
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 10 May 2022
Sec. Computational Physiology and Medicine
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.898251
The COVID-19 disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has become one of the worst global pandemics of the century. Wearable devices are well suited for continuously measuring heart rate. Here we show that the Resting Heart Rate is modified for several weeks following a COVID-19 infection. The Resting Heart Rate shows 3 phases: 1) elevated during symptom onset, with average peak increases relative to the baseline of 1.8% (3.4%) for females (males), 2) decrease thereafter, reaching a minimum on average ≈13 days after symptom onset, and 3) subsequent increase, reaching a second peak on average ≈28 days from symptom onset, before falling back to the baseline ≈112 days from symptom onset. All estimates vary with disease severity1.
Cardiac complications are known to be associated with the COVID-19 disease caused by the novel Severe Acute Respiratory Syndrome—Coronavirus 2 (SARS-CoV-2) (Cizgici et al., 2020; Huang et al., 2020; Kochi et al., 2020; Long et al., 2020; Shi et al., 2020; Siripanthong et al., 2020; Wang et al., 2020; Coromilas et al., 2021). An unusual feature that is sometimes seen in patients diagnosed with COVID-19 is the appearance of bradycardia, i.e., slow heart rate, or heart rate not increasing as expected with body temperature (Ikeuchi et al., 2020; Capoferri et al., 2021; Douedi et al., 2021; Oliva et al., 2021). Amaratunga et al. (2020) found bradycardia in a study of 4 patients with confirmed COVID-19, with minimum pulse rates in the range 42–49 beats per minute. Amir et al. (2021) reported 6 cases of bradycardia among patients diagnosed with COVID-19, with 4 patients developing complete atrioventricular block. Elikowski et al. (2021) presented clinical data of 19 patients diagnosed with COVID-19 who exhibited sinus bradycardia, in some cases showing heart rates as low as 32 bpm during daily hours. Srinivasan et al. (2021) discussed 6 cases of patients who were diagnosed with COVID-19 and admitted with normal sinus rhythm, and who subsequently developed sinus bradycardia with daytime heart rates ranging from 35–48 bpm. In a study of 97 patients with a non-severe presentation of COVID-19, Zhou et al. (2021) found significant sinus bradycardia (below 50 bpm) in 7.2% of cases.
In addition to bradycardia, COVID-19 is known to cause a number of other cardiac anomalies. In a study of 140 individuals diagnosed with COVID-19 and 281 individuals diagnosed with influenza, Lampert et al. (2021) found that diminished QRS amplitude was dynamic during the course of illness, and was an independent predictor of mortality with both COVID-19 and influenza, but was more prevalent in the case of COVID-19. Cardiac injury, heart failure, and arrhythmias have been recorded in patients diagnosed with COVID-19 (Linschoten et al., 2020; Shi et al., 2020; Coromilas et al., 2021; Sahranavard et al., 2021). Guo et al. (2020) reported ventricular tachycardia (VT) or ventricular fibrillation (VF) in 11 out of 187 patients with confirmed COVID-19 and found that elevated levels of troponin T were correlated with VT/VF. Inflammatory damage due to cytokines has been suggested as a possible explanation for cardiac involvement with COVID-19 (Bhatla et al., 2020; Dherange et al., 2020; Peigh et al., 2020; Elikowski et al., 2021). In a retrospective analysis of 3,970 patients admitted with COVID-19 and 1,420 patients admitted with influenza, Musikantow et al. (2021) found similar incidences of atrial fibrillation/atrial flutter with COVID-19 (10%) and influenza (12%). The presence of atrial fibrillation was associated with increased mortality in both COVID-19 and influenza (Musikantow et al., 2021). New-onset atrial fibrillation was found to be influenced most by markers of inflammation such as Interleukin 6 and C-reactive protein (Musikantow et al., 2021). Pardo Sanz et al. (2021) found that new-onset atrial fibrillation is associated with worse outcomes than in patients with existing atrial fibrillation.
The presence of atrial fibrillation in patients with severe COVID-19 was found to be 6 times higher compared to patients with non-severe COVID-19 (Li et al., 2021). Possible mechanisms for the development of atrial fibrillation in patients diagnosed with COVID-19 have been discussed by Stone et al. (2020) and Gawałko et al. (2020). Turagam et al. (2020) studied the occurrence of malignant arrhythmias and found that VT/VF and AV block were more commonly associated with mortality, and that tachyarrhythmias such as VT/VF occurred in the setting of severe metabolic stress. In a comparison of the mortality group with the discharged group, it was found that serum creatinine, peak troponin, C-reactive protein, Procalcitonin, and interleukin 6 levels were all significantly elevated in the mortality group (Turagam et al., 2020). COVID-19 sometimes presents with rare symptoms such as syncope (Oates et al., 2020).
Commercially available wearable devices have been shown to be useful in early detection of COVID-19 and for monitoring symptoms (Miller et al., 2020a; Miller et al., 2020b; Mishra et al., 2020; Natarajan et al., 2020b; Natarajan et al., 2021; Quer et al., 2021). Radin et al. (2021) studied resting heart rate (henceforth RHR) data from Fitbit devices to investigate long term changes following symptom onset. RHR is typically elevated around symptom onset. We use the term “relative” to indicate that the RHR is elevated/decreased relative to the baseline value for that individual, although the RHR is not necessarily above/below the clinical threshold guideline (Ostchega et al., 2011). They also found the RHR exhibits a dip which we refer to as transient relative bradycardia provided the RHR is below the baseline value. The RHR dip was followed by a second elevated RHR peak. They found that the RHR was elevated for up to 79 days from symptom onset.
In this article, we obtain results consistent with the findings of Radin et al., and expand upon existing work in a number of ways. We study a much larger sample size than previously considered. We investigate how the RHR changes, for male and female individuals, and for individuals with severe, mild or asymptomatic presentations of COVID-19. We also consider individuals diagnosed with the seasonal influenza (henceforth “flu”). We tabulate the expected amplitudes of the maxima/minima, as well as the time taken to reach these maxima/minima, and the estimated widths of the peaks/troughs. We examine how these parameters vary with age, sex, and disease severity. We also study heart rate variability and respiratory rate and how these metrics vary with time.
The Fitbit COVID-19 survey was conducted from 21 May 2020 to 10 June 2021, and collected data from participants residing in the United States or Canada. Participants provided information on whether they were diagnosed with COVID-19 or flu, as well as the test date, symptoms, and the start date of symptoms. Participants could optionally provide information about their age, sex, body mass index, and information on underlying conditions. Individuals diagnosed with COVID-19 also indicated the severity of the disease which could be 1) severe, indicating that they required hospitalization, 2) mild, indicating that they recovered at home, or 3) asymptomatic. The survey and associated marketing and recruitment materials were approved by an Institutional Review Board (Advarra). The participants provided electronic informed consent for their data to be used for research. In this study, we consider data from Fitbit users who reported testing positive for COVID-19 in the date range March 1—31 Dec 2020, as well as users who reported testing positive for flu in the date range January 1—31 Dec 2020. There were 11,918 participants who tested positive for COVID-19 (mean age = 40.8 years, std. dev. = 12.4 years, 79.0% female) in our dataset, and 865 participants who tested positive for flu (mean age = 41.7 years, std. dev. = 13.3 years, 78.2% female). We randomly selected 1,000 users who did not report a positive test for either COVID-19 or flu as a control group (mean age = 45.3 years, std. dev. = 13.9 years, 71.6% female). Table 1 shows the prevalence of symptoms (self reported) for COVID-19 and flu, for male and female participants (for COVID-19, we also separate by disease severity). Some symptoms such as fatigue, headache, and body ache are common for both COVID-19 and flu. By contrast, a decrease in taste and/or smell is more likely in the case of COVID-19 (72.6% female, 59.8% male) compared to flu (21.2% female, 11.6% male). Fever is more common with flu (81.3% female, 76.8% male) compared to COVID-19 (51.4% female, 58% male). Figure 1 shows the distribution of positive cases for flu and COVID-19, where the horizontal axis is the test date, and only positive cases are shown. Cases of flu peaked in March 2020, while COVID-19 cases peaked much later.
