- Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Numerous studies have reported that transfer of blastocysts derived from monopronuclear (1PN) zygotes achieved live births. However, the potential value of morphology grading for the prediction of 1PN blastocyst viability is unclear, and the blastocyst selection criterion for successful pregnancy has not been set up yet. The aim of this study is to assess the ability of the blastocyst morphology grading system based on three parameters, namely, inner cell mass (ICM), trophectoderm (TE), and expansion degree and to predict outcomes of a cycle with single 1PN blastocyst transfer.
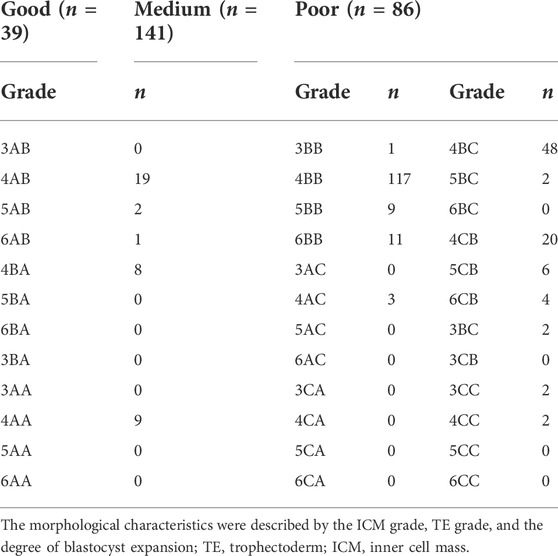
Methods: A total of 266 vitrified-warmed 1PN-derived blastocyst transfer cycles for IVF treatment at Shanghai Ninth People’s Hospital between 2007 and 2020 were included. The study was performed on single blastocyst transfers. Electronic records of patients were retrospectively analyzed. In the current study, the blastocysts were classified into three groups: “good,” 3-6AA, 3-6AB, 3-6BA; “medium,” 3-6BB, 3-6AC, 3-6CA; and “poor,” 3-6BC, 3-6CB, 3-6CC. The basal characteristics, embryo grading, and clinical outcomes were compared between the three groups. The association of morphology parameters with pregnancies and live births was analyzed. Logistic regression was adopted to set up a prediction model of live births.
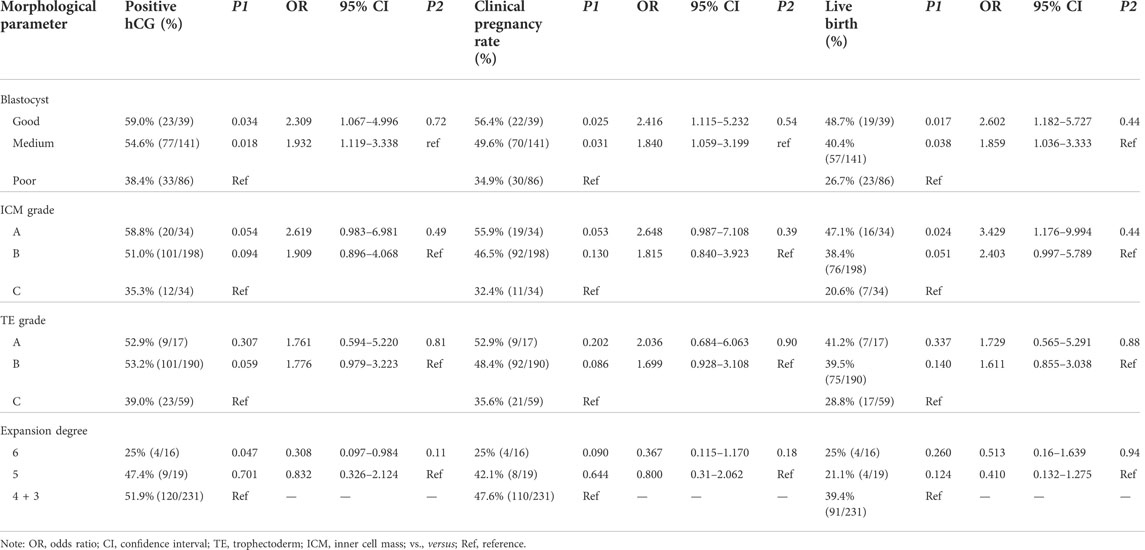
Results: Transfer of the good-quality blastocysts achieved significant higher pregnancies (biochemical pregnancy: 59%; clinical pregnancy: 56.4%, and live birth 48.7%) than those in the group of the medium (biochemical pregnancy: 59%; clinical pregnancy: 49.6%; live birth: 40.4%) or poor-quality (biochemical pregnancy: 38.4%; clinical pregnancy: 34.9%; live birth: 26.7%) blastocysts (p < 0.05). There was a significant association between ICM and live birth. A prediction model of live births involving ICM, TE, and expansion degree was set up.
