- 1Vector Management Division, Defence R&D Establishment, Gwalior, India
- 2Synthetic Chemistry, Defence R&D Establishment, Gwalior, India
- 3Pharma and Toxicology Division, Defence R&D Establishment, Gwalior, India
Cockroach species Periplaneta americana and Blattella germanica potentially survive in locations close to human activity. Besides spoiling food material, cockroaches also transfer pathogens of different diseases among human beings. Since the insecticides have been used extensively to control cockroaches, information on their insecticide susceptibility and toxicity at the cellular level may be crucial. In the study, deltamethrin toxicity as well as the deltamethrin-mediated cytomorphological changes in the brain, ovary and midgut of the two important cockroach species have been assessed. Different concentrations [0.00025% (0.0025 mg/ml), 0.0025% (0.025 mg/ml), 0.025% (0.25 mg/ml), 0.25% (2.5 mg/ml), 0.5% (5 mg/ml), 1% (10 mg/ml)] of deltamethrin in acetone were used to expose test species in WHO bottle assay. Knockdown was recorded after 5 min interval while delayed mortality was observed after 24 h. Brain, ovary and gut were dissected post 1 h exposure and 24 h holding (for 0.25, 0.5 and 1% concentration), and tissues were processed for microscopic analysis. Deltamethrin exposed cockroaches and dissected tissues were used to estimate deltamethrin using HPLC. At 0.00025% (lowest concentration), the percentage knock-down observed was 66.7% for P. americana and 80% B. germanica respectively (R2 = 0.78; p = 0.0001) in 1 h. KDT50 value was found to be 8.7 min (95% CI: 7.3–10.2), while KDT99 was 20.7 min (95% CI: 16.0–35.7) in P. americana at 1% concentration. Whereas, the KDT50 and KDT99 values for B. germanica were 7.4 min (95% CI: 5.4–9.1) and 27.4 min (95% CI: 18.2–80.0) at a similar concentration. LD50 and LD95 values (for 60 min standard exposure) were 0.0006% (95% CI: 0.00–0.001) and 0.034% (95% CI: 0.013–0.49) respectively for P. americana, while these values were 0.0005 (95% CI: 0.00–0.001) and 0.04 (95% CI: 0.01–0.23) for B. germanica. Exposure to 1% deltamethrin induced a considerable toxic effect in the epithelial cells in the midgut. HPLC estimated 0.21 ± 0.05 mg (95% CI: 0.18–0.25; CoV 23.9%) deltamethrin in P. americana post 1% exposure. Even short term exposure to a low concentration of synthetic pyrethroid deltamethrin displayed immediate knockdown and delayed mortality in both the test species. Considerable histological damage was observed in both the insects at 1% exposure. In India, resistance to deltamethrin may have been reported among different insects due to its extensive use. However, the formulations such as insecticide paints, attractant baits etc. developed using deltamethrin as an active ingredient could be useful in cockroach control operations.
1 Introduction
Both Periplaneta americana and Blattella germanica species of cockroaches are important peridomestic pests in urban communities in the majority of Asian countries. These potentially survive in locations close to humans or human activity. Therefore this arthropod pest is primarily found in residential areas, sewage systems, farm produce markets, grain stores, trains and different commercial establishments. Cockroaches are gregarious and it has been found that different species are able to aggregate at the same location (Ame et al., 2004).
P. americana (American cockroach) is generally reddish brown, large and may measure up to 34–53 mm in length. This species is highly mobile and studies conducted have indicated the movement to several hundred meters through sewer systems and channels into the neighboring homes. On the other hand B. germanica (German cockroach) is a small brown to black coloured species and may measure about 11–16 mm in length (Weaving et al., 2003). Of the few cockroach species that are domestic pests, it probably is the most widely troublesome of all (Bonnefoy et al., 2008).
Cockroaches have been regarded as major pest species, which are both a nuisance and can cause severe health problems. These not only spoil the food materials, but also transfer pathogens of different diseases and may cause allergic reactions, and in some cases psychological distress too (Brenner et al., 1995). Although cockroach’s role in direct pathogen transmission has not been much established, the studies have shown that many pathogenic organisms such as poliomyelitis viruses, protozoa, bacteria, fungi, and helminths have been associated with the cockroaches (Fathpour et al., 2003; Tatfeng et al., 2005; Saichua et al., 2008). These have been reported as vectors of nosocomial infections, while both these species have been found to carry antibiotic resistance bacteria (Pai et al., 2004; Pai et al., 2005a). B. germanica regularly inhabits the food preparation areas during the night thus such areas may become contaminated. A study by Tachbele et al. (2006) has identified a species of Salmonella, Shigella flexneri, Escherichia coli, Staphylococcus aureus, and Bacillus cereus from B. germanica in Ethiopia. These studies indicate that cockroach species may act as possible reservoirs and vectors of many pathogens of human importance. In addition to this, both these species of cockroaches cause allergies to human beings (Sheehan et al., 2016). The major allergens, Bla g1, Bla g2, and Per a1, have been found in the saliva, fecal material, secretions, cast skins, and debris. Studies have also demonstrated the relationship between cockroach exposure and poor asthma outcomes among those who are exposed to high levels of cockroach allergens (Do et al., 2016). Therefore the households can have allergic reactions due to exposure to cockroach body parts and feces, which in most cases are asthmatic, but can be life threatening in some cases.
The effective control of cockroaches can reduce the diseases associated with allergen spread and associated mortality and morbidity among humans (Rabito et al., 2017). The control of cockroaches mostly relies on using insecticides, however it is crucial to delineate the effectiveness of these insecticides against different cockroach species in an area of interest. The extensive use of different insecticide groups in the control of virtually all harmful arthropod pests has raised serious concerns about their efficacy at a relatively low and sustainable concentration. Like many arthropod vectors, cockroaches have shown resistance to different insecticides (Pai et al., 2005b; Gondhalekar and Scharf, 2012; Zhu et al., 2016). This may be because cockroaches live in relatively close and large populations, thus facilitating rapid selection for different insecticides they are exposed. However, another study (Syed et al., 2014) has shown that many synthetic pyrethroids were effective against American cockroaches. The study further suggested that insecticide efficacy differs among the locations. Therefore estimation of insecticide susceptibility of cockroach species to prominently used insecticides may guide substantial use of such insecticides for the control in an area of interest.
