- 1The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Hebei Provincial Center for Clinical Laboratories, Shijiazhuang, Hebei, China
- 3Graduate School of Hebei Medical University, Shijiazhuang, Hebei, China
As a marker of inflammation, calprotectin has potential application value in a variety of inflammatory diseases, such as arthritis and bacterial infections. Clostridioides difficile infection (CDI) is an infectious disease that causes intestinal damage and inflammation. This systematic review aims to determine whether fecal calprotectin has application value in CDI. Nine databases were searched from inception to 6 June 2022, and 17 studies were included. These studies were divided into four groups according to their content. Generally speaking, fecal calprotectin is not an ideal indicator for the diagnosis and prognosis prediction of CDI but may serve as a potential indicator for assessing disease severity and as a readily detectable marker for CDI screening. In addition, patients in need of treatment or with detectable toxins in stool may tend to have higher levels of fecal calprotectin. In summary, fecal calprotectin has some potential application value in CDI. However, further studies are needed to verify these findings and determine the reliability of calprotectin as a biomarker for CDI.
1 Introduction
Clostridioides difficile is an anaerobic, spore-forming, Gram-positive bacterium that is considered to be the main cause of antibiotic-associated diarrhea (AAD) and healthcare-associated infections (Khanna and Pardi, 2012). Various clinical manifestations have been reported for C. difficile infection (CDI) from asymptomatic colonization to mild and self-limiting diarrhea to severe fulminant colitis characterized by hypotension, shock, megacolon or intestinal obstruction (McDonald et al., 2018). In the United States, CDI affected 224,000 people and caused approximately 13,000 deaths in 2017 alone, with medical costs estimated at $1 billion (Guh et al., 2020). Therefore, the accurate diagnosis and prevention of CDI are of high importance.
CDI is characterized by three unformed stools in 24 h and the confirmation of the presence of toxigenic C. difficile through laboratory testing (McDonald et al., 2018). Currently, commonly used laboratory assays for diagnosing CDI include toxin-producing cultures, glutamate dehydrogenase (GDH), nucleic acid amplification assays (NAAT) and toxin A/B enzyme immunoassays (EIA) (Lee et al., 2021). Although easy to use and affordable, these tests have limitations. In particular, because the results can only qualitatively indicate the existence of GDH and toxin A/B but cannot provide quantitative measurements, they cannot be used to judge the severity of CDIs (Hassanain et al., 2021). In addition, a positive C. difficile test does not always indicate a clinical infection that requires treatment. The fact that the asymptomatic colonization rate of C. difficile is 3.4–8.1% upon admission further challenges the diagnosis of CDI (Zacharioudakis et al., 2015; Longtin et al., 2016; Meltzer et al., 2019). In a single-center retrospective study (Kelly et al., 2016), only 19.6% of C. difficile detection were considered appropriate, with uncertain and inappropriate detection rates of 65.5% and 14.8%, respectively. Therefore, it is necessary to find new biomarkers for differential diagnosis and severity assessments of CDI.
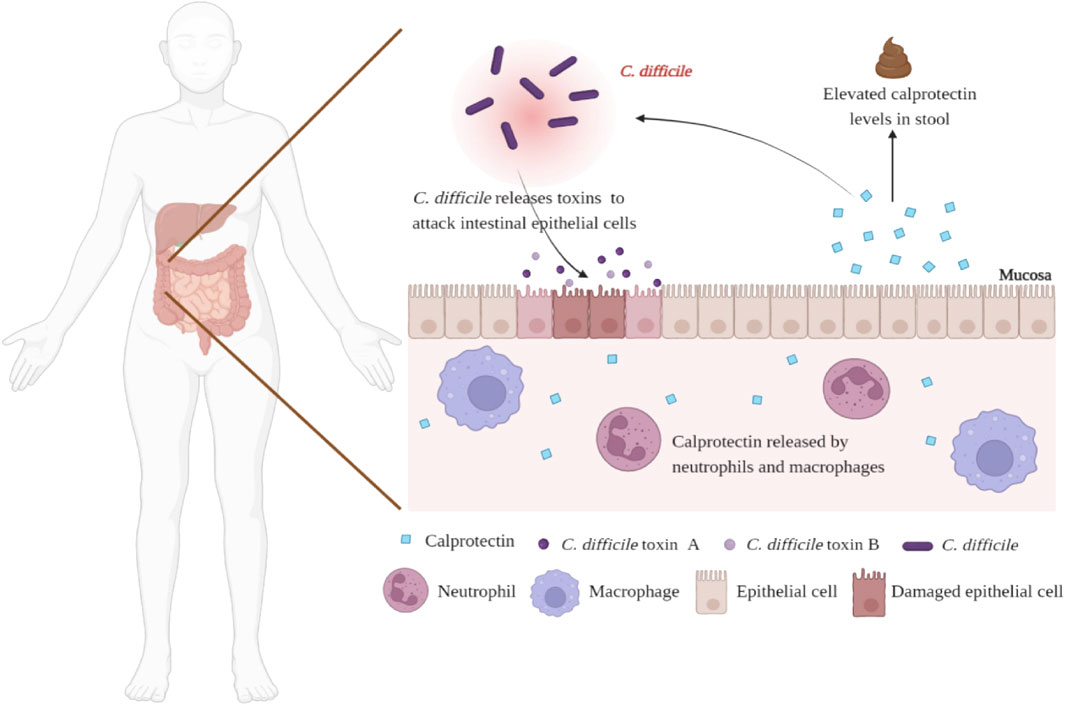
Calprotectin is a 36 kDa member of the S100 protein family, secreted by neutrophils, macrophages, and monocytes. (Khaki-Khatibi et al., 2020). As markers of inflammation, serum and salivary calprotectin have potential applications in a variety of inflammatory diseases, such as arthritis and bacterial infections (Decembrino et al., 2015; Guo et al., 2016; Bartáková et al., 2019). In addition to serum and saliva, calprotectin is also present in feces. Under normal circumstances, the concentration of calprotectin in feces is six times higher than in plasma and is stable at room temperature, giving it an advantage as a biomarker of gastrointestinal inflammation. (Naess-Andresen et al., 1995). The efficacy of fecal calprotectin (fCP) in the diagnosis and prognosis prediction of inflammatory bowel disease (IBD) has been evaluated, including differentiating IBD and irritable bowel syndrome (IBS), predicting disease recurrence and treatment response and evaluating endoscopic activity and disease histological activity (Kalantari et al., 2015; Kalla et al., 2016; Moein et al., 2017; Mak et al., 2018; Reenaers et al., 2018). Notably, CDI can also promote the activation and recruitment of neutrophils and cause inflammation. (Figure 1). Therefore, from this point of view, fCP levels in CDI patients may be elevated and proportional to the degree of intestinal inflammation. In 2008, Shastri et al. (2008) evaluated the role of fCP in the diagnosis of acute diarrhea for the first time and found that patients with CDI had the highest levels of fCP compared with patients with other causes of diarrhea, suggesting that fCP may have value in auxiliary diagnosis of CDI. In recent years, scholars have further explored the characteristics of fCP in CDI patients to examine its potential value. To this end, this review systematically retrieved and summarized relevant studies to comprehensively assess the potential value of fCP in CDI.