Conclusion: In 1PN transfer cycles, a higher overall blastocyst quality is shown to correlate most strongly with optimal pregnancy and live birth outcomes. The selection of high-quality blastocysts for transfer should consider the ICM score first. The prediction model of live births based on ICM, TE, and expansion degree may help predict successful pregnancy in 1PN single-blastocyst transfer cycles.
Introduction
In the field of in vitro fertilization–embryo transfer (IVF-ET), transfer of embryos originating from zygotes with normal chromosomal constitution is required for the satisfying clinical outcomes. Generally, the presence of two pronuclear (2PN) and bodies is the symbol of normal fertilization, indicating the normal chromosomal constitution, while the presence of non-pronuclear (0PN) or monopronuclear (1PN) is typically the abnormal fertilization. Although the embryos originated from normal fertilization zygotes were optimally selected for transfer, those 1PN-derived blastocysts were also the choice due to the incidence of diploid rates, especially in cases where no normal fertilization embryos were originated (Staessen and Van Steirteghem, 1997; Bradley et al., 2017; Capalbo et al., 2017).
Many studies reported that transferring 1PN-derived blastocysts also obtained the similar outcomes as the transfer of 2PN-derived blastocysts, including pregnancies and live births (Bradley et al., 2017; Si et al., 2019; Chen et al., 2020; Li et al., 2020; Li et al., 2021). If we discarded those embryos, some patients, especially older patients or patients with decreased ovarian reserve, will miss an opportunity for pregnancy. In clinical practice, some intervention, such as blastocyst culture for transfer, actually improved the potential of successful pregnancy after the transfer of the 1PN-derived embryos (Bradley et al., 2017; Si et al., 2019). To avoid the risk of adverse developmental outcomes of aneuploidy embryos, some genetic techniques, such as preimplantational genetic screening (PGS) with comprehensive chromosome screening (CCS) and time-lapse embryo monitoring, were applied to assess the implantation potential of 1PN-derived embryos (Mateo et al., 2017; Destouni et al., 2018). However, as a non-invasive, convenience, and economic method, the traditional morphology evaluation was still irreplaceable in clinical practice. So far, the accurate prediction of the developmental potential of the 1PN-derived blastocysts based on morphology is still lacking.
Embryo quality is considered a major predictor of implantation and pregnancy (Kemper et al., 2021; Anagnostopoulou et al., 2022). The blastocyst grade system based on blastocyst expansion, inner cell mass (ICM), and trophectoderm (TE) development provides a powerful prediction between the morphology quantitative measurement and pregnancy and live birth in normal fertilization embryo transfer (Gardner and Schoolcraft, 1999; Firmin and Maitre, 2021). Accumulating evidence proved the relationship of pregnancy and live birth with the composite scores of the different morphology parameters in multiple or single 2PN-derived blastocyst transfer (Guo et al., 2020; Maheshwari et al., 2022). Also, their correlations and the potential of each morphology parameter at the blastocyst stage in live birth prediction were also explored in 2PN-derived blastocyst transfer (Ahlstrom et al., 2016; Girard et al., 2018). Nevertheless, the values of the ICM, TE, and expansion degree in prediction of clinical outcomes have not been reported in 1PN-derived single blastocyst transfer yet.
In the current study, we retrospectively analyzed the clinical outcomes of 1PN-derived blastocyst transfer in frozen–thawed IVF cycles from 266 patients between April 2007 and May 2020 in Ninth People’s Hospital affiliated to Shanghai Jiao Tong University. First, the study aimed to investigate the relationship between individual morphology parameters and biochemical pregnancy, clinical pregnancy, and live birth using data on 1PN-derived single blastocyst transfers. Second, we developed a multiple logistic regression model for the prediction of the probability of live birth after 1PN-derived single blastocyst transfers based on blastocyst morphology grading for clinical practices.
Materials and methods
Patients and cycles
The present retrospective study was conducted at the Department of Assisted Reproduction of Ninth People´s Hospital affiliated to Shanghai Jiao Tong University School of Medicine involving transfer of 266 vitrified-warmed 1PN-derived blastocysts in IVF cycles during April 2007–May 2020. The study was performed on single blastocyst transfers. The patients lost to follow-up were excluded.
Ethics statement
Approval for human retrospective analysis was obtained from the Institutional Ethics Committee of Shanghai Ninth People’s Hospital. All participants provided informed consent after counseling for infertility treatments and routine IVF procedures.
