- 1Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi, China
- 2Wuxi Fisheries College, Nanjing Agricultural University, Wuxi, China
- 3Freshwater Fisheries Research Institute of Jiangsu Province, Nanjing, China
- 4College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, China
Eriocheir sinensis is widely appreciated by the surrounding population due to its culinary delicacy and rich nutrients. The E. sinensis breeding industry is very prosperous and molting is one of the important growth characteristics. Research on the regulation of molting in E. sinensis is still in the initial stages. There is currently no relevant information on the regulatory mechanisms of heart development following molting. Comparative transcriptome analysis was used to study developmental regulation mechanisms in the heart of E. sinensis at the post-molt and inter-molt stages. The results indicated that many regulatory pathways and genes involved in regeneration, anti-oxidation, anti-aging and the immune response were significantly upregulated after molting in E. sinensis. Aside from cardiac development, the differentially expressed genes (DEGs) were relevant to myocardial movement and neuronal signal transduction. DEGs were also related to the regulation of glutathione homeostasis and biological rhythms in regard to anti-oxidation and anti-aging, and to the regulation of immune cell development and the immune response. This study provides a theoretical framework for understanding the regulation of molting in E. sinensis and in other economically important crustaceans.
Introduction
The Yangtze River is 6,397 km long and ranks third in the world in both length and water flow. The Yangtze River basin has very rich biodiversity and contains 424 different fish species and more than 29,000 kinds of phytoplankton and benthic organisms, making it an important region for global biodiversity conservation (Lv et al., 2016; Jin et al., 2022; Zhang et al., 2022). E. sinensis is an economically valuable catadromous species found in the Yangtze River. Due to its high mobility and ability for osmotic regulation, E. sinensis now has a global distribution that includes Europe and America (Gillard et al., 2017; Spiridonov and Zalota, 2017). E. sinensis is rich in nutrients and flavor and is known as “one of the three delicacies of the Yangtze River”. It has high economic value and the E. sinensis breeding industry has developed rapidly (Song and Zhao, 2018; Wang et al., 2018). Molting is a typical characteristic of crustaceans including crabs and shrimps, allowing them to grow discontinuously throughout their life cycle. Study of the regulatory mechanism of E. sinensis molting is important for the protection of wild crab resources and for the development of an economically viable crab breeding industry.
Based on the morphological characteristics of the setae and on the retraction degree of the epidermis, the molting cycle can be divided into four stages: pre-molting (D), inter-molting (C), post-molting (AB) and molting (E). During the post-molt stage, water is quickly absorbed and the exoskeleton gradually hardens. During the inter-molt stage, the exoskeleton continues to harden and mineralize, while the muscle gradually enlarges and the water content decreases. During the pre-molt period, the old skeleton decomposes and is absorbed, while a new skeleton and pigment layer begin to form (Kang et al., 2012). Molting appears to be coordinated by several hormones produced in the central nervous and endocrine systems. This occurs mainly through secretion of the molting inhibition hormone (MIH) by the X-organ/sinus gland complex in the eye-stalk. In addition, a transcription factor composed of the ecdysone receptor and retinoid X receptor acts on Y organs to regulate the synthesis and secretion of the hormone ecdysone by these organs. These two antagonistic hormones act jointly to regulate the molting process of E. sinensis (Das et al., 2018).
Current research on the regulation of E. sinensis molting focuses mainly on the influence of external environmental factors and nutritional elements, including temperature, pH, vitamins, etc. (Yu et al., 2018; Chen et al., 2019; Wang et al., 2020a; Liu et al., 2021a; Zhang et al., 2021). Moreover, the role of several regulatory genes such as V-ATPase subunit B (VATB), transforming growth factor-beta type I receptor (TGFBR1) and S6 kinase has also been studied (Tian et al., 2019; Hou et al., 2020; Tian et al., 2020). The results indicated that V-ATPase subunit B plays essential roles in the cuticle formation process of Eriocheir sinensis. Transforming growth factor-β type I receptor regulates gonad and muscle development of E. sinensis. S6 kinase also plays an important regulatory role in muscle growth during E. sinensis molting process (Tian et al., 2019; Hou et al., 2020; Tian et al., 2020).
Following molting, the newly formed epidermis is soft and therefore prone to invasion and infection by pathogens. Frequent death after molting greatly influences the survival rate of adult E. sinensis and is one of the main problems of the E. sinensis culture industry (Wang et al., 2015). So far there have been few reports on the regulation of E. sinensis at the post-molting stage, with only one study reporting on the structure and composition of the exoskeleton during the molting process (Tian et al., 2013).
As one of the critical organs and a core component of the circulatory system, the heart plays an important role in various life activities such as development and reproduction (Goepel and Wirkner, 2020). Currently, only a few studies have been published on the heart in crabs and these involve the influence of different environmental factors such as hypoxia and temperature on the heart rate (Kushinsky et al., 2019; Zainal and Noorani, 2019; Singh et al., 2020a; Singh et al., 2020b; Levinton et al., 2020). The results indicated that crab performed cardiac compensation in response to declining dissolved oxygen. They had different strategies on heart rate under water, air and different temperature condition. Their heart rate had strong dependence to temperature. Their heart rhythm stability was better than polyric rhythm under high temperature condition (Kushinsky et al., 2019; Zainal and Noorani, 2019; Singh et al., 2020a; Singh et al., 2020b; Levinton et al., 2020). In contrast to vertebrates, the hearts of crustaceans have strong regenerative potential. After each molting, the hearts of crustaceans such as crabs and shrimps can regenerate (Xiong, 2020). It is therefore interesting to explore the regulatory mechanism of heart development in E. sinensis after molting. High-throughput sequencing allows molecular analysis to be carried out on a broader and more profound level (Min et al., 2019; Avarre, 2020; Liu et al., 2021b; Feng et al., 2022). Studies on the regulation of molting in E. sinensis are still at the preliminary stage and hence transcriptome analysis should allow rapid screening of the regulatory pathways and genes involved.
In the present study, comparative transcriptome analysis was performed on the heart tissue of E. sinensis at the post-molt and inter-molt stages. The aim was to identify critical regulatory pathways and genes during the post-molt developmental stage of E. sinensis, thereby providing a theoretical framework for better understanding of this process. This study should also provide a theoretical basis for use in the breeding industries of E. sinensis and other crustaceans, as well as for further research into organ regeneration in vertebrates.
Materials and methods
Ethics statement
The study was approved by the Animal Care and Use Committee of the Freshwater Fisheries Research Center at the Chinese Academy of Fishery Sciences. All the experiments conformed to the Guidelines for the Care and Use of Laboratory Animals set by the Animal Care and Use Committee of the Freshwater Fisheries Research Center (2003WXEP61, Jan 6th of 2003), and the study was carried out under a field permit (No. 20182AC1699).
Experimental crabs and sample collection
One-year-old juvenile E. sinensis crabs (average body weight of (11.6 ± 0.68 g)) were obtained from the Jiangsu Noah’s Ark Agricultural Science and Technology Co. Ltd. Animals at the same developmental stage were selected and cultured in three aquariums. Twenty female E. sinensis crabs and the same number of male juveniles were grown in the same aquarium. The aquariums were continuously aerated and the water quality monitored every day, Water temperature was (19 ± 0.5)°C, pH was (7.5 ± 0.2), concentration of dissolved oxygen was (6 ± 0.3) mg/L, concentration of NH3-N and NO2− was lower than 0.1 mg/L and 0.005 mg/L, respectively. E. sinensis were given a compound feed each day at 14:00 and 17:00. The molting stage was determined according to Kang et al. (Wang et al., 2018). Cameras were installed in each aquarium and the molting process was observed continuously for 24 h each day. The heart was collected within 30 min after molting, with one male sample and one female sample collected from each tank. The same number of heart samples was also collected from crabs at the inter-molt phase. The body size parameters of all E. sinensis crabs were measured before heart collection.