2 Materials and methods
2.1 Definition
CDI is defined as a patient with: (1) presence of diarrhea, defined as 3 or more unformed stools within 1–8 h in 24 or less consecutive hours; (2) positive stool test results in the presence of toxigenic C. difficile or its toxins, or colonoscopy or histopathology showing pseudomembranous colitis (Debast et al., 2014). Recurrent CDI (rCDI) was defined as the development of subsequent CDI episodes up to a period of 60 or 90 days following treatment of the initial episode. C. diffcile colonization patients was defined as the patients were admitted for at least 72 h, who had received at least 1 dose of an antibiotic within the past 7 days, and did not have diarrhea, on the premise of positive NAAT (Kelly et al., 2020). Treatment response was defined as a decrease in stool frequency or improvement in stool consistency and improvement in disease severity parameters (clinical, laboratory, radiological) after treatment without new signs of severe disease (Debast et al., 2014).
2.2 Data sources and search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Moher et al., 2009), using the databases PubMed, Scopus, Ovid, Embase, Cochrane, CNKI, Wanfang, VIP and Siomed. The last search was performed on 6 June 2022, the search formula included “Leukocyte L1 Antigen Complex” and “Clostridioides difficile” as medical subject heading (MeSH) terms that were combined in the PubMed advanced search generator. In other databases, combinations of the following keywords were used: “Clostridium difficile” or “Clostridioides difficile” and “Leukocyte L1 Antigen Complex” or “Calcium-Binding Myeloid Protein P8,14” or “Calcium Binding Myeloid Protein P8,14” or “Calgranulin” or “Calprotectin” or “Migratory Inhibitory Factor-Related Protein MRP” or “Migratory Inhibitory Factor Related Protein MRP” or “Myelomonocytic Antigen L1” or “Antigen L1, Myelomonocytic” or “L1 Antigen” or “Antigen, L1” or “27E10 Antigen” or “Antigen, 27E10” or “Leukocyte L1 Protein” or “L1 Protein, Leukocyte”.
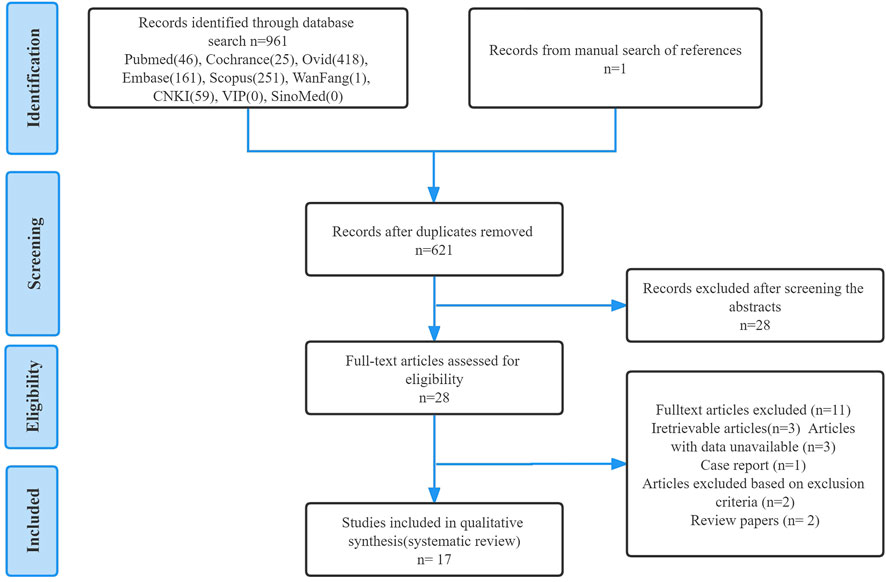
Titles and abstracts were independently screened using selection criteria to identify eligible studies. Then, the full text of the study was carefully evaluated and the study was included or excluded accordingly. For any papers with contentious content, consensus discussions were had and agreements were reached to eliminate any ambiguity. Finally, a manual search was performed for any articles in the reference lists of included studies that were missed during the electronic search process. The detailed search flowchart is presented in Figure 2 (in the Results section).
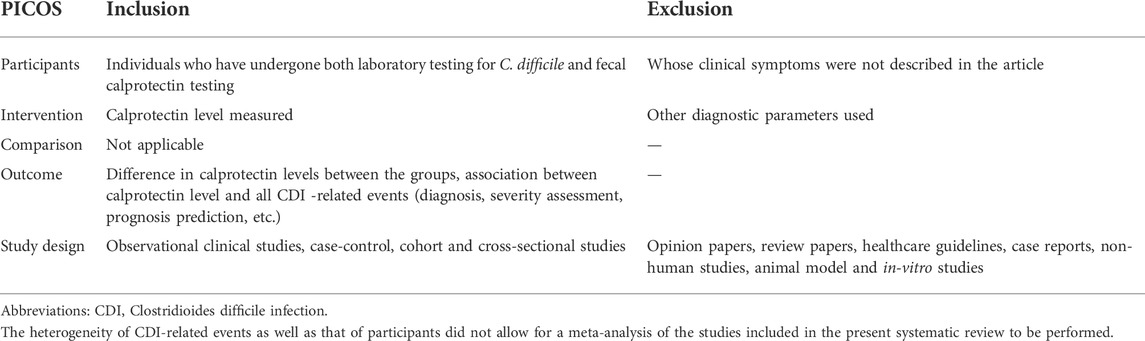
2.3 Eligibility criteria and data extraction
Full article studies were included if they met the following criteria: (a) written in English or Chinese, (b) included individuals who were positive for toxigenic C. difficile or its toxin or toxin gene and had fCP levels tested, (c) were observational studies, including case-control, cohort and cross-sectional studies. The outcomes of interest included correlations between fCP levels and all CDI-related events (diagnosis, severity assessment, prognosis prediction, etc.) and differences in fCP concentrations in different patient groups. Articles that did not describe clinical symptoms (e.g., diarrhea) in individuals who provided stool samples were excluded. (Table 1).
Two study investigators extracted the data independently. Data extraction was conducted for study characteristics (author name, year, study design, comparison groups, topics covered, outcomes measures, fCP detection methods and kits, cutoffs recommended by the kits and whether the CDI meets the definition) (Table 2). In the study by Han et al. (Han et al., 2020) we only extracted data from Group III because only this group included patients with CDI.
