- 1Department of Public Health and Infectious Diseases, Microbiology Section, Sapienza University of Rome, Rome, Italy
- 2Department of Experimental Medicine, Sapienza University of Rome, Rome, Italy
- 3Department of Maternal and Child Health and Urology, Sapienza University of Rome, Rome, Italy
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS- CoV-2) represents an emerging infection that is spreading around the world. Among susceptible patients, pregnant women are more likely to develop serious complications and negative obstetric outcomes. Vertical transmission constitutes a debating issue which has not been completely understood. This review aims at describing the currently available evidence on SARS-CoV2 vertical transmission. We carried out a computerized literature search in the Cochrane Library, PubMed, Scopus and Web of Science, selecting the most relevant studies on vertical transmission from the outbreak onset until February 2022. The analysis of the available literature identifies the presence of SARS-CoV2 genome in different biological specimens, confirming the hypothesis that a transplacental infection can occur. In spite of the high number of infected people around the world, mother-to-child infections have been infrequently reported but it can be observed under certain biologic conditions. A deep knowledge of the underlying mechanisms of SARS-CoV2 vertical transmission is of paramount importance for planning an adequate management for the affected mothers and newborns.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was first identified in Wuhan region, China, on 31 December 2019, and the number of reported cases grew exponentially throughout the world. On 30 January 2020, the World Health Organization (WHO) declared a state of public emergency (World Health Organization, 2020).
The most typical symptoms include fatigue, headache, cough, fever and diarrhea, which can be worsened by preexisting risk factors such as hypertension, diabetes, cardiovascular and respiratory diseases (Huang et al., 2020). Among the susceptible patients, pregnant women are more likely to have serious complications and an increased mortality rate (Hantoushzadeh et al., 2020; Villar et al., 2021). The physiological adaptations of the respiratory, cardiovascular, and immune systems during pregnancy may be responsible for a high risk of developing a severe illness and acute response to viral infections (Piccinni and Romagnani, 1996; Ramsey and Ramin, 2001; Druckmann and Druckmann, 2005; Hall and Klein, 2017). Although CT scan represents the gold standard to evaluate the pulmonary involvement, in pregnant women lung ultrasound is considered a reliable method, minimizing the exposure to ionizing radiations. (Buonsenso et al., 2020; Fang et al., 2020; Gargani et al., 2020; Moro et al., 2020; Porpora et al., 2021).
Nowadays, a variety of therapeutic options exists, including antiviral anti-inflammatory and anti-oxidative drugs, monoclonal antibodies, and immunomodulatory agents (Filardo et al., 2020; Coopersmith et al., 2021; Cascella et al., 2022). To date, the most crucial step in containing SARS-CoV-2 pandemic is vaccination, which can markedly reduce adverse outcomes, hospitalization and deaths (Abu-Raya et al., 2021; Weinberger, 2021; Cascella et al., 2022).
The possibility of vertical transmission represents one of the most debated topics. Deep knowledge of this issue is of paramount importance for planning adequate management for the affected mothers and newborns.
To assess the biological mechanisms underlying the possible vertical transmission, we carried out a computerized literature search in the Cochrane Library, PubMed, Scopus and Web of Science databases, identifying the most relevant articles written in English from the onset of SARS-CoV-2 outbreak.
Pregnancy Outcomes After SARS-CoV-2 Infection
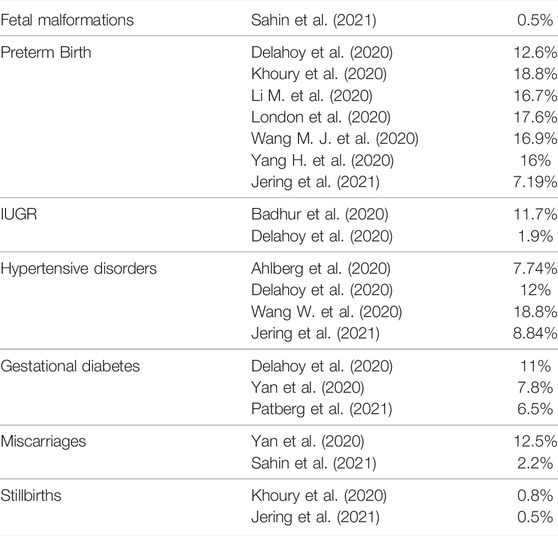
Several studies have found a clear association between the infection of SARS-CoV-2 in pregnancy and the increasing risk of fetal malformations, preterm birth, intrauterine growth restriction, hypertensive disorders, and new onset gestational diabetes, as reported in Table 1 (Dashraath et al., 2020; Thomas et al., 2020; Timircan et al., 2021; Jamieson and Rasmussen, 2022), while a clear correlation between infection, stillbirths and miscarriages is still debated (Dashraath et al., 2020; Karimi-Zarchi et al., 2020; Khalil et al., 2020; Mendoza et al., 2020; Smith et al., 2020; Jamieson and Rasmussen, 2022). The pathophysiological mechanisms on pregnancy outcomes seem to involve different mediators.