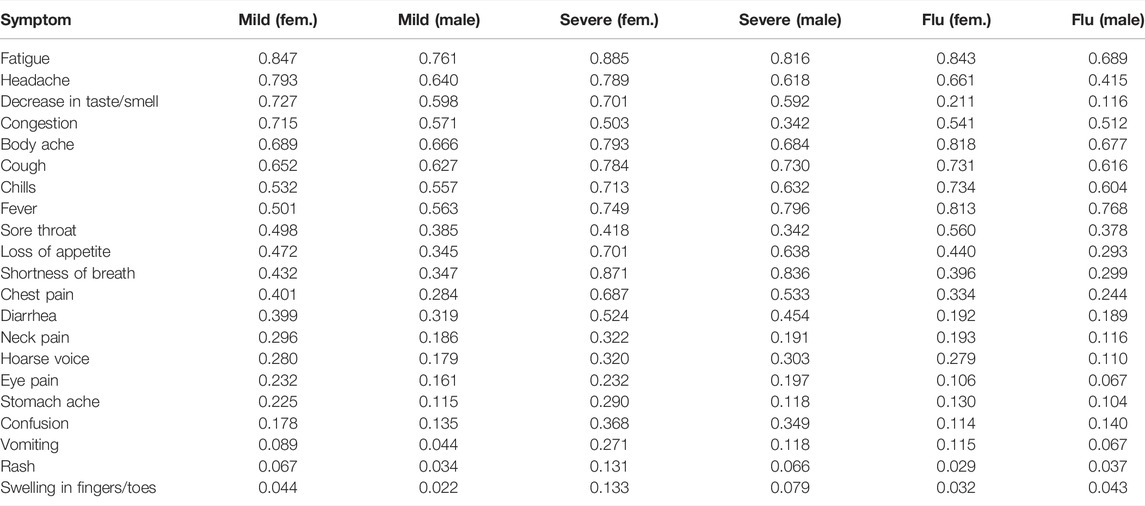
TABLE 1. Prevalence of symptoms (expressed as a fraction), for COVID-19 (mild/severe) and flu, for male and female participants.
We measure the RHR from participants in our study, collected using Fitbit devices. RHR is computed using heart rate data during sleep when sleep data is available, and a proprietary algorithm is utilized to predict the RHR from the time series heart rate data during sleep. If sleep data is unavailable, RHR is computed using wake time heart rate data, at times when no activity is detected. The RHR is also processed with a Kalman filter which serves to smooth the waveform. The RHR as defined by Fitbit is the value closest to the heart rate measured when lying down just before waking up in the morning. Details of how Fitbit measures resting RHR may be found in Russell et al. (2019). Note that the RHR is not the minimum value of heart rate.
Let D0 be the date of symptom onset for symptomatic individuals (and the test date for asymptomatic individuals). Thus for symptomatic individuals, D−1 is one day prior to the appearance of symptoms, and D+1 is one day post appearance of symptoms. We computed the mean value ⟨RHR⟩ for each individual by averaging the RHR values from D−90 to D−15. We discard data from participants who have fewer than 30 measurements in this time window. With this data sufficiency condition, our dataset contains 463 individuals with the flu, and 7,200 individuals with COVID-19 (6,606 symptomatic, 594 asymptomatic, see Figure 2). We compute the fractional change in RHR ξ from days D−14 to D+180 as:
where d is a variable indicating day index.
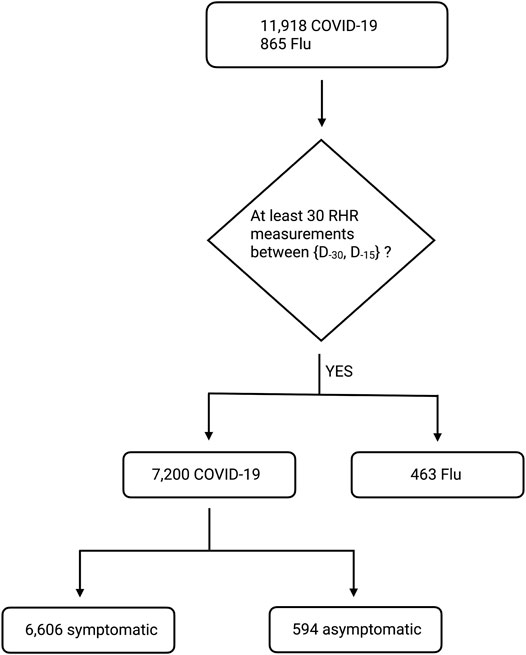
FIGURE 2. Block diagram showing the number of survey participants with COVID-19 or flu. We start with all participants with diagnosed COVID-19 between {2020-03-01, 2020-12-31}, or diagnosed flu between {2020-01-01, 2020-12-31}. Note that D0 is the date of symptom onset for symptomatic individuals (and the test date for asymptomatic individuals). We then discard individuals with fewer than 30 RHR measurements between D−90 and D−15. Our final dataset contains 463 individuals with flu, 6,606 individuals with symptomatic COVID-19, and 594 individuals with asymptomatic COVID-19.
The RHR has a known seasonal modulation. In a study of 200,000 individuals wearing Fitbit devices and residing in the United States, Quer et al. (2020) found a change in the population’s average RHR by 2 beats per minute (bpm). In the Northern hemisphere, the RHR peaks in the first week of January and reaches its minimum at the end of July.
Since all participants in our study reside in the United States or Canada, we assume that they are subject to the same seasonal trends. We use the control group to estimate how RHR varies with the time of year, by applying Eq. 1 to obtain ξcontrol as follows:
1) Randomly sample a date from the COVID-19 or flu distribution and set to D0 (date of symptom onset).
2) Compute ξcontrol(d) using Eq. 1 from the control group for dates d relative to D0.
We may then subtract out the seasonality to find the effect of illness on RHR:
where α may be flu or COVID-19. Note that ξcontrol must be computed twice: once for the COVID-19 distribution, and once for the flu distribution. To compute Δξ(d) for a given individual, we would use the appropriate ξcontrol,α depending on whether the participant was diagnosed with flu or COVID-19.