2.4 Quality assessment
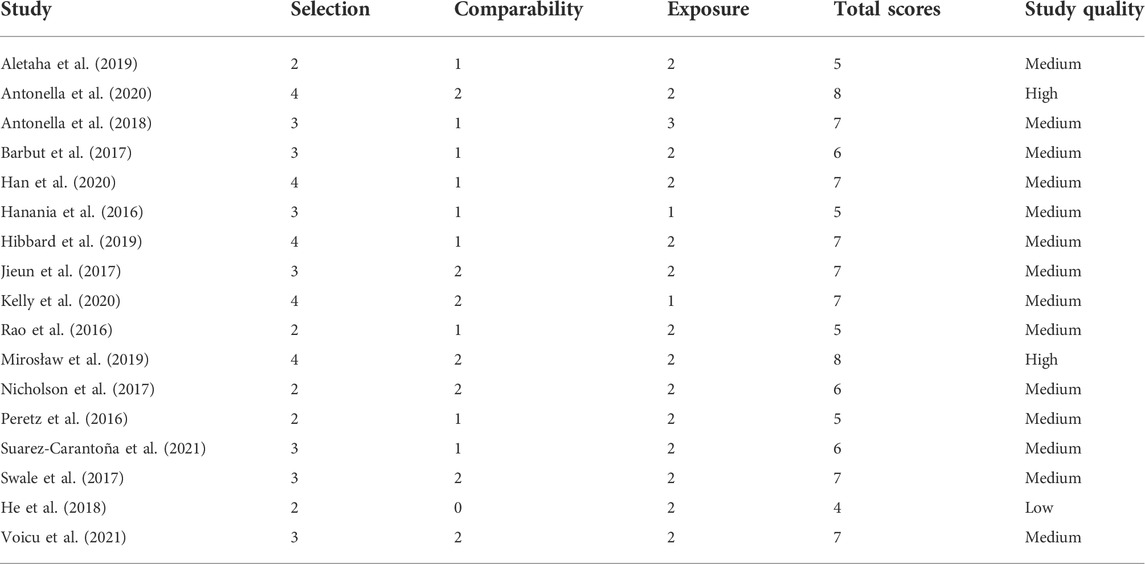
Each of the included studies was independently assessed for quality by two authors using the Newcastle-Ottawa scale (Stang, 2010), and disagreements were resolved by discussion between them. This scale is a validated tool to evaluate the risk of bias in non-randomized studies, including case-control and cohort studies. It comprises three main parameters: selection, comparability and exposure/outcome. The ratings and overall scores of each study are presented in Table 3. Each study was scored as low (<5), medium (5–7) or high (>7) quality.
3 Results
3.1 Search results
As outlined in Figure 2, a total of 962 studies were identified through database and manual searches, of which 934 studies were excluded after title and abstract screening. The remaining 28 studies were further assessed for eligibility by reading the full text. Finally, 17 studies met all inclusion criteria and were included in the systematic review.
3.2 Characteristics and quality of included studies
Table 2 summarizes the characteristics of the included studies. Of the 17 studies, 11 were cohort studies and 6 were case-control studies. Based on the content of these studies, we divided them into 4 topics. Topic 1: fCP in differentiating patients with CDI from other populations. Topic 2: fCP in assessing the severity of CDI. Topic 3: fCP in predicting the prognosis of CDI patients. Topic 4: fCP in other aspects of CDI. Overall, 9 studies focused on topic 1, 8 studies focused on topic 2, 9 studies included topic 3, and 4 studies addressed topic 4. All studies used EIA to measure fCP levels, except for one study that was not reported. Additionally, patients with CDI in 13 studies met our defined criteria. Based on quality scores, two studies were considered high quality, one study was low quality, and the remaining studies were identified as medium quality (Table 3).
3.3 Fecal calprotectin in differentiating patients with Clostridioides difficile infection from other populations
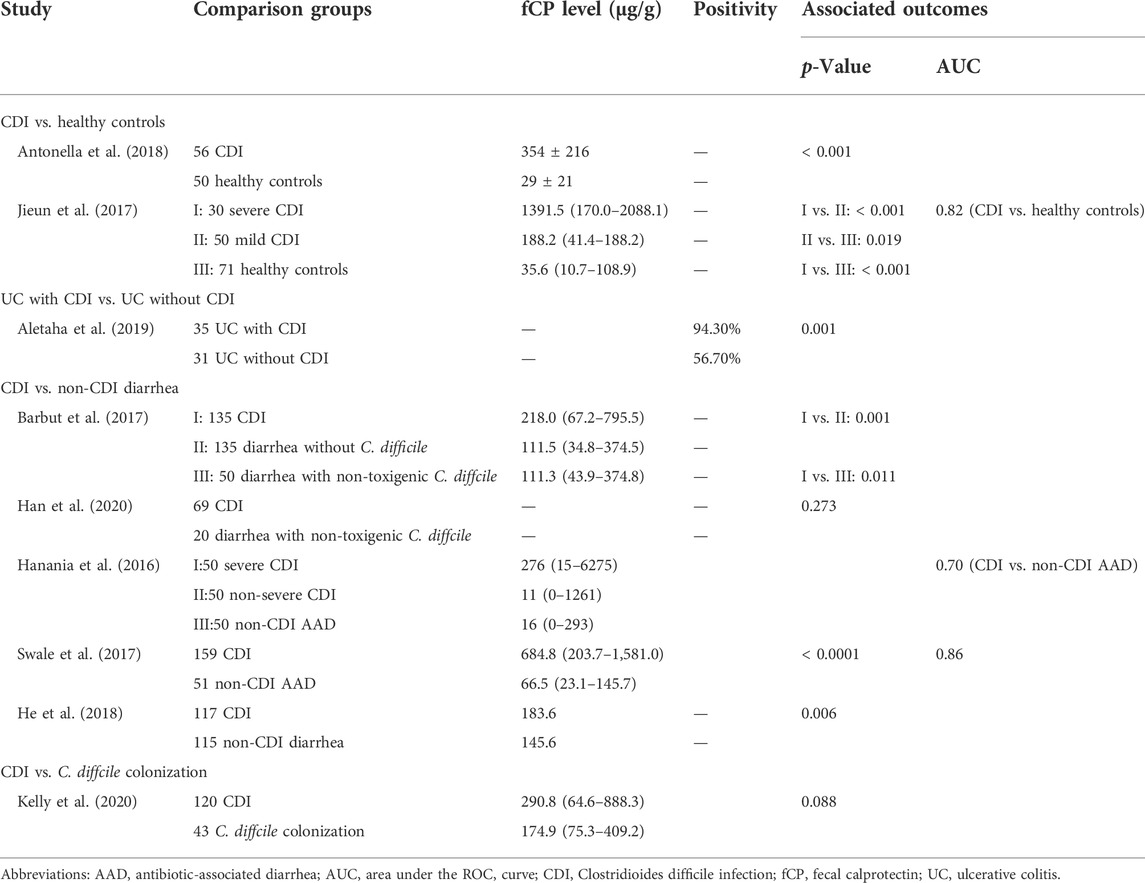
Nine studies assessed differences in fCP concentrations between patients with CDI and other populations (Table 4).
3.3.1 Clostridioides difficile infection vs. healthy controls
Two studies showed that patients with CDI had significantly higher fCP levels than healthy subjects. Receiver operating characteristics (ROC) curves showed that the best functional connectivity (FC) value for distinguishing between CDI and healthy subjects was 112.5 μg/g, the area under the curve (AUC) was 0.821, sensitivity was 75% and specificity was 79%.
3.3.2 Ulcerative colitis with Clostridioides difficile infection vs. Ulcerative colitis without Clostridioides difficile infection
Aletaha et al. (Aletaha et al., 2019) found that UC patients with CDI had a higher rate of fCP positivity compared with UC patients without CDI, but this study did not report a threshold for fCP positivity.
3.3.3 Clostridioides difficile infection vs. C. diffcile colonization
One study (Kelly et al., 2020) observed higher fCP levels in CDI patients than in patients with asymptomatic colonization with C. difficile, but this difference was not statistically significant.
