- 1Department of Physical Education, Catholic University of Brasília, Brasília, Brazil
- 2School of Physical Education and Sport of Ribeirão Preto, University of São Paulo (USP), Ribeirão Preto, São Paulo, Brazil
- 3Department of School of Kinesiology Recreation and Sport, Western Kentucky University, Bowling Green, FL, United States
The current manuscript reviews the literature on the health effects of resistance training (RT) for individuals with Down syndrome (DS), focusing on this training modality’s methodology, application, and safety. The literature has mentioned that early aging in this population is associated with loss of muscle strength, lower lean and bone mass, and increased obesity. It is necessary to propose non-pharmacological measures for prevention and health promotion. Thus, this review suggests a current research-based RT guide for individuals with DS. This review is divided into three sections: Section 2 briefly reviews DS and the effects on structural and functional decline and how exercise and physical activity can influence health aspects in this population; Section 3 summarizes the evidence for RT prescription; Section 4 briefly reviews the health and potential benefits of RT in individuals with DS. The findings from this review suggest that most individuals with DS should engage in moderate-intensity RT at least 2 days a week and perform RT on the major muscle groups and include balance training. The RT program should be modified and adapted according to individuals’ characteristics and limitations. RT promotes positive, health-related benefits such as increasing strength, improving body composition, improving functional capacity and balance, reducing inflammatory status and oxidative stress, and improving the immune system. The RT protocols summarized in this current review provide guidance, critical conclusions, and novel research settings, which could be useful to coaches, clinicians, and researchers to effectively design RT program for individuals with DS.
Introduction
Approximately 1.5 billion people worldwide live with disabilities (PLWD); PLWD are less likely to meet the recommended physical activity guidelines (Ginis et al., 2021). Recognizing the disparity between PLWD and able-bodied populations, the World Health Organization (WHO) created the first set of recommendations for physical activity and sedentary behavior for this particular group (Bull et al., 2020). A common disability that affects activity behavior is Down syndrome (DS). DS is a chromosomal abnormality that occurs in a total or partial tripling of chromosome 21; intellectual disability (ID) represents a main characteristic of the condition (Bull, 2020). Individuals with DS are exposed to premature aging, high risk for congenital heart disease, atlantoaxial instability, Alzheimer’s disease (AD), thyroid disease, early muscle mass loss, and lower muscle strength (Barnhart and Connolly, 2007; Capone et al., 2018; Coelho-Junior et al., 2019; Foley and Killeen, 2019).
Life expectancy of individuals with DS is progressively increasing over time, from 25 years in 1983 to 60 years in 2020 (Bull, 2020; Guthold et al., 2020) due to improvements in medical technology, social care, and healthcare systems (Presson et al., 2013). Yet, the literature demonstrates that adults and older adults with DS are hospitalized more often than the general population and for a more extended period of time (Tenenbaum et al., 2012).
Compared to typically developing peers, people with DS have lower physical fitness and physical activity, with a higher prevalence of sedentary behavior regardless of age (Esposito et al., 2012; Suarez-Villadat et al., 2019; Oreskovic et al., 2020; Suarez-Villadat et al., 2021; Forseth et al., 2022). Adults with DS have increased adiposity, decreased bone mineral density, lean mass, and physical performance similar to or lower than older adults with sarcopenia (Coelho-Junior et al., 2019). These characteristics likely explain why people with DS exhibit lower levels of cardiovascular fitness, a higher prevalence of obesity, and lower muscular strength compared to the general population (Cowley et al., 2010; Fernhall et al., 2013; Bertapelli et al., 2016).
Individuals with DS consistently display reduced upper- and lower-body muscular strength compared to the general population (Pitetti et al., 1992; Cowley et al., 2011). The lower extremity weakness directly influences the ability to perform activities of daily living, such as walking, work-related tasks, going up and downstairs, or getting up and sitting on a chair (Shields and Taylor, 2010; Cowley et al., 2011; Lin and Wuang, 2012). This reduced muscular strength may be associated with attenuated lean body mass. The prevalence of sarcopenia is 14.3% in older adults with ID, with 12% of the sample having DS (Bastiaanse et al., 2012). Sarcopenia is positively associated with impaired mobility and inflammation (Bastiaanse et al., 2012). Lower lean body mass in adolescents with DS was related to reduced functional capacity and maximum oxygen consumption (a common metric for cardiorespiratory fitness) (González-Agüero et al., 2011a; González-Agüero et al., 2011b).
Resistance training (RT) is a non-pharmacological modality that potently attenuates muscle strength losses, improves body composition, and promotes functional capacity in young people and adults with DS (Shields et al., 2008; Cowley et al., 2010; Shields and Taylor, 2010; Cowley et al., 2011). While the number of studies investigating the effects of RT in individuals with DS has grown, the number of practitioners who use this training mode is still small (Sugimoto et al., 2016; Ginis et al., 2021). Cunningham et al. (2022) suggests that practitioners may not feel safe and are unsure about how to apply RT in individuals with an ID (Duplanty et al., 2014).
The purpose of this review was to provide scientific, evidence-based recommendations to health and fitness professionals regarding individualized prescription RT for individuals with DS, which has a high relevance in the exercise physiology and sports medicine. Our remarks may yield clinically useful information and help to effectively design RT interventions with safety accounted for. It is hoped that this review will inform directions for future research into RT monitoring and management, as well as stimulate a more open debate about the beneficial implications of RT on the long-term health in the DS context.
Strength of evidence
The strength of evidence was based on and adapted from the American College of Sports Medicine (ACSM) position stand guideline (Chodzko-Zajko et al., 2009), Health Care Research and Quality (AHRQ) (West et al., 2002), and the evidence rating system of the National Heart Lung and Blood Institute (1998); this was used to evaluate published manuscripts and to determine the most appropriate RT studies with DS. The recommendations of this narrative review followed this classification:
1. Evidence level A (rich body of data): Randomized controlled trials that filled addressed the key questions of this study and population with randomization, blinding, interventions, outcomes, statistical analysis, results, discussion and funding with a large sample and results pointing in the same direction. Also displayed a substantial number of studies involving substantial numbers of participants.
2. Evidence level B (limited body of data): Limited number of randomized controlled trials or post-hoc of subgroup analysis of RCTs, or meta-analysis of RCTs that fulfilled all the key questions as study question, study population, randomization, blinding, interventions, outcomes, statistical analysis, results, discussion, and funding.
3. Evidence level C (nonrandomized trials and observational studies): Observational and uncontrolled studies.
4. Evidence level D (panel consensus judgment): Expert consensus opinion, case studies and consensus of panel members based on clinical experience or knowledge that does not meet the above-listed criteria.
Down Syndrome
Down Syndrome and effects on structural and functional decline
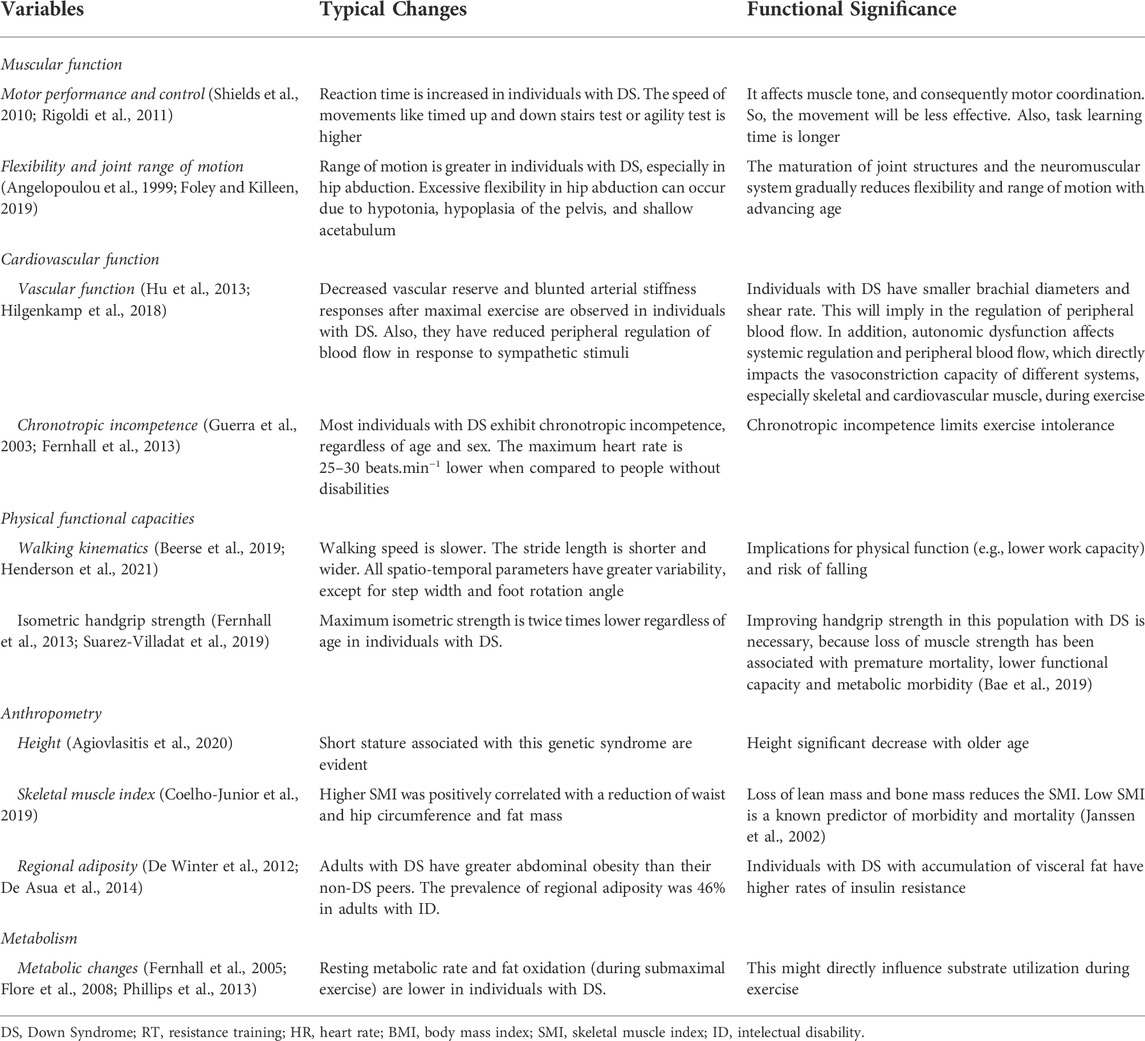
Due to the chromosomal anomaly, individuals with DS show a structural modification and functional decline in most physiological systems, even in the absence of disease (Roizen and Patterson, 2003; Bull, 2020). Among the functional declines, the loss of muscle strength and lower work capacity can be highlighted, which starts early and continues with advancing age (Cioni et al., 1994; Carmeli et al., 2002; Fernhall et al., 2013; Capone et al., 2018). However, the literature emphasizes that good physical fitness is essential for the proper performance of physical exercise because the individual becomes more tolerant to exercise and improves their functional abilities (Dodd and Shields, 2005; Sugimoto et al., 2016; Suarez-Villadat et al., 2021). Unfortunately, previous studies have shown that individuals with ID have low physical fitness which results in increased obesity, risk of falls, and chronic and neurodegenerative diseases (Rimmer et al., 2004; De Winter et al., 2009; Bayen et al., 2018; Suarez-Villadat et al., 2019; Kovačič et al., 2020). It was no surprise that lower baseline values of oxygen consumption (VO2) and a higher body mass to height ratio (BMI) were observed in individuals with DS across different age groups (Fernhall et al., 2013; Wee et al., 2015).
