
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 05 August 2022
Sec. Vascular Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.917808
Purpose: Self-reported snoring has been reported to influence nerves and vessels. However, there are few direct evidences of snoring related to nerves and microvessels defects. Therefore, we evaluated the association of self-reported snoring with retinal structure and microcirculation.
Methods: A total of 2,622 participants were recruited from the Jidong eye cohort study (JECS). Physical examinations, laboratory tests, and questionnaires were recorded. We also used optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) to assess the retinal structure and microvascular network. Snoring was defined as “never,” “occasionally,” and “frequently or more severe” according to self-reported frequency.
Results: The prevalence of snoring were 84.6% (n = 983) and 45.0% (n = 657) in males and females, respectively. Compared with never snoring group, the retinal thickness increased in “occasionally” (p < 0.001) and “frequently or more severe” groups (p = 0.001), while no difference was found between snoring groups (p = 0.14). Superficial retinal capillary plexus (RCP) vessel density was lower in “frequently or more severe” group than in “never” (p < 0.001) and “occasionally” snoring groups (p < 0.001). After adjusting for confounders, “frequently or more severe” snoring was significantly associated with thinner total retinal thickness [β = −2.79 (95% CI: −5.27, −0.30)] and lower superficial RCP vessel density [β = −0.71 (95% CI: −1.19, −0.23)].
Conclusion: Our research showed self-reported snoring was associated with thinner retinal thickness and lower superficial RCP vessel density. The findings of our study emphasize the need for self-reported snoring assessments in determining retinal structure and microcirculation impairment.
Snoring is a common harmful sleep habit that is characterized as breathing sounds produced by the vibration of the pharyngeal wall and its posterior associated structures (de Felício et al., 2018). Several studies reported the prevalence of snoring ranged from 50 to 94% in adults (Xiong et al., 2016; Zhang et al., 2017; De Meyer et al., 2019; Rosen et al., 2019; Jiang et al., 2021). Snoring is also a common early symptom of obstructive sleep apnea (OSA) (Young et al., 2002), and OSA has been confirmed to be associated with diabetes (Reutrakul and Mokhlesi, 2017), metabolic disorders (Gaines et al., 2018), cardiovascular events (Bradley and Floras, 2009) and all-cause mortality (Lechat et al., 2021). Many snorers are not diagnosed with OSA, and snoring without an OSA diagnosis has long been considered a social nuisance and brought relevant burden (Deary et al., 2014; Brockmann et al., 2015). Recently, a growing number of studies have shown that snoring is also a risk factor for the aforementioned diseases and is independent of OSA (Lee et al., 2008; Zou et al., 2019; Taylor et al., 2021). The vessel vibration caused by snoring may induce arterial endothelial injury and promote the formation of atherosclerosis (Li et al., 2012; Behl et al., 2014). Snoring with airway stenosis and hypoxia may lead to nerve impairment (Whyte and Gibson, 2020). However, there was no direct evidence of peripheral nerve and microvascular changes in people who snore.
The retina reflects ocular health status, and previous studies have observed changes of the peripheral nervous system and vascular diseases in the retina (Wang et al., 2018; Wylęgała, 2018; Tang et al., 2020; István et al., 2021). In recent years, optical coherence tomography (OCT) technology has allowed a high-resolution and noninvasive visualization of the retinal structure. Optical coherence tomography angiography (OCTA) develops from OCT and focuses on vascular imaging, which can quantitatively analyze the retinal microcirculation network. These two technologies have been widely used and recognized in ophthalmology. A few studies have found ocular changes in patients with OSA using OCT and OCTA (Lee et al., 2019; Venkatesh et al., 2021). Nevertheless, to our knowledge, snoring related retinal changes have not yet been reported. Therefore, we aimed to investigate the association of self-reported snoring with retinal structure and microcirculation using OCT and OCTA in a large community-based population.
The data in this study were derived from the Jidong eye cohort study (JECS), which was a community-based study that has been described in detail (Yang et al., 2020). In brief, the JECS was established in 2019 and aimed to assess ocular biological indicators and their association with cardiovascular and neurological diseases. A total of 3,377 participants with complete examination records were enrolled, leaving 2,622 for analysis after excluding individuals with ocular history or surgery (n = 19), cerebral/cardiovascular and neurological conditions (n = 102), missing questionnaires (n = 42), axial length ≥26 mm (n = 371), and substandard OCTA images (n = 221).
This study followed the tenets of the Declaration of Helsinki and received approval from the Ethics Committee of the Staff Hospital of the Jidong Oil-Field of Chinese National Petroleum. Informed consent was obtained from each participant.
Baseline characteristics, including demographic data, clinical and laboratory examinations, and ocular biological parameters, were collected. The body mass index was calculated using weight (kg) divided by height squared (m2). Blood pressure was obtained with a digital automatic blood pressure monitor after adequate rest. Complete blood count, biochemistry and lipid profile were tested after at least 8 h of fasting. Participants were regarded as having hypertension, diabetes mellitus, and dyslipidemia if any of the following criterion was met: 1) self-reported history or 2) self-reported medication history or 3) clinical or laboratory examination (blood pressure ≥140/90 mmHg for hypertension, fasting glucose level ≥7.0 mmol/L for diabetes mellitus, serum triglyceride ≥1.76 mmol/L or low-density lipoprotein cholesterol ≥3.37 mmol/L or high-density lipoprotein cholesterol ≤1.04 mmol/L for dyslipidemia).
