
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 22 July 2022
Sec. Exercise Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.899670
 Henning Wackerhage1*†
Henning Wackerhage1*† Sebastian Gehlert2*†
Sebastian Gehlert2*† Henry Schulz3
Henry Schulz3 Sebastian Weber4
Sebastian Weber4 Susanne Ring-Dimitriou5
Susanne Ring-Dimitriou5 Oliver Heine6
Oliver Heine6Today, researchers, practitioners, and physicians measure the concentration of lactate during a graded exercise test to determine thresholds related to the maximal lactate steady state (maxLass) as a sensitive measure of endurance capacity. In the 1970s and 1980s, a group of Cologne-based researchers around Wildor Hollmann, Alois Mader, and Hermann Heck developed the methodology for systematic lactate testing and introduced a 4 mmol.L−1 lactate threshold. Later, they also developed the concept of the maxLass, and Mader designed a sophisticated mathematical model of human energy metabolism during exercise. Mader`s model simulates metabolic responses to exercise based on individual variables such as maximum oxygen uptake (
In the 1970s and 1980s, a group of Cologne-based physicians and exercise physiologists made major contributions to lactate testing and the modeling of human exercise metabolism (Wackerhage, 2021). Arguably, their work is under-appreciated outside German-speaking countries. This is largely due to the fact that the Cologne group published many of their studies in German. Moreover, several important publications by the Cologne group are not listed on Medline and so are hard to find. As several of these publications contain major conceptual advances in relation to lactate testing and human exercise metabolism, we decided to summarize the work of the Cologne group in this review.
Before discussing the contributions of the Cologne group, we will first clarify the terminology in relation to threshold concepts. This is necessary, as for example, Heck et al. already listed no less than nine terms for thresholds in 1985 and the number has grown since (Faude et al., 2009). Generally, thresholds are defined either based on standard gas exchange and ventilatory measurements as pioneered by Karlman Wasserman and colleagues (Poole et al., 2020; Keir et al., 2022) or on lactate measurements which was introduced by the Cologne group (Mader et al., 1976; Faude et al., 2009).
There are two types of ventilatory and lactate-based thresholds for which the following terms have been used:
1) Thresholds terms describing the first, exercise-related rise of the concentration of lactate typically during a graded exercise tests. Terms used to describe this include “lactate threshold 1” (LT1) (Jamnick et al., 2020), “anaerobic threshold” (Wasserman et al., 1973), and “aerobic threshold” (Kindermann et al., 1979).
2) Thresholds terms linked to the maximal lactate steady state (maxLass or MLSS, (Heck et al., 1985). In addition to “maximal lactate steady state”, other terms used include “lactate threshold” 2 (LT2) (Jamnick et al., 2020), “aerobic-anaerobic transition or threshold” (Mader et al., 1976), “anaerobic threshold” (Kindermann et al., 1979), or “individual anaerobic threshold” (Stegmann et al., 1981). The maxLass is also related to the concept of “critical power” (Iannetta et al., 2022) which we do not discuss further here.
In this review, we only refer to the second threshold, i.e., the maximal lactate steady state. For this, we use the acronym “maxLass” throughout. However, the maxLass is difficult to measure in practice and before Heck et al. (1985) introduced the concept of the maxLass, Mader et al. (1976) proposed a “4 mmol.L−1 lactate threshold” essentially as an estimate for the maxLass. In our review, we therefore also use the term “4 mmol.L−1 lactate threshold” to specifically refer to the 4 mmol.L−1 lactate estimate of the maxLass. Occasionally, we also use other threshold terms used in specific studies that we cite.
In 1807/1808, Jöns Jacob Berzelius detected lactate in hunted stags (Gladden, 2008), suggesting that lactate had increased during exercise. In the period that followed, researchers including Nobel laureates August Krogh (Krogh and Lindhard, 1920) and Archibald Vivian Hill (Hill and Lupton, 1923) characterized the response of lactate during exercise. However, this work was descriptive and did not result in standardized, lactate-based, submaximal tests of endurance capacity. Instead, Hill and Lupton conceptualized the
The two major limitations of the
The first major study by the Cologne group on lactate testing was published in 1976 in Sportarzt und Sportmedizin (Mader et al., 1976), which was the house journal of the West German Sportärzte (sports physicians). What was the background of the 1976 study? The first author was Alois Mader, a physician who in 1974 had fled from East to West Germany. In the West, he soon became a member of Wildor Hollmann’s sports medicine group in Cologne. With him he brought a method to measure the concentration of lactate in 20 μl of blood which he had developed together with the chemist Peter Haase in Eastern Germany in 1970. At that time, advances in spectrophotometric and fluorometric methods (Lowry, 1972) made it possible to quickly measure the concentration of lactate enzymatically in microliters of capillary blood from the earlobe. This greatly improved the measurement of lactate as it was previously measured chemically over several hours in milliliters of arterial blood obtained by more risky arterial puncturing (Hollmann, 2001). The 1976 study also gives detailed advice on how to conduct lactate testing by stating that the ergometry should be sports-specific (i.e., cycle ergometry for cyclists, treadmill running for runners, and canoe ergometry for canoeists), the stages during a graded lactate exercise test should last at least 4 min, and the workload or velocity at the 4 mmol.L−1 lactate threshold reacts more sensitively to changes in sports-specific endurance performance than the