Laboratory protocols
Oocytes were retrieved approximately 36 h after hCG administration. All the aspirated oocytes were transferred to the G-IVF PLUS culture media (Vitrolife, Sweden) and fertilized with the use of conventional insemination (IVF). In case of IVF, oocytes were inseminated with approximately 50,000 progressively motile spermatozoa/ml which were harvested with the use of density gradient and swim-up methods in the insemination dish. Zygotes were transferred to the dish containing preequilibrated G-1™ cleavage culture media (Vitrolife, Sweden) 16–18 h after insemination. Fertilization was assessed according to the presence of the pronuclear. Embryos obtained from 1PN zygotes were downgraded on day 3, and all were placed in the G-2™ blastocyst culture media (Vitrolife, Sweden) until the blastocyst stage was reached. Then, the blastocysts were vitrified and thawed in FET cycles. The vitrification procedure was conducted using the Cryotop carrier system (KITAZATO Biopharma Co.). The cryoprotectant solution (KITAZATO@ Vitrification Kit, Japan) consisted of 15% ethylene glycol, 15% dimethyl sulfoxide, and 0.5 M sucrose. We usually used laser objective and positioned the laser pointer on the zona pellucida at the opposite side of the ICM and drilled the zona pellucida through two to three laser pulses. The vitrification procedure collapsed the blastocysts within 30 min to prevent their re-expansion. When thawing blastocysts, the cryoprotectant dilutions (KITAZATO@ Vitrification Kit, Japan) were sequential: 1, 0.5, and 0 M sucrose solutions. All the steps were carried out at room temperature except the first warming step (37°C) (Shen et al., 2020). The blastocysts were usually thawed at approximately 9:30 a.m. and transplanted at 2:00 p.m.
On days 5, 6, and 7 of in vitro cultivation, the blastocysts were evaluated morphologically according to Gardner and Schoolcraft´s classification (Gardner and Schoolcraft, 1999). In our center, blastocyst grading was performed by experienced embryologists, which lowered embryologist variation. The blastocysts were assigned 3, 4, 5, and 6 numeric score based on the degree of expansion and hatching status. Stage 3 blastocysts: full blastocysts; the embryo was completely filled with blastocoel; Stage 4 blastocysts: expanded blastocysts; the blastocoel was larger and the zona was thinner; Stage 5 blastocysts: hatching blastocysts; the TE began to herniate through the zona; Stage 6 blastocysts: hatched blastocysts; the blastocysts completely escaped from the zona. In our center, the blastocysts graded three to six were used for FET. Then, the blastocyst morphology was assessed according to inner cell mass and TE. The ICM grade was evaluated as follows: A, tightly packed with many cells; B, loosely grouped with several cells; and C, few cells. The TE grade was evaluated as follows: A, formed a cohesive epithelium with many cells; B, formed a loose epithelium with few cells; and C, few large cells (Schoolcraft et al., 1999; Zhao et al., 2018).
In this study, the blastocysts were classified into three groups: “good,” 3-6AA, 3-6AB, 3-6BA; “media,” 3-6BB, 3-6AC, 3-6CA; and “poor,” 3-6BC, 3-6CB, 3-6CC (Capalbo et al., 2014; Irani et al., 2017; Zhao et al., 2018). All embryos were cultured in a benchtop incubator (ASTEC) at 37°C with 5% O2 and 6% CO2 concentration. The embryo check-out time was usually between 8 a.m. and 10 a.m. on days 5, 6, and 7. Sometimes, the observation time of the blastocysts on day 5 extends till afternoon.
Embryo transfer and luteal support
In this study, no 1PN-derived blastocysts were transferred in fresh cycles because in our center a freeze-all strategy was adopted. FET was performed via a natural cycle or an artificial cycle according to the individual condition of the patient. Women with menstrual regularity underwent a natural cycle in which human chorionic gonadotropin (hCG, 5000IU; Lizhu Pharmaceutical Trading Co., Shanghai, China) was used as a trigger. Luteal support included Femoston tablets (4 mg/d, Abbott Healthcare Products B.V.) and soft vaginal progesterone capsules (0.4 g/d, Utrogestan, Laboratories Besins Iscovesco, France). Women with menstrual irregularity or abnormal vaginal bleeding history underwent an artificial cycle in which from the third day onward, oral 17ß-estradiol (Fematone 2 mg, three times daily) was commenced for 14 days. Luteal supplement was described as mentioned previously.
Clinical outcomes
Biochemical pregnancy was defined as hCG-positive cycles. Clinical pregnancy was defined as the presence of a gestational sac with cardiac pulse on ultrasound examination, and the clinical pregnancy rate of analyzed cycles was calculated as the intrauterine and ectopic pregnancies divided by the number of embryos transferred cycles.
