
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 28 June 2022
Sec. Lipid and Fatty Acid Research
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.917084
 Chuanyi Huo1†
Chuanyi Huo1† Zikai Song2†
Zikai Song2† Jianli Yin1
Jianli Yin1 Ying Zhu1
Ying Zhu1 Xiaohan Miao1
Xiaohan Miao1 Honghao Qian1
Honghao Qian1 Jia Wang1
Jia Wang1 Lin Ye1*
Lin Ye1* Liting Zhou1*
Liting Zhou1*Background: The benefit of cold exposure for humans against obesity has brought the energy metabolism and activity of brown adipose tissue (BAT) induced by cold into focus. But the results are inconsistent. This review is aimed to systematically explore the effect of cold exposure on the activity of BAT and energy metabolism in humans.
Methods: We searched relevant papers that were published from 1990 to 2021 and were cited in PubMed Central, Web of science, Embase and Cochrane Library databases to conduct this systematic review and meta-analysis. Energy metabolism, BAT volume, BAT activity and non-esterified fatty acids (NEFA) data reported in eligible researches were extracted. Meta-analysis was applied to combine the mean difference or standard mean difference with their 95% confidence intervals (95%CI). RevMan 5.3 software was used for meta-analysis and evaluating the risk of bias. Stata 16.0 was used for evaluating the publication bias.
Results: Ten randomized controlled trials were included in meta-analysis. Compared with human exposed in room temperature at 24°C, the energy expenditure (EE) was increased after acute cold exposure at 16∼19°C (Z = 7.58, p < 0.05, mean different = 188.43kal/d, 95% CI = 139.73–237.13); BAT volume (Z = 2.62, p < 0.05; standard mean different = 0.41, 95% CI = 0.10–0.73); BAT activity (Z = 2.05, p = 0.04, standard mean difference = 1.61, 95% CI = 0.07–3.14) and the intake of BAT NEFA (Z = 2.85, p < 0.05; standard mean different = 0.53, 95% CI = 0.17–0.90) also increased.
Conclusion: Acute cold exposure could improve the energy expenditure and BAT activity in adults, which is beneficial for human against obesity.
Nowadays, overweight and obesity have become the greatest health challenges worldwide. It was estimated that the percentage of overweight or obese adults was 39% for men and 40% for women; over 340 million children and adolescents aged 5–19 were overweight or obese in 2016. Moreover, World Health Organization (WHO) had pointed out that the epidemic of obesity is on the rise worldwide. Obesity affects nearly all physiological functions of the body and increases the risk of many diseases, such as diabetes mellitus, cardiovascular disease, musculoskeletal disorders and some cancers (including endometrial, breast, ovarian, kidney, and colon cancers) (Anandacoomarasamy et al., 2008; Singh et al., 2013; Lauby-Secretan et al., 2016).
Obesity is a state of energy imbalance, which means much more energy intake than energy expenditure, leading to excess energy stored as fat. Reducing energy intake and increasing energy consumption are effective ways to combat obesity. Brown adipose tissue (BAT), owing to its unique capacity to change excess energy into thermal energy, is considered an effective potential target against obesity and related metabolic diseases (Cypess and Kahn, 2010; Tseng et al., 2010). BAT is mainly regulated by the sympathetic nervous system (SNS) that releases norepinephrine to activate β-adrenergic receptor (β-AR) on the surface of brown adipocytes (Oelkrug et al., 2015). Subsequently, uncoupling protein 1 (UCP1) uses the proton gradient created by nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) instead of the decomposition of adenosine triphosphate (ATP) to generate heat (Oelkrug et al., 2015). When UCP1 is expressed in the BAT, it can invigorate the mitochondrial respiration to the most extreme sum in arrange to compensate for the gradient loss, in which process leading to energy consumption increasing (Cannon and Nedergaard, 2004). The BAT activity is higher in normal weight people than that in obese or overweight people. BAT activity is inversely correlated with body mass index (BMI), age and fat content in humans (Cypess et al., 2009; Yoneshiro et al., 2011b; Dinas et al., 2015).
At present, several mechanisms affecting the activity and metabolism of BAT, including diet, exercise, medicine and cold exposure, have been proved (Cannon and Nedergaard, 2004; Marlatt and Ravussin, 2017; Yau and Yen, 2020). Among them, cold exposure is the foremost well-studied method to activate BAT, as the primary role of BAT is to convert glucose and fat into heat by non-shivering thermogenesis (NST). Acute cold exposure (1–48 h) increased glucose uptake and improved insulin sensitivity; and free fatty acids (FFA) uptake and metabolism also increased in BAT(Ouellet et al., 2012; Leitner et al., 2017). Amid continuous cold stimulation, adipose tissue was remodeled to activate the thermogenic potential of both BAT and white adipose tissue (WAT). Incessant cold exposure causes metabolic changes within the BAT to maximize β-oxidation of fat acid from human cells and blood, electron transport action, and Ucp1 expression to produce heat (Cannon and Nedergaard, 2004; Blondin et al., 2014; Blondin et al., 2017a).
A few studies carried out in rodents had suggested that prolonged exposure to cold environment could activate the BAT further improve diet-induced obesity and its related complications, such as disturbed glucose and lipid homeostasis (Vallerand et al., 1986; Bartelt et al., 2011). Studies carried out in humans had also indicated that cold-induced BAT activation could enhance glucose uptake and improve whole-body glucose disposal and insulin sensitivity (Cypess et al., 2009; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009; Yoneshiro et al., 2013b; Blondin et al., 2015). After cold exposure, the resting metabolic rate in healthy people with detectable BAT levels increased by 14% (Chondronikola et al., 2014). However, studies carried out in aborigines living in the deserts of Australia and Bushmen population in the Kalahari Desert of southern Africa indicated that repeated nocturnal cold exposure did not increase the energy expenditure (EE) (Scholander et al., 1958; Wyndham and Morrison, 1958). Besides, no increase in EE was observed in obese individuals exposed to a short-term acute cold due to the small number of BAT activations (Hanssen et al., 2016). Similarly, sleeping in a room at 19°C for 1 month did not alter the cold-induced thermogenesis (CIT) (Lee et al., 2014). To date, due to the limited sample size of the population, the evidence of the effects of cold exposure on BAT activity and energy metabolism in humans is limited and inconsistent. Therefore, the present systematic review is carried out to assess the effect of acute cold exposure on human BAT activity and energy metabolism, and it is very crucial to provide accurate evidence to combat obesity and related metabolic diseases.