BMI might be a key factor in fitness levels and people with DS. When classified by BMI, those with normal body weight had better values of VO2 max and heart rate compared to those classified as obese (Wee et al., 2015). The change in body composition is another hallmark of individuals aging with DS; people with DS have lower lean mass and bone mineral density and a higher percentage of fat, regardless of sex, when compared to the general population (Angelopoulou et al., 2000; González-Agüero et al., 2011a). This has profound effects on the physical health and function of this population. Specific examples include the accumulation of body fat and its redistribution to central and visceral stores, increased risk of cardiometabolic and cardiovascular disease, and early sarcopenia (Bastiaanse et al., 2012; De Asua et al., 2014; Coelho-Junior et al., 2019; Kelly et al., 2019; Magge et al., 2019). A summary of these and other DS effects on structural and functional decline are provided in Table 1.
Down Syndrome and decreased physical activity
PLWD are less physically active than the general population, especially in low and middle-income countries (Bull et al., 2020; Ginis et al., 2021). A recent review found that by increasing the level of physical activity, there were significant improvements in the musculoskeletal and cardiovascular systems, mental and brain health among children, adolescents, and adults with disabilities (Ginis et al., 2021). Similar results were found in children with DS; 58% do not meet the 90 min per week minimum recommendations of daily physical activity (Shields et al., 2009) to promote benefits to adolescents with DS (Izquierdo-Gomez et al. (2015).
The rate of adults with ID meeting physical activity guidelines (13.5%) was less than half of the general population (30.8%) (Stancliffe and Anderson, 2017). Being physically active may be more difficult for individuals with DS due to genetic and phenotypic characteristics. Physical activity involves overcoming social, environmental, and physical barriers such as musculoskeletal disorders and cardiovascular changes that can influence physical and behavioral abilities (Mahy et al., 2010; Barr and Shields, 2011; Pitetti et al., 2013; Shields et al., 2017).
It may be difficult to motivate someone with DS to meet the 90-min activity threshold; fortunately, individuals with DS who performed 12 min/day of physical exercise displayed a higher level of physical fitness when compared with the control group and sedentary adolescents with DS (Izquierdo-Gomez et al., 2015). Physical activity is particularly important for people with DS due to the higher prevalence of mental health conditions (e.g., depression and anxiety) and risk of chronic health conditions such as stroke, diabetes, hypothyroidism, and AD (80% by the age of 65y) (Mccarron et al., 2017; Tsou et al., 2020).
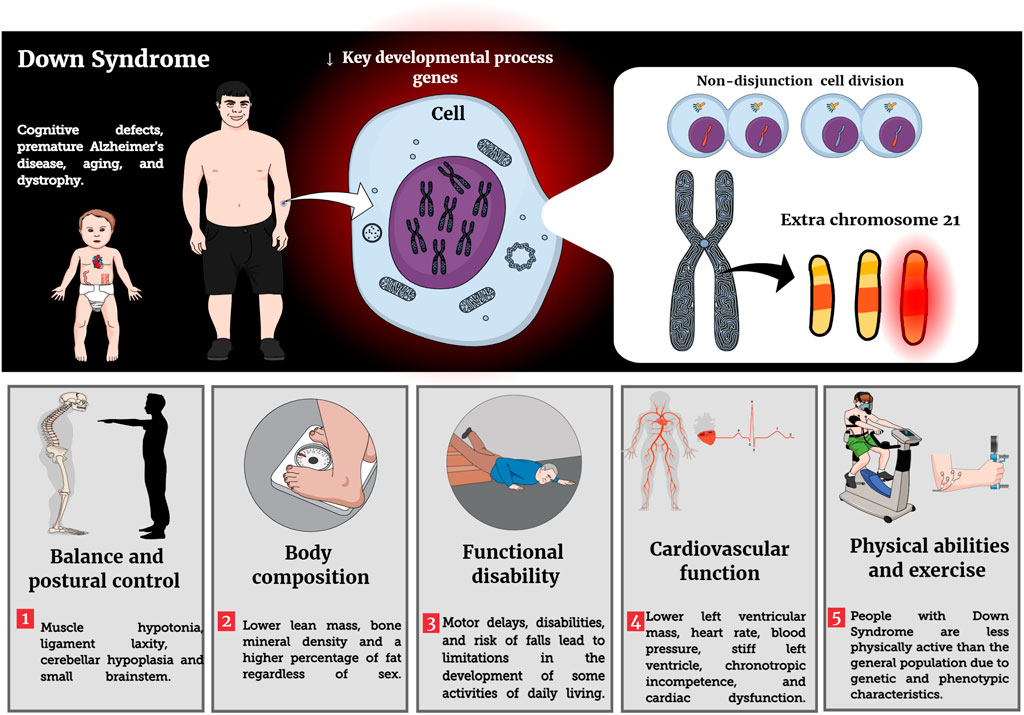
Recent guidelines recommend that PLWD should perform physical activity as well as physical exercise (e.g., RT and balance exercise) at least twice a week, totaling 150 min of moderate physical activity (Naidoo et al., 2021). PLWD should consider adding RT to their regular activity profiles as it improves physical fitness, mental health, quality of life, increases autonomy and independence in activities of daily living, creates opportunities for affective and social relationships in the community, prevents chronic diseases (e.g., hypertension), improves mobility, balance and strengthens muscles and bones (Naidoo et al., 2021). To the authors’ knowledge, however, there is no summative work that has evaluated the literature on RT recommendations and its potential benefits in individuals with DS. Main impacts of DS condition on phenotypic characteristics are reported in Figure 1.
Evidence for prescribing resistance training in individuals with Down Syndrome
The following section overviews RT programs in individuals with DS with specific focus toward the administration of safe, effective, and enjoyable schemes. Table 2 provide an overview of the recommendations for the application of RT in people with DS.

TABLE 2. Evidence statements and summary of recommendations for the individualized resistance training prescription in individuals with DS.
Exercise load
The RT programs presented different evaluations of the training intensity depending on the purpose of each study. Intensity was evaluated by a 1-repetition maximum (1RM) test (Weber and French, 1988; Shields et al., 2008; Shields and Taylor, 2010; Gupta et al., 2011; Rosety-Rodriguez et al., 2013; Shields et al., 2013; Dilek et al., 2018; Rosety-Rodriguez et al., 2021), eight repetitions maximum (8RM) (Davis and Sinning, 1987; Florentino Neto et al., 2010; Fornieles et al., 2014; Diaz et al., 2021), 10 repetitions maximum (10RM) (Cowley et al., 2011) or 12 repetitions maximum (12RM) (Florentino Neto et al., 2010; Seron et al., 2014; Seron et al., 2015; Seron et al., 2017). After application of the different RM tests, the intensity was expressed through the percentage of that workload which varied between 50 and 80% of 1RM (Weber and French, 1988; Shields et al., 2008; Gupta et al., 2011; Shields et al., 2013; Dilek et al., 2018; Rosety-Rodriguez et al., 2021), and 40–65% 8RM (Rosety-Rodriguez et al., 2013; Fornieles et al., 2014; Diaz et al., 2021). Despite presenting a range of different intensities, the summative results identified beneficial effects of RT in people with DS.
Volume
The number of sets per exercise ranged from one to six (Davis and Sinning, 1987; Weber and French, 1988; Tsimaras and Fotiadou, 2004; Shields et al., 2008; Florentino Neto et al., 2010; Shields and Taylor, 2010; González-Agüero et al., 2011b; Cowley et al., 2011; Gupta et al., 2011; González-Agüero et al., 2012; Rosety-Rodriguez et al., 2013; Shields et al., 2013; Fornieles et al., 2014; Seron et al., 2014; Ghaeeni et al., 2015; Seron et al., 2015; Seron et al., 2017; Dilek et al., 2018; Diaz et al., 2021; Rosety-Rodriguez et al., 2021; Shin and Jeong, 2021); it varied according to the training periodization proposed by the objectives of the studies. However, most studies implemented two or three sets of each exercise (Davis and Sinning, 1987; Shields et al., 2008; Florentino Neto et al., 2010; Shields and Taylor, 2010; Cowley et al., 2011; Gupta et al., 2011; Rosety-Rodriguez et al., 2013; Shields et al., 2013; Fornieles et al., 2014; Seron et al., 2014; Seron et al., 2015; Seron et al., 2017; Dilek et al., 2018; Diaz et al., 2021; Rosety-Rodriguez et al., 2021; Shin and Jeong, 2021), with only one study using six sets (Ghaeeni et al., 2015). Most studies prescribed 6 to 12 repetitions (Davis and Sinning, 1987; Shields et al., 2008; Florentino Neto et al., 2010; Shields and Taylor, 2010; Cowley et al., 2011; Gupta et al., 2011; Rosety-Rodriguez et al., 2013; Shields et al., 2013; Fornieles et al., 2014; Seron et al., 2014; Seron et al., 2015; Seron et al., 2017; Diaz et al., 2021; Rosety-Rodriguez et al., 2021). However, the number of repetitions per set varied between 6 and 30. The prescription of 6–12 repetitions was related to the evaluation method of 1RM and maximum repetitions.
Some studies applied circuit exercises that used the time in each stage to perform maximum repetitions (Weber and French, 1988; Tsimaras and Fotiadou, 2004; González-Agüero et al., 2011b; González-Agüero et al., 2012; Shin and Jeong, 2021). Thus, the intensity and volume of training depended on the number of repetitions and sets, exercise order, and weekly frequency.
Rest periods
The duration of the rest interval between sets ranged from 10 to 120 s (s), with most studies showing a rest interval of 90s (Rosety-Rodriguez et al., 2013; Fornieles et al., 2014; Diaz et al., 2021; Rosety-Rodriguez et al., 2021).
Duration
Sessions were structured starting with a warm-up, main phase and return to calm/cool down. Time, or duration, of the sessions varied between 20 and 50 min, as suggested by the ACSM guidelines (Riebe et al., 2018). It should be noted that many studies failed to mention session duration (Davis and Sinning, 1987; Weber and French, 1988; Shields et al., 2008; Shields and Taylor, 2010; Cowley et al., 2011; Gupta et al., 2011; Rosety-Rodriguez et al., 2013; Shields et al., 2013; Fornieles et al., 2014; Diaz et al., 2021; Rosety-Rodriguez et al., 2021) as it is more typical to report sets and repetition schemes opposed to session durations.
Frequency
Frequency varied between two and three times per week, with more leaning toward three sessions (Davis and Sinning, 1987; Weber and French, 1988; Tsimaras and Fotiadou, 2004; Florentino Neto et al., 2010; Gupta et al., 2011; Rosety-Rodriguez et al., 2013; Fornieles et al., 2014; Ghaeeni et al., 2015; Dilek et al., 2018; Diaz et al., 2021; Rosety-Rodriguez et al., 2021; Shin and Jeong, 2021). It has been shown that training twice per week leads to excellent results in muscle strength gains in upper and lower limbs, improvement in functional capacity, reduction of fat percentage, and increases in lean mass in a population with DS (Shields et al., 2008; Shields and Taylor, 2010; González-Agüero et al., 2011b; Cowley et al., 2011; González-Agüero et al., 2012; Shields et al., 2013).
