- 1Department of Biomedical Informatics, Columbia University Irving Medical Center, New York, NY, United States
- 2Division of Pediatric Surgery, Department of Surgery, Columbia University Irving Medical Center, New York, NY, United States
- 3Division of Pediatric Endocrinology, Metabolism, and Diabetes, Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, United States
- 4Department of Bioengineering, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 5Department of Biomedical Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
Type 2 diabetes mellitus is a complex and under-treated disorder closely intertwined with obesity. Adolescents with severe obesity and type 2 diabetes have a more aggressive disease compared to adults, with a rapid decline in pancreatic β cell function and increased incidence of comorbidities. Given the relative paucity of pharmacotherapies, bariatric surgery has become increasingly used as a therapeutic option. However, subsets of this population have sub-optimal outcomes with either inadequate weight loss or little improvement in disease. Predicting which patients will benefit from surgery is a difficult task and detailed physiological characteristics of patients who do not respond to treatment are generally unknown. Identifying physiological predictors of surgical response therefore has the potential to reveal both novel phenotypes of disease as well as therapeutic targets. We leverage data assimilation paired with mechanistic models of glucose metabolism to estimate pre-operative physiological states of bariatric surgery patients, thereby identifying latent phenotypes of impaired glucose metabolism. Specifically, maximal insulin secretion capacity, σ, and insulin sensitivity, SI, differentiate aberrations in glucose metabolism underlying an individual’s disease. Using multivariable logistic regression, we combine clinical data with data assimilation to predict post-operative glycemic outcomes at 12 months. Models using data assimilation sans insulin had comparable performance to models using oral glucose tolerance test glucose and insulin. Our best performing models used data assimilation and had an area under the receiver operating characteristic curve of 0.77 (95% confidence interval 0.7665, 0.7734) and mean average precision of 0.6258 (0.6206, 0.6311). We show that data assimilation extracts knowledge from mechanistic models of glucose metabolism to infer future glycemic states from limited clinical data. This method can provide a pathway to predict long-term, post-surgical glycemic states by estimating the contributions of insulin resistance and limitations of insulin secretion to pre-operative glucose metabolism.
1 Introduction
As obesity rates rise in the United States, so too does the prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents (Skelton et al., 2009; Hales et al., 2017h). While a number of pharmacotherapies exist to treat T2DM, there are few options approved for use in younger patients, who typically have more aggressive disease (TODAY Study Group, 2021; American Diabetes Association Professional Practice Committee, 2022). As such, bariatric surgery is increasingly used as a treatment for severe obesity and prevention or reversal of T2DM, despite risk of operative complications (Hsia et al., 2012; Aung et al., 2016; Beamish and Reinehr, 2017; Inge et al., 2018a; Armstrong et al., 2019; Bolling et al., 2019; Karasko, 2019; Khattab and Sperling, 2019; American Diabetes Association Professional Practice Committee Draznin et al., 2022). Many patients benefit with significant, sustained weight loss, improvement in quality of life, and improvement of obesity-related comorbidities (Inge et al., 2016; Rubino et al., 2016; Beamish and Reinehr, 2017; Pedroso et al., 2018; Inge et al., 2019). However, an ill-defined subset of this population have sub-optimal outcomes (Montero et al., 2011; Livhits et al., 2012; Toh et al., 2017; Inge et al., 2018a; Inge et al., 2018b). Predicting which patients are most likely to benefit from surgery and how they will benefit is a current challenge aimed to minimize unnecessary risk during a critical period of growth and development.
The prevalence of impaired glucose metabolism (IGM)—here referring to a heterogenous population with impaired glucose tolerance (IGT), impaired fasting glucose (IFG), prediabetes (preDM), or T2DM— is increasing in pediatric populations, and may be underestimated (Sinha et al., 2002; Lee et al., 2006; Nowicka et al., 2011; Buse et al., 2013; Dabelea et al., 2014). IGM is progressive and often goes undiagnosed until later in disease history. It is associated with insulin resistance (IR), the need for more insulin to achieve physiologic effects, i.e., peripheral glucose uptake and suppression of hepatic glucose production (HGP). In the obese state, IR is almost guaranteed as it is directly related to visceral adiposity (Kahn and Flier, 2000), but the extent to which the pancreas can compensate exists on a spectrum. Insulin sensitivity (SI) is a measure of the effectiveness of insulin in promoting glucose uptake. It is reciprocally related to insulin resistance. In this context, T2DM is an example extreme IGM, with significant IR coupled with β cell dysfunction and resultant hyperglycemia (Prentki and Nolan, 2006; American Diabetes Association Professional Practice Committee, 2022). The extent to which function can be rescued after progression to T2DM is modifiable to some degree (Lim et al., 2011; Pajvani and Accili, 2015; Taylor et al., 2019; Richter et al., 2020; Holst and Madsbad, 2021; Bartolomé et al., 2022). Duration of disease negatively impacts the probability of resolution, and lifestyle interventions resulting in weight loss during the early prediabetic phase are more likely to prevent progression (Knowler et al., 2002).
Reversal of preDM and prevention of T2DM are therefore considered to be one of the major benefits of bariatric surgery in this age group for which there are limited other options (Aung et al., 2016; Armstrong et al., 2019; Bolling et al., 2019; Khattab and Sperling, 2019; American Diabetes Association Professional Practice Committee Draznin et al., 2022). Indeed, when compared to pharmacologic or lifestyle interventions, bariatric surgery is overall the most successful intervention with respect to glycemic improvements and sustained weight loss (Schauer et al., 2012; Courcoulas et al., 2014; Mingrone et al., 2015). The specific surgery has clear impact on outcomes. The three most prevalent bariatric surgeries in the U.S. are adjustable gastric banding (AGB), Roux-en-Y gastric bypass (RYGB), and vertical sleeve gastrectomy (VSG). In AGB, an inflatable band is placed around the upper part of the stomach creating a small pouch. In RYGB, the jejunum is directly connected to a remnant small pouch of stomach, thereby bypassing the majority of the stomach and the duodenum. In VSG, the majority of the stomach is removed along the greater curvature, creating a narrow tube or sleeve. While AGB is thought to act through purely restrictive mechanisms, VSG and RYGB restrict food intake and increase malabsorption, alter secretion of gut hormones related to satiety and insulin secretion, e.g., glucagon-like peptide-1 (GLP-1) and ghrelin, and change bile acid composition through the change in macronutrients present in areas of the small intestine (Seeley et al., 2015; Mulla et al., 2018; Akalestou et al., 2022). These differing effects can have profound impact on insulin resistance in particular, and the success of VSG and RYGB in comparison to AGB has led to their increased usage (Mulla et al., 2018). Depending on how remission is defined, meta-analyses have shown that 20–80% of adults will have some degree of improvement in T2DM at medium-to-long term follow up (Yip et al., 2013; Elbahrawy et al., 2018; Tsilingiris et al., 2019; Purnell et al., 2021), although there is suggestion of continued impaired β cell function and overestimation of success (Ramos-Levi et al., 2013a; Laferrère and Pattou, 2018). Smaller prospective studies focusing on adolescents suggest T2DM remission may occur in up to 80–90% of patients and improvement of preDM may occur in 70–80% of patients following Roux-en-Y gastric bypass (RYGB), the most drastic surgery that is recommended for adolescents with respect to metabolic intervention and risk of complications (Inge et al., 2014; Inge et al., 2017; Olbers et al., 2017; Stefater and Inge, 2017). However, small sample sizes and relatively homogenous populations (> 70% non-Hispanic white) limit generalizability of results. Predicting which patients are likely to have remission or partial remission of IGM as a result of surgery and thus not develop T2DM remains a challenging task (Ramos-Levi et al., 2013b; Wang et al., 2015; Tsilingiris et al., 2019).
Given the complexity of medical decision making in adolescent bariatric surgery, accurately assessing benefits vs. risks for an individual is critical for patients and their care teams. Glucose dysregulation and obesity can compound existing surgical risks and increase the chance of complications. Although adolescents have lower complication rates compared to their adult counterparts, they may still experience wound infections, anastomotic strictures, leaks, wound dehiscence, abdominal hernias, dehydration, and venous thromboembolism (Lamoshi et al., 2020). In patients with diabetes, poor wound healing and risk of infection are serious considerations and can directly impact the success of the surgery and need for revision (Keidar, 2011). In the long term, patients also have significant malabsorption post-bariatric surgery, resulting in multiple vitamin and mineral deficiencies requiring lifelong supplementation (Bal et al., 2012; Lamoshi et al., 2020). The consequences of this decreased nutrition can be great in adolescents who are still undergoing their growth spurts and accruing bone during their pubertal years (Lamoshi et al., 2020; Weiner et al., 2020; Ou et al., 2022). Although advancements have been made to minimize risk of post-operative complications, improve wound healing, and optimize post-operative nutrition, bariatric surgery remains an aggressive measure taken to improve a patient’s health (Crossan and Sheer, 2022; Dewberry et al., 2022). Therefore, providing accurate information about a patient’s current diabetic state the probability of improvement with surgery can allow for more informed decision making.
Previous studies using statistical or traditional machine learning techniques to predict T2DM outcomes have generally focused on relatively homogenous adult populations who underwent RYGB, with or without genetic information included in analysis. Because available data are sparse, these approaches are subject to error and are not often validated in pediatric populations (Aminian et al., 2017; Cao et al., 2020; Kam et al., 2020). Varying definitions of what successful glycemic outcomes mean also complicate prediction (Buse et al., 2009; Holst and Madsbad, 2021). Outside of surgery type, other potential predictors include anthropometrics (weight, height, body mass index [BMI]), pre-operative disease severity, use of anti-diabetic medications, and presence of comorbidities (DeMaria et al., 2007; Livhits et al., 2012; Dixon et al., 2013; Robert et al., 2013; Panunzi et al., 2015; Wang et al., 2015; Shen et al., 2019). Associations are also seen with baseline biomarkers such as fasting glucose, insulin, C-peptide, triglycerides (TG), C-reactive protein (CRP), and hemoglobin A1c (HbA1c) levels (Ortega et al., 2012; Courcoulas et al., 2013; Inge et al., 2014; Pedersen et al., 2016; Yan et al., 2017). These features, particularly pre-operative disease severity (as measured by duration of disease, labs, and medications) are more homogenous in adolescents, who frequently have preDM rather than T2DM, and are therefore on fewer medications, if any. Additionally, adolescents tend to have lower HbA1c values at baseline prior to undergoing bariatric surgery compared to their adult counterparts. As such, features that are useful predictors in adult surgical response are not necessarily translatable to adolescents.
To provide more personalized predictions, Pedersen, et al. incorporated genetic and clinical information in an artificial neural network to accurately predict short-term discontinuation of diabetes medications at 30 days (Pedersen et al., 2016). The majority of candidate genetic markers were associated with insulin secretion, glucose clearance, or insulin sensitization. While genetics certainly play a role in T2DM, pre-operative genetic analyses are not currently practical for every patient (Hatoum et al., 2011; Okser et al., 2013; Rouskas et al., 2014).
These prior studies provide evidence that an individual’s underlying physiology has long-term implications for treatment outcomes, but existing methods to approximate these physiological pathways may not be practical for use in adolescents (Lee, 2007; Brown and Yanovski, 2014). Glucose tolerance and insulin resistance are frequently estimated using point or dynamic lab proxies due to the expensive and invasive nature of the gold standard for measurement, the hyperinsulinemic-euglycemic clamp (Muniyappa et al., 2018). Oral glucose tolerance tests (OGTTs) are one such approximation used to screen for and diagnose dysglycemia (Olson et al., 2010; Muniyappa et al., 2018; American Diabetes Association Professional Practice Committee, 2022). After fasting (usually overnight), patients are given a fixed dose of liquid glucose (typically 75 g) after which glucose levels are measured at timed intervals. OGTTs can vary in the types of labs drawn, the frequency of sampling, and the duration of the procedure (Muniyappa et al., 2018). In common clinical practice, glucose is measured over two or three hours. Measurements of insulin and C-peptide are not the standard of care; their assays are relatively costly and lack of standardization makes their interpretation less straightforward (Manley et al., 2007; Little et al., 2008; Miller et al., 2009; Tohidi et al., 2017). As such, these labs are typically only collected in research settings.