All participants had a complete record of ophthalmic examination. Best corrected visual acuity (BCVA) was assessed using a 5-meter-distance standard logarithmic visual acuity chart. Refractive error was represented with spherical equivalent (SE, sphere plus one-half cylinder), which was measured using an autorefractor (KR800; Topcon; Tokyo, Japan). Axial length was assessed by ocular biometry (Lenstar 900 Optical Biometer; Hagg-Streit, Koeniz, Switzerland). The ocular anterior segment was estimated with a slit-lamp biomicroscope, and the posterior segment was imaged with digital photography (CR2AF; Canon, Tokyo, Japan). All above-mentioned examinations were performed in 1 day.
Face-to-face interviews were conducted by trained research coordinators to collect questionnaire information. The frequency of snoring was assessed by the question “Do you snore when you sleep?” The individuals were grouped according to their responses (“never,” “occasionally,” “frequently or more severe”). Information on snoring was collected with the help of participant’s spouse or relatives. Nonsmokers and nondrinkers were identified by negative responses to the following questions: “Have you ever smoked” and “Have you ever drunk?”
The evaluation process of the retinal structure and microcirculation was performed using the spectral-domain OCTA device (RTVue XR Avanti with AngioVue; Optovue, Inc., Fremont, CA, United States). Details of the procedures and technology has been previously provided (Laíns et al., 2021). In brief, this scanning and analysis system used low-coherence near-infrared light to image biological tissues and obtained retinal cross-sectional structural images and a list of en face vascular projections. The scan mode centered on the macula with 3 × 3 mm2 area and had 304 horizontal A-scans per vertical B-scan line. Completed images were further assessed by built-in software (Optovue, Inc., Fremont, CA, United States) to obtain correct retinal segmentation: the total retinal thickness was defined from the inner limiting membrane (ILM) to Bruch’s membrane (BM): retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), external limiting membrane (ELM), photoreceptor layer (PR), retinal pigment epithelium (RPE), and BM. It was a complex tissue that contained retinal neuroepithelial layer, pigment epithelium, and the hyaline membrane. The superficial retinal capillary plexus (RCP) was from the ILM to the inner plexiform layer (IPL), and the deep RCP was from the IPL to the outer plexiform layer. The Early Treatment Diabetic Retinopathy Study (ETDRS) map was used to present the processed components, and the map contains a central circle and a concentric ring with diameters of 1 and 1-3 mm, respectively. The outer ring was continuously divided into four quadrants (temporal, superior, nasal and inferior). 3D-projection artifact removal algorithms (Optovue, Inc., Fremont, CA, United States) were used to remove superficial middle vessel signals from the deep layer. The accuracy of the measurement of retinal thickness and vessel density had been proved in previous studies. Figure 1 showed the representative OCTA images of the self-reported “never” snoring and “frequently or more severe” snoring.
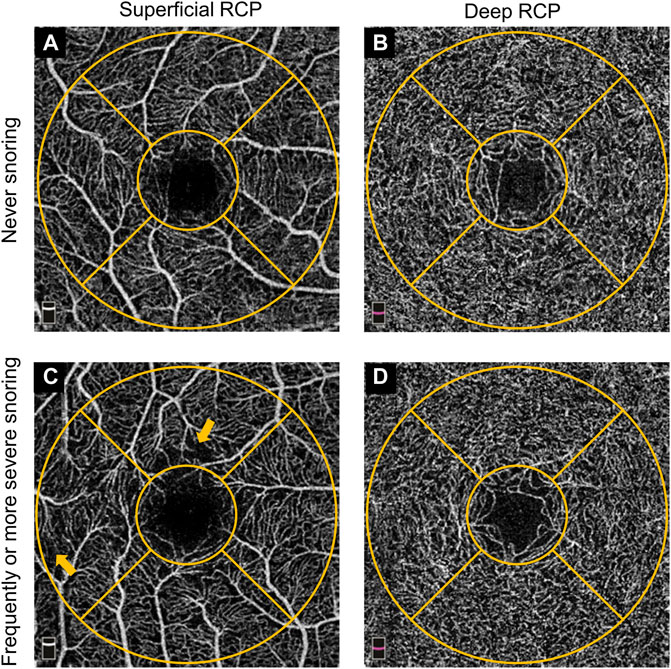
FIGURE 1. Representative OCTA images of self-reported never snoring and frequently or more severe snoring participants. (A). Superficial RCP image from a never snoring participant. (B). Deep RCP image from a never snoring participant. (C). Superficial RCP image from a frequently or more severe snoring participant. (D). Deep RCP image from a frequently or more severe snoring participant. The orange-colored concentric circle represented the ETDRS grid with 1 mm and 1–3 mm diameters, respectively. Origin arrows showed the superficial RCP vessel lost. OCTA, optical coherence tomography angiography; RCP, retinal capillary plexus; ETDRS, Early Treatment of Diabetic Retinopathy Study.
