- 1Faculty of Medicine, Memorial University of Newfoundland, St. John’s, NL, Canada
- 2Space Biomedicine Systematic Review Methods Group, Wylam, United Kingdom
- 3Department of Vascular Medicine, Sainte Musse Hospital, Toulon La Seyne Hospital Centre, Toulon, France
- 4European Astronaut Centre (EAC), European Space Agency, Space Medicine Team (HRE-OM), Cologne, Germany
- 5KBR GmbH, Cologne, Germany
- 6King’s College London, Centre of Human & Applied Physiological Sciences, London, United Kingdom
- 7Division of Physiology, Otto Löwi Research Center for Vascular Biology, Immunity and Inflammation, Medical University of Graz, Graz, Austria
- 8Mohammed Bin Rashid University of Medicine and Applied Health Sciences, Dubai, United Arab Emirates
- 9Institute of Aerospace Medicine, German Aerospace Center, Cologne, Germany
- 10Division of Trauma, Acute Care Surgery, and Surgical Critical Care, Baystate Medical Center, Springfield, MA, United States
- 11Department of Angiology, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
- 12Mechanical and Aerospace Engineering, University of California, San Diego, San Diego, CA, United States
- 13Department of Radiology, University of California, San Diego, San Diego, CA, United States
Background: The recent discovery of a venous thrombosis in the internal jugular vein of an astronaut has highlighted the need to predict the risk of venous thromboembolism in otherwise healthy individuals (VTE) in space. Virchow’s triad defines the three classic risk factors for VTE: blood stasis, hypercoagulability, and endothelial disruption/dysfunction. Among these risk factors, venous endothelial disruption/dysfunction remains incompletely understood, making it difficult to accurately predict risk, set up relevant prophylactic measures and initiate timely treatment of VTE, especially in an extreme environment.
Methods: A qualitative systematic review focused on endothelial disruption/dysfunction was conducted following the guidelines produced by the Space Biomedicine Systematic Review Group, which are based on Cochrane review guidelines. We aimed to assess the venous endothelial biochemical and imaging markers that may predict increased risk of VTE during spaceflight by surveying the existing knowledge base surrounding these markers in analogous populations to astronauts on the ground.
Results: Limited imaging markers related to endothelial dysfunction that were outside the bounds of routine clinical practice were identified. While multiple potential biomarkers were identified that may provide insight into the etiology of endothelial dysfunction and its link to future VTE, insufficient prospective evidence is available to formally recommend screening potential astronauts or healthy patients with any currently available novel biomarker.
Conclusion: Our review highlights a critical knowledge gap regarding the role biomarkers of venous endothelial disruption have in predicting and identifying VTE. Future population-based prospective studies are required to link potential risk factors and biomarkers for venous endothelial dysfunction to occurrence of VTE.
Introduction
The recent incidental discovery of an internal jugular vein thrombosis in an astronaut during long duration spaceflight on the International Space Station (Auñón-Chancellor et al., 2020) has brought predicting the risk of VTE in otherwise healthy individuals to the forefront of the space medicine research community’s interests. While the VTE in question was treated successfully in space with enoxaparin (Auñón-Chancellor et al., 2020), a similar situation during a mission beyond low Earth orbit, far from ground-based medical intervention, could result in significant mission disruption or possible loss of crew life. Therefore, predicting the baseline VTE risk of any given astronaut candidate during medical screening has high value as a tool for prevention of VTE in space. While known thrombophilia disorders are already screened for and are cause for medical exclusion, the complex etiology of VTE touts the potential for novel biomarkers to be used to assess risk. Furthermore, identifying biomarkers that could indicate the precursor processes of VTE in early stages onboard future diagnostic assays in space could be used to justify prophylactic measures such as low dose anticoagulants.
Virchow’s triad outlines the classic risk factors for clot formation: blood stasis, hypercoagulability, and endothelial disruption/dysfunction (Vazquez-Garza et al., 2017). Immobilizations such as bed rest, pregnancy, and plaster casts, all associated with blood flow stasis, are among known common risk factors for venous thromboembolism (VTE) (Reitsma et al., 2012), and spaceflight arguably constitutes another (Kim et al., 2021). Established risks such as malignancies, chemotherapy, oral contraceptives, hormone replacement therapy, obesity, infection, inflammatory disease, lupus, and genetic factors predispose to VTE at least in part through hypercoagulability due to increased clotting factor concentrations or reduced fibrinolytic factor concentrations and innate immunity. Others, such as venous catheters, trauma, or surgery may primarily affect endothelial integrity (Reitsma et al., 2012; Gordon and Lombard, 2017). The mechanisms mediating spaceflight-associated VTE are still elusive, however, all components of Virchow’s triad have been implicated (Limper et al., 2021).
Among the components of Virchow’s triad, which collectively determine VTE risk, endothelial disruption/dysfunction of the venous endothelium received the least attention (Yau et al., 2015). The arterial endothelial function has been studied in great detail due to the association with atherosclerosis and cardiac events (Tsai et al., 2002) much less is known about the venous endothelium. While atherosclerosis has also been linked with occurrence of VTE (Poredos and Jezovnik, 2011), not all predictors for atherosclerotic disease cross over to VTE. Therefore, the etiological differences between the two systems, such as lower shear stress and differing clot composition in the venous system, must be considered (Tsai et al., 2002; Vazquez-Garza et al., 2017).
The endothelium together with the smooth muscle cells controls blood flow and vascular tone, is targeted by pro-inflammatory cytokines and leukocytes, and provides a surface for the expression of molecules triggering the coagulation cascade (Yau et al., 2015). A schematic summary of the coagulation cascade is provided in Figure 1 to frame the discussion in this review.
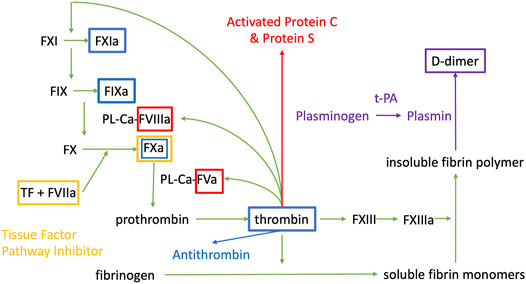
FIGURE 1. Coagulation cascade. PL are phospholipids, Ca is Calcium, F is for Factor, a indicates that it is the activated form of the factor. t-PA is tissue plasminogen activator. The colour of the boxes correspond to the substance that is inhibiting the factor contained in the box.