Duration of training programs
The duration of the programs ranged from 6 to 24 weeks, but most studies averaged at least 12 weeks (Tsimaras and Fotiadou, 2004; Florentino Neto et al., 2010; Rosety-Rodriguez et al., 2013; Fornieles et al., 2014; Seron et al., 2014; Seron et al., 2015; Seron et al., 2017; Diaz et al., 2021; Rosety-Rodriguez et al., 2021). Short-term intervention programs (lasting up to 6 weeks) had positive effects on upper limb strength and balance in adolescents with DS (Weber and French, 1988; Gupta et al., 2011). However, studies with a longer duration (21 and 24 weeks) resulted in additional positive adaptations to body composition in adolescents with DS (González-Agüero et al., 2011b; González-Agüero et al., 2012; Dilek et al., 2018).
Types of exercise
Programs included in this review worked the upper and lower limbs’ muscles including both multi-joint and single joint exercises. These main exercises included the leg press, seated leg press, jumps, squat, deadlift; standing leg curl with ankle weights, one-sided stroke, seated row, lat pull-down, upright row, latissimus dorsi pull down, front pull-down, lateral rows, frontal rows, bench press, seated chest press, press-ups, press-ups on the wall, and abdominal exercises in their different variants.
The included investigations also implemented single-joint exercises for the biceps (bicep curl; arm curl and cable biceps curl), triceps (triceps extension; triceps curl, triceps pushdown; cable triceps extension; triceps French), shoulders (shoulder press; dumbbell front raise), calves (seated calf raise; calf raise; calf raises with ankle weights; heel lift), quadriceps (leg extension), hamstrings (leg curl), and hips (standing hip flex with ankle weights; flexors, abductors, extensors). In addition, three studies included plyometrics with different forms of execution (standing vertical jump, jump with run-in, drop jump, drop jump, and horizontal jump) for the lower limbs (Tsimaras and Fotiadou, 2004; González-Agüero et al., 2011b; González-Agüero et al., 2012; Dilek et al., 2018; Shin and Jeong, 2021).
In most studies, exercise on machines was chosen because it guides the movement and is safer in the initial phase of training (Weber and French, 1988; Shields et al., 2008; Shields and Taylor, 2010; Cowley et al., 2011; Rosety-Rodriguez et al., 2013; Shields et al., 2013; Fornieles et al., 2014; Seron et al., 2014; Seron et al., 2015; Seron et al., 2017; Diaz et al., 2021; Iversen et al., 2021; Rosety-Rodriguez et al., 2021). Most exercise prescriptions ranged from three to eleven exercises.
The literature recommends that exercises selected be simple and easy to understand for PLWD. In addition, exercise leaders should have a personalized follow-up to instruct and demonstrate each exercise in different ways so that the practitioner can verify if the individual can perform it correctly (Duplanty et al., 2014; Jacinto et al., 2021). Therefore, a period of familiarization with the exercises is essential for individuals with DS to learn the proper execution of the movement, eliminate the fear of using the equipment and perform the movement so that there are positive effects of the RT (Duplanty et al., 2014; Jacinto et al., 2021).
Health and potential benefits of RT in individuals with Down Syndrome
Muscle strength. The literature has shown that RT is a safe, socially desirable, and viable option for individuals with DS across the lifespan (Davis and Sinning, 1987; Weber and French, 1988; Shields et al., 2008; Shields and Taylor, 2010; Shields et al., 2013; Sugimoto et al., 2016). RT improves muscle strength in individuals with DS represented by increases in maximal handgrip strength, isokinetic knee extension and flexion strength that had a magnitude of change of 19 and 27%, respectively, when compared to control group (Cowley et al., 2011; Rosety-Rodriguez et al., 2021).
Shields et al. (2013) reported a 21–30% increase in muscle strength among children and young adults with DS after 10 weeks of RT. These results were corroborated in adolescents (Shields and Taylor, 2010) and adults with DS (Shields et al., 2008; Cowley et al., 2011) who used similar RT programs (twice a week for 10 weeks). Adolescents experienced a 42% increase from baseline in lower limb muscle strength (Shields and Taylor, 2010) and adults gained 25% strength in the upper extremities (Shields et al., 2008). These positive findings after RT are witnessed in other studies noting improvements in muscle strength in the upper limbs (Davis and Sinning, 1987; Weber and French, 1988) and in the lower limbs with training (Weber and French, 1988). It is worth mentioning that RT is an important exercise modality that does more than simply impact muscular strength. Stronger extremities have been shown to improve functional capacity, and consequently, daily tasks and physical activities (Shields and Taylor, 2010). RT is suitable for individuals with DS as it is an activity that involves repetitive skills, similar to typical employment opportunities for PLWD.
Body composition
Obesity is common in individuals with DS with approximately 50% of adults being obese. BMI and weight consistently increase between the age groups 18–29 and 30–39 and decrease after the age of 40, which may indicate the disease process (e.g., Alzheimer’s disease) in individuals with DS. Thus, it is necessary to be proactive in the prevention of muscle wasting and hypotonia that result in increased adiposity and decreased bone mineral density (Barnhart and Connolly, 2007; Presson et al., 2013; Capone et al., 2018; Coelho-Junior et al., 2019). Coelho-Junior et al. (2019) found that total lean mass is negatively correlated with age in individuals with DS, regardless of the muscle mass index.
González-Agüero et al. (2011b) found that 21 weeks of RT combined with plyometrics increased total upper and lower limbs lean mass and bone mass with no change in fat mass in children and adolescents with DS. These findings can be corroborated in the literature (Florentino Neto et al., 2010; Rosety-Rodriguez et al., 2013; Diaz et al., 2021). Interestingly, one study reported that a 12 week RT program was sufficient to reduce fat mass in adults with DS (Florentino Neto et al., 2010). This is not a universally supported finding as others failed to report a change in percentage body fat after 12 weeks of RT; alternatively, this ‘failure to change’ may have been a positive outcome as the control group increased the percentage of fat by 2.3% in the same period (Seron et al., 2014).
In addition, the active group experienced an increase in total bone mineral content (BMC), hip and lumbar region (6.7, 14.6 and 6.4%, respectively), when compared to the control group (González-Agüero et al., 2012). These promising results may have occurred due to the inclusion of plyometric jumps with RT, which was a strategy to improve BMC in young people with DS (González-Agüero et al., 2012). In general, there is a positive effect of plyometric exercise on osteogenesis (Vicente-Rodríguez, 2006). The connection may lie in the direct relationship between bone mass and total lean mass (Coelho-Junior et al., 2019; Kim et al., 2020). The practice of impact exercise such as running, plyometrics, and RT is associated calcium and vitamin D uptake to attenuate or prevent osteoporosis in individuals with DS (Angelopoulou et al., 2000; González-Agüero et al., 2012; Reza et al., 2013).
Muscle damage
It is relevant to note that RT can induce muscle damage, especially with inappropriate prescription and unaccustomed exercise due to limited motor control, incorrect technique or a large amount of eccentric contractions. Excessive eccentric muscle actions can result in exercise-induced muscle damage or delayed onset muscle soreness in athletes, untrained and trained individuals (Clarkson and Hubal, 2002).
In this context, Diaz et al. (2021) evaluated the impact of RT on markers of muscle damage (creatine kinase activity, myoglobin concentration, and lactate dehydrogenase activity) in adults with DS. It was observed that there were no significant changes in the markers of muscle damage after 12 weeks or at any time during the intervention program. Another point worth mentioning is adherence to RT, which was 96% across 12 weeks. In addition, the participants did not present sports-related injuries or dropouts, as already demonstrated in the literature (Shields et al., 2008;Cowley et al., 2011; Shields et al., 2013; Diaz et al., 2021).
Functional capacity
A number of assessments were used to evaluate functional tasks for lower-limb physical function in adolescents and adults with DS; the following tests were used: chair rise; ascend and descend 10 steps; walk speed; timed up and down stairs test, and timed get-up-and-go test (Shields et al., 2008; Shields and Taylor, 2010; Cowley et al., 2011; Rosety-Rodriguez et al., 2013). After performing the RT program (10 and 12 weeks), the participants showed a significant improvement in performance in all physical function scores of the lower limb muscles, except for the timed up and down stairs test and upper-body functional activities (e.g., grocery shelving task) (Shields et al., 2008; Shields and Taylor, 2010). Intensity and volume of training may have been insufficient to increase the functional capacity of both upper and lower limbs, despite having increased 21–30% of muscle strength (Shields et al., 2013).
Shields et al. (2013) reported that specificity of the training and duration of the RT program may have influenced the findings. For example, RT for 12 weeks improved the repetitive weighted box-stacking test (19.1 ± 3.0 vs. 23.3 ± 2.7 boxes/min; p = 0.0141) and pail-carry test scores were also significantly improved in the intervention group with DS (33.1 ± 5.7 m vs. 41.8 ± 6.0 m; p = 0.004) (Fornieles et al., 2014; Diaz et al., 2021). Though the duration might have played a factor in the divergent results, one could also argue training model adopted between studies could have influenced the findings. Shields et al. (2013) opted for the traditional RT model (e.g., performing less exercise in a higher amount of time) while Fornieles et al. (2014) and Diaz et al. (2021) increased intensity and volume every 2 weeks. The improvement of work task performance is of paramount importance for job prospects for adults with DS, as their activities in the workplace often require both physical and non-cognitive skills (Shields et al., 2008).
Balance
Individuals with DS are deficient in postural balance, which can be explained by anatomical changes such as flat feet, muscle hypotonia, ligament laxity, cerebellar hypoplasia and small brainstems that generate disturbances in the balance regulation system or postural control (Lauteslager et al., 1998; Guzmán-Muñoz et al., 2017; Kim et al., 2017; Foley and Killeen, 2019; Shiohama et al., 2019). To perform movements of daily living or other motor tasks safely, it is essential to have postural control (Paillard, 2017). However, the literature has shown that the balance deficit in young people with DS can increase motor delays and disabilities, risk of falls and lead to limitations in the development of some activities of daily living (Lauteslager et al., 1998; Jung et al., 2017; Kim et al., 2017; Paillard, 2017; Maïano et al., 2019). There appear to be positive effects of different exercises (e.g., e-games, whole-body vibration, stretching, strength, and balance) on the static balance and dynamic balance of children and adolescents with DS (Maïano et al., 2019). Exercise as simple as walking on the treadmill and core strengthening improves static and dynamic balance in children with DS (Alsakhawi and Elshafey, 2019). These findings provide a powerful argument that people with DS should start and/or maintain regular resistance exercises.
Various RT programs were effective at improving balance. Gupta et al. (2011) reported a significant improvement in the Bruininks Osteresky Test of Motor Proficiency (BOTMP) balance subscale scores from 10.50 to 19.50 in the experimental group after strengthening the muscles of the pelvic girdle and lower limbs for 6 weeks (Gupta et al., 2011).
Blood pressure
About 45–65% of people with DS have a congenital heart disease that affects the structure and function of the heart and vascular system (Capone et al., 2018). As a result, previous studies have shown that people with DS have chronotropic incompetence, lower heart rate (HR), altered cardiac autonomic function during exercise, lower blood pressure (BP) and resting heart rate when compared to individuals without DS (Fernhall and Otterstetter, 2003; Iellamo et al., 2005; Agiovlasitis et al., 2010; Fernhall et al., 2013; De Carvalho et al., 2015). According to De Carvalho et al. (2015) autonomic dysfunction occurs due to changes in sympathetic and parasympathetic tone that are associated with early morbidity and mortality. Hence, one study found that increased BP in adults with DS was associated with higher body mass index (Pucci et al., 2016).