Heart Rate Variability, as the name implies, refers to the variance in the heart rate. Heart Rate Variability is usually a good thing (with the exception of heart arrhythmias) since a healthy heart does not beat like a metronome. Heart Rate Variability allows the cardiovascular system to adjust to physical and psychological challenges to homeostasis (Shaffer and Ginsberg, 2017). Here, we consider the root mean square of the successive differences (RMSSD) between heart beats as the HRV metric of importance. The RMSSD is computed in 5 min windows between the hours of midnight and 7 a.m. The median of the measurements is then reported. Fitbit does not measure interbeat intervals when movement is detected, and therefore Fitbit devices primarily measure HRV during sleep. Further details of how Fitbit devices compute RMSSD have been described in Natarajan et al. (2020a). For the respiratory rate calculation, we first compute the power spectral density of interbeat intervals in 5 min windows between the hours of midnight and 7 a.m. The measurements are then averaged, and the respiratory rate is estimated from the averaged power spectral density. We subtract out the seasonality of the RMSSD and respiratory rate in a similar manner to the RHR, with the addition of a median filter (with a period of 7 days). The median filter is necessary because the RMSSD and respiratory rate waveforms are not smoothed by a Kalman filter, and are hence noisier than the RHR. The effect of illness on the RMSSD and respiratory rate may be estimated as:
where ρ and ρcontrol may be RMSSD or respiratory rate, and the angle brackets denote the median filter. Further details of how Fitbit devices compute the respiratory rate during sleep have been described in Natarajan et al. (2021).
All analyses were performed using standard Python libraries. For parameter estimation, the waveforms for ξ and ξcontrol were linearly interpolated, and then smoothed with a Savitzky − Golay filter with a window length of 7 days, and cubic polynomial smoothing. For the mean parameters tabulated in Tables 4, 5, we used a step size of 0.2 days for the linear interpolation. The standard error of the parameter values was estimated using the jackknife technique (Miller, 1974; Efron, 1981). For the mean and jackknife error estimated parameters shown in Table 3, we used a finer step size of 0.01 days. We computed Δξ from the smoothed waveforms, and estimated the peaks/troughs by means of a peak detection algorithm. For estimation of p − values, we used a one sided t − test. For estimation of correlation, we used the Pearson r coefficient.
Figure 3 shows the fractional change (ξ) in RHR for flu and COVID-19, for symptomatic individuals, along with the fractional change in the Control group (ξcontrol). Subplot 1) compares ξ and ξcontrol for flu, while subplot 2) compares ξ and ξcontrol for COVID-19. The horizontal axis Dn is the date relative to the date when symptoms first appear (n < 0 are prior to symptom onset, while n > 0 are after symptom onset. D0 is the date when symptoms first present). ξ and ξcontrol are averaged over all individuals for a specific Dn. Subplot 3) shows Δξ, i.e. the excess fractional change in RHR after the seasonal variation has been subtracted, while subplot 4) shows the parameters At1, Ab, At2, Tt1, Tb, Tt2, Wt1, Wb, and Wt2. The parameters to be estimated are summarized in Table 2. From subplot (c), we infer the following:
1) The excess fractional change in RHR, i.e., Δξ is elevated on average during the onset of symptoms. Δξ reaches a peak value At1 at a time Tt1 days from the onset of symptoms. Wt1 is the full width at half maximum. This is the first transient relative tachycardia.
2) Following the peak, Δξ decreases, reaching a minimum value Ab at a time Tb days from the onset of symptoms. Wb is the full width at half minimum (only defined when Ab < 0). If Ab < 0, we refer to the trough as transient relative bradycardia.
3) Following the minimum, Δξ increases again, reaching a second peak At2 at a time Tt2 days from symptom onset. Wt2 is the full width at half maximum.
4) Past Tt2, Δξ decreases and eventually falls to zero, indicating that the RHR variation is no longer due to illness.
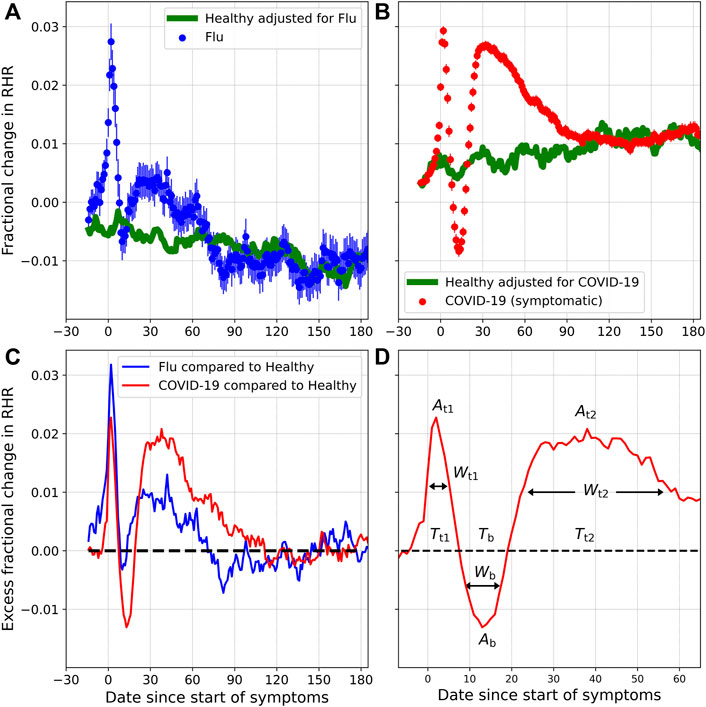
FIGURE 3. Fractional change in RHR (ξ), for individuals diagnosed with flu (A) and COVID-19 (B), along with the expected seasonal variation (ξcontrol). (C) shows the excess fractional change in RHR (Δξ) for flu and COVID-19, once the contribution of seasonality has been subtracted. (D) shows the various parameters to be estimated.
Figure 4 shows the excess fractional change in RHR (Δξ) for COVID-19 and flu, for varying severity, and for male and female individuals. Subplot (a) shows the effect of severity on Δξ. The magenta curve is plotted for severe cases (i.e., cases that required hospitalization). The brown curve shows mild cases, while the green curve is plotted for asymptomatic cases. The blue curve shows Δξ for flu. The amplitudes of the two relative tachycardia peals At1 and At2 are much larger in the case of severe COVID-19. The bradycardia dip (Ab) is more pronounced in the case of mild COVID-19. The first relative tachycardia peak amplitude (At1) is larger for flu compared to mild or asymptomatic COVID-19, while the second tachycardia peak is similar in the cases of flu and asymptomatic COVID-19. For the cases of severe, mild, asymptomatic, and flu respectively, we find Δξ falls to zero approximately 118, 112, 79, and 71 days after the onset of symptoms (or test date for asymptomatic cases).