Total RNA extraction and illumina sequencing
Total RNA was extracted with RNAiso reagent according to the manufacturer’s instructions (TaKaRa, Japan). Equal amounts of total RNA from the heart of one female crab and one male crab at the same developmental stage and from each tank were pooled to form one sample. In total, three samples were obtained from the post-molt stage (MP) and three from the inter-molt stage (MI). RNA samples were checked for quality and the quantification of extracted total RNA, construction of cDNA library, and high-throughput sequencing were performed according to the methods reported in our previous study (Wang et al., 2020b). The raw data generated in this study was submitted to the NCBI (NCBI, United States) with accession number PRJNA836628.
Data filtering and assembly
Raw data were filtered using the NGS QC TOOLKIT V2.3.3 software (Roche, United States). Some low quality reads, contaminated reads, and primer and adapter sequences were removed (Patel and Jain, 2012). The filtered clean data was assembled using Trinity software (v2.2.0) (Grabherr et al., 2011).
Transcriptome annotation
Unigenes were aligned in accordance with the following databases: non-redundant protein (Nr), non-redundant nucleotides (Nt), Swiss-prot (http://www.uniprot.org/downloads), clusters of orthologous groups for eukaryotic complete genomes (KOG, ftp://ftp.ncbi.nih.gov/pub/COG/KOG/kyva), and the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/pathway.html) (Altschul et al., 1990; Kanehisa et al., 2008). Gene ontology (GO) homology annotation was carried out using Blast2GO software (Conesa et al., 2005).
Differential gene expression analysis
Differential gene expression analysis was carried out using the DESeq R package (1.18.0) (Anders and Huber, 2012). Fold-change was calculated as the ratio of the expression level of genes in the MI sample to the MP sample. |log2foldchange| > 1 and padj < 0.05 (adjusted p value) were set as the cutoff thresholds for DEGs. The detailed method for DGE analysis was described in our previous study (Wang et al., 2020b). GO and KEGG enrichment analyses were carried out on DEGs (padj <0.05). Finally, the top 30 GO terms and top 30 KEGG pathways were identified using methods described in our previous study (Wang et al., 2020b).
Quantitative real-time PCR (qPCR) validation
The accuracy of high-throughput sequencing data was validated with qPCR. Ten DEGs were randomly selected from the transcriptome data for qPCR analysis using the ABI 7500 real-time PCR system (ABI, United States). Primers were designed with Primer Premier six software and the primer sequences are shown in Supplementary Material S1. Beta-actin was used as the internal reference. Amplifications were performed with the following program: 95°C for 30 s, 40 cycles of 95°C for 5 s, 60 °C for 35 s, and 72°C for 52 s. Each sample was studied in triplicate and gene expression levels were calculated with the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Statistical analysis
Statistical significance (p < 0.05) was calculated using one-way ANOVA and Duncan’s multiple range tests (SPSS 21.0). Values were shown as (Mean ± Standard Error). The minimum significance level was set to 0.05. When distribution of data was skewed, the Dunn–Bonferroni post hoc method following Kruskal–Wallis test was used (Cai et al., 2020a).
Results
Sequencing and assembly of the E. sinensis heart transcriptome
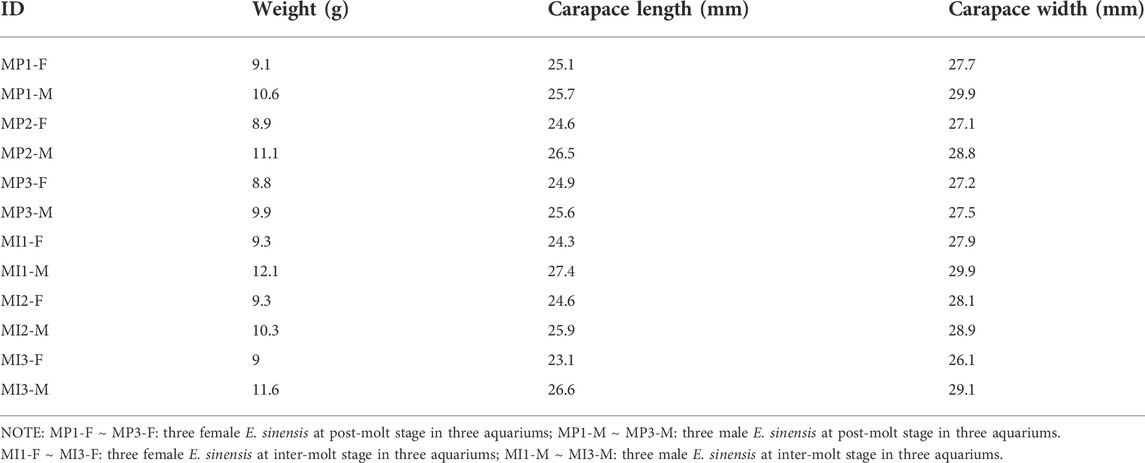
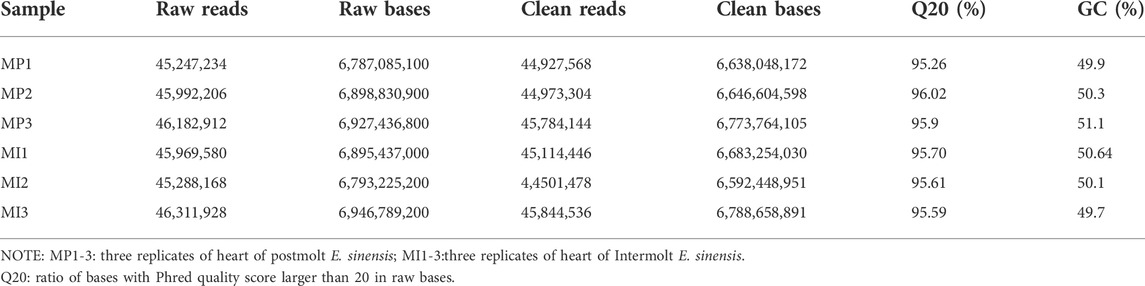
Body size parameters for E. sinensis at post-molt and inter-molt stages are shown in Table 1. In total, 271,145,476 clean data were generated (Table 2). In this study, Q20 value were more than 95%, these indicated that base calling accuracy for more than 95% data reached 99% and thus met the requirement for further analysis. After assembly, 169,812 unigenes were obtained. Of these, 103,924 unigenes ranged from 501 to 1000bp, 6,113 unigenes were grater than 1000bp in length. The average length was 961bp, N50 was 1109bp.
Top 30 GO enrichment analysis of DEGs at the post-molt and inter-molt stages
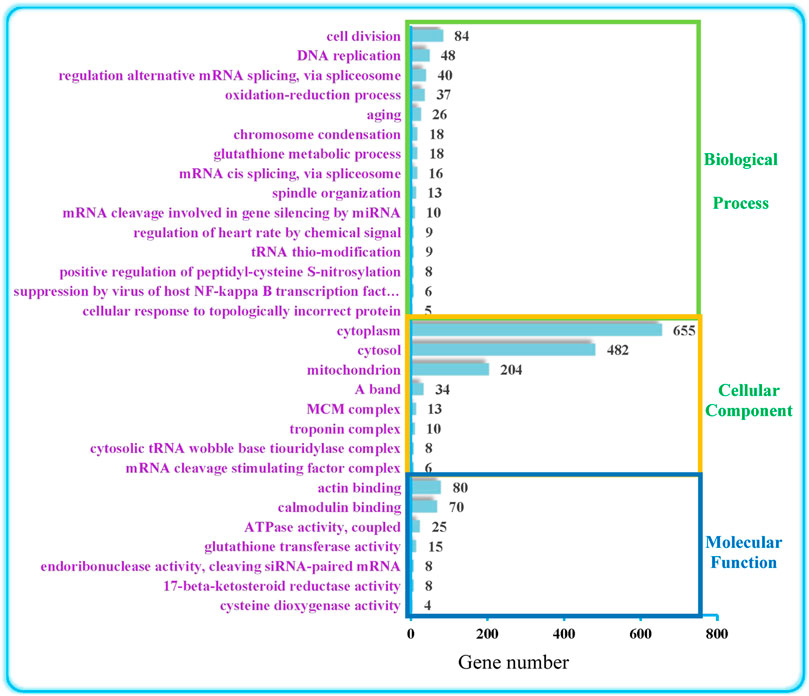
As shown in Figure 1, GO can be divided into the three levels of Biological Process (BP), Molecular Function (MF), and Cellular Component (CC). These were further classified using the online program Ontobee analysis. The results indicated that BP was mainly involved in the regulation of anti-oxidation (cellular response to topologically incorrect protein, positive regulation of peptidyl-cysteine S-nitrosylation, glutathione metabolic process, aging, oxidation-reduction process), circulatory system regulation (regulation of heart rate by chemical signal) and nucleic acid metabolic processes (tRNA thio-modification, mRNA cleavage involved in gene silencing by miRNA, mRNA cis splicing via spliceosome, regulation of alternative mRNA splicing via spliceosome, DNA replication), cytoskeleton and organelle organization (spindle organization, chromosome condensation). CC mainly involved the regulation of myocardial movement (troponin complex, A band), organelle relevant to energy metabolism (mitochondrion) and organelle relevant to nucleic acid metabolism (mRNA cleavage stimulating factor complex, cytosolic tRNA wobble base tiouridylase complex). MF involved mainly binding activity relevant to cytoskeleton protein and signal transduction regulatory protein (calmodulin binding, actin binding), antioxidant enzyme regulation (cysteine dioxygenase activity, 17-beta-ketosteroid reductase activity, glutathione transferase activity), energy metabolism relevant enzyme activity (ATPase activity, coupled) and nuclease activity (endoribonuclease activity, cleaving siRNA-paired mRNA).