Two trained ophthalmologists reviewed all OCT and OCTA images and excluded defective images if they met any of the following criteria: 1) incorrect segmentation; 2) obvious decentration misalignment; 3) serious motion artifacts; and 4) signal index <7. By standard, we included the right eye of each participant for analysis.
Continuous variables were described as the mean [standard deviation (SD)]. Categorical variables were summarized using counts (percentage). One-way analysis of variance (ANOVA) and the χ2 test were applied to analyze the difference among self-reported snoring groups.
We employed a multivariable generalized linear model (GLM) to explore the association of snoring with retinal parameters, and confounders (age, sex, body mass index, education level, history of diabetes, hypertension, dyslipidemia, smoking and drinking, creatinine level, number of red blood cells, and hemoglobin level) were adjusted in this model, considering the statistical significance in baseline and clinical related or cared. Never snoring was set as a reference. Multicollinearity was checked using variance inflation factors. We also performed stratified analysis to determine the potential modification effects of hypertension. The association was presented as β coefficients with 95% confidence intervals (CI). A two-tailed P level <0.05 was defined as statistically significant. All analyses were performed using SPSS (version 26.0; IBM; NY, United States).
A total of 2,622 eligible participants were enrolled in this study, and the mean (SD) age was 44.0 (11.6) years. There were 1,162 men, and 983 (84.6%) of them reported different frequencies of snoring. There were also 657 (45.0%) females who snored. Compared with those in the “never” snoring group, participants with more frequent snoring were more likely to be smokers and drinkers. In addition, participants in the snore groups had a higher prevalence of hypertension, diabetes and dyslipidemia, and a higher BMI, serum creatinine level, red blood cell count and hemoglobin level. However, there was no significant difference in education among snore groups. Table 1 shows the baseline characteristics of the groups with different snoring frequencies.
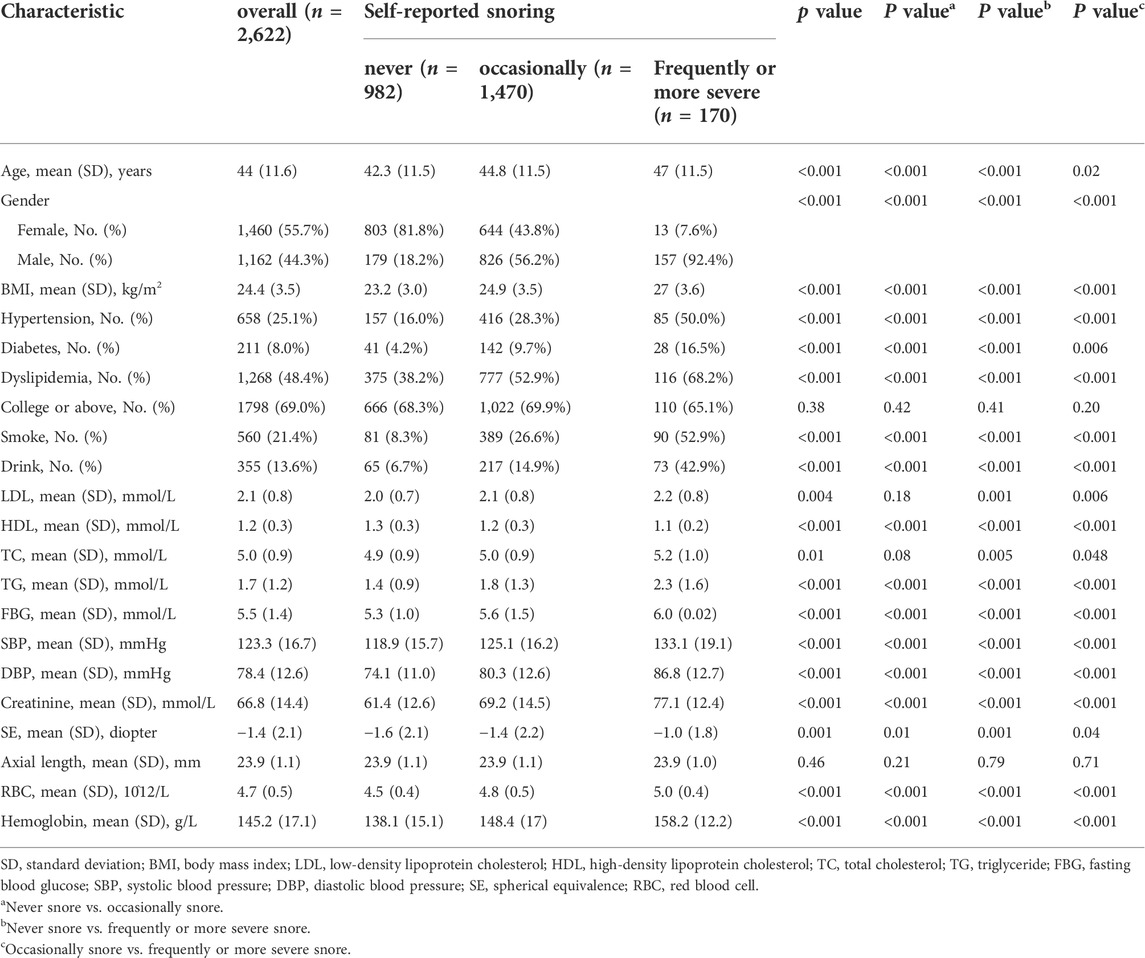
TABLE 1. Baseline characteristics of eligible participants according to frequency of self-reported snoring.