The present study was undertaken with the objective to understand the effectiveness of synthetic pyrethroid deltamethrin against two prominent cockroach species P. americana and B. germanica under laboratory conditions. The study presents an effort to generate deltamethrin sensitivity data and assess whether deltamethrin as a single active ingredient could be used to effectively control of cockroaches.
2 Materials and Methods
2.1 Test Organism
The culture of both the cockroach species were maintained in the insectary of Defence Research and Development Establishment, Gwalior. However for the present study, the cockroach species were collected from the kitchens, basements, cupboards and related areas in Gwalior, Madhya Pradesh (India) (Latitude: 26°13′5.8332″N and longitude: 78°10′58.1916″E) and the colonies were maintained in 3 L capacity glass jar fitted with specially designed metal steps inside the jar for movement at 25 ± 2°C temperature, 60 ± 5% relative humidity and exposed to a photoperiod of 12:12 (L:D) (Syed et al., 2014). The insects were provided water, wheat flour and powdered dog biscuits ad libitum. Adult cockroaches of 5–10 days old (almost equal size) from the reared generations were used for the experiments (WHO, 1970; Snoody and Appel, 2014).
2.2 Knockdown and Mortality Bioassays
Technical grade deltamethrin (98% purity) obtained from M/S Tagros Chemical India, Chennai was used for the experimental exposure. The insecticide in different concentrations [0.00025% (0.0025 mg/ml), 0.0025% (0.025 mg/ml), 0.025% (0.25 mg/ml), 0.25% (2.5 mg/ml), 0.5% (5 mg/ml), 1% (10 mg/ml)] were made in acetone. Two ml of concentration was applied uniformly on the surface of the glass bottle jar (500 ml capacity; area 487.8 cm2) and left to dry for 1 h in a fume hood. For each experiment five adult cockroaches were exposed for 1 h, and at least 30 cockroaches were taken for each exposure. In the control treatment, only acetone was used. Knockdown was observed after 5 min interval. After the exposure, the cockroaches were transferred to holding plates and provided with ample food and water. Mortality was recorded after 24, 48, and 72 h post exposure. Lethal doses (LD) were determined by exposing the cockroaches at different concentrations for a given time; whereas lethal time (LT) was estimated by exposing the cockroaches at a given concentration for different periods. Petroleum jelly mixed with mineral oil was a thin layer coated at the open end of the jar to prevent the escape of insects during experiments. The experiments were performed as described previously (WHO, 1970; Scharf et al., 1995; Snoody and Appel, 2014).
2.3 Histological Analysis
For histopathological evaluation, cockroach species P. americana was exposed to 0.25% (group I; N = 5), 0.5% (group II; N = 5), and 1% (group III; N = 5) of deltamethrin for 1 h. Whereas the insects exposed to acetone (group IV; N = 5) were taken as control. Brain, ovary and gut were dissected in saline solution (0.13 M NaCl; 0.01 M Na2HPO4; 0.02 M KH2PO4; pH 7.2) post 24 h of exposure and fixed in 10% neutral buffered formalin solution for another 24 h at room temperature. After fixation and overnight washing in distilled water, the extracted tissues were processed for dehydration in graded series of alcohol and toluene in an auto-tissue processor (Leica TP-1020, Germany). Processed tissues were embedded in paraffin wax (Leica, Germany). Multiple sections of 2.5–3 μm thickness from each block were cut on a rotatory microtome (Thermo Scientific, Microm, United States), mounted on pre-coated glass slide and air dried overnight. The sections were deparaffinised and stained with haematoxylin and eosin (McMannus and Mowry, 1965) in auto-stainer (Leica, Germany) and cover slipped by auto cover slipper (Leica, Germany). After drying, sections were photographed under a light microscope (Leica DMLB, Germany) using DM-500 camera (Leica, Germany).
2.4 HPLC Estimation of Deltamethrin
P. americana adults exposed to 1% deltamethrin were weighted and taken (N = 10) for HPLC quantification of deltamethrin on cockroach surface. Whereas, the brain, gut and ovary tissues dissected from the cockroaches exposed to 0.25, 0.5, and 1% deltamethrin were used for estimation. For quantification from the whole cockroach, each sample was taken into 5 ml ice-cold acetonitrile (HPLC grade), homogenized for 10 min and sonicated for 15 min. Similarly, internal tissues were homogenized in 500 μl acetonitrile, sonicated for 15 min and filtered before using for HPLC. The samples were filtered through 0.22 μ PVDF membrane filter and stored at −4°C for use in HPLC. Active compound deltamethrin was separated on a C18 reverse-phase column (4.6 × 250 mm, particle size 5 μm; Waters XTerra™) maintained at room temperature (25°C). Methanol and water (80:20 v/v) were used as mobile phase with a flow rate of 1 ml/min using a binary pump (Waters, 1225). Detection was performed using a dual absorbance detector (Waters, 2487) at 280 nm. Prepared samples were injected through Rheodyne injector (injection volume 20 μl). The HPLC instrument used was controlled by Empower computer programme (Europa Science, Ltd., Cambridge, UK) for data collection and overall instrument control during the experiments.
2.5 Statistical Analysis
Knockdown at different time intervals was observed and presented in percent knockdown (% KD), whereas mortality was scored after 24 h of exposure and presented as corrected mortality. Spearman correlation was performed to assess the knockdown for both the species at a different interval, while using that the data did not follow Gaussians distribution. Log dose probit method has been used to estimate the KD, LD, and LT values using LdP Software (Ihabsoft, Turkey). Results were considered significant when p < 0.05 at 95% confidence intervals.