Statistical analysis
All data statistical analyses were performed using SPSS software. All continuous data were tested for normality. Those normally distributed data were presented as mean ± standard deviation and were analyzed using Student´s t-test. Those non-normally distributed data were presented as the mean (interquartile range) and were subjected to the Mann–Whitney U-test. Categorical variables were expressed as percentages and were analyzed using the chi-squared test or Fisher´s exact test. The relationship between parameters and outcomes were analyzed via a simple logistic regression analysis. A multiple logistic regression model of the live birth rate was conducted using the three parameters: expansion, ICM grade, and TE grade entering into the model. Based on the model, the predicted probabilities of live birth accompanied with 95% Wald confidence limits were reported. A value of p < 0.05 was referred to as a statistical significance.
Results
Association of blastocyst grades with clinical outcomes of embryos originated from monopronuclear zygotes
A total of 266 frozen–thawed blastocysts originated from monopronuclear zygotes in IVF cycles were included in the current study. These blastocysts were divided into three groups according to morphological grades (good, medium, and poor) as showed in Table 1. There was no significant difference in patient age, BMI, years of infertility, type of infertility, and endometrial thickness between the three groups (Table 2).

TABLE 2. Patient characteristics of embryos with different morphological grades between 2007 and 2020.
We compared clinical embryo outcomes of transfer of single 1PN-derived blastocysts among the three groups. Transfer of the good-quality blastocysts achieved significant higher biochemical and clinical pregnancy ratios than those in the group of the poor-quality blastocysts (59% vs. 38.4%; p = 0.032 and 56.4% vs. 34.9%; p = 0.024, respectively, Figure 1A). Also, transfer of the medium-quality blastocysts achieved significant higher biochemical and clinical pregnancy ratios than those achieved in the poor-quality blastocyst group (54.6% vs. 38.4%; p = 0.018; 49.6% vs. 34.9%; p = 0.03, respectively, Figure 1B). However, there was no significant difference between the good-quality blastocysts and the medium-quality blastocysts in biochemical and clinical pregnancy ratios (59% vs. 54.6%; p = 0.627; 56.4% vs. 49.6%; p = 0.454). Furthermore, the live birth rates in groups of good, medium, and poor quality were 48.7%, 40.4% and 26.7%, respectively, exhibiting a blastocyst quality relative decrease (p = 0.353, p = 0.016, and p = 0.036, respectively, Figure 1C). Also, 1PN-derived blastocysts could be used for ET and cryopreservation since their use in FET cycles resulted in a high pregnancy and live birth rates. However, the clinical outcome significantly differed between various 1PN-derived blastocyst morphology groups. Our data indicated that the optimal clinical outcomes might result from the transferring of higher quality blastocysts (Table 3).
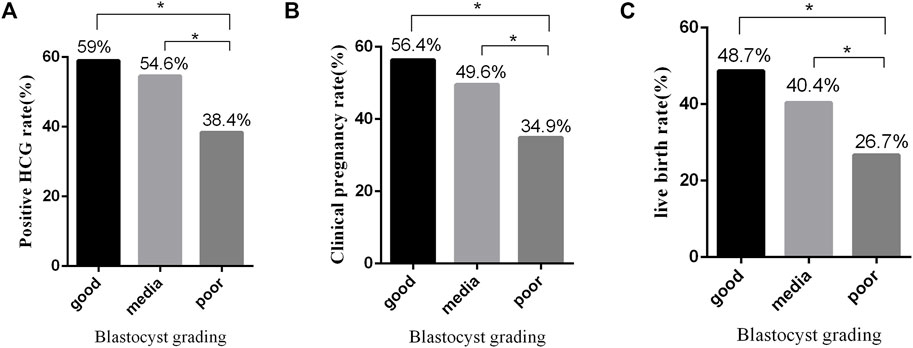
FIGURE 1. Effects of blastocyst grades on clinical outcomes of embryos. (A) Positive hCG rate from FET using embryos with different morphological grades. (B) Clinical pregnancy rate from FET using embryos with different morphological grades. (C) Live birth rate from FET using embryos with different morphological grades. *p ≤ 0.05 (chi-squared test).
Reference values of inner cell mass, trophectoderm development, and the expansion degree of blastocysts for clinical outcomes
Since TE, ICM, and the degree of blastocyst expansion are important in morphological grades, we next explored their reference values for embryo clinical outcomes. Although there was a tendency of decreases in biochemical and clinical pregnancy by ICM degrading, no statistical differences were observed (p > 0.05, Table 3). Only the live birth rate significantly decreased with the decline in the ICM grade (p < 0.05, Table 3). The group with A grade of ICM achieved a much higher live birth rate of 47.1%, while the group with C grade of ICM achieved a low live birth rate of 20.6% [p = 0.024, odds ratio (OR), 3.429; 95% CI, 1.176–9.994, Table 3]. It was also observed that the degree of blastocyst expansion was related with the biochemical rate but not the clinical pregnancy and live birth rate. Transferring of blastocysts with an expansion degree 6 yielded a lower biochemical rate than that of the blastocysts with an expansion degree 3–4 (25% vs. 51.9%; p = 0.047; odds ratio [OR], 0.308; 95% CI, 0.097–0.984, Table 3). However, the TE grade was found to have no effect on either the biochemical pregnancy rate, the clinical pregnancy rate, or the live birth rate (Table 3).