Table 2 shows the ocular characteristics in different snoring groups. The whole total retinal thickness was higher in participants who self-reported “occasionally” and “frequently or more severe” snoring compared with those in “never” snoring group (p < 0.001 and p = 0.001, respectively.) In addition, there was no statistical difference between participants in “occasionally” and “frequently or more severe” snoring groups: (p = 0.14) In superficial retina, RCP vessel density of participants with “frequently or more severe” snoring was lower than those who “never” and “occasionally” snore (p < 0.001 and p < 0.001, respectively.) however, we did not observe a significant difference between participants in “never” and “occasionally” snoring groups (p = 0.57) In deep retinal, participants with higher frequency snoring had lower RCP vessel density (all p < 0.001) and foveal avascular zone (FAZ) area (all p < 0.05). The retinal characteristics of other quadrants were listed in Table 2.
We show the relationships between retinal thickness and snore in Table 3. We added age, sex, BMI, education level, history of diabetes, hypertension, dyslipidemia, smoking and drinking, creatinine level, red blood cell count, and hemoglobin level to the GLM. Compared with the “never” snoring group, the “frequently or more severe” snoring group was associated with thinner total retinal thickness in the whole [β = −2.79 (95% CI: −5.27, −0.30)], parafovea [β = −2.94 (95% CI: −5.49, −0.38)] and in the four quadrants (Table 3). However, no significant association of retinal thickness was found in the “occasionally” snoring group (Table 3).
The relationship of superficial and deep RCP vessel density with snoring groups in different retinal regions were represented in Table 3. After adjusting for the confounders, we found that in superficial RCP, compared with the “never” snoring group, the “frequently or more severe” snoring group was associated with lower vessel density in the whole [β = −0.71 (95% CI: −1.19, −0.23)] and parafovea [β = −0.71 (95% CI: −1.23, −0.19)] and in other quadrants (Table 3). There was no statistical significance in the “occasionally” snoring group. In the deep RCP vessel density and FAZ area, compared with the “never” snoring group, all snore groups showed insignificant associations (Table 3).
Table 4 showed the associations of self-reported snoring with retinal RCP vessel density stratified by hypertension. After adjusting for the confounders, we did not observe interactions of self-reported with hypertension for both superficial and deep RCP vessel density (all interaction-p > 0.05).
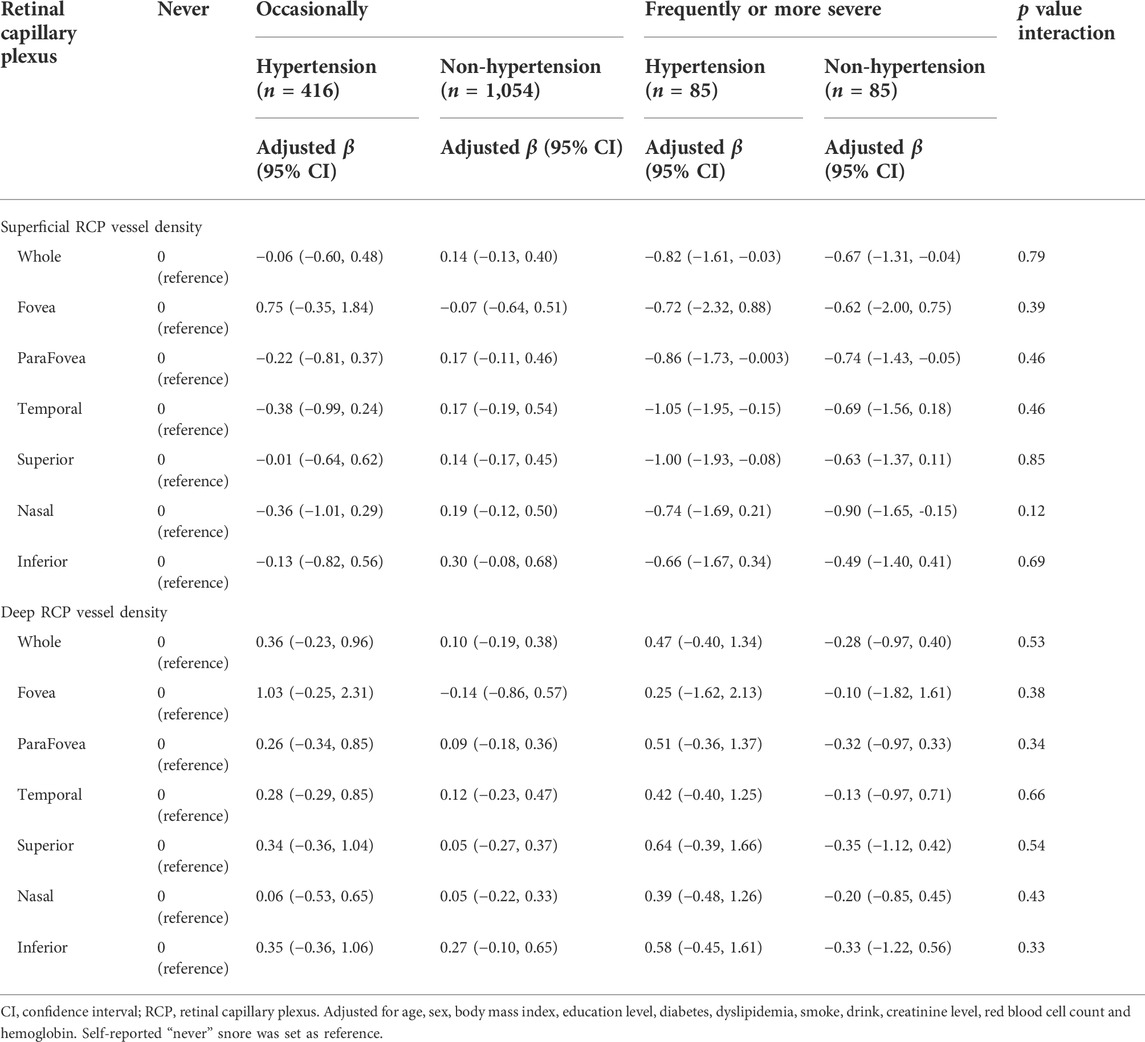
TABLE 4. Associations of self-reported snoring with retinal capillary plexus stratified by hypertension.