Fasting insulin and glucose measurements can be used to calculate indices such as the homeostatic model assessment of insulin resistance (HOMA-IR), which can approximate parameters such as peripheral and hepatic insulin sensitivity
Several mechanistic models of glucose and insulin metabolism have been empirically developed and validated against clamp or FSIVGTT studies to mathematically describe glucose metabolism. Models such as the meal model (Dalla Man et al., 2007), oral minimal model (Cobelli et al., 2014), and ultradian model (Sturis et al., 1991) incorporate insulin secretion rates and glucose elimination to estimate states over varying timescales. Topp et al. developed a mechanistic model that incorporates more granular β cell dynamics, which was extended by Ha et al. to model the development of type 2 diabetes over time and quantify the extent to which different insults in the system contribute to disease (Topp et al., 2000; Ha et al., 2016; Ha and Sherman, 2020). Represented as a system of ordinary differential equations (ODEs), these models have the potential to allow for patient-level characterization of glucose metabolism.
Extracting clinical knowledge from these models is not straightforward, as both the models themselves and their results are often viewed as too abstract for application in clinical practice. However, within these mechanistic models are clinically meaningful physiologic parameters when applied to the appropriate problems, e.g. assessing insulin sensitivity’s relationship with lipoprotein metabolism (Chung et al., 2022). Furthermore, while clamp studies represent a patient’s physiologic state and glucose excursions at a specific point in time, mechanistic models have the potential to elucidate more long-term physiologic states that are difficult to capture clinically.
Knowledge within mechanistic models of glucose metabolism can be exploited via data assimilation, a family of methods frequently used in meteorology and aerospace science (Evensen, 2009; Law et al., 2015). Data assimilation leverages the underlying information about the system contained in these mechanistic models to update current and past states using filtering and smoothing, updates that in turn provide the ability to forecast future states by running the estimated model forward in time. Various filters exist that allow for parameterization and propagation of state uncertainty for non-linear systems such as glucose homeostasis (Kalman, 1960; Wan et al., 2001; Julier and Uhlmann, 2004). In previous work, we used the ultradian and meal models with an unscented Kalman filter (UKF), a sequential method that propagates uncertainty in non-linear systems, as well as deterministic and stochastic optimization methods using techniques such as Markov chain Monte Carlo (MCMC) to estimate both states and parameters, generating a real-time, personalized forecast from free-living data (Albers et al., 2017; Levine et al., 2017). In the free-living data context where data are sparse, we developed a constrained (Albers et al., 2019) version of the ensemble Kalman filter (EnKF) method (Evensen, 2009) that made state and parameter estimates more robust. We also successfully applied these methods to real-time glucose forecasting in the setting of T2DM with OGTT data (Mulgrave et al., 2020). Whereas in the free-living case the focus was accurate glucose state determination, the OGTT case focused more on parameter estimates as a marker for underlying disease.
Here, we take this prior work to further demonstrate the validity of using physiologic parameters inferred from mechanistic models of glucose metabolism to predict impaired glucose metabolism (IGM) in adolescents at 12-months post-bariatric surgery as compared to other clinical information. We use data assimilation to estimate parameters from an extension of the model initially proposed by Topp et al. incorporating β cell mass (Ha and Sherman, 2020), partially represented in Figure 1. We then train logistic regression models leveraging these parameter estimates derived from data assimilation on a cohort of adolescents who underwent vertical sleeve gastrectomy (VSG) or laparascopic adjustable gastric banding (AGB) at our institution between 2006 and 2020.
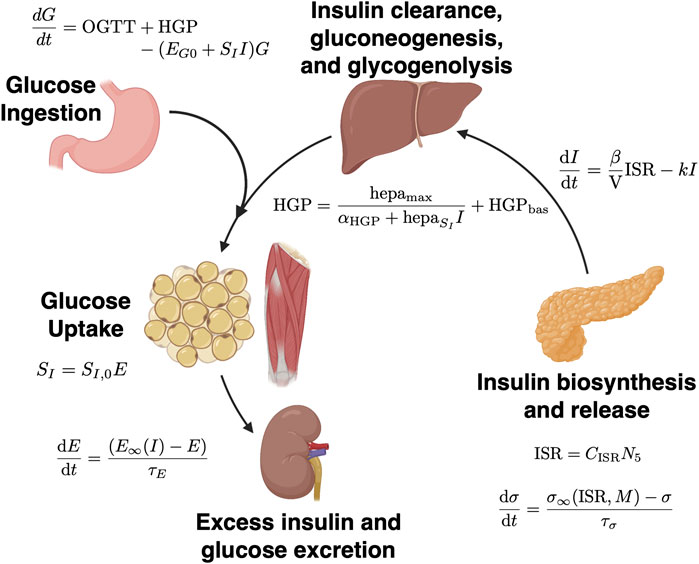
FIGURE 1. Schematic of mechanistic models of glucose metabolism. Underlying physiologic processes are represented by a series of ordinary differential equations (ODEs). Solutions lie on discrete spaces based on patient physiology at the time of measurements. In data assimilation, after reverse parameter estimation at discrete time points (i.e., insulin secretion capacity, σ, and insulin sensitivity, SI), the system state is updated and the ODEs are solved again. Figure adapted from Tokarz et al. (Tokarz et al., 2018) and created with BioRender.com. Relevant equations are outlined in Methods and in Ha and Sherman 2020 (Ha and Sherman, 2020).
2 Materials and methods
2.1 Extraction of clinical data
Data were collected retrospectively from Columbia University Irving Medical Center (CUIMC) from adolescent patients aged 10–21 who had bariatric surgery between 2006 and 2020. Records were first selected based on the presence of bariatric surgery procedure codes with a diagnosis of obesity on the same day (n = 396), a 120-minute OGTT measuring glucose and insulin (at 0, 30, 60, and 120 min) within one year prior to surgery (n = 202), a pre-operative HbA1c within 120 days of the OGTT (n = 202), and at least one post-operative outcome documented within 6–18 months post-surgery (n = 176). These patients were seen through the Center for Adolescent Bariatric Surgery (CABS) at CUIMC. Patients with diganosis codes associated with type 1, cystic fibrosis-related, or gestational diabetes mellitus were excluded. Features as outlined above were extracted for patients who met criteria.
2.2 Data pre-processing and manual feature selection
In addition to features necessary to include patients (OGTT glucose and insulin measurements and HbA1c), additional laboratory variables were selected a priori. These labs were pre-operative thyroid stimulating hormone (TSH), thyroxine (T4), free T4 (FT4), triiodothyronine (T3), aspartate transaminase (AST), alanine transaminase (ALT), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and C-peptide. Age was used as a continuous variable. Categorical features included surgery type, demographic data (self-reported race, ethnicity, and sex), and pre-operative presence of specific comorbidities associated with insulin resistance and T2DM: liver disease (including non-alcoholic steatohepatitis [NASH] and non-alcoholic fatty liver disease [NAFLD]), polycystic ovary syndrome (PCOS), dyslipidemia, hypertension (HTN), obstructive sleep apnea (OSA), thyroid disease, metabolic syndrome (MetS), T2DM (by diagnosis code), and abnormal glucose metabolism (by diagnosis code). To be coded as having the comorbidity of interest without manual chart review, at least 25% of all encounters within the appropriate time window had to contain a related diagnosis code (5 years pre-operative; 6–18 months post-operative) (Perotte and Hripcsak, 2013). Features missing in more than 25% of the sample were removed from analysis. The closest labs obtained prior to surgery and the pre-operative OGTT were included as features if there were multiple lab results within the appropriate time window. HOMA-IR was calculated using mass units (Matthews et al., 1985) as follows in Eq. 1:
where G0 is fasting glucose in mg/dL and I0 is fasting insulin in µIU/mL.
For continuous variables, outliers were defined as those values outside 1.5× the interquartile range (IQR). However, after manual chart review, none of the outliers were removed as none were found to be spurious measurements.
Due to non-normal distributions of the majority of continuous variables, numeric features were scaled and standardized using power transformation with the Box-Cox method (Box and Cox, 1964) prior to hyperparameter tuning. Missing values (n = 7, all thyroid function tests) were imputed using iterative imputation using five nearest features with sampling from the prior distribution (Buck, 1960; Buuren and Groothuis-Oudshoorn, 2011; Pedregosa et al., 2011).
In total, we used 21 continuous clinical variables, 23 categorical variables (three multinomial, 11 binary), and six continuous parameter estimates from data assimilation (mean and standard deviations of maximal insulin secretion capacity [σ], insulin sensitivity [SI], and their product [σ*SI] for an individual’s estimated distributions). Models including HOMA-IR as a feature did not include data assimilation estimates. After data pre-processing, a total of 49 features were included in the most comprehensive model.
2.3 Outcome labelling
Patient post-operative outcome classification was coded as a binary variable indicating the presence or absence of impaired glucose metabolism (IGM) at 12 months. To be classified as having IGM, patients could have any one of the following criteria occur in the 6–18-month post-operative window: ≥ 2 elevated HbA1c values, post-operative OGTT G0 ≥ 100 mg/dL, post-operative OGTT G120 ≥ 140 mg/dL, anti-diabetic drug use (including metformin), or presence of diagnosis codes for T2DM or abnormal glucose metabolism in ≥ 25% of encounters in the post-op window (American Diabetes Association Professional Practice Committee, 2022). If multiple specimens for the same lab were collected within the 6-18-month window, the latest labs were used. Outcome definitions are shown in Table 1.
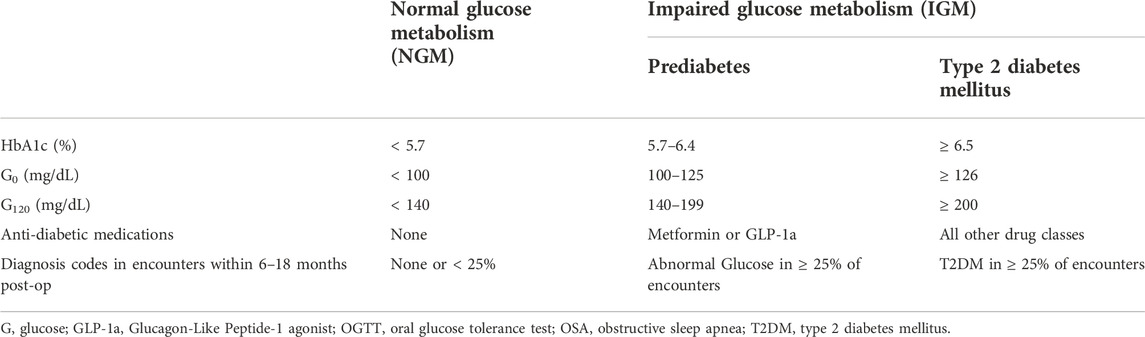
TABLE 1. Outcome definitions for classification as having impaired glucose metabolism (IGM) or normal glucose metabolism (NGM) at 12 months post-bariatric surgery.
2.4 Predictive model training and evaluation
Regularized logistic regression models were trained to predict impaired glucose metabolism (IGM) as a binary outcome on varied subsets of the features as input. The data were first split into 70–30 train-test sets. Hyperparameters were tuned on the train set with stratified nested k-fold cross-validation. Tuned hyperparameters included regularization method (L2 vs. L1), regularization constant (λ = α*n), learning rate [f(α)], and max iterations. The loss function was binary cross-entropy loss with balanced class weight. The optimizer was stochastic gradient descent with an adaptive learning rate. To ensure model robustness to random data splitting, we performed 30 independent train-test splits and the results were averaged. The hyperparameters associated with the minimum loss in training were selected for model evaluation.
Because our dataset was imbalanced with respect to outcomes, we chose class weights in the logistic regression models that were inversely proportional to the class frequency. To evaluate the performance of the prediction models, we computed area under the Receiver Operating Characteristic curve (AUROC), precision, recall, and average precision (AP) on out-of-bag estimates from 1,000 bootstrapped samples (n = 176). Average precision refers to the weighted mean of precision with respect to recall at all probability thresholds for classification and focuses on how well the models predict the positive class (in our case, post-operative IGM). It is analogous to the area under the precision-recall curve (AUPRC) and better suited for imbalanced datasets compared to accuracy. Baseline performance of a naïve classifier would have an AUPRC equal to the proportion of the positive class in the data (here, 0.318).
2.5 Mechanistic glucose metabolism modeling
Relevant equations used in our study are briefly outlined below, with a full description of the model available in the supplemental materials of Ha and Sherman 2020 (Ha and Sherman, 2020; Sherman, 2022).
Eq. 2 describes change in glucose over time as a function of the glucose flux during the OGTT (OGTT), hepatic glucose production (HGP), insulin sensitivity (SI), the insulin-independent effectiveness of glucose (EG0), current glucose (G), and insulin (I). It is given as:
where HGP is a decreasing function of I and hepatic insulin sensitivity (hepaSI) and EG0 represents insulin-independent glucose uptake by peripheral tissues, here fixed at 0.0118 min−1.