3 Results
3.1 Knock-Down Bioassays
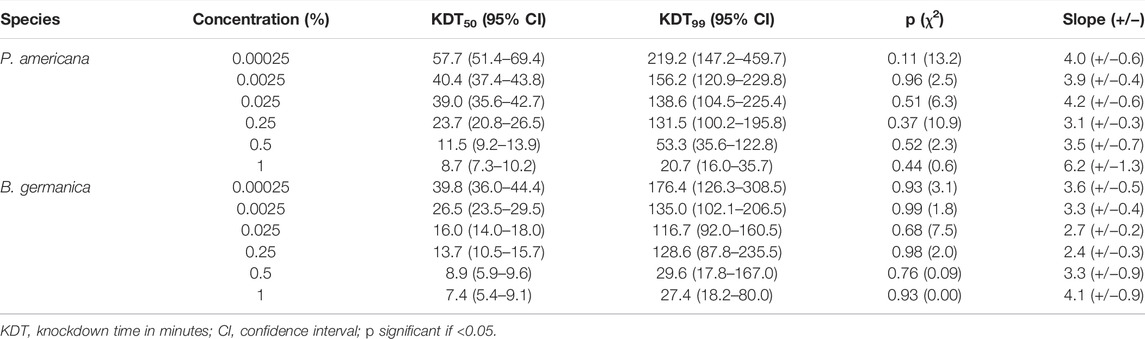
The percent knock-down achieved at different time intervals in cockroach species P. americana and B. germanica after 1 h of exposure to different concentrations of deltamethrin has been depicted in Figure 1, whereas the overall knock-down effect observed has been presented in Table 1. At the lowest concentration of 0.00025%, the percentage knock-down observed was 66.7% for P. americana and 80% B. germanica respectively (R2 = 0.78; p = 0.0001) in 1 h. On the other hand after 20 min of the exposure at 1% deltamethrin concentration, both the species exhibited 100% knockdown (Figure 1). The KDT50 value was 8.7 min (95% CI: 7.3–10.2) whereas KDT99 was 20.7 min (95% CI: 16.0–35.7) in P. americana at 1% concentration. Nevertheless both KDT50 and KDT99 values increased at 0.00025% and recorded to be 57.7 min (95% CI: 51.4–69.4) and 219.2 min (95% CI: 147.2–459.7) respectively. On the other hand for B. germanica, the KDT50 and KDT99 values were 7.4 min (95% CI: 5.4–9.1) and 27.4 min (95% CI: 18.2–80.0) at 1% concentration, while found to be 39.8 min (95% CI: 36.0–44.4) and 176.4 min (95% CI: 126.3–308.5) at 0.00025%, respectively (Table 1). The probit analysis used for determining the knock-down percent at different time intervals for all the treatments displayed normal distribution and did not deviate from the linearity (p ≤ 0.11; χ2 ≥ 13.2).
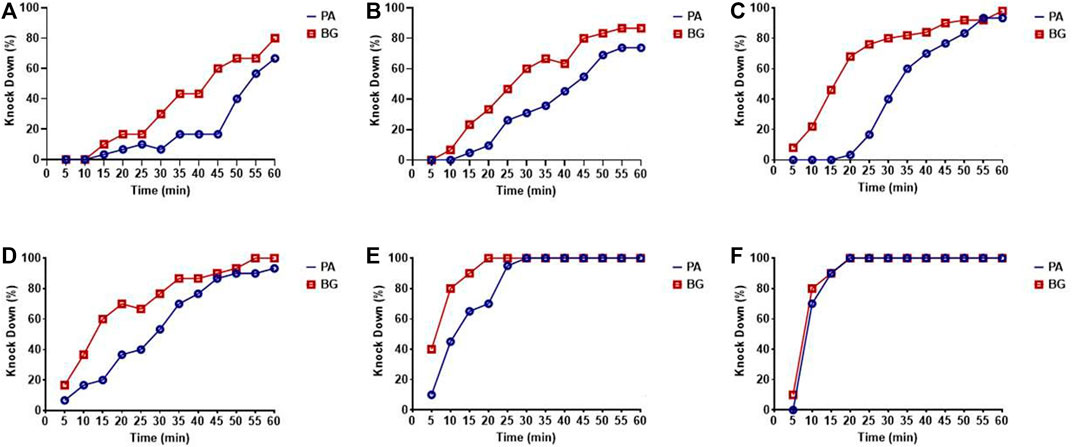
FIGURE 1. Knock-down rate for P. americana (PA) and B. germanica (BG) for different concentrations of deltamethrin. (A) 0.00025%, (B) 0.0025%, (C) 0.025%, (D) 0.25%, (E) 0.5%, and (F) 1%.
3.2 Time-Concentration Mortality
The exposure time mortality data has been shown in Table 2. It was found that post 10 min exposure of deltamethrin and 24 h holding the LD50 and LD95 values were 1.06% (95% CI: 0.44–7.07) and 117.15% (95% CI: 13.18–62,099.0), respectively, while for 60 min standard exposure these values were found to be 0.0006% (95% CI: 0.00–0.001) and 0.034% (95% CI: 0.013–0.49) respectively for P. americana species. Furthermore LD50 and LD95 values for B. germanica species were found to be 0.35% (0.16–1.60) and 100.6 (9.2–3,09,939.6) after 10 min exposure while 0.0005 (95% CI: 0.00–0.001) and 0.04 (95% CI: 0.01–0.23) after 60 min exposure, respectively. Furthermore the mortality values obtained 48 and 72 h holding post 1 h exposure have been displayed in Table 3. It was observed that the LD99 values were 0.01% (p = 0.88) and 0.02% (p = 0.27), respectively for P. americana and B. germanica species 72 h holding post 1 h of exposure (Table 3). Probit model used to determine the lethal time values by using exposure for different time periods at a concentration showed that the LT50 and LT99 values were 72.5 and 199.6 min, respectively for P. americana at the lowest concentration used (Table 4). Similarly, these values were 75.9 and 380.8 min respectively for B. germanica at a similar concentration. It was found that the corrected mortality after 24, 48, and 72 h holding time post 1 h exposure was 100% each in 0.25% deltamethrin in both the species, whereas it varied from 40 to 70% in P. americana and 43%–70% in B. germanica for 0.00025% deltamethrin (Supplementary File 1).