To our knowledge, this is the first study assessing the association of self-reported snoring with retinal thickness and RCP vessel density in a large community-based population using OCT and OCTA. We found that snoring was associated with thin retinal thickness and low superficial RCP vessel density. Unfortunately, data on clinical diagnosis of OSA were not available in present study. Given snoring is an early symptom of OSA, effect of OSA-related pathology on retinal health could not be rule out from the present study.
We found compared with non-snorers, subjects who frequently or more severe snored had thinner total retinal thickness. Snoring is considered a common symptom and early marker of OSA, and previous studies have reported that OSA is associated with neuro-ophthalmologic diseases (Wong and Fraser, 2019). Ching et al. (Lin et al., 2013) found that the hazard ratio of open-angle glaucoma in subjects with OSA was 1.67 higher than those of the comparison group in the 5-years follow-up period. Fan et al. (2019) also reported that severe OSA is significantly associated with the progressive decrease in RNFL thickness in patients with glaucoma. Furthermore, several studies had found that retinal thickness is decreased in patients with different severities of OSA (Casas et al., 2018; Kısabay Ak et al., 2020; Naranjo-Bonilla et al., 2021). However, obtaining an accurate diagnosis of OSA is sometimes difficult. Polysomnography is considered the gold-standard in clinical practice and requires patients to complete an overnight examination. Therefore, snoring assessment is a more intuitive and convenient method with greater economic benefits for large-scale clinical surveys. In this study, we also observed that people with snoring had significant decreased total retinal thickness, which may share the same pathogenic mechanism with OSA. Hypoxia caused by intermittent upper airway obstruction during sleep results in an increase in PaCO2 and a decrease in PaO2 (Prabhakar et al.,2020). This paroxysmal vascular insufficiency may impair optic nerve perfusion and oxygenation, and result in further optic neuropathy (Lin et al., 2013).
We found that compared with people who do not snore, subjects who self-reported that they “frequently or more severe” snored had lower superficial RCP vessel density. Some studies have reported that people who snore have a higher risk of cardiovascular diseases and adverse events than those in comparison. (Nagayoshi et al., 2012; Li et al., 2020). Muskaan et al. (Behl et al., 2014) observed that snorers had lower flow-mediated dilation of side branch vessels on ultrasound. Arrigo et al. (Cicero et al., 2016) found the arterial stiffness markers (pulse wave velocity and augmentation index) were significantly higher in those who snore than in controls. Li et al. (2012) investigated the carotid artery using ultrasonography in people who are self-reported snorers and imaging showed that the maximum intima-media thickness increased not only in common but also in bifurcation carotid arteries in those who snore. Furthermore, the increased thickness odds ratios of those who habitually snore were 1.71 and 3.63, respectively. In addition, current studies mostly focus on the cardiovascular field, while microvascular changes have not yet been reported in snorers.
Several OCTA studies have reported decreasing retinal vessel density in OSA patients (Ucak and Unver, 2020; Ye et al., 2021; Çolak et al., 2021; Ava et al., 2022). Our research supported these findings as we also observed lower superficial RCP vessel density in those who snore according to self-reported information. In previous studies, snoring was only considered a marker of OSA (Rosen et al., 2019; Li et al., 2020). Thus, our findings expanded the relationship between snoring and vascular impairment in the community-based population. Potentially, the reduced RCP vessel density in subjects who snore may be explained by sympathetic activation, oxidative stress and intimal injury caused by hypoxia (Behl et al., 2014; Prabhakar et al.,2020). However, further research is required to explore the internal mechanism.