We conducted the standard method according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Shamseer et al., 2015). Two independent authors (HCY and SZK) searched for related articles about the effects of cold exposure on BAT activity and EE in humans published from 1 January 1990 to 31 May 2021 in PubMed, Web of Science, Embase and Cochrane library databases. The search strategy in the PubMed database can be found in Appendix A. Following the different retrieval requirements of different databases, the connectives were appropriately adjusted. In addition, the reference lists of the included studies in systematic reviews searched out were searched to supplement the literature of this study that had not been initially searched out. The disagreement between two independent authors in the searching strategy was resolved by consensus.
The articles were included or excluded by two independent authors (HCY and SZK), and the differences between the two authors were resolved through consensus or the third author (ZY). The studies we included were randomized controlled trials (RCT) carried out in humans with clear data of the sample size, the brown adipose tissue activity and energy metabolism before and after cold exposure. We excluded the studies with the influence of other intervention factors besides cold exposure, the studied carried out in animals, or lack the data of BAT activity and energy metabolism before and after cold exposure. For studies from the same research group, we only included the latest research report. Our research does not include reviews, editorials, letters, magazine articles and meeting minutes.
We imported all articles searched from various databases into EndNote and removed duplicates. Two authors (HCY and YJL) independently reviewed the title, abstract, and full text that met the inclusion criteria. For any discrepancies, the third author (ZY) will make the final decision. For the included studies, two authors (HCY and QHH) extracted the following information: 1) the name of the first author; 2) the year of publication; 3) the country; 4) the year(s) of study; 5) the type of study; 6) the number of participants; 7) the average age (if the mean was not available, using the median instead); 8) baseline data such as BMI. Cold exposure intervention data were also extracted. Finally, we extracted the main results data of cold exposure on BAT activity and energy metabolism measured by 18 F-Fluorodeoxyglucose (FDG), Positron Emission Tomography (PET), Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and energy expenditure.
Two authors (HCY and QHH) independently conducted the risk of bias in the included studies that met the systematic review standards, and any differences in the risk of bias assessment were adjusted by the third author (ZY). For the qualified randomized controlled trials, we used the Cochrane Library bias assessment tool to assess the risk of bias (Higgins et al., 2011).
RevMan 5.3 and Stata 16.0 software were used to conduct the meta-analysis. Continuous, inverse variance were selected to summarize energy metabolism difference (kcal/d), BAT volume, BAT activity and non-esterified fatty acids (NEFA). The means and standard deviations were used to represent the difference in energy expenditure between participants who were exposed to cold environment and participants who did not expose to cold environment or were controlled, studies only provided subgroup means and standard deviations were analyzed after merging. Cochran’s Q test and I2 statistics were used to assess the heterogeneity among studies, and I2 > 50% indicates significant heterogeneity. Subgroup analysis was used for heterogeneity analysis. When I2 ≤ 50%, the fixed-effect model was used for meta-analysis; when I2 > 50%, the random effect model was used. We estimated the 95% confidence interval (CI) of the study and used the Z value and p value to test the statistical significance. When p < 0.05, it was considered to be statistically different. Where appropriate, we used the following formula to convert standard error (SE) into standard deviation (SD): SD = SE×
A total of 2089 documents were searched out initially. 307 duplicate articles, 1750 unrelated articles based on the review of the titles and abstracts were excluded. Of the remaining 32 articles, 23 were removed because some of them applied other interventions or did not clearly report EE, BAT volume, BAT activity or BAT NEFA. One study was manually included via searching the reference list. Finally, 10 RCT articles were included for meta-analysis. All of the 10 articles were in English. All participants in the 10 RCTs were healthy. After the qualification assessment, 10 papers reporting the BAT activity or energy expenditure after cold exposure were retained for quantitative analysis, 2 in Finland, 2 in the United States, 1 in Australia, 1 in Switzerland, 2 in the Netherlands and 2 in Japan. The research selection process and flowchart of the literature search were shown in Figure 1.
In general, the average age of participants was mainly 20∼40 years old, and the number of male participants was more than females. These studies all used FDG-PET/CT or MRI to evaluate the BAT activity before and after cold exposure, as well as the energy metabolism. Others baseline information can be found in Table 1.
The bias risk assessment on the included RCTs was summarized in Figure 2A. 65% of the included RCTs showed a low risk in selection bias, and 35% showed an unclear risk of bias. In performance bias, 70% showed low risk, 30% showed an unclear risk of bias. In the detection bias, 90% showed a low risk, and 10% showed an unclear risk of bias. All of the included RCTs were low-risk in attrition bias and reporting bias. In the other bias, 90% displayed low risk, while 10% showed high risk. The detailed risk of bias for each study included in the systematic review was shown in Figure 2B.