Eqs. 3, 4 describe change in insulin over time as a function of β cell mass (β), volume of distribution (V), insulin secretion rate (ISR), and insulin (I). ISR is a function of calcium ion concentration in the β cell cytosol (CISR) and the number of primed insulin vesicles at the β cell membrane (N5). They are given as:
where k is a rate constant of insulin clearance. The precise form of CISR as a function of glucose (G) and ATP-dependent potassium ion channel (K+-ATP) density (γ) can be determined by expanding the equations derived from a slight modification of the steady state of the previously published exocytosis model (Chen et al., 2008).
Eq. 5 shows the change in γ, the density of the β cell membrane ATP-dependent potassium ion channel (K+-ATP), as an increasing function of glucose (G) on a scale of hours to days. This describes the shift in glucose-dependent insulin secretion in the setting of chronic hyperglycemia, where increases in channel density lead to decreases in insulin secretion. Because of the short time scale of the OGTT, γ was fixed at –0.076 as in prior work (Sherman, 2022) and this equation was not explicitly solved in our methods. It is provided here for clarity on how chronic hyperglycemia affects β cell structure and is given as:
where γ∞(G) is an increasing sigmoidal function of glucose and τγ is a time constant.
From the insulin exocytosis model, Eqs. 6, 7 describe the change in the number of vesicles in the β cell granule-membrane complex during docking and priming (N6 and N5, respectively) as functions of K+-ATP channel density (γ), glucose (G), maximal insulin secretion capacity (σ), and the baseline insulin vesicle priming rate, r20 (Chen et al., 2008). These equations are given as:
where Ci,j represents the cytosol calcium ion concentration at a given state in exocytosis: C5,5 is a function of G and γ; C5,6 and C6,6 are functions of G, γ, and r20; C6,0 is a function of G, γ, and σ, and; C6,5 is a constant.
Eq. 8 describes the change in the maximal insulin secretion capacity, σ, to compensate for chronic hyperglycemia on a scale of hours to days. It is a unitless scale factor. During the OGTT, σ is assumed to be at steady state. σ is a function of insulin secretion rate (ISR) and β cell metabolism (M), where σ increases with increases in ISR and decreases as M increases.
Parameters and non-estimated initial states were set according to their values in previous research (Ha and Sherman, 2020; Sherman, 2022). In experiments where measured insulin values were not included in the estimation optimization, I0 was set as 5.63 µIU/mL. The parameters estimated without using a patient's insulin values are denoted with an,
2.6 Estimating parameters using data assimilation
In our methods, we estimated two parameters via data assimilation, insulin sensitivity, SI, and maximal insulin secretion capacity, σ. Ordinary differential equations were solved using a Rosenbrock-W method (Rosenbrock23) (Rackauckas and Nie, 2017) within the bounds of [0.005, 3] for SI and [0.01, 10] for σ. The posterior distributions of the parameters and the product σ*SI were estimated based on a 500,000 iteration Random Walk Metropolis-Hastings MCMC (Hastings, 1970) chain (excluding a burn-in of 50,000 iterations), assuming a normal distribution error model and uniform priors. A decrease in autocorrelation approaching 0 was appreciated with increasing lags (k) near k = 500 to support chain convergence, and the number of iterations and burn-in were selected to be orders of magnitude larger than k (Roy, 2019). Acceptance rate varied with each patient between 0.2–0.5. Multiple independent chains for a subset of 10 patients provided confidence in the reproducibility of the estimates. The means and standard deviations of the parameters were calculated from the remaining 450,000 iterations (Ge et al., 2018) in an individual’s chain, and these summary statistics were included in logistic regression models.
2.7 Descriptive statistics
The majority of continuous features were not normally distributed, so non-parametric methods were used to calculate descriptive statistics on non-transformed data. These data are reported as medians with 95% confidence intervals around the median. The Mann-Whitney-U tests were used to compare continuous variables under these circumstances. χ2 or Fisher’s exact tests were used to compare categorical variables depending on the frequencies of the categories. Two-tailed Student’s t-tests were used to compare means and 95% confidence intervals of bootstrapped coefficient estimates and scoring metrics.
3 Results
3.1 Description of data
Out of 396 adolescents who underwent bariatric surgery, 248 had pre-operative OGTTs. Of 202 patients without any missing values in their pre-operative OGTT glucose and insulin measurements, 176 had follow-up within the appropriate time window and were further analyzed. All potential classification methods for impaired glucose metabolism (IGM) demonstrated class imbalance, with ∼35% (n = 56) of patients with meeting post-operative criteria for IGM as outlined above. Pre-operative characteristics by group are shown in Tables 2, 3. Using the same criteria to classify IGM post-operatively, 101 of the 176 (57.4%) met criteria for IGM pre-operatively. At baseline, patients who had higher probabilities of having IGM post-operatively were more insulin resistant as measured by HOMA-IR, had higher G30, G60, and G120 values, and had higher I120 values.
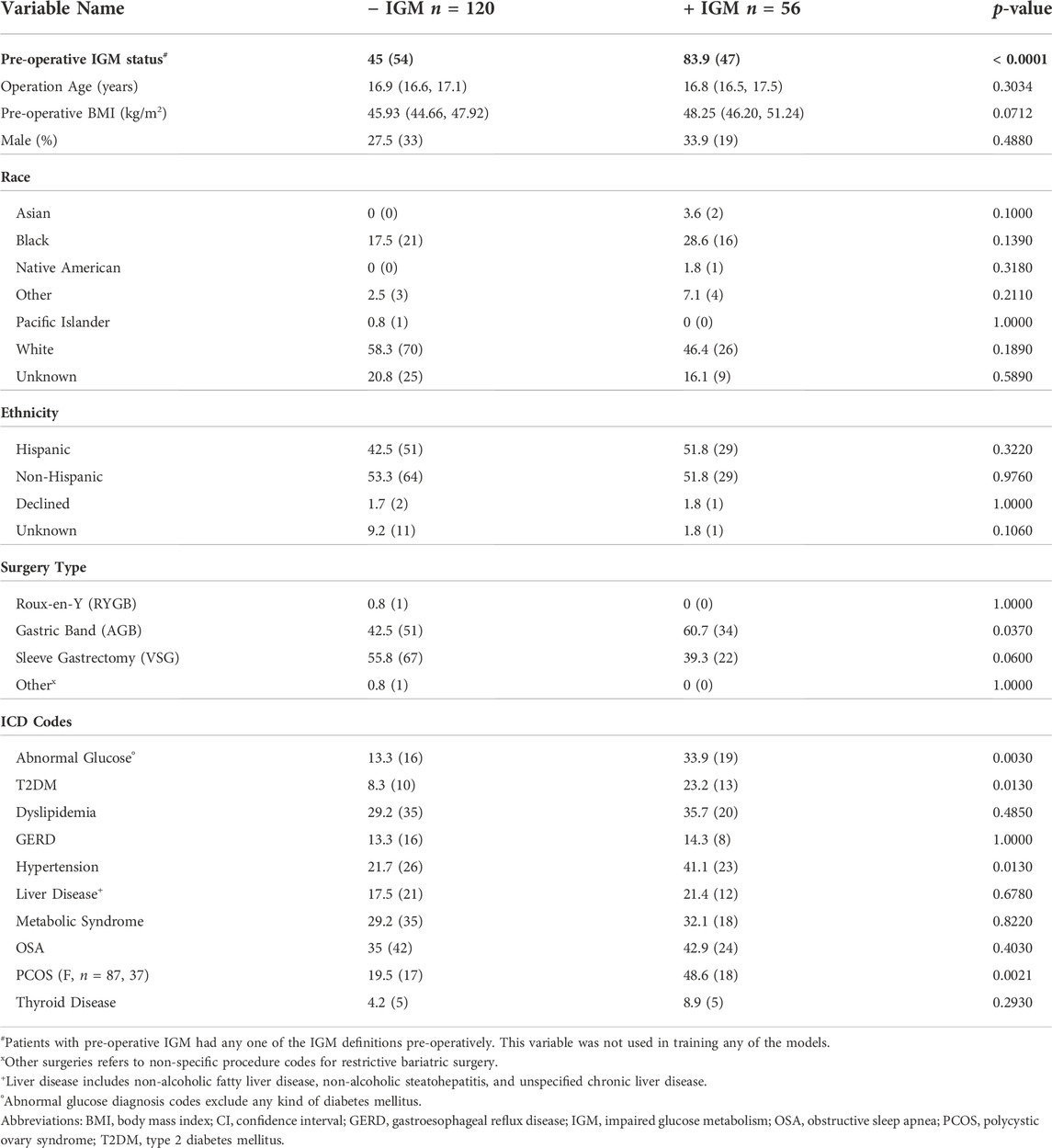
TABLE 2. Pre-operative demographic comparisons by binary post-operative outcome at 12 months. For continuous variables, medians with their 95% CI are shown. Categorical variables are shown as a percentage and count. Significant p-values for Mann-Whitney-U tests after Bonferroni correction are in bold (p < 0.001).
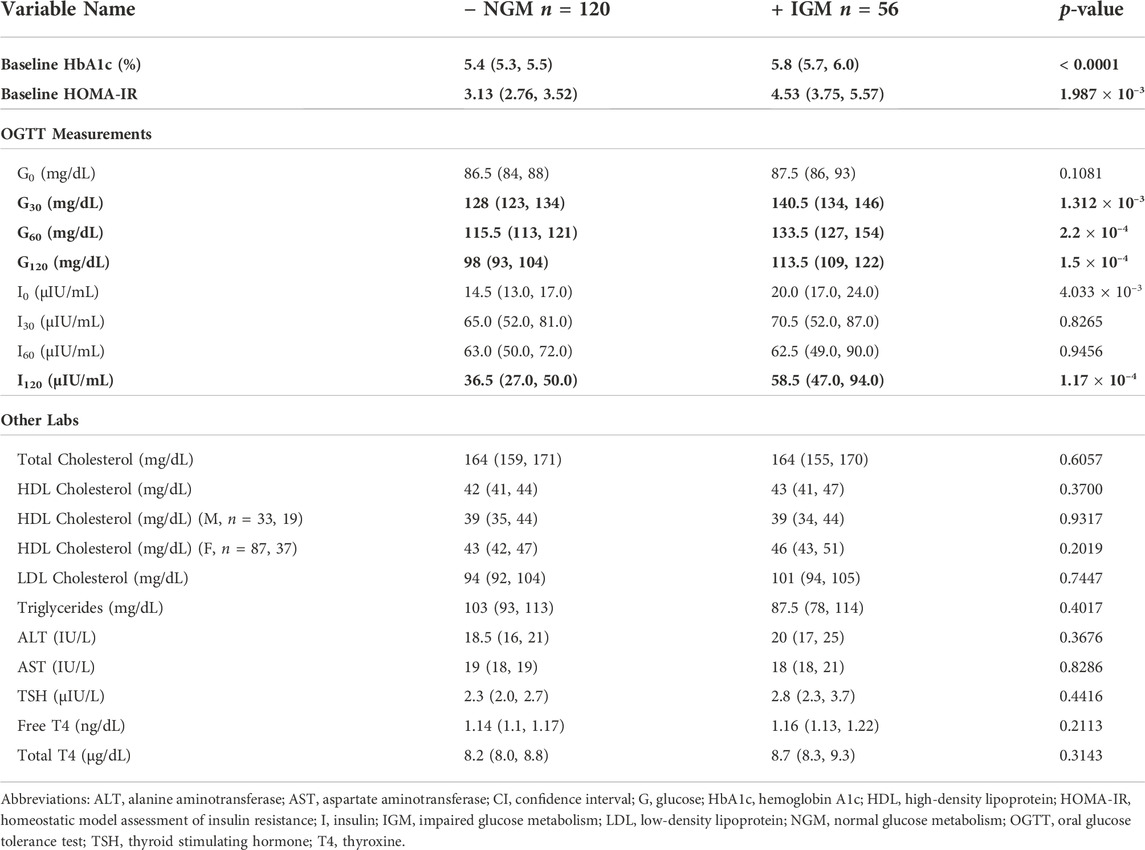
TABLE 3. Pre-operative laboratory value comparisons by binary post-operative outcome at 12 months. Medians with their 95% CI are shown. Significant p-values for Mann-Whitney-U tests after Bonferroni correction are in bold (p < 0.002).