3.3.4 Clostridioides difficile infection vs. non-Clostridioides difficile infection diarrhea
Three studies observed higher fCP levels in patients with CDI compared to patients with diarrhea from other causes (Barbut et al., 2017; Swale et al., 2017; He et al., 2018). Another study (Han et al., 2020) did not observe a significant difference between the two. In addition, two studies conducted ROC analysis on the ability of fCP to distinguish CDI patients from patients with non-CDI AAD and found AUC values of 0.70 and 0.86, respectively (Hanania et al., 2016; Swale et al., 2017).
3.4 Fecal calprotectin in assessing the severity of Clostridioides difficile infection
As shown in the Table 5, there are eight studies exploring the feasibility of using fCP to assess the severity of CDI. The study of Jieun et al. (2017) showed that the area under the ROC curves were 0.821 and 0.746 with a sensitivity of 75% and 70% and specificity of 79% and 80%, for severe versus mild cases, respectively. Another study yielded sensitivity and specificity of fCP for distinguishing severe CDI from non-severe CDI and non-CDI AAD are 57% and 88% (Hanania et al., 2016). Voicu et al. (2021) suggested a cut-off of 290.09 μg/g for the predictive marker of fCP, which permitted to identify patients with severe and mild CDI, having 100% sensitivity and 76% specificity. Only two studies showed no correlation between fCP levels and patients’ clinical scores. However, one of them found a trend for higher fCP levels in patients with a higher Clostridium severity score index (p = 0.0633). Other studies show higher fCP levels in severe CDI patients compared to non-severe CDI patients, although one of these studies did not reach statistical significance.
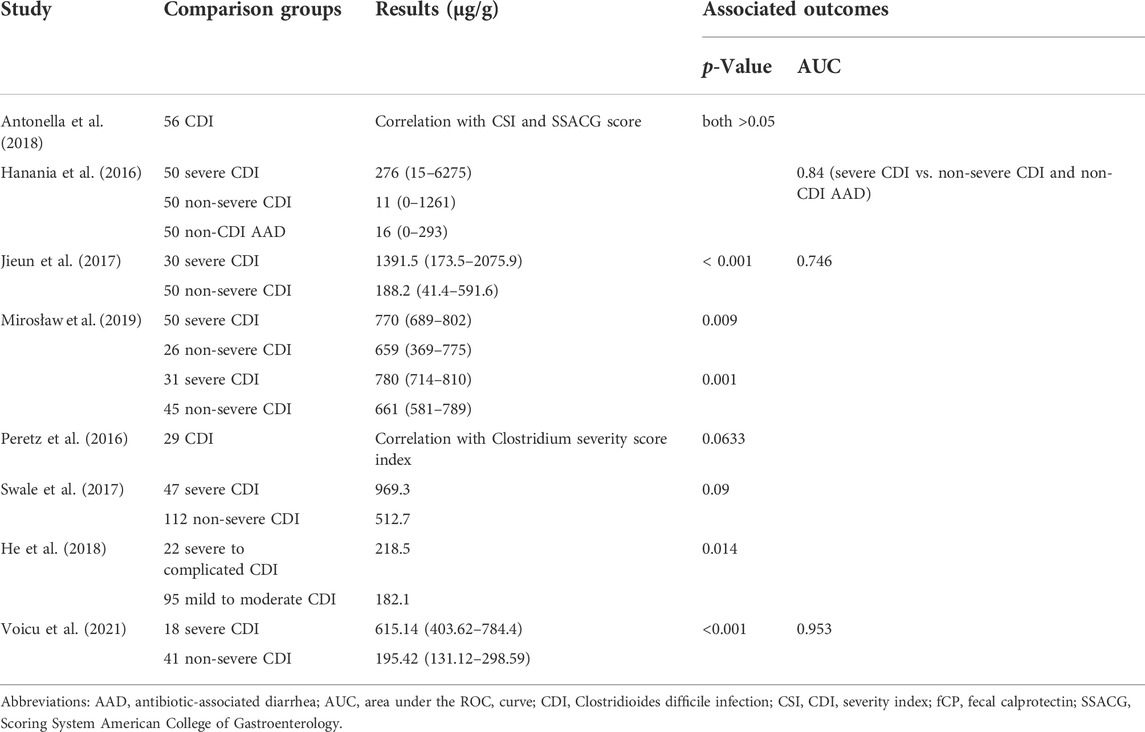
TABLE 5. Main results of studies that explored the relationship between fCP and the severity of CDI.
3.5 Fecal calprotectin in predicting the prognosis of Clostridioides difficile infection patients
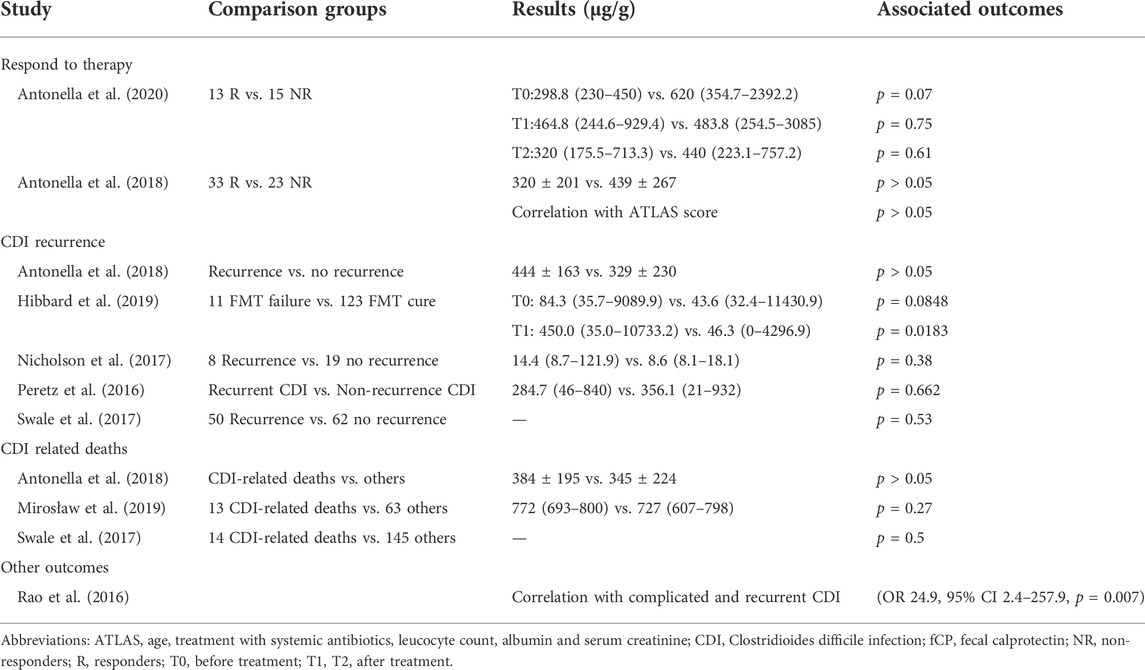
Eight studies assessed the prognostic value (response to therapy, disease recurrence, death, etc.) of fCP in patients with CDI, as shown in Table 6.
3.5.1 Response to therapy
There are two studies compared concentrations of fCP between patients who did and did not respond to treatment. The results showed no significant difference in fCP levels between responders and non-responders, either before or after treatment, although responders had lower fCP levels. Additionally, the study by Antonella et al. (2018) showed no correlation between fCP levels and ATLAS scores, which assess treatment response.