In Figure 1, we reproduce a figure of the 1976 study which is largely unknown in the English-speaking world. It shows the effect of a ≈6-week endurance training on heart volume measured by X-ray, the
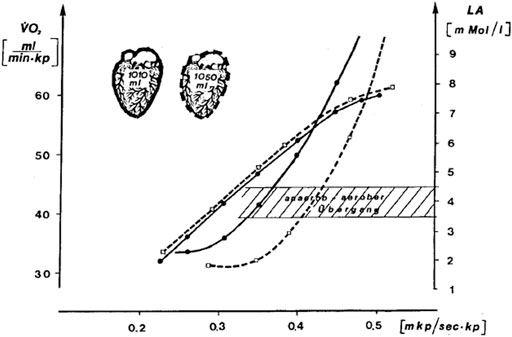
FIGURE 1. Effect of a ≈6-week endurance training by one individual at the intensity of the 4 mmol.L−1 lactate threshold (Mader et al. termed it “aerobic-anaerobic transition”) on heart volume measured by X-ray,
In the years after 1976, researchers in German-speaking countries and elsewhere routinely performed lactate exercise tests and learned how to interpret the resultant data. At that time, several groups proposed strategies to determine an “individual” lactate threshold instead of a fixed 4 mmol.L−1 lactate threshold. To find out whether the 4 mmol.L−1 lactate threshold is at the transition from purely aerobic to partially anaerobic metabolism, Heck and colleagues measured lactate during 30 min exercise bouts at different constant workloads. This work led to the term “maximum lactate steady state”, abbreviated as maxLass or as MLSS by others. Their criterion for the maxLass was the “[maximal] treadmill speed [where] the lactate level does not increase […] more than 1 mmol.L−1 during the last 20 min [of exercise]” (Heck et al., 1985). In contrast to the 1976 study, the 1985 study and the concept of the maxLass (or MLSS) are discussed today in the English-speaking literature.
At the beginning of the 1980s, Mader then started to develop a model of human energy metabolism during exercise. He first introduced his model in his habilitation, a type of thesis that used to be a prerequisite for a professorship, in 1984. It described a mathematical model that simulates the exercise response of ATP, phosphocreatine, lactate, pH, glycolytic ATP resynthesis, and ATP resynthesis by oxidative phosphorylation in humans (Mader, 1984). Mader not only described the underlying formulas that connect the pathways of human energy metabolism but also used them to create a computer model.
What inspired Alois Mader to develop such a mathematical model of human exercise metabolism, what influenced its design and how did it work? Mader realized that human energy metabolism in the 1980s was a black box. Specifically, researchers investigated the effect of exercise on the concentration of blood lactate but the mechanisms by which an exercise input causes a metabolic output were unclear. The challenge was to come up with a set of equations that linked input and output.
Key ideas for his model also originate from the Austrian scientist Ludwig von Bertalanffy, one of the founders of systems biology (von Bertalanffy, 1950). Bertalanffy had developed a theory for open systems such as living organisms. Such open systems exchange food and gases with the environment and maintain a steady state. This is different from closed systems that do not exchange molecules with the environment (von Bertalanffy, 1950). Mader then applied the principles of open systems and his knowledge of metabolic reactions to quantitatively model the behavior of human energy metabolism in response to exercise. When designing his model, he also followed Occam’s razor principle which requires choosing the simplest theory to try to explain a phenomenon. This implies using as few variables and equations as possible and to link them to each other through clear, logical, mathematical relationships (Walsh, 1979).
Here, we describe Mader’s mathematical model of human exercise metabolism. Mader first published this model in German (Mader, 1984), then together with Heck in English (Mader and Heck, 1986), and a revised English version in 2003 (Mader, 2003). While the latter studies were published in English, they are hard to follow as, for example, the 2003 study contains no less than 33 mathematical equations. To make Mader’s reasoning and model accessible, we will now discuss the main features of Mader’s model without mathematical formulas.
The starting point for Mader’s mathematical model was to convert exercise intensity or power output in Watts into the rate of ATP hydrolysis in mmol.L−1 of ATP per second, assuming a constant work efficiency. Because there is only ≈5–8 mmol ATP per kg muscle (Harris et al., 1974; Dawson, 1983), any exercise-induced rise in the rate of ATP hydrolysis must immediately be matched by increases in the overall rate of ATP resynthesis to avoid ATP depletion and rigor mortis.
ATP resynthesis occurs via three reactions which are
1) ATP resynthesis from phosphocreatine (PCr) via the Lohmann reaction (ADP + phosphocreatine ↔ ATP + creatine),
2) ATP resynthesis via glycolysis which leads to pyruvate and lactate synthesis and a reduction of the pH,
3) ATP resynthesis from oxidative phosphorylation which is linked to oxygen uptake.
Moreover, Mader has calculated the rate of lactate oxidation as the group of George Brooks had earlier reported that lactate oxidation or clearance increases in response to endurance training in rats (Donovan and Brooks, 1983). An assumption of the model is that muscular energy metabolism controls itself via autoregulatory mechanisms. This means that exercise-modulated metabolites such as ADP activate or inhibit key regulatory enzymes such as phosphofructokinase and the pyruvate dehydrogenase complex (Baker et al., 2010). In Mader’s model, ADP and AMP activate metabolic enzymes whereas hydrogen ions and ATP inhibit them (Mader, 2003). Mader’s challenge was to come up with a set of equations that could realistically model the rates of these reactions and the concentration of metabolites depending on an individual’s variables such as muscle mass, maximal glycolytic rate (termed by Mader the maximal rate of lactate formation, abbreviated as νLa.max), and oxidative capacity. A comparatively simple task was to simulate the relationship between ATP, ADP, AMP, and phosphocreatine via reactions such as the Lohmann reaction as McGilvery and Murray had already modeled this in the mid-1970s (McGilvery and Murray, 1974).