Whenever the endothelium is damaged, the initial step is vasoconstriction to limit blood flow and therefore hemorrhage. The ruptured endothelial barrier exposes prothrombic subendothelial factors such as Von Willebrand Factor (vWF) and collagen to blood promoting platelet adhesion (Palta et al., 2014). In turn, activated coagulation factors form complexes on the platelets, promoting the formation of a cross linked fibrin clot (Palta et al., 2014).
Once the coagulation cascade is activated, the anticoagulant and fibrinolytic systems are also activated, giving rise to complex interactions. Upon thrombin activation, a negative feedback loop induces activation of natural anticoagulants Protein C (PC) and Protein S (PS). PC and PS regulate the activity of activated factor V (FVa) and activated factor VIII (FVIIIa) (Palta et al., 2014). The fibrinolytic system is also activated during coagulation and tissue factor plasminogen inhibitor (TFPI) inhibits activated factor X, tissue factor, and activated factor VII complex (Palta et al., 2014). Thrombin generates antithrombin, which inhibits thrombin and Factors IXa, Xa, and XIa, while endothelial cells produce prostaglandin and nitric oxide (NO) which inhibit vasoconstriction and platelet aggregation (Palta et al., 2014). Plasmin is formed from plasminogen via tissue plasminogen activator (tPA), which then lyses the insoluble fibrin polymer to D-dimer and fibrin degradation products. Thrombomodulin binds to thrombin to prevent clot formation on undamaged endothelium (Palta et al., 2014).
The multitude of factors and co-factors involved in the regulation of coagulation provide many possibilities for VTE risk assessment and diagnosis based on factor levels. While D-dimers (fibrin degradation products, increased following thrombus formation) are traditionally used in the algorithm of preclinical assessment of VTE suspicion because of their high negative predictive value, their lack of specificity does not allow their use for VTE diagnosis which necessitates imaging studies (Barnes et al., 2008; Jacobs et al., 2016). Barnes et al. (Barnes et al., 2008) conducted a narrative review focused on potential biomarkers for the specific diagnosis of VTE, such as P-selectin, E-selectin, microparticles, thrombin, fibrin monomers, and interleukin 10 (IL-10). They found that several studies determined that P-selectin was elevated in patients with deep venous thrombosis (DVT), which may decrease after the acute event (Barnes et al., 2008).
Microparticles have less evidence, but are also elevated in acute settings (Barnes et al., 2008). Genetic mutations associated with the E-selectin and IL-10 genes additionally predicted increased risk of DVT. Looking forward, the authors suggested that these biomarkers may be able to help predict recurrence of VTE and the severity of thrombosis (Barnes et al., 2008). A recent follow up review by Jacobs et al. (Jacobs et al., 2016) came to similar conclusion regarding P-selectin and D-dimer.
The purpose of the present systematic review is primarily to identify potential biological and imaging markers related to endothelial dysfunction or VTE events in terrestrial medicine that could be utilized to elucidate VTE mechanisms in space. Subsequently, these biomarkers could be systematically tested to determine their performance in identifying spaceflight-associated VTE risk early on. On potential application is risk prediction during space flight crew selection. Moreover, biomarker testing could be utilized to guide preventive measures in space, such as low dose anticoagulation, before overt VTE occurs. The present study seeks to synthesize themes linking markers for endothelial disruption and/or dysfunction with the occurrence of VTE and generate recommendations for future research.
Methods
Search Strategy
A range of terms (Supplementary Table S1) were used in combinations to search PubMed, Web of Science, and Cochrane Reviews and Trials databases between June 24th-25th 2021. Initial pre-scoping was performed to determine appropriate search terms that would capture an adequate number of papers to reach knowledge saturation. In databases where Medical Subject Headings (MeSH) terms cannot be used, the same terms were searched without the MeSH identifier. The European Space Agency (ESA) Topical Team on VTE contributed with their expertise to ensure all appropriate keywords were included. The qualitative systematic review analysis method was chosen due to high heterogeneity of available evidence, and the lack of consistent study protocols and outcomes that would allow metanalysis of previously published findings. The population of interest was healthy individuals with no prior risk factors for VTE, either as main study subjects or controls. The Interest was linking VTE with the venous endothelial system. No limitation on controls were specified. The Outcomes included structural biomarkers, venous mechanical properties, venous flow properties, symptoms, circulating biomarkers, endothelial markers, blood cell counts, thrombelastometry, platelet aggregation, coagulation times, thrombin generation, fibrinolytic values/endothelial activation, and pro- and anti-coagulatory factors. The full Population, Interest, Control, and Outcome (PICO) table used to define the present search criteria, along with the search strategy implemented can be found in the Supplementary Material as Table 1.
Inclusion Criteria
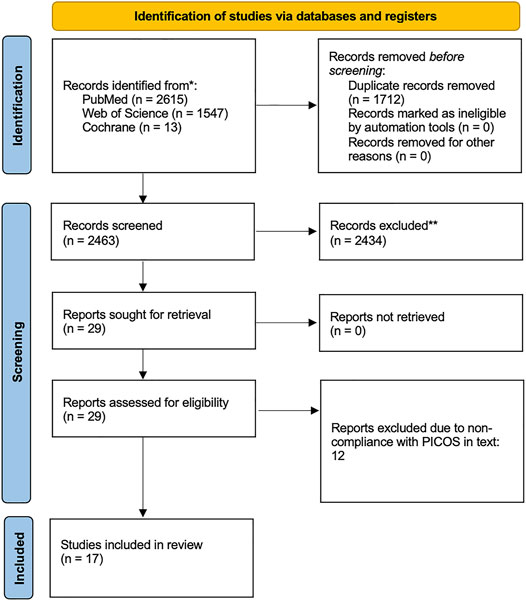
Any studies that did not meet the inclusion criteria were excluded. Included papers were original studies that were available as full texts and published in English. No limitation on publication period was used. The PRISMA standards (Preferred Reporting Items for Systematic reviews and Meta Analyses) checklist moher et al. was followed to ensure gold standard, transparent, and complete reporting of results. The PRISMA flow diagram is shown in Figure 2.
