
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 26 September 2022
Sec. Physio-logging
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.933987
This article is part of the Research TopicWearable Sensing of Movement Quality after Neurological Disorders.View all 7 articles
 Johannes Pohl1,2*†
Johannes Pohl1,2*† Alain Ryser3†
Alain Ryser3† Janne Marieke Veerbeek4
Janne Marieke Veerbeek4 Geert Verheyden2
Geert Verheyden2 Julia Elisabeth Vogt3
Julia Elisabeth Vogt3 Andreas Rüdiger Luft1,5‡
Andreas Rüdiger Luft1,5‡ Chris Awai Easthope6‡
Chris Awai Easthope6‡Background: Stroke leads to motor impairment which reduces physical activity, negatively affects social participation, and increases the risk of secondary cardiovascular events. Continuous monitoring of physical activity with motion sensors is promising to allow the prescription of tailored treatments in a timely manner. Accurate classification of gait activities and body posture is necessary to extract actionable information for outcome measures from unstructured motion data. We here develop and validate a solution for various sensor configurations specifically for a stroke population.
Methods: Video and movement sensor data (locations: wrists, ankles, and chest) were collected from fourteen stroke survivors with motor impairment who performed real-life activities in their home environment. Video data were labeled for five classes of gait and body postures and three classes of transitions that served as ground truth. We trained support vector machine (SVM), logistic regression (LR), and k-nearest neighbor (kNN) models to identify gait bouts only or gait and posture. Model performance was assessed by the nested leave-one-subject-out protocol and compared across five different sensor placement configurations.
Results: Our method achieved very good performance when predicting real-life gait versus non-gait (Gait classification) with an accuracy between 85% and 93% across sensor configurations, using SVM and LR modeling. On the much more challenging task of discriminating between the body postures lying, sitting, and standing as well as walking, and stair ascent/descent (Gait and postures classification), our method achieves accuracies between 80% and 86% with at least one ankle and wrist sensor attached unilaterally. The Gait and postures classification performance between SVM and LR was equivalent but superior to kNN.
Conclusion: This work presents a comparison of performance when classifying Gait and body postures in post-stroke individuals with different sensor configurations, which provide options for subsequent outcome evaluation. We achieved accurate classification of gait and postures performed in a real-life setting by individuals with a wide range of motor impairments due to stroke. This validated classifier will hopefully prove a useful resource to researchers and clinicians in the increasingly important field of digital health in the form of remote movement monitoring using motion sensors.
Motor impairment after stroke reduces the level of daily physical activities and mobility and negatively affects social participation (de Rooij et al., 2021). In comparison to healthy controls, stroke survivors show significantly lower energy expenditure, more time spent sedentary, and less stepping activity in the acute (Kramer et al., 2013; Moore et al., 2013), subacute and chronic phases of motor recovery after stroke (Rand et al., 2009; English et al., 2016; Ezeugwu and Manns, 2017). Continuous inactivity and sedentary behavior are associated with an increased risk of recurrent stroke events (Wendel-Vos, 2004). Consequently, monitoring physical activities and implementing secondary prevention strategies are one of the main targets of health care providers.
According to the World Health Organization, daily physical activity measures should be structured in four components: frequency, intensity, time, and type (FITT) (Cavill et al., 2006). The quantitative and qualitative analysis of physical activities requires a hypothesis-driven set of outcome metrics considered relevant for the studied population. Regarding monitoring motor recovery and movement behavior in individuals with stroke, a minimal set of relevant outcome metrics which addresses upper and lower limb performance was proposed by van Meulen et al. (2016). Sitting, standing, and walking were considered types of physical activities of which duration, intensity, and quality of movement should be analyzed (van Meulen et al., 2016). Movement sensors are increasingly used as an unobtrusive approach to continuously monitor real-life physical activity in various populations with and without neurologic diseases (Block et al., 2016; Del Din et al., 2016). Especially neurologic populations returning from in-patient rehabilitation are a prime target for monitoring daily physical activity (Prajapati et al., 2011). Gains in motor function that are achieved during in-patient therapy are frequently not successfully translated into home routines (Thilarajah et al., 2016, 2018). Many patients with persistent motor impairment consult outpatient rehabilitation interventions that support them in maintaining and successfully integrating new motor capabilities into their home routine (Ostwald et al., 2009). Early identification of a lack of transfer of in-patient motor gains to home routine would allow for active referral of at-risk patients to avoid long-term complications or functional decline.
Movement sensors deployed in home environments generate large-scale unstructured and unlabeled time series data (Ehatisham-ul-Haq and Azam, 2020). The extraction of metrics corresponding to the most relevant outcomes proposed by van Meulen is not trivial (van Meulen et al., 2016). Thus, a significant body of research has emerged dedicated to the automated classification of physical activity types (e.g., walking and standing) and to calculating intensity and movement quality metrics once the activity type is determined (e.g., step count, energy expenditure, and stride symmetry in a walking period). The accurate classification of physical activity is paramount to analysis, as it provides metrics of frequency and duration and identifies the data sequences that enable subsequent evaluation of activity-specific metrics. Although neurologic populations are a primary target group for remote monitoring in home environments, most activity classification algorithms have been developed and validated for healthy populations within laboratory conditions (Gyllensten and Bonomi, 2011; O’Brien et al., 2017). Healthy populations arguably demonstrate a higher signal-to-noise ratio, characterized by greater movement speeds and lower movement variability (Balasubramanian et al., 2009; Stergiou and Decker, 2011; Atrsaei et al., 2020; Atrsaei et al., 2021). Applying these algorithms to neurologic populations in home environments substantially reduces activity classification accuracy (Jayaraman et al., 2018). To our knowledge, only a few studies have attempted to develop and validate physical activity classification algorithms specifically for neurologic populations such as stroke (Leuenberger et al., 2014; Massé et al., 2015; Derungs et al., 2020). In these studies, movement tasks were predefined and performed in standard clinical environments. To confidently apply these classification algorithms in home environments, the ecological validity must be demonstrated in real-life settings.
Accuracy of activity classification depends heavily on the sensor technology (e.g., modalities, dimensionality, sampling rate), data processing (preprocessing and classification algorithm), the number and location of sensors, and the characteristics of targeted physical activity types (set of activities, patient-specific movement characteristics, environment) (Clark et al., 2017; Clark et al., 2018; Allahbakhshi et al., 2019; Rast and Labruyère, 2020; Wu et al., 2021; Boukhennoufa et al., 2022) Although all mentioned components are important, the optimal number and location of sensors are essential to accurately detect diverse types of physical activity. This is especially relevant as a low number of sensors contributes strongly to participant comfort and adherence in remote settings and reduces the technological complexity of the recording setup. In healthy individuals, a minimal sensor setup for basic activities such as sitting, standing, and walking requires two sensors, ideally placed on the waist and ankle (Allahbakhshi et al., 2019). However, additional sensors are desirable to increase the number of detectable activities and provide a basis for movement intensity and quality, such as real-world gait analysis (Renggli et al., 2020; Werner et al., 2021).
Accordingly, our study has the following objectives: firstly, we compare the performance of three frequently used machine learning algorithms to classify gait and posture in real-world environments for subjects with motor impairment after stroke. Secondly, we compare the performance across five typical sensor configurations.