In addition, we did not find any association between snoring and deep RCP vessel density. Amatoury et al. (2006) and Howitt et al. (2007) found that in rabbits, the carotid artery wall and the artery lumen could be affected by the vibration of snoring. The mechanical vibration may not propagate to the capillaries. This hypothesis may explain the association only presented in the superficial RCP, rather than in deep RCP. Compared with the deep retina, the superficial retina had branch blood vessels with larger diameters and was more vulnerable to the effect of snoring.
The associations of the both superficial and deep RCP vessel density with self-reported snoring were consistent across the subgroup based on hypertension. Although previous study had found the effect of hypertension to OCTA metrics (Peng et al., 2020), this interactions seem to be absent in our study. This might attribute to regularly antihypertensive drugs taking. Participants in our study had five types of antihypertensive agents: Betablockers, Ca antagonists, diuretic, angiotensin converting enzyme inhibitor, and angiotensin receptor blockers, and all subjects reported a once-daily intake.
The major strength of our study was the simultaneous assessment of retinal structure and microcirculation based on a self-reported snoring assessment. However, some limitations also apply. First, this is a single cross-sectional study, the internal causal relationships would be indistinguishable and further multicenter study is needed to expand the results to other population. Second, self-reported snoring information was collected from the questionnaire, so there may be bias and misclassification between actual and self-reported snoring. Third, we could not distinguish those with OSA from those who snore, which requires complex and time-consuming examination. Thus, the possibility would not be excluded that OSA, as a confounder, might have affected the association. However, the major purpose of this study was to analyze the association of retinal parameters with easily accessible self-reported snoring indicator, and its benefit ratio and enforceability could be expected.
In conclusion, we found that snoring was associated with decreased retinal thickness and lower RCP vessel density. Our study suggests that, despite the insufficiency of clinical diagnosis of OSA, self-reported snoring information was also a marker for retinal structure and microcirculation impairment. This research also supported the use of OCT and OCTA to help evaluate the severity of peripheral nerve and systemic microvascular injury in people who snore.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Staff Hospital of the Jidong Oil-Field of Chinese National Petroleum. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LC, ML, JQ, and FL designed and conceptualized the study and interpreted the data. YX analyzed the data. YX, KS, CL, KY, XZ, BS, and YJ had a major role in the acquisition of data. YX drafted the manuscript. LC, ML, JQ, and FL revised the manuscript for intellectual content. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (No. 81900903), the Zhejiang Provincial Natural Science Foundation of China (No. LY22H120007), National Key R&D Program of China (Nos. 2020YFC2008200 and 2019YFC0840708), the Medical and Health Science and Technology Program of Zhejiang Province (Nos. 2017KY113 and 2018KY543) and Wenzhou Science and Technology Program (Nos. 2021Y0908).
We are grateful to the cooperation of Jidong community, Tangshan City, Hebei Province, China, especially the dedicated participants and all professional research staff involved in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amatoury J., Howitt L., Wheatley J. R., Avolio A. P., Amis T. C. (2006)., 100. Bethesda, Md, 1547–1553. doi:10.1152/japplphysiol.01439.2005Snoring-related energy transmission to the carotid artery in rabbitsJ. Appl. Physiol.5
Ava S., Erdem S., Karahan M., Dursun M. E., Hazar L., Sen H. S. (2022). Evaluation of the effect of obstructive sleep apnea syndrome on retinal microvascularity by optical coherence tomography angiography. Photodiagnosis Photodyn. Ther. 38, 102761. doi:10.1016/j.pdpdt.2022.102761
Behl M., Bliwise D., Veledar E., Cunningham L., Vazquez J., Brigham K., et al. (2014). Vascular endothelial function and self-reported sleep. Am. J. Med. Sci. 347 (6), 425–428. doi:10.1097/MAJ.0b013e31829bc950
Bradley T. D., Floras J. S. (2009). Obstructive sleep apnoea and its cardiovascular consequences. Lancet (London, Engl. 373 (9657), 82–93. doi:10.1016/S0140-6736(08)61622-0
Brockmann P. E., Schlaud M., Poets C. F., Urschitz M. S. (2015). Predicting poor school performance in children suspected for sleep-disordered breathing. Sleep. Med. 16 (9), 1077–1083. doi:10.1016/j.sleep.2015.03.021
Casas P., Ascaso F. J., Vicente E., Tejero-Garces G., Adiego M. I., Cristobal J. A. (2018). Visual field defects and retinal nerve fiber imaging in patients with obstructive sleep apnea syndrome and in healthy controls. BMC Ophthalmol. 18 (1), 66. doi:10.1186/s12886-018-0728-z
Cicero A. F. G., Morbini M., Urso R., Rosticci M., Parini A., Grandi E., et al. (2016). Association between self-reported snoring and arterial stiffness: Data from the brisighella heart study. Intern. Emerg. Med. 11 (1), 77–83. doi:10.1007/s11739-015-1310-9
Çolak M., Ozek D., Ozcan K. M., Eravci F. C., Karakurt S. E., Karakus M. F., et al. (2021). Evaluation of retinal vessel density and foveal avascular zone measurements in patients with obstructive sleep apnea syndrome. Int. Ophthalmol. 41 (4), 1317–1325. doi:10.1007/s10792-020-01690-0