Top 30 KEGG enrichment analysis
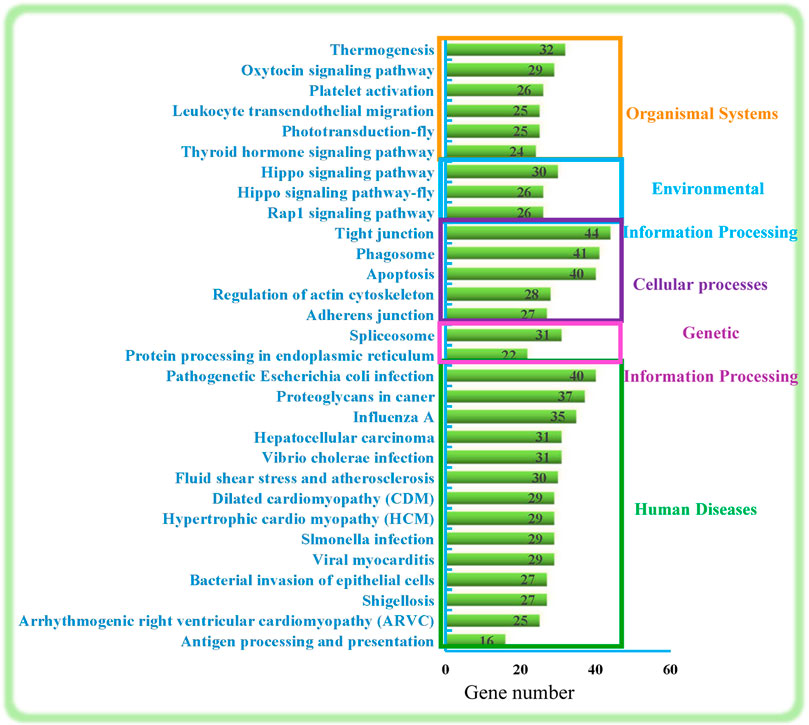
As shown in Figure 2, the top 30 KEGG pathways were classified into five categories: Organismal Systems, Environmental Information Processing, Cellular processes, Genetic Information Processing, and Human Diseases. In general, these mainly involve regulation of the immune system (Leukocyte transendothelial migration, the phagosome, and antigen processing and presentation), regulation of regeneration and development (Rap1 signaling pathway, Hippo signaling pathway-fly, Hippo signaling pathway, Thyroid hormone signaling pathway, regulation of actin cytoskeleton and apoptosis) and genetic information processing and signal transduction (protein processing in endoplasmic reticulum, spliceosome, adherens junction, tight junction).
Regulatory network between post-molt and inter-molt stages in the heart of E. sinensis
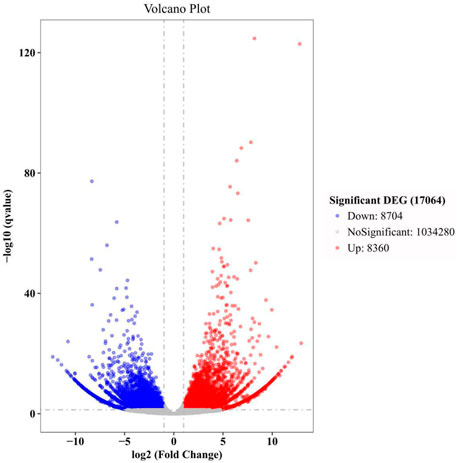
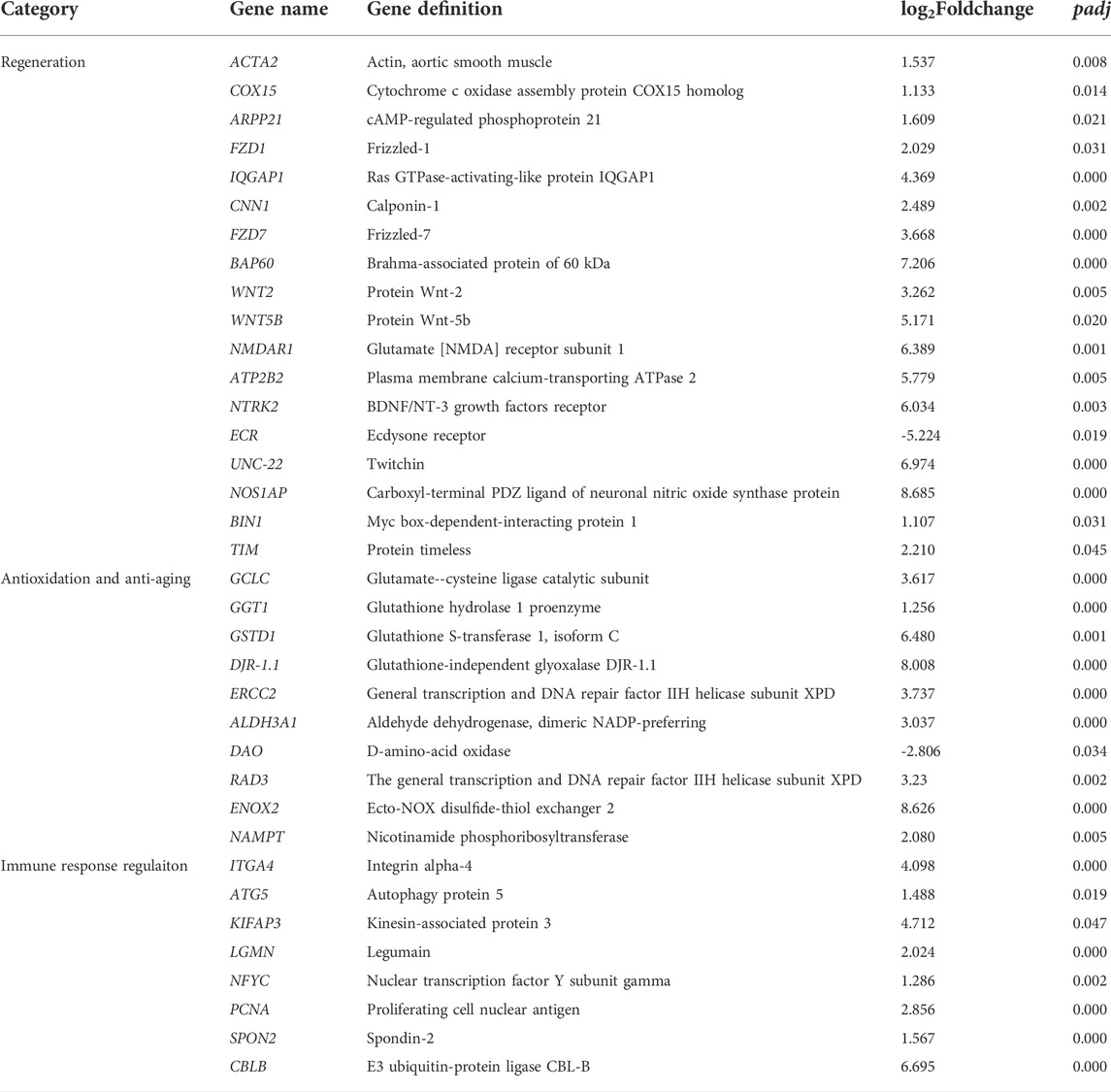
In this study, there were 17,064 DEGs in total (Figure 3). The top 30 GO terms, top 30 KEGG pathways, and key DEGs can be classified into three categories: regulation of regeneration, regulation of anti-oxidation and anti-aging, and regulation of the immune response. The key functional DEGs identified in this study are listed in Table 3, and all DEGs are shown in Supplementary Material S1. The regulatory network in the heart of E. sinensis between the post-molt and inter-molt stages is shown in Figure 4.