Subjects enrolled in a prospective observational study at the University Hospital Zurich were invited to participate if individuals met the following inclusion criteria: mono- or hemiparesis after stroke, ability to walk independently (with or without walking aid) with a Functional Ambulation Categories (FAC) score ≥3/5, living at home, aged above 18 years. Subjects were informed regarding the goal and procedure of the study and provided written informed consent for their participation. Ethical clearance to conduct the study was provided by the cantonal ethics committee Zurich Switzerland (BASEC No. Req-2020-00947).
Five movement sensors developed for research purposes (https://zurichmove.com/) were attached with elastic straps, one on the dorsal side of the wrists, at the lateral malleolus of the ankles, and on the chest below the sternum (Figure 1A). These inertial measurement units (IMU) include a 3-axis accelerometer, a 3-axis gyroscope, a 3-axis digital compass, an altimeter (10 cm resolution), a storage capacity of 4 GB, and a rechargeable battery that enables recordings of up to 72 h. Sensor configuration was adjusted to a sampling frequency of 50 Hz, and synchronization between modules was achieved via a radio frequency syncing protocol.
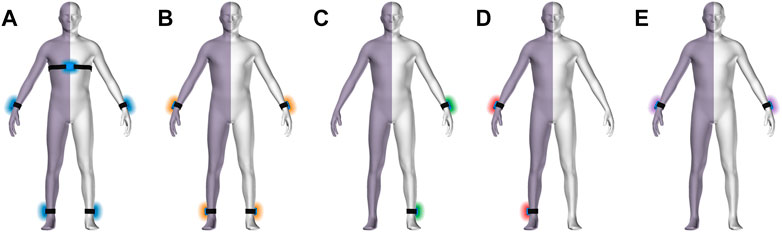
FIGURE 1. Body scheme with hemi-paretic side symbolized by violet shade. Sensor configurations (A–D) with sensors locations: all sensors (A), no chest sensor, bilateral wrist and ankle (B), unilateral wrist and ankle on the non-affected side (C), unilateral wrist and ankle on the affected side (D), and wrist sensors only (E).
A semi-structured protocol containing routine daily life activities was developed following the recommendations for standardized validation procedures for activity and posture classification (Lindemann et al., 2014). The subjects were visited at home, where they were asked to perform tasks that they would typically perform throughout a regular day, beginning with getting up (e.g., morning routine, grooming, dressing). The activity plot started with an open question, e.g., “What do you typically do first after getting up in the morning? Please proceed with this activity if I were not present.” Individuals subsequently performed their routine activities triggered by similar questions involving kitchen work, desk work, setting up a table, eating, preparing coffee, and cleaning up. These activities involved various uninstructed upper limb activities, body postures, walking, and stair ascent/descent. Participants were also asked to perform their typical leisure activities, which included reading, writing, watching TV, exercising, or playing a musical instrument. Thereby the observers remained reserved, leaving the order of actions or way how to perform a task to the individual’s preference. Exceptionally for stair walking activities, instructions were given to perform stair ascent and descent using both step-over-step and step-by-step patterns if the participant was capable to perform both patterns. Individuals with muscle weakness due to hemiplegia typically adopt the step-by-step pattern. For safety reasons, participants were supervised or assisted if they had difficulties performing a step-over-step pattern. Physical activities were recorded using a conventional video camera (GoPro Hero7, GoPro. Inc., San Mateo, United States) with 30 frames per second (fps). Synchronization between video and sensor data was obtained by video-recording an instructed knocking and turning sequence of the unaffected wrists, which provided salient signals in both systems.
Video data were recorded with a framerate of 30 fps, whereas time-series from the IMU were collected with 50 fps. A single experimenter labeled videos in a frame-by-frame manner, and the labels were subsequently resampled to match the frequency of the synchronized IMUs. For quality assessment, a random sample of 33.3% of data was labeled by a second experimenter. The labeling procedure and quality assessment were conducted using Labelbox online software (https://labelbox.com).
Labeling criteria were defined for start-to-end conditions of three body postures (lying down, sitting, standing) and two gait types (walking and stair ascent/descent). Additionally, we annotated three transition labels between two corresponding posture or gait types (lying down/sit, sit/stand, stand/walk) without specifying directionality (e.g., sit-to-stand or stand-to-sit). This labeling scheme resulted in a discrete label for every frame of the recording. Labeling criteria are presented in detail in the Supplementary Table S1.
To remove noisy and irrelevant frequencies from the collected IMU time-series, we incorporated the preprocessing steps suggested in the work of Moncada-Torres et al. (2014). The proposed collection of filter operations for IMU data suppress noise and extract frequency bands corresponding to subject posture and activity and can be found in full detail in the original work (Moncada-Torres et al., 2014). This process results in four types of time-series, namely the three triaxial signals posture acceleration, activity acceleration, and gyroscope, as well as the filtered barometric signal, containing data related to posture/orientation, movement, position, and altitude, respectively. Feature characteristics are presented in the Supplementary Table S2. These signals were computed for each sensor location and subsequently split into windows of 128 time samples with an overlap of 64 samples as described in previous work (Bonomi et al., 2009; Preece et al., 2009). For subsequent sliding-window-based analysis, the majority label was assigned for each time window.
To fit a model to the data, we extracted a series of features from each of the four time-series. For each window of the posture and activity acceleration, gyroscope, and barometric signals, we extracted the features suggested by Moncada-Torres et al. (2014). This amounted to 134 features per sensor, resulting in feature vectors with 670 dimensions considering all sensors. To handle intra-subject variability, we standardized each feature per subject to zero mean and unit variance over each window, which was previously shown to increase separability, repeatability, and clustering (Krausz et al., 2019). This entails the advantage that each feature’s magnitude is relative to its magnitudes in other windows of the same subject, effectively negating the effect of varying magnitude across subjects.
More specifically, for a subject with
The labeled dataset described in the previous sections was used to solve two classification tasks. First, we aggregated labels for walking and stair ascent/descent as “gait,” whereas lying, sitting, and standing were aggregated into the unified label “no gait.” These aggregated labels were used to solve a binary classification task, namely Gait classification. Our second classification task was appointed Gait and posture classification included all five gait activity and body posture labels to solve a multiclass classification task. Note that for both tasks, we removed all windows labeled with transition classes from the training set but not from the validation set to evaluate our method’s generalization to real-life data where transitions are present. Validation, including transition done as follows: Let A be a specific posture or activity and B the posture or activity following A. The time window containing the transition from class A to B is then correctly classified if a model predicts either A or B. Evaluation of our method was done in a nested leave-one-subject-out fashion. This protocol estimates how well model scores generalize to unseen data by defining two loops: the outer and inner loop. In every outer iteration, the outer loop splits the dataset into an outer training and test set, where the test set consists of the data of one patient, and the training set contains the rest of the data. The inner loop then takes the outer training set and performs leave-one-subject-out cross-validation. In every inner iteration, we defined an inner test set over the data of one patient of the outer training set and assigned the rest to the inner training set. Each model is fitted on this training set multiple times with different hyperparameter configurations, subsequently computing scores on the inner test set. After we computed scores for each individual, we aggregated scores across all inner splits and retrained the configuration with the best scores on the whole outer training set. Finally, we recomputed all scores on the outer test set with the retrained models. We do this for every patient and report the results by aggregating scores across all outer splits. We further compared cross-validation performance between experiments when including and excluding transitions from validation sets for both classification tasks. Model performance was evaluated by computing sensitivity, specificity, positive predictive value (PPV), and accuracy in a one vs. rest fashion (Ballabio et al., 2018). As these metrics are sensitive to class imbalance, we also evaluated balanced accuracy, defined as the arithmetic mean between sensitivity and specificity (Ballabio et al., 2018). A potential relationship between classification performance and functional motor impairment (Berg Balance Score; 10-m walking speed) was analyzed for the full sensor setup, the unilateral setup (non-affected side), and the wrists-only setup (see Figures 1A, C, E). Normality was determined by the Shapiro-Wilks test, and Pearson or Spearman correlation was applied accordingly.