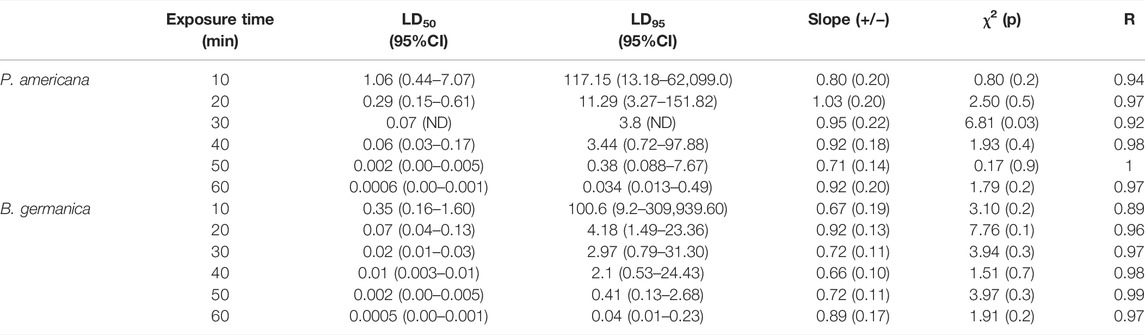
TABLE 2. Lethal dose (LD) values of P. americana and B. germanica for deltamethrin exposure to different time periods.
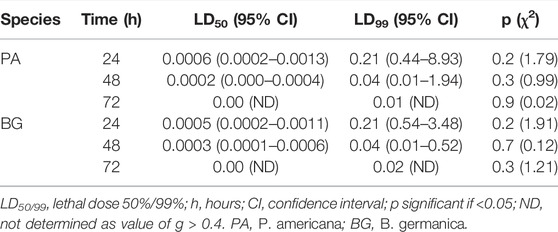
TABLE 3. Mortality effect on P. americana and B. germanica species post 24, 48, and 72 h after 1 h deltamethrin exposure.
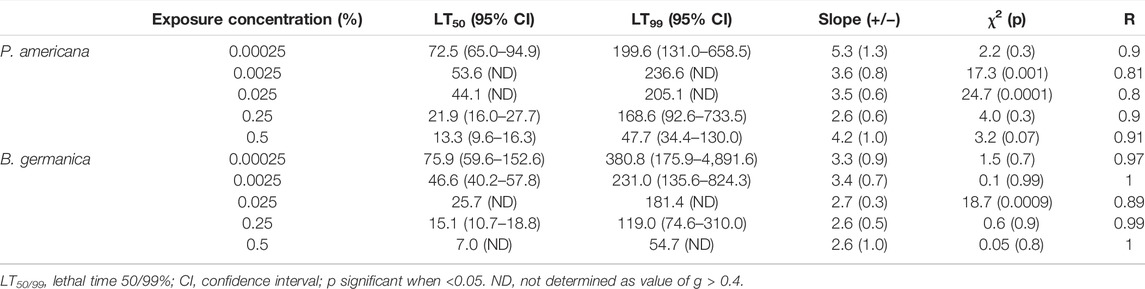
TABLE 4. Lethal time (LT) values of P. americana and B. germanica for different concentration of deltamethrin exposure.
3.3 Histological Analysis
3.3.1 Brain
The brain of cockroach species P. americana consisted of well—defined regions with condensed neuropiles in the central region while neural cell bodies in the periphery. The histological examination of brain tissue from control and treatment did not show any considerable differences and was normal in histology with intact neurons. There was no abnormality in the treated samples compared to the control (Figures 2A–D).
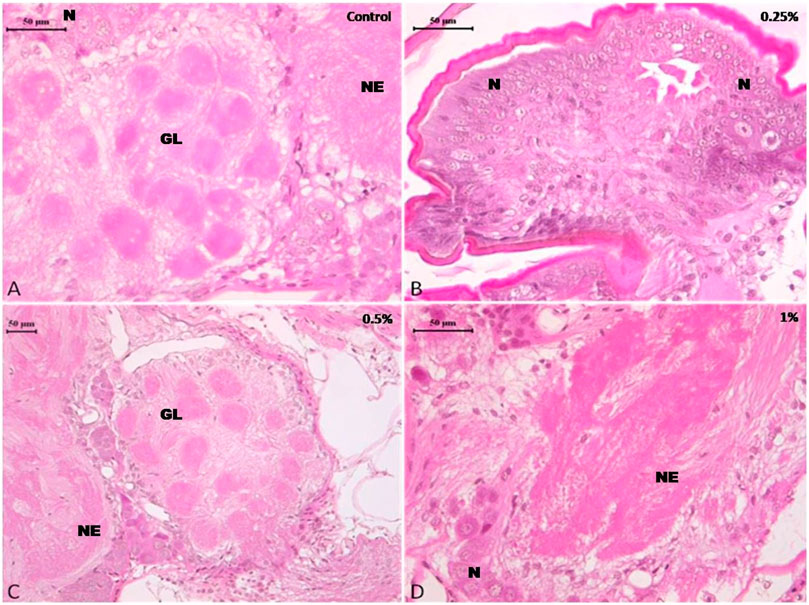
FIGURE 2. Photomicrograph of control and deltamethrin treated cockroach (P. americana) brain: (A) Control; (B) treated with 0.25% deltamethrin; (C) treated with 0.5% deltamethrin; (D) treated with 1% deltamethrin. GL, glomeruli; N, neurons; NE, neuropile.
3.3.2 Ovary
The histopathological examination of the ovary of both control as well as deltamethrin exposed (0.25, 0.5, and 1%) cockroach species P. americana showed normal ovarian follicles with no deteriorating effects. The developing ovarioles exhibited normal development and yolk construction. In general, the yolk material in oocytes was homogenous and the yolk bodies were surrounded by clear rings, with no cracks and fissures (Figures 3A–D). However non-significant distortion in the yolk was observed in 1% treatment (Figure 3D).