3.5.2 Clostridioides difficile infection recurrence
Five studies compared fCP levels in rCDI patients with those without recurrence. Only one study showed significantly higher fCP levels in patients with rCDI after fecal microbiota transplantation (FMT) than in patients without recurrence, and results from other studies showed no statistically significant difference between the two groups of patients.
3.5.3 Clostridioides difficile infection related death
In all three studies, there were no statistically significant differences in fCP levels in CDI-related deaths compared with surviving subjects.
3.5.4 Other outcomes
Rao et al. (2016) found that patients with complicated/recurrent CDI (adverse outcomes) have higher normalized fCP levels. Further modeled as a diagnostic test, a high normalized fCP was 38.5% sensitive and 91.9% specific for complicated/recurrent CDI, suggesting that high fCP levels were associated with adverse outcomes in CDI.
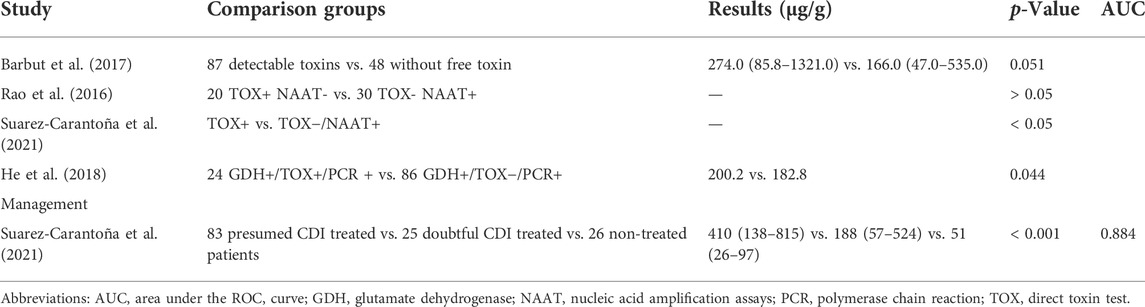
3.6 Fecal calprotectin in other aspects of Clostridioides difficile infection
Four studies compared fCP levels in stool samples that were positive for direct toxin testing and those with no detectable toxin. All of these results showed higher levels of fCP in samples positive for direct toxin assays, even though two of the studies did not reach statistical differences (Table 7).
Suarez-Carantoña et al. (2021) divided patients into three groups: group I, recommended treatment for hypothetical CDI; group II, uncertain diagnosis but patients treated for CDI; and group III, assumed C. difficile colonization or self-limiting CDI that did not require treatment according to the recommendations of clinicians and professional consultants. After comparing the fCP levels of the three groups of patients, it was found that the fCP levels of the patients in group I were significantly higher than those in the other two groups. At the same time, the fCP level of patients in group II was significantly higher than that in group III (Table 7).
4 Discussion
Currently, there has been a lack of suitable biomarkers for the diagnosis, disease severity assessment and prognosis prediction of CDI patients. In recent years, several studies have explored the potential application value of fCP in CDI. However, there is conflict and controversy among their results. This review comprehensively summarizes the relevant studies in this field. Overall, a certain degree of inconsistency among study results was observed across topics. Nonetheless, we analyze the results and present our own insights based on their study design and methodology.
4.1 The low application value of fecal calprotectin in the diagnosis of Clostridioides difficile infection
To date, research on fCP in the diagnosis of CDI has mainly focused on four issues. The first is whether fCP can distinguish CDI patients from healthy controls. Studies by Antonella et al. (2018) and Jieun et al. (2017) both showed that levels of fCP in CDI patients were significantly higher than those in healthy subjects. Average fCP levels of healthy controls in the two studies were below 50 μg/g, which was consistent with previous fCP data reported in other studies. The ROC curve showed good discriminative ability of fCP for CDI patients and healthy subjects, indicating that fCP has the basic condition as an inflammatory marker. However, it may not be of much help to clinicians because most of the time the problem is to distinguish patients with CDI from those with diarrhea from other causes, rather than healthy individuals. The second issue is whether fCP can distinguish CDI patients from IBD patients. Only one study focused on this issue and found that the positivity rate of fCP in UC patients with positive CDI test was significantly higher than that in patients with negative CDI test. It should be noted that even in UC patients with negative CDI test, the fCP positive rate reached 56%, and the cut-off value of fCP was not mentioned in the study. Therefore, further studies are needed to assess the ability of fCP to differentiate CDI from IBD patients. The third issue is whether fCP can distinguish CDI patients from non-CDI patients with diarrhea. Three of the four studies observed significantly higher levels of fCP in CDI patients compared with non-CDI diarrhea patients. Two studies conducted ROC analysis on the ability of fCP to distinguish CDI patients from non-CDI AAD patients and found AUC values of 0.70 and 0.86, respectively. These results suggest that fCP has some utility in distinguishing CDI patients from non-CDI diarrhea patients, and may be useful for screening patients with diarrhea for CDI, but would not add much value to the currently available diagnostic paradigm. The fourth issue is whether fCP can distinguish CDI patients from those colonized by C. difficile. One study evaluated differences in fCP levels between CDI and toxigenic C. difficile-colonized patients, and no significance was observed. To date, we have not found any studies evaluating the ability of fCP to differentiate C. difficile infection from colonization by ROC curve.
Judging from the current data, although the level of fCP in CDI patients is higher than that in other populations, and fCP has shown good discriminative ability in some studies, its value for improving current CDI diagnosis methods may be very limited. On one hand, we observed that fCP levels vary widely, and there was significant overlap between CDI patients and control groups, making it difficult to determine optimal cut-off values for fCP and reducing the accuracy of CDI predictions. Even though part of the reason for the large inter-individual variability may be due to differences in the kits and methods used to detect fCP. On the other hand, other intestinal inflammatory diseases can also lead to elevated fCP (Kopylov et al., 2014), which is especially important for CDI because infected patients are usually elderly and accompanied by multiple comorbidities. Nevertheless, a study by Whitehead et al. (2014) reported a sensitivity of 96% for fCP >50 mg/g to discriminate C. difficile-positive samples in stool samples from a cohort of patients with diarrhea. Therefore, fCP may have some value for screening CDI patients with diarrhea. Finally, further studies are needed to evaluate the ability of fCP to distinguish CDI patients from those with asymptomatic colonization and IBD.
4.2 The relationship between fecal calprotectin levels and the severity of Clostridioides difficile infection
According to guideline recommendations (Van Prehn et al., 2021), there are different treatment options for CDI patients of different severity. Therefore, it is important to use reliable biomarkers to confirm the severity of infections. However, current diagnostic methods for CDI are still unable to determine the severity of CDI. Clinicians make condition assessments mainly on the clinical manifestations and risk factors of patients. To distinguish mild from severe CDI, the 2010 Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Clinical Practice Guidelines (Cohen et al., 2010) and the European Society of Clinical Microbiology and Infectious Diseases guidelines define criteria based on patient age, physical signs and complications as well as serum albumin, creatinine and leukocytes. Neutrophils, a major leukocyte, play an important role in the pathogenesis of CDI, and fCP secreted by neutrophils is considered by some to be a potential biomarker of disease activity.
