- 1Department of Nephrology, The Second Hospital of Anhui Medical University, Hefei, China
- 2Blood Purification Center, No. 2 People’s Hospital of Fuyang City, Fuyang, China
- 3Department of Nephrology, The First Affiliated Hospital of Anhui University of Science and Technology, Huainan, China
- 4Department of Nephrology, The Second People’s Hospital of Lu’an City, Lu’an, China
- 5Department of Nephrology, Maanshan People’s Hospital, Maanshan, China
- 6Blood Purification Center, Bozhou People’s Hospital, Bozhou, China
- 7Department of Nephrology, Tongling People’s Hospital, Tongling, China
- 8Department of Nephrology, Lujiang County People’s Hospital, Lujiang, China
- 9Department of Nephrology, Shouxian County Hospital, Shouxian County, China
- 10Department of Nephrology, Hefei Jinnan Kidney Hospital, Hefei, China
- 11Department of Nephrology, Funan County People’s Hospital, Funan County, China
- 12Department of Nephrology, Anqing Municipal Hospital, Anqing, China
- 13Department of Nephrology, Anhui Wanbei Coal-Electricity Group General Hospital, Suzhou, China
- 14Department of Nephrology, The First People’s Hospital of Hefei, Hefei, China
- 15Department of Nephrology, The People’s Hospital of Taihu, Taihu County, China
- 16Blood Purification Center, Huangshan City People’s Hospital, Huangshan, China
- 17Department of Nephrology, Huainan Chao Yang Hospital, Huainan, China
- 18Department of Nephrology, Lixin County People’s Hospital, Lixin County, China
- 19Department of Nephrology, Dongzhi County People’s Hospital, Dongzhi County, China
- 20Department of Nephrology, Tianchang City People’s Hospital, Tianchang, China
- 21Department of Nephrology, Xiaoxian People’s Hospital, Xiaoxian County, China
- 22Department of Nephrology, The Second Affiliated Hospital of Bengbu Medical College, Bengbu, China
- 23Department of Nephrology, The Second People’s Hospital of Hefei, Hefei, China
- 24Department of Nephrology, Huaibei People’s Hospital, Huaibei, China
- 25Department of Nephrology, The People’s Hospital of Xuancheng City, Xuancheng, China
- 26Department of Nephrology, Lujiang County Hospital of TCM, Lujiang County, China
- 27Department of Nephrology, The Fifth People’s Hospital of Hefei, Hefei, China
Objective: Serum magnesium (Mg2+) levels are associated with insulin resistance, hypertension, lipid abnormalities, and inflammation. However, limited studies have indicated the relationship between Mg2+ and multiple system indexes. The purpose of this study was to investigate the association between Mg2+ and allostatic load (AL) in hemodialysis patients.
Methods: A cross-sectional survey was conducted on hemodialysis patients from different centers in Anhui Province, China, between January and December 2020. A total of 3,025 hemodialysis patients were recruited. Their clinical data were measured before hemodialysis. Information was collected by an online self-reported questionnaire and medical record. Serum Mg2+ was divided into three groups by tertiles. A score of AL greater than or equal to 3 was defined as high AL. A binary logistic regression model was applied to examine the relationship between serum Mg2+ and AL.
Results: A total of 1,222 patients undergoing hemodialysis were included, 60% of whom were males (733/1,222). The mean (standard deviation) age of patients was 55.90 (12.75). The median level of serum Mg2+ was 1.22 mmol/L. The rate of high AL levels was 23.4%. Serum Mg2+ was negatively correlated with body mass index, fasting blood glucose (Glu), and C-reactive protein and positively correlated with high-density lipoprotein, low-density lipoprotein, total cholesterol, diastolic blood pressure (DBP), and serum phosphorus. After adjusting for gender, anxiety, diabetes, family residence, lipid-lowering agents, antihypertensive medications, albumin, and Glu, the binary logistic regression model showed that patients with lower levels of serum Mg2+ were more likely have high AL (OR for the T1 group of serum Mg2+:1.945, 95% CI: 1.365–2.773, and OR for the T2 group of serum Mg2+:1.556, 95% CI: 1.099–2.201).
Conclusion: Our data support the hypothesis that higher serum Mg2+ concentrations may contribute to lower health risk in hemodialysis populations. Further randomized controlled trials and cohort studies are warranted to verify whether Mg2+ supplementation could be part of routine examinations in hemodialysis populations.
Introduction
End-stage renal disease (ESRD) represents the final phase of kidney deterioration and is characterized by an estimated GFR of <15 ml/min/1.73 m2, with the occurrence of signs or symptoms of uremia (Carlo et al., 2015). Hemodialysis is the main alternative therapy for sustaining life in ESRD patients (Ahmadmehrabi and Tang, 2018). Every year, about 1 million patients with ESRD are undergoing hemodialysis around the world. The number of hemodialysis patients has been increasing at a global average annual rate of 7% (Shinkawa et al., 2019). More than 1.5 million ESRD patients were reported in China, and 100,000 to 150,000 new cases are reported each year (Xiao et al., 2011). Poor prognosis and high medical costs for hemodialysis patients have become a global public health problem, which seriously puts a heavy burden on health conditions and economic status. In addition, hemodialysis often aggravates physiological and psychological problems, such as calcium and phosphorus metabolism disorders, bone abnormalities, and psychological stress. Some studies showed that stress played an important role in health among hemodialysis patients (García-Llana et al., 2014). However, few studies have comprehensively assessed stress in the hemodialysis population by objective measures.