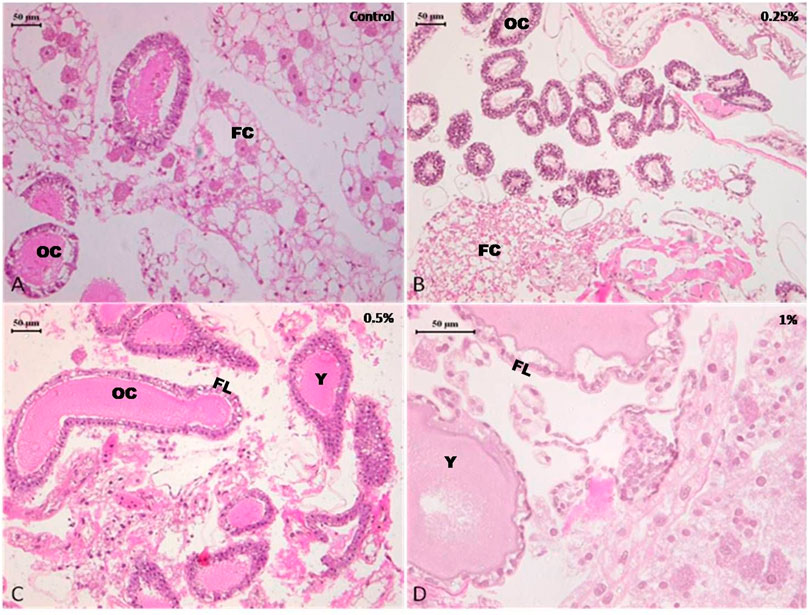
FIGURE 3. Photomicrograph of control and deltamethrin treated cockroach (P. americana) ovary: (A) Control; (B) treated with 0.25% deltamethrin; (C) treated with 0.5% deltamethrin; (D) treated with 1% deltamethrin. FC, fat cells; OC, oocytes; FL, follicular cells; Y, yolk granules.
3.3.3 Midgut
The midgut of control and 0.25 and 0.5% deltamethrin treated cockroaches displayed normal epithelium cells having a striated border with well—defined nucleus. The epithelium cells rest upon a basement membrane followed by a normal inner layer of circular muscles and a regular outer layer of longitudinal muscles. The midgut contained a normal peritrophic membrane within its central lumen and is present as a thin transparent membrane. The histology of the midgut of group I and II insects was normal and did not show a considerable difference from the control (Figures 4A–C). After treatment with 1% of deltamethrin (group III) disorganization and disintegration of epithelial cells were observed in the midgut of the cockroach (Figure 4D). Degenerated cytoplasm with distortion in circular and longitudinal muscle, the disappearance of cell boundaries of the epithelial cells and vacuolization were some common histopathological changes seen in group III as compared to control.
FIGURE 4. Photomicrograph of control and deltamethrin treated cockroach (P. americana) mid gut: (A) Control; (B) treated with 0.25% deltamethrin; (C) treated with 0.5% deltamethrin; (D) treated with 1% deltamethrin. EL, epithelial cells; CL, central lumen; PM, peritrophic membrane.
3.4 Deltamethrin Quantification
HPLC method could estimate the deltamethrin on the exposed body of P. americana during the experiments. It was found that the average deltamethrin was 0.21 ± 0.05 mg/cockroach (95% CI: 0.18–0.25; CoV 23.9%) (Supplementary File 2 shows median weight of exposed cockroaches and deltamethrin extracted using HPLC). The HPLC chromatograms of insecticide mixture (including deltamethrin) were used as standard and deltamethrin extracted from the P. americana surface has been shown in Figures 5A,B. HPLC could not detect deltamethrin in the brain, gut and ovary tissue of the exposed cockroaches.
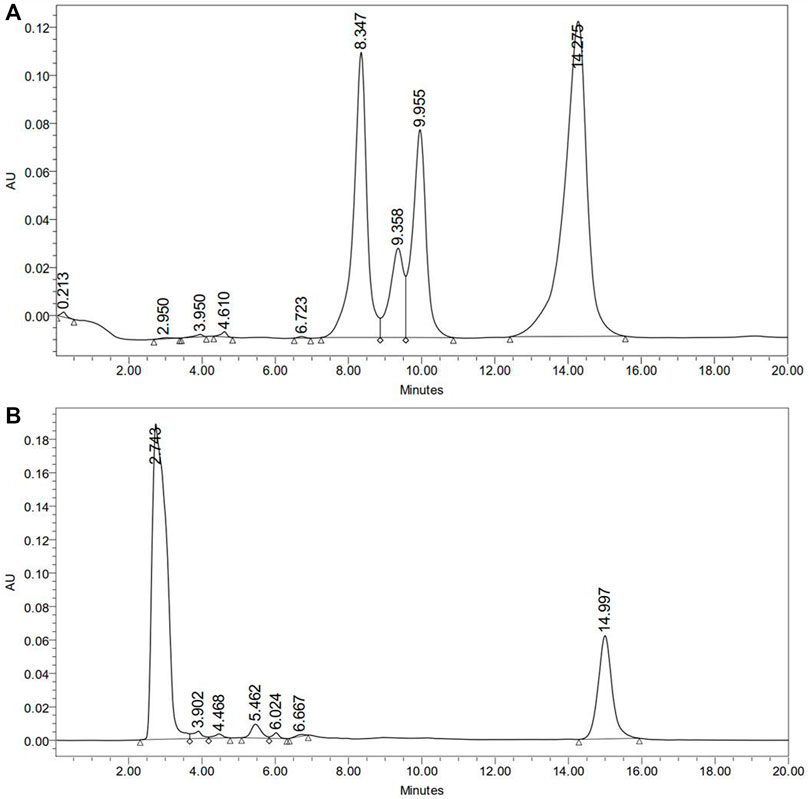
FIGURE 5. HPLC estimation of deltamethrin on P. americana exposed to 1% deltamethrin. (A) HPLC-UV chromatogram of insecticides mixture including deltamethrin used as standard; Pyriproxifen (9.358 min), chloropyriphos (9.995 min), and deltamethrin (14.275 min); (B) HPLC-UV chromatogram for deltamethrin extracted from whole cockroach; deltamethrin (14.997 min).