In this review, 5 relevant studies all showed higher fCP levels in patients with severe CDI, although a statistical difference was not reached in one of the studies. Moreover, based on the AUC values, sensitivity, and specificity reported in 3 studies, fCP showed a relatively strong ability to distinguish patients with severe CDI from patients with non-severe CDI or non-CDI AAD. However, we also found inconsistencies in their results. For example, the median fCP in patients with severe CDI in different studies ranged from a minimum of 218.5 µg/g to a maximum of 1391.5 µg/g. This discrepancy can be attributed in part to differences in fCP detection kits and in part to differences in the criteria for assessing the severity of CDI. In addition, two studies failed to observe a correlation between fCP levels and three index scores reflecting the severity of CDI. Overall, although the criteria for assessing the severity of CDI differed in different studies, most studies supported the potential value of fCP for assessing disease severity. Current studies have found that fCP levels are significantly related to higher peripheral blood white blood cell counts, and the higher the intensity of CDI inflammation, the greater the increase in neutrophil counts, which may reflect the relationship between fCP levels and the degree of intestinal inflammation. Therefore, from this point of view, fCP may play a role in assessing the severity of CDI. However, it should be noted that large variability in observed fCP levels may also complicate the formulation of optimal cut-off values for severe and non-severe CDI. Therefore, more prospectively designed studies with large sample sizes are needed to further evaluate the ability of fCP to differentiate patients with severe CDI. In addition, prior to this, it is important to unify the criteria for defining severe CDI, as this will improve comparability between different studies.
4.3 Single fecal calprotectin may not predict prognosis in patients with Clostridioides difficile infection
So far, the clinical scoring system that has been proposed to predict the prognosis of CDI is mainly based on a combination of clinical, laboratory and radiology/endoscopic parameters (Belmares et al., 2007; Fujitani et al., 2011; Miller et al., 2013). However, colonoscopy and abdominal CT examination are neither commonly performed on these patients nor are they easily obtained, so the effectiveness of these scores in clinical practice is still limited. Here we describe several studies that have evaluated the prognostic value of fCP in CDI.
Two studies evaluated the value of fCP in predicting patient response to treatment. One study classified patients into “responders” and “non-responders” based on the presence or absence of diarrhea relief and improvement in clinical picture, and assessed treatment response by ATLAS scores based on patient age, antibiotic treatment, white blood cell count, albumin, and serum creatinine. Another study classified patients as “responders” and “non-responder” based on whether they had diarrhea at 8 weeks. Neither study observed a difference in fCP levels between “responders” and “non-responders” or a correlation between treatment response and fCP levels, even when patients received different treatment options. The level of fCP may be related to the patient’s disease state at the time of stool collection, as levels of fCP may be higher during an acute CDI episode. In the study of Antonella et al. (2020), they measured the fCP levels of patients before treatment (T0) and after treatment (T1, T2). However, no differences were observed between responders and non-responders. One possibility is that the clinical response could be related to the difference in fCP levels before and after treatment. Perhaps it is more appropriate to evaluate patients’ responses to treatment in conjunction with the degree of reduction in fCP after treatment.
Based on current data, there is insufficient evidence that fCP levels at the time of CDI diagnosis predict disease recurrence and related death. In the study by Hibbard et al. (2019), there was no significant difference in fCP levels before FMT between FMT-cured (no episodes of CDI during the 60 days after FMT) and FMT-failure patients. Relatively speaking, fCP levels on day 7 after FMT were more valuable in predicting response to FMT. At the same time, one study showed that elevated fCP is a risk factor for patients with adverse outcomes (complexity and recurrence of CDI). We do not believe that fCP levels at the time of diagnosis are suitable for predicting patient outcomes because the time span between the measurement of fCP and the appearance of adverse outcomes is too long. Patients should be followed for a longer period of time and their fCP levels should be continuously measured to better evaluate the value and potential of fCP in the prognosis prediction of CDI. We look forward to more rigorously designed studies with larger sample sizes evaluating this in the future.
4.4 Higher fecal calprotectin levels may indicate detectable toxins in stool and a need for treatment in the patient
The use of NAAT in the diagnosis of CDI has resulted in a significant increase in the documented incidence of CDI due to its higher sensitivity. Data have shown that individuals who test positive for both NAAT and direct toxin assays have longer duration of symptoms and hospitalization, as well as higher mortality, than individuals who test positive for NAAT alone. Meanwhile, the duration of symptoms and mortality in NAAT-positive/toxin-negative patients were similar to those in both NAAT- and toxin-negative patients. Therefore, some scholars have questioned the clinical significance of only NAAT positive. Considerable debate remains about how to interpret and manage NAAT-positive/toxin-negative patients. As per the European guidelines for patients with evidence of C. difficile but negative toxin test results, patients need to be evaluated clinically as they may have undetectable toxin levels or false negative toxin results or may be potential carriers of toxigenic C. difficile (Crobach et al., 2016). Hogan et al. (2022) observed similar clinical outcomes in treated and untreated C. difficile NAAT-positive/toxin-negative adult hospitalized patients. These data support the view that a positive direct toxin test is more closely related to infection than a positive toxin gene test. Four studies in this review showed higher levels of fCP in samples positive for direct toxin assays than in samples positive for toxin genes alone or in which toxin was not directly detectable, although no statistical difference was observed in two of them. From this point of view, a high fCP level may indicate a positive direct toxin test and an infection in the patient. In addition, even a positive toxin test result does not always mean a patient’s need for treatment. Suarez-Carantoña et al. (2021) divided patients into those with hypothetical CDI for whom treatment was recommended, those with indeterminate diagnosis but received CDI therapy, and those with C. difficile colonization or self-limiting CDI who did not require treatment. After comparing levels of fCP in the three groups of patients, it was found that fCP levels were significantly higher in patients who required treatment than in those who did not. Therefore, fCP should be investigated as a potentially useful marker to indicate whether patients with toxin-producing C. difficile require treatment.
4.5 Limitations and recommendations for future studies
Several limitations were observed in this systematic review. Firstly, the guidelines and standards (including CDI diagnosis, severity assessment and prognosis assessment) used in various studies were inconsistent, which was a major source of heterogeneity. Secondly, the selection of subjects in some studies were not rigorous enough, which may lead to the inclusion of patients with other underlying diseases that affect the level of fCP. In addition, there were differences in the outcome measures chosen in the studies. Due to this heterogeneity, a meta-analysis was difficult to conduct, and we could not determine calprotectin cut-off values for distinguishing between patients.
Therefore, more high-quality studies are needed to further explore the value of fCP in CDI. Here, we offer some suggestions. First of all, the diagnosis and severity assessment of CDI should strictly follow the criteria prescribed by the guidelines. Second, it is better if the selected control group is matched with the experimental group in terms of age, gender, underlying diseases, etc. Third, we encourage future studies to use ROC curves to assess the discriminative power of fCP in different patients. Finally, multicenter studies with large sample sizes may provide more reliable and convincing results.