The concept of allostatic load (AL) was proposed by McEwen and Stellar (1993). AL is defined as the cumulative burden of chronic stress and life events (Guidi et al., 2021). It is also a comprehensive index that reflects multiple biological system disorders and can be used as an early predictor of health risks (Guidi et al., 2021; Nelson et al., 2021). The algorithms of a multisystemic AL index have been developed in some epidemiological studies, which was the sum of various clinical indications of multiple physiological systems that reached subclinical thresholds (Seeman et al., 1997). In recent studies, AL is commonly assessed by three biological systems, namely, metabolic system, autonomic nervous system, and immune system. A higher level of AL is commonly connected to chronic disease (Sabbah et al., 2008; Duru et al., 2012) and it has also been proven that it had a negative impact on chronic kidney disease (Bruce et al., 2015; Norton et al., 2016). In addition, some clinical indicators may also affect AL.
Magnesium (Mg2+) is the fourth most abundant cation in the human body, which is found primarily in bone and skeletal muscle (Navarro-González et al., 2009). The importance of Mg2+ on health has been emphasized by Kruse et al. (1934). Clinical and experimental evidence suggested that serum Mg2+ concentrations declined in humans or animals with chronic disorders (Romani, 2011). Hypomagnesemia was a common complication in dialysis patients with ESRD. Serum Mg2+ levels exceeding 0.9 mmol/L were found in 31.5% of hospitalized patients, whereas levels below 0.7 mmol/L were found in 20.2% of patients (Cheungpasitporn et al., 2015). Dietary limitations, use of loop and thiazide diuretics, and a reduced Mg2+ concentration in the dialyzate all contribute to Mg2+ loss (Navarro-González et al., 2009). Mg2+ may play a vital role in the literature linking magnesium derangements with bone disease, cardiovascular disease, sudden cardiac death, and mortality (Misra and Nessim, 2017; Floege, 2018). A low serum Mg2+ level was thought to contribute to endothelial dysfunction, soft tissue calcification, and cardiac arrhythmias (Shechter et al., 2000; Kanbay et al., 2012). However, the effects of magnesium homeostasis disturbances on multiple biological system disorders in hemodialysis patients are still not well-recognized.
Some studies have attempted to confirm the effects of different Mg2+ levels on health and its potential pathway among patients with chronic diseases. However, the associations between serum Mg2+ and AL are not well-established, particularly in patients undergoing hemodialysis. Therefore, we hypothesize that patients undergoing hemodialysis with low levels of serum Mg2+ may have a greater risk of high-level AL. The present study was conducted with a sample size of 1,222 patients in 27 blood purification centers, which could be beneficial in providing evidence for the clinical effectiveness of magnesium supplementation.
Materials and methods
Participants
A multi-center, cross-sectional study was conducted among patients undergoing hemodialysis from 27 blood purification centers of Anhui Province located in East China between 1 January and 31 December 2020. A total of 3,025 patients were recruited. Inclusion criteria for participants were age ≥18 years old with regular hemodialysis for more than 3 months and provided informed consent. Patients who were <18 years old or refused to participate or were defined as follows: heart failure with NYHA Ⅲ grade or above; complicated with severe liver, lung, brain, and other organ failure diseases, such as cirrhosis, chronic respiratory failure, and hemiplegia; HIV infection or AIDS; complications with malignant tumor psychosis were excluded. A total of 1,803 patients were excluded without C-reactive protein (CRP) data, which was a biological marker of AL. Finally, 1,222 patients were analyzed in the study.
This study was approved by the Ethics Committee of the Second Hospital of Anhui Medical University (No. PJ-YX2020-006). Electronic informed consent was obtained from all participants before completing the survey. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Basic information
An online platform named “h6world” supported by the Clinical Big Data Platform Research Group of Peking University was used to collect data. Before the survey, doctors, nurses, or graduate students of each center were trained. Each center logged into the platform for investigation data entry. All data were summarized to the project leader and were checked by a quality controller, including the demographic characteristics (e.g., gender, age, family residence, family income, etc.), behaviors (e.g., smoking, drinking, etc.), hemodialysis status, psychological states, medications, comorbidities, and clinical data.
Physical examination
For all patients, anthropometric parameters such as body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), and blood samples were collected before hemodialysis. Height measurement is accurate to 0.01 m, and weight measurement is accurate to 0.1 kg, measured with a calibrated electronic scale. The BMI was calculated as weight (kg) divided by height squared (square meters) (kg/m2).