4 Discussion
Both P. americana as well as B. germanica are extremely common and among the most adapted detrimental pests that survive almost any environment around humans (Bonnefoy et al., 2008; Fakoorziba et al., 2010). Besides being vectors of commonly occurring human pathogenic bacteria, cockroaches have been found to host many antibiotic-resistant bacteria in their digestive system (Fardisi et al., 2019). Control of cockroaches may be difficult due to several reasons, including the development of resistance to repeatedly used insecticides, nevertheless, resistance impact can be minimized through pre-assessment of efficacy in addition to the rotational use of different insecticides.
The present research has been focused on evaluating deltamethrin mediated toxicity (including knock-down, delayed mortality and tissue level degeneration in critical internal organs) through time bound contact exposure to understand the effectiveness of deltamethrin as an active ingredient (AI) in controlling cockroaches. Both the species showed high knock-down sensitivity to the insecticide as the knock-down was found ranging from 66.7% (at 0.00025%) to 100% (at 1%) during the short term continuous exposure of 1 h. The results have suggested the concentration and time dependent knock-down and 24 h delayed mortality in both the tested cockroach species. Therefore, the LD values were found to be comparatively higher when exposure was given for a lower time period. Both the species displayed about 60% survivability for 24 h holding while about 30% survivability for 72 h holding at the lowest concentration. On the other hand 100% reduction in tested species was recorded at the concentration above 0.25%. This shows that probably a sufficient amount of insecticide could not be absorbed through the cuticle at a lower concentration.
A variety of insecticides has been evaluated against cockroaches, and many of them have been found effective at low concentrations. A study conducted in Iran has shown that cockroach strains which were not much exposed to synthetic pyrethroids displayed >80% mortality as compared to those which were exposed due to irregular use of insecticides, mainly pyrethroids (Shahi et al., 2008). Synthetic pyrethroids have been shown to reduce the population of German cockroaches by >80% in India by the first week of treatment (Agrawal et al., 2005). The toxic effects are inherent capability of an insecticide and largely vary among different species of insects and even similar but geographically isolated species for various reasons (Dhiman et al., 2013; Yadav et al., 2015). Different methods have been used to assess the toxicity of insecticides, however topical application remains the most sensitive method to ascertain the effectiveness (Holbrook et al., 2003). Many studies have demonstrated moderate to high resistance in cockroach species to different insecticides. Chai and Lee (Chai and Lee, 2010) have reported heavy resistance for deltamethrin and cypermethrin against both P. americana and B. germanica in Singapore. In another study, it was found that there was only 20% reduction in number when cockroaches were exposed to different pyrethroids (Fardisi et al., 2017). The resistance to insecticides in cockroaches develops faster probably because these inhabit relatively closely to large populations unlike mosquitoes and bugs, hence experiencing high selection pressure. In the present study, both the tested species were sensitive to deltamethrin and recorded >96% mortality in 0.025% deltamethrin suggesting that the deltamethrin resistance levels are extremely low in the test populations. Fardisi et al. (2019) have reported that against cockroaches an insecticide can effectively reduce the populations in areas with low starting resistance due to limited or no historical exposure of insecticides. Furthermore the study also suggested that the insecticides may exert a repellency effect if they fail to produce mortality due to increased resistance levels.
Present results suggested that short term (24 h) holding post 1 h exposure of 0.25 and 0.5% deltamethrin did not cause considerable degeneration in the brain and ovary tissue. The brain showed normal morphology with distinct neuron cell bodies. It has been well documented that synthetic pyrethroids normally damage the nerve cells (Soderlund, 2012; Gutiérrez et al., 2016), but no such damage was found in the present study. Furthermore the ovary also displayed normal panostic architecture with distinct oocytes and ovarian follicles without any fissure. Insecticide indoxacarb has been found to cause malformation in the oocytes displaying abnormal yolk and vacuolated follicular epithelium (Razik and El- Raheem, 2019). However, similar to the present results, pyrethroid lambda-cyhalothrin exposure did not significantly alter the ovarian structure (Razik and El- Raheem, 2019). Nevertheless, exposure to 1% deltamethrin caused degeneration in gut epithelium affecting overall cytomorphology. This suggested that the survival deficits observed might have been resulted from a considerable disturbance in the physiological processes such as cellular metabolism and signal transmission in addition to the tissue damage. The sections did not show cytoplasmic granulation indicating that short time exposure was insufficient to allow deltamethrin accumulation or its by-products in the tissue system. However, it has been suggested that 24 h continuous exposure to deltamethrin caused its bio-accumulation in the midgut cytoplasm of C. radiatus nymph in the form of basophilic granules (Gutiérrez et al., 2016). The vacuolization in the midgut could be the initiation of deltamethrin mediated degeneration process. In an earlier study it was observed that cytoplasmic vacuolization was associated with deltamethrin-mediated initial changes, which resulted in autophagy and apoptosis (Gutiérrez et al., 2016). The toxic effect of deltamethrin may not necessarily be due to direct toxicity (Gondhalekar et al., 2013; Acharya et al., 2021) but deltamethrin exposure may result in excito-repellency (Dhiman et al., 2021), homeostasis imbalance in the midgut thereby reducing the ability to digest food for nutrients (Huang et al., 2015), dysregulation of the breathing activities (Unkiewicz-Winiarczyk and Gromysz -K, 2012) and also limiting in the production of signaling molecules that regulate its own physiology (Caccia et al., 2019). Deltamethrin was detected on the body surface of cockroach but not found in brain, ovary and gut tissue using HPLC. This suggests that deltamethrin up-taken through the oral or cuticular route in the used concentrations was insufficient enough to reach and accumulate in the internal tissues.
Resistance to insecticides continues to exacerbate the impact of cockroaches on public health and overall hygiene. It is obvious that resistance to one or another class of insecticides is ubiquitous among the cockroach populations due to use of different insecticides based products. Still, it may not be logical to simply guess the level of resistance at any place. Therefore efficacy assessment of an insecticide, because of its ability to identify suitable insecticide, is vital for choosing an appropriate insecticide for control success. Deltamethrin, at present, is among the most used insecticides in different intervention programmes and was able to provide efficacy against two cockroach species in the present study.