Conde-Agudelo and Romero described the pathophysiological processes causing hypertensive complications and preeclampsia/eclampsia in patients with SARS-CoV-2. The viral binding process to the host cells led to a reduced activation of the renin angiotensin system: this phenomenon predisposes to the onset of preeclampsia, by decreasing the vasodilatory action of angiotensin 1 to 7 and increasing the vasoconstriction and inflammation.(Conde-Agudelo and Romero, 2022). Moreover, this proinflammatory environment is further enhanced by SARS-CoV-2 ability to shatter the syncytiotrophoblast, with a consequent increase of soluble fms-like tyrosine kinase-1 (sflt-1) which is strictly associated to the onset of this disease (Conde-Agudelo and Romero, 2022). Seethy et al. reported alterations in several placental proteins, such as MFGE8, PLAT, or PAR2, involved in trophoblastic invasion, proliferation and differentiation. These mechanisms define a direct correlation between a severe course of the disease and an increased risk of hypertensive complications (Seethy et al., 2021). Eberle et al. reported a possible association of SARS-CoV-2 and new-onset gestational diabetes: the viral entrance in the pancreatic beta cells through the angiotensin-converting enzyme 2 (ACE2) receptors, may cause insulin deficiency and an increased risk of keto-acydosis (Eberle et al., 2021). Furthermore, it seems that SARS-CoV-2 might cause pleiotropic alterations of glucose metabolism in patients with pre-existing diabetes or insulin-resistance (Rubino et al., 2020). Maternal infection seems to have a negative impact on neonatal health and an increased rate of preterm birth, stillbirth and low birth weight was reported, regardless of the mode of delivery (Kyle et al., 2022), probably due to the massive inflammatory response and to the suboptimal environment for the fetal growth (Wei et al., 2021).
Due to the negative effect of this infection on pregnancy, considerable support to pregnant patients comes from the implementation of the vaccination campaign. The American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine, the Centers for Disease Control and Prevention (CDC), and the Italian Society Of Gynecology and Obstetrics (SIGO) encourage SARS-CoV-2 vaccination in pregnancy (ACOG, 2018; CDC, 2021; Sigo Position Paper, 2021; European Center for Disease Prevention and Control, 2020), which also provides an immunological protection for the newborn (Jorgensen et al., 2022). In fact, pregnant women generate robust humoral immunity after the mRNA anti SARS-CoV2 vaccination (Gray et al., 2021; Shimabukuro et al., 2021). SARS-CoV-2 specific antibodies are present in both maternal and cord blood, suggesting that antibodies elicited by SARS-CoV-2 immunization cross the placenta (Beharier et al., 2021; Collier et al., 2021; Gray et al., 2021; Mithal et al., 2021). Both spike (S) and receptor binding domain (RBD) IgG antibodies as well as neutralizing antibodies are transplacentally transferred to the newborns, suggesting vaccines as the most important intervention to protect pregnant and breastfeeding women and their offspring from the infection (Jorgensen et al., 2022). To date, one of the most debating issues is to determine the most appropriate timing to assure an effective protection for this dyad. Recent data have, indeed, suggested that receiving a 2-dose mRNA COVID-19 vaccine during pregnancy can protect infants aged <6 months against the risk of COVID-19 hospitalization: this phenomenon seems to be enhanced among mothers vaccinated later in pregnancy (Dick et al., 2022). On this regard, a considerable decrease in anti-SARS-CoV-2 antibody levels has been observed throughout pregnancy in women vaccinated at early gestation, hinting at a relevant impact of the antenatal immunization timing on SARS-CoV-2 transplacental antibody transfer, which can potentially influence neonatal seroprotection (Rottenstreich et al., 2022).
Placental Involvement in the Vertical Transmission of SARS-CoV2
Given the high number of cases of SARS-CoV-2 infection, it is essential to clarify the pathophysiological mechanisms that predispose to viral infection or protect the maternal-fetal interface. SARS-CoV-2 shares some structural characteristics with SARS and MERS, such as a positive single-stranded RNA virus with a nucleocapsid and an envelope and four structural proteins: spike (S), envelope (E), nucleocapsid (N) and membrane (M) (Guo et al., 2020; Villalain et al., 2020). N protein is essential to protect the RNA, whereas S, M, and E proteins compose the viral envelope (Kumar and Al Khodor, 2020).