Allostatic load
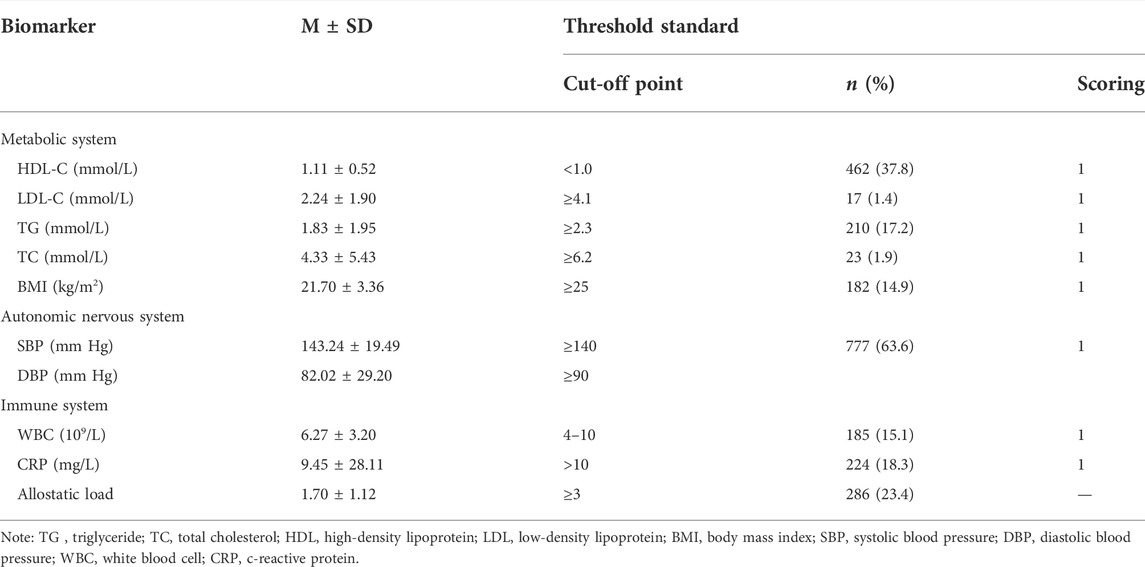
Laboratory analyses were performed on fasting venous blood samples collected before dialysis. AL levels in the hemodialysis population were comprehensively assessed using three biological systems: the metabolic system which contains total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), and BMI; autonomic nervous system (SBP and DBP); immune system containing CRP and white blood cells (WBCs). The cut-off points for blood pressure and BMI were based on the KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients (K/DOQI Workgroup, 2005) and WHO Expert Consultation about the Asian criteria of obesity (WHO Expert Consultation, 2004). The cut-off points for blood lipids in the metabolic system were determined according to the 2016 Chinese guideline for the management of dyslipidemia in adults (Joint committee issued Chinese guideline for the management of dyslipidemia in adults, 2016). The normal range for WBC count was 4–10 × 106/L and for CRP was 0–10 mg/L, which was determined based on clinical laboratory criteria (Davarpanah and Eberhardt, 2020). These data were measured by standard methods in the department of biochemistry. The specific threshold standards are listed in Table 1.
Each biomarker was assigned a value according to the threshold value standard, and the value exceeding the threshold value (HDL was below the threshold value) is assigned a value of 1, otherwise, it is assigned a value of 0. The total scores of AL were obtained by adding up all the indexes, ranging from 0 to 8. High scores mean that patients were suffering a more serious imbalance of the biological system. The 75th percentile of the AL scores was 2. Therefore, participants with total scores ≥3 are defined as the high-AL group, as found in previous studies (Peek et al., 2010; Frei et al., 2015).
Statistical analysis
The measurement data were represented by the mean and standard deviation (SD) for normally distributed data and by median and quartile for skewed distribution data. Two independent t-tests and rank sum tests were used to examine the difference in clinical information between high-AL and low-AL groups. Categorical data were expressed as frequencies (n) and percentages (%). The chi-square test was used to calculate the difference in the percentage of high AL between different groups. Spearman’s correlations were applied to analyze the correlations between serum Mg2+ and clinical index in patients undergoing hemodialysis. AL scores were counted by the cut-off points of eight biomarkers and calculated by numerical transformations. It was not a distribution of probability and was presented as a dichotomous variable. Therefore, binary logistic regression was used to analyze the association between serum Mg2+ and AL. These data were processed by SPSS 26.0. All statistical tests were two-sided, and the significance was set at p < 0.05.