To solve the two classification tasks, we explored three different classifiers, namely support vector machine (SVM), logistic regression (LR), and k-nearest neighbor (kNN), for comparison. Each of these methods required us to set a combination of several hyperparameters, which is non-trivial, as different sensor setups (Figures 1A–E) and classification tasks require different settings. Consequently, we performed a cross-validated grid search (Sunkad and Soujanya, 2016; Rabbi et al., 2021). To this end, we defined a list of possible hyperparameters for each model (Table 1) and sensor configuration within a classification task. To evaluate the generalizability of the cross-validated grid-search, we computed the nested leave-one-subject-out cross-validation scores, where we performed the inner loop using each hyperparameter combination. To decide which model to retrain in the inner loop, we used the configuration achieving the best balanced accuracy as it accounts for class imbalance, thus ensuring that models that consistently predict the same class are not selected. Optimal hyperparameters regarding classification tasks and sensor configuration are presented in the Supplementary Table S3.
To explore misclassification rates across classes for the optimal classification setup, we mapped true classes versus predicted classes by confusion matrices. All experiments were conducted using Python (v3.7, Python Software Foundation, https://www.python.org/), and model implementations were imported from the Scikit-Learn library (https://scikit-learn.org/).
Real-life data of fourteen individuals with mild-to-moderate mobility impairments were visited and recorded in their home environment. Participants’ characteristics and scores of clinical gait and balance assessments can be seen in Table 2. The agreement rate between labelers was 93.5%. The total volume of labeled video data amounted to 379 min containing an imbalance of classes ranging from 4.8% (lying) to 32.8% (standing) of averaged cumulative duration (Table 3). The average bout duration of activity from start to end was shortest for transitions (range 2.7–4.7 s) and longest for lying.
Gait classification performed consistently well across the classification models (Figure 2), achieving accuracy above 90% in most configurations (Figure 3). Across sensor setups (Figures 1A–E), SVM achieved slightly superior performance, improving in balanced accuracy over kNN by 0.9%–3.5% and over LR by 0.2%–1.0% (Table 4). Misclassifications were low using all sensors (6%) and the unilateral configuration (7%) but were increased to 15% using only wrist sensors (Figure 4).
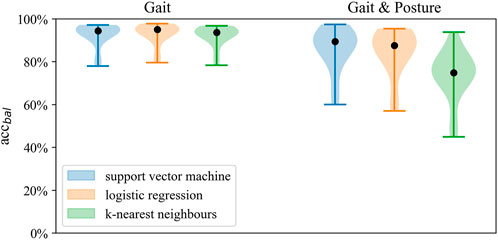
FIGURE 2. Distribution of balanced accuracy across models for Gait classification and Gait and posture classification on the full sensor setup.
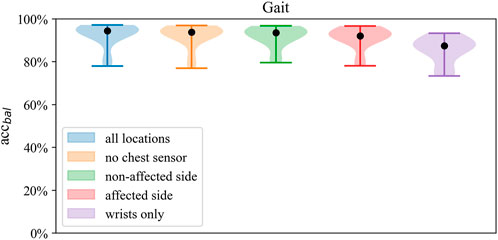
FIGURE 3. Distributions of balanced accuracy of the SVM in Gait classification across sensor setups.
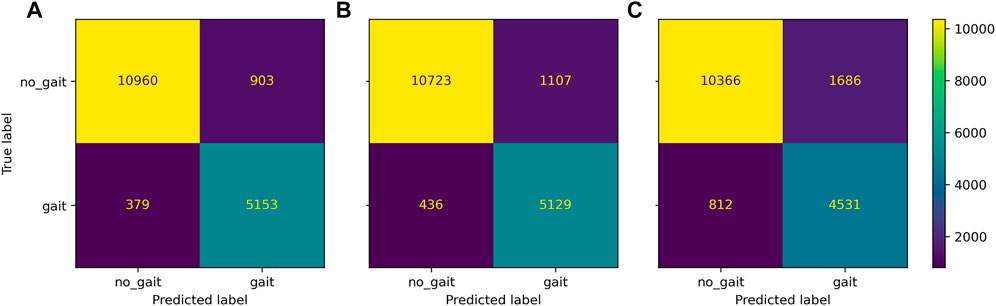
FIGURE 4. Confusion matrix of Gait classification task for the three district sensor setups: bilateral setup all sensors, (A), unilateral setup non-affected ankle and wrist sensor, (B), wrists-only setup (C).
Across classifiers, performance measures marginally decreased when reducing the number of sensors, ranging from best balanced accuracy of 92.6% (all sensors, SVM) to 63.9% balanced accuracy (wrists-only, kNN). Compared to the wrists-only setup, balanced accuracy improved by 5%–8% with a unilateral setup of one wrist and ankle sensor on the affected side. Minor differences of 0.3%–0.8% within models were found between the affected and non-affected unilateral setup and between the full setup and the bilateral setup of wrists and ankles (Table 4). Misclassification frequencies for a bilateral unilateral and wrists-only setup are presented in Figure 4. The correlation between gait classification performance and functional impairment was non-significant (p > 0.05) across sensor configurations. Gait classification performance across classifiers on individual participant level is presented for the all sensors setup (Supplementary Figure S1; Supplementary Table S7), unilateral non-affected (Supplementary Figure S2; Supplementary Table S8), and the wrists-only setup (Supplementary Figure S3; Supplementary Table S9) in the supplement.
Model performance of the gait classification task was robust across validation tasks, including and excluding transitions (Figure 5). Gait classification performance validated with and without transitions can be seen in the online Supplementary Table S4.
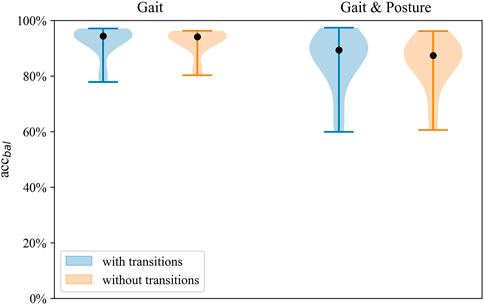
FIGURE 5. Distribution of balanced accuracy of the SVM with and without transitions on the full sensor setup for both classification tasks.
Best performance on Gait and Posture Classification was obtained by SVM using the full sensor setup resulting in balanced accuracy (SD) of 84.7% (9.2) followed by logistic regression with 82.9% (7.6). Performance varied considerably between models where SVM and LR showed higher balanced accuracy by 10%–14% compared to kNN across sensor configurations. Balanced accuracy scores by the SVM classifier are presented in Table 5 and Figure 6, whereas a comparison of performance between classifiers is presented in Figure 3. Detailed Gait and posture performance measures of all classifiers are presented in the online Supplementary Table S5.