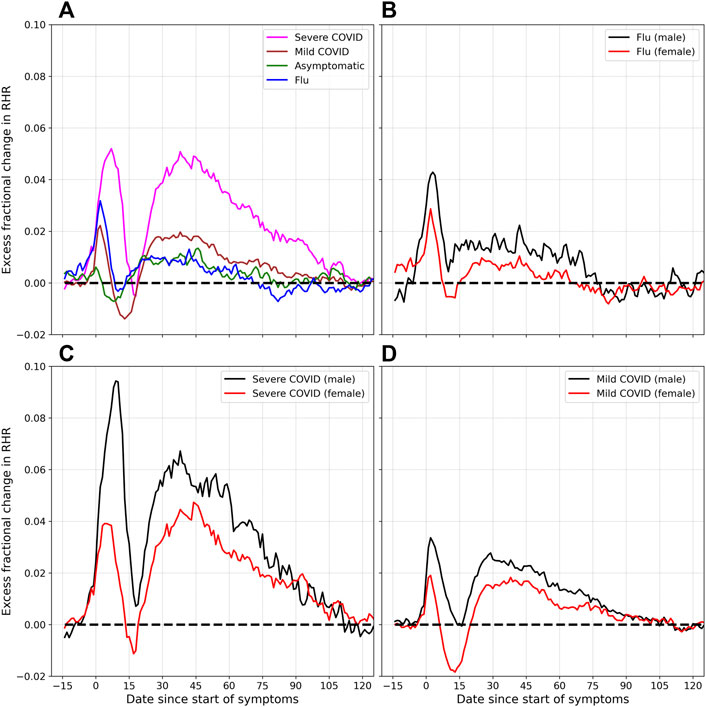
FIGURE 4. Excess fractional change in RHR (Δξ), and variation with severity and sex: (A) shows Δξ for severe, mild, and asymptomatic COVID-19, as well as flu. (B) shows Δξ for male and female individuals diagnosed with flu. (C) and (D) show Δξ for male and female participants, for the cases for severe and mild COVID-19 respectively.
Subplot (b) shows the difference between male and female individuals who were diagnosed with flu. Similar to the case of COVID-19, the peak amplitudes At1 and At2 are higher for males compared to females. The trough amplitude Ab is lower for females. Subplots (c) and (d) show the variation of Δξ for male and female individuals, for severe and mild COVID-19 cases respectively. In both cases, the peak amplitudes At1 and At2 are higher for males, for both severe and mild cases of COVID-19. The minimum amplitude Ab on the other hand is lower in females than in males, and is more negative for mild cases of COVID-19 compared to severe cases.
Table 3 shows the estimated values of the amplitudes for the RHR minimum (Ab) and the two maxima (At1 and At2), along with jackknife estimated standard errors. Table 4 shows the estimated values of the various parameters, for female and male participants, and for different ages. Each peak/trough is characterized by 3 parameters: the amplitude, time from symptom onset, and full width at half maximum/minimum. The 2 relative tachycardia peaks are parameterized by (At1, Tt1, Wt1) and (At2, Tt2, Wt2), while the minimum is parameterized by (Ab, Tb, Wb). Note that Wb is only defined when Ab < 0 indicating relative bradycardia. Table 5 shows the estimated values of the parameters for male and female participants, for severe, mild, and asymptomatic COVID-19 presentations, as well as for flu.
Let us now quantify the prevalence of relative bradycardia and relative tachycardia. To do this, we define the following 4 windows each of which are 15 days long (starting and ending dates inclusive, and D0 is the date when symptoms present, only symptomatic individuals included):
• “Control” window from D−45 to D−31.
• Presumed “healthy” window from D−30 to D−16.
• Presumed “relative bradycardia” window from D+7 to D+21.
• Presumed “relative tachycardia” window from D+22 to D+36.
The healthy and control windows are expected to contain data when participants are healthy. The RHR is averaged over each window, and we only consider participants who have 15 days of data in each window. Let ΔRHR be the difference between the RHR averaged over window w and the RHR averaged over the control window:
We compute the fraction of participants who have ΔRHR
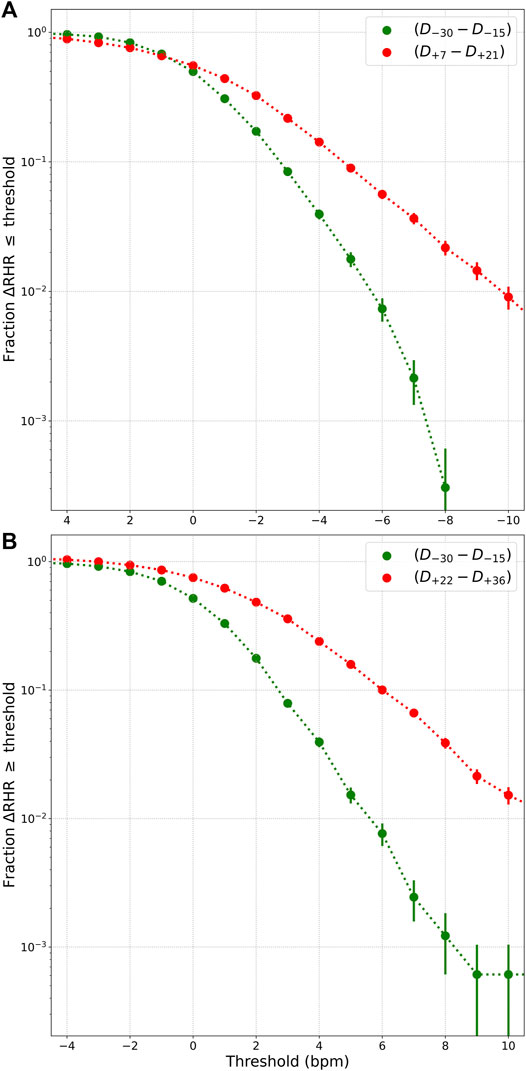
FIGURE 5. (A) Fraction of individuals with ΔRHR
Interestingly, there is a correlation between the peak value of ΔRHR measured during symptom onset (we use a 15 days window from D−5 to D+9) and the peak and minimum values of ΔRHR measured in the second relative tachycardia window (D+22 to D+36) and the relative bradycardia window (D+7 to D+21). Figure 6 shows this correlation. The horizontal axis is ΔRHR during symptom onset, while the vertical axis shows ΔRHR during either the second tachycardia phase (red) or the bradycardia phase (blue) (the points are the mean values, while the shaded contours show the one standard deviation range). We see that ΔRHRtachy measured in the second relative tachycardia phase is positively correlated with ΔRHRsymptom, the peak value observed at symptom onset. Similarly, ΔRHRbrady, the minimum value observed during the relative bradycardia phase is positively correlated with ΔRHRsymptom. This means that a low ΔRHR at symptom onset is indicative of a decreased RHR in the relative bradycardia phase. An approximate estimate of ΔRHRtachy and ΔRHRbrady may be obtained by the following linear relations (with all measurements in bpm):
where we obtained the intercept and slope by fitting a linear line to the mean values.
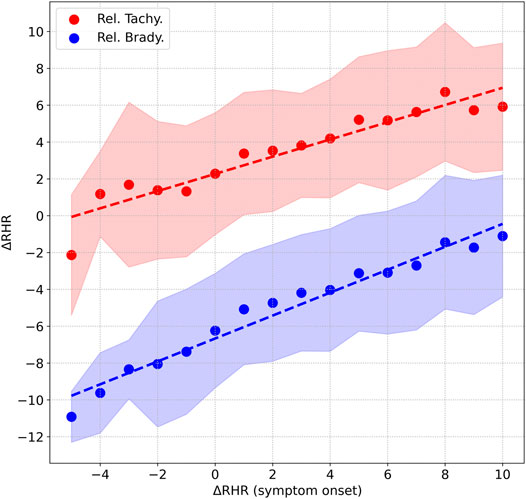
FIGURE 6. Correlation between the peak value of ΔRHR measured during symptom onset, and 1) peak value of ΔRHR in the second relative tachycardia window shown in red, 2) minimum value of ΔRHR measured in the relative bradycardia window shown in blue. The shaded areas represent the 1 standard deviation range.