A total of 202 patients met inclusion criteria, but 26 of them did not have follow-up within 6–18 months of surgery. One patient was lost to follow-up due to death within three months of surgery. The proportions of patients lost to follow-up were statistically different between those with pre-operative IGM (Fisher’s exact test p = 0.035): nine met two or more pre-operative IGM criteria (three with elevated G0, one with elevated G120, six with multiple elevated HbA1c values, one on anti-diabetic medication).
3.2 Data assimilation results
Parameter estimates from data assimilation are summarized in Table 4 and the probability distribution of their means across the cohort as estimated by kernel density estimation are shown in Figure 2. The post-operative + IGM group had statistically significantly lower baseline insulin sensitivity (SI) and maximal insulin secretion capacity (σ) values compared to the post-operative − IGM group, irrespective of inclusion of insulin in the data assimilation.
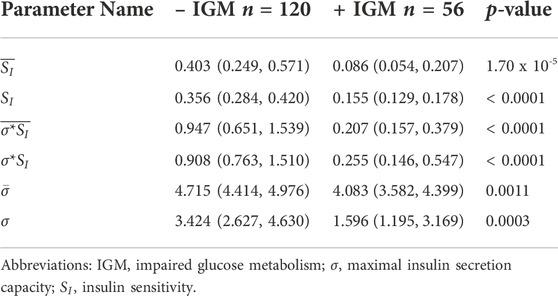
TABLE 4. Data assimilation-derived mechanistic model parameter estimate comparisons by binary post-operative outcome at 12 months. The parameters SI, σ, and σ*SI refer to the means of the posterior distributions for each patient. Overlined parameters were estimated without insulin values. For comparisons, medians and 95% confidence intervals of the median are shown. All comparisons had significant p-values for Mann-Whitney-U test after Bonferroni correction (p < 0.008).
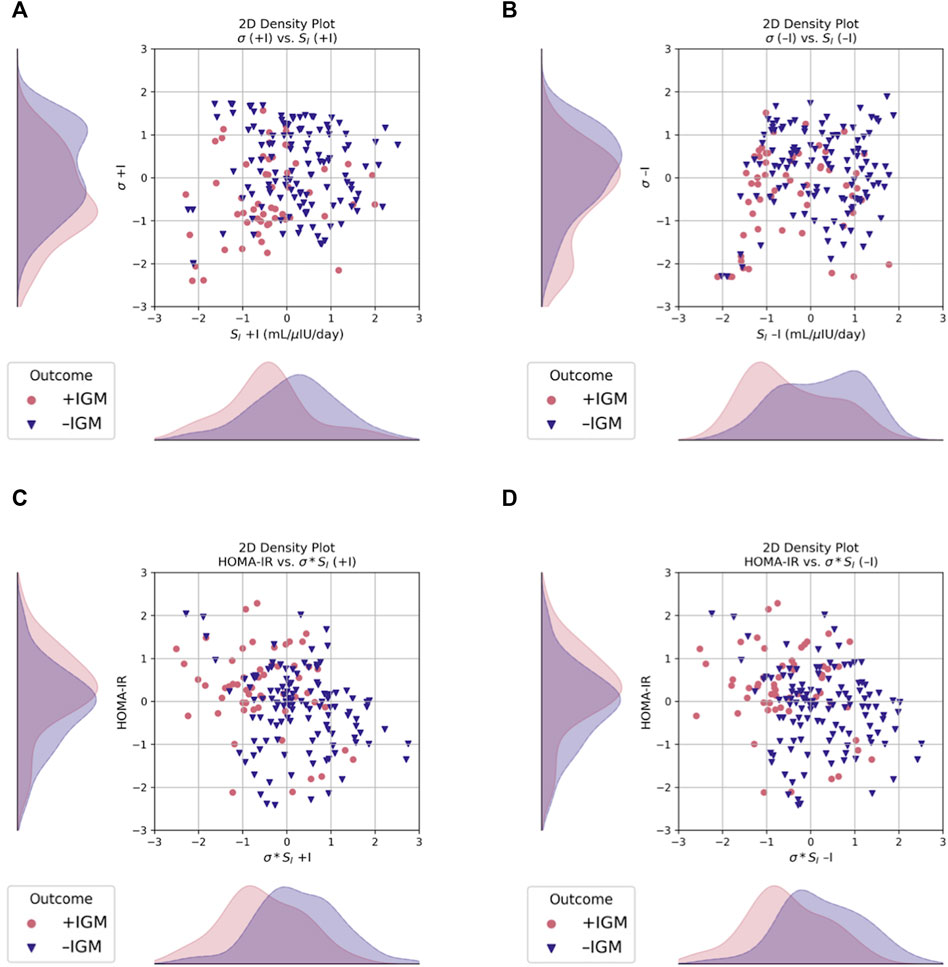
FIGURE 2. Scatter and 2D kernel density estimation plots, stratified by post-operative outcome at 12 months. Data are shown after Box-Cox transformation for visualization. (A) Incorporating measured insulin measurements in the data assimilation estimations increases separation of the estimated probability distributions of σ between groups, as compared to (B) without insulin, where SI is more separated but σ has more overlap. (C) σ*SI had better separation compared to HOMA-IR when estimated both with and (D) without insulin. Blue triangles, normal glucose metabolism (–IGM); red circles, impaired glucose metabolism (+IGM).
The marginal posterior densities of the parameters were estimated for each individual patient using MCMC. The marginal posterior density of SI was sharply peaked away from its bounds on manual inspection for a random subset of patients, giving us confidence in its mean estimator. The density for σ*SI was also sharply peaked away from its bounds (not shown).
3.3 Prediction model results
For the most complex model, data assimilation estimates were negatively correlated with the probability of having post-operative impaired glucose metabolism (IGM) at 12 months. Across models that contained them, HbA1c and I120 were positively correlated with the probability of having post-operative IGM at 12 months. However, the model coefficients were overall not significantly different from zero for all models (not shown). When ranked by magnitude, the largest coefficients, when included, were coefficients for HbA1c, I120, and G60.
Selected AUROC and average precision score comparisons are shown in Tables 5, 6, respectively.
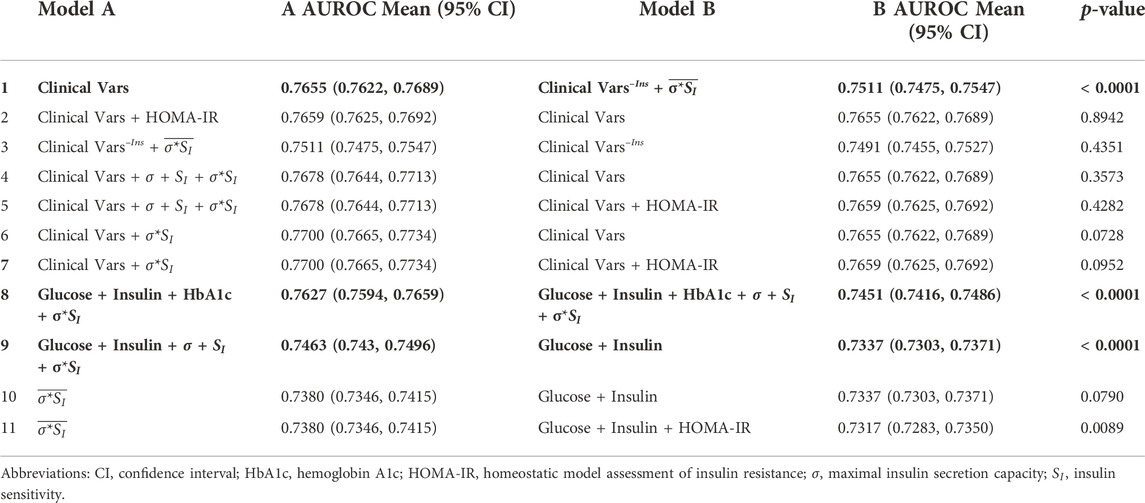
TABLE 5. AUROC comparisons between models trained on subsets of features with and without data assimilation. Models are presented in alphabetical order with the better performing model on the left (Model A). Clinical Vars refers to all clinical features in Tables 2, 3 except pre-operative IGM status and HOMA-IR. Clinical Vars–Ins refers to the same set of features in Clinical Vars after removing insulin measurements. Overlined parameters were estimated without insulin values. Significant p-values for two-tailed Student’s t-test after Bonferroni correction are shown in bold (p < 0.004).
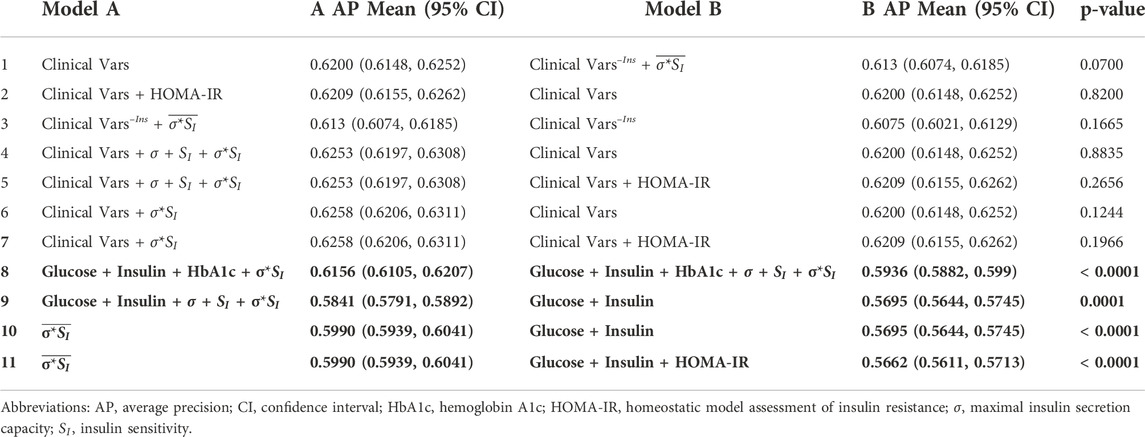
TABLE 6. Average precision comparisons between models trained on subsets of features with and without data assimilation. Models are presented in the same order as in Table 5. Clinical Vars refers to all clinical features in Tables 2, 3 except pre-operative IGM status and HOMA-IR. Clinical Vars–Ins refers to the same set of features in Clinical Vars after removing insulin measurements. Overlined parameters were estimated without insulin values. Significant p-values for two-tailed Student’s t-test after Bonferroni correction are shown in bold (p < 0.004).
The best performing model used all available clinical variables (n features = 43) and σ*SI with an AUROC of 0.77 (95% CI 0.7665, 0.7734) and average precision of 0.6258 (95% CI 0.6206, 0.6311). Our most comprehensive models using all clinical data had similar performances regardless of whether data assimilation estimates or HOMA-IR were included (Tables 5, 6, rows 1–7).
4 Discussion
4.1 Data assimilation estimates can add clinical information that improves prediction
Our best-performing model used the aforementioned clinical variables combined with the product of maximal insulin secretion capacity and insulin sensitivity, σ*SI, with an AUROC of 0.77 (95% CI 0.7665, 0.7734) and average precision of 0.6258 (95% CI 0.6206, 0.6311). This model was nominally better than one using clinical variables alone with an AUROC of 0.7655 (95% CI 0.7622, 0.7689) and average precision of 0.6200 (95% CI 0.6148, 0.6252), but the differences were not significant at p = 0.0728 and 0.1244, respectively (Tables 5, 6, row 6).
4.2 Data assimilation estimates can infer missing information encoded in insulin measurements
The comparability of our most comprehensive models suggests that the information added by data assimilation is captured in an extensive, but not exhaustive, clinical dataset. Embedded in the electronic health record (EHR) data was a powerful experiment where the effects of bariatric surgery could be thoroughly investigated. Models incorporating insulin, in general, outperformed models that did not include it. However, when insulin measurements are missing, data assimilation can add physiologic information that approaches the predictive ability of the full clinical dataset. For example, when using all other clinical features except insulin, the model using the product
Our models further suggest that σ*SI, even when estimated sans insulin, can represent the information within an OGTT using glucose and insulin. When compared, our model trained on only
4.3 The mechanistic models were validated using clinical data
The mechanistic models were able to be well estimated, achieving a stable solution with a unique minimum, using our clinical dataset. Furthermore, the mechanistic model output did not contradict nor add unvalidated information, in that the parameters estimated corresponded to variables associated with glucose and insulin metabolism, and not to other clinical variables (e.g., demographics, thyroid function).