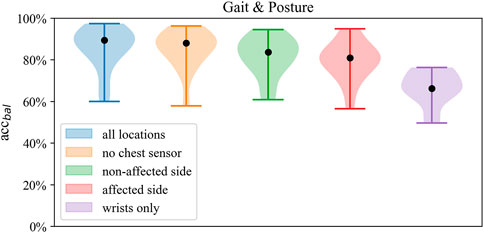
FIGURE 6. Distribution of balanced accuracy of the SVM in Gait and posture classification across sensor configurations.
Across sensor configurations, around 80% of balanced accuracy was obtained when at least one wrist and one ankle sensor were included, which was reduced by 12%–15% when using only wrist sensor data. Compared to the affected side, the unilateral configuration of the non-affected side improved balanced accuracy by 0.1%–1.8%, whereas compared to both unilateral setups, the bilateral setup (no chest) improved balanced accuracy by 2%–5%. Within both bilateral sensor configurations, there was no improvement in performance by the chest sensor.
Regarding the prediction of Gait and posture classes, lying was detected with the highest sensitivity (93%) and specificity (99.0%), whereas stair walking showed the lowest sensitivity (74.8%) using the full sensor configuration. When classifying lying, sitting, and standing, positive predictive values ranged from 80.8% to 78.8%. Classification of walking exhibited a positive predictive value of 81.1%, whereas, for stair walking, PPV was 68.7%. Robust performance was found in nested leave-one-subject-out cross-validation of the Gait and posture classification task irrespective of the inclusion or exclusion of transitions in the validation data set (Figure 5). Detailed performance across classifiers, excluding transitions, is shown in the Supplementary Table S6.
Misclassification frequencies are displayed in Figure 7 for a bilateral (A, all sensors), unilateral (B, non-affected), and wrist-only setup (C). Regarding the full sensor configuration, misclassifications were highest by predicting standing instead of sitting (11.0%), sitting instead of standing (9.4%), and walking instead of stair walking (13.8%) but were low, when predicting walking instead of standing (6.2%) and vice versa (6.2%). Using the unilateral setup, misclassification increased by 3.0%–3.8% between standing and walking and by 1.1%–2.4% between walking and stair walking. By using the wrists-only setup, misclassifications 11.2%–17.6% across the classed lying, sitting, standing, and walking. Spearman correlations between classification performance and impairment scores were not statistically significant for the bilateral and unilateral setup (p > 0.05). Using the wrists-only sensors, a significant negative correlation was found between walking speed and overall Gait and posture classification performance (ρ = −0.54 to −0.76; p < 0.05) and classification of walking (ρ = −0.53 to −0.60; p < 0.05). Relationships between classification performance walking and walking speed are presented across sensor setups in the Supplementary Figure S1. Gait and posture classification performance across classifiers on individual participant level is presented for the all sensors setup (Supplementary Figure S4; Supplementary Table S10), unilateral non-affected (Supplementary Figure S5; Supplementary Table S11), and the wrists-only setup (Supplementary Figure S6; Supplementary Table S12) in the supplement.

FIGURE 7. Confusion matrix Gait and Posture classification task for the three district sensor setups: bilateral setup all sensors, (A), unilateral setup (non-affected ankle and wrist sensor, (B), wrists-only setup (C).
This study provides insight into real-life Gait and Postures Classification accuracy in individuals with varying motor impairment due to stroke. We compared the performance of state-of-the-art machine learning algorithms on Gait Classification and Gait and Posture Classification including transitions across five sensor configurations. All sensor configurations, including at least one ankle sensor, resulted in high accuracy for both classification tasks. The level of accuracy was consistent in the nested cross-validation independent of the exclusion of transitions, indicating that this algorithm is sufficiently robust to handle comprehensive real-life data. In the following section, we discuss the main performance characteristics of each classification task and address their practical implications for research and clinical rehabilitation settings.
Human movement behavior is complex and shows high variability across age groups and health conditions (Stergiou and Decker, 2011; König et al., 2016; Stergiou et al., 2016). Despite this, we applied a severe simplification by dividing basic activities into the two classes of walking and non-walking. This distinction is especially relevant for activity classification for two reasons: Firstly, it creates categories to quantify physical activity by whole-body movements during Gait as opposed to low activity/inactivity in static conditions during Non-Gait bouts. Secondly, it enables the extraction of walking periods for qualitative spatiotemporal gait analysis. The distinction between walking and non-walking is one of the main contributors to estimating energy expenditure, widely considered a benchmark outcome for monitoring physical activities (Kristoffersson and Lindén, 2020; Kristoffersson and Lindén, 2022; Boukhennoufa et al., 2022).
In our sample, a very high proportion, above 90% of recordings, was correctly classified as walking and non-walking using at least one ankle and wrist sensor. Similar results were shown by Leuenberger et al. (2014), who achieved equivalent sensitivity and specificity in detecting walking activities with all sensors compared to ankle and chest sensors (Moncada-Torres et al., 2014). Sensor location showed a clear effect on performance measures. In particular, omitting ankle sensors reduced accuracy, which plicates previous studies’ conclusions (Giggins et al., 2017). Real-life gait classification accuracy relies on arm swing patterns which might have led to a significant reduction in accuracy and precision in our sample when using only wrist sensors. However, a still acceptable level with a PPV of 73% was maintained compared to a PPV of 80% recently achieved in free-living conditions of elderly individuals using wrist sensors (Soltani et al., 2020). The overall lower accuracy and precision for the wrist-only setup could be due to very slow walking speeds (median 0.8 m/s; range 0.3–1.4), using a walking aid, or having severe arm paresis in three participants, affecting arm swing patterns. It is important to note that our classification task for Gait activities included overground walking, stair ascent, and descent where arm swing was absent due to asymmetric stepping pattern, holding on to rail and walker, or even a very low cadence. These pathognomonic movement patterns with hemiparesis have likely reduced the classification performance of all sensor configurations—especially the wrist-only configuration. In line with this hypothesis, Fulk et al. (2014) found step detection accuracy by wearing one wrist sensor to be reduced when post-stroke individuals had higher levels of motor impairment measured by the Berg Balance Scale (>49/56 points) and gait speed (<0.7 m/s) measured by the 2 Minute Walk Test. Using only a single sensor for gait classification has been reported as a viable option in healthy populations (Lee and Kwan, 2018); performance significantly degrades when applied in neurological populations (Capela et al., 2016) such as stroke.
The classification of Gait and Non-Gait is only considered a first step in enabling a more detailed analysis of motion, which often requires additional sensors. Detailed analysis of walking motion in individuals with stroke and spinal cord injury using a single sensor resulted in considerable measurement errors of walking distance and step count when using wrist or arm-mounted accelerometers (Jayaraman et al., 2018; Compagnat et al., 2019). Measurement errors were smaller when locating the sensor at the hips, at the ankles, or when applying multiple sensors (Werner et al., 2021).
Our sample is small but comprises a wide range of motor and mobility impairments ranging from dependency on supervision for overground walking and dependency on walking aids to normal walking ability. With most PPVs above 80%, the proportion of false-positive detected walking remains small, which is essential when investigating gait bout frequency throughout the day or creating summary intensity and quality metrics from gait sequences.