Prediction of the live birth rate
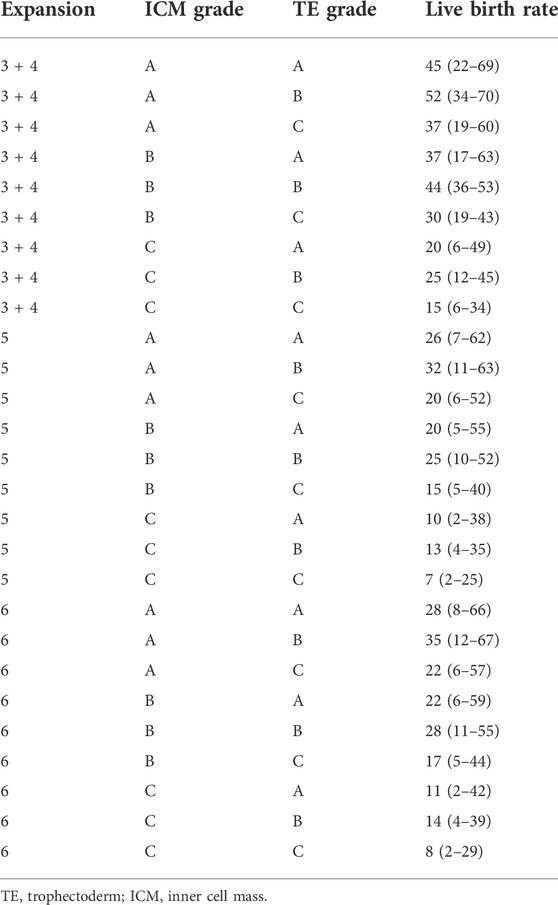
To evaluate the prediction potential of blastocyst quality in the live birth rate, the blastocyst morphological parameters, ICM, TE, and expansion degree, were included in the multiple logistic regression analysis. As shown in Table 4, the prediction model provides the estimated probabilities of achieving a live birth depending on the composite quality score of the blastocysts. As examples for the model, a patient with transfer of a blastocyst with a score of 3-4AB, 5BB, or 6CC is predicted to have a probability of achieving a live birth of 52%, 25%, or 8%, respectively.
Discussion
Until now, 1PN-derived blastocyst transfer has been applied in multiple centers (Staessen et al., 1993; van der Heijden et al., 2009; Azevedo et al., 2014; Bradley et al., 2017; Capalbo et al., 2017; Si et al., 2019; Li et al., 2021), but the association between morphology parameters and clinical outcomes has not been elucidated clearly. To the best of our knowledge, this study was the first report to present the association of ICM, TE, and expansion degree grading with the clinical outcomes and the prediction value of these parameters for achieving live birth in 1PN-derived blastocyst transfer. Our data showed that the good-quality blastocysts obtained higher biochemical and clinical pregnancy and live birth, indicating that the conventional criteria of the morphological grading system (Gardner and Schoolcraft, 1999; Gardner et al., 2000) were also valuable for 1PN-derived blastocyst selection for transfer. Another strength of this study is that we provide the prediction model based on ICM grades, TE grades, and expansion degree, which simultaneously assess all developmental variables that can be used in embryo selection.
The correlation between blastocyst morphology and outcome of single 2PN-derived blastocyst transfers was evaluated in a large number of reports (Hill et al., 2013; Van den Abbeel et al., 2013; Rienzi et al., 2019) using the criteria of Gardner and Schoolcraft (Gardner et al., 2000). It was proved that the scores of ICM grades, TE grades, and expansion degree were significantly associated with pregnancies and live birth rates. Consistently, our data showed that the good-quality blastocysts achieved the highest pregnancy and live birth rate, and the medium blastocysts also owned better clinical outcomes than the poor blastocysts. Therefore, the overall grading of blastocyst quality based on the scores of ICM, TE, and expansion degree was still appropriate for 1PN-derived blastocyst transfer selection for higher pregnancy and live birth.