de Felício C. M., da Silva Dias F. V., Trawitzki L. V. V. (2018). Obstructive sleep apnea: Focus on myofunctional therapy. Nat. Sci. Sleep. 10, 271–286. doi:10.2147/NSS.S141132
De Meyer M. M. D., Jacquet W., Vanderveken O. M., Marks L. A. M. (2019). Systematic review of the different aspects of primary snoring. Sleep. Med. Rev. 45, 88–94. doi:10.1016/j.smrv.2019.03.001
Deary V., Ellis J. G., Wilson J. A., Coulter C., Barclay N. L. (2014). Simple snoring: Not quite so simple after all? Sleep. Med. Rev. 18 (6), 453–462. doi:10.1016/j.smrv.2014.04.006
Fan Y.-Y., Su W. W., Liu C. H., Chen H. S. L., Wu S. C., Chang S. H. L., et al. (2019). Correlation between structural progression in glaucoma and obstructive sleep apnea. Eye Lond. Engl. 33 (9), 1459–1465. doi:10.1038/s41433-019-0430-2
Gaines J., Vgontzas A. N., Fernandez-Mendoza J., Bixler E. O. (2018). Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep. Med. Rev. 42, 211–219. doi:10.1016/j.smrv.2018.08.009
Howitt L., Kairaitis K., Kirkness J. P., Garlick S. R., Wheatley J. R., Byth K., et al. (2007)., 103. Bethesda, Md, 1622–1627. doi:10.1152/japplphysiol.01413.2006Oscillatory pressure wave transmission from the upper airway to the carotid arteryJ. Appl. Physiol.5
István L., Czako C., Elo A., Mihaly Z., Sotonyi P., Varga A., et al. (2021). Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. GeroScience 43 (4), 1703–1723. doi:10.1007/s11357-021-00392-4
Jiang Z., Qin J., Liang K., Zhao R., Yan F., Hou X., et al. (2021). Self-reported snoring is associated with chronic kidney disease in obese but not in normal-weight Chinese adults. Ren. Fail. 43 (1), 709–717. doi:10.1080/0886022X.2021.1915332
Kısabay Ak A., Batum M., Goktalay T., Mayali H., Kurt E., Selcuki D., et al. (2020). Evaluation of retinal fiber thickness and visual pathways with optic coherence tomography and pattern visual evoked potential in different clinical stages of obstructive sleep apnea syndrome. Doc. Ophthalmol. 141 (1), 33–43. doi:10.1007/s10633-020-09749-0
Laíns I., Wang J. C., Cui Y., Katz R., Vingopoulos F., Staurenghi G., et al. (2021). Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 84. 100951. doi:10.1016/j.preteyeres.2021.100951
Lechat B., Appleton S., Melaku Y. A., Hansen K., McEvoy R. D., Adams R., et al. (2021). Co-morbid insomnia and obstructive sleep apnoea is associated with all-cause mortality. Eur. Respir. J. Prepr., 2101958. doi:10.1183/13993003.01958-2021
Lee S. A., Amis T. C., Byth K., Larcos G., Kairaitis K., Robinson T. D., et al. (2008). Heavy snoring as a cause of carotid artery atherosclerosis. Sleep 31 (9), 1207–1213.
Lee S. S. Y. Y., McArdle N., Sanfilippo P. G., Yazar S., Eastwood P. R., Hewitt A. W., et al. (2019). Associations between optic disc measures and obstructive sleep apnea in young adults. Ophthalmology 126 (10), 1372–1384. doi:10.1016/j.ophtha.2019.04.041
Li J., McEvoy R. D., Zheng D., Loffler K. A., Wang X., Redline S., et al. (2020). Self-reported snoring patterns predict stroke events in high-risk patients with OSA: Post hoc analyses of the SAVE study. Chest 158 (5), 2146–2154. doi:10.1016/j.chest.2020.05.615
Li Y., Liu J., Wang W., Yong Q., Zhou G., Wang M., et al. (2012). Association of self-reported snoring with carotid artery intima-media thickness and plaque. J. Sleep. Res. 21 (1), 87–93. doi:10.1111/j.1365-2869.2011.00936.x
Lin C.-C. C., Hu C. C., Ho J. D., Chiu H. W., Lin H. C. (2013). Obstructive sleep apnea and increased risk of glaucoma: A population-based matched-cohort study. Ophthalmology 120 (8), 1559–1564. doi:10.1016/j.ophtha.2013.01.006
Nagayoshi M., Tanigawa T., Yamagishi K., Sakurai S., Kitamura A., Kiyama M., et al. (2012). Self-reported snoring frequency and incidence of cardiovascular disease: The circulatory risk in communities study (CIRCS). J. Epidemiol. 22 (4), 295–301. doi:10.2188/jea.JE20110109
Naranjo-Bonilla P., Munoz-Villanueva M. C., Gimenez-Gomez R., Jurado-Gamez B. (2021). Retinal and choroidal thickness measurements in obstructive sleep apnea: Impacts of continuous positive airway pressure treatment. Graefe’s archive Clin. Exp. Ophthalmol. = Albrecht von Graefes Archiv fur klinische und Exp. Ophthalmol. 259 (11), 3381–3393. doi:10.1007/s00417-021-05322-w
Peng Q., Hu Y., Huang M., Wu Y., Zhong P., Dong X., et al. (2020). Retinal neurovascular impairment in patients with essential hypertension: An optical coherence tomography angiography study. Invest. Ophthalmol. Vis. Sci. 61 (8), 42. doi:10.1167/iovs.61.8.42
Prabhakar N. R., Peng Y.-J., Nanduri J. (2020). Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Invest. 130 (10), 5042–5051. doi:10.1172/JCI137560
Reutrakul S., Mokhlesi B. (2017). Obstructive sleep apnea and diabetes: A state of the art review. Chest 152 (5), 1070–1086. doi:10.1016/j.chest.2017.05.009
Rosen D. M., Kundel V., Rueschman M., Kaplan R., Guo N., Wilson J. G., et al. (2019). Self-reported snoring and incident cardiovascular disease events: Results from the jackson heart study. Sleep Breath. = Schlaf Atmung 23 (3), 777–784. doi:10.1007/s11325-018-01776-1
Tang F. Y., Chan E. O., Sun Z., Wong R., Lok J., Szeto S., et al. (2020)., 7. London, England, 7. doi:10.1186/s40662-019-0173-yClinically relevant factors associated with quantitative optical coherence tomography angiography metrics in deep capillary plexus in patients with diabetesEye Vis.