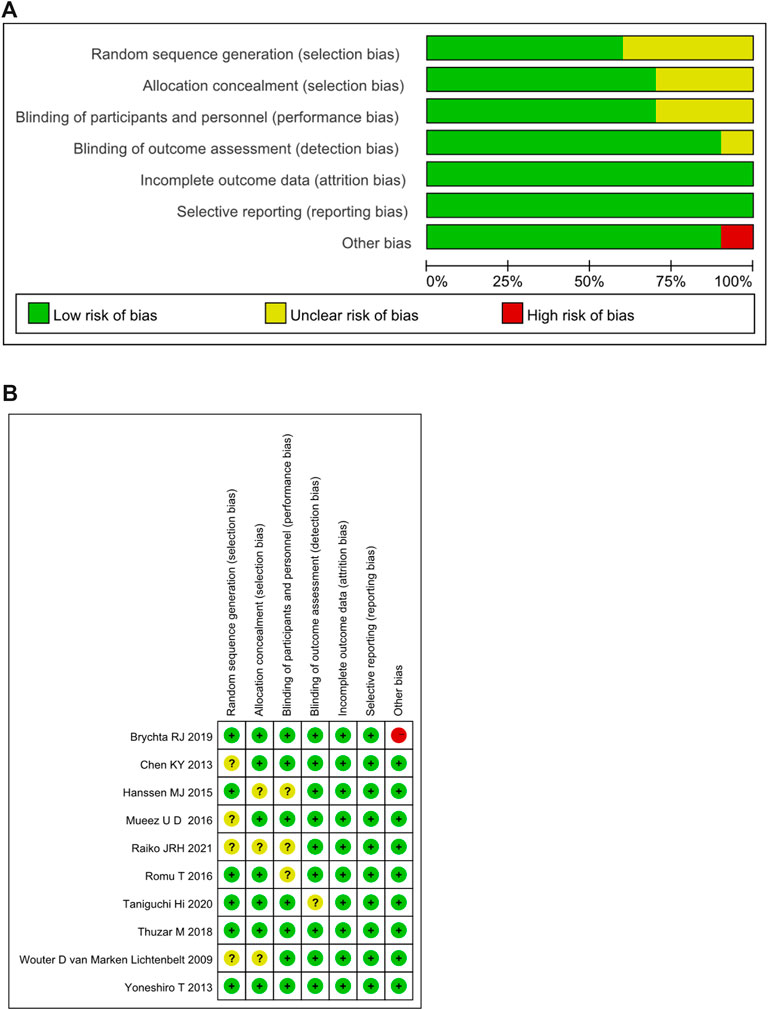
FIGURE 2. (A) Summary of risk of bias assessment for randomized controlled trials.(B) Risk of bias assessment for randomized controlled trials. +: low risk of bias; -: high risk of bias; ?: unclear risk of bias.
As for BAT volume, BAT activity and BAT NEFA, standard mean difference that can eliminate the influence of the absolute value of a study and the influence of measurement unit on the results was adopted. As for EE, mean difference was adopted.
In the ten studies, eight studies reported clearly data of body energy expenditure before and after cold exposure (Taniguchi et al., 2020; Brychta et al., 2019; Thuzar et al., 2018; Romu et al., 2016; M et al., 2016; Hanssen MJ. et al., 2015; Chen et al., 2013; van Marken Lichtenbelt et al., 2009). Since I2 < 50%, no obvious heterogeneity was observed. The results of the effect of cold exposure on body energy metabolism showed that the energy metabolism of subjects in the cold exposure group was significantly higher than that in the control group (Z = 7.58, p < 0.05, mean different = 188.43kal/d, 95% CI = 139.73–237.13). The result was shown in Figure 3.
The changes in BAT volume were evaluated in 4 studies (Raiko et al., 2021; Romu et al., 2016; M et al., 2016; Chen et al., 2013). Since I2 < 50%, no obvious heterogeneity was observed. The results indicated that the volume of BAT was higher after cold exposure than that of room temperature (Z = 2.62, p < 0.05, standard mean difference = 0.41, 95% CI = 0.10–0.73). The result was shown in Figure 4.
As for BAT activity, two studies reported relevant data (Yoneshiro et al., 2013a; Chen et al., 2013). Because I2 = 76% and p < 0.05, high heterogeneity was observed. Subgroup analysis was used for heterogeneity analysis. The results showed that the activity of BAT was improved after cold exposure (Z = 2.05, p = 0.04, standard mean difference = 1.61, 95% CI = 0.07–3.14). The result was shown in Figure 5.
Three studies reported the intake of BAT NEFA (Raiko et al., 2021; M et al., 2016; Hanssen MJ. et al., 2015). Due to I2 < 50%, no obvious heterogeneity was observed. The results showed that the intake of BAT NEFA was increased after cold exposure (Z = 2.85, p < 0.05, mean difference = 0.53, 95% CI = 0.17–0.90). The result was shown in Figure 6.
We performed sensitivity analyses via omitting 1 study each time. The analysis results indicated that the meta-analysis results for EE, BAT volume and BAT NEFA did not alter when each study was removed in turn, so that the findings were robust. The result was shown in Table 2.
There is some heterogeneity for the BAT activity, thus subgroup analysis is used to detect the heterogeneity. Only gender subgroup was analyzed. Other subgroups were not conducted because of under-representation in number of trials. The BAT activity was improved in males (standard mean difference = 1.58, 95% CI = 0.66–2.50) and females (standard mean difference = 1.26, 95% CI = 0.28–2.24). The result was shown in Figure 7.
Visual inspection of funnel plots revealed no asymmetry (Figure 8), and the results from Egger’s and Begg’s test indicated that no evidence for publication bias was detected for energy expenditure (Begg’s test, p = 0.386; Egger’s test, p = 0.521) and BAT volume (Begg’s test, p = 0.308; Egger’s test, p = 0.174).
Since BAT was “rediscovered” in humans, it has attracted great attention due to its potential ability to fight against obesity and related metabolic disorders (van Marken Lichtenbelt et al., 2009).