The full sensor configuration achieved the best classification performance across all Gait and Posture classes was achieved using the full sensor configuration. The classes lying, sitting, standing, and walking were classified with a good overall accuracy of 85%. The performance was relatively robust and remained independent from functional impairment when using at least one ankle and wrist sensor. When reducing the sensor configuration to wrists-only, the overall accuracy declined to about 70% and showed an interrelation with individual impairment levels (see Supplementary Figure S1). Consequently, using our algorithm for the wrist-only setup, performance might be more susceptive to functional impairment.
Accurate classification of body postures and physical activity types allows valuable insight into profiles of individual behavior and lays a crucial foundation for type quantification and subsequent qualitative analysis of human movement behavior. Walking and stair walking are two critical activities that define independent mobility and are associated with higher levels of physical activity and energy expenditure than static postures (Pinheiro MB et al., 2013; Creasy et al., 2016). Using the bilateral and unilateral sensor setups, walking was detected in about 87%, whereas the sensitivity for stair walking was lower. Accurately predicting walking activities by only wrist sensors remains highly challenging since hemiparetic arm swing patterns, even in standardized conditions, show great variability (Kahn et al., 2019) on one hand, and voluntary arm activity (such as grasping, transporting, and gesticulating) are present during walking in real life conditions. This mixture of walking-related and task-related upper limb movements might differ by individual ability and hence the level of functional impairment after stroke. We found an inverse relationship using only wrist sensor data, indicating higher classification performance in participants with lower self-selected walking speeds. One possible explanation for this phenomenon could be that participants with higher functional impairment were rarely able to perform upper limb tasks during gait but performed a distinguishable arm swing pattern to maintain dynamic balance. Arm swing during hemiplegic gait is highly relevant for stride synchronization (Kloter et al., 2011; Ustinova et al., 2017) and associated with increased angular motion in the mediolateral and front dorsal plane (Kahn et al., 2019), which could have generated discriminative sensor signals by wrist sensors. However, it is advisable to increase classification performance by adding at least one ankle sensor to obtain a walking-characteristic reference.
The stair walking class was generally underrepresented, and its slightly lower sensitivity might be due to the variability in stepping patterns (e.g., step-by-step and step-over-step patterns) performed by all participants. Detecting stair ascent and stair descent in participants with stroke seems to be a persisting challenge, as previous studies also reported low sensitivities, around 70%, with a full sensor configuration (Leuenberger et al., 2014). Specified mobility impairment modifies movement behavior and is highly relevant to achieving generalizability but is often underreported in activity classification studies. In some participants of our sample, impairment-associated stair walking behavior was present: low stepping speeds ascending or descending, longer pauses between steps, and holding onto the rail bilaterally. However, across sensor setups, we did not find a relationship between classification performance of stair walking and functional impairment, which might be the abovementioned variability of stepping patterns within participants.
It is a significant advantage for the discrimination between body postures when distinct sensor orientation by a key sensor location is typical for that posture. In our study, the horizontal orientation of one axis of at least one ankle and the chest sensor might have led to a sensitivity above 90% for detecting the horizontal body position while lying down. The observed slight increase of sensitivity in the classification of lying, which occurs when the chest sensor is omitted, may seem paradoxical initially. We consider the increased noise introduced by the chest due to changes in lying position or misclassification as sitting when the trunk is inclined to sitting or lying as one reasonable explanation. In our clinical experience, the application of chest sensors has been associated with discomfort and requires higher levels of patient supervision to maintain wearing compliance. Therefore, we consider it a meaningful advantage to omit the chest sensor while still maintaining excellent performance when discriminating between lying and sitting. Compared to our results, other studies in healthy adults (Horemans et al., 2020) and individuals with stroke (Fanchamps et al., 2018) have reported lower performance discriminate the two classes sitting and lying with a single thigh-mounted sensor because thigh orientation is similar in both postures. We explored misclassification incidences between postures and walking activities and found the highest misclassifications between sitting/standing and standing/walking. Although classification accuracy is high, specific information on false-positive proportions is valuable in the context of outcome interpretation. Accurate classification of physical activity types in real-life environments remains challenging because the distinction of predefined classes is impeded in natural movement containing a continuous flow of physical activities and transitions. Our results showed good performance for classifying lying, sitting, and walking, including movement transitions in a real-life environment, which is essential for further analysis and valid outcome measures.
The quantification of physical activity can be split into classification methods that categorize physical activity into discrete classes allowing for a more detailed level of physical activity and posture analysis. We introduced two classification tasks that can reliably extract specified physical activities from a continuous IMU time-series acquired from stroke patients. Both classification tasks can be applied to enable a quantitative and qualitative analysis of mobility and upper limb activities.
The first classification task broadly categorizes sensor data into walking and non-walking activities serving two purposes. Firstly, it can be used to extract walking sequences to quantify spatiotemporal gait parameters. Reduced walking speed, deviations in stride length, and stride asymmetry are characteristic of hemiplegic Gait (Viccaro et al., 2011; Mohan et al., 2021) and are associated with fall events (Moreira et al., 2015). Our highly accurate walking classification algorithm, validated under real-life conditions, might increase the reliability of step detection-based gait analysis algorithms primarily developed under laboratory-based conditions (Renggli et al., 2020; Felius et al., 2022). Secondly, our algorithm shows very low misclassification rates and can be implemented for walking exclusion to quantify upper limb movement during more static body postures. Gait-related whole-body movement was recently shown to account for 30%–40% of changes in sensor-based upper limb outcomes (Regterschot et al., 2021b). Therefore, correcting upper limb outcomes for gait-related whole-body movement is essential to increasing content validity.
Our second classification scheme branches out the classes of lying, sitting, standing, walking, and stair walking, which is opportune for two purposes. Firstly, it discriminates walking from stair walking featuring district demands on energy expenditure and energy cost in individuals with stroke (Polese et al., 2017). Since sensitivity was low for the classification of stair walking, only walking classification might be used for subsequent spatiotemporal analysis as described above. Secondly, this classification scheme allows for quantifying upper limb activity, differentiating body postures, and excluding whole-body movements. Post-stroke arm use patterns differ between sitting and standing postures (Michielsen et al., 2012), but evidence is scarce, and mechanisms remain unclear. Regterschot et al. (2021a) recently investigated longitudinal trajectories of upper limb outcome during sitting and standing, which resulted in lower change rates than studies that included whole-body movements. In the Gait and posture task, misclassification occurred mainly between sitting and standing but was low between static and dynamic conditions. Therefore, this algorithm can be implemented for the computation of continuous real-life measurements to analyze outcome of the time, frequency, and intensity domain. Implementation of physical activity classification algorithms is needed to expand the knowledge of motor recovery and movement behavior after stroke.
In a clinical setting, the location and number of sensors to wear might also influence patients’ compliance and wear time. Skin-mounted sensors attached to the trunk, thigh, or hip have been reported as less comfortable and are associated with increased proportions of missing data (Duncan and Murray, 2012; Troiano et al., 2014; Howie and Straker, 2016). The presented comparison of sensor configurations provides a basis for selecting a minimal yet sufficient sensing configuration for a given clinical application. We only present accuracies for classifying types of specific physical activities—sensing configurations will take into account the full analysis pipeline and the clinical end goal. For instance, although we compared bilateral and unilateral sensor configurations to classify body postures and physical activities, quantifying movement symmetry in hemiplegic individuals requires a bilateral sensor configuration.