5 Conclusion
A low concentration of deltamethrin was effective against two important field collected laboratory reared cockroach species. Even short term exposure displayed immediate knock—down, delayed mortality and considerable histological damage in the gut of tested species. The resistance to deltamethrin may be widespread among various public health importance insects due to its use in various “over the counter” available products, however the suitable formulations such as insecticide paints, attractant baits etc. made using deltamethrin as an active ingredient could be useful in the control operations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author Contributions
SD: Developed the study idea, performed laboratory experiments, and prepared the manuscript. KY: Involved in dissection, culture maintenance, performed the experiments. BA: Involved in HPLC estimation and drafted the manuscript. RG: HPLC experiments. DN: Histological assessment and manuscript editing. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors are thankful to Director, Defence Research Development Establishment, Gwalior, for supporting the work. The help of technical staff during the experiments is acknowledged.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.926267/full#supplementary-material
References
Acharya B. N., Ahirwar R., Dhiman S., Yadav K., Pandey P., Sukumaran D. (2021). Deltamethrin Microencapsulation in Emulsion Paint Binder and its Long-Term Efficacy against Dengue Vector Aedes aegypti. Front. Public Health 9, 686122. doi:10.3389/fpubh.2021.686122
Agrawal V. K., Tilak R., Gupta K. K. (2005). Efficacy of Synthetic Pyrethroid and Propoxur Aerosol in the Control of German Cockroaches (Dictyoptera: Blatellidae) in Cookhouses. J. Vector Borne Dis. 42, 117–121.
Ame J. M., Rivault C., Deneubourg J. L. (2004). Cockroach Aggregation Based on Strain Odour Recognition. Anim. Behav. 68 (4), 793–801. doi:10.1016/j.anbehav.2004.01.009
Bonnefoy X., Kampen H., Sweeney K.. Public health significance of urban pests. Geneva, Switzerland: World Health Organization. (2008) 54–84. ISBN 978-92-890-7188-8.
Brenner R. J. (1995). “Economics and Medical Importance of German Cockroaches,” in Understanding and Controlling the German Cockroach. Editors M. K. Rust, J. M. Owens, and D. A. Reierson (New York, USA: Oxford University Press), 77–92.
Caccia S., Casartelli M., Tettamanti G. (2019). The Amazing Complexity of Insect Midgut Cells: Types, Peculiarities, and Functions. Cell. Tiss. Res. 377, 505–525. doi:10.1007/s00441-019-03076-w
Chai R. Y., Lee C. Y. (2010). Insecticide Resistance Profiles and Synergism in Field Populations of the German Cockroach (Dictyoptera: Blattellidae) from Singapore. J. Econ. Entomol. 103 (2), 460–471. doi:10.1603/ec09284
Dhiman S., Rabha B., Talukdar P. K., Das N. G., Yadav K., Baruah I., et al. (2013). DDT & Deltamethrin Resistance Status of Known Japanese Encephalitis Vectors in Assam, India. Indian J. Med. Res. 138, 988–994.
Dhiman S., Yadav K., Acharya B. N., Ahirwar R. K., Sukumaran D. (2021). Behavioural Response of Mosquito Vectors Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus to Synthetic Pyrethroid and Organophosphorus-Based Slow-Release Insecticidal Paint. Parasites Vectors 14, 259. doi:10.1186/s13071-021-04746-x
Do D. C., Zhao Y., Gao P. (2016). Cockroach Allergen Exposure and Risk of Asthma. Allergy 71 (4), 463–474. doi:10.1111/all.12827
Fakoorziba M. R., Eghbal F., Hassanzadeh J., Moemenbellah-Fard M. D. (2010). Cockroaches (Periplaneta americana and Blattella germanica) as Potential Vectors of the Pathogenic Bacteria Found in Nosocomial Infections. Ann. Trop. Med. Parasitol. 104, 521–528. doi:10.1179/136485910x12786389891326
Fardisi M., Gondhalekar A. D., Ashbrook A. R., Scharf M. E. (2019). Rapid Evolutionary Responses to Insecticide Resistance Management Interventions by the German Cockroach (Blattella germanica L.) Sci. Rep. 9, 8292. doi:10.1038/s41598-019-44296-y
Fardisi M., Gondhalekar A. D., Scharf M. E. (2017). Development of Diagnostic Insecticide Concentrations and Assessment of Insecticide Susceptibility in German Cockroach (Dictyoptera: Blattellidae) Field Strains Collected From Public Housing. J. Econ. Entomol. 110, 1210–1217. doi:10.1093/jee/tox076
Fathpour H., Emtiazi G., Ghasemi E. (2003). Cockroaches as Reservoirs and Vectors of Drug Resistant Salmonella Spp. Iran. Biomed. J. 7 (1), 35–38.
Gondhalekar A. D., Scharf M. E. (2012). Mechanisms Underlying Fipronil Resistance in a Multiresistant Field Strain of the German Cockroach (Blattodea: Blattellidae). J. Med. Entomol. 49, 122–131. doi:10.1603/me11106
Gondhalekar A. D., Scherer C. W., Saran R. K., Scharf M. E. (2013). Implementation of an Indoxacarb Susceptibility Monitoring Program Using Field-Collected German Cockroach Isolates from the United States. J. Econ. Entom. 106 (2), 945–953. doi:10.1603/ec12384
Gutiérrez Y., Santos H. P., Serrão J. E., Oliveira E. E. (2016). Deltamethrin-Mediated Toxicity and Cytomorphological Changes in the Midgut and Nervous System of the Mayfly Callibaetis Radiatus. PLoS One 11 (3), e0152383. doi:10.1371/journal.pone.0152383
Holbrook G. L., Roebuck J., Moore C. B., Waldvogel M. G., Schal C. (2003). Origin and Extent of Resistance to Fipronil in the German Cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae). J. Econ. Entomol. 96 (5), 1548–1558. doi:10.1093/jee/96.5.1548
Huang J. H., Jing X., Douglas A. E. (2015). The Multi-Tasking Gut Epithelium of Insects. Insect Biochem. Mol. Biol. 67, 15–20. doi:10.1016/j.ibmb.2015.05.004
McMannus J. F. A., Mowry R. W. (1965). “General Methods for Study of the Cell and its Structure,” in Staining Methods: Histologic and Histochemical (New York: Harper), 73–90.