With respect to the association of morphological parameters with the clinical outcomes, there are many controversial studies. Goto et al. (2011) reported that the significant increases in the clinical pregnancy rate, viable pregnancy rate, and delivery rate were achieved with high-quality blastocyst transfer in 1,488 transfer cycles, while neither ICM nor TE affected the pregnancy outcome within the same blastocyst expansion. However, the extent of blastocyst expansion was found to be the most important parameter in another study (Van den Abbeel et al., 2013). It was also reported that an ICM containing many cells contributed to vital implantation (Richter et al., 2001; Kovacic et al., 2004) and may reduce the risk of pregnancy loss (Hill et al., 2013). However, the relationship of the morphological parameters with the clinical outcomes has not been clarified separately in those 1PN-derived blastocysts. In the current study, we found a significant association between the ICM grading and live birth. Our data showed that the live birth rate reached 47.1% in 1PN-derived blastocysts with ICM A, much higher than that of blastocysts with ICM C. Here, our data showed that the ICM grading, neither TE nor expansion degree, seemed the most important factor to influence live birth in 1PN-derived blastocyst transfer. Thus, the quality grade and ICM should be preferentially considered when transferring the vitrified-warmed blastocysts derived from monopronuclear zygotes. The same results but for 2PN-derived fresh blastocysts were presented by Kovacic et al. (2004).
Even though we did not find a correlation between TE grade and pregnancy potential and live births, its importance in euploid embryo competence has been reported due to its crucial role in the process of implantation in fresh or vitrified-warmed cycles (Norwitz et al., 2001; Ahlstrom et al., 2011). Also, the blastocyst expansion degree has been previously reported as predictive of implantation for euploid embryos (Van den Abbeel et al., 2013). Our data showed that they could not be assessed as a significant parameter for pregnancy or live birth after 1PN-derived blastocyst transfer. The TE and expansion degree were proposed as associated directly because a high-quality TE might involve a more efficient pumping of the ions, which in turn prompts the blastocyst expansion (Ahlstrom et al., 2011). However, both TE and expansion degree lost their significance in the analysis of the pure association with the clinical outcomes in our study, which might be resulted from the correction by the other morphological parameters. Nonetheless, all of these parameters were included in the prediction model of live birth, since the overall grading of blastocysts involving ICM, TE, and expansion degree showed a significant value for pregnancies and live births. Interestingly, the CC blastocysts, such as 3 + 4 CC, 5CC, and 6CC grade, still have 15%, 7%, and 8% live birth, respectively. It is important to recognize that despite little priority for these low-quality blastocysts, they could be a candidate for transfer, especially in those patients without competent embryos.
Our study is limited by the retrospective nature, and the embryo grading is inevitably subjected to intra- and inter-observer variations. The small number of groups of expansion degree 6 and 5 might weaken the conclusions regarding its association with clinical outcomes. Further prospective studies with larger sample sizes of transfer cycles to assess the prediction efficacy of our live birth model for transfer of 1PN-derived blastocysts are required.
Although there are many novel methods that are developed to select the high-competent embryos, such as genetic screening and time-lapse systems, embryo morphology evaluation under the microscope at different stages still remains valuable for the easiness, convenience, and invasiveness. In the current study, we present the valuable reference of traditional morphology grading in 1PN-derived blastocyst transfer. Our data supported that the overall quality grading was significantly associated with pregnancies and live births, and ICM seemed the most important factor in regard to live births. The strengths of the study are the performance of single blastocyst transfers and using live births as the measurement endpoint. The prediction model of live births will promote the selection of 1PN-derived blastocysts with high-developmental capacity. Although the clinical outcomes and live birth rates are high with good-quality 1PN-derived blastocysts, the blastocysts from normal fertilization (2PN) should be prioritized when selecting an embryo for transfer.
Conclusion
1PN-derived blastocysts have a high potential to result in live births and should not be discarded. In 1PN transfer cycles, a higher overall blastocysts quality is shown to correlate most strongly with optimal pregnancy and live birth outcomes. The selection of high-quality blastocysts for transfer should consider the ICM score first. The prediction model of live births based on ICM, TE, and expansion degree may help predict successful pregnancy in 1PN single-blastocyst transfer cycles.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Ninth People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HL and QL developed the experimental designs. TW and JS wrote the manuscript. HL and BW revised the manuscript and contributed to data interpretation. MY, WY, and WJ participated in data analysis and critical discussion. All the authors read and approved the final manuscript.
Funding
This study was financially supported by the Natural Science Foundation of Shanghai (Grant Number. 19ZR1429300, awarded to HL).