Results
Characteristics of patients undergoing hemodialysis
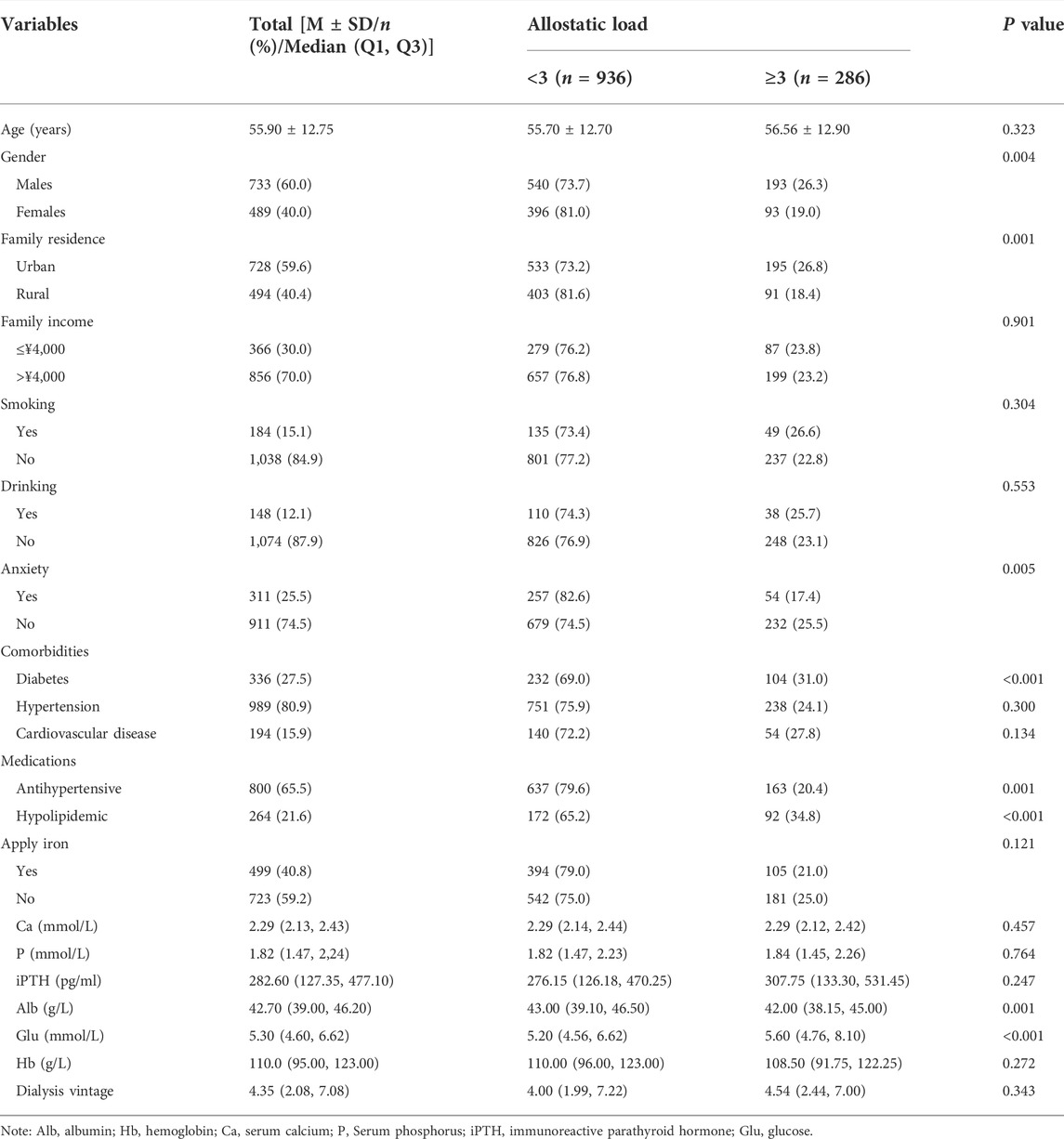
Table 2 lists the demographic and clinical characteristics of 1,222 patients recruited in this study, including 733 males (60.0%) and 489 females (40.0%). The mean age of hemodialysis patients was 55.9 (SD: 12.75), and the percentage of the high level of AL was 23.4% (286/1,222). The median (Q1 and Q3) hemodialysis vintage was 4.35 (2.08 and 7.08) years. The proportion of high AL level was higher in males (26.3%) and lower in females (19.0%). The proportion of high AL level was higher in urban population (26.8%) than in rural population (18.4%). In addition, patients without anxiety disorder reported a higher rate of high AL levels (26.5%). Patients with diabetes reported a higher rate of high AL levels (31.0%). Patients with diabetes reported a higher rate of high AL level (31.0%) than patients without diabetes (20.5%). Patients taking antihypertensive medications reported a lower rate of high AL levels (20.4%) than patients not taking antihypertensive medications (29.1%). However, patients taking hypolipidemic medications reported a higher rate of high AL levels (34.8%) than patients not taking hypolipidemic medications (20.3%). In addition, a lower level of Alb and higher level of Glu were found in the high-AL group. All the aforementioned indexes had statistical significance (p < 0.05).
Spearman’s correlations between serum Mg2+ and clinical data
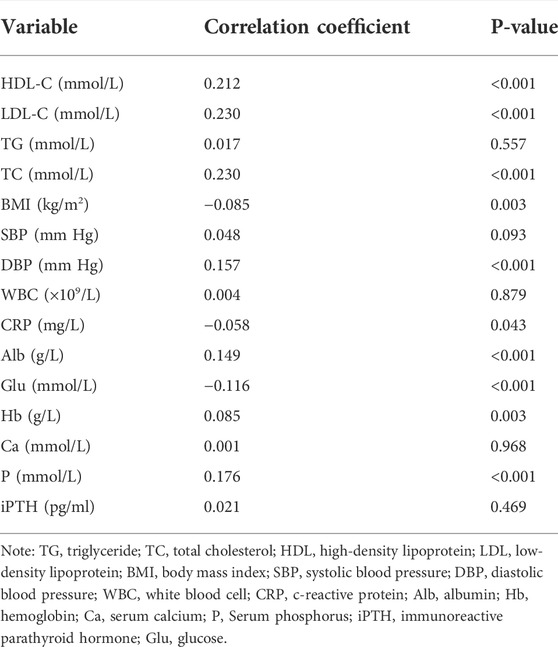
Table 3 shows the results of Spearman’s correlations between serum Mg2+ and clinical indexes in patients undergoing hemodialysis. Serum Mg2+ was positively correlated with HDL (r = 0.212, p < 0.001), LDL (r = 0.230, p < 0.001), TC (r = 0.230, p < 0.001), DBP (r = 0.157, p < 0.001), and P (r = 0.176, p < 0.001) and negatively correlated with Glu (r = −0.116, p < 0.001), BMI (r = −0.085, p = 0.003), and CRP (r = −0.058, p = 0.043). All the aforementioned indexes had statistical significance (p < 0.05). There was no correlation between serum Mg2+ and TG, Ca, iPTH, WBC, or SBP.
The percentage of allostatic load levels in different groups of serum Mg2+ among patients undergoing hemodialysis
As seen in Table 4, serum Mg2+ was divided into three groups by tertiles. The T1 group was defined as having serum Mg2+ concentration <1.00 mmol/L, the T2 group was defined as having serum Mg2+ concentration of 1.00–1.28 mmol/L, and the T3 group for serum Mg2+ concentration ≥1.28 mmol/L. The proportion of high AL levels in the T1 group (34.0%) was higher than that of the T2 (25.4%) and T3 (16.6%) groups, and the difference was statistically significant (p < 0.001).
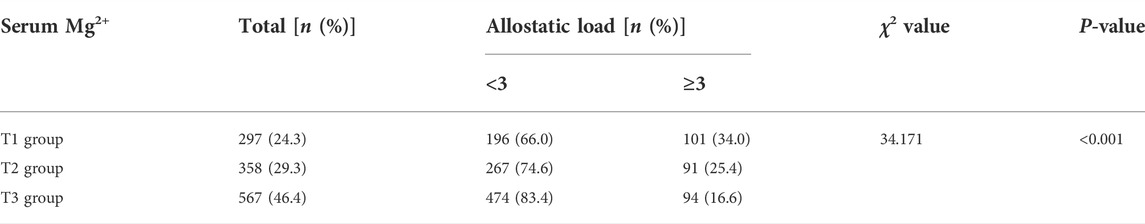
TABLE 4. Percentage of AL levels in different groups of serum Mg2+ among patients undergoing hemodialysis.