Although Wijers et al. reported that EE was significantly elevated in healthy lean subjects after cold exposure the present meta-analysis confirms that human energy expenditure is increased after acute cold exposure (van Marken Lichtenbelt, 2009; Wijers et al., 2010). Similarly, we found that the intake of NEFA is increased after acute cold exposure. Ouellet et al. also proved that plasma NEFA uptake is increased in cold-activated BAT compared with resting skeletal muscles and subcutaneous adipose tissues (Ouellet et al., 2012). Those evidences suggest that acute cold exposure plays an important role in enhancing energy metabolism. Even though the mechanism of cold-induced thermogenesis (CIT) is not fully defined, it has been previously indicated that glucose treatment, plasma glucose oxidation, and insulin sensitivity were improved effectively after acute cold exposure (Chondronikola et al., 2014). This mechanism is achieved by enhancing the expression of glucose transporter type 4 (GLUT4) or removing the triacylglycerol (TAG) in serum (Shibata et al., 1989; Shimizu et al., 1993; Baba et al., 2007; Bartelt et al., 2011; Nedergaard et al., 2011). Cold-induced increasing energy expenditure is related to the 18FDG positive BAT(Yoneshiro et al., 2011a; Vijgen et al., 2011). Whole body energy expenditure caused by cold in 18FDG BAT-positive subjects is greatly affected by seasonal changes (Yoneshiro et al., 2016). Free fatty acids as reaction substrates were used by BAT to generate heat (Cannon and Nedergaard, 2004). After cold exposure, NEFA from WAT lipolysis and lipoproteins (TRL) from triglyceride-rich lipoproteins enter BAT (Heeren and Scheja, 2018). Cold exposure promotes BAT activation to stimulate TAG lipolysis, then long-chain fatty acids were released and UCP1 was activated, leading to rodent and human mitochondrial thermogenesis (Bartelt et al., 2011; Nie et al., 2015; Blondin et al., 2017b). Acute cold exposure leads to an increase NEFA levels in plasma. And the genes related to lipid utilization are upregulated with cold exposure in humans. It is worth noting that activated BAT is correlated to cold-induced lipolysis, increased FFA re-esterification, FFA oxidation and energy expenditure compared to both lean and obese individuals with no or negligible BAT activity (Chondronikola et al., 2014; Blondin et al., 2015; Chondronikola et al., 2016). The reason for the difference in fatty acid intake in BAT may be related to the basal level of BAT and the low oxidative metabolism capacity in the activated state (Saari et al., 2020). However, during a 4-month mild cold exposure in healthy lean participants, BAT recruitability was confirmed by cold exposure, but significant CIT response augmentation did not be observed (Lee et al., 2014). This may be due to a slight increase in ambient temperature during the day, which damages BAT and blunts previous metabolic benefits. A 10-day cold acclimation period in patients with type 2 diabetes resulted in only a minor increase in metabolic activity of the supraclavicular BAT region (Hanssen M. J. W. et al., 2015). In addition to being overweight, these participants were older and already had low activity in this BAT region at baseline.
Moreover, our results show that both BAT activity and volume are improved after acute cold exposure. Our findings are agreement with the study of Hanssen et al., who reported obese subjects acquired large amounts of BAT during a short-term cold exposure periods (Hanssen et al., 2016). After cold exposure, oxygen consumption and blood perfusion in BAT are increased, which may be responsible for the increase of BAT volume. Although, the large number of specialized studies had investigated cold-activated BAT in humans, BAT volume is reported in few studies. Since a threshold based on PET SUV alone leads to an overestimation of BAT numbers, and a threshold based on SUV and CT HU leads to an underestimation of total BAT activity, different analyzing techniques need to be used to get BAT activity and BAT volume. FDG PET-CT is considered as a standard tool to identify human BAT, however, it has serious limitations that only tissues that actively ingest glucose can be detected (Cypess et al., 2014). However, instead of glucose, fatty acids were the main substrate of BAT function. As a result, bias may exist in the estimation of the quantification in BAT activity. Indeed, BAT activity is related to age, sex, environmental temperature and body fat content. A study showed that BAT was detected more than 50% in subjects aged 20–29 years, and less than 10% aged 50 and older (Yoneshiro et al., 2011b). In addition, some studies have found that PET/CT studies have observed a higher prevalence of BAT in winter than in summer, possibly due to the changes in environmental temperature (Cypess et al., 2009; Saito et al., 2009; Lee et al., 2010; Ouellet et al., 2011). BAT activity is negatively correlated with BMI, and the activity of healthy people is greater than that of overweight or obese people. The reason for this fact was that the supraclavicular fat depot in obese people is dominated by white fat, which leads to the weakening of BAT activation ability (van Marken Lichtenbelt et al., 2009).
The effects of cold exposure and BAT on energy metabolism have been intensively studied in animal models, but some points should be noted when we apply these studies to humans. Firstly, the fat depots in mice mainly located in the interscapular region and in the cervical spine, around the heart and kidneys, while in adult humans they mainly located in cervical, paravertebral, axillary and clavicular regions (Townsend and Tseng, 2015; Ikeda et al., 2018). Secondly, studies in rodents proved two type of BAT, “classical and brite”, which differ in development origin (Petrovic et al., 2010; Wu et al., 2012). Brown adipocytes, located in the interscapular region, originate from Myf5-positive myoblastic cells that called classical BAT(Timmons et al., 2007; Seale et al., 2008). Brite cells, named “beige or brite adipocytes”, originate from a Myf5-negative precursor cells, and/or the formation of brite cells within the white adipose depot is referred to as “browning” (Walden et al., 2012; Wu et al., 2012). BAT depots around the neck and in the supraclavicular regions of humans may comprise both classical brown and brite/beige cells, but its distribution may be affected by age and region (Jespersen et al., 2013; Lidell et al., 2013). Therefore, further studies are needed to distinguish the types of human BAT more clearly, to elucidate their role and mechanism in whole-body energy. When exposure to low temperature for a long time, it can promote browning of white adipose tissue, and increases the amount of brown adipocytes and the number of UCP1 through proliferation of interstitial preadipocytes and matured adipocytes of animals (Bukowiecki et al., 1986; Okamatsu-Ogura et al., 2017). Although experiments in rodents have shown the profound effects of cold on process of browning, the results of human experiments remain inconclusive. Studies in healthy human scWAT cells suggested that long-term cold exposure increased UCP1 expression and mitochondrial activity, which are characteristic of beige cells (Kern et al., 2014; Finlin et al., 2017). But a cold-adaptation study in healthy humans did not show scWAT beiging (van der Lans et al., 2013). Besides, 2–6 weeks cold exposure increases the volume of BAT, but it is not possible whether the increase volume was from enhanced activity or from an expanded cell mass (Blondin et al., 2014; Lee et al., 2014). While long-term cold exposure can promote beiging in cells, further research is needed to determine the temperature and time to induce beiging in humans, and the feasibility of these results applying. Furthermore, Compared with rodents, human have relatively less BAT, with 0.02% of body weight in humans and 0.4–1% of body weight in rodents (Geisler, 2011). Besides, mice are often housed at 22°C, a temperature different from the thermoneutrality (29–30°C) (de Jong et al., 2019). Such adaptation renders their BAT chronically activated, which may influence their energy metabolic behavior. Humans can protect themselves against a cold challenge, leading to BAT at a lower level. This may explain the differences in BAT activity and energy metabolism caused by cold exposure in animals and humans.