Pai H. H., Chen W. C., Peng C. F. (2004). Cockroaches as Potential Vectors of Nosocomial Infections. Infect. Control Hosp. Epidemiol. 25 (11), 979–984. doi:10.1086/502330
Pai H. H., Chen W. C., Peng C. F. (2005). Isolation of Bacteria with Antibiotic Resistance from Household Cockroaches (Periplaneta americana and Blattella germanica). Acta Trop. 93 (3), 259–265. doi:10.1016/j.actatropica.2004.11.006
Pai H. H., Wu S. C., Hsu E. L. (2005). Insecticide Resistance in German Cockroaches (Blattella germanica) from Hospitals and Households in Taiwan. Int. J. Environ. Health Res. 15 (1), 33–40. doi:10.1080/09603120400018816
Rabito F. A., Carlson J. C., He H., Werthmann D., Schal C. (2017). A Single Intervention for Cockroach Control Reduces Cockroach Exposure and Asthma Morbidity in Children. J. Allergy Clin. Immunol. 140, 565–570. doi:10.1016/j.jaci.2016.10.019
Razik M. A. R. A. M. A., El-Raheem A. M. A. (2019). Biochemical and Histological Effects of Lambdacyhalothrin, Emamectin Benzoate and Indoxacarb on German Cockroach Blatella Germanics L. Am. J. Biochem. Mol. Biol. 9, 7–16.
Saichua P., Pinmai K., Somrithipol S., Tor-Udom S. (2008). Isolation of Medically Important Fungi from Cockroaches Trapped at Thammasat Chalermprakiat Hospital. TMJ 8 (3), 345–351.
Scharf M. E., Bennett G. W., Reid B. L., Qui C. (1995). Comparisons of Three Insecticide Resistance Detection Methods for the German Cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 88 (3), 536–542. doi:10.1093/jee/88.3.536
Shahi M., Hanafi-Bojd A. A., Vatandoost H. (2008). Evaluation of Five Local Formulated Insecticides against German Cockroach (Blattella germanica L.) in Southern Iran. Iran. J. Arthropod Borne Dis. 2 (1), 21–27.
Sheehan W. J., Phipatanakul W., Phipatanakul W. (2016). Indoor Allergen Exposure and Asthma Outcomes. Curr. Opin. Pediatr. 28 (6), 772–777. doi:10.1097/MOP.0000000000000421
Snoody E. T., Appel A. G. (2014). Field and Laboratory Efficacy of Three Insecticides for Population Management of the Asian Cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 107 (1), 326–332.
Soderlund D. M. (2012). Molecular Mechanisms of Pyrethroid Insecticide Neurotoxicity: Recent Advances. Arch. Toxicol. 86, 165–181. doi:10.1007/s00204-011-0726-x
Syed R., Manzoor F., Adalat R., Abdul-Sattar A., Syed A. (2014). Laboratory Evaluation of Toxicity of Insecticide Formulations from Different Classes against American Cockroach (Dictyoptera: Blattdiae). J. Arthropod Borne Dis. 8 (1), 21–34.
Tachbele E., Erku W., Gebre-Michael T., Ashenafi M. (2006). Cockroach-associated Food-Borne Bacterial Pathogens from Some Hospitals and Restaurants in Addis Ababa, Ethiopia: Distribution and Antibiograms. J. Rural. Trop. Pub Health 5, 34–41.
Tatfeng Y. M., Usuanlele M. U., Orukpe A., Digban A. K., Okodua M., Oviasogie F., et al. (2005). Mechanical Transmission of Pathogenic Organisms: the Role of Cockroaches. J. Vector Borne Dis. 42 (4), 129–134.
Unkiewicz-Winiarczyk A., Gromysz-K K. (2012). Effect Of Temperature on Toxicity of Deltamethrin and Oxygen Consumption by Porcellio scaber Latr (Isopoda). Bull. Environ. Contam. Toxicol. 89, 960–965. doi:10.1007/s00128-012-0814-5
Weaving A., Griffiths P. M., Llewellyn C. (2003). Field Guide to Insects of South Africa. New South Wales: New Holland Publishers, Ltd. ISBN 1-86872-713-0.
WHO (1970). Instructions for Determining the Susceptibility or Resistanceof Cockroaches to Insecticides. Geneva: World Health Organization, 130–133. Technical Report Series No. 443.
Yadav K., Rabha B., Dhiman S., Veer V. (2015). Multi-insecticide Susceptibility Evaluation of Dengue Vectors Stegomyia Albopicta and St. Aegypti in Assam, India. Parasites Vectors 8, 143. doi:10.1186/s13071-015-0754-0
Keywords: deltamethrin, Periplaneta americana, Blattella germanica, knock-down, mortality, toxicity
Citation: Dhiman S, Yadav K, Acharya BN, Nagar DP and Rao Ghorpade R (2022) Deltamethrin Contact Exposure Mediated Toxicity and Histopathological Aberrations in Tissue Systems of Public Health Importance Cockroach Species Periplaneta americana and Blattella germanica. Front. Physiol. 13:926267. doi: 10.3389/fphys.2022.926267
Received: 22 April 2022; Accepted: 07 June 2022;
Published: 18 July 2022.
Edited by:
Jose Eduardo Serrão, Universidade Federal de Viçosa, BrazilReviewed by:
Jalal Jalali Sendi, University of Guilan, IranMuhammad Qasim, Zhejiang University, China
Copyright © 2022 Dhiman, Yadav, Acharya, Nagar and Rao Ghorpade. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunil Dhiman, c3VuaWxkaGltYW44MUBnbWFpbC5jb20=
 Sunil Dhiman
Sunil Dhiman Kavita Yadav1
Kavita Yadav1 B. N. Acharya
B. N. Acharya D. P. Nagar
D. P. Nagar