Next, Mader had to calculate ATP resynthesis via glycolysis based on studies that identified regulators of glycolysis. To model this, Mader assumed ADP and AMP as activators and a low pH as an inhibitor of glycolytic ATP resynthesis (Mader, 1984; Mader, 2003), red curves in Figure 2. This glycolytic ATP resynthesis equation models two important features of exercise metabolism:
1) It models that at low intensities, the rate of pyruvate synthesis by glycolysis is below the rate of pyruvate oxidation, that is, theoretically possible at such intensities. At exercise intensities above the maxLass, glycolytic pyruvate synthesis exceeds the amount of pyruvate that can be oxidized by mitochondria. Therefore, pyruvate and lactate, which are linked via the lactate dehydrogenase reaction, rise continuously.
2) It models inhibition of glycolytic ATP resynthesis by a low, acidic pH. Mechanistically, an acid pH inhibits phosphofructokinase which is the rate-limiting enzyme of glycolysis (Trivedi and Danforth, 1966; Dobson et al., 1986).
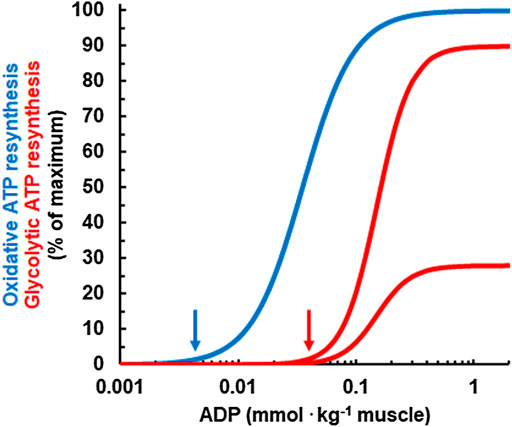
FIGURE 2. Relationship between the concentration of ADP and the simulated, relative rates of oxidative (blue) and glycolytic ATP resynthesis (red) for a pH of 7 (upper red curve) and for a pH of 6.4 (lower red curve). Note that ADP needs to rise to higher concentrations for glycolytic ATP resynthesis to kick in than for oxidative ATP resynthesis. This is an important feature for the regulation of human energy metabolism and explains why oxidative ATP resynthesis is dominant at rest and during low intensity exercise. Moreover, as an acid pH inhibits phosphofructokinase, the rate of glycolytic ATP resynthesis at a given ADP concentration is lower if the pH is in reduced. In the model, the maximal rate of glycolytic ATP resynthesis is reached at a pH of 7.4 which is not shown in the figure.
Finally, Mader used the concentration of ADP to calculate the rate of ATP resynthesis from oxidative phosphorylation. In this model, the activation of oxidative metabolism is described by a Hill equation of 2nd order (blue curve in Figure 2), while the activation of glycolysis is described by a 3rd order equation. Such a sigmoid equation was first used by Archibald V. Hill to describe oxygen binding to hemoglobin (Hill, 1910). Mader deemed this necessary to model “realistic” rates of oxidative ATP resynthesis and V̇O2. Later, other studies experimentally confirmed a sigmoid equation for the regulation of oxidative ATP resynthesis (Jeneson et al., 1996; Cieslar and Dobson, 2000).
In the “engine room” of Mader’s human exercise metabolism model lies the “crossing point” where pyruvate synthesis equals pyruvate oxidation. Figure 3A illustrates the relevant metabolic pathways and Figure 3B the crossing point curves. At low exercise intensities, the concentration of ADP is still comparatively low. Based on the equation for glycolytic ATP resynthesis, the rate of glycolytic pyruvate synthesis (red line in Figure 3B) is lower than the amount of pyruvate that can be oxidized by mitochondria (blue line in Figures 3B–[1]). At such low intensities, glycolysis synthesizes less pyruvate and acetyl-CoA than the mitochondria need for oxidative phosphorylation (i.e., the red pyruvate synthesis line is below the blue pyruvate oxidation line) and so the model assumes that the “missing” acetyl-CoA is synthesized mainly from acetyl CoA generated by the β-oxidation of fatty acids. We discuss this assumption below.