Study Selection and Data Extraction
The initial screening of documents, using abstracts and titles, was carried out using the Rayyan systematic review online application (Ouzzani et al., 2016). Two authors screened the papers for adherence to the search criteria together (unblinded), using a third author as a tie breaker for any papers which they could not agree upon. Included papers were original studies on healthy humans (either as the main population or control group) that focused on linking endothelial biomarkers to the venous system in terms of VTE risk. Common reasons for papers to be excluded were that they were only performed in vitro or in cell models, that the study population lacked healthy controls or healthy subjects (ie. no known risk factors for VTE), or that the papers were reviews. Twelve papers were further excluded upon reading the full texts. If it was unclear from the initial screening whether a study met the inclusion criteria, the full text of the document was obtained. NVivo12 (QSR International Pty Ltd. (2018) NVivo (Version 12), https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home) was used to extract data from each manuscript.
Quality Assessment
The quality of included studies was assessed using the Risk of Bias Assessment Tool for Nonrandomized Studies RoBANS (Kim et al., 2013). RoBANS assesses the quality of non-randomized literature across six domains: the selection of participants; confounding variables; measurement of exposure; blinding of outcome assessments; incomplete outcome data; and selective outcome reporting, with each study ranking either low, unclear or high risk (Kim et al., 2013). The ranking of the included papers is shown above in Table 1.
Data Analysis
Thematic analysis (Braun and Clarke, 2006; Braun et al., 2019) and thematic synthesis (Thomas and Harden, 2008) following the qualitative systematic review methods guide by Winnard et al. (2021) were used. Thematic analysis is split into 6 stages: The first stage involved reading all the included papers to identify relevant concepts and themes. The second stage was initial coding of included papers using NVivo12 (QSR International Pty Ltd. (2018) NVivo (Version 12), https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home). The third was the grouping of codes in broader themes, the fourth reviewing the themes. The fifth step involved defining criteria for each theme, before the sixth and final step of creating a thematic analysis map.
Results
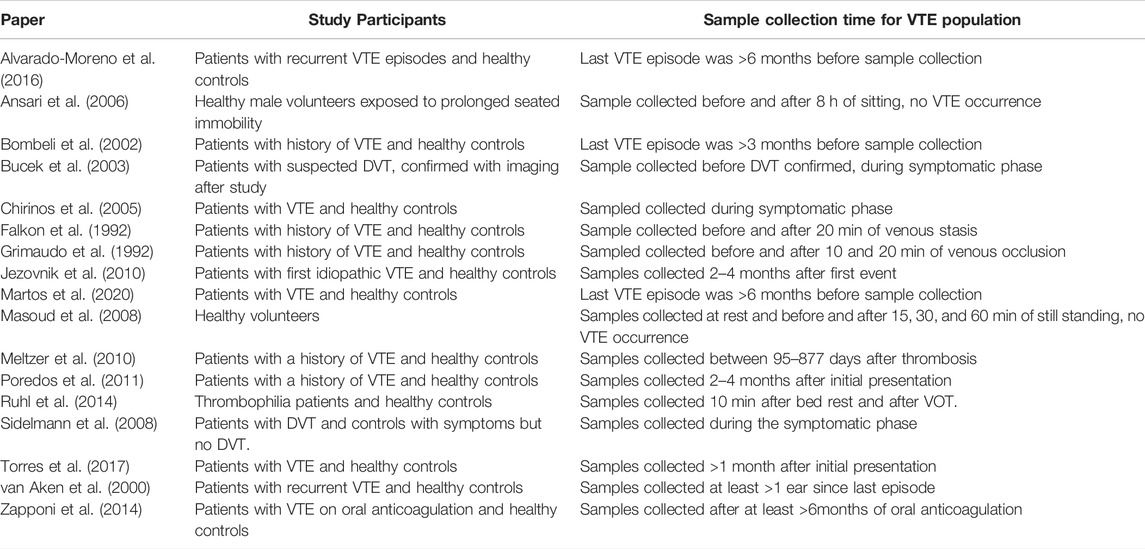
The study population and sample collection time for each of the included studies is shown in Table 2. While all included papers looked at healthy populations either as the cohort or as the control group, the sample collection time for the participants affected by VTE in studies which had a case group varied widely, and was primarily after an acute VTE event, rather than before. This resulted in very little predictive value in the biomarkers studied. The two prospective studies, Ansari et al. (Ansari et al., 2006) and Masoud et al. (Masoud et al., 2008) did not result in any VTE occurrence with their relative interventions.
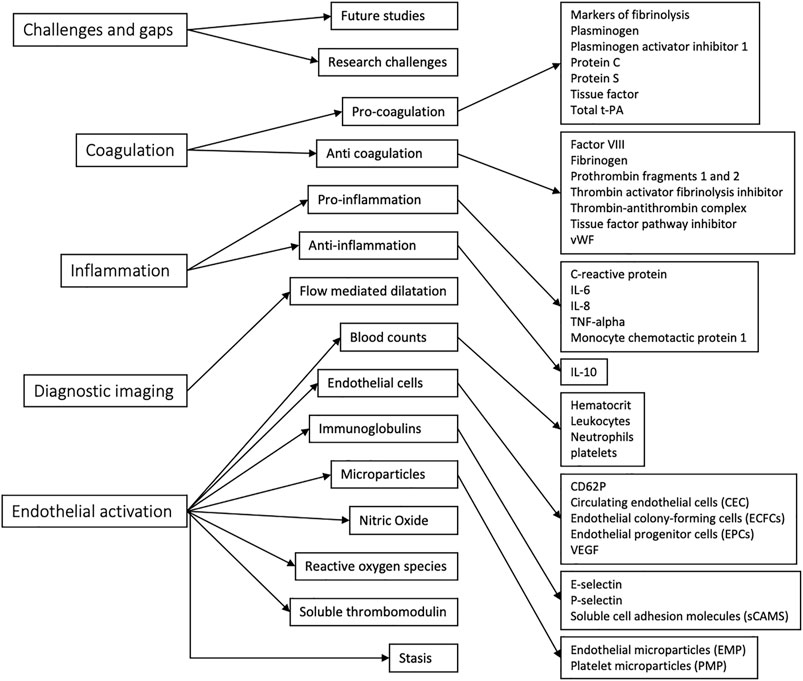
The thematic map generated through NVivo analysis is shown in Figure 3. Individual themes were first identified, then grouped into larger themes for aid analysis. While these themes loosely followed the PICOS distribution of search terms, coagulation, inflammation, and endothelial activation arose as the most appropriate umbrella themes for the key points of focus in the included papers. The criteria for inclusion of each code in the respective themes are explained in Table 3.