The practice of physical exercise is essential to improve body composition and cardiovascular health. Seron et al. (2015) demonstrated that after 12 weeks of RT overweight adults with DS had a significant reduction in BP levels (systolic BP = -6.2 mmHg; diastolic BP = -4.8 mmHg; mean BP = -4.2 mmHg). These results are promising considering that individuals with DS have a higher incidence of cardiovascular risk factors such as low cardiorespiratory capacity, loss of muscle strength and obesity (Hu et al., 2013). Thus, numerous studies have demonstrated the beneficial effects of physical exercise that result in improving cardiopulmonary capacity, strength and body composition, and consequently improves cardiovascular parameters (González-Agüero et al., 2011b; Fernhall et al., 2013; Wee et al., 2015; Sugimoto et al., 2016; Jacinto et al., 2021).
Cardiorespiratory fitness
Persons with DS have lower peak and submaximal exercise capacity as a result of numerous factors, among which are a lower level of physical fitness, alteration of autonomic cardiac function and catecholamine responses, prevalence of heart and pulmonary disease and chronotropic incompetence (Guerra et al., 2003; Iellamo et al., 2005; Fernhall et al., 2009; Mendonca G. V. et al., 2010). In addition, joint laxity and muscle hypotonia may influence poor exercise economy as it alters gait kinetics and kinematics (Mendonca G. et al., 2010).
On the other hand, a recent meta-analysis demonstrated that RT improved functional exercise capacity, endurance and peak exercise capacity in older adults with chronic obstructive pulmonary disease (Li et al., 2020). In addition to delaying the onset of neuromuscular fatigue in a submaximal aerobic test, RT improves strength and muscle functionality in aging adults (Emerson et al., 2015).
Seron et al. (2017) demonstrated that 12 weeks of RT increased pulmonary ventilation (VE) and total test time (TTT) in the treadmill test in young adults with DS. On the other hand, there was no significant difference in VO2peak after aerobic or RT interventions. However, the increase in maximum VE is of paramount importance for people with DS, as they present anatomical changes in the respiratory tract, such as a small nasal passage, which can hinder respiratory mechanics during exercise (Mendonca G. V. et al., 2010). In addition, the same study showed that increased time to exhaustion can increase cardiorespiratory endurance during exercise (Dodd and Shields, 2005).
Oxidative stress
Oxidative stress can occur when there is an imbalance between generation and removal of reactive oxygen species (ROS) in the body. Unfortunately, oxidative stress is elevated from birth in people with DS (Jovanovic et al., 1998; Muchová et al., 2014).
A recent study in adults with DS showed that RT improved the antioxidant defense system and reduction in oxidative damage (Rosety-Rodriguez et al., 2021). After 12 weeks of RT (3x per week; 40–50% 8RM) active people with DS increased plasma total antioxidant status (TAS), erythrocyte glutathione reductase (GR) activity, and plasma levels of glutathione when compared control group with DS (Rosety-Rodriguez et al., 2021). The same study found a reduction in both markers of oxidative damage (malondialdehyde (MDA) and reduced carbonyl groups) in the intervention group. Despite the positive results of RT and oxidative damage, more work is needed to understand the effect of RT exercise on oxidative stress responses in different age groups, hence the potential role for RT to act as a stimulus for adaptation.
Testosterone hormones and immunoglobulin A
People with DS have a higher prevalence of diseases related to the immune system when compared to the general population, due to low cellular and humoral immunity (Illouz et al., 2021). This occurs, in part, to the severe impairment of immunoglobulin (Ig) secretion in saliva (Chaushu et al., 2002). Thus, saliva is a predictor of susceptibility to respiratory tract infections (Chaushu et al., 2002).
Studies have demonstrated the positive effects of RT on the mucosal immune response as well as on salivary hormone levels in adults (Tsai et al., 2012; Schwanbeck et al., 2020). In more detail, Tsai et al. (2012) reported that RT promotes cumulative effects on IgA and cortisol responses in elite weightlifters. Likewise, RT with free weights significantly increased salivary testosterone and cortisol levels in male adults (Schwanbeck et al., 2020). Similar results were found in adults with DS after performing 12 weeks of RT (3x per week, 40–65% 8RM) such that there was an improvement in mucosal immunity response by increasing IgA concentration in the exercising group. Also, there was an increase in testosterone levels (Fornieles et al., 2014). However, no significant changes were observed in cortisol concentration after the RT program (Fornieles et al., 2014). It seems that short-term RT is a non-pharmacological alternative to improve the mucosal immune response as well as the salivary hormonal profile in individuals with DS (Fornieles et al., 2014). However, further studies are needed to confirm these results in different age groups.
Inflammation
A meta-analysis evaluating inflammation reviewed 19 studies involving 957 children with DS and 541 controls. The investigation found significantly higher levels of Il-1β, TNF-α, IFN-γ and neopterin in individuals with DS (Zhang et al., 2017). However, the same study showed that circulating levels of interleukin (IL)-4, IL-6, IL-8 and IL-10 were not significantly different between those with DS and control groups (Zhang et al., 2017). Given these findings, RT has shown positive results to improve low-grade systemic inflammatory status after 12 weeks (3 days per week/40–65% 8RM). Male adults with DS experienced significantly decreased plasma levels of leptin, TNF- α and IL-6 while in the control group with DS had no change (Rosety-Rodriguez et al., 2013). The same study also highlights that there is a positive association between IL-6 and TNF-α and waist circumference (Rosety-Rodriguez et al., 2013).
The anti-inflammatory effects of RT may be mediated either by a reduction in visceral fat mass (with a subsequent decrease in adipokine release) or by an induction of an anti-inflammatory environment at each exercise session that will result in the production of IL-10, which counterbalances the pro-inflammatory state (Strasser et al., 2012). In addition, it should be noted that changes in body composition can directly influence adipokines, hence the importance of physical exercise in this physiological context (Ordonez et al., 2013).
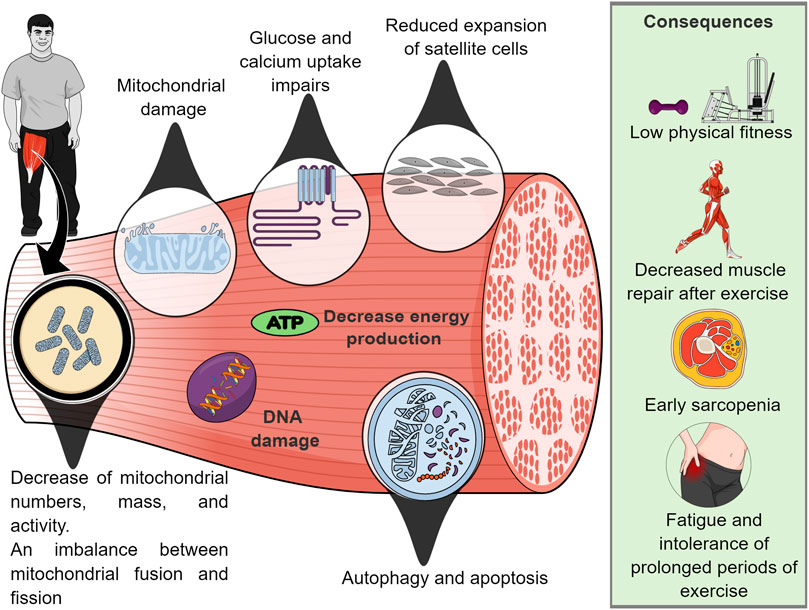
Mitochondrial dysfunction. Mitochondrial dysfunction may contribute to premature aging, low levels of physical activity and AD in individuals with DS (Valenti et al., 2018). This is due primarily to the mitochondrial defect (in mitochondrial numbers or intrinsic mitochondrial activity) that leads to an imbalance between mitochondrial fusion and fission that results in inadequate mitochondrial energy production, impaired exercise tolerance, as well as intrinsic bioenergetic and metabolic dysfunction in skeletal muscle (Valenti et al., 2018; Pecze et al., 2020).
In this context, the literature has shown that after an acute session of maximal isokinetic strength testing in the quadriceps muscle, post-exercise phosphocreatine resynthesis (PCr) assessed by phosphorus magnetic resonance spectroscopy was 16% slower in adults with DS compared with non-DS, ID controls (Phillips et al., 2013). This result indicates a defect in mitochondrial respiratory function and alteration in biochemical/bioenergetic mechanisms in the skeletal muscle of this population (Phillips et al., 2013).
In addition, a recent study carried out in DS mice (model Ts65Dn) showed that 1 month of training (adapted aerobic exercise) did not improve body composition, however, they found that trisomy directly affects the metabolic response of skeletal muscle to exercise (Cisterna et al., 2022). The same study demonstrated that euploid mice have a better ability to restore energy storage (e.g., PCr) than trisomic mice. Unfortunately, it was observed that muscle regeneration in trisomic mice is attenuated due to reduced expansion of satellite cells, which maintain skeletal muscle, they are essential for muscle repair after exercise (Pawlikowski et al., 2018).
Furthermore, Cowley et al. (2012) demonstrated that DS mice (model Ts65Dn) showed progressive skeletal muscle fatigue in repeated activation/recovery protocols. This contractile muscle weakness has been associated with alteration of different metabolic mechanisms (e.g. glucose and calcium uptake) as well as mitochondrial dysfunction (Hawli et al., 2009; Pecze et al., 2020). Thus, individuals with DS are more prone to fatigue and are intolerant to prolonged periods of exercise (Aguiar et al., 2008; Pecze et al., 2020). A summary of mitochondrial mechanisms that potentially mediate muscle dysfunction is reported in Figure 2.
Discussion
The evidence collected and reported in this review show promising health benefits of RT for individuals with DS. There is data to support that the RT increases muscle strength, and improves body composition, functional capacity, and balance, while reducing inflammatory status and oxidative stress and improving the immune system. Such effects can be important for health promotion, well-being, and longevity in individuals with DS. Furthermore, an emerging body of compelling data demonstrates that different RT protocols might induce beneficial effects on distinct tissues, organs, and physiological systems in individuals with DS (Figure 3). However, the overall methodological quality of the current literature is heterogenic in several key fields. To improve evidence quality, well-designed and standardized investigations with large samples are needed to establish the optimal parameters to exercise prescription.