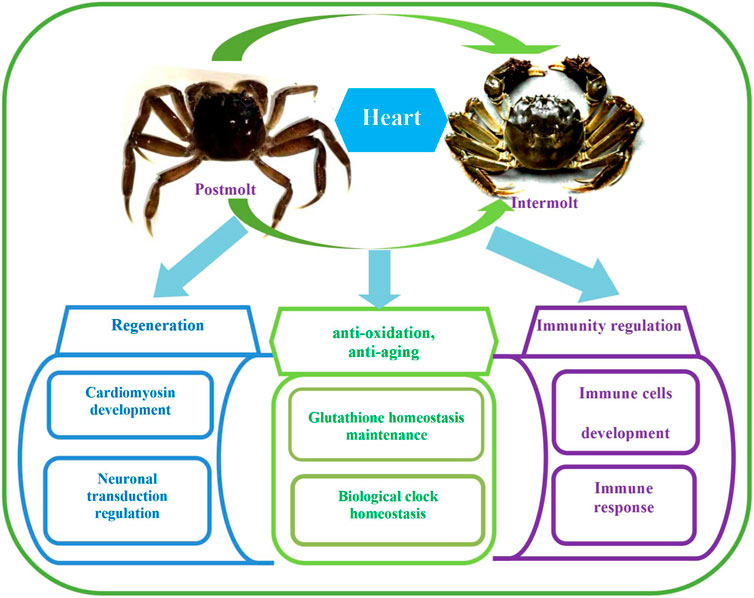
FIGURE 4. Regulatory network in the heart of E. sinensis identified between the post-molt and inter-molt stages.
Validation of transcriptome data by qPCR
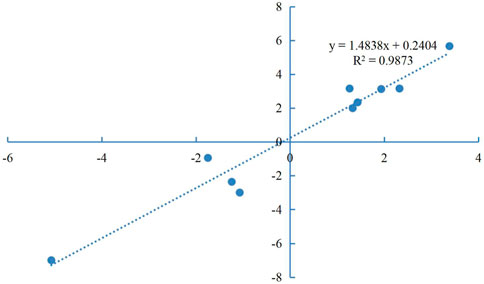
Primers for 10 of the DEGs identified here are shown in Supplementary Material S2. The relative expression levels of these DEGs as measured by qRT-PCR were consistent with those determined by high-throughput sequencing (Figure 5), indicating the reliability of the transcriptome data. Correlation analysis was shown in Figure 6.
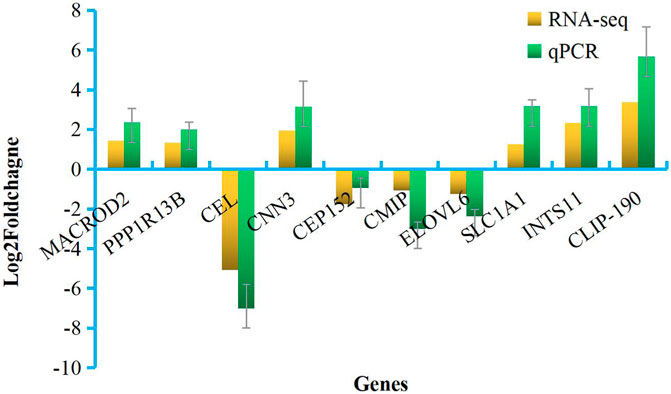
FIGURE 5. Validation of RNA-seq data by qPCR. X-axis, detected gene names; Y-axis, the relative expression level was expressed as log2 (fold change) in gene expression.
Discussion
This study identified regulatory pathways and functional genes relevant to tissue regeneration, anti-oxidation, anti-aging and immune regulation that were differentially expressed after E. sinensis molting compared to the inter-molt stage.
Tissue regeneration in the post-molt stage
Several pathways involved in tissue regeneration including the Hippo signaling pathway, Rap1 signaling pathway, thyroid hormone signaling pathway and apoptosis were significantly upregulated at the post-molt stage of E. sinensis.
The Hippo signaling pathway is highly conserved among different species and was first discovered in drosophila, where it plays an important regulatory role in cell proliferation, differentiation and migration, and organ size control (Fulford et al., 2018; Hong et al., 2018). The Wnt pathway is also highly conserved. Relevant studies have mainly been conducted on vertebrate species and indicate the Wnt signaling pathway plays an important regulatory role in cardiac development. Wnt binds to Frizzled receptor, with both molecules being essential regulators of cardiac development. Inhibition of the Wnt signaling pathway can block cardiac development during the early differentiation of human pluripotent stem cells (Pahnke et al., 2016; Guo et al., 2019; Kaplan et al., 2019). Rap1 is a small GTPase protein with high homology to Ras protein. It acts as a molecular switch and plays an important role in modulating cell movement and the formation of cellular connections. Rap1 can participate in the regulation of tight connections and in the formation of adhesion connections between epithelial cells and endothelial cells, thus affecting the integrity of barrier functions (Yang et al., 2021). Apoptosis is the spontaneous and orderly death of cells required to maintain internal environmental homeostasis. It plays an important regulatory role in the evolution of internal homeostasis and in the development of many organ systems. The release of cytochrome C from mitochondria is a key step in apoptosis. Caspase can act on several enzymes related to cytoskeleton regulation and hence alter the cell structure (Klemm et al., 2021). Damaged cells undergo apoptosis to clear out irreparable cells, thereby initiating tissue regeneration (Onishchenko et al., 2021).
In the present study we found differential expression of hippo, Rap1 and apoptosis pathway genes, as well as some regulatory genes relevant to cardiac development, heart rate regulation and calcium signal transduction in the neuronal system. The WNT-5B developmental protein together with frizzled receptors play a modulatory role in tissue morphogenesis (Ghosh et al., 2008; Yu et al., 2010; Sunkara et al., 2021). Studies on Xenopus have shown that FZD7 is required for heart development (Abu-Elmagd et al., 2017). In the present study, WNT-5B, FZD1 and FZD7 were all up-regulated after molting, suggesting they play a synergistic regulatory role in the cardiac development of E. sinensis during the post-molt period. COX15 is essential for the synthesis of heme A and plays a regulatory function in the blood circulatory system (De Oliveira et al., 2021). Myocytes invaginate to form T-tubes and prevent the negative effects of rapid changes in extracellular fluid induced by calcium. BIN1 plays a regulatory role in T-tube formation (Draeger et al., 2017). BIN1 also modulates calcium flow and cardiac myocyte movement. The function of UNC-22 is to modulate muscle contraction and relaxation (Matsunaga et al., 2015). CNN1 plays a regulatory role in muscle contraction via binding to actin and calmodulin (Feng et al., 2019). ARPP21 has a negative regulatory role for calmodulin-dependent enzymes. In the present study, upregulation of ARPP21 expression may help to maintain the homeostasis of cardiac myocyte contraction (Chen et al., 2018). IQGAP1 plays a modulatory role in cytoskeleton assembly of actin (Cheung et al., 2013). ATP2B2 has an active regulatory role in calcium homeostasis in neuronal systems (Martin-De-Saavedra et al., 2022). Glutamate is an important excitatory neurotransmitter that together with its receptor has a regulatory role in autonomy, conductivity and self-discipline (Hearn et al., 2022). Research on cultured rat myocardial cells indicate that glutamate can increase the concentration of calcium, thus increasing the contraction rate of cardiac myocytes (Hasan and Nabika, 2021). GRIN1 plays a positive role in regulating myocardial contraction. BAP60 participates in the regulation of neurogenesis (Koe et al., 2014). NTRK2 plays a regulatory role in the development and maturation of central and peripheral nervous systems and synaptic plasticity (Pattwell et al., 2021).