The association between serum Mg2+ and allostatic load among patients undergoing hemodialysis
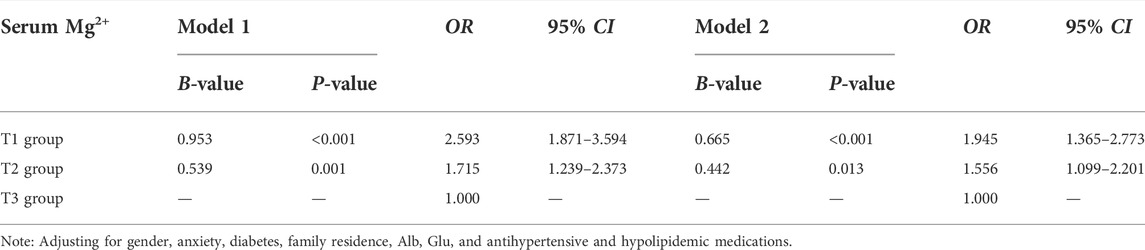
Table 5 depicts the crude and adjusted OR (95% CI) for serum Mg2+ in comparison with that of the reference group (T3 group) for AL. In model 1, the univariate logistic regression results showed that compared with the T3 group, the ORs for the T1 and T2 groups were 2.593 (95% CI: 1.871–3.594) and 1.715 (95% CI: 1.239–2.373), respectively. In model 2, after adjusting for gender, anxiety, diabetes, family residence, antihypertensive and hypolipidemic medications, and Alb and Glu, the results showed that compared with the T3 group, the ORs for the T1 and T2 groups were 1.945 (95% CI: 1.365–2.773) and 1.556 (95% CI: 1.099–2.201), respectively.
Sensitivity analysis of characteristics between the included sample and excluded sample
A sensitivity analysis was performed on the excluded samples and included samples. As shown in Supplementary Table S1, older and more urban patients were included in the analyzed samples. Additionally, the included sample showed greater rates of diabetes (27.5%), hypertension (80.9%), cardiovascular disease (15.9%), and hypolipidemic drugs (21.6%) but a lower rate of antihypertensive medications (73.0%).
Discussion
This is the first study to analyze the correlation between serum Mg2+ and AL. The results showed that a high AL level was common in patients undergoing hemodialysis and was inversely related to serum Mg2+ concentration. The proportion of patients with high levels of AL was 23.4% in our study. Compared with other studies, patients undergoing hemodialysis had almost the same proportion in high levels of AL. For example, a recent pilot study found that the percentage of higher AL levels was 25% among female outpatients with fibromyalgia (Leombruni et al., 2019). However, the percentage of high AL levels may vary in different standards of AL. The percentage of high AL in adolescents was 35% according to the cut-off point of 2 (Theall et al., 2012). AL is the active process that leads to adaptation to a stressor and is defined as “achieving stability via change” (McEwen and Stellar, 1993). Previous findings indicated that higher allostatic load or allostatic overload was associated with poorer health outcomes in both general and clinical populations, such as poor physical health in re-employed populations with no good jobs (Chandola and Zhang, 2018), unexplained infertility in fertile women (Barrett et al., 2018), and overall mortality in patients with metastatic non-small cell lung cancer (Obeng-Gyasi et al., 2022), which supported the clinical efficacy of the trans-diagnostic identification of allostatic load and allostatic overload (Guidi et al., 2021). Thus, we believe that maintaining a low level of AL might be helpful for patients undergoing hemodialysis to decrease risks to health.
It has been found that lower serum Mg2+ was negatively correlated to AL levels in patients undergoing hemodialysis, which was the main novel finding in our study. Mg2+ loss in patients undergoing hemodialysis is less likely to attract the attention of clinicians, which has also been called a “neglected cation” (Alhosaini and Leehey, 2015). In fact, Mg2+ plays an important role in the homeostasis of organisms. It is not only a cofactor for several enzymes involved in most cellular processes (Jahnen-Dechent and Ketteler, 2012). Changes in the level of serum Mg2+ are also involved in a variety of pathological conditions (Tangvoraphonkchai and Davenport, 2018). Due to the controversy over the clinical significance of disturbances in magnesium homeostasis and the true risk of hypomagnesemia and hypermagnesemia, this “neglect” was proposed (Alhosaini and Leehey, 2015). A large study in Japan showed that there was a relationship between low magnesium concentrations and mortality, which means that hypomagnesemia was an important predictor of cardiovascular or non-cardiovascular mortality. Hypermagnesemia is also associated with higher mortality, but the intensity of correlation was smaller than that of hypomagnesemia (Sakaguchi et al., 2014).