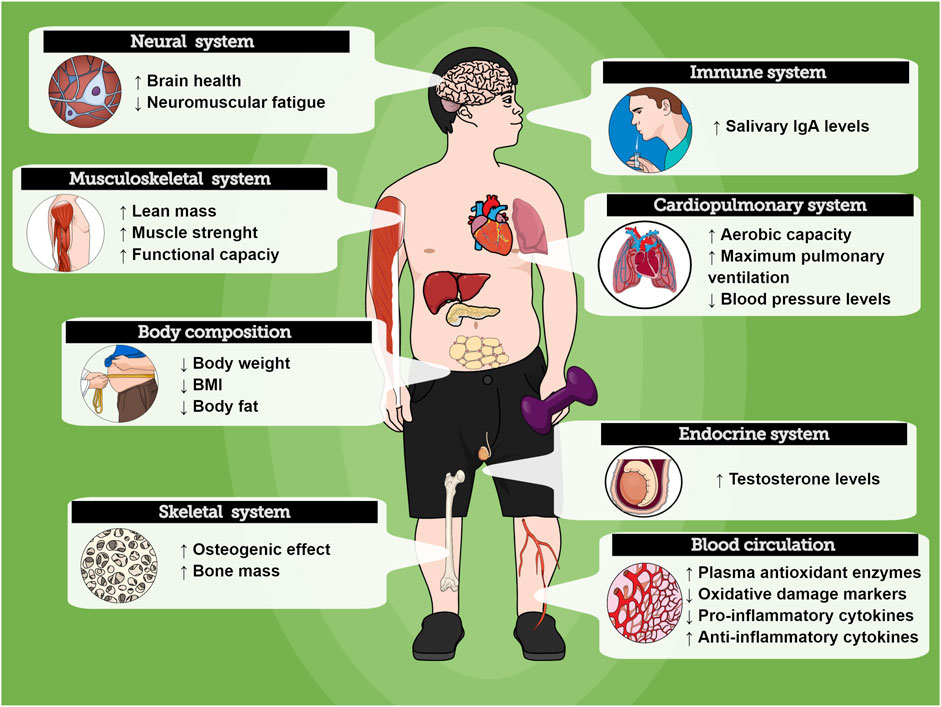
FIGURE 3. Overview of the RT effects on physiological systems in people with Down Syndrome. Exercise might promote positive health responses on different organs.
The authors attempted to summarize the strength of the available scientific evidence on RT and individuals with DS noted in sections two and three of this narrative review. Although the narrative review does not use systematic research and analytical protocols, it is fundamental for understanding the mechanisms and suggesting possible gaps on the theme developed throughout this writing (West et al., 2002). Also, it is important to consider that a significant challenge arises in evaluating a body of knowledge comprising observational and randomized clinical trials (RCTs), and this important limitation should be considered in this review (West et al., 2002).
It was observed that there are still gaps in the literature regarding the effects of RT on cardiovascular responses, muscle damage, oxidative stress, inflammation, testosterone hormones and immunoglobulin A, considering that among these topics the authors found in the literature only one study, some of which were not RCTs (Rosety-Rodriguez et al., 2013; Fornieles et al., 2014; Seron et al., 2015; Diaz et al., 2021; Rosety-Rodriguez et al., 2021). In addition, most of these studies were in young adults with DS, except for the study on cardiovascular responses and RT (Seron et al., 2015).
Furthermore, future studies should evaluate the association between muscle mass/strength and chronic disease risk factors (inflammatory biomarkers, cholesterol level, insulin, systolic BP, diastolic BP and HR) in individuals with DS. We believe high priority should be placed on this issue because large cohorts showed that a decrease in muscle mass and strength is a predictor of chronic disease risk factors and all-cause mortality (Ruiz et al., 2008; Ruiz et al., 2009; Volaklis et al., 2015; Garcia-Hermoso et al., 2018; Jochem et al., 2019). This relevant topic might add new concepts and implications for public health in DS conditions.
Regardless of the advantages of RT, participation of people with DS in regular programs remains low in clinical practice, likely due to several aspects, such as time constraints, a high-perceived difficulty, and limited access to gyms, trained professionals, and equipment. Thus, the identification of exercise approaches that limit barriers to involvement may encourage engagement in RT and consequently enhance health outcomes are necessary. It was proposed that minimal doses of exercise, characterized by lower session volumes than in traditional RT guidelines, can improve muscle mass, strength, and functional capacity in younger and older people (Fyfe et al., 2022). Such minimal-dose strategies to RT have the capacity to reduce many barriers to exercise participation and may have beneficial implications for the feasibility and scalability in DS context.
Individuals with DS possess varying degrees of cognitive delays, from very mild to severe. Moreover, visual and hearing impairments vary greatly among individuals with DS, which can impact exercise learning. Associated with these factors, 70% of adults with DS develop AD-like neuropathology by 40 years of age and exercise adherence for persons with AD can be very challenging given their unique AD symptoms combinations (e.g., behavioral, and psychological symptoms of dementia) (Yu et al., 2017; Antonarakis et al., 2020). Unfortunately, there are no effective interventions to significantly delay or prevent the onset of AD, but people with DS need to be physically active as part of a healthy aging plan (Pape et al., 2021). On another hand, previous investigations have shown that physical exercise can prevent and promote improvements in the treatment of Alzheimer’s disease, as exercise improves modulation amyloid β turnover, inflammation, synthesis, and release of neurotrophins, and cerebral blood flow in elderly without DS (De La Rosa et al., 2020).
Optimal training prescription requires a person-centered approach using individualized strategies, with an appropriate balance of exercise stimulus, recovery, and optimal periodization to attain optimal physiological outcomes. From a clinical point of view, it seems to be important to start RT as early as possible to obtain the best physical performance, health outcomes, and quality of life. However, to aid the successful transfer to RT for individuals with DS, adherence, adhesion, and dropout reasons for an exercise program should be considered. Furthermore, one possibility that should not be ruled out is that individuals with DS must be supervised by a multidisciplinary team with long-term multifactor method (cognitive-behavioral therapies, individual diet, and medical intervention), in an attempt to comprehend the real impacts of RT on daily lives.
We propose that training acute variables (intensity, duration, volume, frequency, and interval) be controlled, addressed, and successfully integrated to create an effective intervention, regardless of the resistance exercise types. The optimal interplay of these variables can deeply potentiate training outcomes and requires attention, mainly in sedentary individuals with DS. We recommend the inclusion of progressive overload in order to maximize adaptions. However, there is a need for additional studies to understand the possible DS severity, age, and sex discrepancies in response to different RT protocols, besides the effects of interrupting exercise or detraining, and the impact of long-term training (>6 months) and lifelong RT, not just for a predetermined time.
Due to hypermobility of the joints, low muscle quality, and bone density related to DS, the prescription should be with higher caution than in younger healthy adults. Primary attention should be given to correct movement instruction and assertive verbal reports are essential. The selection of resistance exercises for individuals with DS should be based on specific needs, but that aim to improve static and dynamic balance, motor coordination, functional mobility, and consequently independence. Individuals that can tolerate bilateral closed kinetic chain exercises represent a great indication of intermuscular coordinated strength capacity (Fragala et al., 2019). Exercise training should reflect all major muscle groups in the upper and lower extremities with a priority for multijoint movements. For older adults with DS, a multicomponent exercise intervention program can be an alternative strategy for improving gait, balance, and strength, as well as reducing the falls rate (Fragala et al., 2019).
Given the complexity of biological systems in DS, the use of animal experimental and basic science might provide a meaningful understanding of the several adaptive mechanisms undergoing acute and chronic resistance exercise, particularly when ethical considerations make the use of human models unfeasible (Herault et al., 2017). Functional assessments, morphological, biochemical, and molecular approaches might aid understanding of the full picture of cellular adaptation in response to the RT program, besides the further discovery of potential therapeutic targets. A mechanistic framework of these responses could give valuable insights for therapeutic approaches to development and treatment guidance in humans, as well as clarify the main adverse and beneficial effects of RT in different physiological systems, tissues, and organs.
It is important for future research to verify the effects of RT in individuals with DS on variables highlighted in this narrative review with different age groups, especially after 40 years of age. People with DS are living longer lives and are exposed to early aging that may increase the risk of comorbidities and mortality in this population (Tenenbaum et al., 2012; Tsou et al., 2020). Finally, given the rapid development of research in this area, annual updates of this review are needed to keep pace with the latest findings regarding the RT prescription and safety for individuals with DS.
Conclusion
The objective of this narrative review was to give an overview of the adaptations and benefits generated by RT with different intensities, volume, and application methods. The authors recommend the use of RT, as well as RT combined with different forms of exercise (aerobic, balance, plyometrics, and isometric), considering the volume and intensity, as well as the duration of training, type of exercise applied (e.g., machine exercise, bodyweight and/or free weights). Table 2 sets out the parameters by which professionals should use RT based on up-to-date and current research in the area. The RT protocols summarized in this current review provide guidance, critical conclusions, and novel research settings, which could be useful to coaches, clinicians, and researchers to effectively design RT program for individuals with DS.
Author contributions
GM and DN conceived, designed the study, and conducted the reference review. EF and GM wrote the methodology GM, IN, and DN contributed to the written initial draft preparation. WS revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are also thankful to Coordination of Superior Level Staff Improvement—Brazil.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agiovlasitis S., Collier S. R., Baynard T., Echols G. H., Goulopoulou S., Figueroa A., et al. (2010). Autonomic response to upright tilt in people with and without Down syndrome. Res. Dev. Disabil. 31, 857–863. doi:10.1016/j.ridd.2010.03.002
Agiovlasitis S., Jin J., Yun J. (2020). Age-group differences in body mass index, weight, and height in adults with down syndrome and adults with intellectual disability from the United States. Adapt. Phys. Act. Q. 38, 79–94. doi:10.1123/apaq.2020-0004
Aguiar A. S., Tuon T., Albuquerque M. M., Rocha G. S., Speck A. E., Araújo J. C., et al. (2008). The exercise redox paradigm in the down’s syndrome: Improvements in motor function and increases in blood oxidative status in young adults. J. Neural Transm. 115, 1643–1650. doi:10.1007/s00702-008-0120-x
Alsakhawi R. S., Elshafey M. A. (2019). Effect of core stability exercises and treadmill training on balance in children with down syndrome: Randomized controlled trial. Adv. Ther. 36, 2364–2373. doi:10.1007/s12325-019-01024-2
Angelopoulou N., Matziari C., Tsimaras V., Sakadamis A., Souftas V., Mandroukas K. (2000). Bone mineral density and muscle strength in young men with mental retardation (with and without Down syndrome). Calcif. Tissue Int. 66, 176–180. doi:10.1007/s002230010035
Angelopoulou N., Tsimaras V., Christoulas K., Mandroukas K. (1999). Measurement of range of motion in individuals with mental retardation and with or without Down syndrome. Percept. Mot. Ski. 89, 550–556. doi:10.2466/pms.1999.89.2.550
Antonarakis S. E., Skotko B. G., Rafii M. S., Strydom A., Pape S. E., Bianchi D. W., et al. (2020). Down syndrome. Nat. Rev. Dis. Prim. 6, 9. doi:10.1038/s41572-019-0143-7
Bae E.-J., Park N.-J., Sohn H.-S., Kim Y.-H. (2019). Handgrip strength and all-cause mortality in middle-aged and older Koreans. Int. J. Environ. Res. Public Health 16, 740. doi:10.3390/ijerph16050740
Barnhart R. C., Connolly B. (2007). Aging and down syndrome: Implications for physical therapy. Phys. Ther. 87, 1399–1406. doi:10.2522/ptj.20060334
Barr M., Shields N. (2011). Identifying the barriers and facilitators to participation in physical activity for children with Down syndrome. J. Intellect. Disabil. Res. 55, 1020–1033. doi:10.1111/j.1365-2788.2011.01425.x
Bastiaanse L. P., Hilgenkamp T. I., Echteld M. A., Evenhuis H. M. (2012). Prevalence and associated factors of sarcopenia in older adults with intellectual disabilities. Res. Dev. Disabil. 33, 2004–2012. doi:10.1016/j.ridd.2012.06.002
Bayen E., Possin K. L., Chen Y., De Langavant L. C., Yaffe K. (2018). Prevalence of aging, dementia, and multimorbidity in older adults with Down syndrome. JAMA Neurol. 75, 1399–1406. doi:10.1001/jamaneurol.2018.2210
Beerse M., Henderson G., Liang H., Ajisafe T., Wu J. (2019). Variability of spatiotemporal gait parameters in children with and without Down syndrome during treadmill walking. Gait Posture 68, 207–212. doi:10.1016/j.gaitpost.2018.11.032
Bertapelli F., Pitetti K., Agiovlasitis S., Guerra-Junior G. (2016). Overweight and obesity in children and adolescents with down syndrome—prevalence, determinants, consequences, and interventions: A literature review. Res. Dev. Disabil. 57, 181–192. doi:10.1016/j.ridd.2016.06.018
Blood Institute (1998). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. National institutes of health. Obes. Res. 6 (2), 51S–209S. http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf
Bull F. C., Al-Ansari S. S., Biddle S., Borodulin K., Buman M. P., Cardon G., et al. (2020). World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462. doi:10.1136/bjsports-2020-102955
Capone G. T., Chicoine B., Bulova P., Stephens M., Hart S., Crissman B., et al. (2018). Co‐occurring medical conditions in adults with down syndrome: A systematic review toward the development of health care guidelines. Am. J. Med. Genet. A 176, 116–133. doi:10.1002/ajmg.a.38512
Carmeli E., Ayalon M., Barchad S., Sheklow S. L., Reznick A. Z. (2002). Isokinetic leg strength of institutionalized older adults with mental retardation with and without Down's syndrome. J. Strength Cond. Res. 16, 316–320. doi:10.1519/1533-4287(2002)016<0316:ilsoio>2.0.co;2
Chaushu S., Yefenof E., Becker A., Shapira J., Chaushu G. (2002). A link between parotid salivary Ig level and recurrent respiratory infections in young Down's syndrome patients. Oral Microbiol. Immunol. 17, 172–176. doi:10.1034/j.1399-302x.2002.170306.x
Chodzko-Zajko W. J., Proctor D. N., Singh M. a. F., Minson C. T., Nigg C. R., Salem G. J., et al. (2009). Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 41, 1510–1530. doi:10.1249/mss.0b013e3181a0c95c
Cioni M., Cocilovo A., Di Pasquale F., Araujo M., Siqueira C. R., Bianco M. (1994). Strength deficit of knee extensor muscles of individuals with Down syndrome from childhood to adolescence. Am. J. Ment. Retard. 99, 166–174.