The current meta-analysis presents a response to the view that acute cold exposure to BAT is beneficial to human health, and there is also an evidence that chronic cold exposure can confer benefits on metabolic health. For example, sustained 6-week cold exposure resulted in increased BAT activity and CIT, along with decreased body fat mass (Yoneshiro et al., 2013a). Although, the potential of BAT activation to increase energy consumption inducing weight loss is exciting. Unfortunately, BAT activation through CIT in humans has just increased the energy expenditure and decreased the fat mass without eliciting any loss in body weight (Yoneshiro et al., 2013b). A study showed that BAT prevalence increased as an acute response to cold environment (less than 7 days), and the effect was neutralized with delayed of low temperatures (Kim et al., 2008). Moreover, maximal activation of BAT for extended periods is a difficult feat, particularly in humans. Compared with rodents, exposure humans to a severe and delayed environment is impractical. On the other hand, prolonged cold stimulation leads to a compensatory increase in people’s food intake, which to some extent counteracts the effect of BAT activation against obesity (Ravussin et al., 2014). Therefore, chronic cold exposure may cannot promote weight loss. Conversely, acute cold exposure holds a great potential for combating with obesity due to its ease of implementation. In the future, combining acute cold exposure with other modalities such as exercise, diet, and medication could be considered as a meaningful contribution to addressing obesity and metabolic disorder.
BAT activity and volume had been increased after acute cold exposure. BAT also played an important role in regulating metabolism. After cold exposure, the body’s energy metabolic increases as well as NEFA intake, both of which indicated that it could regulate metabolism and increase heat production. BAT thermogenesis may be available as therapies to against obesity in the near future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
LZ, CH, and LY were involved in study conceptualization and design that led to this article. CH, JY, YZ, and JW were involved in the methodology. ZS, XM, and HQ were responsible for the formal analysis. CH, ZS, and LZ were responsible for writing and preparing the original draft. All authors participated in the writing, review, and editing of the manuscript.
This work was sponsored by the Science and Technology Projects of Jilin provincial science and Technoogy Department (20200201523JC) and the Education Department of Jilin Province (JJKH20201052KJ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
WHO, world health organization; BAT, brown adipose tissue; SNS, sympathetic nervous system; β-AR, β-adrenergic receptor; UCP1, uncoupling protein one; NADH, nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide; ATP, adenosine triphosphate; BMI, body mass index; NST, non-shivering thermogenesis; FFA, free fatty acids; WAT, white adipose tissue; EE, energy expenditure; CIT, cold-induced thermogenesis; RCT, randomized controlled trials; FDG, 18 F-fluorodeoxyglucose; PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography; NEFA, non-esterified fatty acids; CI, confidence interval; SE, standard error; SD, standard deviation; GLUT4, glucose transporter type 4; TAG, removing triacylglycerol; TRL, lipolysis and lipoproteins.
{[“brown adipose tissue” (Title/Abstract) OR “brown fat” (Title/Abstract) OR “brown like adipose tissue” (Title/Abstract) OR “beige” (Title/Abstract) OR “brown like fat” (Title/Abstract) OR “brown adipose like phenotype” (Title/Abstract) OR “browning process” (Title/Abstract) AND “hypothermia” (Title/Abstract)] OR “hypothermia induced” [ Title/Abstract] OR “induced mild hypothermia” [Title/Abstract]} AND 1990/01/01:2021/5/31 [Date-Publication].
Anandacoomarasamy A., Caterson I., Sambrook P., Fransen M., March L. (2008). The Impact of Obesity on the Musculoskeletal System. Int. J. Obes. 32 (2), 211–222. doi:10.1038/sj.ijo.0803715
Baba S., Engles J. M., Huso D. L., Ishimori T., Wahl R. L. (2007). Comparison of Uptake of Multiple Clinical Radiotracers into Brown Adipose Tissue under Cold-Stimulated and Nonstimulated Conditions. J. Nucl. Med. 48 (10), 1715–1723. doi:10.2967/jnumed.107.041715
Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., et al. (2011). Brown Adipose Tissue Activity Controls Triglyceride Clearance. Nat. Med. 17 (2), 200–205. doi:10.1038/nm.2297
Blondin D. P., Daoud A., Taylor T., Tingelstad H. C., Bézaire V., Richard D., et al. (2017a). Four-week Cold Acclimation in Adult Humans Shifts Uncoupling Thermogenesis from Skeletal Muscles to Brown Adipose Tissue. J. Physiol. 595 (6), 2099–2113. doi:10.1113/jp273395
Blondin D. P., Labbé S. M., Noll C., Kunach M., Phoenix S., Guérin B., et al. (2015). Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects with Type 2 Diabetes. Diabetes 64 (7), 2388–2397. doi:10.2337/db14-1651
Blondin D. P., Labbé S. M., Tingelstad H. C., Noll C., Kunach M., Phoenix S., et al. (2014). Increased Brown Adipose Tissue Oxidative Capacity in Cold-Acclimated Humans. J. Clin. Endocrinol. Metab. 99 (3), E438–E446. doi:10.1210/jc.2013-3901
Blondin D. P., Tingelstad H. C., Noll C., Frisch F., Phoenix S., Guérin B., et al. (2017b). Dietary Fatty Acid Metabolism of Brown Adipose Tissue in Cold-Acclimated Men. Nat. Commun. 8, 14146. doi:10.1038/ncomms14146
Brychta R. J., Huang S., Wang J., Leitner B. P., Hattenbach J. D., Bell S. L., et al. (2019). Quantification of the Capacity for Cold-Induced Thermogenesis in Young Men with and without Obesity. J. Clin. Endocrinol. Metab. 104 (10), 4865–4878. doi:10.1210/jc.2019-00728
Bukowiecki L. J., Géloën A., Collet A. J. (1986). Proliferation and Differentiation of Brown Adipocytes from Interstitial Cells during Cold Acclimation. Am. J. Physiol. 250 (6 Pt 1), C880–C887. doi:10.1152/ajpcell.1986.250.6.C880
Cannon B., Nedergaard J. (2004). Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 84 (1), 277–359. doi:10.1152/physrev.00015.2003