Increasing risks for AL were connected to low serum Mg2+. Mg2+ was strongly linked to the cardiovascular system, inflammation, insulin resistance, and metabolic syndrome (Belin and He, 2007). Some studies found that magnesium deficiency is associated with an increased risk of hypertension (Liu and Dudley, 2020), diabetes (Piuri et al., 2021), and mortality (Sakaguchi et al., 2014). As mentioned previously, Mg2+ is an essential cofactor for many enzymes involved in glucose metabolism. In animal experiments, the onset of diabetes appears to be delayed in model rats supplemented with Mg2+, whereas animals with low Mg2+ levels show elevated serum glucose levels and altered lipid patterns (Mancuso et al., 2020). Other studies showed that magnesium supplementation improves the insulin resistance index and beta cell function and reduces hemoglobin A1c (HbA1c) levels in patients with type 2 diabetes mellitus (Rodrı´guez-Mora´n and Guerrero-Romero, 2003; Guerrero-Romero and Rodrı´guez-Mora´n, 2011). In our study, a strong negative correlation between Mg2+ and blood glucose was found, which is consistent with previous findings. In addition, Mg2+ has been found to be associated with dyslipidemia in the general population, and our study also indicated that Mg2+ was strongly correlated to related blood lipid indexes in the hemodialysis population. Our results also proved the evidence for the effect of Mg2+ on dyslipidemia, similar to a previous study (He et al., 2006). Moreover, it has been found that serum Mg2+ also had a strong correlation with diastolic blood pressure. However, no studies have focused on the connection between Mg2+ and blood pressure, but there are some studies reporting the effects of different magnesium concentrations in the dialyzate on blood pressure. For example, a study in Greece showed that hypotensive episodes during dialysis were least common in using the highest magnesium dialyzate and most common in using the lowest magnesium dialyzate. In addition, studies in Egypt and Iran also support this view (Kyriazis et al., 2004; Elsharkawy et al., 2006; Pakfetrat et al., 2010). However, there are also some opposite results. Different serum Mg2+ concentrations in the dialyzate have no significant effect on blood pressure in dialysis patients (Deng et al., 2015). Future studies are needed to verify whether a higher serum Mg2+ concentration has a protective effect on blood pressure control. Interestingly, we found that CRP was negatively correlated with Mg2+. Low Mg2+ status was accompanied by an elevation of CRP, and Mg2+ supplementation reduces CRP levels (Mahabir, 2014; Mazidi et al., 2018; Nielsen, 2018).
The present large-scale, multi-center study contributed to high reliability of the findings. However, some limitations should be addressed. First, a cross-sectional design could not provide causal associations, and prospective cohort studies are needed to verify causal associations between serum Mg2+ and AL in the future. Second, patients without complete biomarkers of AL were excluded, which may influence the results. To determine the impact of the excluded samples on the results, a sensitivity analysis was performed. The results revealed that there were some differences in age, family residence, hypertension, diabetes, cardiovascular disease, and antihypertensive and hypolipidemic medication. Consistent with the findings, participants included in this study may have overestimated the percentage of high AL. Therefore, the inclusion criteria which strictly controlled and stratified the analysis of patients with comorbidity are warranted. Third, the components of AL in different studies were not unified, which commonly consisted of the metabolic system, autonomic nervous system, and immune system. The neuroendocrine index of AL was lacking in the present study, but the indicators selected in our study were well-validated in the different biological systems, and the results were in line with those of our original hypothesis and previous studies. At last, it should also be mentioned that the generalizability of our findings to different ethnicities is unknown due to significant worldwide disparities in the clinical pattern and prognosis of hemodialysis patients.
Conclusion
This is the first study to examine serum Mg2+ and AL in the hemodialysis population. Patients undergoing hemodialysis with lower levels of serum Mg2+ are more likely to be at higher health risks. In addition, this study also provides evidence for the effects of serum Mg2+ on blood lipids, glucose metabolism, blood pressure, and immunity. The findings could provide empirical evidence for the intervention of Mg2+ supplements on reducing the health risk of the hemodialysis population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by No. PJ-YX2020-006. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ was responsible for data analysis and writing. ZY, HY, XiuL, ZL, YB, GQ, HaW, JL, YG, SY, LC, JiaY, JH, SM, JinY, LY, RS, XJ, HoW, FZ, TC, XinL, XZ, LL, JF, WW, YZ, and GS were responsible for investigation and data collection. DW was responsible for funding acquisition. ST was responsible for project administration.
Funding
The study was supported by the Science Foundation of Anhui Medical University (2019xkj140); the Clinical Research Incubation Program of the Second Hospital of Anhui Medical University (2020LCZD01); the Co-construction Project of Clinical and Preliminary Disciplines of Anhui Medical University in 2020 (2020lcxk022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.963914/full#supplementary-material
SUPPLEMENTARY TABLE S1Sensitivity analysis of characteristics between the included sample and excluded sample.
References
Ahmadmehrabi S., Tang W. H. W. (2018). Hemodialysis-induced cardiovascular disease. Semin. Dial. 31 (3), 258–267. doi:10.1111/sdi.12694
Alhosaini M., Leehey D. J. (2015). Magnesium and dialysis: The neglected cation. Am. J. Kidney Dis. 66 (3), 523–531. doi:10.1053/j.ajkd.2015.01.029
Barrett E. S., Vitek W., Mbowe O., Thurston S. W., Legro R. S., Alvero R., et al. (2018). Allostatic load, a measure of chronic physiological stress, is associated with pregnancy outcomes, but not fertility, among women with unexplained infertility. Hum. Reprod. 33 (9), 1757–1766. doi:10.1093/humrep/dey261
Belin R. J., He K. (2007). Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes. Res. 20 (2), 107–129.