This study has several limitations. Firstly, the sample was relatively small for its heterogeneity in age and motor impairment, which might have expanded the dispersion of performance metrics. A larger sample might have generated more robust results. Secondly, we included only basic physical activities in our protocol. Classification of transportation such as taking an elevator, riding by car, or public transport occurs in static body positions, although whole-body movement is detected by movement sensors which could lead to misclassification. Thirdly, we are not able to automatically predict transitions based on the IMU data. This is typically not a requirement but may be desirable in cases where transition periods need to be removed from a time-series or where the transition itself is the label of interest (Novak et al., 2013). Nevertheless, we observed that transitions in continuous data can be handled well by our method when only labels of the adjoining classes are defined to be correct.
This work compares the performance of two Gait and Posture classification tasks with different levels of complexity that are ecologically validated in stroke survivors and real-life conditions. We achieved accurate classification of naturalistic body postures and walking activities performed by individuals with a wide range of motor impairment after stroke, representing a target population for the deployment of remote monitoring. This not only enables the determination of activity bout frequency, duration and intensity but also allows an in-depth analysis of movement quality. The provided classification performances across different sensor configurations enable clinicians and researchers to make informed choices using our algorithm to optimally adapt the sensor configuration to their targeted outcome of interest. The implementation of our algorithms to identify physical activity classes accurately and reliably provides a cornerstone for the research community to apply more detailed analysis algorithms to continuous data collected without contextual information. We herewith hope to contribute to the establishment of digital biomarkers derived from continuous sensing of movement with wearables for individuals with stroke.
The classification algorithms of this study are available open source: https://github.com/StimuLOOP/activity-detection.
Ethical clearance to conduct the study was provided by the cantonal ethics committee Zurich Switzerland (BASEC No. Req-2020-00947). The patients/participants provided their written informed consent to participate in this study.
JP conceptualized the study and collected and labeled the data. AR performed data processing and analysis. Results were interpreted, and the manuscript was drafted by JP and AR. Supervision was provided by CE. The authors AR, AL, CE, GV, JV, EV, and JP revised the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
This work was financially supported by the P & K Pühringer Foundation, the medical research center “The Loop Zurich,” and the Vontobel Foundation.
We thank Arturo Moncada Torres for support regarding feature extraction and Yannik Rottenberger for his engagement in labeling work. Thanks to Patrick Sequeira for support in preliminary laboratory tests and Stefan Schneller for support regarding the graphical design of sensor configurations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.933987/full#supplementary-material
Allahbakhshi H., Hinrichs T., Huang H., Weibel R. (2019). The key factors in physical activity type detection using real-life data: A systematic review. Front. Physiol. 10, 75. doi:10.3389/fphys.2019.00075
Atrsaei A., Corrà M. F., Dadashi F., Vila-Chã N., Maia L., Mariani B., et al. (2021). Gait speed in clinical and daily living assessments in Parkinson’s disease patients: Performance versus capacity. NPJ Park. Dis. 7, 24. doi:10.1038/s41531-021-00171-0
Atrsaei A., Dadashi F., Hansen C., Warmerdam E., Mariani B., Maetzler W., et al. (2020). Postural transitions detection and characterization in healthy and patient populations using a single waist sensor. J. Neuroeng. Rehabil. 17, 70. doi:10.1186/s12984-020-00692-4
Balasubramanian C. K., Neptune R. R., Kautz S. A. (2009). Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture 29, 408–414. doi:10.1016/j.gaitpost.2008.10.061
Ballabio D., Grisoni F., Todeschini R. (2018). Multivariate comparison of classification performance measures. Chemom. Intell. Lab. Syst. 174, 33–44. doi:10.1016/j.chemolab.2017.12.004
Block V. A. J., Pitsch E., Tahir P., Cree B. A. C., Allen D. D., Gelfand J. M. (2016). Remote physical activity monitoring in neurological disease: A systematic review. PLOS ONE 11, e0154335. doi:10.1371/journal.pone.0154335
Bonomi A. G., Goris A. H. C., Yin B., Westerterp K. R. (2009). Detection of type, duration, and intensity of physical activity using an accelerometer. Med. Sci. Sports Exerc. 41, 1770–1777. doi:10.1249/MSS.0b013e3181a24536
Boukhennoufa I., Zhai X., Utti V., Jackson J., McDonald-Maier K. D. (2022). Wearable sensors and machine learning in post-stroke rehabilitation assessment: A systematic review. Biomed. Signal Process. Control 71, 103197. doi:10.1016/j.bspc.2021.103197
Capela N. A., Lemaire E. D., Baddour N., Rudolf M., Goljar N., Burger H. (2016). Evaluation of a smartphone human activity recognition application with able-bodied and stroke participants. J. Neuroeng. Rehabil. 13, 5. doi:10.1186/s12984-016-0114-0
Cavill N., Kahlmeier S., Racioppi F. (2006). Physical activity and health in europe: Evidence for action. Copenhagen: World Health Organisation, p9.
Clark C. C. T., Barnes C. M., Stratton G., McNarry M. A., Mackintosh K. A., Summers H. D. (2017). A review of emerging analytical techniques for objective physical activity measurement in humans. Sports Med. 47, 439–447. doi:10.1007/s40279-016-0585-y
Clark C. C. T., Nobre G. C., Fernandes J. F. T., Moran J., Drury B., Mannini A., et al. (2018). Physical activity characterization: Does one site fit all? Physiol. Meas. 39, 09TR02. doi:10.1088/1361-6579/aadad0
Compagnat M., Batcho C. S., David R., Vuillerme N., Salle J. Y., Daviet J. C., et al. (2019). Validity of the walked distance estimated by wearable devices in stroke individuals. Sensors 19, 2497. doi:10.3390/s19112497
Creasy S. A., Rogers R. J., Byard T. D., Kowalsky R. J., Jakicic J. M. (2016). Energy expenditure during acute periods of sitting, standing, and walking. J. Phys. Act. Health 13, 573–578. doi:10.1123/jpah.2015-0419
de Rooij I. J. M., Riemens M. M. R., Punt M., Meijer J.-W. G., Visser-Meily J. M. A., van de Port I. G. L. (2021). To what extent is walking ability associated with participation in people after stroke? J. Stroke Cerebrovasc. Dis. 30, 106081. doi:10.1016/j.jstrokecerebrovasdis.2021.106081
Del Din S., Godfrey A., Galna B., Lord S., Rochester L. (2016). Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J. Neuroeng. Rehabil. 13, 46. doi:10.1186/s12984-016-0154-5
Derungs A., Schuster-Amft C., Amft O. (2020). Wearable motion sensors and digital biomarkers in stroke rehabilitation. Curr. Dir. Biomed. Eng. 6, 229–232. doi:10.1515/cdbme-2020-3058
Duncan E. A., Murray J. (2012). The barriers and facilitators to routine outcome measurement by allied health professionals in practice: A systematic review. BMC Health Serv. Res. 12, 96. doi:10.1186/1472-6963-12-96
Ehatisham-ul-Haq M., Azam M. A. (2020). Opportunistic sensing for inferring in-the-wild human contexts based on activity pattern recognition using smart computing. Future Gener. Comput. Syst. 106, 374–392. doi:10.1016/j.future.2020.01.003
English C., Healy G. N., Coates A., Lewis L., Olds T., Bernhardt J. (2016). Sitting and activity time in people with stroke. Phys. Ther. 96, 193–201. doi:10.2522/ptj.20140522