4.4 Uncertainty of data assimilation estimates results in reduced performance in logistic regression
When included in the model, HbA1c and insulin measures (particularly I120) were frequently ranked as the most important predictors by magnitude. The improved prediction ability using A1c or insulin was not replicated by substituting them with data assimilation estimates not using insulin (not shown). While not a complete substitute for the information contained in HbA1c or insulin, when maximal insulin secretion capacity (σ), insulin sensitivity (SI), or σ*SI are estimated using insulin measurements, they still improve performance when added to models containing OGTT insulin (AUROC 0.7463 [0.743, 0.7496] vs. 0.7337 [0.7303, 0.7371], p < 0.0001; AP 0.5841 [0.5791, 0.5892] vs. 0.5695 [0.5644, 0.5745], p = 0.0001) (Tables 5, 6, row 9).
Although in most models the feature coefficients were not statistically different from zero (not shown), the standard deviation of maximal insulin secretion capacity (σ) is given more importance than the actual estimated parameters themselves and HbA1c in the models using all three data assimilation estimates. Furthermore, in the presence of insulin, regularization consistently shrinks the coefficient for insulin sensitivity (SI), whereas the coefficient for maximal insulin secretion capacity (σ) increases. The variance in σ's estimation and its overlapping distributions between groups compared to that of SI, even when estimated using insulin measurements, likely contributes to the loss of predictive power (Figure 2). Using σ*SI in lieu of σ and SI separately improves model performance (AUROC 0.7627 [0.7594, 0.7659] vs. 0.7451 [0.7416, 0.7486], p < 0.0001; AP 0.6156 [0.6105, 0.6207] vs. 0.5936 [0.5882, 0.599], p < 0.0001) (Tables 5, 6, row 8).
4.5 Disease subtypes can be described by parameter estimates
Differences in pre-operative insulin sensitivity (SI) and maximal insulin secretion capacity (σ) estimates were seen between outcome groups when estimated both with and without measured insulin (p < 0.05, Figure 2 and Table 4). Regardless of a patient’s pre-operative glycemic status, SI better distinguished those patients who would have post-operative impaired glucose metabolism (IGM) compared to either σ or σ*SI (Figure 2).
Those with post-operative IGM tended to have lower baseline SI and σ values compared to those without it, demonstrating that both may contribute to a patient’s disease, although not necessarily equally. Additionally, those with post-operative IGM had higher pre-operative HbA1c, G30, G60, and G120 and I120 values (p < 0.05, Table 3). This may reflect defects in second phase insulin secretion, which is associated with decreases in insulin secretion capacity (Ha and Sherman, 2020). Notably, σ*SI and SI values were not correlated with baseline BMI values, demonstrating a seeming disconnect between whole-body adiposity and insulin sensitivity (not shown). Other physiologic parameters that were not estimated using data assimilation in this study, such as insulin secretion rate (ISR) and hepatic insulin sensitivity (
4.6 Fixed mechanistic model parameters may differ between adolescents and adults
Not all parameters in the mechanistic models can be estimated simultaneously. All models have potential limitations in generalizability beyond the populations studied during initial development. Use of fixed parameters with values derived from clinical studies in adults may not optimally estimate parameters during pubertal states, where hormonal crosstalk greatly influences energy homeostasis. For example, adolescence is marked by a drastic decrease in insulin sensitivity independent of adiposity; euglycemia is achieved by a compensatory and proportional increase in insulin secretion (Hannon et al., 2006). Insulin secretion rates will therefore be elevated in this age group compared to their adult counterparts, and estimated parameter bounds may differ considerably.
Other parameters which may be relatively constant in adulthood might be more dynamic in adolescents. Obesity and age impact β cell mass and proliferation, which were set as fixed components of these mechanistic models (Saisho et al., 2013; Michaliszyn et al., 2014). The fasting value of γ, representing the K+-ATP channel density on the β cells, was set to its example value of –0.076 (Sherman, 2022). However, γ plays an important role in regulating β cell physiology and glucose-mediated insulin secretion. Not properly tuning the fasting value of γ may adversely affect the estimates of the parameters regulating β cell physiology such as maximal insulin secretion capacity (σ) and insulin priming rate (r20). These effects likely reduced the prediction capabilities of our estimates.
Finally, the assumption that estimates based on the 120-min-OGTT approximate parameters as they would be estimated by a hyperinsulinemic euglycemic clamp may be violated in adolescents. Recently, validation of the oral minimal model in adolescents showed that the 120-min-OGTT underestimates insulin sensitivity compared to longer OGTTs, which is not the case for adults (Bartlette et al., 2021). Complicating this suboptimal approximation is the erratic behavior of OGTT measurements, even in euglycemic patients. Glucose and insulin assays can be imprecise, particularly in periods where glucose and insulin are changing rapidly (i.e., after a meal), and removing spurious OGTT measurements can improve performance (Abohtyra et al., 2022).
4.7 Binary classification of outcomes obscures a heterogenous population
Because of the relatively rare frequency of overt diabetes pre-operatively and varying sensitivity of common measurements in adolescents, the post-operative glucose metabolism outcome was coded as a binary variable: normal glucose metabolism (NGM) or impaired glucose metabolism (IGM). However, there are likely multiple sub-phenotypes present in these groups, which would negatively impact prediction models.
Though the diagnostic threshold for T2DM is HbA1c ≥ 6.5%, we used HbA1c ≥ 5.7%, the threshold for preDM, to indicate any IGM to improve sensitivity for detecting T2DM in adolescents (Nowicka et al., 2011). The exact interpretation of HbA1c must be considered along with an individual’s hemoglobin concentration and structure (Radin, 2014). In particular, studies have shown that traditional HbA1c thresholds for T2DM (≥ 6.5%) are imperfect for diagnosis in adults and may significantly underestimate the prevalence of T2DM in adolescents (Nowicka et al., 2011; American Diabetes Association Professional Practice Committee Draznin et al., 2022). Furthermore, given the frequency of anemia in bariatric surgery patients, HbA1c may misrepresent average blood glucose levels and thus, normal values are insufficient to exclude IGM phenotypes. As such, HbA1c was used as a feature in a logistic regression rather than as the outcome in a linear regression.
All patients were included for analysis regardless of pre-operative IGM status. Notably, neither hyperinsulinism nor insulin resistance were used to code IGM. As insulin resistance is tightly coupled with visceral adiposity, the likelihood that all patients had insulin resistance as the only manifestation of their IGM phenotype is high; this is supported by the lower SI values and elevated HOMA-IR calculations of patients at baseline (Kahn and Flier, 2000; Stern et al., 2005). Nonetheless, the decision to focus on glucose rather than insulin perturbations in outcome labeling likely impaired the ability of our models to identify those with IGM-like phenotypes post-operatively, particularly as the outcome is not granular enough to distinguish this heterogenous population.
4.8 Limitations
4.8.1 Small sample size
Our methods were hindered by a small, imbalanced, and homogenous sample from a single institution. In addition to increasing sample size by using incomplete OGTT data, future work could address class imbalance by under sampling the majority class or removing redundant information. Our sample size relative to features was exacerbated by patient attrition, likely not at random.
4.8.2 Bias in clinical measures
Our cohort is more diverse with respect to ethnicity and race compared to previously reported studies, which may impact the ability of a model to predict outcomes when using estimators such as HbA1c. Race differences in outcomes have been seen in other studies and have been attributed to poor calibration of models or measurements across heterogenous populations (Wallace et al., 2004; Olson et al., 2010; Tharakan et al., 2017). However, race differences between Black and white adolescents have also been noted in hyperinsulinemic-euglycemic clamp studies, so the true difference in T2DM development is still unclear (Michaliszyn et al., 2017).
4.8.3 Complexity and validation of electronic health records
Our study did not occur in the context of a clinical trial and is subject to the constraints of EHR data. The most effective use of EHR data applies knowledge of how data are inputted into the system while also understanding the underlying medical decision making process. Improper automatic encoding of features or outcomes could have negatively impacted our models’ predictive abilities. In the case of our analyses, features such comorbidities and drug information are less reliable than laboratory values, and awareness of missing data is not guaranteed—that is, the EHR datasets are not complete (Weiskopf et al., 2013). For example, formal diagnoses of obesity-related liver diseases (i.e., NAFLD and NASH) use a liver biopsy to confirm pathology, which can be done while a patient is undergoing bariatric surgery. However, the diagnosis codes for suspected liver disease may not be reflected in the EHR, and if they are inputted, they are done so irregularly. This was the case in our patient sample, where many of the comorbidities were mentioned within the patient assessment and/or radiology impressions of notes, but not listed as a visit-associated diagnosis code frequently enough to be marked as present by our criteria. Relatedly, medication data are sparse and often inaccurate, with unreliable start and stop dates. Diet information, which is undoubtedly important in this context, is not typically represented at all in structured datasets. Information about mental health and psychiatric comorbidities is intentionally difficult to access for secondary use, and social stigmas surrounding mental health reduce confidence that, absent documentation, no comorbidities are present. Similarly, social determinants of health such as food insecurity, exposure to discrimination, and exposure to adverse childhood events are not captured in this dataset. Compounding these limitations are existing health disparities in access bariatric surgery, leading to selection bias (Tsui et al., 2021).
Manual chart review is the gold standard for extracting clinical information, but this is time intensive and subject to human error. It also cannot account for truly missing information. As this relates to our methods, to avoid misclassifying patients with post-operative IGM, we erred on the side of underestimating the proportion of patients with specific comorbidities by requiring at least 25% of encounters to contain the relevant diagnosis codes and structured documentation of medications. This may have classified patients as not having IGM post-operatively when in fact they did have some impaired metabolism.
4.8.4 Limitations related to the use of mechanistic models
The use of mechanistic models and oridnary differential equations inherently limits the number of parameters that can be estimated concurrently. Combined with the limitations related to our retrospective, observational analysis, we are limited in our ability to verify the estimated values with respect to a patient’s true physiology. There are several physiologic estimates from data assimilation that could be of use clinically, such as pre-hepatic insulin secretion rate (ISR) or hepatic insulin sensitivity (
Related to the limitations of mechanistic models, our predictive models did not incorporate estimates of hepatic insulin sensitivity (
Also absent in these models are the effects of circulating incretins, i.e., glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide or gastric inhibitory polypeptide (GIP). These gut-secreted, insulinotropic hormones are implicated as one potential therapeutic mechanism of action in metabolic surgeries through their actions on insulin secretion and hepatic insulin clearance (Fetner et al., 2005; Hutch and Sandoval, 2017). Supporting this is the general success of novel classes of anti-diabetic drugs leveraging GLP-1 receptor agonists to manage T2DM and obesity in adults and adolescents (Kelly et al., 2020). However, GLP-1 and GIP are not directly represented in the mechanistic models we used, nor is it currently feasible to directly measure their levels in an outpatient clinical setting. Changes in incretins are indirectly reflected in changes in maximal insulin secretion capacity, σ, where increases in GLP-1 lead to increases in σ through the exocytosis model (Ha and Sherman, 2020). If these mechanistic models are employed to predict future physiologic states on a longer time scale, it is critical to include models which incorporate incretin effects to better model post-surgical physiology.
4.9 Future directions
Our model could not capture all features to confidently predict post-surgical glycemic states as a dichotomous outcome despite an extensive collection of laboratory data. We do not believe that these estimates should be broadly disseminated to preclude or exclude patients from receiving indicated care. Rather, with future validation, parameters like σ and SI could inform expectations with respect to potential outcomes. Patients intending to have their prediabetic or diabetic states completely reversed should be informed of the possibility that they may not completely resolve with surgery alone. Quantifying that uncertainty may be accomplished using models like the ones described here. By providing more informed consent, we hope that more patients will be able to have meaningful discussions with their care teams to improve long-term surgical outcomes.
Future studies can incorporate using more longitudinal data to see the trends in insulin secretion capacity (σ) and insulin sensitivity (SI) in the pre-, peri-, and post-operative periods. Applying more granular outcome definitions on a continuous scale may better capture patients who might improve in the severity of their disease, but not sufficiently to resolve impaired glucose metabolism. Alternatively, unsupervised machine learning methods could be applied on a larger cohort of patients to identify different pre-operative phenotypes.