The placental interface plays an essential role in protecting the fetus from different pathogens. The chorionic villi are composed of three kinds of trophoblasts: syncytiotrophoblast (STB), extravillous trophoblast (EVT), and cytotrophoblast (CTB). STB represents the outer layer and is in direct contact with maternal blood, mediating the exchange between mother and fetus; the CTB is the source of trophoblastic stem cells, and it is in close contact with STB. EVT is composed of cells at the base of stem villi and a disruption of its continuity can lead to the placental passage of pathogens and fetal infection (Maltepe and Fisher, 2015; Pereira, 2018).
Spike glycoprotein is essential to promote the attachment of SARS-CoV-2 to the surface of the host cell and the subsequent fusion of the viral envelope. The target receptor is ACE2 which needs the serine proteases TMPRSS2 as a co-receptor to finalize the cell invasion (Valdés et al., 2006; Kumar and Al Khodor, 2020), considered as one of the most important factors contributing to its pathogenicity (Hoffmann et al., 2020; Zhang et al., 2020). ACE2 receptor is a transmembrane peptidase expressed on the surface of lungs, kidneys, intestinal tract, and placental tissues (Hoffmann et al., 2020). The placental immunohistochemical analysis revealed a different ACE2 and TMPRSS2 distribution through trimesters and trophoblast layers. During the first trimester, ACE2 is preferentially expressed in STB and TMPRSS2 in EVT and CTB. In the second and third trimesters, ACE2 expression also involves EVT, while TMPRSS2 is equally represented in all trophoblast sites (Cui et al., 2021). (Figure 1). The pleiotropic expression of ACE2 and TMPRSS2 hints at the biological basis of SARS-CoV-2 transplacental route. Further evidence reported the presence of SARS-CoV-2 S- and N- protein expression in the STB layer, confirmed by in-situ hybridization techniques, showing the presence of viral RNA (Baud et al., 2020). The low prevalence of SARS-CoV-2 RNA in placental tissue found in some studies could be explained by the focal localization of the virus in this organ, which may influence the detection of viral RNA. This problem could be overcome by testing more samples from the same placenta. A co-expression of ACE2 and TMPRSS2 was found in stromal and perivascular decidual cells and in villous CTB and STB (Li M. et al., 2020). Several histologic alterations of placental tissue, such as massive vascular malperfusion, chronic intervillositis, funisitis and villitis seem to be a potential sign of subsequent fetal infection (Schwartz et al., 2020). Shanes et al. reported a high rate of placental vascular malperfusion in 16 placentas of women with COVID-19 who delivered in the third trimester as compared to unaffected patients, despite the negative results of RT-PCR for SARS-CoV-2 (Shanes et al., 2020).
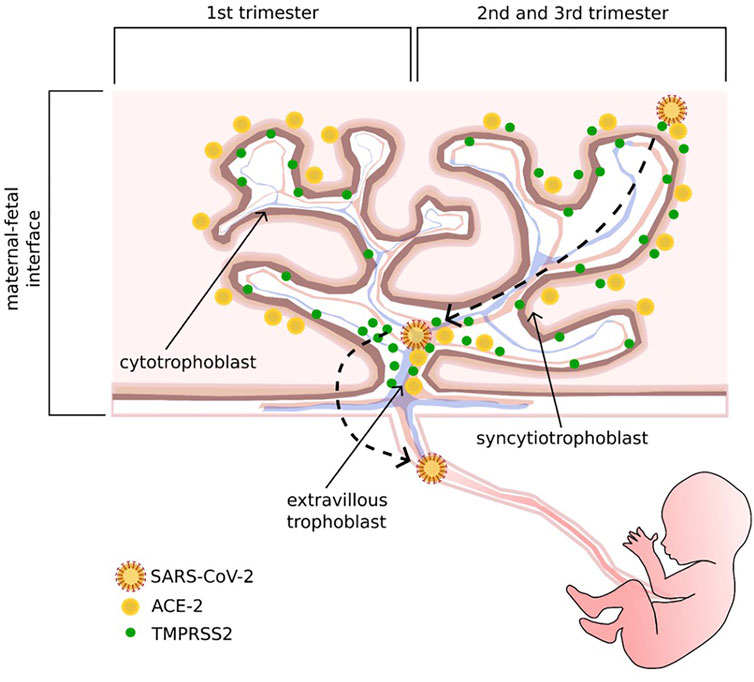
FIGURE 1. Schematic representation of ACE 2 and its co-receptor, TMPRSS 2, distribution through trimesters and trophoblast layers, and the potential adhesion and invasion pathways of SARS-CoV-2. In the first trimester, ACE2 is preferentially expressed in the syncytiotrophoblast layer, while TMPRSS2 is mostly localized on the cytotrophoblast and extravillous trophoblast layers; in the second and third trimesters, ACE2 is also expressed on the extravillous trophoblast layer, and TMPRSS2 can be found on all trophoblast sites.