Ezeugwu V. E., Manns P. J. (2017). Sleep duration, sedentary behavior, physical activity, and quality of life after inpatient stroke rehabilitation. J. Stroke Cerebrovasc. Dis. 26, 2004–2012. doi:10.1016/j.jstrokecerebrovasdis.2017.06.009
Fanchamps M., Horemans H., Ribbers G., Stam H., Bussmann J. (2018). The accuracy of the detection of body postures and movements using a physical activity monitor in people after a stroke. Sensors 18, 2167. doi:10.3390/s18072167
Felius R. A. W., Geerars M., Bruijn S. M., van Dieën J. H., Wouda N. C., Punt M. (2022). Reliability of IMU-based gait assessment in clinical stroke rehabilitation. Sensors 22, 908. doi:10.3390/s22030908
Fulk G. D., Combs S. A., Danks K. A., Nirider C. D., Raja B., Reisman D. S. (2014). Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys. Ther. 94, 222–229. doi:10.2522/ptj.20120525
Giggins O. M., Clay I., Walsh L. (2017). Physical activity monitoring in patients with neurological disorders: A review of novel body-worn devices. Digit. Biomark. 1, 14–42. doi:10.1159/000477384
Gyllensten I. C., Bonomi A. G. (2011). Identifying types of physical activity with a single accelerometer: Evaluating laboratory-trained algorithms in daily life. IEEE Trans. Biomed. Eng. 58, 2656–2663. doi:10.1109/TBME.2011.2160723
Horemans H., Kooijmans H., van den Berg-Emons R., Bussmann H. (2020). The Activ8 activity monitor: Validation of posture and movement classification. J. Rehabil. Assist. Technol. Eng. 7, 2055668319890535. doi:10.1177/2055668319890535
Howie E. K., Straker L. M. (2016). Rates of attrition, non-compliance and missingness in randomized controlled trials of child physical activity interventions using accelerometers: A brief methodological review. J. Sci. Med. Sport 19, 830–836. doi:10.1016/j.jsams.2015.12.520
Jayaraman C., Mummidisetty C. K., Mannix-Slobig A., McGee Koch L., Jayaraman A. (2018). Variables influencing wearable sensor outcome estimates in individuals with stroke and incomplete spinal cord injury: A pilot investigation validating two research grade sensors. J. Neuroeng. Rehabil. 15, 19. doi:10.1186/s12984-018-0358-y
Kahn M. B., Clark R. A., Williams G., Bower K. J., Banky M., Olver J., et al. (2019). The nature and extent of upper limb associated reactions during walking in people with acquired brain injury. J. Neuroeng. Rehabil. 16, 160. doi:10.1186/s12984-019-0637-2
Kloter E., Wirz M., Dietz V. (2011). Locomotion in stroke subjects: Interactions between unaffected and affected sides. Brain 134, 721–731. doi:10.1093/brain/awq370
König N., Taylor W. R., Baumann C. R., Wenderoth N., Singh N. B. (2016). Revealing the quality of movement: A meta-analysis review to quantify the thresholds to pathological variability during standing and walking. Neurosci. Biobehav. Rev. 68, 111–119. doi:10.1016/j.neubiorev.2016.03.035
Kramer S. F., Cumming T., Churilov L., Bernhardt J. (2013). Measuring activity levels at an acute stroke ward: Comparing observations to a device. Biomed. Res. Int. 2013, 460482–460488. doi:10.1155/2013/460482
Krausz N. E., Hu B. H., Hargrove L. J. (2019). Subject- and environment-based sensor variability for wearable lower-limb assistive devices. Sensors 19, 4887. doi:10.3390/s19224887
Kristoffersson A., Lindén M. (2022). A systematic review of wearable sensors for monitoring physical activity. Sensors 22, 573. doi:10.3390/s22020573
Kristoffersson A., Lindén M. (2020). A systematic review on the use of wearable body sensors for health monitoring: A qualitative synthesis. Sensors 20, 1502. doi:10.3390/s20051502
Labelbox.com (2022). Labelbox. [online]. Available at: https://labelbox.com> (Accessed May 1, 2022).
Lee K., Kwan M.-P. (2018). Physical activity classification in free-living conditions using smartphone accelerometer data and exploration of predicted results. Comput. Environ. Urban Syst. 67, 124–131. doi:10.1016/j.compenvurbsys.2017.09.012
Leuenberger K., Gonzenbach R., Wiedmer E., Luft A., Gassert R. (2014). “Classification of stair ascent and descent in stroke patients,” in 2014 11th International Conference on Wearable and Implantable Body Sensor Networks Workshops (Zurich, Switzerland: IEEE), 11–16. doi:10.1109/BSN.Workshops.2014.10
Lindemann U., Zijlstra W., Aminian K., Chastin S., de Bruin E., Helbostad J., et al. (2014). Recommendations for standardizing validation procedures assessing physical activity of older persons by monitoring body postures and movements. Sensors 14, 1267–1277. doi:10.3390/s140101267
Massé F., Gonzenbach R. R., Arami A., Paraschiv-Ionescu A., Luft A. R., Aminian K. (2015). Improving activity recognition using a wearable barometric pressure sensor in mobility-impaired stroke patients. J. Neuroeng. Rehabil. 12, 72. doi:10.1186/s12984-015-0060-2
Michielsen M. E., Selles R. W., Stam H. J., Ribbers G. M., Bussmann J. B. (2012). Quantifying nonuse in chronic stroke patients: A study into paretic, nonparetic, and bimanual upper-limb use in daily life. Arch. Phys. Med. Rehabil. 93, 1975–1981. doi:10.1016/j.apmr.2012.03.016
Mohan D. M., Khandoker A. H., Wasti S. A., Ismail Ibrahim Ismail Alali S., Jelinek H. F., Khalaf K. (2021). Assessment methods of post-stroke gait: A scoping review of technology-driven approaches to gait characterization and analysis. Front. Neurol. 12, 650024. doi:10.3389/fneur.2021.650024
Moncada-Torres A., Leuenberger K., Gonzenbach R., Luft A., Gassert R. (2014). Activity classification based on inertial and barometric pressure sensors at different anatomical locations. Physiol. Meas. 35, 1245–1263. doi:10.1088/0967-3334/35/7/1245
Moore S. A., Hallsworth K., Plötz T., Ford G. A., Rochester L., Trenell M. I. (2013). Physical activity, sedentary behaviour and metabolic control following stroke: A cross-sectional and longitudinal study. PLoS ONE 8, e55263. doi:10.1371/journal.pone.0055263
Moreira B. S., Sampaio R. F., Kirkwood R. N. (2015). Spatiotemporal gait parameters and recurrent falls in community-dwelling elderly women: A prospective study. Braz. J. Phys. Ther. 19, 61–69. doi:10.1590/bjpt-rbf.2014.0067
Novak D., Reberšek P., De Rossi S. M. M., Donati M., Podobnik J., Beravs T., et al. (2013). Automated detection of gait initiation and termination using wearable sensors. Med. Eng. Phys. 35, 1713–1720. doi:10.1016/j.medengphy.2013.07.003
O’Brien M. K., Shawen N., Mummidisetty C. K., Kaur S., Bo X., Poellabauer C., et al. (2017). Activity recognition for persons with stroke using mobile phone technology: Toward improved performance in a home setting. J. Med. Internet Res. 19, e184. doi:10.2196/jmir.7385
Ostwald S. K., Godwin K. M., Cheong H., Cron S. G. (2009). Predictors of resuming therapy within four weeks after discharge from inpatient rehabilitation. Top. Stroke Rehabil. 16, 80–91. doi:10.1310/tsr1601-80
Pinheiro Mb P. J., Machado G. C., Faria C. D. C. M., Hirochi T. L., Teixeira-Salmela L. F. (2013). The ability to manage stairs for chronic stroke survivors improves with increases in physical activity levels. J. Nov. Physiother. 3. doi:10.4172/2165-7025.1000159
Polese J. C., Ribeiro-Samora G. A., Lana R. C., Rodrigues-De-Paula F. V., Teixeira-Salmela L. F. (2017). Energy expenditure and cost of walking and stair climbing in individuals with chronic stroke. Braz. J. Phys. Ther. 21, 192–198. doi:10.1016/j.bjpt.2017.04.001
Prajapati S. K., Gage W. H., Brooks D., Black S. E., McIlroy W. E. (2011). A novel approach to ambulatory monitoring: Investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehabil. Neural Repair 25, 6–14. doi:10.1177/1545968310374189
Preece S. J., Goulermas J. Y., Kenney L. P. J., Howard D., Meijer K., Crompton R. (2009). Activity identification using body-mounted sensors—A review of classification techniques. Physiol. Meas. 30, R1–R33. doi:10.1088/0967-3334/30/4/R01
Python.org (2022). Python software foundation. [online] Available at: https://www.python.org/> (Accessed April 2, 2022).