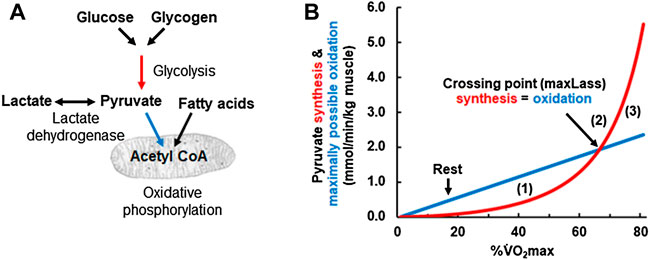
FIGURE 3. (A) Schematic drawing of the pathways simulated in Panel 3 (B). Red arrow refers to glycolytic ATP resynthesis or pyruvate synthesis, the glycolytic flux rate νLa. The blue arrows refer to the oxidative ATP resynthesis or the maximally possible rate of pyruvate oxidation, the oxidative flux (V̇O2). (B) Calculated glycolytic pyruvate (and lactate) synthesis [red arrow in (A) and red line in (B)] and maximally possible rate of pyruvate (and lactate) oxidation at different exercise intensities expressed as a percentage of the
At higher exercise intensities, the rate of glycolysis rises to a point where the rate of pyruvate formation by glycolysis equals the rate of pyruvate oxidation (i.e., the red pyruvate synthesis line meets the blue pyruvate oxidation line in Figures 3B–[2]). At the crossing point, pyruvate synthesis reaches the maximally possible rate of pyruvate oxidation by mitochondria. As pyruvate is in equilibrium with lactate via the lactate dehydrogenase reaction, the turning point for pyruvate will be the turning point for lactate and the maxLass (Heck et al., 1985). If the power output rises further, then pyruvate synthesis exceeds the maximally possible rate of pyruvate oxidation and so both pyruvate and lactate will rise with time (Figures 3B–[3]).
The “crossing point” figure also gives a simple explanation for the regulation of fat oxidation, that is, consistent with current thought. To the left of the “crossing point” in Figure 3B, glycolytic pyruvate synthesis is insufficient to provide all of the acetyl-CoA needed for oxidative ATP resynthesis (i.e., the red line is below the blue line). Already in 1986, Mader and Heck proposed that “the lack of pyruvate [due to insufficient pyruvate synthesis by glycolysis] is covered by fatty acid oxidation” (Mader and Heck, 1986). This is consistent with the observation that fat oxidation is highest at low and medium intensity (i.e., intensity to the left of the crossing point or maxLass) but approaches zero at intensities at and above the maxLass (van Loon et al., 2001). This interpretation also agrees with the recent conclusion by the Kiens group that “the rate of glycolysis seems thus to be central to mitochondrial acetyl-CoA availability and the regulation of fatty acid oxidation” (Lundsgaard et al., 2018). In summary, Mader designed already in the 1980s a mathematical model of human metabolism that realistically simulates human metabolic responses to exercise and explains the metabolic basis for the maxLass and the regulation of fat metabolism.
In this review, we will not attempt to provide evidence for all the assumptions underlying Mader’s original and extended model as this has been done elsewhere (Mader, 1984; Mader and Heck, 1986; Mader, 2003). Instead, we will use computer simulations made with a current version of Mader’s human exercise model to both demonstrate that it sufficiently models known human exercise phenomena and to highlight insights that can be gained by its use. First, we will show that Mader’s model simulates the V̇O2 slow component and thereby provides a simple, mechanistic explanation for it. Second, we will use the simulations to suggest that a high maximal glycolytic rate νLa.max results in a lower power at the maxLass and reduces endurance performance compared to an athlete with the same
In 1986, Brian Whipp and Karlman Wasserman described a “second, slower component of
1) During exercise above the maxLass the lactate concentration increases continuously in skeletal muscle and blood.
2) Because of the rise of lactate, the pH will decrease.
3) A low pH inhibits phosphofructokinase (Trivedi and Danforth, 1966; Dobson et al., 1986) and thereby reduces the rate of glycolytic ATP resynthesis and lactate formation after its initial peak.
4) If the ATP cost of exercise remains constant, then the pH-dependent reduction of glycolytic ATP resynthesis must be compensated by a rise in the rate of oxidative ATP resynthesis which manifests itself as the
5) If efficiency would worsen (Jones et al., 2011), which is not modeled by Mader’s model, then the
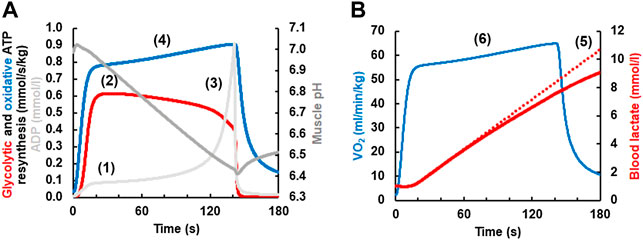
FIGURE 4. (A) Simulation of the V̇O2 slow component simulated with a current version of the Mader human exercise metabolism model. At the onset of exercise, the concentrations of ADP and AMP (not shown) increase (1). This stimulates phosphofructokinase and thereby increases the rate of glycolytic ATP resynthesis and lactate formation, reaching a maximum in-between 20–40 s (2). As the concentration of muscle lactate increases (B), muscle pH becomes increasingly acid from ≈20 s onwards (3). As an acid pH inhibits phosphofructokinase (Panel 4A) and thereby glycolytic ATP resynthesis, the rate of glycolytic ATP resynthesis declines after its maximum in-between ≈ 20–40 s even though the concentrations of ADP and AMP (not shown) rise greatly just before fatigue. Because glycolytic ATP resynthesis declines, oxidative ATP resynthesis must rise to meet the constant ATP hydrolysis during constant load exercise (4) (the contribution of ATP resynthesis from phosphocreatine is incorporated in the model but not shown). If the efficiency would worsen, then the slow component would increase further. Panel 4B On a whole body level, the reduction in the rate of glycolytic ATP resynthesis and lactate formation is seen (5) as a less than linear increase of the lactate concentration (the dotted line plots an increase of lactate at the rate that is reached in-between 20–40 s). The slow component of oxidative ATP resynthesis in (A) is seen as a corresponding slow component of the V̇O2 in (B) (6).