The heart of crustaceans is neurogenic. Cardiac ganglion in the heart modulates cardiac signal transduction and initiates and regulates cardiac myocyte contraction (Garcia-Crescioni et al., 2010). In the present study, some of the regulatory DEGs involved in heart development after molting were related to neuronal signal transduction, myocardial movement, heart development and apoptosis.
Anti-oxidation and anti-aging at the post-molt stage
Aging is a complex natural phenomenon that manifests as a decline in physiological function, weakened resistance to the environment, slower metabolism and slower response to stress. The free radical theory is one of the most convincing modern theories to explain the aging mechanism (Cai et al., 2020b). High concentrations of free radicals and their derivatives in tissues have harmful effects on biological macromolecules and can accelerate the aging process. The scavenging of free radicals and subsequent prevention of lipid peroxidation can improve the anti-oxidation capacity, thus causing a delay in aging (Fernandez-Marcos and Nobrega-PereiraNADPH, 2016; Hajam et al., 2022).
Glutathione (GSH) is an important non-enzymatic antioxidant and efficient nucleophile. GSH reacts with electrophiles to remove harmful metabolites such as free radicals. Its concentration is an important indicator of the antioxidant capacity of the body (Lopez-Navarro et al., 2020). In the present study, some of the DEGs were related to the “glutathione metabolic process/glutathione transferase activity/oxidation-reduction process/anti-aging”. GCLC is an essential component for GSH biosynthesis (Fu et al., 2020). GGT1 also plays an active role in the regulation of cysteine homeostasis and glutathione homeostasis (West et al., 2013). DJR-1.1 participates in the detoxification of endogenous glyoxal and in the protection of cell death induced by glyoxal (Hasim et al., 2014). The NMDA receptor is an excitatory neurotransmitter with a critical role in regulating synaptic plasticity, memory, etc. DAO can degrade D–serine, thereby inhibiting NMDA (Lin et al., 2017). In the present study, downregulation of DAO had a positive role in the delay of aging. ALDH3A1 inhibits lipid peroxidation (Black et al., 2012). Upregulation of ALDH3A1 observed in this study enhances the removal of toxic substances and strengthens the anti-aging capability. The accumulation of protein synthesis errors is also an important contributor to aging. Upregulation of RAD3 is conducive to the fidelity of DNA replication, which ultimately benefits protein synthesis (Yeo et al., 2020). Thus, RAD3 can delay the aging process. Many physiological activities have circadian rhythms and the biological clock is closely related to aging. Dysfunction of the biological clock seriously affects the physiological and behavioral rhythms of organisms, leading to endocrine disorders and the acceleration of aging (Radman, 2012). ENOX2 has a positive regulatory role in the organism’s biological clock (Morre and Morre, 2008). NAMPT acts to maintain biological clock homeostasis and thus prevent aging (Khaidizar et al., 2021).
Surprisingly, some of the pathways and regulatory genes involved in anti-oxidation (regulation of GSH homeostasis, inhibition of lipid peroxidation), anti-aging (regulation of biological clock homeostasis) were found in this study to be upregulated during the post-molt stage of E. sinensis. In contrast to vertebrates, the heart of E. sinensis has strong regenerative ability. Regeneration is the opposite process to aging and hence this study could provide a theoretical framework for research into the anti-aging molecular mechanism in vertebrates.
Immune regulation during the post-molt stage
The migration of white blood cells from the blood to tissues (leukocyte transendothelial migration) is essential for immune surveillance and inflammation. Inflammatory cells migrate from peripheral blood vessels to inflammatory sites under the stimulation of inflammatory factors, resulting in an immune response (Van Steen et al., 2021). Antigen processing and presentation is the process by which antigen molecules are captured by antigen presenting cells, digested into peptides and then combined with MHC molecules to form complexes that are presented at the cell surface and recognized by immunoactive cells (Gannage et al., 2019).
In this study, some regulatory genes related to immune cell development and immune response regulation were differentially expressed after molting. CBLB has a negative regulatory role with regard to lymphocyte receptors (Nanjappa et al., 2020). The down-regulation of CBLB observed here may help to maintain immune response homeostasis.
During their lifetime, cells face a variety of endogenous and exogenous stresses, including protein misfolding, organelle damage, nutrient deficiency and pathogen invasion. Autophagy is an important way for cells to respond to these stresses. The substances to be removed are wrapped and then transported to lysosomes for degradation (Rakesh et al., 2022; Zhou et al., 2022). ATG5 is an essential component in the formation of autophagy vesicles and plays a key regulatory role in many aspects of lymphocyte development and proliferation (Kim et al., 2020). Upregulation of ATG5 has a positive regulatory role in immune system development and in the immune response. ITGA4 triggers the aggregation of homogenous leukocyte lines onto activated endothelial cells and is involved in T-cell interactions with target cells. In the present study, ITGA4 contributed to enhancement of the immune response. Asparagine is an essential component for the assembly of MHC class I molecules (Fu et al., 2022). LGMN has strict specificity for the hydrolysis of asparagine bonds and participates in the processing of MHC Class II antigen-presenting proteins in the lysosomal/endosomal system. Research conducted in vertebrates has shown that LGMN can promote cardiac repair (Jia et al., 2022). In the present study, LGMN was beneficial for antigen processing and presentation during the immune response and may also play a positive role in tissue repair and regeneration after E. sinensis molting. SPON2 acts as an opsonin for macrophages. It binds directly to bacteria and is critical for initiating innate immune responses (Zhou et al., 2021). SPON2 may have a positive regulatory role by enhancing resistance to pathogenic microorganisms during the post-molt period of E. sinensis.
Conclusion
In this study, comparative transcriptome analysis was carried out on the heart of E. sinensis at the post-molt and inter-molt stages. The results showed significant differential expression of many regulatory pathways and genes involved in regeneration, antioxidation, anti-aging and the immune response. Aside from cardiac development and with regard to the regulation of regeneration, these DEGs were relevant to myocardial movement and to neuronal signal transduction. With regard to antioxidation and anti-aging, the DEGs were involved with regulation on GSH homeostasis and biological rhythms. With regard to the immune response, the DEGs were involved in the regulation of immune cell development and the immune response. This study provides a theoretical background for further research into regulatory mechanisms in E. sinensis and other economically valuable crustaceans (eg. procambarus clarkii) and the crustacean breeding industry in general. In contrast to vertebrates, the heart of E. sinensis has strong regenerative potential. This study may provide a theoretical framework for further research into the regulatory mechanisms of organ regeneration and anti-aging in vertebrates.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the Freshwater Fisheries Research Center at the Chinese Academy of Fishery Sciences. All the experiments conformed to the Guidelines for the Care and Use of Laboratory Animals set by the Animal Care and Use Committee of the Freshwater Fisheries Research Center (2003WXEP61, Jan 6th of 2003), and the study was carried out under a field permit (No. 20182AC1699).
Author contributions
MW designed the research study. MW, YT, GX, and SS performed the research. PX, JG, and XM provided advice on the research. CT, JL, FY, HL, CS and JG provided help on the sampling and parameters measurement. MW analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the Natural Science Foundation for Young Scholars in Jiangsu Province of China (SBK2020044520); the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD36); the Key Project for Jiangsu Agricultural New Variety Innovation (PZCZ201749); the Jiangsu Revitalization of Seed Industry (JBGS(2021)031); the Jiangsu Modern Agricultural Industry Technology System (JFRS-01-01); the “JBGS” Project of Seed Industry Revitalization in Jiangsu Province [JBGS(2021)125]; the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2021XT0702).