Let us now briefly consider 2 more health metrics: the heart rate variability quantified by the RMSSD and the respiratory rate. Figure 7 shows the excess fractional change Δξ in RMSSD [subplot (a)] and the respiratory rate [subplot (b)], plotted for symptomatic COVID-19. Also shown for comparison in dashed lines is the fractional change in RHR. The fractional change in RMSSD shows a similar time evolution as the RHR, except with opposite phase, i.e., when the RHR is elevated, the RMSSD is decreased and vice-versa. The relative bradycardia phase is seen to be accompanied by elevated heart rate variability, while the second relative tachycardia phase is associated with reduced heart rate variability. The variation is also of greater amplitude with the RMSSD compared to the RHR. The fractional change in the respiratory rate, on the other hand, does not show a similar time evolution. The respiratory rate is elevated during symptom onset, in response to the illness. It then decreases sharply, and then gradually returns to normal. Thus there is no equivalent of the relative bradycardia and second relative tachycardia phases with the respiratory rate.
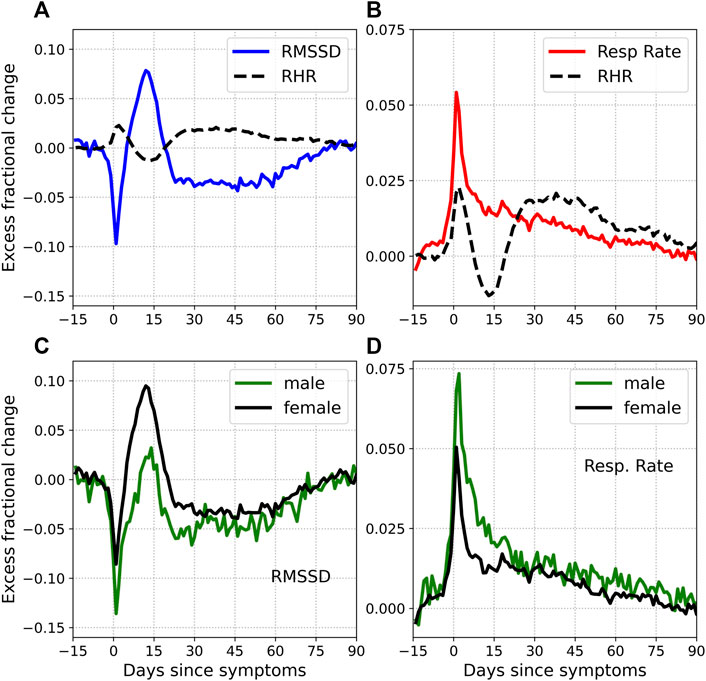
FIGURE 7. Excess fractional change in RMSSD and respiratory rate. Subplots (A) and (B) also show the excess fractional change in RHR (dashed black lines). Note that the RHR waveform is smoothed over time, resulting in a more rounded peak. Subplots (C) and (D) show the RMSSD and respiratory rate for male (green) and female (black) subjects respectively.
The growing popularity of wearable devices such as smart watches and trackers, and their increasing capability in measuring health biometrics makes them important tools in the field of digital health. From a national survey of 4,551 respondents conducted from January 2019 − April 2019, it was estimated that about 30% of adults in the United States use wearable devices, and over 82% are willing to share their health data with their care providers (Chandrasekaran et al., 2020). Telehealth can facilitate access to care, reduce the risk of transmission of COVID-19, and reduce strain on health care capacity and facilities (Demeke et al., 2021). Smartwatches and trackers that measure biomarkers that respond to illness such as heart rate, heart rate variability, respiratory rate, oxygen saturation, etc. can provide valuable information to health care providers. The market size for global wearable medical devices was nearly 30 billion in 2019, and is projected to reach ∼ 196 billion by 2027 (Fortune Business Insights, 2020). The COVID-19 pandemic has accelerated the popularity of telemedicine, with telehealth visits increasing from approximately 840,000 in 2019, to 52.7 million in 2020 (Suran, 2022).
A persistent problem with COVID-19 is the appearance of “long COVID” or “post COVID” symptoms for weeks or months after acquiring a COVID-19 infection, and can affect a whole spectrum of people, from those with very mild disease, to the most severe (Greenhalgh et al., 2020; Crook et al., 2021; Raveendran et al., 2021). It can also affect multiple organ systems, and can present with a wide array of symptoms (Greenhalgh et al., 2020; Crook et al., 2021; Raveendran et al., 2021). It is estimated that 10% of patients who test positive for COVID-19 remain unwell beyond 3 weeks (Greenhalgh et al., 2020). A complication to effective treatment for long COVID is that patients usually test negative for COVID-19 (Raveendran et al., 2021), yet show symptoms. Long COVID or post COVID thus refers to the time between microbiological recovery and clinical recovery (Raveendran et al., 2021). Long COVID might be considered post-acute COVID if symptoms persist beyond 3 weeks but less than 12 weeks, and chronic COVID when symptoms exist beyond 12 weeks (Greenhalgh et al., 2020). It is therefore not surprising that physiological biomarkers are affected for several months following a COVID-19 diagnosis. Wearable devices can play an important role in monitoring long COVID by measuring biomarkers indicative of illness. Radin et al. (2021) used data from Fitbit devices to show that the RHR is affected for 2–3 months following symptom onset. In this article, we confirmed the findings of Radin et al. (2021) using a large dataset of 7,200 COVID-19 positive and 463 flu positive individuals. Consistent with the findings of Radin et al. (2021), we find that the RHR can be modified for up to 3 months following symptom onset. Interestingly, this phenomenon is also seen in cases of flu. Both COVID-19 and flu cases show 3 distinct phases:
• First relative tachycardia phase during symptom onset when the RHR is elevated above normal, reaching a local peak value At1 at a time Tt1 days after symptom onset, with a full width at half maximum Wt1. The peak value At1 is higher in males compared to females, for COVID-19 (p − value
• Following this peak, the RHR decreases, and reaches a local minimum value Ab at a time Tb days after symptom onset. If Ab < 0, we refer to this phase as relative bradycardia, and define a full width at half minimum Wb. The condition Ab < 0 is satisfied for females (averaged over all ages), for COVID-19 (p < 0.0001), and for flu (p = 0.042). The condition Ab < 0 is not statistically significant for males (averaged over all ages), for either COVID-19 or flu.
• Following the minimum, the RHR increases, reaching a second local maximum At2 at a time Tt2 days from symptom onset, with a full width at half maximum Wt2. The peak value At2 is higher in males compared to females, for COVID-19 (p < 0.0001) and flu (p = 0.0667).