Recent studies concluded that the rate of glycolytic ATP resynthesis decreases with time during exercise at an intensity higher than the maxLass, as modeled in Figure 4. For example, O’Connell and colleagues suggested “that the rate of ATP replenishment by anaerobic metabolism might actually continue to decrease after the time when a steady state for V̇O2 should be achieved” (O’Connell et al., 2017). Similarly, Colosio et al. also suggested that “the emergence of a V̇O2 slow component is ascribable to a ‘metabolic shift’ between aerobic and anaerobic metabolisms” (Colosio et al., 2020), which is what Mader`s model essentially predicted in the mid-1980s.
Intuitively, one might assume that the ultimate endurance athlete would combine a high glycolytic and oxidative ATP resynthesis. However, glycolysis is a self-limiting system as it causes lactate to accumulate which in turn will cause the pH to fall which will inhibit phosphofructokinase and glycolytic ATP resynthesis (Figure 3) (Trivedi and Danforth, 1966; Dobson et al., 1986). Moreover, Mader`s model predicts that athletes with a higher νLa.max generally have higher lactate concentrations at the same workload, reach their maxLass at a lower workload and, due to higher carbohydrate usage, perform less well at long distance endurance events even if their
Using Mader’s equations, we have modeled a graded exercise test of two athletes, both with a
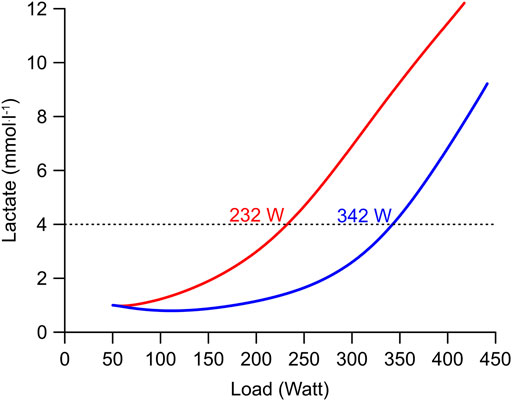
FIGURE 5. Simulation of a graded exercise test of two subjects with extreme differences of their νLa.max but equal
The idea that a high νLa.max can hinder endurance performance is consistent with the observation that endurance athletes have high percentages of type 1 fibers (Costill et al., 1976; Gehlert et al., 2012), which usually contain low concentrations of anaerobic enzymes (Murgia et al., 2017). Because of these insights, some sports scientists and practitioners especially in German-speaking countries now consider the νLa.max as an important variable when analysing the metabolism of an endurance athlete (Weber, 2003; Nitzsche et al., 2018).
Finally, a rightward shift of the lactate curve after, for example, several weeks of endurance training (see e.g., Figure 1) can potentially be explained either by a drop of the νLa.max or by an increase of lactate oxidation, or both. Here, George Brooks and coworkers have demonstrated early on that the rate of lactate oxidation increases after endurance training (Donovan and Brooks, 1983) whereas the νLa.max does not decrease (Hommel et al., 2019). Having said that, chronic 10 Hz electrical stimulation of a rabbit fast-twitch tibialis anterior muscle reduces the expression of glycolytic enzymes, such as phosphofructokinase (Henriksson et al., 1986), which will reduce the νLa.max. Therefore, the rightward shift of the lactate curve after endurance training is currently mainly explained by an increase of lactate oxidation but more research is needed to find out whether changes of the νLa.max can contribute to the rightward shift of the lactate curve.
In many sports, power outputs that exceed 1000 W for several seconds to more than a minute are required (Sahlin, 2014). Such disciplines include games such as football, handball, basketball or rugby, martial arts, 400 m sprints and for example, 1,000 m track cycling. To determine how a high νLa.max affects performance in such events, we have simulated the metabolic response to the measured power output of an elite track cyclist who cycled 1,000 m in a time of 1 min and 1 s with a mean power output of 950 watts and a starting power output of nearly 2000 watts. Figure 6A shows a simulation for a relative
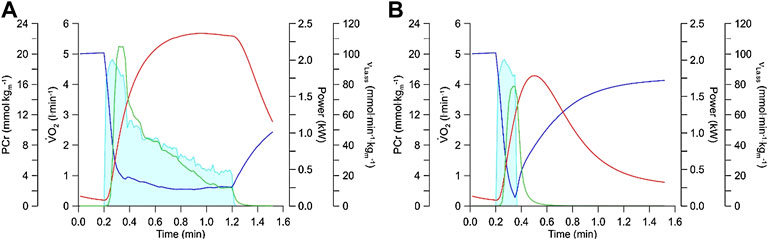
FIGURE 6. (A) Simulation of energy metabolism during a 1,000 m time trial of a track cyclist. This calculation is based on the recorded power output from an Olympic track cyclist using the equations from the Mader model, the computer simulation calculates the behavior of glycolytic flux (green line; expressed per kg muscle), PCr levels (blue line), and oxygen uptake (red line) in dependency of the power output (light blue shade). Physiological variables used in the simulation were
The simulation demonstrates the importance of a high νLa.max (which is essentially a high activity of glycolytic enzymes) for events where the power output reaches very high values for a few seconds to just more than a minute in elite athletes.
