROKEAHA.110.596841
Wang W., Jiang B., Sun H., Ru X., Sun D., Wang L., et al. (2017). Prevalence, incidence, and mortality of stroke in China: Results from a nationwide population-based survey of 480 687 adults. Circulation 135 (8), 759–771. doi:10.1161/CIRCULATIONAHA.116.025250
Wolf D., Ley K. (2019). Immunity and inflammation in atherosclerosis. Circ. Res. 124 (2), 315–327. doi:10.1161/CIRCRESAHA.118.313591
Yamagami H., Kitagawa K., Nagai Y., Hougaku H., Sakaguchi M., Kuwabara K., et al. (2004). Higher levels of interleukin-6 are associated with lower echogenicity of carotid artery plaques. Stroke 35 (3), 677–681. doi:10.1161/01.STR.0000116876.96334.82
Yi X., Liao D., Wu L., Chen H., Li J., Wang C. (2016). CYP genetic variants, CYP metabolite levels, and symptomatic carotid stenosis in ischemic stroke patients. J. Atheroscler. Thromb. 23, 621–631. doi:10.5551/jat.32714
Yi X., Zhu L., Sui G., Li J., Luo H., Yu M., et al. (2020). Inflammation and endothelial function relevant genetic polymorphisms and carotid plaque in Chinese population[J]. J. Atheroscler. Thrombosis 27, 978–994. doi:10.5551/jat.53074
Zhang M., Bai Y., Wang Y., Cui H., Tang M., Wang L., et al. (2022). Cumulative evidence for associations between genetic variants in interleukin 6 receptor gene and human diseases and phenotypes. Front. Immunol. 13, 860703. doi:10.3389/fimmu.2022.860703
Zhao J., Cheema F. A., Bremner J. D., Goldberg J., Su S., Snieder H., et al. (2008). Heritability of carotid intima-media thickness: A twin study. Atherosclerosis 197 (2), 814–820. doi:10.1016/j.atherosclerosis.2007.07.030
Keywords: high-risk stroke population, plaque vulnerability, inflammation, genetic polymorphism, sex
Citation: Li J, Zhang P, Yi X, Luo H, Yu M, Chen H and Wang C (2022) Sex-specific association between inflammation and endothelial function relevant gene and vulnerable carotid plaque. Front. Physiol. 13:977578. doi: 10.3389/fphys.2022.977578
Received: 24 June 2022; Accepted: 27 July 2022;
Published: 19 August 2022.
Edited by:
Luciana Venturini Rossoni, University of São Paulo, BrazilReviewed by:
Jamaira A. Victorio, State University of Campinas, BrazilTiago J. Costa, Johns Hopkins Medicine, United States
Copyright © 2022 Li, Zhang, Yi, Luo, Yu, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, bGlqaWU4NjAxMTRAMTYzLmNvbQ==
 Jie Li
Jie Li Ping Zhang
Ping Zhang Xingyang Yi
Xingyang Yi Hua Luo2
Hua Luo2 Chun Wang
Chun Wang