Taylor C., Kline C. E., Rice T. B., Duan C., Newman A. B., Barinas-Mitchell E. (2021). Snoring severity is associated with carotid vascular remodeling in young adults with overweight and obesity. Sleep. health 7 (2), 161–167. doi:10.1016/j.sleh.2020.12.004
Ucak T., Unver E. (2020). Alterations in parafoveal and optic disc vessel densities in patients with obstructive sleep apnea syndrome. J. Ophthalmol. 4034382. doi:10.1155/2020/4034382
Venkatesh R., Pereira A., Aseem A., Jain K., Sangai S., Shetty R., et al. (2021). Association between sleep apnea risk score and retinal microvasculature using optical coherence tomography angiography. Am. J. Ophthalmol. 221, 55–64. doi:10.1016/j.ajo.2020.08.037
Wang L., Murphy O., Caldito N. G., Calabresi P. A., Saidha S. (2018)., 5. London, England, 11. doi:10.1186/s40662-018-0104-3Emerging applications of optical coherence tomography angiography (OCTA) in neurological researchEye Vis.
Whyte A., Gibson D. (2020). Imaging of sleep-disordered breathing in adults. Clin. Radiol. 75 (12), 960e1–960. e16. doi:10.1016/j.crad.2020.05.017
Wong B., Fraser C. L. (2019). Obstructive sleep apnea in neuro-ophthalmology. J. Neuroophthalmol. 39 (3), 370–379. doi:10.1097/WNO.0000000000000728
Wylęgała A. (2018). Principles of OCTA and applications in clinical neurology. Curr. Neurol. Neurosci. Rep. 18 (12), 96. doi:10.1007/s11910-018-0911-x
Xiong X., Zhong A., Xu H., Wang C. (2016). Association between self-reported habitual snoring and diabetes mellitus: A systemic review and meta-analysis. J. Diabetes Res. 2016. 1958981. doi:10.1155/2016/1958981
Yang K., Cui L., Zhang G., Wang X., Zhu X., Xiao Y., et al. (2020). The Jidong eye cohort study: Objectives, design, and baseline characteristics. Eye Vis. 7 (1), 58. doi:10.1186/s40662-020-00223-1
Ye H., Jin C., Li X., Zhao L., Li Y., Qiao T. (2021). OCT-angiography comparison between obstructive sleep apnea children and normal subjects in China. Curr. Eye Res. 46 (3), 355–360. doi:10.1080/02713683.2020.1801757
Young T., Peppard P. E., Gottlieb D. J. (2002). Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 165 (9), 1217–1239. doi:10.1164/rccm.2109080
Zhang N., Chen Y., Chen S., Jia P., Guo X., Sun G., et al. (2017). Self-reported snoring is associated with dyslipidemia, high total cholesterol, and high low-density lipoprotein cholesterol in obesity: A cross-sectional study from a rural area of China. Int. J. Environ. Res. Public Health 14 (1), E86. doi:10.3390/ijerph14010086
Keywords: optical coherence tomography, optical coherence tomography angiography, self-reported snoring, macular microcirculation, macular structure
Citation: Xiao Y, Shi K, Li C, Yang K, Zhu X, Su B, Ju Y, Lu F, Qu J, Li M and Cui L (2022) Association of self-reported snoring with decreased retinal thickness and vessel density. Front. Physiol. 13:917808. doi: 10.3389/fphys.2022.917808
Received: 11 April 2022; Accepted: 07 July 2022;
Published: 05 August 2022.
Edited by:
Eliete Bouskela, Rio de Janeiro State University, BrazilReviewed by:
Honghua Yu, Guangdong Provincial People’s Hospital, ChinaCopyright © 2022 Xiao, Shi, Li, Yang, Zhu, Su, Ju, Lu, Qu, Li and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Qu, amlhLnF1QDE2My5jb20=; Ming Li, bG1AZXllLmFjLmNu; Lele Cui, Y2xsQGV5ZS5hYy5jbg==
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.