One insight from Mader’s model is that the νLa.max is an important, additional variable that influences endurance performance. Mader proposed to estimate the maximum rate of glycolysis by lactate measurements before and after an all-out sports-specific sprint test using the following equation (Mader, 1984):
where
In practice, measuring the νLa.max is challenging. The test setup must allow on the one hand an effort that maximally activates glycolysis during, for example, a 10 s effort while on the other hand limiting the activation of oxidative metabolism as much as possible to reduce intramuscular lactate oxidation (Heck et al., 2003).
Further considerations concerning the νLa.max assume that individuals with a higher amount of muscle mass and more fast, glycolytic type 2A and 2X muscle fibers produce generally higher lactate levels during aerobic and anaerobic exercise than subjects with fewer type 2A and 2X fibers (Ivy et al., 1980). Importantly, those athletes produce more lactate at every exercise intensity and reach higher maximal lactate concentrations in a graded exercise test (Mader and Heck, 1986; Mader, 2003). As a result, the exercise intensity at maxLass is lower and absolute carbohydrate usage higher, which is detrimental during long distance endurance events (Jones, 2006). In contrast, athletes participating, for example, in glycolytic and intermittent sports, must rely on a high glycolytic in combination with a high oxidative capacity.
To us, Mader’s model of human energy metabolism was far ahead of its time when it was published in 1984 and is has arguably stood the test of time. But what are its benefits? First, Mader’s model gives a plausible, mechanistic explanation of how human exercise metabolism works. This is, for example, evident from the simulation of the
In summary, the Cologne group has introduced and simplified lactate testing, defined the 4 mmol.L−1 lactate threshold, maxLass and νLa.max, and has generated the first, comprehensive simulation model of human exercise metabolism. Some of these conceptual advances are underappreciated outside German-speaking countries especially because some seminal publications are not easily accessible. We therefore hope that this article will help to raise the awareness of the Cologne group and their contributions.
On the 13.5.2021, Prof Dr Wildor Hollmann passed away. He founded the Institute of Sports Medicine in Cologne in 1958, led the Cologne group and motivated and taught generations of students who loved his lectures. Prof. Dr. Claude Bouchard considers Wildor Hollmann as his first mentor (Bouchard, 2022). As students of Wildor Hollmann, we dedicate this article to his memory.
HW and SG wrote and drafted the manuscript and gave intellectual content; HS and SRD wrote the manuscript and gave intellectual content; SW, SRD, and OH gave intellectual content.
Author SW is employed by INSCYD GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Hermann Heck (Ruhr-Universität Bochum, emeritus) for his outstanding support in writing this manuscript. As one of our teachers, as a member of the Cologne group, developer of the maxLass concept, and as the author of a German language textbook on lactate, he gave us essential insights into the history of the Cologne group, provided us with the Mader model simulation data, and extensively commented on the text. Finally, we also thank Claude Bouchard (Pennington Biomedical Research Center), Mike Joyner (Mayo Clinic), and David Bishop (Victoria University) for informal discussions and for providing an international perspective. Bouchard stayed in Cologne with the Cologne group but before the lactate testing era (personal note).
Baker J. S., Mccormick M. C., Robergs R. A. (2010). Interaction Among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 905612. doi:10.1155/2010/905612
Bouchard C. (2022). The Study of Human Variability Became a Passion. Eur. J. Clin. Nutr. 76, 631–636. doi:10.1038/s41430-021-00871-z
Cieslar J. H., Dobson G. P. (2000). Free [ADP] and Aerobic Muscle Work Follow at Least Second Order Kinetics in Rat Gastrocnemius In Vivo. J. Biol. Chem. 275, 6129–6134. doi:10.1074/jbc.275.9.6129
Colosio A. L., Caen K., Bourgois J. G., Boone J., Pogliaghi S. (2020). Bioenergetics of the VO2 Slow Component between Exercise Intensity Domains. Pflugers Arch. - Eur. J. Physiol. 472, 1447–1456. doi:10.1007/s00424-020-02437-7
Costill D. L., Daniels J., Evans W., Fink W., Krahenbuhl G., Saltin B. (1976). Skeletal Muscle Enzymes and Fiber Composition in Male and Female Track Athletes. J. Appl. Physiology 40, 149–154. doi:10.1152/jappl.1976.40.2.149
Dawson M. J. (1983). “Phosphorus Metabolites and the Control of Glycolysis Studied by Nuclear Magnetic Resonance,” in Biochemistry of Exercise. International Series of Sport Sciences. Editors J. Knuttgen, J. Vogel, and J. Poortmans (Champaign, Illinois: Human Kinetics Publishers, Inc.).