Chen K. Y., Brychta R. J., Linderman J. D., Smith S., Courville A., Dieckmann W., et al. (2013). Brown Fat Activation Mediates Cold-Induced Thermogenesis in Adult Humans in Response to a Mild Decrease in Ambient Temperature. J. Clin. Endocrinol. Metab. 98 (7), E1218–E1223. doi:10.1210/jc.2012-4213
Chondronikola M., Volpi E., Børsheim E., Porter C., Annamalai P., Enerbäck S., et al. (2014). Brown Adipose Tissue Improves Whole-Body Glucose Homeostasis and Insulin Sensitivity in Humans. Diabetes 63 (12), 4089–4099. doi:10.2337/db14-0746
Chondronikola M., Volpi E., Børsheim E., Porter C., Saraf M. K., Annamalai P., et al. (2016). Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 23 (6), 1200–1206. doi:10.1016/j.cmet.2016.04.029
Cypess A. M., Haft C. R., Laughlin M. R., Hu H. H. (2014). Brown Fat in Humans: Consensus Points and Experimental Guidelines. Cell Metab. 20 (3), 408–415. doi:10.1016/j.cmet.2014.07.025
Cypess A. M., Kahn C. R. (2010). Brown Fat as a Therapy for Obesity and Diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17 (2), 143–149. doi:10.1097/med.0b013e328337a81f
Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., et al. (2009). Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 360 (15), 1509–1517. doi:10.1056/nejmoa0810780
de Jong J. M. A., Sun W., Pires N. D., Frontini A., Balaz M., Jespersen N. Z., et al. (2019). Human Brown Adipose Tissue Is Phenocopied by Classical Brown Adipose Tissue in Physiologically Humanized Mice. Nat. Metab. 1 (8), 830–843. doi:10.1038/s42255-019-0101-4
Dinas P. C., Nikaki A., Jamurtas A. Z., Prassopoulos V., Efthymiadou R., Koutedakis Y., et al. (2015). Association between Habitual Physical Activity and Brown Adipose Tissue Activity in Individuals Undergoing PET-CT Scan. Clin. Endocrinol. 82 (1), 147–154. doi:10.1111/cen.12620
Finlin B. S., Zhu B., Confides A. L., Westgate P. M., Harfmann B. D., Dupont-Versteegden E. E., et al. (2017). Mast Cells Promote Seasonal White Adipose Beiging in Human. Diabetes 66 (5), 1237–1246. doi:10.2337/db16-1057
Geisler J. G. (2011). Targeting Energy Expenditure via Fuel Switching and beyond. Diabetologia 54 (2), 237–244. doi:10.1007/s00125-010-1932-4
Hanssen M. J., Wierts R., Hoeks J., Gemmink A., Brans B., Mottaghy F. M., et al. (2015b). Glucose Uptake in Human Brown Adipose Tissue Is Impaired upon Fasting-Induced Insulin Resistance. Diabetologia 58 (3), 586–595. doi:10.1007/s00125-014-3465-8
Hanssen M. J. W., Hoeks J., Brans B., van der Lans A. A. J. J., Schaart G., van den Driessche J. J., et al. (2015a). Short-term Cold Acclimation Improves Insulin Sensitivity in Patients with Type 2 Diabetes Mellitus. Nat. Med. 21 (8), 863–865. doi:10.1038/nm.3891
Hanssen M. J. W., van der Lans A. A. J. J., Brans B., Hoeks J., Jardon K. M. C., Schaart G., et al. (2016). Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes 65 (5), 1179–1189. doi:10.2337/db15-1372
Heeren J., Scheja L. (2018). Brown Adipose Tissue and Lipid Metabolism. Curr. Opin. Lipidol. 29 (3), 180–185. doi:10.1097/mol.0000000000000504
Higgins J. P. T., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Ikeda K., Maretich P., Kajimura S. (2018). The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metabolism 29 (3), 191–200. doi:10.1016/j.tem.2018.01.001
Jespersen N. Z., Larsen T. J., Peijs L., Daugaard S., Homøe P., Loft A., et al. (2013). A Classical Brown Adipose Tissue mRNA Signature Partly Overlaps with Brite in the Supraclavicular Region of Adult Humans. Cell Metab. 17 (5), 798–805. doi:10.1016/j.cmet.2013.04.011
Kern P. A., Finlin B. S., Zhu B., Rasouli N., McGehee R. E., Westgate P. M., et al. (2014). The Effects of Temperature and Seasons on Subcutaneous White Adipose Tissue in Humans: Evidence for Thermogenic Gene Induction. J. Clin. Endocrinol. Metabolism 99 (12), E2772–E2779. doi:10.1210/jc.2014-2440
Kim S., Krynyckyi B. R., Machac J., Kim C. K. (2008). Temporal Relation between Temperature Change and FDG Uptake in Brown Adipose Tissue. Eur. J. Nucl. Med. Mol. Imaging 35 (5), 984–989. doi:10.1007/s00259-007-0670-4
Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K., et al. (2016). Body Fatness and Cancer - Viewpoint of the IARC Working Group. N. Engl. J. Med. 375 (8), 794–798. doi:10.1056/nejmsr1606602
Lee P., Greenfield J. R., Ho K. K. Y., Fulham M. J. (2010). A Critical Appraisal of the Prevalence and Metabolic Significance of Brown Adipose Tissue in Adult Humans. Am. J. Physiology-Endocrinology Metabolism 299 (4), E601–E606. doi:10.1152/ajpendo.00298.2010
Lee P., Smith S., Linderman J., Courville A. B., Brychta R. J., Dieckmann W., et al. (2014). Temperature-acclimated Brown Adipose Tissue Modulates Insulin Sensitivity in Humans. Diabetes 63 (11), 3686–3698. doi:10.2337/db14-0513
Leitner B. P., Huang S., Brychta R. J., Duckworth C. J., Baskin A. S., McGehee S., et al. (2017). Mapping of Human Brown Adipose Tissue in Lean and Obese Young Men. Proc. Natl. Acad. Sci. U.S.A. 114 (32), 8649–8654. doi:10.1073/pnas.1705287114
Lidell M. E., Betz M. J., Leinhard O. D., Heglind M., Elander L., Slawik M., et al. (2013). Evidence for Two Types of Brown Adipose Tissue in Humans. Nat. Med. 19 (5), 631–634. doi:10.1038/nm.3017
M U. D., Raiko J., Saari T., Kudomi N., Tolvanen T., Oikonen V., et al. (2016). Human Brown Adipose Tissue [(15)O]O2 PET Imaging in the Presence and Absence of Cold Stimulus. Eur. J. Nucl. Med. Mol. Imaging 43 (10), 1878–1886. doi:10.1007/s00259-016-3364-y
Marlatt K. L., Ravussin E. (2017). Brown Adipose Tissue: an Update on Recent Findings. Curr. Obes. Rep. 6 (4), 389–396. doi:10.1007/s13679-017-0283-6
Nedergaard J., Bengtsson T., Cannon B. (2011). New Powers of Brown Fat: Fighting the Metabolic Syndrome. Cell Metab. 13 (3), 238–240. doi:10.1016/j.cmet.2011.02.009
Nie Y., Yan Z., Yan W., Xia Q., Zhang Y. (2015). Cold Exposure Stimulates Lipid Metabolism, Induces Inflammatory Response in the Adipose Tissue of Mice and Promotes the Osteogenic Differentiation of BMMSCs via the P38 MAPK Pathway In Vitro. Int. J. Clin. Exp. Pathol. 8 (9), 10875–10886.