Figure 3 shows the fractional change in RHR ξ for flu and COVID-19, along with the control. Subtracting the variation for the control group allows us to calculate the effect due to illness Δξ. Figure 4 shows Δξ for male and female participants for different cases of COVID-19 severity, and for flu. Since these phases occur with both COVID-19 and flu, they are not novel to COVID-19. They can however, be more prominent in the case of COVID-19. It should be noted that flu has also been associated with cardiovascular disease (Nguyen et al., 2016). It is also known that the autonomic nervous system plays a key role in sensing and responding to infection, with pathogens activating vagal signaling causing bradyarrhythmias (Fairchild et al., 2011). Dysregulation of autonomic function has been reported in the weeks/months following a COVID-19 infection (Baker et al., 2022). It is intriguing that we see a statistically significant difference between male and female individuals, in the time variation of the RHR (Figure 4) and the time variation of the RMSSD (Figure 7). If the modification of the RHR and the RMSSD are indeed signs of autonomic dysregulation, it is intriguing that there is a statistically significant dependence on sex.
The parameters that describe the time evolution of the RHR depend on sex, age, and disease severity and are tabulated in Tables 3–5. The relative bradycardia minimum Ab and the time to the minimum Tb are correlated with age (The Pearson r for the correlation between Ab and age was found to be r = 0.80 for females and r = 0.97 for males. The Pearson r for the correlation between Tb and age was found to be r = 0.98 for females and r = 0.77 for males). The width of the first relative tachycardia peak Wt1 is also correlated with age (r = 0.83 for females, and r = 0.94 for males).
Among the 3 phases, the relative tachycardia at symptom onset is the easiest to understand, and may be interpreted as an immune response to infection. More unexpected is the decrease in RHR, possibly resulting in transient relative bradycardia. Approximately 1% of individuals experience a value of RHR that is at least 10 bpm lower than normal (see Figure 5). Sinus bradycardia is known to occur during sleep in the case of COVID-19 (Hu et al., 2020). Since Fitbit’s measurement of RHR is not the lowest value of heart rate, this raises the possibility that the heart rate during sleep may reach dangerously low values. It is important to be aware of this transient relative bradycardia phase, since certain medications used in the treatment of COVID-19 such as Remdesivir have been known to cause bradycardia (Gupta et al., 2020; Jacinto et al., 2021; Sanchez-Codez et al., 2021).
Interestingly, mild cases of COVID-19 are more likely to present with relative bradycardia. It is also more likely to present in females compared to males. A possible explanation for this may be obtained from Figure 6 and Table 1. Figure 6 shows that the peak RHR during symptom onset is positively correlated with the magnitude of the dip, i.e., a smaller peak RHR during symptom onset is associated with a larger (and possibly negative) dip. It is well known the heart rate is strongly linked to temperature, increasing by 8.5 bpm per 1°C increase in temperature, as found in one study (Karjalainen and Viitasalo, 1986). As seen from Table 1, males are more likely to present with a fever compared to females. Similarly, fever is more common in severe cases compared to mild cases. Thus, females as well as mild cases presenting with a small RHR increase (or no increase) are more likely to experience a bradycardia dip compared to males, or severe cases. Similarly, Figure 6 shows that the peak RHR during symptom onset is positively correlated with the height of the second tachycardia peak. As a result, the second tachycardia peak is higher in males, and for severe cases.
It is interesting to ask whether heart rate variability is altered in a way similar to the RHR. The RMSSD reflects beat-to-beat variance in the heart rate and estimates vagally mediated changes (Shaffer and Ginsberg, 2017). We found that the RMSSD shows a similar time evolution as the RHR, except with opposite phase. Figure 7 shows the time evolution of the RMSSD [subplot (a)] and the respiratory rate [subplot (b)] with time. Unlike the RMSSD, the respiratory rate decreases monotonously past the initial peak. Subplots (c) and (d) show the variation of RMSSD and respiratory rate, for male and female subjects.
Limitations: There are several limitations to the current work. A major concern is that we do not know whether the individuals who were diagnosed with COVID-19 and flu received medications to treat their illness, and what effects those medications may have had on their heart rate. Nevertheless, treatment for COVID-19 (especially for mild presentations which are most commonly seen in our data) tends to be symptomatic, e.g., antipyretics to reduce fever, analgesic medications to relieve pain, etc. It is very unlikely that patients were prescribed medications weeks following a diagnosis. The symptoms, severity, as well as symptom start date were all obtained from a survey, and could not be independently verified. We have also assumed that the participants were healthy prior to being diagnosed with COVID-19 or flu, and that the control group consisted of healthy individuals. These concerns are partially mitigated by our large sample size so that a small number of outliers are not expected to skew results. An independent investigation by clinical personnel, of the resting heart rate and heart rate variability changes in the months following a COVID-19 diagnosis will yield further credibility to our findings. Despite these limitations, the work presented in this article shows how COVID-19 and flu can alter the resting heart rate in the weeks/months following infection. This knowledge can be crucial in the case of patients with cardiac complications, or when patients are being treated with medication known to have cardiac effects.
The datasets presented in this article are not readily available because Fitbit’s privacy policy does not permit us to make the raw data available to third parties including researchers, outside of our web API Oauth 2.0 consent process. For specific questions, contact Fitbit at https://healthsolutions.fitbit.com/contact/.
The studies involving human participants were reviewed and approved by Advarra IRB. The patients/participants provided their written informed consent to participate in this study.
AN was responsible for planning the work, for part of the scientific analysis, and for preparing the first version of the manuscript. H-WS was responsible for part of the scientific analysis. CH led the Fitbit COVID-19 study, and provided valuable insights and feedback on the analysis and presentation of results.
This study received funding from Fitbit Research, Google LLC. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All authors declare no other competing interests.
Authors AN, H-WS, and CH were employed by Fitbit Research, Google Inc.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank members of the Fitbit Research team for many helpful discussions.
1This feature is not intended to diagnose or treat any medical condition and should not be relied on for any medical purposes. It is provided to Fitbit users to help manage well-being.