Cisterna B., Bontempi P., Sobolev A. P., Costanzo M., Malatesta M., Zancanaro C. (2022). Quantitative magnetic resonance characterization of the effect of physical training on skeletal muscle of the Ts65Dn mice, a model of Down syndrome. Quant. Imaging Med. Surg. 12 (3), 2066–2074. doi:10.21037/qims-21-729
Clarkson P. M., Hubal M. J. (2002). Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 81, S52–S69. doi:10.1097/00002060-200211001-00007
Coelho-Junior H. J., Villani E. R., Calvani R., Carfì A., Picca A., Landi F., et al. (2019). Sarcopenia-related parameters in adults with down syndrome: A cross-sectional exploratory study. Exp. Gerontol. 119, 93–99. doi:10.1016/j.exger.2019.01.028
Cowley P. M., Keslacy S., Middleton F. A., Deruisseau L. R., Fernhall B., Kanaley J. A., et al. (2012). Functional and biochemical characterization of soleus muscle in down syndrome mice: Insight into the muscle dysfunction seen in the human condition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R1251–R1260. doi:10.1152/ajpregu.00312.2012
Cowley P. M., Ploutz-Snyder L. L., Baynard T., Heffernan K., Jae S. Y., Hsu S., et al. (2010). Physical fitness predicts functional tasks in individuals with Down syndrome. Med. Sci. Sports Exerc. 42, 388–393. doi:10.1249/MSS.0b013e3181b07e7a
Cowley P. M., Ploutz-Snyder L. L., Baynard T., Heffernan K. S., Young Jae S., Hsu S., et al. (2011). The effect of progressive resistance training on leg strength, aerobic capacity and functional tasks of daily living in persons with Down syndrome. Disabil. Rehabil. 33, 2229–2236. doi:10.3109/09638288.2011.563820
Cunningham C., O’donoghue G., Cosgrave S., Sands T., Leacy B., Markievicz I., et al. (2022). Gym staff perspectives on disability inclusion: A qualitative study. Disabil. Rehabil. 1, 1–8. doi:10.1080/09638288.2022.2036826
Davis W. E., Sinning W. E. (1987). Muscle stiffness in down syndrome and other mentally handicapped subjects: A research note. J. Mot. Behav. 19, 130–144. doi:10.1080/00222895.1987.10735404
De Asua D. R., Parra P., Costa R., Moldenhauer F., Suarez C. (2014). Evaluation of the impact of abdominal obesity on glucose and lipid metabolism disorders in adults with Down syndrome. Res. Dev. Disabil. 35, 2942–2949. doi:10.1016/j.ridd.2014.07.038
De Carvalho T. D., De Abreu L. C., Mustacchi Z., Vanderlei L. C. M., Godoy M. F., Raimundo R. D., et al. (2015). Cardiac autonomic modulation of children with Down syndrome. Pediatr. Cardiol. 36, 344–349. doi:10.1007/s00246-014-1012-5
De La Rosa A., Olaso-Gonzalez G., Arc-Chagnaud C., Millan F., Salvador-Pascual A., García-Lucerga C., et al. (2020). Physical exercise in the prevention and treatment of Alzheimer's disease. J. Sport Health Sci. 9, 394–404. doi:10.1016/j.jshs.2020.01.004
De Winter C. D., Bastiaanse L., Hilgenkamp T., Evenhuis H., Echteld M. (2012). Overweight and obesity in older people with intellectual disability. Res. Dev. Disabil. 33, 398–405. doi:10.1016/j.ridd.2011.09.022
De Winter C. F., Magilsen K. W., Van Alfen J. C., Penning C., Evenhuis H. M. (2009). Prevalence of cardiovascular risk factors in older people with intellectual disability. Am. J. Intellect. Dev. Disabil. 114, 427–436. doi:10.1352/1944-7558-114.6.427
Diaz A. J., Rosety I., Ordonez F. J., Brenes F., Garcia-Gomez N., Castejon-Riber C., et al. (2021). Effects of resistance training in muscle mass and markers of muscle damage in adults with down syndrome. Int. J. Environ. Res. Public Health 18, 8996. doi:10.3390/ijerph18178996
Dilek G., Öztürk C., Hepguler A., Ozkinay F., Dilek M. (2018). The effect of exercise on bone mineral density in patients with Down syndrome. jpr. 5, 118–123. doi:10.4274/jpr.66588
Dodd K. J., Shields N. (2005). A systematic review of the outcomes of cardiovascular exercise programs for people with Down syndrome. Arch. Phys. Med. Rehabil. 86, 2051–2058. doi:10.1016/j.apmr.2005.06.003
Duplanty A., Vingren J., Keller J. (2014). Exercise training recommendations: Working with individuals with intellectual disabilities. Strength & Cond. J. 36, 29–31. doi:10.1519/ssc.0000000000000040
Emerson N. S., Stout J. R., Fukuda D. H., Robinson E. H., Scanlon T. C., Beyer K. S., et al. (2015). Resistance training improves capacity to delay neuromuscular fatigue in older adults. Arch. Gerontol. Geriatr. 61, 27–32. doi:10.1016/j.archger.2015.04.002
Esposito P. E., Macdonald M., Hornyak J. E., Ulrich D. A. (2012). Physical activity patterns of youth with Down syndrome. Intellect. Dev. Disabil. 50, 109–119. doi:10.1352/1934-9556-50.2.109
Fernhall B., Baynard T., Collier S. R., Figueroa A., Goulopoulou S., Kamimori G. H., et al. (2009). Catecholamine response to maximal exercise in persons with Down syndrome. Am. J. Cardiol. 103, 724–726. doi:10.1016/j.amjcard.2008.10.036
Fernhall B., Figueroa A., Collier S., Goulopoulou S., Giannopoulou I., Baynard T. (2005). Resting metabolic rate is not reduced in obese adults with Down syndrome. Ment. Retard. 43, 391–400. doi:10.1352/0047-6765(2005)43[391:RMRINR]2.0.CO;2
Fernhall B., Mendonca G. V., Baynard T. (2013). Reduced work capacity in individuals with down syndrome: A consequence of autonomic dysfunction? Exerc. Sport Sci. Rev. 41, 138–147. doi:10.1097/JES.0b013e318292f408
Fernhall B., Otterstetter M. (2003). Attenuated responses to sympathoexcitation in individuals with Down syndrome. J. Appl. Physiol. 94, 2158–2165. doi:10.1152/japplphysiol.00959.2002
Flore P., Bricout V.-A., Biesen D. V., Guinot M., Laporte F., Pépin J.-L., et al. (2008). Oxidative stress and metabolism at rest and during exercise in persons with Down syndrome. Eur. J. Cardiovasc. Prev. Rehabil. 15, 35–42. doi:10.1097/HJR.0b013e3282f2bff3
Florentino Neto J., Pontes L. M. D., Fernandes Filho J. (2010). Alterações na composição corporal decorrentes de um treinamento de musculação em portadores de síndrome de Down. Rev. Bras. Med. Esporte 16, 09–12. doi:10.1590/s1517-86922010000100001
Foley C., Killeen O. G. (2019). Musculoskeletal anomalies in children with down syndrome: An observational study. Arch. Dis. Child. 104, 482–487. doi:10.1136/archdischild-2018-315751
Fornieles G., Rosety M., Elosegui S., Rosety J., Alvero-Cruz J., Garcia N., et al. (2014). Salivary testosterone and immunoglobulin A were increased by resistance training in adults with Down syndrome. Braz. J. Med. Biol. Res. 47, 345–348. doi:10.1590/1414-431x20143468
Forseth B., Carlson J. A., Willis E. A., Helsel B. C., Ptomey L. T. (2022). A comparison of accelerometer cut-points for measuring physical activity and sedentary time in adolescents with Down syndrome. Res. Dev. Disabil. 120, 104126. doi:10.1016/j.ridd.2021.104126
Fragala M. S., Cadore E. L., Dorgo S., Izquierdo M., Kraemer W. J., Peterson M. D., et al. (2019). Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 33, 2019–2052. doi:10.1519/JSC.0000000000003230
Fyfe J. J., Hamilton D. L., Daly R. M. (2022). Minimal-dose resistance training for improving muscle mass, strength, and function: A narrative review of current evidence and practical considerations. Sports Med. 52, 463–479. doi:10.1007/s40279-021-01605-8
Garcia-Hermoso A., Cavero-Redondo I., Ramirez-Velez R., Ruiz J. R., Ortega F. B., Lee D. C., et al. (2018). Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 99, 2100–2113. e2105. doi:10.1016/j.apmr.2018.01.008
Ghaeeni S., Bahari Z., Khazaei A. A. (2015). Effect of core stability training on static balance of the children with Down syndrome. Phys. Treatments-Specific Phys. Ther. 5, 49–54.