Bruce M. A., Griffith D. M., Thorpe R. J. (2015). Stress and the kidney. Adv. Chronic Kidney Dis. 22 (1), 46–53. doi:10.1053/j.ackd.2014.06.008
Carlo J. O., Phisitkul P., Phisitkul K., Reddy S., Amendola A. (2015). Perioperative implications of end-stage renal disease in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 23 (2), 107–118. doi:10.5435/JAAOS-D-13-00221
Chandola T., Zhang N. (2018). Re-Employment, job quality, health and allostatic load biomarkers: Prospective evidence from the UK household longitudinal study. Int. J. Epidemiol. 47 (1), 47–57. doi:10.1093/ije/dyx150
Cheungpasitporn W., Thongprayoon C., Qian Q. (2015). Dysmagnesemia in hospitalized patients: Prevalence and prognostic importance. Mayo Clin. Proc. 90 (8), 1001–1010. doi:10.1016/j.mayocp.2015.04.023
Davarpanah A. H., Eberhardt L. W. (2020). Case 282. Radiology 295 (3), 733–735. doi:10.1148/radiol.2020190435
Deng F., Hong D. Q., Pu L. (2015). Impact of different magnesium concentrations in dialysate on serum magnesium normalization and blood pressure in hemodialysis patients. Pract. J. Clin. Med. 12 (3), 28–30.
Duru O. K., Harawa N. T., Kermah D., Norris K. C. (2012). Allostatic load burden and racial disparities in mortality. J. Natl. Med. Assoc. 104 (1-2), 89–95. doi:10.1016/s0027-9684(15)30120-6
Elsharkawy M. M., Youssef A. M., Zayoon M. Y. (2006). Intradialytic changes of serum magnesium and their relation to hypotensive episodes in hemodialysis patients on different dialysates. Hemodial. Int. 10 (2), S16–S23. doi:10.1111/j.1542-4758.2006.00120.x
Floege J. (2018). Magnesium concentration in dialysate: Is higher better? Clin. J. Am. Soc. Nephrol. 13 (9), 1309–1310. doi:10.2215/CJN.08380718
Frei R., Haile S. R., Mutsch M., Rohrmann S. (2015). Relationship of serum vitamin D concentrations and allostatic load as a measure of cumulative biological risk among the us population: A cross-sectional study. PLoS One 10 (10), e0139217. doi:10.1371/journal.pone.0139217
García-Llana H., Remor E., Del Peso G., Selgas R. (2014). The role of depression, anxiety, stress and adherence to treatment in dialysis patients’ health-related quality of life: A systematic review of the literature. Nefrologia 34 (5), 637–657. doi:10.3265/Nefrologia.pre2014.Jun.11959
Guerrero-Romero F., Rodrı´guez-Mora´n M. (2011). Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: Double-blind, randomized clinical trial. Eur. J. Clin. Invest. 41 (4), 405–410. doi:10.1111/j.1365-2362.2010.02422.x
Guidi J., Lucente M., Sonino N., Fava G. A. (2021). Allostatic load and its impact on health: A systematic review. Psychother. Psychosom. 90 (1), 11–27. doi:10.1159/000510696
He K., Liu K., Daviglus M. L., Morris S. J., Loria C. M., Van Horn L., et al. (2006). Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 113 (13), 1675–1682. doi:10.1161/CIRCULATIONAHA.105.588327
Jahnen-Dechent W., Ketteler M. (2012). Magnesium basics. Clin. Kidney J. 5 (1), i3–i14. doi:10.1093/ndtplus/sfr163
Joint committee issued Chinese guideline for the management of dyslipidemia in adults. (2016). Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 44 (10), 833–853. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
K/DOQI Workgroup (2005). K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 45 (3), S1–S153.
Kanbay M., Yilmaz M. I., Apetrii M., Saglam M., Yaman H., Unal H. U., et al. (2012). Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am. J. Nephrol. 36 (3), 228–237. doi:10.1159/000341868
Kruse H. D., Schmidt M. M., Mccollum E. V. (1934). Studies on magnesium deficiency in animals 5 Changes in the mineral metabolism of animals following magnesium deprivation. J. Biol. Chem. 106, 553–572. doi:10.1016/s0021-9258(18)75432-x
Kyriazis J., Kalogeropoulou K., Bilirakis L., Smirnioudis N., Pikounis V., Stamatiadis D., et al. (2004). Dialysate magnesium level and blood pressure. Kidney Int. 66 (3), 1221–1231. doi:10.1111/j.1523-1755.2004.00875.x
Leombruni P., Zizzi F., Pavan S., Fusaro E., Miniotti M. (2019). Allostatic overload in patients with fibromyalgia: Preliminary findings. Psychother. Psychosom. 88 (3), 180–181. doi:10.1159/000496229
Liu M., Dudley S. C. (2020). Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants (Basel) 9 (10), 907. doi:10.3390/antiox9100907
Mahabir S. (2014). Dietary magnesium and inflammation. Eur. J. Clin. Nutr. 68 (8), 970. doi:10.1038/ejcn.2014.110
Mancuso E., Perticone M., Spiga R., Averta C., Rubino M., Fiorentino T. V., et al. (2020). Association between serum Mg2+ concentrations and cardiovascular organ damage in a cohort of adult subjects. Nutrients 12 (5), 1264. doi:10.3390/nu12051264
Mazidi M., Rezaie P., Banach M. (2018). Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. 14 (4), 707–716. doi:10.5114/aoms.2018.75719
McEwen B. S., Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 153 (18), 2093–2101. doi:10.1001/archinte.1993.00410180039004
Misra P. S., Nessim S. J. (2017). Clinical aspects of magnesium physiology in patients on dialysis. Semin. Dial. 30 (5), 438–445. doi:10.1111/sdi.12613