Acknowledgments
Thanks to editors and all the peer reviewers for their opinions and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.948511/full#supplementary-material
Abbreviations
E. sinensis, Eriocheir sinensis; DEGs, differentially expressed genes; VATB, V-ATPase subunit B; TGFBR1, transforming growth factor-beta type I receptor; MP, post-molt stage; MI, inter-molt stage; Nr, Non-redundant protein; Nt, Non-redundant nucleotides; KOG, Clusters of orthologous groups for eukaryotic complete genomes; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; qPCR, Quantitative real-time polymerase chain reaction; BP, biological process; MF, molecular function; CC, cellular component; GSH, Glutathione.
References
Abu-Elmagd M., Mulvaney J., Wheeler G. N. (2017). Frizzled-7 is required for Xenopus heart development. Biol. Open 6 (12), 1861–1868. doi:10.1242/bio.026963
Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 (3), 403–410. doi:10.1016/S0022-2836(05)80360-2
Anders S., Huber W. (2012). Differential expression of RNA-Seq data at the gene level–the DESeq package. Heidelberg: European Molecular Biology, 6.
Avarre J. C. (2020). Next-generation sequencing: a revolution in the field of fish diseases. B Eur. Assoc. Fish. Pa 40 (2), 62–69. doi:10.1177/1176934319892284
Black W., Chen Y., Matsumoto A., Thompson D. C., Lassen N., Pappa A., et al. (2012). Molecular mechanisms of aldh3a1-mediated cellular protection against 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 52 (9), 1937–1944. doi:10.1016/j.freeradbiomed.2012.02.050
Cai M., Liu Z., Chen M., Zhang M., Jiao Y., Chen Q., et al. (2020). Comparative proteomic analysis of senescence in the freshwater cladoceran Daphnia pulex. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 239, 110352. doi:10.1016/j.cbpb.2019.110352
Cai M., Liu Z., Yu P., Jiao Y., Chen Q., Jiang Q., et al. (2020). Circadian rhythm regulation of the oxidation–antioxidant balance in Daphnia pulex. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 240, 110387. doi:10.1016/j.cbpb.2019.110387
Chen H., Gu X., Zeng Q., Mao Z., Liang X., Martyniuk C. (2019). Carbamazepine disrupts molting hormone signaling and inhibits molting and growth of Eriocheir sinensis at environmentally relevant concentrations. Aquat. Toxicol. 208, 138–145. doi:10.1016/j.aquatox.2019.01.010
Chen P., Ruan X., Chen Y., Chu S., Mo K., Wu C., et al. (2018). Generating a reporter mouse line marking medium spiny neurons in the developing striatum driven by Arpp21 cis-regulatory elements. J. Genet. Genomics 45 (12), 673–676. doi:10.1016/j.jgg.2018.09.007
Cheung K. L., Lee J. H., Shu L., Kim J-H., Sacks D. B., Kong A-N. T. (2013). The Ras GTPase-activating-like protein IQGAP1 mediates Nrf2 protein activation via the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK pathway. J. Biol. Chem. 288 (31), 22378–22386. doi:10.1074/jbc.M112.444182
Conesa A., Gotz S., Garcia-Gomez J. M., Terol J., Talon M., Robles M. (2005). Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21 (18), 3674–3676. doi:10.1093/bioinformatics/bti610
Das S., Vraspir L., Zhou W., Durica D. S., Mykles D. L. (2018). Transcriptomic analysis of differentially expressed genes in the molting gland (Y-organ) of the blackback land crab, Gecarcinus lateralis, during molt-cycle stage transitions. Comp. Biochen Phys. D. 28, 37–53. doi:10.1016/j.cbd.2018.06.001
De Oliveira M. G., Tengan C., Micheletti C., De Macedo P. R., Soares P., Cernach M. C., et al. (2021). A novel variant in the COX15 gene causing a fatal infantile cardioencephalomyopathy: A case report with clinical and molecular review. Eur. J. Med. Genet. 64 (5), 104195. doi:10.1016/j.ejmg.2021.104195
Draeger N. M., Nachman E., Winterhoff M., Bruehmann S., Shah P., Katsinelos T., et al. (2017). Bin1 directly remodels actin dynamics through its BAR domain. EMBO Rep. 18 (11), 2051–2066. doi:10.15252/embr.201744137
Feng H-Z., Wang H., Takahashi K., Jin J. P. (2019). Double deletion of calponin 1 and calponin 2 in mice decreases systemic blood pressure with blunted length-tension response of aortic smooth muscle. J. Mol. Cell. Cardiol. 129, 49–57. doi:10.1016/j.yjmcc.2019.01.026
Feng Q., Liu M., Cheng Y., Wu X. (2022). Comparative transcriptome analysis reveals the process of ovarian development and nutrition metabolism in Chinese mitten crab, Eriocheir sinensis. Front. Genet. 13, 910682. doi:10.3389/fgene.2022.910682
Fernandez-Marcos P. J., Nobrega-PereiraNADPH S. (2016). Nadph: New oxygen for the ROS theory of aging. Oncotarget 7 (32), 50814–50815. doi:10.18632/oncotarget.10744
Fu N., Li D., Li W., Zhao W., Zhang S., Liu L., et al. (2020). Glutamate-cysteine ligase catalytic subunit attenuated hepatitis C virus-related liver fibrosis and suppressed endoplasmic reticulum stress. Front. Mol. Biosci. 7, 199. doi:10.3389/fmolb.2020.00199
Fu Y., Chen W., He M., Tang L., Guo S., Zhang Q. (2022). An integrin alpha 4 (ChInt alpha 4) from oyster Crassostrea hongkongensis mediates the hemocytes phagocytosis towards Vibrio alginolyticus. Fish. Shellfish Immunol. 122, 246–256. doi:10.1016/j.fsi.2022.02.015
Fulford A., Tapon N., Ribeiro P. S. (2018). Upstairs, downstairs: Spatial regulation of hippo signalling. Curr. Opin. Cell Biol. 51, 22–32. doi:10.1016/j.ceb.2017.10.006
Gannage M., Da Silva R. B., Munz C. (2019). Monitoring antigen processing for MHC presentation via macroautophagy. Methods Mol. Biol. 1988, 357–373. doi:10.1007/978-1-4939-9450-2_25
Garcia-Crescioni K., Fort T. J., Stern E., Brezina V., Miller M. W. (2010). Feedback from peripheral musculature to central pattern generator in the neurogenic heart of the crab Callinectes sapidus: Role of mechanosensitive dendrites. J. Neurophysiol. 103 (1), 83–96. doi:10.1152/jn.00561.2009
Ghosh S., Roy S., Seguin C., Bryant S. V., Gardiner D. M. (2008). Analysis of the expression and function of Wnt-5a and Wnt-5b in developing and regenerating axolotl (Ambystoma mexicanum) limbs. Dev. Growth Differ. 50 (4), 289–297. doi:10.1111/j.1440-169x.2008.01000.x
Gillard M., Thiebaut G., Deleu C., Leroy B. (2017). Present and future distribution of three aquatic plants taxa across the world: Decrease in native and increase in invasive ranges. Biol. Invasions 19 (7), 2159–2170. doi:10.1007/s10530-017-1428-y
Goepel T., Wirkner C. S. (2020). The circulatory system of Penaeus vannamei Boone, 1931-Lacunar function and a reconsideration of the "open vs. closed system" debate. J. Morphol. 281 (4-5), 500–512. doi:10.1002/jmor.21117
Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., Amit I., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29 (7), 644–652. doi:10.1038/nbt.1883