Discussion
In total, 17 papers that linked biomarkers of endothelial disruption to VTE and the venous endothelial system were included. Five broad themes were identified, which were coagulation, endothelial activation, inflammation, the role of diagnostic imaging, and challenges and gaps. All other identified themes were grouped under these categories for thematic analysis.
Relevant Biomarkers for Coagulation, Endothelial Activation, and Inflammation
Coagulation and Endothelial Dysfunction in VTE Risk
The biomarkers related to coagulation are intuitively linked to the prediction of VTE risk. Therefore, it is not surprising that coagulation arose as an umbrella theme for this qualitative analysis. The tissue plasminogen activator/plasminogen activator inhibitor-1 (tPA/PAI-1) ratio is often considered a marker of ongoing fibrinolysis, similar to the D-dimer. Vascular endothelium promotes fibrinolysis due to release of tPA from vessel wall (Falkon et al., 1992), while PAI-1 is produced by endothelium and liver (Falkon et al., 1992). The linkage of the state of the vessel wall to the coagulation factors complicates the picture and makes it difficult to fully separate endothelial dysfunction/activation from coagulation factors, hence the discussion of these two themes together. For example, shear stress has been linked to increased levels of tPA and PAI-1 (Masoud et al., 2008) and enhanced expression of tissue factor (TF).
When measured at different sites (ie. arm and leg) in the same person undergoing venous stasis due to prolonged sitting, the changes in total tPA between the two sites did not correlate, which could be influenced by reduced haptic clearance of tPA or local endothelial activation effects due to localized venous stasis (Ansari et al., 2006), whereas the increases in PAI-1 were strongly correlated between body sites, indicating a more systemic physiological response. Another study found that when a local stimulus is applied to the endothelium through targeted venous stasis, there is only little correlation between tPA and PAI-1, indicating both endothelial and systemic forces working at different secretory levels (Falkon et al., 1992). Baseline values of tPA and plasmin-alpha2-antiplasmin (PAP) were slightly higher for thrombophilic patients than healthy probands in (Rühl et al., 2014), but this study found that the release of tPA from endothelial cells during short stasis was not influenced by presence of thrombophilic risk factors or clinically relevant arterial phenomenon.
One way to determine the contribution of endothelial factors to coagulation is to directly stimulate the endothelium. Studies have found that levels of PAI-1 and tPA were increased in patients with VTE (Bombeli et al., 2002). Patients with DVT were found to have lower mean fibrinolytic response to venous occlusion, higher antigen levels of t-PA and PAI-1. After venous occlusion, healthy controls had excess tPA over PAI-1, whereas only a minority of patients with VTE maintained that ratio (Grimaudo et al., 1992). Normal PAI-1 indicated normal fibrinolytic response in 97% of patients with idiopathic VTE, and can be used to detect 83% of patients with elevated PAI-1 (Grimaudo et al., 1992). Inability of t-PA released during venous occlusion to overcome inhibitory capacity of PAI-1 has been proposed as a biomarker that can predict risk of VTE, however it requires the use of the time-consuming venous occlusion test (Grimaudo et al., 1992).
Other coagulation factors were also discussed as possible biomarkers. Plasma levels of FVIII and vWF have been found to be elevated in patients with a history of at least one unprovoked VTE event (Bombeli et al., 2002), while the respective levels of vWF and factor VIII are well correlated in these patients and healthy controls (Bombeli et al., 2002). However, vWF levels did not correlate well with other endothelial derived proteins like soluble thrombomodulin (sTM), PAI-1, and tPA (Bombeli et al., 2002), which indicates differing secretion patterns.
Venous Thromboembolism Risk and Inflammation
Further complicating the picture is the role of inflammation. PAI-I, t-PA, and thrombin activatable fibrinolysis inhibitor (TAFI) are all associated with VTE, but so is plasminogen. The association of plasminogen with VTE seems to be surprising, since it is a precursor for the anti-fibrinolytic cascade, but plasminogen and t-PA levels may reflect underlying risk factors such as inflammation and endothelial activation (Masoud et al., 2008; Meltzer et al., 2010). This is supported by the findings that adjustment for acute phase protein attenuated the association between VTE and plasminogen, suggesting that plasminogen is a marker of systemic inflammation (Masoud et al., 2008).
The linkage between inflammation and VTE risk and clinical biomarkers does not always hold true. Reduced concentration of TFPI is a risk factor for development of DVT (Sidelmann et al., 2008). However, concentration of free and total TFPI, CRP, vWF and D-dimer were significantly higher in patients with clinically suspected VTE in an acute setting, but the relationship between these markers were not significantly linked with inflammation or endothelial cell disruption. It is believed TFPI may serve as a marker of endothelial cell dysfunction because inflammation and tissue injury are associated with elevated concentrations of TFPI, but the covariation between D-dimer and TFPI found by the above study suggests that D-dimer and TFPI are released from fibrin by degradation of fibrin clot. The TFPI relationship with acute DVT persisted when adjusted for effects of inflammation, which further supports that TFPI and inflammation are unlikely to be related (Sidelmann et al., 2008).