Rabbi J., Fuad M. T. H., Awal M. A. (2021). Human activity analysis and recognition from smartphones using machine learning techniques. ArXiv210316490 Cs. Available at: http://arxiv.org/abs/2103.16490 (Accessed April 21, 2022).
Rand D., Eng J. J., Tang P.-F., Jeng J.-S., Hung C. (2009). How active are people with stroke?: Use of accelerometers to assess physical activity. Stroke 40, 163–168. doi:10.1161/STROKEAHA.108.523621
Rast F. M., Labruyère R. (2020). Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. Neuroeng. Rehabil. 17, 148. doi:10.1186/s12984-020-00779-y
Regterschot G. R. H., Bussmann J. B. J., Fanchamps M. H. J., Meskers C. G. M., Ribbers G. M., Selles R. W. (2021a). Objectively measured arm use in daily life improves during the first 6 months poststroke: A longitudinal observational cohort study. J. Neuroeng. Rehabil. 18, 51. doi:10.1186/s12984-021-00847-x
Regterschot G. R. H., Selles R. W., Ribbers G. M., Bussmann J. B. J. (2021b). Whole-body movements increase arm use outcomes of wrist-worn accelerometers in stroke patients. Sensors 21, 4353. doi:10.3390/s21134353
Renggli D., Graf C., Tachatos N., Singh N., Meboldt M., Taylor W. R., et al. (2020). Wearable inertial measurement units for assessing gait in real-world environments. Front. Physiol. 11, 90. doi:10.3389/fphys.2020.00090
Scikit-learn.org (2022). scikit-learn: machine learning in Python — scikit-learn 0.16.1 documentation. [online] Available at: https://scikit-learn.org/> (Accessed May 2, 2022).
Soltani A., Paraschiv-Ionescu A., Dejnabadi H., Marques-Vidal P., Aminian K. (2020). Real-world gait bout detection using a wrist sensor: An unsupervised real-life validation. IEEE Access 8, 102883–102896. doi:10.1109/ACCESS.2020.2998842
Stergiou N., Decker L. M. (2011). Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 30, 869–888. doi:10.1016/j.humov.2011.06.002
Stergiou N., Kent J. A., McGrath D. (2016). Human movement variability and aging. Kinesiol. Rev. (Champaign). 5, 15–22. doi:10.1123/kr.2015-0048
Sunkad Z. A., Soujanya (2016). “Feature selection and hyperparameter optimization of SVM for human activity recognition,” in 2016 3rd International Conference on Soft Computing & Machine Intelligence (ISCMI). Dubai, United Arab Emirates: IEEE, 104–109. doi:10.1109/ISCMI.2016.30
Thilarajah S., Clark R. A., Williams G. (2016). Wearable sensors and mobile health (mHealth) technologies to assess and promote physical activity in stroke: A narrative review. Brain Impair. 17, 34–42. doi:10.1017/BrImp.2016.1
Thilarajah S., Mentiplay B. F., Bower K. J., Tan D., Pua Y. H., Williams G., et al. (2018). Factors associated with post-stroke physical activity: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 99, 1876–1889. doi:10.1016/j.apmr.2017.09.117
Troiano R. P., McClain J. J., Brychta R. J., Chen K. Y. (2014). Evolution of accelerometer methods for physical activity research. Br. J. Sports Med. 48, 1019–1023. doi:10.1136/bjsports-2014-093546
Ustinova K. I., Langenderfer J. E., Balendra N. (2017). Enhanced arm swing alters interlimb coordination during overground walking in individuals with traumatic brain injury. Hum. Mov. Sci. 52, 45–54. doi:10.1016/j.humov.2017.01.001
van Meulen F. B., Klaassen B., Held J., Reenalda J., Buurke J. H., van Beijnum B.-J. F., et al. (2016). Objective evaluation of the quality of movement in daily life after stroke. Front. Bioeng. Biotechnol. 3, 210. doi:10.3389/fbioe.2015.00210
Viccaro L. J., Perera S., Studenski S. A. (2011). Is timed up and Go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 59, 887–892. doi:10.1111/j.1532-5415.2011.03336.x
Wendel-Vos G., Schuit A. J., Feskens E. J. M., Boshuizen H. C., Verschuren W. M. M., Saris W. H. M., et al. (2004). Physical activity and stroke. A meta-analysis of observational data. Int. J. Epidemiol. 33, 787–798. doi:10.1093/ije/dyh168
Werner C., Awai Easthope C., Curt A., Demkó L. (2021). Towards a mobile gait analysis for patients with a spinal cord injury: A robust algorithm validated for slow walking speeds. Sensors 21, 7381. doi:10.3390/s21217381
Wu J., Kuruvithadam K., Schaer A., Stoneham R., Chatzipirpiridis G., Easthope C. A., et al. (2021). An intelligent in-shoe system for gait monitoring and analysis with optimized sampling and real-time visualization capabilities. Sensors 21, 2869. doi:10.3390/s21082869
Zurichmove.com (2022). [online] Available at: https://zurichmove.com (Accessed April 21, 2022).
Keywords: physical activity, body posture, gait, real-life, movement sensor, stroke
Citation: Pohl J, Ryser A, Veerbeek JM, Verheyden G, Vogt JE, Luft AR and Easthope CA (2022) Accuracy of gait and posture classification using movement sensors in individuals with mobility impairment after stroke. Front. Physiol. 13:933987. doi: 10.3389/fphys.2022.933987
Received: 01 May 2022; Accepted: 29 August 2022;
Published: 26 September 2022.
Edited by:
Mohamed Irfan Mohamed Refai, University of Twente, NetherlandsReviewed by:
Mannes Poel, University of Twente, NetherlandsCopyright © 2022 Pohl, Ryser, Veerbeek, Verheyden, Vogt, Luft and Easthope. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Pohl, am9oYW5uZXMucG9obEB1c3ouY2g=
†These authors share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.