Guo X., Gu X., Hareshwaree S., Rong X., Li L., Chu M. (2019). Induced pluripotent stem cell-conditional medium inhibits H9C2 cardiomyocytes apoptosis via autophagy flux and Wnt/β-catenin pathway. J. Cell. Mol. Med. 23 (6), 4358–4374. doi:10.1111/jcmm.14327
Hajam Y. A., Rani R., Ganie S. Y., Sheikh T. A., Javaid D., Qadri S. S., et al. (2022). Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 11 (3), 552. doi:10.3390/cells11030552
Hasan M. Z., Nabika T. (2021). RVLM microinjection to explain the role of metabotropic glutamate receptors in the regulation of baseline blood pressure and heart rate in SHRSP. Circulation 144, 9101. doi:10.1161/circ.144.suppl_1.9101
Hasim S., Hussin N. A., Alomar F., Bidasee K. R., Nickerson K. W., Wilson M. A. (2014). A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J. Biol. Chem. 289 (3), 1662–1674. doi:10.1074/jbc.M113.505784
Hearn J. I., Green T. N., Hisey C. L., Bender M., Josefsson E. C., Knowlton N., et al. (2022). Deletion of Grin1 in mouse megakaryocytes reveals NMDA receptor role in platelet function and proplatelet formation. Blood 139 (17), 2673–2690. doi:10.1182/blood.2021014000
Hong L., Li X., Zhou D., Geng J., Chen L. (2018). Role of Hippo signaling in regulating immunity. Cell. Mol. Immunol. 15 (12), 1003–1009. doi:10.1038/s41423-018-0007-1
Hou X., Chen X., Yang H., Yue W., Wang J., Han H., et al. (2020). V-ATPase subunit B plays essential roles in the molting process of the Chinese mitten crab, Eriocheir sinensis. Biol. Open 9 (5), bio048926. doi:10.1242/bio.048926
Jia D., Chen S., Bai P., Luo C., Liu J., Sun A., et al. (2022). Cardiac resident macrophage-derived legumain improves cardiac repair via promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation 145 (20), 1542–1556. doi:10.1161/CIRCULATIONAHA.121.057549
Jin B., Winemiller K., Ren W., Tickner D., Wei X., Guo L., et al. (2022). Basin-scale approach needed for Yangtze River fisheries restoration. Fish Fish. 23, 1009–1015. doi:10.1111/faf.12657
Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., et al. (2008). KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484. doi:10.1093/nar/gkm882
Kang X. J., Tian Z. H., Wu J. L., Mu S. M. (2012). Molt stages and changes in digestive enzyme activity in hepatopancreas during molt cycle of Chinese mitten crab, Eriocheir sinensis. J. Fish. Sci. China 19, 806–812. doi:10.3724/sp.j.1118.2012.00806
Kaplan N. A., Wang W., Christiaen L. (2019). Initial characterization of Wnt-Tcf functions during Ciona heart development. Dev. Biol. 448 (2), 199–209. doi:10.1016/j.ydbio.2018.12.018
Khaidizar F. D., Bessho Y., Nakahata Y. (2021). Nicotinamide phosphoribosyltransferase as a key molecule of the aging/senescence process. Int. J. Mol. Sci. 22 (7), 3709. doi:10.3390/ijms22073709
Kim C., Kim W., Lee H., Ji E., Choe Y., Martindale J., et al. (2020). Correction: The RNA-binding protein HuD regulates autophagosome formation in pancreatic β cells by promoting autophagy-related gene 5 expression. J. Biol. Chem. 295 (38), 13408. doi:10.1074/jbc.AAC120.015676
Klemm J., Stinchfield M. J., Harris R. E. (2021). Necrosis-induced apoptosis promotes regeneration in Drosophila wing imaginal discs. Genetics 219 (3), iyab144. doi:10.1093/genetics/iyab144
Koe C. T., Li S., Rossi F., Wong J. J. L., Wang Y., Zhang Z., et al. (2014). The Brm-HDAC3-Erm repressor complex suppresses dedifferentiation in Drosophila type II neuroblast lineages. Elife 3, e01906. doi:10.7554/eLife.01906
Kushinsky D., Morozova E. O., Marder E. (2019). In vivo effects of temperature on the heart and pyloric rhythms in the crab Cancer borealis. J. Exp. Biol. 222 (5), jeb199190. doi:10.1242/jeb.199190
Levinton J. S., Volkenborn N., Gurr S., Correal K., Villacres S., Seabra R., et al. (2020). Temperature-related heart rate in water and air and a comparison to other temperature-related measures of performance in the fiddler crab Leptuca pugilator (Bosc 1802). J. Therm. Biol. 88, 102502. doi:10.1016/j.jtherbio.2019.102502
Lin C-H., Yang H-T., Chiu C-C., Lane H-Y. (2017). Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci. Rep. 7, 14849. doi:10.1038/s41598-017-13951-7
Liu S., Wang X., Bu X., Lin Z., Li E., Shi Q., et al. (2021). Impact of dietary vitamin D-3 supplementation on growth, molting, antioxidant capability, and immunity of juvenile chinese mitten crabs (Eriocheir sinensis) by metabolites and vitamin D receptor. J. Agric. Food Chem. 69 (43), 12794–12806. doi:10.1021/acs.jafc.1c04204
Liu Z., Li Y., Pérez E., Jiang Q., Chen Q., Jiao Y., et al. (2021). Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: Application of transcriptome profiling in risk assessment of nanoplastics. J. Hazard. Mat. 402, 123778. doi:10.1016/j.jhazmat.2020.123778
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25 (4), 402–408. doi:10.1006/meth.2001.1262
Lopez-Navarro M. E., Jarquin-Martinez M., Sanchez-Labastida L. A., Ramirez-Rosales D., Godinez-Victoria M., Quintas-Granados L. I., et al. (2020). Decoding aging: Understanding the complex relationship among aging, free radicals, and GSH. Oxid. Med. Cell. Longev. 2020, 3970860. doi:10.1155/2020/3970860
Lv W., Liu Z., Yang Y., Huang Y., Fan B., Jiang Q., et al. (2016). Loss and self-restoration of macrobenthic diversity in reclamation habitats of estuarine islands in Yangtze estuary, China. Mar. Pollut. Bull. 103, 128–136. doi:10.1016/j.marpolbul.2015.12.030
Martin-De-Saavedra M. D., Dos Santos M., Culotta L., Varea O., Spielman B. P., Parnell E., et al. (2022). Shed CNTNAP2 ectodomain is detectable in CSF and regulates Ca2+ homeostasis and network synchrony via PMCA2/ATP2B2. Neuron 110 (4), 627–643. doi:10.1016/j.neuron.2021.11.025
Matsunaga Y., Qadota H., Furukawa M., Choe H., Benian G. M. (2015). Twitchin kinase interacts with MAPKAP kinase 2 in Caenorhabditis elegans striated muscle. Mol. Biol. Cell 26 (11), 2096–2111. doi:10.1091/mbc.E14-05-1009
Min P. T., Li L. W., Razali S. A., Afiquah-Aleng N., Nor S. A. M., Sung Y. Y., et al. (2019). Applications of next-generation sequencing technologies and computational tools in molecular evolution and aquatic Animals conservation studies: A short review. Evol. Bioinform. Online 15, 1176934319892284. doi:10.1177/1176934319892284
Morre D. J., Morre D. M. (2008). ENOX proteins, copper hexahydrate-based ultradian oscillators of the cells' biological clock. Netherlands: Springer, 84.
Nanjappa S. G., Mudalagiriyappa S., Sharma J., Vieson M. (2020). Decoding the role of CBLB for innate immune responses regulating systemic dissemination during non-tuberculous mycobacteria infection. J. Immunol. 204 (1).
Onishchenko N., Fomenko E., Nikolskaya A., Gonikova Z., Shagidulin M., Sevastyanov V., et al. (2021). Total RNA from bone marrow cells induces liver regeneration by co-activating early apoptosis and proliferation processes. Transpl. Int. 34, 213–214.