More direct indicators of inflammation, such as C-reactive protein (CRP), interleukin-6 (IL-6), and interleukin-8 (IL-8) are increased in patients with acute DVT (Bucek et al., 2002). Along the same line, high levels of markers of endothelial dysfunction such as vWF, P-selectin, and vascular cell adhesion protein (VCAM-1) are found in DVT patients (Jezovnik and Poredos, 2010). Van Aken et al. (Van Aken et al., 2000) found that the thrombosis risk for humans with IL-8 in upper quartile compared to lowest quartile was 9.4 (Van Aken et al., 2000). Interrelationship between inflammatory markers and markers of endothelial dysfunction favors the idea that inflammation could be involved in etiopathogenesis of idiopathic venous thrombosis. Another study found that patients with idiopathic VT had high levels of IL-6 and IL-8, as well as lower levels of the anti-inflammatory interleukin-10 (IL-10) during the stable phase of their disease (Poredos and Jezovnik, 2011). However, previous studies indicated that CRP levels do not seem to correlate with increased or decreased risk of VTE (Falkon et al., 1992), while others found that risk of VTE decreased after adjustment for markers of inflammation (Meltzer et al., 2010).
Chronic low-grade inflammation may be a risk factor for development of VTE as well as atherosclerotic disease, through endothelial dysfunction in the vasculature (Jezovnik and Poredos, 2010). The inflammatory process might not just be through alteration of procoagulant and antifibrinolytic activity but actual compromised endothelial integrity of the vessel wall that could be present in the stable phase of the disease as well. Inflammation could therefore be the inciting endothelial injury (Gordon and Lombard, 2017), or it could be a consequence of the disease, not a cause. There is a possibility that the coagulant process or thrombus itself induces inflammatory responses (Van Aken et al., 2000). Endothelial cell (EC) activation is associated with endothelial microparticle (EMP) release, and it has been found that patients with VTE had higher levels of EMP-62E (Chirinos et al., 2005). Elevated P-selectin, which mediates platelet endothelial interaction, shows that damage to the venous wall could be the inciting event (Jezovnik and Poredos, 2010).However, other studies found no difference concerning concentration of four major soluble cell adhesion molecules (sCAMS) between patients with DVT and controls in the acute phase (Bucek et al., 2003), which would indicate activation of vascular endothelial cells does not play a central role, at least acutely (Bucek et al., 2003).
Most studies look at these markers after patients have presented with their first VTE event, which does not provide any information about their premorbid state. Too much inflammation could reduce bioavailability of nitric oxide (NO), also reducing functional capacity of the vessel (Poredos and Jezovnik, 2011). If endothelial cell (EC) dysfunction is present, the antithrombotic potential of EC falls (Alvarado-Moreno et al., 2016). Activation of ECs due to cytokines may induce production of free radicals that allow ECs to malfunction (Alvarado-Moreno et al., 2016).
Relevant Imaging and Diagnostic Techniques
All imaging described in the included studies focused on diagnosis of VTE, as opposed to establishing risk for VTE. Compression venous ultrasound (CUS) is considered the gold standard imaging for DVT (Bombeli et al., 2002; Bucek et al., 2002; Chirinos et al., 2005). CUS imaging is based on identifying deep venous incompressibility and thrombus visualization. The only imaging focusing on endothelial clotting risk was ultrasound measurement of flow mediated dilatation (FMD) of the brachial artery (Poredos and Jezovnik, 2011). FMD is a marker of pre-clinical arterial vessel wall remodeling processes and endothelial dysfunction (Raitakari and Celermajer, 2000). FMD has not been studied in the venous endothelial system.
Identified Biomarkers
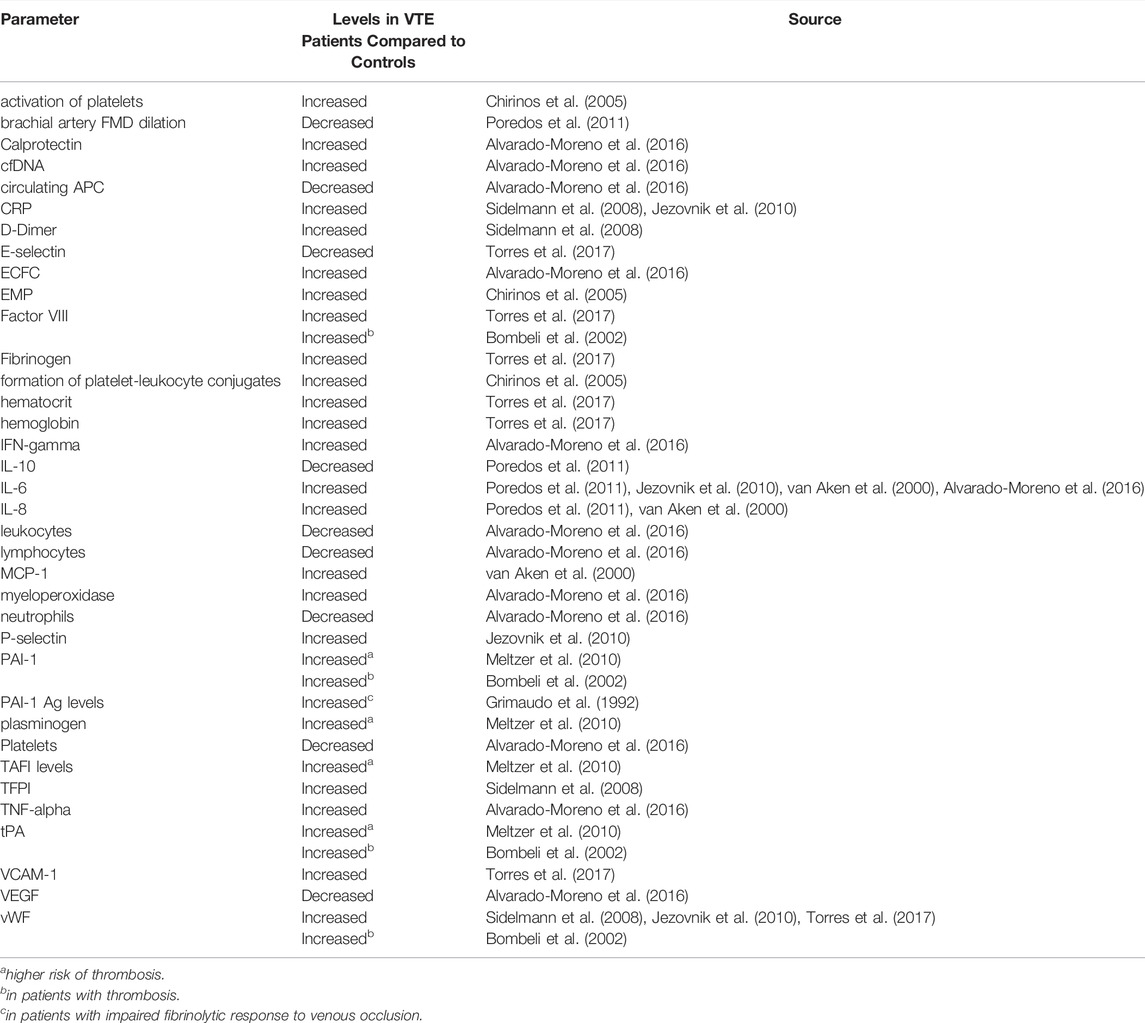
Several possible identifiers of biomarkers which are increased or decreased in the context of VTE were identified throughout this review. A summary in shown in Table 4. As highlighted in Table 2 above, most studies focused on the stable phase of VTE after the acute event had already occurred, therefore most of the below biomarkers are not predictive but rather reactive.