Amaratunga E. A., Corwin D. S., Moran L., Snyder R. (2020). Bradycardia in Patients with COVID-19: A Calm before the Storm? Cureus 12, e8599. doi:10.7759/cureus.8599
Amir M., Yoseph H., Farisi A. T. A., Phie J. K. P., Adam A. T. S. (2021). Symptomatic Bradycardia in Covid-19 Hospitalized Patients: A Case Series. Int. J. Infect. Dis. 111, 1–4. doi:10.1016/j.ijid.2021.07.068
Baker A. M. E., Maffitt N. J., Del Vecchio A., McKeating K. M., Baker M. R., Baker S. N., et al. (2022). Neural Dysregulation in Post-Covid Fatigue. medRxiv. doi:10.1101/2022.02.18.22271040
Bhatla A., Mayer M. M., Adusumalli S., Hyman M. C., Oh E., Tierney A., et al. (2020). COVID-19 and Cardiac Arrhythmias. Heart Rhythm 17, 1439–1444. doi:10.1016/j.hrthm.2020.06.016
Capoferri G., Osthoff M., Egli A., Stoeckle M., Bassetti S. (2021). Relative Bradycardia in Patients with COVID-19. Clin. Microbiol. Infect. 27, 295–296. doi:10.1016/j.cmi.2020.08.013
Chandrasekaran R., Katthula V., Moustakas E. (2020). Patterns of Use and Key Predictors for the Use of Wearable Health Care Devices by US Adults: Insights from a National Survey. J. Med. Internet Res. 22, e22443. doi:10.2196/22443
Cizgici A. Y., Zencirkiran Agus H., Yildiz M. (2020). COVID-19 Myopericarditis: It Should Be Kept in Mind in Today's Conditions. Am. J. Emerg. Med. 38, 1547–e6. doi:10.1016/j.ajem.2020.04.080
Coromilas E. J., Kochav S., Goldenthal I., Biviano A., Garan H., Goldbarg S., et al. (2021). Worldwide Survey of COVID-19-Associated Arrhythmias. Circ. Arrhythm Electrophysiol. 14, e009458. doi:10.1161/CIRCEP.120.009458
Crook H., Raza S., Nowell J., Young M., Edison P. (2021). Long Covid-Mechanisms, Risk Factors, and Management. BMJ 374, n1648. doi:10.1136/bmj.n1648
Demeke H. B., Merali S., Marks S., Pao L. Z., Romero L., Sandhu P., et al. (2021). Trends in Use of Telehealth Among Health Centers during the COVID-19 Pandemic - United States, June 26-November 6, 2020. MMWR Morb. Mortal. Wkly. Rep. 70, 240–244. doi:10.15585/mmwr.mm7007a3
Dherange P., Lang J., Qian P., Oberfeld B., Sauer W. H., Koplan B., et al. (2020). Arrhythmias and COVID-19: A Review. JACC Clin. Electrophysiol. 6, 1193–1204. doi:10.1016/j.jacep.2020.08.002
Douedi S., Mararenko A., Alshami A., Al‐Azzawi M., Ajam F., Patel S., et al. (2021). COVID‐19 Induced Bradyarrhythmia and Relative Bradycardia: An Overview. J. Arrhythmia 37, 888–892. doi:10.1002/joa3.12578
Efron B. (1981). Nonparametric Estimates of Standard Error: The Jackknife, the Bootstrap and Other Methods. Biometrika 68, 589–599. doi:10.1093/biomet/68.3.589
Elikowski W., Fertała N., Zawodna-Marszałek M., Rajewska-Tabor J., Świdurski W., Wajdlich D., et al. (2021). Marked Self-Limiting Sinus Bradycardia in COVID-19 Patients Not Requiring Therapy in the Intensive Care Unit - Case Series Report. Pol. Merkur Lekarski 49, 295–302.
Fairchild K. D., Srinivasan V., Randall Moorman J., Gaykema R. P. A., Goehler L. E. (2011). Pathogen-Induced Heart Rate Changes Associated with Cholinergic Nervous System Activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R330–R339. doi:10.1152/ajpregu.00487.2010
Fortune Business Insights (2020). Wearable Medical Devices Market Size, Share and Industry Analysis by Product (Diagnostic & Patient Monitoring, Therapeutics), by Application (Remote Patient Monitoring and Home Healthcare, Sports and Fitness), by Distribution Channel (Retail Pharmacies, Online Pharmacies, Hypermarkets) and Regional Forecast 2020-2027. Report ID: FBI101070. Fortune Business Insights.
Gawałko M., Kapłon-Cieślicka A., Hohl M., Dobrev D., Linz D. (2020). COVID-19 Associated Atrial Fibrillation: Incidence, Putative Mechanisms and Potential Clinical Implications. Int. J. Cardiol. Heart Vasc. 30, 100631. doi:10.1016/j.ijcha.2020.100631
Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. (2020). Management of Post-Acute Covid-19 in Primary Care. BMJ 370, m3026. doi:10.1136/bmj.m3026
Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. (2020). Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 5, 811–818. doi:10.1001/jamacardio.2020.1017
Gupta A. K., Parker B. M., Priyadarshi V., Parker J. (2020). Cardiac Adverse Events with Remdesivir in COVID-19 Infection. Cureus 12, e11132. doi:10.7759/cureus.11132
Hu L., Gong L., Jiang Z., Wang Q., Zou Y., Zhu L. (2020). Clinical Analysis of Sinus Bradycardia in Patients with Severe COVID-19 Pneumonia. Crit. Care 24, 257. doi:10.1186/s13054-020-02933-3
Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 395, 497–506. doi:10.1016/s0140-6736(20)30183-5
Ikeuchi K., Saito M., Yamamoto S., Nagai H., Adachi E. (2020). Relative Bradycardia in Patients with Mild-To-Moderate Coronavirus Disease, Japan. Emerg. Infect. Dis. 26, 2504–2506. doi:10.3201/eid2610.202648
Jacinto J. P., Patel M., Goh J., Yamamura K. (2021). Remdesivir-Induced Symptomatic Bradycardia in the Treatment of COVID-19 Disease. HeartRhythm Case Rep. 7, 514–517. doi:10.1016/j.hrcr.2021.05.004
Karjalainen J., Viitasalo M. (1986). Fever and Cardiac Rhythm. Arch. Intern. Med. 146, 1169–1171. doi:10.1001/archinte.1986.00360180179026
Kochi A. N., Tagliari A. P., Forleo G. B., Fassini G. M., Tondo C. (2020). Cardiac and Arrhythmic Complications in Patients with COVID‐19. J. Cardiovasc. Electrophysiol. 31, 1003–1008. doi:10.1111/jce.14479
Lampert J., Miller M., Halperin J. L., Oates C., Giustino G., Nelson K., et al. (2021). Prognostic Value of Electrocardiographic QRS Diminution in Patients Hospitalized with COVID-19 or Influenza. Am. J. Cardiol. 159, 129–137. doi:10.1016/j.amjcard.2021.07.048
Li Z., Shao W., Zhang J., Ma J., Huang S., Yu P., et al. (2021). Prevalence of Atrial Fibrillation and Associated Mortality Among Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8, 720129. doi:10.3389/fcvm.2021.720129
Linschoten M., Peters S., van Smeden M., Jewbali L. S., Schaap J., Siebelink H.-M., et al. (2020). Cardiac Complications in Patients Hospitalised with COVID-19. Eur. Heart J. Acute Cardiovasc. Care 9, 817–823. doi:10.1177/2048872620974605
Long B., Brady W. J., Koyfman A., Gottlieb M. (2020). Cardiovascular Complications in COVID-19. Am. J. Emerg. Med. 38, 1504–1507. doi:10.1016/j.ajem.2020.04.048
Miller D. J., Capodilupo J. V., Lastella M., Sargent C., Roach G. D., Lee V. H., et al. (2020a). Analyzing Changes in Respiratory Rate to Predict the Risk of COVID-19 Infection. PLoS One 15, e0243693. doi:10.1371/journal.pone.0243693