Dobson G. P., Yamamoto E., Hochachka P. W. (1986). Phosphofructokinase Control in Muscle: Nature and Reversal of pH-dependent ATP Inhibition. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 250, R71–R76. doi:10.1152/ajpregu.1986.250.1.r71
Donovan C. M., Brooks G. A. (1983). Endurance Training Affects Lactate Clearance, Not Lactate Production. Am. J. Physiology-Endocrinology Metabolism 244, E83–E92. doi:10.1152/ajpendo.1983.244.1.e83
Faude O., Kindermann W., Meyer T. (2009). Lactate Threshold Concepts. Sports Med. 39, 469–490. doi:10.2165/00007256-200939060-00003
Gehlert S., Weber S., Weidmann B., Gutsche K., Platen P., Graf C., et al. (2012). Cycling Exercise-Induced Myofiber Transitions in Skeletal Muscle Depend on Basal Fiber Type Distribution. Eur. J. Appl. Physiol. 112, 2393–2402. doi:10.1007/s00421-011-2209-4
Gladden L. B. (2008). 200th Anniversary of Lactate Research in Muscle. Exerc. Sport Sci. Rev. 36, 109–115. doi:10.1097/jes.0b013e31817c0038
Harris R. C., Hultman E., Nordesjö L.-O. (1974). Glycogen, Glycolytic Intermediates and High-Energy Phosphates Determined in Biopsy Samples of Musculus Quadriceps Femoris of Man at Rest. Methods and Variance of Values. Scli 33, 109–120. doi:10.3109/00365517409082477
Heck H., Mader A., Hess G., Mücke S., Müller R., Hollmann W. (1985). Justification of the 4-mmol/l Lactate Threshold. Int. J. Sports Med. 06, 117–130. doi:10.1055/s-2008-1025824
Heck H., Schulz H., Bartmus U. (2003). Diagnostics of Anaerobic Power and Capacity. Eur. J. Sport Sci. 3, 1–23. doi:10.1080/17461390300073302
Henriksson J., Chi M. M., Hintz C. S., Young D. A., Kaiser K. K., Salmons S., et al. (1986). Chronic Stimulation of Mammalian Muscle: Changes in Enzymes of Six Metabolic Pathways. Am. J. Physiology-Cell Physiology 251, C614–C632. doi:10.1152/ajpcell.1986.251.4.c614
Hill A. V., Lupton H. (1923). Muscular Exercise, Lactic Acid, and the Supply and Utilization of Oxygen. Qjm os-16, 135–171. doi:10.1093/qjmed/os-16.62.135
Hill A. V. (1910). The Possible Effects of the Aggregation of the Molecules of Haemoglobin on its Dissociation Curves. J. Physiology.
Hollmann W. (2001). 42 Years Ago ??? Development of the Concepts of Ventilatory and Lactate Threshold. Sports Med. 31, 315–320. doi:10.2165/00007256-200131050-00002
Hommel J., Öhmichen S., Rudolph U. M., Hauser T., Schulz H. (2019). Effects of Six-Week Sprint Interval or Endurance Training on Calculated Power in Maximal Lactate Steady State. bs 36, 47–54. doi:10.5114/biolsport.2018.78906
Iannetta D., Ingram C. P., Keir D. A., Murias J. M. (2022). Methodological Reconciliation of CP and MLSS and Their Agreement with the Maximal Metabolic Steady State. Med. Sci. Sports Exerc 54, 622–632. doi:10.1249/mss.0000000000002831
Ivy J. L., Withers R. T., Van Handel P. J., Elger D. H., Costill D. L. (1980). Muscle Respiratory Capacity and Fiber Type as Determinants of the Lactate Threshold. J. Appl. Physiology 48, 523–527. doi:10.1152/jappl.1980.48.3.523
Jamnick N. A., Pettitt R. W., Granata C., Pyne D. B., Bishop D. J. (2020). An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 50, 1729–1756. doi:10.1007/s40279-020-01322-8
Jeneson J. L., Wiseman R. W., Westerhoff H. V., Kushmerick M. J. (1996). The Signal Transduction Function for Oxidative Phosphorylation Is at Least Second Order in ADP. J. Biol. Chem. 271, 27995–27998. doi:10.1074/jbc.271.45.27995
Jones A. M., Grassi B., Christensen P. M., Krustrup P., Bangsbo J., Poole D. C. (2011). Slow Component of V˙O2 Kinetics. Med. Sci. Sports Exerc 43, 2046–2062. doi:10.1249/mss.0b013e31821fcfc1
Jones A. M. (2006). The Physiology of the World Record Holder for the Women's Marathon. Int. J. Sports Sci. Coach. 1, 101–116. doi:10.1260/174795406777641258
Joyner M. J., Coyle E. F. (2008). Endurance Exercise Performance: the Physiology of Champions. J. physiology 586, 35–44. doi:10.1113/jphysiol.2007.143834
Keir D. A., Iannetta D., Mattioni Maturana F., Kowalchuk J. M., Murias J. M. (2022). Identification of Non-invasive Exercise Thresholds: Methods, Strategies, and an Online App. Sports Med. 52, 237–255. doi:10.1007/s40279-021-01581-z
Kindermann W., Simon G., Keul J. (1979). The Significance of the Aerobic-Anaerobic Transition for the Determination of Work Load Intensities during Endurance Training. Eur. J. Appl. Physiol. 42, 25–34. doi:10.1007/bf00421101
Krogh A., Lindhard J. (1920). The Changes in Respiration at the Transition from Work to Rest. J. Physiol. 53, 431–439. doi:10.1113/jphysiol.1920.sp001889
Loon L. J. C., Greenhaff P. L., Constantin‐Teodosiu D., Saris W. H. M., Wagenmakers A. J. M. (2001). The Effects of Increasing Exercise Intensity on Muscle Fuel Utilisation in Humans. J. physiology 536, 295–304. doi:10.1111/j.1469-7793.2001.00295.x
Lundsgaard A.-M., Fritzen A. M., Kiens B. (2018). Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol. Metabolism 29, 18–30. doi:10.1016/j.tem.2017.10.011
Mader A., Heck H. (1986). A Theory of the Metabolic Origin of "anaerobic Threshold". Int. J. Sports Med. 7 (Suppl. 1), 45–65. doi:10.1055/s-2008-1025802
Mader A. (1984). Eine Theorie zur Berechnung der Dynamik und des steady state von Phosphorylierungsszzustand und Stoffwechselaktivität der Muskelzelle als Folge des Energiebedarfs. Köln: Deutscher Sporthochschule.