5 Conclusions
Overall, although the current studies on fCP in CDI are small and preliminary, we still obtained some valuable information. We observed a trend towards higher fCP levels in patients with CDI compared to healthy individuals and patients with diarrhea of other causes. Maybe it can be used for CDI screening but its application value in CDI diagnosis may be low. The potential role of fCP in the assessment of CDI severity warrants further evaluation. In addition, high levels of fCP may indicate the need for treatment. Unfortunately, there is insufficient evidence to suggest that fCP has a prognostic value in CDI. The results analyzed in this systematic review should be interpreted with caution because of differences between study results. Meanwhile, more high-quality studies are needed to further comprehensively evaluate the application value and potential of fCP in CDI.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
J-HZ, X-XL, and B-JW were responsible for the idea and concept of the paper. B-JW and L-GT performed the literature search, study screening and data collection. B-JW and L-GT wrote the paper. J-HZ and X-XL, critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This present work was funded by the grants of the International Scientific and Technology Corporation Program of Hebei Provincial Department of Science and Technology (183977118D) and the Supported by Foundation of Hebei Provincial Department of Finance (361004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aletaha N., Dadvar Z., Salehi B., Ketabi M. P., Niksirat A., Jowkar A., et al. (2019). Clinical and pathological features of ulcerative colitis in patients with and without clostridium difficile infection; an observational study. Middle East J. Dig. Dis. 11 (1), 17–23. doi:10.15171/mejdd.2018.123
Antonella G., Carla V., Ludovico S., Giulio V., Marcello C., Giovanni C., et al. (2018). Fecal calprotectin in management of Clostridium difficile infection: A longitudinal study. Scand. J. Gastroenterol. 53 (5), 567–572. doi:10.1080/00365521.2017.1392598
Antonella G., Cristina C., Emily C., Marcello C., Ettore C., Krizia P., et al. (2020). Fecal calprotectin and need of multiple microbiota trasplantation infusions in Clostridium difficile infection. J. Gastroenterol. Hepatol. 35 (11), 1909–1915. doi:10.1111/jgh.15072
Barbut F., Gouot C., Lapidus N., Syed-Zaidi L., Eckert C., Lalande V., et al. (2017). Faecal lactoferrin and calprotectin in patients with Clostridium difficile infection: A case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 36 (12), 2423–2430. doi:10.1007/s10096-017-3080-y
Bartáková E., Štefan M., Stráníková A., Pospíšilová L., Arientová S., Beran O., et al. (2019). Calprotectin and calgranulin C serum levels in bacterial sepsis. Diagn. Microbiol. Infect. Dis. 93 (3), 219–226. doi:10.1016/j.diagmicrobio.2018.10.006
Belmares J., Gerding D. N., Parada J. P., Miskevics S., Weaver F., Johnson S., et al. (2007). Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J. Infect. 55 (6), 495–501. doi:10.1016/j.jinf.2007.09.015
Cohen S. H., Gerding D. N., Johnson S., Kelly C. P., Loo V. G., McDonald L. C., et al. (2010). Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect. Control Hosp. Epidemiol. 31 (5), 431–455. doi:10.1086/651706
Crobach M. J., Planche T., Eckert C., Barbut F., Terveer E. M., Dekkers O. M., et al. (2016). European society of clinical Microbiology and infectious diseases: Update of the diagnostic guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 22 (Suppl. 4), S63–S81. doi:10.1016/j.cmi.2016.03.010
Debast S. B., Bauer M. P., Kuijper E. J. (2014). European society of clinical Microbiology and infectious diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 20 (Suppl. 2), 1–26. doi:10.1111/1469-0691.12418
Decembrino L., De Amici M., Pozzi M., De Silvestri A., Stronati M. (2015). Serum calprotectin: A potential biomarker for neonatal sepsis. J. Immunol. Res. 2015, 147973. doi:10.1155/2015/147973
Fujitani S., George W. L., Murthy A. R. (2011). Comparison of clinical severity score indices for Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 32 (3), 220–228. doi:10.1086/658336
Guh A. Y., Mu Y., Winston L. G., Johnston H., Olson D., Farley M. M., et al. (2020). Trends in u.s. Burden of clostridioides difficile infection and outcomes. N. Engl. J. Med. 382 (14), 1320–1330. doi:10.1056/NEJMoa1910215
Guo Q., Zha X., Li C., Jia Y., Zhu L., Guo J., et al. (2016). Serum calprotectin--a promising diagnostic marker for adult-onset Still's disease. Clin. Rheumatol. 35 (1), 73–79. doi:10.1007/s10067-015-3108-6
Han S., Yi J., Kim J., Moon A. H. (2020). Investigation of intestinal microbiota and fecal calprotectin in Non-Toxigenic and toxigenic clostridioides difficile colonization and infection. Microorganisms 8, E882. doi:10.3390/microorganisms8060882
Hanania A., Jiang Z., Smiley C., Lasco T., Garey K., DuPont H., et al. (2016). Fecal calprotectin in the diagnosis of Clostridium difficile infection. Infect. Dis. Clin. Pract. Balt. Md. 24 (1), 31–34. doi:10.1097/ipc.0000000000000312
Hassanain W. A., Spoors J., Johnson C. L., Faulds K., Keegan N., Graham D., et al. (2021). Rapid ultra-sensitive diagnosis of clostridium difficile infection using a SERS-based lateral flow assay. Analyst 146 (14), 4495–4505. doi:10.1039/d1an00726b
Hibbard J., Jiang Z. D., DuPont H. L. (2019). Fecal calprotectin and fecal indole predict outcome of fecal microbiota transplantation in subjects with recurrent Clostridium difficile infection. Anaerobe 56, 102–105. doi:10.1016/j.anaerobe.2019.03.006
Hogan C. A., Hitchcock M. M., Frost S., Kapphahn K., Holubar M., Tompkins L. S., et al. (2022). Clinical outcomes of treated and untreated C. difficile PCR-positive/toxin-negative adult hospitalized patients: A quasi-experimental noninferiority study. J. Clin. Microbiol. 60 (6), e218721. doi:10.1128/jcm.02187-21
Jieun K., Heejung K., Ju O. H., Sun K. H., Jee H. Y., Dongeun Y., et al. (2017). Fecal calprotectin level reflects the severity of Clostridium difficile infection. Ann. Lab. Med. 37 (1), 53–57. doi:10.3343/alm.2017.37.1.53
Kalantari H., Taheri A., Yaran M. (2015). Fecal calprotectin is a useful marker to diagnose ulcerative colitis from irritable bowel syndrome. Adv. Biomed. Res. 4, 85. doi:10.4103/2277-9175.156647
Kalla R., Kennedy N. A., Ventham N. T., Boyapati R. K., Adams A. T., Nimmo E. R., et al. (2016). Serum calprotectin: A novel diagnostic and prognostic marker in inflammatory bowel diseases. Am. J. Gastroenterol. 111 (12), 1796–1805. doi:10.1038/ajg.2016.342
Kelly C. P., Chen X., Williams D., Xu H., Cuddemi C. A., Daugherty K., et al. (2020). Host immune markers distinguish clostridioides difficile infection from asymptomatic carriage and non-C. Difficile diarrhea. Clin. Infect. Dis. 70 (6), 1083–1093. doi:10.1093/cid/ciz330