Oelkrug R., Polymeropoulos E. T., Jastroch M. (2015). Brown Adipose Tissue: Physiological Function and Evolutionary Significance. J. Comp. Physiol. B 185 (6), 587–606. doi:10.1007/s00360-015-0907-7
Okamatsu-Ogura Y., Fukano K., Tsubota A., Nio-Kobayashi J., Nakamura K., Morimatsu M., et al. (2017). Cell-cycle Arrest in Mature Adipocytes Impairs BAT Development but Not WAT Browning, and Reduces Adaptive Thermogenesis in Mice. Sci. Rep. 7 (1), 6648. doi:10.1038/s41598-017-07206-8
Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., et al. (2012). Brown Adipose Tissue Oxidative Metabolism Contributes to Energy Expenditure during Acute Cold Exposure in Humans. J. Clin. Invest. 122 (2), 545–552. doi:10.1172/jci60433
Ouellet V., Routhier-Labadie A., Bellemare W., Lakhal-Chaieb L., Turcotte E., Carpentier A. C., et al. (2011). Outdoor Temperature, Age, Sex, Body Mass Index, and Diabetic Status Determine the Prevalence, Mass, and Glucose-Uptake Activity of 18F-FDG-Detected BAT in Humans. J. Clin. Endocrinol. Metab. 96 (1), 192–199. doi:10.1210/jc.2010-0989
Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2010). Chronic Peroxisome Proliferator-Activated Receptor γ (PPARγ) Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-Containing Adipocytes Molecularly Distinct from Classic Brown Adipocytes. J. Biol. Chem. 285 (10), 7153–7164. doi:10.1074/jbc.m109.053942
Raiko J. R. H., Saari T., Orava J., Savisto N., Parkkola R., Haaparanta-Solin M., et al. (2021). Changes in Electrocardiogram Parameters during Acute Nonshivering Cold Exposure and Associations with Brown Adipose Tissue Activity, Plasma Catecholamine Levels, and Brachial Blood Pressure in Healthy Adults. Physiol. Rep. 9 (3), e14718. doi:10.14814/phy2.14718
Ravussin Y., Xiao C., Gavrilova O., Reitman M. L. (2014). Effect of Intermittent Cold Exposure on Brown Fat Activation, Obesity, and Energy Homeostasis in Mice. PLoS One 9 (1), e85876. doi:10.1371/journal.pone.0085876
Romu T., Vavruch C., Dahlqvist-Leinhard O., Tallberg J., Dahlström N., Persson A., et al. (2016). A Randomized Trial of Cold-Exposure on Energy Expenditure and Supraclavicular Brown Adipose Tissue Volume in Humans. Metabolism 65 (6), 926–934. doi:10.1016/j.metabol.2016.03.012
Saari T. J., Raiko J., M U. D., U-Din M., Taittonen M., Laine J., et al. (2020). Basal and Cold-Induced Fatty Acid Uptake of Human Brown Adipose Tissue Is Impaired in Obesity. Sci. Rep. 10 (1), 14373. doi:10.1038/s41598-020-71197-2
Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., et al. (2009). High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans. Diabetes 58 (7), 1526–1531. doi:10.2337/db09-0530
Scholander P. F., Hammel H. T., Hart J. S., Lemessurier D. H., Steen J. (1958). Cold Adaptation in Australian Aborigines. J. Appl. Physiology 13 (2), 211–218. doi:10.1152/jappl.1958.13.2.211
Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., et al. (2008). PRDM16 Controls a Brown Fat/skeletal Muscle Switch. Nature 454 (7207), 961–967. doi:10.1038/nature07182
Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 349, g7647. doi:10.1136/bmj.g7647
Shibata H., Pérusse F., Vallerand A., Bukowiecki L. J. (1989). Cold Exposure Reverses Inhibitory Effects of Fasting on Peripheral Glucose Uptake in Rats. Am. J. Physiol. 257 (1 Pt 2), R96–R101. doi:10.1152/ajpregu.1989.257.1.R96
Shimizu Y., Nikami H., Tsukazaki K., Machado U. F., Yano H., Seino Y., et al. (1993). Increased Expression of Glucose Transporter GLUT-4 in Brown Adipose Tissue of Fasted Rats after Cold Exposure. Am. J. Physiol. 264 (6 Pt 1), E890–E895. doi:10.1152/ajpendo.1993.264.6.E890
Singh G. M., Danaei G., Farzadfar F., Stevens G. A., Woodward M., Wormser D., et al. (2013). The Age-specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: a Pooled Analysis. PLoS One 8 (7), e65174. doi:10.1371/journal.pone.0065174
Taniguchi H., Shimizu K., Wada S., Nirengi S., Kataoka H., Higashi A. (2020). Effects of Beta-Conglycinin Intake on Circulating FGF21 Levels and Brown Adipose Tissue Activity in Japanese Young Men: a Single Intake Study and a Randomized Controlled Trial. J. Physiol. Anthropol. 39 (1), 17. doi:10.1186/s40101-020-00226-w
Thuzar M., Law W. P., Ratnasingam J., Jang C., Dimeski G., Ho K. K. Y. (2018). Glucocorticoids Suppress Brown Adipose Tissue Function in Humans: a Double-Blind Placebo-Controlled Study. Diabetes Obes. Metab. 20 (4), 840–848. doi:10.1111/dom.13157