Navarro-González J. F., Mora-Fernández C., García-Pérez J. (2009). Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin. Dial. 22 (1), 37–44. doi:10.1111/j.1525-139X.2008.00530.x
Nelson B. W., Sheeber L., Pfeifer J., Allen N. B. (2021). Psychobiological markers of allostatic load in depressed and nondepressed mothers and their adolescent offspring. J. Child. Psychol. Psychiatry 62 (2), 199–211. doi:10.1111/jcpp.13264
Nielsen F. H. (2018). Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 11, 25–34. doi:10.2147/JIR.S136742
Norton J. M., Moxey-Mims M. M., Eggers P. W., Narva A. S., Star R. A., Kimmel P. L., et al. (2016). Social determinants of racial disparities in CKD. J. Am. Soc. Nephrol. 27 (9), 2576–2595. doi:10.1681/ASN.2016010027
Obeng-Gyasi S., Li Y., Carson W. E., Reisenger S., Presley C. J., Shields P. G., et al. (2022). Association of allostatic load with overall mortality among patients with metastatic non-small cell lung cancer. JAMA Netw. Open 5 (7), e2221626. doi:10.1001/jamanetworkopen.2022.21626
Pakfetrat M., Roozbeh Shahroodi J., Malekmakan L., Zare N., Hashemi Nasab M., Hossein Nikoo M. (2010). Is there an association between intradialytic hypotension and serum magnesium changes? Hemodial. Int. 14 (4), 492–497. doi:10.1111/j.1542-4758.2010.00477.x
Peek M. K., Cutchin M. P., Salinas J. J., Sheffield K. M., Eschbach K., Stowe R. P., et al. (2010). Allostatic load among non-hispanic whites, non-hispanic blacks, and people of Mexican origin: Effects of ethnicity, nativity, and acculturation. Am. J. Public Health 100 (5), 940–946. doi:10.2105/AJPH.2007.129312
Piuri G., Zocchi M., Della Porta M., Ficara V., Manoni M., Zuccotti G. V., et al. (2021). Magnesium in obesity, metabolic syndrome, and type 2 diabetes. Nutrients 13 (2), 320. doi:10.3390/nu13020320
Rodrı´guez-Mora´n M., Guerrero-Romero F. (2003). Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: A randomized double-blind controlled trial. Diabetes Care 26 (4), 1147–1152. doi:10.2337/diacare.26.4.1147
Romani A. M. (2011). Cellular magnesium homeostasis. Arch. Biochem. Biophys. 512 (1), 1–23. doi:10.1016/j.abb.2011.05.010
Sabbah W., Watt R. G., Sheiham A. (2008). Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: Evidence from the third national health and nutrition examination survey. J. Epidemiol. Community Health 62 (5), 415–420. doi:10.1136/jech.2007.064188
Sakaguchi Y., Fujii N., Shoji T., Hayashi T., Rakugi H., Isaka Y. (2014). Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 85 (1), 174–181. doi:10.1038/ki.2013.327
Seeman E., Singer B. H., Rowe J., Horwitz R. I., McEwen B. S. (1997). Price of adaptation-allostatic load and its health consequences. MacArthur studies of successful aging. Arch. Intern. Med. 157, 2259–2268. doi:10.1001/archinte.157.19.2259
Shechter M., Sharir M., Labrador M. J., Bairey Merz C. N., Silver B., Bairey Merz C. N. (2000). Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 102 (19), 2353–2358. doi:10.1161/01.cir.102.19.2353
Shinkawa H., Yasunaga H., Hasegawa K., Matsui H., Michihata N., Fushimi K., et al. (2019). Mortality and morbidity after pancreatoduodenectomy in patients undergoing hemodialysis: Analysis using a national inpatient database. Surgery 165 (4), 747–750. doi:10.1016/j.surg.2018.10.009
Tangvoraphonkchai K., Davenport A. (2018). Magnesium and cardiovascular disease. Adv. Chronic Kidney Dis. 25 (3), 251–260. doi:10.1053/j.ackd.2018.02.010
Theall K. P., Drury S. S., Shirtcliff E. A. (2012). Cumulative neighborhood risk of psychosocial stress and allostatic load in adolescents. Am. J. Epidemiol. 176 (7), S164–S174. doi:10.1093/aje/kws185
WHO Expert Consultation (2004). Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363 (9403), 157–163. doi:10.1016/S0140-6736(03)15268-3
Keywords: allostatic load, magnesium, hemodialysis, clinical, stress
Citation: Zhang Y, Yang Z, Yang H, Li X, Liu Z, Bai Y, Qian G, Wu H, Li J, Guo Y, Yang S, Chen L, Yang J, Han J, Ma S, Yang J, Yu L, Shui R, Jin X, Wang H, Zhang F, Chen T, Li X, Zong X, Liu L, Fan J, Wang W, Zhang Y, Shi G, Wang D and Tao S (2022) A multi-center study on the association between serum magnesium levels and allostatic load in hemodialysis patients. Front. Physiol. 13:963914. doi: 10.3389/fphys.2022.963914
Received: 23 June 2022; Accepted: 12 September 2022;
Published: 03 October 2022.
Edited by:
Ana Cusumano, Centro de Educación Médica e Investigaciones Clínicas Norberto Quirno (CEMIC), ArgentinaReviewed by:
Jeffrey Howard, University of Texas at San Antonio, United StatesRoland Wohlgemuth, Lodz University of Technology, Poland
János Nemcsik, Semmelweis University, Hungary
Copyright © 2022 Zhang, Yang, Yang, Li, Liu, Bai, Qian, Wu, Li, Guo, Yang, Chen, Yang, Han, Ma, Yang, Yu, Shui, Jin, Wang, Zhang, Chen, Li, Zong, Liu, Fan, Wang, Zhang, Shi, Wang and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deguang Wang, d2FuZ2RlZ3VhbmdAYWhtdS5lZHUuY24=; Shuman Tao, c2h1bWFudGFvQDEyNi5jb20=
†These authors have contributed equally to this work
 Yingxin Zhang
Yingxin Zhang Zhengling Yang1†
Zhengling Yang1† Shuman Tao
Shuman Tao