Pahnke A., Conant G., Huyer L. D., Zhao Y., Feric N., Radisic M. (2016). The role of Wnt regulation in heart development, cardiac repair and disease: A tissue engineering perspective. Biochem. Biophys. Res. Commun. 473 (3), 698–703. doi:10.1016/j.bbrc.2015.11.060
Patel R. K., Jain M. (2012). NGS QC toolkit: A toolkit for quality control of next generation sequencing data. Plos One 7 (2), e30619. doi:10.1371/journal.pone.0030619
Pattwell S., Arora S., Nuechterlein N., Zager M., Loeb K., Cimino P., et al. (2021). NTRK2 splice variant, TRKB.T1, links neurobiology, embryonic development, and oncogenesis. Neuro-Oncology 23, 222–22. doi:10.1093/neuonc/noab196.891
Radman M. (2012). Aging: the chemistry of a flexible biological clock. Med. Sci. 28 (3), 231–233. doi:10.1051/medsci/2012283001
Rakesh R., PriyaDharshini L., Sakthivel K., Rasmi R. (2022). Role and Regulation of Autophagy in Cancer Biochimica et Biophysica Acta. BBA-Mol Basis Dis. 1868 (7), 166400. doi:10.1016/j.bbadis.2022.166400
Singh D. S., Alkins-Koo M., Rostant L. V., Mohammed A. (2020). Heart rate responses to different temperatures in juvenile Poppiana dentata ( ). Braz J. Biol. 80 (1), 30–38. doi:10.1590/1519-6984.188457
Singh D. S., Alkins-Koo M., Rostant L. V., Mohammed A. (2020). The effects of acute oxygen changes on heart rate in the freshwater crab Poppiana dentata (Randall, 1840). Lat. Am. J. Aquat. Res. 48 (3), 480–487. doi:10.3856/vol48-issue3-fulltext-2397
Song Q. H., Zhao Y. F. (2018). Analysis on current situation and criteria for Eriocheir sinensis culturing industry. Sci. Fish. Farming 10, 13–16.
Spiridonov V. A., Zalota A. K. (2017). Understanding and forecasting dispersal of non-indigenous marine decapods (Crustacea: Decapoda) in East European and north asian waters. J. Mar. Biol. Assoc. U. K. 97 (3), 591–611. doi:10.1017/s0025315417000169
Sunkara R. R., Koulgi S., Jani V., Gadewal N., Sonavane U., Joshi R., et al. (2021). Understanding the binding affinities between SFRP1CRD, SFRP1Netrin, Wnt5B and frizzled receptors 2, 3 and 7 using MD simulations. J. Biomol. Struct. Dyn. 40, 1–14. doi:10.1080/07391102.2021.1890219
Tian Z., Lin G., Jiao C. (2019). Identification of a S6 kinase transcript in the Chinese mitten crab Eriocheir sinensis and its molting-related expression in muscle tissues. Fish. Sci. 85, 737–746. doi:10.1007/s12562-019-01326-y
Tian Z., Peng H., Deng W., Jiao C. (2020). Identification of a transforming growth factor-beta type I receptor transcript in Eriocheir sinensis and its molting-related expression in muscle tissues. Mol. Biol. Rep. 47 (1), 77–86. doi:10.1007/s11033-019-05108-8
Tian Z. H., Kang X. J., Jiao C. Z. (2013). Structural and constituent changes in integument during the molt cycle of chinese mitten crab Eriocheir sinensis. Acta Hydrobiol. Sin. 37, 899–904.
Van Steen A. C. I., Kempers L., Schoppmeyer R., Blokker M., Beebe D. J., Nolte M. A., et al. (2021). Transendothelial migration induces differential migration dynamics of leukocytes in tissue matrix. J. Cell Sci. 134 (21), jcs258690. doi:10.1242/jcs.258690
Wang M., Tang Y., Yu J., Su S., Li J., Yu F., et al. (2020). Molecular insights into the sex-differential regulation of signal transduction in the cerebral ganglion and metabolism in the hepatopancreas of Eriocheir sinensis during reproduction. Genomics 112 (1), 71–81. doi:10.1016/j.ygeno.2019.10.014
Wang Q., Wu X., Long X., Zhu W., Ma T., Cheng Y. (2018). Nutritional quality of different grades of adult male Chinese mitten crab, Eriocheir sinensis. J. Food Sci. Technol. 55, 944–955. doi:10.1007/s13197-017-3002-0
Wang X., Huang Z., Wang C., Qi C., Gu Z., Li E., et al. (2020). A comparative study on growth and metabolism of Eriocheir sinensis juveniles under chronically low and high pH stress. Front. Physiol. 11, 885. doi:10.3389/fphys.2020.00885
Wang Y., Wang S., Chen J., Ji J. (2015). Prevention measures of molting failure of adult Eriocheir sinensis. Sci. Fish. Farming 6, 57–59.
West M. B., Chen Y., Wickham S., Heroux A., Cahill K., Hanigan M. H., et al. (2013). Novel insights into eukaryotic gamma-glutamyltranspeptidase 1 from the crystal structure of the glutamate-bound human enzyme. J. Biol. Chem. 288 (44), 31902–31913. doi:10.1074/jbc.M113.498139
Xiong J. W. (2020). Evolutionary insights into heart regeneration. Cell Regen. 9 (1), 23. doi:10.1186/s13619-020-00069-x
Yang H., Xue W., Ding C., Wang C., Xu B., Chen S., et al. (2021). Vitexin mitigates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction via epac1-rap1 signaling. Oxid. Med. Cell. Longev. 2021, 9921982. doi:10.1155/2021/9921982
Yeo C. T., Oleson B., Corbett J. A., Schnuck J. K. (2020). 2118-P: Regulation of ataxia-telangiectasia and rad3-related (ATR)–Dependent DNA damage response by nitric oxide in ß-cells. Diabetes 69. doi:10.2337/db20-2118-P
Yu H., Smallwood P. M., Wang Y., Vidaltamayo R., Reed R., Nathans J. (2010). Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development 137 (21), 3707–3717. doi:10.1242/dev.052001
Yu P., Liu Z., Wu D., Chen M., Lv W., Zhao Y. (2018). Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 200, 28–36. doi:10.1016/j.aquatox.2018.04.015
Zainal K., Noorani A. (2019). Temperature dependence of the heart rates in the blue swimming crab portunus segnis (forskal, 1775). Arab. J. Sci. Eng. 44 (7), 6259–6265. doi:10.1007/s13369-019-03826-6
Zhang K. J., Wang J. A., Ni K. D., Chen H. H., Chen X. W., Wang J., et al. (2021). Effects of different water temperatures on molting, growth and feeding rates of Juvenile Eriocheir sinensis cultured in individual culture system. Freshw. Fish. 51, 100–105.
Zhang W., Bussmann R. W., Li J., Liu B., Xue T., Yang X., et al. (2022). Biodiversity hotspots and conservation efficiency of a large drainage basin: Distribution patterns of species richness and conservation gaps analysis in the Yangtze River basin, China. Conserv. Sci. Pract. 4 (4), e12653. doi:10.1111/csp2.12653
Zhou A., Zhang W., Dong X., Liu M., Chen H., Tang B. (2022). The battle for autophagy between host and influenza A virus. Virulence 13 (1), 46–59. doi:10.1080/21505594.2021.2014680
Keywords: Eriocheir sinensis, heart, regeneration, post-molt stage, comparative transcriptome
Citation: Wang M, Ge J, Ma X, Su S, Tian C, Li J, Yu F, Li H, Song C, Gao J, Xu P, Tang Y and Xu G (2022) Exploration of the regulatory mechanisms of regeneration, anti-oxidation, anti-aging and the immune response at the post-molt stage of Eriocheir sinensis. Front. Physiol. 13:948511. doi: 10.3389/fphys.2022.948511
Received: 20 May 2022; Accepted: 22 August 2022;
Published: 21 September 2022.
Edited by:
Sanjay K. Gupta, Indian Institute of Agricultural Biotechnology (ICAR), IndiaReviewed by:
Zhihao Jia, Purdue University, United StatesZhiquan Liu, East China Normal University, China
Copyright © 2022 Wang, Ge, Ma, Su, Tian, Li, Yu, Li, Song, Gao, Xu, Tang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongkai Tang, dGFuZ3lrQGZmcmMuY24=; Gangchun Xu, eHVnY0BmZnJjLmNu
 Meiyao Wang
Meiyao Wang Jiachun Ge3
Jiachun Ge3