Challenges of Studying Venous Endothelial Dysfunction
Fibrinolytic investigations are not commonplace because: 1) it is unclear what to look for to qualify as a fibrinolytic deficiency, 2) simulation tests are time consuming, and 3) there is no consensus on abnormal values (Grimaudo et al., 1992).
The study of venous endothelial dysfunction in vivo is not standardized; hence the proxy measurement of arterial dysfunction is often used instead (Poredos and Jezovnik, 2011). Endothelial dysfunction may not be restricted to the arterial system (Rühl et al., 2014), however dedicated study of venous endothelial dysfunction is not currently carried out even when investigating venous pathologies such as VTE.
The present review focused on biomarkers reflecting disturbance, activation, or inflammation of the venous wall. Unfortunately, due to the nature of the measurement methods used in the studies, the venous system was not particularly distinct from the arterial one or from systemic markers of inflammation, such as CRP.
Status of the Current Evidence Base
The lack of longitudinal studies related specifically to the biomarkers outlined above is another weakness of the included studies. Most published studies focus either on the immediate acute biomarkers present in patients presenting with VTE, or in the chronic phase of the disease, as seen in Table 2. The proceeding state of the patients, aside from the exclusion of patients based on known risk factors such as hormonal birth control, thrombophilia, and atherosclerotic disease, was unknown. The small number of studies in this area limited our analysis of each specific biomarker and its broader clinical context.
The causal nature of venous wall inflammation and VTE is yet to be elucidated (Van Aken et al., 2000). More studies are required to explore the link between neutrophil adhesiveness and VTE recurrence (Zapponi et al., 2011). Further studies should also be dedicated to variation in fibrinolysis with time of day (Ansari et al., 2006).
Further predictive value for each of the biomarkers outlined in Table 4 above for long term risk as well as acute incidence of VTE needs to be assessed. A prospective study focusing on each of the above biomarkers would further clarify their role as diagnostic and/or predictive markers of VTE, and contribute to the understanding of the etiology of VTE and the role of the various factors.
With a greater understanding of the roles that possible predictive biomarkers may play in the pathogenesis of VTE, targeted therapies may be developed significantly reducing the risk of VTE (Martos et al., 2020).
Conclusion
This review sought to identify biomarkers linked to venous endothelial dysfunction on Earth that can be used to elucidate mechanisms of spaceflight-associated VTE. The analysis of the 17 included papers found that distinction between the arterial and venous systems, as well as between local and systemic inflammation was lacking. Routine imaging to identify risk of VTE was not well studied. Most available studies examined biomarkers after the acute event, which provided limited predictive value for each of the biomarkers identified.
Future work should focus on conducting prospective cohort studies to identify levels of specific endothelial biomarkers that can accurately assess risk of VTE to enable better patient management before exposure to known or potential triggers of thromboembolic events, such as spaceflight. Future studies should additionally focus on the differences between the arterial and venous systems to further understand the pathogenesis of VTE.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
KH assisted in designing the search strategy, conducted the search, screened the papers, conducted the thematic analysis, and wrote the manuscript. JL and AW led the design of the search strategy and the methods. AE, NG, TW and DG assisted in defining the PICOS, TW and DG were the second and third authors involved in screening. AE, NG, JJ, TH, LM, LP and TW reviewed the findings of the thematic analysis, verified scientific merit, and contributed to the discussion. All authors reviewed and approved the final manuscript.
Funding
The European Space Agency provided funding to cover the open access cost of this publication. Publication costs were covered specifically from the ESA-sponsored Topical Team on “Pathophysiology, risk and clinical presentation of venous thromboembolism (VTE) and its evaluation of its prevention, diagnosis, mitigation and management strategies in spaceflight” (Grant number 4000131108/20/NL/PG/pt) and NASA Grant number 80NSSC19K0020, PI.
Conflict of Interest
DG and TW were employed by the company KBR GmbH.
The authors declare that this study received funding from KBR GmbH. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the members of the European Space Agency Venous Thromboembolism Topical Team for their input and advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.885183/full#supplementary-material
Abbreviations
CRP, C-reactive protein; CUS, Compression venous ultrasound; DVT, Deep vein thrombosis; EC, Endothelial cell; EMP, Endothelial microparticle; ESA, European Space Agency; FMD, Flow mediated dilatation; FVa, Activated Factor V, FVIIIa; Activated Factor VIII; IL-10, Interleukin 10; IL-6, Interleukin-6; IL-8, Interleukin-8; MeSH, Medical subject headings; PAI-1, Plasminogen activator inhibitor 1; PAP, Plasmin-alpha2-antiplasmin; PC, Protein C; PICO, Population, Interest, Control, and Outcome; PS, Protein S; sCAMS, Soluble cell adhesion molecule; sTM, Soluble thrombomodulin; TAFI, Thrombin activatable fibrinolysis inhibitor; TF, Tissue factor; TFPI, Tissue factor plasminogen inhibitor; tPA, Tissue plasminogen activator; VCAM-1, Vascular cell adhesion protein 1; VTE, Venous thromboembolism; vWF, Von Willebrand factor.