Mader A. (2003). Glycolysis and Oxidative Phosphorylation as a Function of Cytosolic Phosphorylation State and Power Output of the Muscle Cell. Eur. J. Appl. Physiol. 88, 317–338. doi:10.1007/s00421-002-0676-3
Mader A., Liesen H., Heck H., Philippi H., Rost R., Schürch P., et al. (1976). Zur Beurteilung der sportartspezifischen Ausdauerleistungsfähigkeit im Labor. Sportarzt Sportmed. 27 (4), 80–88.
Mcgilvery R. W., Murray T. W. (1974). Calculated Equilibria of Phosphocreatine and Adenosine Phosphates during Utilization of High Energy Phosphate by Muscle. J. Biol. Chem. 249, 5845–5850. doi:10.1016/s0021-9258(20)79895-9
Murgia M., Toniolo L., Nagaraj N., Ciciliot S., Vindigni V., Schiaffino S., et al. (2017). Single Muscle Fiber Proteomics Reveals Fiber-type-specific Features of Human Muscle Aging. Cell Rep. 19, 2396–2409. doi:10.1016/j.celrep.2017.05.054
Nitzsche N., Baumgärtel L., Schulz H. (2018). Comparison of Maximum Lactate Formation Rates in Ergometer Sprint and Maximum Strength Loads. Dtsch. Z Sportmed 2018, 13–18. doi:10.5960/dzsm.2017.312
O’connell J. M., Weir J. M., Macintosh B. R. (2017). Blood Lactate Accumulation Decreases during the Slow Component of Oxygen Uptake without a Decrease in Muscular Efficiency. Pflügers Archiv - Eur. J. Physiology 469, 1257–1265. doi:10.1007/s00424-017-1986-y
Poole D. C., Rossiter H. B., Brooks G. A., Gladden L. B. (2020). The Anaerobic Threshold: 50+ Years of Controversy. J. Physiology 599. doi:10.1113/jp279963
Sahlin K. (2014). Muscle Energetics during Explosive Activities and Potential Effects of Nutrition and Training. Sports Med. 44 (Suppl. 2), S167–S173. doi:10.1007/s40279-014-0256-9
Stegmann H., Kindermann W., Schnabel A. (1981). Lactate Kinetics and Individual Anaerobic Threshold*. Int. J. Sports Med. 02, 160–165. doi:10.1055/s-2008-1034604
Trivedi B., Danforth W. H. (1966). Effect of pH on the Kinetics of Frog Muscle Phosphofructokinase. J. Biol. Chem. 241, 4110–4114. doi:10.1016/s0021-9258(18)99819-4
Von Bertalanffy L. (1950). The Theory of Open Systems in Physics and Biology. Science 111, 23–29. doi:10.1126/science.111.2872.23
Wackerhage H. (2021). Contributions by the Cologne Group to the Development of Lactate Exercise Testing and Anaerobic Threshold Concepts in the 1970s and 1980s. J. Physiology 599, 1713–1714. doi:10.1113/jp281142
Walsh D. (1979). Occam's Razor: A Principle of Intellectual Elegance. Am. Philosophical Q. 16, 241–244.
Wasserman K., Mcilroy M. B. (1964). Detecting the Threshold of Anaerobic Metabolism in Cardiac Patients during Exercise. Am. J. Cardiol. 14, 844–852. doi:10.1016/0002-9149(64)90012-8
Wasserman K., Whipp B. J., Koyl S. N., Beaver W. L. (1973). Anaerobic Threshold and Respiratory Gas Exchange during Exercise. J. Appl. Physiology 35, 236–243. doi:10.1152/jappl.1973.35.2.236
Weber S. (2003). Calculation of performance-determining parameters of metabolic activity at the cellular level by means of cycle ergometry [Berechnung leistungsbestimmender Parameter der metabolischen Aktivität auf zellulärer Ebene mittels fahrradergometrischer Untersuchungen]. Dipon Diplomarb. Dtsch. Sporthochsch. Köln.
Keywords: simulating energy metabolism, lactate treshold testing, slow component, pyruvate deficit, maximal rate of glycolysis, V̇O2max, νLa max, Cologne group
Citation: Wackerhage H, Gehlert S, Schulz H, Weber S, Ring-Dimitriou S and Heine O (2022) Lactate Thresholds and the Simulation of Human Energy Metabolism: Contributions by the Cologne Sports Medicine Group in the 1970s and 1980s. Front. Physiol. 13:899670. doi: 10.3389/fphys.2022.899670
Received: 19 March 2022; Accepted: 09 June 2022;
Published: 22 July 2022.
Edited by:
Olivier Girard, University of Western Australia, AustraliaReviewed by:
Danilo Iannetta, The University of Utah, United StatesCopyright © 2022 Wackerhage, Gehlert, Schulz, Weber, Ring-Dimitriou and Heine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henning Wackerhage, aGVubmluZy53YWNrZXJoYWdlQHR1bS5kZQ==; Sebastian Gehlert, Z2VobGVydEB1bmktaGlsZGVzaGVpbS5kZQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.