Investigation into additional features that may improve predictive performance can also inform future work. Focusing on the direct effects of surgery itself may provide more insight into patient outcomes, especially when certain surgeries (e.g., sleeve gastrectomy or RYGB) have larger metabolic impacts as compared to relatively metabolically modest restrictive procedures like gastric banding. Using models that directly incorporate the effects of GLP-1 and its secretion in response to glucose ingestion, such as that developed by De Gaetano et al., (De Gaetano et al., 2013) should be included in future work, as should assessment of the change in GLP-1 secretion patterns post-operatively. Prospective studies with larger sample sizes or measurement of more stable insulin byproducts such as C-peptide during OGTTs could improve model performance both by adding a more specific feature and through improvement of data assimilation estimates. Manual chart review can provide information about pre- and post-operative anthropometrics to better quantify adiposity. Alternatively, methods to estimate fat-free body mass using more readily available clinical data could be explored. Inclusion of other candidate biomarkers associated with glucose and insulin metabolism such as incretins, growth factors, inflammatory markers, and carrier proteins could be added. Variables related to behaviors (including diet), mental health, and social determinants of health should also be included in future studies attempting prediction in this same population.
This research is a starting point for further investigation into the use of mechanistic models and data assimilation applied to clinical problems. In addition to application of similar techniques to clinical problems outside of prediction, research focusing on solving strategies with sparse or irregularly sampled clinical data could provide robust and reliable methods for future studies.
5 Conclusion
We demonstrate that data assimilation captures predictive information about glucose metabolism that is not readily apparent from OGTT measurements alone. Further, we validated that our chosen mechanistic model does not add any additional information than it is meant to represent. The clinical variables combined with the product of maximal insulin secretion capacity and insulin sensitivity, σ*S1, produced the best-performing model with AUROC = 0.77 and average precision = 0.6258. This model was nominally better than one using clinical variables alone with AUROC = 0.7655, but the difference was not significant at p = 0.07. In some cases, using the individual components of insulin secretion capacity (σ) and insulin sensitivity (SI) along with their product reduced prediction model performance.
Looking at whether insulin measurement can be replaced by data assimilation, we found that the model using clinical variables with insulin (AUROC = 0.7655) performed better than the models using clinical variables without insulin but combined with
If we limit our model inputs to OGTT glucose and insulin values, we found adding data assimilation estimates of insulin secretion capacity and insulin sensitivity (σ, SI, and σ*S) significantly increased performance (p < 0.001). Models using
While data assimilation alone does not significantly improve prediction ability compared to a maximal dataset, the separation of parameter distributions may provide insight into how underlying physiologic processes contribute to a patient’s disease. In this adolescent cohort, low insulin sensitivity and low maximal insulin secretion capacity distinguish those patients who are less likely to see glycemic benefits from bariatric surgery. While knowing the extent to which defects in glucose-insulin metabolism contribute to disease is not sufficient to confidently predict surgical outcomes, future research can leverage mechanistic models to infer a patient’s physiology even when certain data are absent.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
LR, IF, BA, DA, and GH contributed to the conception and design of the study. LR and AO extracted structured and unstructured clinical data from the EHR. IF and JZ provided clinical care for patients in the Center for Adolescent Bariatric Surgery. LR performed manual chart review where necessary. LR cleaned and pre-processed data. BA and DA coded the mechanistic models, and BA performed MCMC fitting. LR and LZ developed the logistic regression models. LR wrote the first draft of the manuscript. BA, LZ, DA, and GH wrote sections of the manuscript. All others contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by NIH Grant Nos. R01 LM006910 (GH, LZ, AO), R01 LM012734 (GH and DA), and T15 LM007079 (LR and BA).
Acknowledgments
We acknowledge the excellent support, guidance, and advice from Arthur S. Sherman and Joon Ha to ensure sound use and encoding of their models in this clinical application.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor CDB declared a past co-authorship with authors DA and GH.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abohtyra R. M., Chan C. L., Albers D. J., Gluckman B. J. (2022). Inferring insulin secretion rate from sparse patient glucose and insulin measures. Endocrinol. Incl. Diabetes Mellitus Metabolic Dis. doi:10.1101/2022.03.10.22272234
Akalestou E., Miras A. D., Rutter G. A., le Roux C. W. (2022). Mechanisms of weight loss after obesity surgery. Endocr. Rev. 43, 19–34. doi:10.1210/endrev/bnab022
Albers D. J., Blancquart P-A., Levine M. E., Seylabi E. E., Stuart A. (2019). Ensemble kalman methods with constraints. Inverse Probl. 35, 095007. doi:10.1088/1361-6420/ab1c09
Albers D. J., Levine M., Gluckman B., Ginsberg H., Hripcsak G., Mamykina L. (2017). Personalized glucose forecasting for type 2 diabetes using data assimilation. PLoS Comput. Biol. 13, e1005232. doi:10.1371/journal.pcbi.1005232
American Diabetes Association Professional Practice Committee Draznin B., Aroda V. R., Bakris G., Benson G., Brown F. M. (2022). 14. Children and adolescents: Standards of medical Care in diabetes—2022. Diabetes Care 45, S208–S231. doi:10.2337/dc22-S014
American Diabetes Association Professional Practice Committee (2022). 2. Classification and diagnosis of diabetes: Standards of medical Care in diabetes—2022. Diabetes Care 45, S17–S38. doi:10.2337/dc22-S002
Aminian A., Brethauer S. A., Andalib A., Nowacki A. S., Jimenez A., Corcelles R., et al. (2017). Individualized metabolic surgery score: Procedure selection based on diabetes severity. Ann. Surg. 266, 650–657. doi:10.1097/SLA.0000000000002407
Armstrong S. C., Bolling C. F., Michalsky M. P., Reichard K. W. (2019). Pediatric metabolic and bariatric surgery: Evidence, barriers, and best practices. Pediatrics 144, e20193223. doi:10.1542/peds.2019-3223
Arslanian S., El ghormli L., Young Kim J., Bacha F., Chan C., Ismail H. M., et al. (2019). The shape of the glucose response curve during an oral glucose tolerance test: Forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care 42, 164–172. doi:10.2337/dc18-1122
Aung L., Lee W-J., Chen S. C., Ser K. H., Wu C. C., Chong K., et al. (2016). Bariatric surgery for patients with early-onset vs late-onset type 2 diabetes. JAMA Surg. 151, 798–805. doi:10.1001/jamasurg.2016.1130
Bal B. S., Finelli F. C., Shope T. R., Koch T. R. (2012). Nutritional deficiencies after bariatric surgery. Nat. Rev. Endocrinol. 8, 544–556. doi:10.1038/nrendo.2012.48
Bartlette K., Carreau A-M., Xie D., Garcia-Reyes Y., Rahat H., Pyle L., et al. (2021). Oral minimal model-based estimates of insulin sensitivity in obese youth depend on oral glucose tolerance test protocol duration. Metabol. Open 9, 100078. doi:10.1016/j.metop.2021.100078
Bartolomé A., Suda N., Yu J., Zhu C., Son J., Ding H., et al. (2022). Notch-mediated Ephrin signaling disrupts islet architecture and β cell function. JCI Insight 7, e157694. doi:10.1172/jci.insight.157694
Beamish A. J., Reinehr T. (2017). Should bariatric surgery be performed in adolescents? Eur. J. Endocrinol. 176, D1-D15. D1–D15. doi:10.1530/EJE-16-0906
Bergman R. N., Prager R., Volund A., Olefsky J. M. (1987). Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J. Clin. Investig. 79, 790–800. doi:10.1172/JCI112886
Bolling C. F., Armstrong S. C., Reichard K. W., Michalsky M. P. (2019). Metabolic and bariatric surgery for pediatric patients with severe obesity. Pediatrics 144, e20193224. doi:10.1542/peds.2019-3224
Box G. E. P., Cox D. R. (1964). An analysis of transformations. J. R. Stat. Soc. Ser. B 26, 211–243. doi:10.1111/j.2517-6161.1964.tb00553.x
Brown R. J., Yanovski J. A. (2014). Estimation of insulin sensitivity in children: Methods, measures and controversies. Pediatr. Diabetes 15, 151–161. doi:10.1111/pedi.12146
Bryant M., Ashton L., Brown J., Jebb S., Wright J., Roberts K., et al. (2014). Systematic review to identify and appraise outcome measures used to evaluate childhood obesity treatment interventions (CoOR): Evidence of purpose, application, validity, reliability and sensitivity. Health Technol. Assess. 18, 1–380. doi:10.3310/hta18510
Buck S. F. (1960). A method of estimation of missing values in multivariate data suitable for use with an electronic computer. J. R. Stat. Soc. Ser. B 22, 302–306. doi:10.1111/j.2517-6161.1960.tb00375.x
Buse J. B., Caprio S., Cefalu W. T., Ceriello A., Del Prato S., Inzucchi S. E., et al. (2009). How do we define cure of diabetes? Diabetes Care 32, 2133–2135. doi:10.2337/dc09-9036
Buse J. B., Kaufman F. R., Linder B., Hirst K., El Ghormli L., Willi S., et al. (2013). Diabetes screening with hemoglobin A1c versus fasting plasma glucose in a multiethnic middle-school cohort. Diabetes Care 36, 429–435. doi:10.2337/dc12-0295
Buuren S. van, Groothuis-Oudshoorn K. (2011). Mice : Multivariate imputation by chained equations in R. J. Stat. Softw. 45. doi:10.18637/jss.v045.i03
Cao Y., Näslund I., Näslund E., Ottosson J., Montgomery S., Stenberg E. (2020). Using convolutional neural network to predict remission of diabetes after gastric bypass surgery: A machine learning study from the scandinavian obesity surgery register. Endocrinol. Incl. Diabetes Mellitus Metabolic Dis. doi:10.1101/2020.11.03.20224956
Cauter E. V., Mestrez F., Sturis J., Polonsky K. S. (1992). Estimation of insulin secretion rates from C-peptide levels: Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41, 368–377. doi:10.2337/diab.41.3.368
Chen Y., Wang S., Sherman A. (2008). Identifying the targets of the amplifying pathway for insulin secretion in pancreatic β-cells by kinetic modeling of granule exocytosis. Biophys. J. 95, 2226–2241. doi:10.1529/biophysj.107.124990
Chung S. T., Katz L. E. L., Stettler-Davis N., Shults J., Sherman A., Ha J., et al. (2022). The relationship between lipoproteins and insulin sensitivity in youth with obesity and abnormal glucose tolerance. J. Clin. Endocrinol. Metabolism 107, 1541–1551. doi:10.1210/clinem/dgac113
Cobelli C., Dalla Man C., Toffolo G., Basu R., Vella A., Rizza R. (2014). The oral minimal model method. Diabetes 63, 1203–1213. doi:10.2337/db13-1198
Courcoulas A. P., Christian N. J., Belle S. H., Berk P. D., Flum D. R., Garcia L., et al. (2013). Weight change and health outcomes at 3 Years after bariatric surgery among individuals with severe obesity. JAMA 310, 2416–2425. doi:10.1001/jama.2013.280928
Courcoulas A. P., Goodpaster B. H., Eagleton J. K., Belle S. H., Kalarchian M. A., Lang W., et al. (2014). Surgical vs medical treatments for type 2 diabetes mellitus: A randomized clinical trial. JAMA Surg. 149, 707–715. doi:10.1001/jamasurg.2014.467
Crossan K., Sheer A. J. (2022). “Surgical options in the treatment of severe obesity,” in StatPearls. StatPearls publishing: Treasure island, FL. Available at: https://www.ncbi.nlm.nih.gov/books/NBK576372/(accessed Sep 13 2022).
Dabelea D., Mayer-Davis E. J., Saydah S., Imperatore G., Linder B., Divers J., et al. (2014). Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311, 1778–1786. doi:10.1001/jama.2014.3201
Dalla Man C., Rizza R. A., Cobelli C. (2007). Meal simulation model of the glucose-insulin system. IEEE Trans. Biomed. Eng. 54, 1740–1749. doi:10.1109/TBME.2007.893506
De Gaetano A., Panunzi S., Matone A., Samson A., Vrbikova J., Bendlova B., et al. (2013). Routine OGTT: A robust model including incretin effect for precise identification of insulin sensitivity and secretion in a single individual. PLoS ONE 8, e70875. doi:10.1371/journal.pone.0070875
DeMaria E. J., Portenier D., Wolfe L. (2007). Obesity surgery mortality risk score: Proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surg. Obes. Relat. Dis. 3, 134–140. doi:10.1016/j.soard.2007.01.005
Dewberry L. C., Niemiec S. M., Hilton S. A., Louiselle A. E., Singh S., Sakthivel T. S., et al. (2022). Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomedicine. 40, 102483. doi:10.1016/j.nano.2021.102483
Dixon J. B., Chuang L-M., Chong K., Chen S. C., Lambert G. W., Straznicky N. E., et al. (2013). Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 36, 20–26. doi:10.2337/dc12-0779
Elbahrawy A., Bougie A., Loiselle S-E., Demyttenaere S., Court O., Andalib A. (2018). Medium to long-term outcomes of bariatric surgery in older adults with super obesity. Surg. Obes. Relat. Dis. 14, 470–476. doi:10.1016/j.soard.2017.11.008
Evensen G. (2009). Data assimilation: The ensemble kalman filter. Berlin, Heidelberg: Springer Berlin Heidelberg Springer e-books.