Ginis K. a. M., Van Der Ploeg H. P., Foster C., Lai B., Mcbride C. B., Ng K., et al. (2021). Participation of people living with disabilities in physical activity: A global perspective. Lancet 398, 443–455. doi:10.1016/S0140-6736(21)01164-8
González-Agüero A., Ara I., Moreno L. A., Vicente-Rodríguez G., Casajús J. A. (2011a). Fat and lean masses in youths with down syndrome: Gender differences. Res. Dev. Disabil. 32, 1685–1693. doi:10.1016/j.ridd.2011.02.023
González-Agüero A., Vicente-Rodríguez G., Gómez-Cabelllo A., Ara I., Moreno L. A., Casajus J. A. (2012). A 21‐week bone deposition promoting exercise programme increases bone mass in young people with Down syndrome. Dev. Med. Child. Neurol. 54, 552–556. doi:10.1111/j.1469-8749.2012.04262.x
González-Agüero A., Vicente-Rodríguez G., Gómez-Cabello A., Ara I., Moreno L. A., Casajús J. A. (2011b). A combined training intervention programme increases lean mass in youths with Down syndrome. Res. Dev. Disabil. 32, 2383–2388. doi:10.1016/j.ridd.2011.07.024
Guerra M., Llorens N., Fernhall B. (2003). Chronotropic incompetence in persons with down syndrome. Arch. Phys. Med. Rehabil. 84, 1604–1608. doi:10.1053/s0003-9993(03)00342-3
Gupta S., Rao B. K., Kumaran S. (2011). Effect of strength and balance training in children with down’s syndrome: A randomized controlled trial. Clin. Rehabil. 25, 425–432. doi:10.1177/0269215510382929
Guthold R., Stevens G. A., Riley L. M., Bull F. C. (2020). Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1· 6 million participants. Lancet. Child. Adolesc. Health 4, 23–35. doi:10.1016/S2352-4642(19)30323-2
Guzmán-Muñoz E., Gutiérrez-Navarro L., Miranda-Díaz S. (2017). Postural control in children, adolescents and adults with Down syndrome. Int. Med. Rev. Down Syndrome 21, 12–16. doi:10.1016/j.sdeng.2016.09.003
Hawli Y., Nasrallah M., Fuleihan G. E.-H. (2009). Endocrine and musculoskeletal abnormalities in patients with Down syndrome. Nat. Rev. Endocrinol. 5, 327–334. doi:10.1038/nrendo.2009.80
Henderson G., Ferreira D., Wu J. (2021). The effects of direction and speed on treadmill walking in typically developing children. Gait Posture 84, 169–174. doi:10.1016/j.gaitpost.2020.11.028
Herault Y., Delabar J. M., Fisher E. M. C., Tybulewicz V. L. J., Yu E., Brault V. (2017). Rodent models in down syndrome research: Impact and future opportunities. Dis. Model. Mech. 10, 1165–1186. doi:10.1242/dmm.029728
Hilgenkamp T. I., Wee S. O., Schroeder E. C., Baynard T., Fernhall B. (2018). Peripheral blood flow regulation in response to sympathetic stimulation in individuals with Down syndrome. Artery Res. 24, 16–21. doi:10.1016/j.artres.2018.10.001
Hu M., Yan H., Ranadive S. M., Agiovlasitis S., Fahs C. A., Atiq M., et al. (2013). Arterial stiffness response to exercise in persons with and without Down syndrome. Res. Dev. Disabil. 34, 3139–3147. doi:10.1016/j.ridd.2013.06.041
Iellamo F., Galante A., Legramante J. M., Lippi M. E., Condoluci C., Albertini G., et al. (2005). Altered autonomic cardiac regulation in individuals with Down syndrome. Am. J. Physiol. Heart Circ. Physiol. 289, H2387–H2391. doi:10.1152/ajpheart.00560.2005
Illouz T., Biragyn A., Iulita M. F., Flores-Aguilar L., Dierssen M., De Toma I., et al. (2021). Immune dysregulation and the increased risk of complications and mortality following respiratory tract infections in adults with down syndrome. Front. Immunol. 12, 621440. doi:10.3389/fimmu.2021.621440
Iversen V. M., Norum M., Schoenfeld B. J., Fimland M. S. (2021). No time to lift? Designing time-efficient training programs for strength and hypertrophy: A narrative review. Sports Med. 51, 2079–2095. doi:10.1007/s40279-021-01490-1
Izquierdo-Gomez R., Veiga Ó. L., Villagra A., Diaz-Cueto M. (2015). Correlates of sedentary behaviour in youths with down syndrome: The UP&DOWN study. J. Sports Sci. 33, 1504–1514. doi:10.1080/02640414.2014.994660
Jacinto M., Oliveira R., Brito J. P., Martins A. D., Matos R., Ferreira J. P. (2021). Prescription and effects of strength training in individuals with intellectual disability—a systematic review. Sports 9, 125. doi:10.3390/sports9090125
Janssen I., Heymsfield S. B., Ross R. (2002). Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 50, 889–896. doi:10.1046/j.1532-5415.2002.50216.x
Jochem C., Leitzmann M., Volaklis K., Aune D., Strasser B. (2019). Association between muscular strength and mortality in clinical populations: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 20, 1213–1223. doi:10.1016/j.jamda.2019.05.015
Jovanovic S. V., Clements D., Macleod K. (1998). Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic. Biol. Med. 25, 1044–1048. doi:10.1016/s0891-5849(98)00137-3
Jung H.-K., Chung E., Lee B.-H. (2017). A comparison of the balance and gait function between children with Down syndrome and typically developing children. J. Phys. Ther. Sci. 29, 123–127. doi:10.1589/jpts.29.123
Kelly A., Gidding S. S., Walega R., Cochrane C., Clauss S., Townsend R. R., et al. (2019). Relationships of body composition to cardiac structure and function in adolescents with down syndrome are different than in adolescents without down syndrome. Pediatr. Cardiol. 40, 421–430. doi:10.1007/s00246-018-2014-5
Kim A., Baek S., Park S., Shin J. (2020). Bone mineral density of femur and lumbar and the relation between fat mass and lean mass of adolescents: Based on korea national health and nutrition examination survey (KNHNES) from 2008 to 2011. Int. J. Environ. Res. Public Health 17, 4471. doi:10.3390/ijerph17124471
Kim H. I., Kim S. W., Kim J., Jeon H. R., Jung D. W. (2017). Motor and cognitive developmental profiles in children with Down syndrome. Ann. Rehabil. Med. 41, 97–103. doi:10.5535/arm.2017.41.1.97
Kovačič T., Kovačič M., Ovsenik R., Zurc J. (2020). The impact of multicomponent programmes on balance and fall reduction in adults with intellectual disabilities: A randomised trial. J. Intellect. Disabil. Res. 64, 381–394. doi:10.1111/jir.12727
Lauteslager P., Vermeer A., Helders P. (1998). Disturbances in the motor behaviour of children with Down's syndrome: The need for a theoretical framework. Physiotherapy 84, 5–13. doi:10.1016/s0031-9406(05)65896-8
Li N., Li P., Lu Y., Wang Z., Li J., Liu X., et al. (2020). Effects of resistance training on exercise capacity in elderly patients with chronic obstructive pulmonary disease: A meta-analysis and systematic review. Aging Clin. Exp. Res. 32, 1911–1922. doi:10.1007/s40520-019-01339-8
Lin H.-C., Wuang Y.-P. (2012). Strength and agility training in adolescents with down syndrome: A randomized controlled trial. Res. Dev. Disabil. 33, 2236–2244. doi:10.1016/j.ridd.2012.06.017
Magge S. N., Zemel B. S., Pipan M. E., Gidding S. S., Kelly A. (2019). Cardiometabolic risk and body composition in youth with down syndrome. Pediatrics 144, e20190137. doi:10.1542/peds.2019-0137
Mahy J., Shields N., Taylor N., Dodd K. (2010). Identifying facilitators and barriers to physical activity for adults with Down syndrome. J. Intellect. Disabil. Res. 54, 795–805. doi:10.1111/j.1365-2788.2010.01308.x
Maïano C., Hue O., Lepage G., Morin A. J., Tracey D., Moullec G. (2019). Do exercise interventions improve balance for children and adolescents with down syndrome? A systematic review. Phys. Ther. 99, 507–518. doi:10.1093/ptj/pzz012
Mccarron M., Mccallion P., Reilly E., Dunne P., Carroll R., Mulryan N. (2017). A prospective 20‐year longitudinal follow‐up of dementia in persons with Down syndrome. J. Intellect. Disabil. Res. 61, 843–852. doi:10.1111/jir.12390
Mendonca G., Pereira F., Morato P., Fernhall B. (2010a). Walking economy of adults with Down syndrome. Int. J. Sports Med. 31, 10–15. doi:10.1055/s-0029-1241211
Mendonca G. V., Pereira F. D., Fernhall B. (2010b). Reduced exercise capacity in persons with down syndrome: Cause, effect, and management. Ther. Clin. Risk Manag. 6, 601–610. doi:10.2147/TCRM.S10235
Muchová J., Zitnanova I., Durackova Z. (2014). Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol. Res. 63, 535–542. doi:10.33549/physiolres.932722
Naidoo R., Smith B., Foster C., Chetty V. (2021). Physical activity for adults with disabilities: Designing a South African infographic to communicate guidelines. BMJ Publ. Group Ltd Br. Assoc. Sport Exerc. Med. 56 (10), 537–538. doi:10.1136/bjsports-2021-104855
Ordonez F. J., Fornieles-Gonzalez G., Camacho A., Rosety M. A., Rosety I., Diaz A. J., et al. (2013). Anti-inflammatory effect of exercise, via reduced leptin levels, in obese women with Down syndrome. Int. J. Sport Nutr. Exerc. Metab. 23, 239–244. doi:10.1123/ijsnem.23.3.239
Oreskovic N. M., Cottrell C., Torres A., Patsiogiannis V., Santoro S., Nichols D., et al. (2020). Physical activity patterns in adults with Down Syndrome. J. Appl. Res. Intellect. Disabil. 33, 1457–1464. doi:10.1111/jar.12773
Paillard T. (2017). Plasticity of the postural function to sport and/or motor experience. Neurosci. Biobehav. Rev. 72, 129–152. doi:10.1016/j.neubiorev.2016.11.015
Pape S. E., Baksh R. A., Startin C., Hamburg S., Hithersay R., Strydom A. (2021). The association between physical activity and CAMDEX-DS changes prior to the onset of alzheimer’s disease in down syndrome. J. Clin. Med. 10, 1882. doi:10.3390/jcm10091882
Pawlikowski B., Betta N. D., Elston T., Williams D. A., Olwin B. B. (2018). Muscle stem cell dysfunction impairs muscle regeneration in a mouse model of Down syndrome. Sci. Rep. 8, 4309–4311. doi:10.1038/s41598-018-22342-5
Pecze L., Randi E. B., Szabo C. (2020). Meta-analysis of metabolites involved in bioenergetic pathways reveals a pseudohypoxic state in Down syndrome. Mol. Med. 26, 102–126. doi:10.1186/s10020-020-00225-8
Phillips A. C., Sleigh A., Mcallister C. J., Brage S., Carpenter T. A., Kemp G. J., et al. (2013). Defective mitochondrial function in vivo in skeletal muscle in adults with down’s syndrome: A 31P-mrs study. PloS one 8, e84031. doi:10.1371/journal.pone.0084031
Pitetti K., Baynard T., Agiovlasitis S. (2013). Children and adolescents with Down syndrome, physical fitness and physical activity. J. Sport Health Sci. 2, 47–57. doi:10.1016/j.jshs.2012.10.004
Pitetti K. H., Climstein M., Mays M. J., Barrett P. J. (1992). Isokinetic arm and leg strength of adults with down syndrome: A comparative study. Arch. Phys. Med. Rehabil. 73, 847–850.