Acknowledgments
The authors gratefully thank the staff of the Department of Assisted Reproduction of Shanghai Ninth People’s Hospital for their contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahlstrom A., Westin C., Reismer E., Wikland M., Hardarson T. (2011). Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum. Reprod. 26, 3289–3296. doi:10.1093/humrep/der325
Ahlstrom A., Park H., Bergh C., Selleskog U., Lundin K. (2016). Conventional morphology performs better than morphokinetics for prediction of live birth after day 2 transfer. Reprod. Biomed. Online 33, 61–70. doi:10.1016/j.rbmo.2016.03.008
Anagnostopoulou C., Rosas I. M., Singh N., Gugnani N., Chockalingham A., Singh K., et al. (2022). Oocyte quality and embryo selection strategies: a review for the embryologists, by the embryologists. Panminerva Med. 64, 171–184. doi:10.23736/S0031-0808.22.04680-8
Azevedo A. R., Pinho M. J., Silva J., Sa R., Thorsteinsdottir S., Barros A., et al. (2014). Molecular cytogenetics of human single pronucleated zygotes. Reprod. Sci. 21, 1472–1482. doi:10.1177/1933719114530185
Bradley C. K., Traversa M. V., Hobson N., Gee A. J., McArthur S. J. (2017). Clinical use of monopronucleated zygotes following blastocyst culture and preimplantation genetic screening, including verification of biparental chromosome inheritance. Reprod. Biomed. Online 34, 567–574. doi:10.1016/j.rbmo.2017.03.013
Capalbo A., Rienzi L., Cimadomo D., Maggiulli R., Elliott T., Wright G., et al. (2014). Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum. Reprod. 29, 1173–1181. doi:10.1093/humrep/deu033
Capalbo A., Treff N., Cimadomo D., Tao X., Ferrero S., Vaiarelli A., et al. (2017). Abnormally fertilized oocytes can result in healthy live births: improved genetic technologies for preimplantation genetic testing can be used to rescue viable embryos in in vitro fertilization cycles. Fertil. Steril. 108, 1007–1015. doi:10.1016/j.fertnstert.2017.08.004
Chen X., Shi S., Mao J., Zou L., Yu K. (2020). Developmental potential of abnormally fertilized oocytes and the associated clinical outcomes. Front. Physiol. 11, 528424. doi:10.3389/fphys.2020.528424
Destouni A., Dimitriadou E., Masset H., Debrock S., Melotte C., Van Den Bogaert K., et al. (2018). Genome-wide haplotyping embryos developing from 0PN and 1PN zygotes increases transferrable embryos in PGT-M. Hum. Reprod. 33, 2302–2311. doi:10.1093/humrep/dey325
Firmin J., Maitre J. L. (2021). Morphogenesis of the human preimplantation embryo: bringing mechanics to the clinics. Semin. Cell Dev. Biol. 120, 22–31. doi:10.1016/j.semcdb.2021.07.005
Gardner D. K., Schoolcraft W. B. (1999). Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 11, 307–311. doi:10.1097/00001703-199906000-00013
Gardner D. K., Lane M., Stevens J., Schlenker T., Schoolcraft W. B. (2000). Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil. Steril. 73, 1155–1158. doi:10.1016/s0015-0282(00)00518-5
Girard J. M., Simorre M., Leperlier F., Reignier A., Lefebvre T., Barriere P., et al. (2018). Association between early βhCG kinetics, blastocyst morphology and pregnancy outcome in a single-blastocyst transfer program. Eur. J. Obstet. Gynecol. Reprod. Biol. 225, 189–193. doi:10.1016/j.ejogrb.2018.04.037
Goto S., Kadowaki T., Tanaka S., Hashimoto H., Kokeguchi S., Shiotani M. (2011). Prediction of pregnancy rate by blastocyst morphological score and age, based on 1, 488 single frozen-thawed blastocyst transfer cycles. Fertil. Steril. 95, 948–952. doi:10.1016/j.fertnstert.2010.06.067
Guo N., Deng T., Jiang H., Gong Y., Yin L., Ren X., et al. (2020). Association between blastocyst morphology and live birth rate following frozen-thawed single blastocyst transfer: Results from a 5-year retrospective analysis of 2593 cryopreserved blastocysts. J. Obstet. Gynaecol. Res. 46, 2314–2322. doi:10.1111/jog.14423
Hill M. J., Richter K. S., Heitmann R. J., Graham J. R., Tucker M. J., DeCherney A. H., et al. (2013). Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil. Steril. 99, 1283–1289. doi:10.1016/j.fertnstert.2012.12.003
Irani M., Reichman D., Robles A., Melnick A., Davis O., Zaninovic N., et al. (2017). Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil. Steril. 107, 664–670. doi:10.1016/j.fertnstert.2016.11.012
Kemper J. M., Liu Y., Afnan M., Hammond E. R., Morbeck D. E., Mol B. W. J. (2021). Should we look for a low-grade threshold for blastocyst transfer? A scoping review. Reprod. Biomed. Online 42, 709–716. doi:10.1016/j.rbmo.2021.01.019