References
Alvarado-Moreno J. A., Hernandez-Lopez R., Chavez-Gonzalez A., Yoder M. C., Rangel-Corona R., Isordia-Salas I., et al. (2016). Endothelial colony-forming Cells: Biological and Functional Abnormalities in Patients with Recurrent, Unprovoked Venous Thromboembolic Disease. Thromb. Res. 137, 157–168. doi:10.1016/j.thromres.2015.11.005
Ansari M. T., Mahmood M. T., Karlberg J. P. (2006). The Association between Seated Immobility and Local Lower-Limb Venous Coagulability in Healthy Adult Volunteers: A Simulation of Prolonged Travel Immobility. Blood Coagul. Fibrinolysis 17 (5), 335–341. doi:10.1097/01.mbc.0000233362.80486.6c
Auñón-Chancellor S. M., Pattarini J. M., Moll S., Sargsyan A. (2020). Venous Thrombosis during Spaceflight. N. Engl. J. Med. 382 (1), 89–90. doi:10.1056/NEJMc1905875
Barnes D. M., Wakefield T. W., Rectenwald J. E. (2008). Novel Biomarkers Associated with Deep Venous Thrombosis: A Comprehensive Review. Biomark Insights 3 (734), 117727190800300. doi:10.1177/117727190800300004
Bombeli T., Jutzi M., De Conno E., Seifert B., Fehr J. (2002). In patients with deep-vein thrombosis elevated levels of factor VIII correlate only with von Willebrand factor but Not other endothelial cell-derived coagulation and fibrinolysis proteins. Blood Coagul. Fibrinolysis 13 (7), 577–581. doi:10.1097/00001721-200210000-00001
Braun V., Clarke V., Hayfield N., Terry G. (2019). “Thematic Analysis,” in Handbook of Research Methods in Health Social Sciences. Editor P. Liamputtong, 843–860. doi:10.1007/978-981-10-5251-4_103
Braun V., Clarke V. (2006). Using Thematic Analysis in Psychology. Qual. Res. Psychol. 3 (2), 77–101. doi:10.1191/1478088706qp063oa
Bucek R. A., Reiter M., Quehenberger P., Minar E., Baghestanian M. (2003). The Role of Soluble Cell Adhesion Molecules in Patients with Suspected Deep Vein Thrombosis. Blood Coagul. Fibrinolysis 14 (7), 653–657. doi:10.1097/00001721-200310000-00006
Bucek R. A., Reiter M., Quehenberger P., Minar E. (2002). C-reactive Protein in the Diagnosis of Deep Vein Thrombosis. Br. J. Haematol. 119 (2), 385–389. doi:10.1046/j.1365-2141.2002.03886.x
Chirinos J. A., Heresi G. A., Velasquez H., Jy W., Jimenez J. J., Ahn E., et al. (2005). Elevation of Endothelial Microparticles, Platelets, and Leukocyte Activation in Patients with Venous Thromboembolism. J. Am. Coll. Cardiol. 45 (9), 1467–1471. doi:10.1016/j.jacc.2004.12.075
Falkon L., Garí M., Borrell M., Fontcuberta J. (1992). The Release of Plasminogen Activators (T-PA and u-PA) and Plasminogen Activator Inhibitor (PAI-1) after Venous Stasis. Blood Coagul. Fibrinolysis 3 (1), 33–38. [Internet]. doi:10.1097/00001721-199202000-00006
Gordon R. J., Lombard F. W. (2017). Perioperative Venous Thromboembolism. Anesth. Analgesia 125 (2), 403–412. doi:10.1213/ane.0000000000002183
Grimaudo V., Bachmann F., Hauert J., Christe M. A., Kruithof E. K. (1992). Hypofibrinolysis in Patients with a History of Idiopathic Deep Vein Thrombosis And/or Pulmonary Embolism. Thromb. Haemost. 67 (4), 397–401. doi:10.1055/s-0038-1648459
Jacobs B., Obi A., Wakefield T. (2016). Diagnostic Biomarkers in Venous Thromboembolic Disease. J. Vasc. Surg. Venous Lymphatic Disord. 4 (4), 508–517. doi:10.1016/j.jvsv.2016.02.005
Jezovnik M. K., Poredos P. (2010). Idiopathic Venous Thrombosis Is Related to Systemic Inflammatory Response and to Increased Levels of Circulating Markers of Endothelial Dysfunction. Int. Angiol 29 (3), 226–231.