Presson A. P., Partyka G., Jensen K. M., Devine O. J., Rasmussen S. A., Mccabe L. L., et al. (2013). Current estimate of Down syndrome population prevalence in the United States. J. Pediatr. 163, 1163–1168. doi:10.1016/j.jpeds.2013.06.013
Pucci F., Machado G., Solera E., Cenovicz F., Arruda C., Braga C., et al. (2016). Blood pressure levels and body mass index in Brazilian adults with Down syndrome. Sao Paulo Med. J. 134, 330–334. doi:10.1590/1516-3180.2016.0057180316
Reza S. M., Rasool H., Mansour S., Abdollah H. (2013). Effects of calcium and training on the development of bone density in children with Down syndrome. Res. Dev. Disabil. 34, 4304–4309. doi:10.1016/j.ridd.2013.08.037
Riebe D., Ehrman J. K., Liguori G., Magal M., Medicine A. C. O. S. (2018). ACSM's guidelines for exercise testing and prescription. Baltimore: Wolters Kluwer.
Rigoldi C., Galli M., Albertini G. (2011). Gait development during lifespan in subjects with Down syndrome. Res. Dev. Disabil. 32, 158–163. doi:10.1016/j.ridd.2010.09.009
Rimmer J. H., Heller T., Wang E., Valerio I. (2004). Improvements in physical fitness in adults with Down syndrome. Am. J. Ment. Retard. 109, 165–174. doi:10.1352/0895-8017(2004)109<165:IIPFIA>2.0.CO;2
Roizen N. J., Patterson D. (2003). Down's syndrome. Lancet 361, 1281–1289. doi:10.1016/S0140-6736(03)12987-X
Rosety-Rodriguez M., Bernardi M., Elosegui S., Rosety I., Diaz A., Rosety M., et al. (2021). A short-term resistance training circuit improved antioxidants in sedentary adults with Down Syndrome. Oxidative Med. Cell. Longev. 2021, 8811153. doi:10.1155/2021/8811153
Rosety-Rodriguez M., Camacho A., Rosety I., Fornieles G., Rosety M. A., Diaz A. J., et al. (2013). Resistance circuit training reduced inflammatory cytokines in a cohort of male adults with Down syndrome. Med. Sci. Monit. 19, 949–953. doi:10.12659/MSM.889362
Ruiz J. R., Sui X., Lobelo F., Lee D. C., Morrow J. R., Jackson A. W., et al. (2009). Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol. Biomarkers Prev. 18, 1468–1476. doi:10.1158/1055-9965.EPI-08-1075
Ruiz J. R., Sui X., Lobelo F., Morrow J. R., Jackson A. W., Sjostrom M., et al. (2008). Association between muscular strength and mortality in men: Prospective cohort study. BMJ 337, a439. doi:10.1136/bmj.a439
Schwanbeck S. R., Cornish S. M., Barss T., Chilibeck P. D. (2020). Effects of training with free weights versus machines on muscle mass, strength, free testosterone, and free cortisol levels. J. Strength Cond. Res. 34, 1851–1859. doi:10.1519/JSC.0000000000003349
Seron B. B., Goessler K. F., Modesto E. L., Almeida E. W., Greguol M. (2015). Blood pressure and hemodynamic adaptations after a training program in young individuals with Down syndrome. Arq. Bras. Cardiol. 104, 487–491. doi:10.5935/abc.20150033
Seron B. B., Modesto E. L., Stanganelli L. C. R., Carvalho E. M. O. D., Greguol M. (2017). Effects of aerobic and resistance training on the cardiorespiratory fitness of young people with Down Syndrome. Rev. Bras. Cineantropom. Desempenho. Hum. 19, 385–394. doi:10.5007/1980-0037.2017v19n4p385
Seron B. B., Silva R. a. C., Greguol M. (2014). Effects of two programs of exercise on body composition of adolescents with Down syndrome. Rev. Paul. Pediatr. 32, 92–98. doi:10.1590/s0103-05822014000100015
Shields N., Dodd K. J., Abblitt C. (2009). Do children with down syndrome perform sufficient physical activity to maintain good health? A pilot study. Adapt. Phys. Act. Q. 26, 307–320. doi:10.1123/apaq.26.4.307
Shields N., Hussey J., Murphy J., Gormley J., Hoey H. (2017). An exploratory study of the association between physical activity, cardiovascular fitness and body size in children with Down syndrome. Dev. Neurorehabil. 20, 92–98. doi:10.3109/17518423.2015.1077901
Shields N., Taylor N. F. (2010). A student-led progressive resistance training program increases lower limb muscle strength in adolescents with down syndrome: A randomised controlled trial. J. Physiother. 56, 187–193. doi:10.1016/s1836-9553(10)70024-2
Shields N., Taylor N. F., Dodd K. J. (2008). Effects of a community-based progressive resistance training program on muscle performance and physical function in adults with down syndrome: A randomized controlled trial. Arch. Phys. Med. Rehabil. 89, 1215–1220. doi:10.1016/j.apmr.2007.11.056
Shields N., Taylor N. F., Fernhall B. (2010). A study protocol of a randomised controlled trial to investigate if a community based strength training programme improves work task performance in young adults with Down syndrome. BMC Pediatr. 10, 17–7. doi:10.1186/1471-2431-10-17
Shields N., Taylor N. F., Wee E., Wollersheim D., O'shea S. D., Fernhall B. (2013). A community-based strength training programme increases muscle strength and physical activity in young people with down syndrome: A randomised controlled trial. Res. Dev. Disabil. 34, 4385–4394. doi:10.1016/j.ridd.2013.09.022
Shin Y.-A., Jeong H.-B., Lee J. S. (2021). The effect of resistance and balance training on postural control and physical fitness in adults with down syndrome. Exerc. Sci. 30, 175–182. doi:10.15857/ksep.2021.30.2.175
Shiohama T., Levman J., Baumer N., Takahashi E. (2019). Structural magnetic resonance imaging-based brain morphology study in infants and toddlers with down syndrome: The effect of comorbidities. Pediatr. Neurol. 100, 67–73. doi:10.1016/j.pediatrneurol.2019.03.015
Stancliffe R. J., Anderson L. L. (2017). Factors associated with meeting physical activity guidelines by adults with intellectual and developmental disabilities. Res. Dev. Disabil. 62, 1–14. doi:10.1016/j.ridd.2017.01.009
Strasser B., Arvandi M., Siebert U. (2012). Resistance training, visceral obesity and inflammatory response: A review of the evidence. Obes. Rev. 13, 578–591. doi:10.1111/j.1467-789X.2012.00988.x
Suarez-Villadat B., Veiga O. L., Villagra A., Izquierdo-Gomez R., Marcos A., Upand Group D.S. (2019). Changes in body composition and physical fitness in adolescents with Down syndrome: The UP&DOWN Longitudinal study. Child. Obes. 15, 397–405. doi:10.1089/chi.2018.0198
Suarez-Villadat B., Villagra A., Veiga O. L., Cabanas-Sanchez V., Izquierdo-Gomez R., Marcos A., Up , et al. (2021). Prospective associations of physical activity and health-related physical fitness in adolescents with down syndrome: The UP&DOWN longitudinal study. Int. J. Environ. Res. Public Health 18, 5521. doi:10.3390/ijerph18115521
Sugimoto D., Bowen S. L., Meehan W. P., Stracciolini A. (2016). Effects of neuromuscular training on children and young adults with down syndrome: Systematic review and meta-analysis. Res. Dev. Disabil. 55, 197–206. doi:10.1016/j.ridd.2016.04.003
Tenenbaum A., Chavkin M., Wexler I. D., Korem M., Merrick J. (2012). Morbidity and hospitalizations of adults with Down syndrome. Res. Dev. Disabil. 33, 435–441. doi:10.1016/j.ridd.2011.09.026
Tsai M.-L., Li T.-L., Chou L.-W., Chang C.-K., Huang S.-Y., Fang S.-H. (2012). Resting salivary levels of IgA and cortisol are significantly affected during intensive resistance training periods in elite male weightlifters. J. Strength Cond. Res. 26, 2202–2208. doi:10.1519/JSC.0b013e31823a4246
Tsimaras V. K., Fotiadou E. G. (2004). Effect of training on the muscle strength and dynamic balance ability of adults with down syndrome. J. Strength Cond. Res. 18, 343–347. doi:10.1519/R-12832.1
Tsou A. Y., Bulova P., Capone G., Chicoine B., Gelaro B., Harville T. O., et al. (2020). Medical care of adults with down syndrome: A clinical guideline. JAMA 324, 1543–1556. doi:10.1001/jama.2020.17024
Valenti D., Braidy N., De Rasmo D., Signorile A., Rossi L., Atanasov A., et al. (2018). Mitochondria as pharmacological targets in Down syndrome. Free Radic. Biol. Med. 114, 69–83. doi:10.1016/j.freeradbiomed.2017.08.014
Vicente-Rodríguez G. (2006). How does exercise affect bone development during growth? Sports Med. 36, 561–569. doi:10.2165/00007256-200636070-00002
Volaklis K. A., Halle M., Meisinger C. (2015). Muscular strength as a strong predictor of mortality: A narrative review. Eur. J. Intern. Med. 26, 303–310. doi:10.1016/j.ejim.2015.04.013
Weber R., French R. (1988). Down’s syndrome adolescents and strength training. Clin. Kinesiol 42, 13–21.
Wee S. O., Pitetti K. H., Goulopoulou S., Collier S. R., Guerra M., Baynard T. (2015). Impact of obesity and Down syndrome on peak heart rate and aerobic capacity in youth and adults. Res. Dev. Disabil. 36, 198–206. doi:10.1016/j.ridd.2014.10.002
West S., King V., Carey T. S., Lohr K. N., Mckoy N., Sutton S. F., et al. (2002). Systems to rate the strength of scientific evidence. Evid. report/technology Assess. Summ. 1, 1–11.
Yu F., Greimel S., Kelly K., Wyman J. F. (2017). Strategies to engage older adults with behavioral and psychological symptoms of dementia in exercise: A multiple case study. Appl. Nurs. Res. 36, 77–80. doi:10.1016/j.apnr.2017.05.002
Keywords: down syndrome, resistance training, intellectual disability, strength training, neuromuscular training, physical exercise program
Citation: Melo GLR, Neto IVdS, Fonseca EFd, Stone W and Nascimento DdC (2022) Resistance training and Down Syndrome: A narrative review on considerations for exercise prescription and safety. Front. Physiol. 13:948439. doi: 10.3389/fphys.2022.948439
Received: 19 May 2022; Accepted: 08 August 2022;
Published: 27 September 2022.
Edited by:
Ahmad Alkhatib, University of Taipei, TaiwanReviewed by:
Alejandro Sal de Rellán Guerra, Universidad Isabel I de Castilla, SpainErsan Arslan, Tokat Gaziosmanpasa University, Turkey
Copyright © 2022 Melo, Neto, Fonseca, Stone and Nascimento. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geiziane Leite Rodrigues Melo, Z2VpemlhbmVtZWxvOTNAZ21haWwuY29t
 Geiziane Leite Rodrigues Melo
Geiziane Leite Rodrigues Melo Ivo Vieira de Sousa Neto
Ivo Vieira de Sousa Neto Eduardo Fernandes da Fonseca1
Eduardo Fernandes da Fonseca1 Whitley Stone
Whitley Stone Dahan da Cunha Nascimento
Dahan da Cunha Nascimento