Kovacic B., Vlaisavljevic V., Reljic M., Cizek-Sajko M. (2004). Developmental capacity of different morphological types of day 5 human morulae and blastocysts. Reprod. Biomed. Online 8, 687–694. doi:10.1016/s1472-6483(10)61650-1
Li M., Dang Y., Wang Y., Li J., Liu P. (2020). Value of transferring embryos derived from monopronucleated (1PN) zygotes at the time of fertilization assessment. Zygote 28, 241–246. doi:10.1017/S096719942000009X
Li M., Huang J., Zhuang X., Lin S., Dang Y., Wang Y., et al. (2021). Obstetric and neonatal outcomes after the transfer of vitrified-warmed blastocysts developing from nonpronuclear and monopronuclear zygotes: a retrospective cohort study. Fertil. Steril. 115, 110–117. doi:10.1016/j.fertnstert.2020.07.019
Maheshwari A., Bari V., Bell J. L., Bhattacharya S., Bhide P., Bowler U., et al. (2022). Transfer of thawed frozen embryo versus fresh embryo to improve the healthy baby rate in women undergoing IVF: the E-freeze RCT. Health Technol. Assess. 26, 1–142. doi:10.3310/AEFU1104
Mateo S., Vidal F., Parriego M., Rodriguez I., Montalvo V., Veiga A., et al. (2017). Could monopronucleated ICSI zygotes be considered for transfer? Analysis through time-lapse monitoring and PGS. J. Assist. Reprod. Genet. 34, 905–911. doi:10.1007/s10815-017-0937-z
Norwitz E. R., Schust D. J., Fisher S. J. (2001). Implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408. doi:10.1056/NEJMra000763
Richter K. S., Harris D. C., Daneshmand S. T., Shapiro B. S. (2001). Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil. Steril. 76, 1157–1167. doi:10.1016/s0015-0282(01)02870-9
Rienzi L., Cimadomo D., Delgado A., Minasi M. G., Fabozzi G., Gallego R. D., et al. (2019). Time of morulation and trophectoderm quality are predictors of a live birth after euploid blastocyst transfer: a multicenter study. Fertil. Steril. 112, 1080–1093. doi:10.1016/j.fertnstert.2019.07.1322
Schoolcraft W. B., Gardner D. K., Lane M., Schlenker T., Hamilton F., Meldrum D. R. (1999). Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil. Steril. 72, 604–609. doi:10.1016/s0015-0282(99)00311-8
Shen X., Long H., Gao H., Guo W., Xie Y., Chen D., Cong Y., et al. (2020). The valuable reference of live birth rate in the single vitrified-warmed BB/BC/CB blastocyst transfer: The cleavage-stage embryo quality and embryo development speed. Front. Physiol. 11, 1102. doi:10.3389/fphys.2020.01102
Si J., Zhu X., Lyu Q., Kuang Y. (2019). Obstetrical and neonatal outcomes after transfer of cleavage-stage and blastocyst-stage embryos derived from monopronuclear zygotes: a retrospective cohort study. Fertil. Steril. 112, 527–533. doi:10.1016/j.fertnstert.2019.04.045
Staessen C., Van Steirteghem A. C. (1997). The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum. Reprod. 12, 321–327. doi:10.1093/humrep/12.2.321
Staessen C., Janssenswillen C., Devroey P., Van Steirteghem A. C. (1993). Cytogenetic and morphological observations of single pronucleated human oocytes after in-vitro fertilization. Hum. Reprod. 8, 221–223. doi:10.1093/oxfordjournals.humrep.a138026
Van den Abbeel E., Balaban B., Ziebe S., Lundin K., Cuesta M. J., Klein B. M., et al. (2013). Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod. Biomed. Online 27, 353–361. doi:10.1016/j.rbmo.2013.07.006
van der Heijden G. W., van den Berg I. M., Baart E. B., Derijck A. A., Martini E., de Boer P. (2009). Parental origin of chromatin in human monopronuclear zygotes revealed by asymmetric histone methylation patterns, differs between IVF and ICSI. Mol. Reprod. Dev. 76, 101–108. doi:10.1002/mrd.20933
Keywords: monopronuclear, morphology grading, pregnancy, live birth, prediction
Citation: Wang T, Si J, Wang B, Yin M, Yu W, Jin W, Lyu Q and Long H (2022) Prediction of live birth in vitrified-warmed 1PN-derived blastocyst transfer: Overall quality grade, ICM, TE, and expansion degree. Front. Physiol. 13:964360. doi: 10.3389/fphys.2022.964360
Received: 08 June 2022; Accepted: 24 October 2022;
Published: 10 November 2022.
Edited by:
Daniela Nogueira, Groupe Inovie, FranceReviewed by:
Andrea Abdala, Art Fertility Clinics, United Arab EmiratesBorut Kovacic, Maribor University Medical Centre, Slovenia
Copyright © 2022 Wang, Si, Wang, Yin, Yu, Jin, Lyu and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qifeng Lyu, bHl1cWlmZW5nQDEyNi5jb20=; Hui Long, bHl1cWlmZW5nX2xoQDEyNi5jb20=
†These authors have contributed equally to this work
 Tiantian Wang
Tiantian Wang Jiqiang Si†
Jiqiang Si† Bian Wang
Bian Wang Mingru Yin
Mingru Yin Hui Long
Hui Long