Fetner R., McGinty J., Russell C., Pi-Sunyer F. X., Laferrère B. (2005). Incretins, diabetes, and bariatric surgery: A review. Surg. Obes. Relat. Dis. 1, 589–597. doi:10.1016/j.soard.2005.09.001
Ge H., Xu K., Ghahramani Z. (2018). Turing: A language for flexible probabilistic inference. In Int. Conf. Artif. Intell. Statistics, 9-11. Spain: AISTATS, 1682–1690.
Gunczler P., Lanes R. (2006). Relationship between different fasting-based insulin sensitivity indices in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 19, 259–265. doi:10.1515/JPEM.2006.19.3.259
Gutch M., Kumar S., Razi S. M., Gupta K. K., Gupta A. (2015). Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 19, 160–164. doi:10.4103/2230-8210.146874
Ha J., Satin L. S., Sherman A. S. (2016). A mathematical model of the pathogenesis, prevention, and reversal of type 2 diabetes. Endocrinology 157, 624–635. doi:10.1210/en.2015-1564
Ha J., Sherman A. (2020). Type 2 diabetes: One disease, many pathways. Am. J. Physiol. Endocrinol. Metab. 319, E410-E426–E426. doi:10.1152/ajpendo.00512.2019
Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L. (2017h). Prevalence of obesity among adults and youth: United States, 2015–2016. Hyattsville, MD: National Center for Health Statistics.//www.cdc.gov/nchs/data/databriefs/db288.pdf.
Hannon T. S., Janosky J., Arslanian S. A. (2006). Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. 60, 759–763. doi:10.1203/01.pdr.0000246097.73031.27
Hastings W. K. (1970). Monte Carlo sampling methods using Markov chains and their applications. Biometrika 57, 97–109. doi:10.1093/biomet/57.1.97
Hatoum I. J., Greenawalt D. M., Cotsapas C., Reitman M. L., Daly M. J., Kaplan L. M. (2011). Heritability of the weight loss response to gastric bypass surgery. J. Clin. Endocrinol. Metab. 96, E1630–E1633. doi:10.1210/jc.2011-1130
Holst J. J., Madsbad S. (2021). What is diabetes remission? Diabetes Ther. 12, 641–646. doi:10.1007/s13300-021-01032-y
Hsia D. S., Fallon S. C., Brandt M. L. (2012). Adolescent bariatric surgery. Arch. Pediatr. Adolesc. Med. 166, 757–766. doi:10.1001/archpediatrics.2012.1011
Hutch C. R., Sandoval D. (2017). The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology 158, 4139–4151. doi:10.1210/en.2017-00564
Inge T. H., Coley R. Y., Bazzano L. A., Xanthakos S. A., McTigue K., Arterburn D., et al. (2018). Comparative effectiveness of bariatric procedures among adolescents: The PCORnet bariatric study. Surg. Obes. Relat. Dis. 14, 1374–1386. doi:10.1016/j.soard.2018.04.002
Inge T. H., Courcoulas A. P., Jenkins T. M., Michalsky M. P., Brandt M. L., Xanthakos S. A., et al. (2019). Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med. 380, 2136–2145. doi:10.1056/NEJMoa1813909
Inge T. H., Courcoulas A. P., Jenkins T. M., Michalsky M. P., Helmrath M. A., Brandt M. L., et al. (2016). Weight loss and health status 3 Years after bariatric surgery in adolescents. N. Engl. J. Med. 374, 113–123. doi:10.1056/NEJMoa1506699
Inge T. H., Jenkins T. M., Xanthakos S. A., Dixon J. B., Daniels S. R., Zeller M. H., et al. (2017). Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): A prospective follow-up analysis. Lancet. Diabetes Endocrinol. 5, 165–173. doi:10.1016/S2213-8587(16)30315-1
Inge T. H., Laffel L. M., Jenkins T. M., Marcus M. D., Leibel N. I., Brandt M. L., et al. (2018). Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 172, 452–460. doi:10.1001/jamapediatrics.2017.5763
Inge T. H., Zeller M. H., Jenkins T. M., Helmrath M., Brandt M. L., Michalsky M. P., et al. (2014). Perioperative outcomes of adolescents undergoing bariatric surgery: The teen–longitudinal assessment of bariatric surgery (Teen-LABS) study. JAMA Pediatr. 168, 47–53. doi:10.1001/jamapediatrics.2013.4296
Julier S. J., Uhlmann J. K. (2004). Unscented filtering and nonlinear estimation. Proc. IEEE 92, 401–422. doi:10.1109/jproc.2003.823141
Kahn B. B., Flier J. S. (2000). Obesity and insulin resistance. J. Clin. Investig. 106, 473–481. doi:10.1172/JCI10842
Kalman R. E. (1960). A new approach to linear filtering and prediction problems. J. Basic Eng. 82, 35–45. doi:10.1115/1.3662552
Kam H., Tu Y., Pan J., Han J., Zhang P., Bao Y., et al. (2020). Comparison of four risk prediction models for diabetes remission after roux-en-Y gastric bypass surgery in obese Chinese patients with type 2 diabetes mellitus. Obes. Surg. 30, 2147–2157. doi:10.1007/s11695-019-04371-9
Karasko D. (2019). Weight loss in adolescents after bariatric surgery: A systematic review. J. Pediatr. Health Care 33, 26–34. doi:10.1016/j.pedhc.2018.05.010
Keidar A. (2011). Bariatric surgery for type 2 diabetes reversal: The risks. Diabetes Care 34, S361–S266. doi:10.2337/dc11-s254
Kelly A. S., Auerbach P., Barrientos-Perez M., Gies I., Hale P. M., Marcus C., et al. (2020). A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128. doi:10.1056/NEJMoa1916038
Khattab A., Sperling M. A. (2019). Obesity in adolescents and youth: The case for and against bariatric surgery. J. Pediatr. 207, 18–22. doi:10.1016/j.jpeds.2018.11.058
Kim J. Y., Tfayli H., Bacha F., Lee S., Michaliszyn S. F., Yousuf S., et al. (2020). β-cell function, incretin response, and insulin sensitivity of glucose and fat metabolism in obese youth: Relationship to OGTT-time-to-glucose-peak. Pediatr. Diabetes 21, 18–27. doi:10.1111/pedi.12940
Kjems L. L., Christiansen E., Vølund A., Bergman R. N., Madsbad S., Volund A. (2000). Validation of methods for measurement of insulin secretion in humans in vivo. Diabetes 49, 580–588. doi:10.2337/diabetes.49.4.580
Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., et al. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403. doi:10.1056/NEJMoa012512
Laferrère B., Pattou F. (2018). Weight-independent mechanisms of glucose control after roux-en-Y gastric bypass. Front. Endocrinol. 9, 530. doi:10.3389/fendo.2018.00530
Lamoshi A., Chernoguz A., Harmon C. M., Helmrath M. (2020). Complications of bariatric surgery in adolescents. Semin. Pediatr. Surg. 29, 150888. doi:10.1016/j.sempedsurg.2020.150888
Law K. J. H., Stuart A. M., Zygalakis K. C. (2015). Data assimilation: A mathematical introduction. arXiv:150607825 [math, stat]Available at: http://arxiv.org/abs/1506.07825 (accessed Mar 15, 2022).
Lee J. M. (2007). Insulin resistance in children and adolescents. Rev. Endocr. Metab. Disord. 7, 141–147. doi:10.1007/s11154-006-9019-8
Lee J. M., Okumura M. J., Davis M. M., Herman W. H., Gurney J. G. (2006). Prevalence and determinants of insulin resistance among U.S. Adolescents: A population-based study. Diabetes Care 29, 2427–2432. doi:10.2337/dc06-0709
Levine M. E., Hripcsak G., Mamykina L., Stuart A., Albers D. J. (2017). Offline and online data assimilation for real-time blood glucose forecasting in type 2 diabetes. arXiv:170900163 [math, q-bio]Available at: http://arxiv.org/abs/1709.00163 (accessed Mar 15, 2022).
Lim E. L., Hollingsworth K. G., Aribisala B. S., Chen M. J., Mathers J. C., TayloR R. (2011). Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54, 2506–2514. doi:10.1007/s00125-011-2204-7
Little R. R., Rohlfing C. L., Tennill A. L., Madsen R. W., Polonsky K. S., Myers G. L., et al. (2008). Standardization of C-peptide measurements. Clin. Chem. 54, 1023–1026. doi:10.1373/clinchem.2007.101287
Livhits M., Mercado C., Yermilov I., Parikh J. A., Dutson E., Mehran A., et al. (2012). Preoperative predictors of weight loss following bariatric surgery: Systematic review. Obes. Surg. 22, 70–89. doi:10.1007/s11695-011-0472-4
Manley S. E., Stratton I. M., Clark P. M., Luzio S. D. (2007). Comparison of 11 human insulin assays: Implications for clinical investigation and research. Clin. Chem. 53, 922–932. doi:10.1373/clinchem.2006.077784
Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. (1985). Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. doi:10.1007/BF00280883
Michaliszyn S. F., Lee S., Bacha F., Tfayli H., Farchoukh L., Mari A., et al. (2017). Differences in β-cell function and insulin secretion in Black vs. White obese adolescents: Do incretin hormones play a role? Race, β-cell function, and incretin response. Pediatr. Diabetes 18, 143–151. doi:10.1111/pedi.12364
Michaliszyn S. F., Mari A., Lee S., Bacha F., Tfayli H., Farchoukh L., et al. (2014). β-Cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 63, 3846–3855. doi:10.2337/db13-1951
Miller W. G., Thienpont L. M., Van Uytfanghe K., Clark P. M., Lindstedt P., Nilsson G., et al. (2009). Toward standardization of insulin immunoassays. Clin. Chem. 55, 1011–1018. doi:10.1373/clinchem.2008.118380
Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Nanni G., et al. (2015). Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386, 964–973. doi:10.1016/S0140-6736(15)00075-6
Montero P. N., Stefanidis D., Norton H. J., Gersin K., Kuwada T. (2011). Reported excess weight loss after bariatric surgery could vary significantly depending on calculation method: A plea for standardization. Surg. Obes. Relat. Dis. 7, 531–534. doi:10.1016/j.soard.2010.09.025
Mulgrave J. J., Levine M. E., et al. (2020). Using data assimilation of mechanistic models to estimate glucose and insulin metabolism. arXiv:200306541 [physics, stat]Available at: http://arxiv.org/abs/2003.06541 (accessed Mar 16, 2021).
Mulla C. M., Middelbeek R. J. W., Patti M-E. (2018). Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 1411, 53–64. doi:10.1111/nyas.13409
Muniyappa R., Madan R. (2018). “Assessing insulin sensitivity and resistance in humans,” in Endotext. Editors K. Feingold, B. Anawalt, and A. Boyce (South Dartmouth, MA. Available at: https://www.ncbi.nlm.nih.gov/books/NBK278954/.
Nathan D. M., Davidson M. B., DeFronzo R. A., Heine R. J., Henry R. R., Pratley R., et al. (2007). Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 30, 753–759. doi:10.2337/dc07-9920
Nowicka P., Santoro N., Liu H., Lartaud D., Shaw M. M., Goldberg R., et al. (2011). Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 34, 1306–1311. doi:10.2337/dc10-1984
Okser S., Pahikkala T., Aittokallio T. (2013). Genetic variants and their interactions in disease risk prediction – machine learning and network perspectives. BioData Min. 6, 5. doi:10.1186/1756-0381-6-5
Olbers T., Beamish A. J., Gronowitz E., Flodmark C. E., Dahlgren J., Bruze G., et al. (2017). Laparoscopic roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): A prospective, 5-year, Swedish nationwide study. Lancet. Diabetes Endocrinol. 5, 174–183. doi:10.1016/S2213-8587(16)30424-7
Olson D. E., Rhee M. K., Herrick K., Ziemer D. C., Twombly J. G., Phillips L. S. (2010). Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 33, 2184–2189. doi:10.2337/dc10-0433
Ortega E., Morínigo R., Flores L., Moize V., Rios M., Lacy A. M., et al. (2012). Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg. Endosc. 26, 1744–1750. doi:10.1007/s00464-011-2104-4
Ou X., Chen M., Xu L., Lin W., Huang H., Chen G., et al. (2022). Changes in bone mineral density after bariatric surgery in patients of different ages or patients with different postoperative periods: A systematic review and meta-analysis. Eur. J. Med. Res. 27, 144. doi:10.1186/s40001-022-00774-0
Pajvani U. B., Accili D. (2015). The new biology of diabetes. Diabetologia 58, 2459–2468. doi:10.1007/s00125-015-3722-5
Panunzi S., De Gaetano A., Carnicelli A., Mingrone G. (2015). Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: Do BMI or procedure choice matter? A meta-analysis. Ann. Surg. 261, 459–467. doi:10.1097/SLA.0000000000000863
Pedersen H. K., Gudmundsdottir V., Pedersen M. K., Brorsson C., Brunak S., Gupta R. (2016). Ranking factors involved in diabetes remission after bariatric surgery using machine-learning integrating clinical and genomic biomarkers. NPJ Genom. Med. 1, 16035. doi:10.1038/npjgenmed.2016.35
Pedregosa F., Varoquaux G., Gramfort A. (2011). Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830.