Kim D. S., Vaquer S., Mazzolai L., Roberts L. N., Pavela J., Watanabe M., et al. (2021). The Effect of Microgravity on the Human Venous System and Blood Coagulation: a Systematic Review. Exp. Physiol. 106 (5), 1149-1158. doi:10.1113/ep089409
Kim S. Y., Park J. E., Lee Y. J., Seo H.-J., Sheen S.-S., Hahn S., et al. (2013). Testing a Tool for Assessing the Risk of Bias for Nonrandomized Studies Showed Moderate Reliability and Promising Validity. J. Clin. Epidemiol. 66 (4), 408–414. doi:10.1016/j.jclinepi.2012.09.016
Limper U., Tank J., Ahnert T., Maegele M., Grottke O., Hein M., et al. (2021). The Thrombotic Risk of Spaceflight: Has a Serious Problem Been Overlooked for More Than Half of a century? Eur. Heart J. 42 (1), 97–100. doi:10.1093/eurheartj/ehaa359
Martos L., Oto J., Fernández-Pardo Á., Plana E., Solmoirago M. J., Cana F., et al. (2020). Increase of Neutrophil Activation Markers in Venous Thrombosis-Contribution of Circulating Activated Protein C. Int. J. Mol. Sci. 21 (16), 1–19. doi:10.3390/ijms21165651
Masoud M., Sarig G., Brenner B., Jacob G. (2008). Orthostatic Hypercoagulability. Hypertension 51 (6), 1545–1551. doi:10.1161/hypertensionaha.108.112003
Meltzer M. E., Lisman T., De Groot P. G., Meijers J. C. M., Le Cessie S., Doggen C. J. M., et al. (2010). Venous Thrombosis Risk Associated with Plasma Hypofibrinolysis Is Explained by Elevated Plasma Levels of TAFI and PAI-1. Blood 116 (1), 113–121. doi:10.1182/blood-2010-02-267740
Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. (2016). Rayyan-a Web and mobile App for Systematic Reviews. Syst. Rev. 5 (1), 210. doi:10.1186/s13643-016-0384-4
Palta S., Saroa R., Palta A. (2014). Overview of the Coagulation System. Indian J. Anaesth. 58 (5), 515. doi:10.4103/0019-5049.144643
Poredos P., Jezovnik M. K. (2011). In Patients with Idiopathic Venous Thrombosis, Interleukin-10 Is Decreased and Related to Endothelial Dysfunction. Heart Vessels 26 (6), 596–602. doi:10.1007/s00380-010-0111-3
Raitakari O. T., Celermajer D. S. (2000). Research Methods in Human Cardiovascular Pharmacology. Br. J. Clin. Pharmacol. 50 (5), 397–404. doi:10.1046/j.1365-2125.2000.00277.x
Reitsma P. H., Versteeg H. H., Middeldorp S. (2012). Mechanistic View of Risk Factors for Venous Thromboembolism. Atvb 32 (3), 563–568. doi:10.1161/atvbaha.111.242818
Rühl H., Müller J., Wäschenbach J., Oldenburg J., Dewald O., Pötzsch B. (2014). Short-term Venous Stasis Induces Fibrinolytic Activation but Not Thrombin Formation. J. Atheroscler. Thromb. 21 (12), 1260–1270.
Sidelmann J. J., Bladbjerg E.-M., Gram J., Münster A.-M. B., Jespersen J. (2008). Tissue Factor Pathway Inhibitor Relates to Fibrin Degradation in Patients with Acute Deep Venous Thrombosis. Blood Coagul. Fibrinolysis 19 (5), 405–409. doi:10.1097/mbc.0b013e3283049639
Thomas J., Harden A. (2008). Methods for the Thematic Synthesis of Qualitative Research in Systematic Reviews. BMC Med. Res. Methodol. 8 (1), 45. doi:10.1186/1471-2288-8-45
Tsai A. W., Cushman M., Rosamond W. D., Heckbert S. R., Polak J. F., Folsom A. R. (2002). Cardiovascular Risk Factors and Venous Thromboembolism Incidence. Arch. Intern. Med. 162 (10), 1182. doi:10.1001/archinte.162.10.1182
Van Aken B. E., Den Heijer M., Bos G. M., Van Deventer S. J., Reitsma P. H. (2000). Recurrent Venous Thrombosis and Markers of Inflammation. Thromb. Haemost. 83 (4), 536–539. doi:10.1055/s-0037-1613858
Vazquez-Garza E., Jerjes-Sanchez C., Navarrete A., Joya-Harrison J., Rodriguez D. (2017). Venous Thromboembolism: Thrombosis, Inflammation, and Immunothrombosis for Clinicians. J. Thromb. Thrombolysis 44 (3), 377–385. doi:10.1007/s11239-017-1528-7
Winnard A., Caplan N., Bruce-Martin C., Swain P., Velho R., Meroni R., et al. (2021). Developing, Implementing, and Applying Novel Techniques during Systematic Reviews of Primary Space Medicine Data. Aerosp Med. Hum. Perform. 92 (8), 681–688. doi:10.3357/amhp.5803.2021
Yau J. W., Teoh H., Verma S. (2015). Endothelial Cell Control of Thrombosis. BMC Cardiovasc. Disord. 15. doi:10.1186/s12872-015-0124-z
Zapponi k. C. S., Bittar L. F., Mazetto B. M., Santiago-Bassora F. D., Orsi F. A., de Paula E. V., et al. (2011). Increased Neutrophil Adhesive Properties in Patients with Venous Thromboembolism and Residual Vein Thrombosis and High D-Dimer Levels. Blood 118 (21), 2301. doi:10.1182/blood.V118.21.2301.2301
Keywords: venous thrombosis, biomarker, Virchow’s triad, venous thromboembolism, VTE, DVT, Endothelial (dys)function, spaceflight
Citation: Harris K, Laws JM, Elias A, Green DA, Goswami N, Jordan J, Kamine TH, Mazzolai L, Petersen LG, Winnard AJ and Weber T (2022) Search for Venous Endothelial Biomarkers Heralding Venous Thromboembolism in Space: A Qualitative Systematic Review of Terrestrial Studies. Front. Physiol. 13:885183. doi: 10.3389/fphys.2022.885183
Received: 27 February 2022; Accepted: 25 March 2022;
Published: 27 April 2022.
Edited by:
Costantino Balestra, Haute École Bruxelles-Brabant (HE2B), BelgiumReviewed by:
Salih Murat Egi, Galatasaray University, TurkeyZhenguo Zhai, China-Japan Friendship Hospital, China
Copyright © 2022 Harris, Laws, Elias, Green, Goswami, Jordan, Kamine, Mazzolai, Petersen, Winnard and Weber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nandu Goswami, bmFuZHUuZ29zd2FtaUBtZWR1bmlncmF6LmF0
 Katie Harris
Katie Harris Jonathan Michael Laws2
Jonathan Michael Laws2 Antoine Elias
Antoine Elias David Andrew Green
David Andrew Green Nandu Goswami
Nandu Goswami Jens Jordan
Jens Jordan Tovy Haber Kamine
Tovy Haber Kamine Lucia Mazzolai
Lucia Mazzolai Lonnie G. Petersen
Lonnie G. Petersen Andrew James Winnard
Andrew James Winnard Tobias Weber
Tobias Weber