Timmons J. A., Wennmalm K., Larsson O., Walden T. B., Lassmann T., Petrovic N., et al. (2007). Myogenic Gene Expression Signature Establishes that Brown and White Adipocytes Originate from Distinct Cell Lineages. Proc. Natl. Acad. Sci. U.S.A. 104 (11), 4401–4406. doi:10.1073/pnas.0610615104
Townsend K. L., Tseng Y. H. (2015). Of Mice and Men: Novel Insights Regarding Constitutive and Recruitable Brown Adipocytes. Int. J. Obes. Suppl. 5 (5Suppl. 1), S15–S20. doi:10.1038/ijosup.2015.5
Tseng Y.-H., Cypess A. M., Kahn C. R. (2010). Cellular Bioenergetics as a Target for Obesity Therapy. Nat. Rev. Drug Discov. 9 (6), 465–482. doi:10.1038/nrd3138
Vallerand A. L., Lupien J., Bukowiecki L. J. (1986). Cold Exposure Reverses the Diabetogenic Effects of High-Fat Feeding. Diabetes 35 (3), 329–334. doi:10.2337/diabetes.35.3.329
van der Lans A. A. J. J., Hoeks J., Brans B., Vijgen G. H. E. J., Visser M. G. W., Vosselman M. J., et al. (2013). Cold Acclimation Recruits Human Brown Fat and Increases Nonshivering Thermogenesis. J. Clin. Invest. 123 (8), 3395–3403. doi:10.1172/jci68993
van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M. A. F. L., Kemerink G. J., Bouvy N. D., et al. (2009). Cold-activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 360 (15), 1500–1508. doi:10.1056/nejmoa0808718
Vijgen G. H. E. J., Bouvy N. D., Teule G. J. J., Brans B., Schrauwen P., van Marken Lichtenbelt W. D. (2011). Brown Adipose Tissue in Morbidly Obese Subjects. PLoS One 6 (2), e17247. doi:10.1371/journal.pone.0017247
Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., et al. (2009). Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 360 (15), 1518–1525. doi:10.1056/nejmoa0808949
Waldén T. B., Hansen I. R., Timmons J. A., Cannon B., Nedergaard J. (2012). Recruited vs. Nonrecruited Molecular Signatures of Brown, "brite," and White Adipose Tissues. Am. J. Physiology-Endocrinology Metabolism 302 (1), E19–E31. doi:10.1152/ajpendo.00249.2011
Wijers S. L. J., Saris W. H. M., Lichtenbelt W. D. v. M. (2010). Cold-induced Adaptive Thermogenesis in Lean and Obese. Obes. (Silver Spring) 18 (6), 1092–1099. doi:10.1038/oby.2010.74
Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A.-H., et al. (2012). Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 150 (2), 366–376. doi:10.1016/j.cell.2012.05.016
Wyndham C. H., Morrison J. F. (1958). Adjustment to Cold of Bushmen in the Kalahari Desert. J. Appl. Physiology 13 (2), 219–225. doi:10.1152/jappl.1958.13.2.219
Yau W. W., Yen P. M. (2020). Thermogenesis in Adipose Tissue Activated by Thyroid Hormone. Int. J. Mol. Sci. 21 (8). doi:10.3390/ijms21083020
Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., et al. (2013a). Recruited Brown Adipose Tissue as an Antiobesity Agent in Humans. J. Clin. Invest. 123 (8), 3404–3408. doi:10.1172/JCI67803
Yoneshiro T., Aita S., Matsushita M., Kameya T., Nakada K., Kawai Y., et al. (2011a). Brown Adipose Tissue, Whole-Body Energy Expenditure, and Thermogenesis in Healthy Adult Men. Obes. (Silver Spring) 19 (1), 13–16. doi:10.1038/oby.2010.105
Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., et al. (2013b). Recruited Brown Adipose Tissue as an Antiobesity Agent in Humans. J. Clin. Invest. 123 (8), 3404–3408. doi:10.1172/jci67803
Yoneshiro T., Aita S., Matsushita M., Okamatsu-Ogura Y., Kameya T., Kawai Y., et al. (2011b). Age-related Decrease in Cold-Activated Brown Adipose Tissue and Accumulation of Body Fat in Healthy Humans. Obes. (Silver Spring) 19 (9), 1755–1760. doi:10.1038/oby.2011.125
Keywords: obesity, brown adipose tissue, hypothermia, energy expenditure, meta-analysis
Citation: Huo C, Song Z, Yin J, Zhu Y, Miao X, Qian H, Wang J, Ye L and Zhou L (2022) Effect of Acute Cold Exposure on Energy Metabolism and Activity of Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Front. Physiol. 13:917084. doi: 10.3389/fphys.2022.917084
Received: 10 April 2022; Accepted: 13 June 2022;
Published: 28 June 2022.
Edited by:
Heather Bradshaw, Indiana University Bloomington, United StatesReviewed by:
Yingyun Gong, Nanjing Medical University, ChinaCopyright © 2022 Huo, Song, Yin, Zhu, Miao, Qian, Wang, Ye and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Ye, amx5ZWxpbkAxNjMuY29t; Liting Zhou, emhvdWx0dGdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.