Miller J. C., Skoll D., Saxon L. A. (2020b). Home Monitoring of Cardiac Devices in the Era of COVID-19. Curr. Cardiol. Rep. 23, 1. doi:10.1007/s11886-020-01431-w
Mishra T., Wang M., Metwally A. A., Bogu G. K., Brooks A. W., Bahmani A., et al. (2020). Pre-Symptomatic Detection of COVID-19 from Smartwatch Data. Nat. Biomed. Eng. 4, 1208–1220. doi:10.1038/s41551-020-00640-6
Musikantow D. R., Turagam M. K., Sartori S., Chu E., Kawamura I., Shivamurthy P., et al. (2021). Atrial Fibrillation in Patients Hospitalized with COVID-19: Incidence, Predictors, Outcomes, and Comparison to Influenza. JACC Clin. Electrophysiol. 7, 1120–1130. doi:10.1016/j.jacep.2021.02.009
Natarajan A., Pantelopoulos A., Emir-Farinas H., Natarajan P. (2020a). Heart Rate Variability with Photoplethysmography in 8 Million Individuals: A Cross-Sectional Study. Lancet Digit. Health 2, e650–e657. doi:10.1016/s2589-7500(20)30246-6
Natarajan A., Su H.-W., Heneghan C. (2020b). Assessment of Physiological Signs Associated with COVID-19 Measured Using Wearable Devices. NPJ Digit Med. 3, 156. doi:10.1038/s41746-020-00363-7
Natarajan A., Su H.-W., Heneghan C., Blunt L., O’Connor C., Niehaus L. (2021). Measurement of Respiratory Rate Using Wearable Devices and Applications to COVID-19 Detection. NPJ Digit Med. 4, 136. doi:10.1038/s41746-021-00493-6
Nguyen J. L., Yang W., Ito K., Matte T. D., Shaman J., Kinney P. L. (2016). Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 1, 274–281. doi:10.1001/jamacardio.2016.0433
Oates C. P., Turagam M. K., Musikantow D., Chu E., Shivamurthy P., Lampert J., et al. (2020). Syncope and Presyncope in Patients with COVID‐19. Pacing Clin. Electrophysiol. 43, 1139–1148. doi:10.1111/pace.14047
Oliva A., Franchi C., Gatto M. C., Galardo G., Pugliese F., Mastroianni C. (2021). Prevalence and Clinical Significance of Relative Bradycardia at Hospital Admission in Patients with Coronavirus Disease 2019 (COVID-19). Clin. Microbiol. Infect. 27, 1185–1187. doi:10.1016/j.cmi.2021.04.013
Ostchega Y., Porter K. S., Hughes J., Dillon C. F., Nwankwo T. (2011). Resting Pulse Rate Reference Data for Children, Adolescents, and Adults: United States, 1999-2008. Natl. Health Stat. Rep. 41 (41), 1–16.
Pardo Sanz A., Salido Tahoces L., Ortega Pérez R., González Ferrer E., Sánchez Recalde Á., Zamorano Gómez J. L. (2021). New-Onset Atrial Fibrillation during COVID-19 Infection Predicts Poor Prognosis. Cardiol. J. 28, 34–40. doi:10.5603/cj.a2020.0145
Peigh G., Leya M. V., Baman J. R., Cantey E. P., Knight B. P., Flaherty J. D. (2020). Novel Coronavirus 19 (COVID-19) Associated Sinus Node Dysfunction: a Case Series. Eur. Heart J. Case Rep. 4, 1–6. doi:10.1093/ehjcr/ytaa132
Quer G., Gouda P., Galarnyk M., Topol E. J., Steinhubl S. R. (2020). Inter- and Intraindividual Variability in Daily Resting Heart Rate and its Associations with Age, Sex, Sleep, BMI, and Time of Year: Retrospective, Longitudinal Cohort Study of 92,457 Adults. PLoS One 15, e0227709. doi:10.1371/journal.pone.0227709
Quer G., Radin J. M., Gadaleta M., Baca-Motes K., Ariniello L., Ramos E., et al. (2021). Wearable Sensor Data and Self-Reported Symptoms for COVID-19 Detection. Nat. Med. 27, 73–77. doi:10.1038/s41591-020-1123-x
Radin J. M., Quer G., Ramos E., Baca-Motes K., Gadaleta M., Topol E. J., et al. (2021). Assessment of Prolonged Physiological and Behavioral Changes Associated with COVID-19 Infection. JAMA Netw. Open 4, e2115959. doi:10.1001/jamanetworkopen.2021.15959
Raveendran A. V., Jayadevan R., Sashidharan S. (2021). Long COVID: An Overview. Diabetes Metab. Syndr. 15, 869–875. doi:10.1016/j.dsx.2021.04.007
Russell A., Heneghan C., Venkatraman S. (2019). Investigation of an Estimate of Daily Resting Heart Rate Using a Consumer Wearable Device. medRxiv. doi:10.1101/19008771
Sahranavard M., Akhavan Rezayat A., Zamiri Bidary M., Omranzadeh A., Rohani F., Hamidi Farahani R., et al. (2021). Cardiac Complications in COVID-19: A Systematic Review and Meta-Analysis. Arch. Iran Med. 24, 152–163. doi:10.34172/aim.2021.24
Sanchez-Codez M. I., Rodriguez-Gonzalez M., Gutierrez-Rosa I. (2021). Severe Sinus Bradycardia Associated with Remdesivir in a Child with Severe SARS-CoV-2 Infection. Eur. J. Pediatr. 180, 1627. doi:10.1007/s00431-021-03940-4
Shaffer F., Ginsberg J. P. (2017). An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 5, 258. doi:10.3389/fpubh.2017.00258
Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. (2020). Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 5, 802–810. doi:10.1001/jamacardio.2020.0950
Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M. Y., et al. (2020). Recognizing COVID-19-Related Myocarditis: The Possible Pathophysiology and Proposed Guideline for Diagnosis and Management. Heart Rhythm 17, 1463–1471. doi:10.1016/j.hrthm.2020.05.001
Srinivasan A., Pansuriya T., Wilson B., Sarva S. T., Jayaram T., Alim S., et al. (2021). Systemic Inflammation-Related Bradycardia in COVID-19. Case Rep. Cardiol. 2021, 9986955. doi:10.1155/2021/9986955
Stone E., Kiat H., McLachlan C. S. (2020). Atrial Fibrillation in COVID‐19: A Review of Possible Mechanisms. FASEB J. 34, 11347–11354. doi:10.1096/fj.202001613
Suran M. (2022). Increased Use of Medicare Telehealth during the Pandemic. JAMA 327, 313. doi:10.1001/jama.2021.23332
Turagam M. K., Musikantow D., Goldman M. E., Bassily-Marcus A., Chu E., Shivamurthy P., et al. (2020). Malignant Arrhythmias in Patients with COVID-19: Incidence, Mechanisms, and Outcomes. Circ. Arrhythm Electrophysiol. 13, e008920. doi:10.1161/CIRCEP.120.008920
Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069. doi:10.1001/jama.2020.1585
Keywords: digital health, heart rate variability, COVID-19, heart rate, bradycardia, tachycardia
Citation: Natarajan A, Su H-W and Heneghan C (2022) Occurrence of Relative Bradycardia and Relative Tachycardia in Individuals Diagnosed With COVID-19. Front. Physiol. 13:898251. doi: 10.3389/fphys.2022.898251
Received: 17 March 2022; Accepted: 08 April 2022;
Published: 10 May 2022.
Edited by:
Rajesh Kumar Tripathy, Birla Institute of Technology and Science, IndiaReviewed by:
Lakhan Dev Sharma, VIT-AP University, IndiaCopyright © 2022 Natarajan, Su and Heneghan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aravind Natarajan, YW5hdGFyYWphbkBnb29nbGUuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.