Pedroso F. E., Angriman F., Endo A., Dasenbrock H., Storino A., Castillo R., et al. (2018). Weight loss after bariatric surgery in obese adolescents: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 14, 413–422. doi:10.1016/j.soard.2017.10.003
Perotte A., Hripcsak G. (2013). Temporal properties of diagnosis code time series in aggregate. IEEE J. Biomed. Health Inf. 17, 477–483. doi:10.1109/JBHI.2013.2244610
Prentki M., Nolan C. J. (2006). Islet cell failure in type 2 diabetes. J. Clin. Investig. 116, 1802–1812. doi:10.1172/JCI29103
Purnell J. Q., Dewey E. N., Laferrère B., Selzer F., Flum D. R., Mitchell J. E., et al. (2021). Diabetes remission status during seven-year follow-up of the longitudinal assessment of bariatric surgery study. J. Clin. Endocrinol. Metab. 106, 774–788. doi:10.1210/clinem/dgaa849
Rackauckas C., Nie Q. (2017). Differentialequations.jl–a performant and feature-rich ecosystem for solving differential equations in julia. J. Open Res. Softw. 5, 15. doi:10.5334/jors.151
Radin M. S. (2014). Pitfalls in hemoglobin A1c measurement: When results may be misleading. J. Gen. Intern. Med. 29, 388–394. doi:10.1007/s11606-013-2595-x
Ramos-Levi A., Sanchez-Pernaute A., Matia P., Cabrerizo L., Barabash A., Hernandez C., et al. (2013). Diagnosis of diabetes remission after bariatic surgery may be jeopardized by remission criteria and previous hypoglycemic treatment. Obes. Surg. 23, 1520–1526. doi:10.1007/s11695-013-0995-y
Ramos-Levi A., Sanchez-Pernaute A., Matia P., Cabrerizo L., Barabash A., Hernandez C., et al. (2013). Diagnosis of diabetes remission after bariatic surgery may be jeopardized by remission criteria and previous hypoglycemic treatment. Obes. Surg. 23, 1520–1526. doi:10.1007/s11695-013-0995-y
Richter L. R., Wan Q., Wen D., Zhang Y., Yu J., Kang J. K., et al. (2020). Targeted delivery of notch inhibitor attenuates obesity-induced glucose intolerance and liver fibrosis. ACS Nano 14, 6878–6886. doi:10.1021/acsnano.0c01007
Robert M., Ferrand-Gaillard C., Disse E., Espalieu P., Simon C., Laville M., et al. (2013). Predictive factors of type 2 diabetes remission 1 Year after bariatric surgery: Impact of surgical techniques. Obes. Surg. 23, 770–775. doi:10.1007/s11695-013-0868-4
Rouskas K., Cauchi S., Raverdy V., Yengo L., Froguel P., Pattou F. (2014). Weight loss independent association of TCF7 L2 gene polymorphism with fasting blood glucose after Roux-en-Y gastric bypass in type 2 diabetic patients. Surg. Obes. Relat. Dis. 10, 679–683. doi:10.1016/j.soard.2013.12.016
Roy V. (2019). Convergence diagnostics for Markov chain Monte Carlo. arXiv:190911827 [stat]Available at: http://arxiv.org/abs/1909.11827 (accessed Apr 13, 2022).
Rubino F., Nathan D. M., Eckel R. H., Schauer P. R., Alberti K. G. M. M., Zimmet P. Z., et al. (2016). Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care 39, 861–877. doi:10.2337/dc16-0236
Saisho Y., Butler A. E., Manesso E., Elashoff D., Rizza R. A., Butler P. C. (2013). β-Cell mass and turnover in humans: Effects of obesity and aging. Diabetes Care 36, 111–117. doi:10.2337/dc12-0421
Schauer P. R., Kashyap S. R., Wolski K., Brethauer S. A., Kirwan J. P., Pothier C. E., et al. (2012). Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 366, 1567–1576. doi:10.1056/NEJMoa1200225
Seeley R. J., Chambers A. P., Sandoval D. A. (2015). The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell. Metab. 21, 369–378. doi:10.1016/j.cmet.2015.01.001
Shaibi G. Q., Davis J. N., Weigensberg M. J., Goran M. I. (2011). Improving insulin resistance in obese youth: Choose your measures wisely. Int. J. Pediatr. Obes. 6, e290–e296. doi:10.3109/17477166.2010.528766
Shen S-C., Wang W., Tam K-W., Chen H. A., Lin Y. K., Wang S. Y., et al. (2019). Validating risk prediction models of diabetes remission after sleeve gastrectomy. Obes. Surg. 29, 221–229. doi:10.1007/s11695-018-3510-7
Sinha R., Fisch G., Teague B., Tamborlane W. V., Banyas B., Allen K., et al. (2002). Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 346, 802–810. doi:10.1056/NEJMoa012578
Skelton J. A., Cook S. R., Auinger P., Klein J. D., Barlow S. E. (2009). Prevalence and trends of severe obesity among US children and adolescents. Acad. Pediatr. 9, 322–329. doi:10.1016/j.acap.2009.04.005
Stefater M. A., Inge T. H. (2017). Bariatric surgery for adolescents with type 2 diabetes: An emerging therapeutic strategy. Curr. Diab. Rep. 17, 62. doi:10.1007/s11892-017-0887-y
Stern S. E., Williams K., Ferrannini E., DeFronzo R. A., Bogardus C., Stern M. P. (2005). Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 54, 333–339. doi:10.2337/diabetes.54.2.333
Sturis J., Polonsky K. S., Mosekilde E., Van Cauter E. (1991). Computer model for mechanisms underlying ultradian oscillations of insulin and glucose. Am. J. Physiol. 260, E801–E809. doi:10.1152/ajpendo.1991.260.5.E801
Tagi V. M., Giannini C., Chiarelli F. (2019). Insulin resistance in children. Front. Endocrinol. 10, 342. doi:10.3389/fendo.2019.00342
Taylor R., Al-Mrabeh A., Sattar N. (2019). Understanding the mechanisms of reversal of type 2 diabetes. Lancet. Diabetes Endocrinol. 7, 726–736. doi:10.1016/S2213-8587(19)30076-2
Tharakan G., Scott R., Szepietowski O., Miras A. D., Blakemore A. I., Purkayastha S., et al. (2017). Limitations of the DiaRem score in predicting remission of diabetes following roux-en-Y gastric bypass (RYGB) in an ethnically diverse population from a single institution in the UK. Obes. Surg. 27, 782–786. doi:10.1007/s11695-016-2368-9
TODAY Study Group Bjornstad P., Drews K. L., Caprio S., Gubitosi-Klug R., Nathan D. M. (2021). Long-term complications in youth-onset type 2 diabetes. N. Engl. J. Med. 385, 416–426. doi:10.1056/NEJMoa2100165
Toh S., Rasmussen-Torvik L. J., Harmata E. E., Pardee R., Saizan R., Malanga E., et al. (2017). The national patient-centered clinical research network (PCORnet) bariatric study cohort: Rationale, methods, and baseline characteristics. JMIR Res. Protoc. 6, e222. doi:10.2196/resprot.8323
Tohidi M., Arbab P., Ghasemi A. (2017). Assay-dependent variability of serum insulin concentrations: A comparison of eight assays. Scand. J. Clin. Lab. Investig. 77, 122–129. doi:10.1080/00365513.2016.1278260
Tokarz V. L., MacDonald P. E., Klip A. (2018). The cell biology of systemic insulin function. J. Cell. Biol. 217, 2273–2289. doi:10.1083/jcb.201802095
Topp B., Promislow K., Devries G., Miura R. M., Finegood D. T. (2000). A model of β -cell mass, insulin, and glucose kinetics: Pathways to diabetes. J. Theor. Biol. 206, 605–619. doi:10.1006/jtbi.2000.2150
Tsilingiris D., Koliaki C., Kokkinos A. (2019). Remission of type 2 diabetes mellitus after bariatric surgery: Fact or fiction? Int. J. Environ. Res. Public Health 16, 3171. doi:10.3390/ijerph16173171
Tsui S. T., Yang J., Zhang X., Tatarian T., Docimo S., Spaniolas K., et al. (2021). Health disparity in access to bariatric surgery. Surg. Obes. Relat. Dis. 17, 249–255. doi:10.1016/j.soard.2020.10.015
Wallace T. M., Levy J. C., Matthews D. R. (2004). Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495. doi:10.2337/diacare.27.6.1487
Wan E. A., van der Merwe R. (2001). “The unscented kalman filter,” in Kalman filtering and neural networks. Editor S. Haykin (New York, USA: John Wiley & Sons), 221–280.
Wang G-F., Yan Y-X., Xu N., Yin D., Hui Y., Zhang J. P., et al. (2015). Predictive factors of type 2 diabetes mellitus remission following bariatric surgery: A meta-analysis. Obes. Surg. 25, 199–208. doi:10.1007/s11695-014-1391-y
Weiner A., Cowell A., McMahon D. J., Tao R., Zitsman J., Oberfield S. E., et al. (2020). The effects of adolescent laparoscopic adjustable gastric band and sleeve gastrectomy on markers of bone health and bone turnover. Clin. Obes. 10, e12411. doi:10.1111/cob.12411
Weiskopf N. G., Hripcsak G., Swaminathan S., Weng C. (2013). Defining and measuring completeness of electronic health records for secondary use. J. Biomed. Inf. 46, 830–836. doi:10.1016/j.jbi.2013.06.010
Yan W., Bai R., Yan M., Song M. (2017). Preoperative fasting plasma C-peptide levels as predictors of remission of type 2 diabetes mellitus after bariatric surgery: A systematic review and meta-analysis. J. Investig. Surg. 30, 383–393. doi:10.1080/08941939.2016.1259375
Yeckel C. W., Weiss R., Dziura J., Taksali S. E., Dufour S., Burgert T. S., et al. (2004). Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J. Clin. Endocrinol. Metab. 89, 1096–1101. doi:10.1210/jc.2003-031503
Keywords: type 2 diabetes, data assimilation, mechanistic models of glucose metabolism, pediatrics, bariatric surgery, machine learning, obesity
Citation: Richter LR, Albert BI, Zhang L, Ostropolets A, Zitsman JL, Fennoy I, Albers DJ and Hripcsak G (2022) Data assimilation on mechanistic models of glucose metabolism predicts glycemic states in adolescents following bariatric surgery. Front. Physiol. 13:923704. doi: 10.3389/fphys.2022.923704
Received: 19 April 2022; Accepted: 11 October 2022;
Published: 28 November 2022.
Edited by:
Cecilia Diniz Behn, Colorado School of Mines, United StatesReviewed by:
Justin R. Ryder, University of Minnesota Twin Cities, United StatesRongjing Ding, Peking University, China
Sushant Singh, Amity University, Raipur, India
Copyright © 2022 Richter, Albert, Zhang, Ostropolets, Zitsman, Fennoy, Albers and Hripcsak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George Hripcsak, aHJpcGNzYWtAY29sdW1iaWEuZWR1
 Lauren R. Richter
Lauren R. Richter Benjamin I. Albert
Benjamin I. Albert Linying Zhang
Linying Zhang Anna Ostropolets
Anna Ostropolets Jeffrey L. Zitsman2
Jeffrey L. Zitsman2 Ilene Fennoy
Ilene Fennoy David J. Albers
David J. Albers George Hripcsak
George Hripcsak