Kelly S. G., Yarrington M., Zembower T. R., Sutton S. H., Silkaitis C., Postelnick M., et al. (2016). Inappropriate clostridium difficile testing and consequent overtreatment and inaccurate publicly reported metrics. Infect. Control Hosp. Epidemiol. 37 (12), 1395–1400. doi:10.1017/ice.2016.210
Khaki-Khatibi F., Qujeq D., Kashifard M., Moein S., Maniati M., Vaghari-Tabari M., et al. (2020). Calprotectin in inflammatory bowel disease. Clin. Chim. Acta. 510, 556–565. doi:10.1016/j.cca.2020.08.025
Khanna S., Pardi D. S. (2012). Clostridium difficile infection: New insights into management. Mayo Clin. Proc. 87 (11), 1106–1117. doi:10.1016/j.mayocp.2012.07.016
Kopylov U., Rosenfeld G., Bressler B., Seidman E. (2014). Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm. Bowel Dis. 20 (4), 742–756. doi:10.1097/01.MIB.0000442681.85545.31
Lee H. S., Plechot K., Gohil S., Le J. (2021). Clostridium difficile: Diagnosis and the consequence of over diagnosis. Infect. Dis. Ther. 10 (2), 687–697. doi:10.1007/s40121-021-00417-7
Longtin Y., Paquet-Bolduc B., Gilca R., Garenc C., Fortin E., Longtin J., et al. (2016). Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: A quasi-experimental controlled study. JAMA Intern. Med. 176 (6), 796–804. doi:10.1001/jamainternmed.2016.0177
Mak W. Y., Buisson A., Andersen M. J., Lei D., Pekow J., Cohen R. D., et al. (2018). Fecal calprotectin in assessing endoscopic and histological remission in patients with ulcerative colitis. Dig. Dis. Sci. 63 (5), 1294–1301. doi:10.1007/s10620-018-4980-0
McDonald L. C., Gerding D. N., Johnson S., Bakken J. S., Carroll K. C., Coffin S. E., et al. (2018). Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA). Clin. Infect. Dis. 66 (7), e1–e48. doi:10.1093/cid/cix1085
Meltzer E., Smollan G., Huppert A., Fluss R., Tal I., Gilboa M., et al. (2019). Universal screening for Clostridioides difficile in a tertiary hospital: Risk factors for carriage and clinical disease. Clin. Microbiol. Infect. 25 (9), 1127–1132. doi:10.1016/j.cmi.2019.02.002
Miller M. A., Louie T., Mullane K., Weiss K., Lentnek A., Golan Y., et al. (2013). Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect. Dis. 13, 148. doi:10.1186/1471-2334-13-148
Mirosław D., Grażyna B., Hanna P., Dorota W., Piotr O., Michał P., et al. (2019). The level of fecal calprotectin significantly correlates with Clostridium difficile infection severity. Folia Med. cracov. 59 (3), 53–65.
Moein S., Qujeq D., Vaghari T. M., Kashifard M., Hajian-Tilaki K. (2017). Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Casp. J. Intern. Med. 8 (3), 178–182. doi:10.22088/cjim.8.3.178
Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Naess-Andresen C. F., Egelandsdal B., Fagerhol M. K. (1995). Calcium binding and concomitant changes in the structure and heat stability of calprotectin (L1 protein). Clin. Mol. Pathol. 48 (5), M278–M284. doi:10.1136/mp.48.5.m278
Nicholson M. R., Crews J. D., Starke J. R., Jiang Z. D., DuPont H., Edwards K., et al. (2017). Recurrent clostridium difficile infection in children: Patient risk factors and markers of intestinal inflammation. Pediatr. Infect. Dis. J. 36 (4), 379–383. doi:10.1097/INF.0000000000001450
Peretz A., Tkhawkho L., Pastukh N., Brodsky D., Halevi C. N., Nitzan O., et al. (2016). Correlation between fecal calprotectin levels, disease severity and the hypervirulent ribotype 027 strain in patients with Clostridium difficile infection. BMC Infect. Dis. 16 (1), 309. doi:10.1186/s12879-016-1618-8
Rao K., Santhosh K., Mogle J. A., Higgins P. D. R., Young V. B. (2016). Elevated fecal calprotectin associates with adverse outcomes from Clostridium difficile infection in older adults. Infect. Dis. 48 (9), 663–669. doi:10.1080/23744235.2016.1186832
Reenaers C., Bossuyt P., Hindryckx P., Vanpoucke H., Cremer A., Baert F., et al. (2018). Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United Eur. Gastroenterol. J. 6 (8), 1117–1125. doi:10.1177/2050640618784046
Shastri Y. M., Bergis D., Povse N., Schäfer V., Shastri S., Weindel M., et al. (2008). Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am. J. Med. 121 (12), 1099–1106. doi:10.1016/j.amjmed.2008.06.034
Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Suarez-Carantoña C., Rodriguez-Torres A., Viteri-Noel A., Pintado V., Garcia-Fernandez S., Mora-Pimentel D., et al. (2021). Usefulness of fecal calprotectin in the management of patients with toxigenic clostridioides difficile. J. Clin. Med. 10 (8), 1627. doi:10.3390/jcm10081627
Swale A., Miyajima F., Roberts P., Hall A., Little M., Beadsworth M. B. J., et al. (2017). Calprotectin and lactoferrin faecal levels in patients with Clostridium difficile infection (CDI): A prospective cohort study. PLoS One 9 (8), e106118. doi:10.1371/journal.pone.0106118
He T., Kaplan SE, Gomez LA, Lu X, Ramanathan LV, Kamboj M, et al. (2018). Fecal calprotectin concentrations in cancer patients with Clostridium difficile infection. Eur. J. Clin. Microbiol. Infect. Dis. 37 (12), 2341–2346. doi:10.1007/s10096-018-3381-9
Van Prehn J., Reigadas E., Vogelzang E. H., Bouza E., Hristea A., Guery B., et al. (2021). European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for clostridioides difficile infection in adults. Clin. Microbiol. Infect. 27 (Suppl. 2), S1–S21. doi:10.1016/j.cmi.2021.09.038
Voicu M. N., Ahmet A. M., Turcu-Stiolica A., Ungureanu B. S., Dragoescu A. N., Popescu F., et al. (2021). Clostridoides difficile infection severity assessment by fecal calprotectin: A pilot study. Curr. Health Sci. J. 47 (2), 204–208. doi:10.12865/CHSJ.47.02.09
Whitehead S. J., Shipman K. E., Cooper M., Ford C., Gama R. (2014). Is there any value in measuring faecal calprotectin in Clostridium difficile positive faecal samples? J. Med. Microbiol. 63 (Pt_4), 590–593. doi:10.1099/jmm.0.067389-0
Keywords: Clostridioides difficile infection, fecal calprotectin, biomarker, value, systematic review
Citation: Wen B-J, Te L-G, Liu X-X and Zhao J-H (2022) The value of fecal calprotectin in Clostridioides difficile infection: A systematic review. Front. Physiol. 13:881816. doi: 10.3389/fphys.2022.881816
Received: 28 February 2022; Accepted: 27 June 2022;
Published: 03 August 2022.
Edited by:
Waddah Alrefai, University of Illinois at Chicago, United StatesReviewed by:
Seema Saksena, University of Illinois at Chicago, United StatesMaribeth Nicholson, Vanderbilt University Medical Center, United States
Abbas Yadegar, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2022 Wen, Te, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Hong Zhao, emhhb2poXzIwMDJAeWFob28uY29t
†These authors have contributed equally to this work and share first authorship
 Bao-Jiang Wen
Bao-Jiang Wen Li-Ger Te3†
Li-Ger Te3†