The histopathologic alterations of the placenta and the presence of viral proteins strongly support the possibility of the transplacental passage of the virus: in fact, placental damage may be considered as an indirect sign of a maternal-fetal response to viral infection (Baud et al., 2020; Hosier et al., 2020).
In support to the link between histopathological damage and SARS-CoV-2 infection of placental tissue, it stands the identification of a strong expression of SARS-CoV-2 S- and N-proteins in the STB layer, with an increased expression of S- protein where the intervillous inflammation was huge (Facchetti et al., 2020). This was confirmed by in-situ hybridization, detecting the presence of viral RNA in the syncytiotrophoblast. At the same time, the placenta showed signs of intervillositis, necrosis of the syncytiotrophoblast, evidence of fetal vascular malperfusion and fibrin deposition (Facchetti et al., 2020).
The cellular apoptosis and the vascular impairment may enhance the placental permeability (Miranda et al., 2019), predisposing to circulatory disturbances that lead to subchorionic and intervillous fibrin deposition (Baergen and Heller, 2020; Shanes et al., 2020).
Evidence of Vertical Transmission and Neonatal Infection
The risk of vertical transmission was analyzed during previous coronavirus outbreaks without conclusive results (Hospital et al., 2003; Wong et al., 2004; Akhtar et al., 2020).
Analyzing different biological specimens and the presence of antibodies in the maternal blood, several studies pointed to the transplacental passage of SARS-CoV-2 viral particles through the maternal-fetal interface, with an estimated rate of vertical transmission ranging from 3 to 8% of cases (Bwire et al., 2021; Kotlyar et al., 2021; Taglauer et al., 2022). Concerns over SARS-CoV-2 vertical transmission are appropriate, due to the tropism for the cells of the maternal-fetal interface (Li N. et al., 2020). It is well-known that a viral infection, acquired during pregnancy, can lead to three different vertical transmissions: intrauterine, intrapartum and postpartum routes (Schwartz, 2020). The transmission in utero occurs when the virus, circulating in the bloodstream, crosses the maternal-placental interface, reaching the umbilical cord and infecting the fetus (Mahyuddin et al., 2020). Intrapartum infection may occur during labor when the fetus is exposed to a pathogen that has colonized vaginal secretions and/or feces (Schwartz et al., 2020). Postpartum transmission happens after delivery, when the infection can spread through fomites and maternal close contact. A retrospective study conducted by Ferrazzi et al., noticed that infected women who breastfed without wearing a mask can facilitate neonatal infection (Ferrazzi et al., 2020). Since the available evidence does not support breast milk as a source of neonatal infection (Marín Gabriel et al., 2020), it seems that, whether hygienic measures are not assured, the close connection between mother and infant after birth might represent a viable way for the transmission of pathogen’s particles (Marín Gabriel, M. Á. et al., 2020, Stiehm and Keller, 2001). In this way the virus can reach the target cells invading the infant’s gastrointestinal and respiratory system. Regarding breastfeeding as a carrier for the infection, a recent review reported no replication-competent SARS-CoV-2 in breastmilk (Jamieson and Rasmussen, 2022).
To assess the most viable route of fetal transmission, Beesley et al. conducted a retrospective study evaluating the gene expression level of ACE2 and TMPRSS2 through PCR analysis of multiple fetal tissues at different gestational ages during the second trimester. According to their results, the assumption for fetal infection is the co-expression of both target proteins, which seems to occur only in the fetal kidney and gastrointestinal tract. Therefore, it is possible to hypothesize a viral entry into the bowel lumen through fetal swallowing of infected amniotic fluid (Beesley et al., 2022).
Alternative mechanisms of cellular entry were also proposed. In particular, an antibody-dependent enhancement (ADE) of the virus infection was suggested. According to this theory, Fc receptors may facilitate cellular entry by inducing a conformational change in S protein, which promotes a proteolytic cleavage. The cleavage, in turn, allows the viral binding and the subsequent activation of dipeptidyl dipeptidase 4 (DDP4) receptors, which fosters cellular entry (Moore and Suthar, 2021).
SARS-CoV-2 virions in the syncytiotrophoblast and in the fetal capillary endothelium have been recently reported, supporting the hypothesis of the pathogen transfer through the entire thickness of the maternal-fetal interface (Di Gioia et al., 2022); however, neonatal infection rarely occurs. The low rate of vertical transmission is not always related to a reduction in ACE2 and TMPRSS2 expression but it is also due to changes occurring after the virus entry (Ouyang et al., 2021). Furthermore, experiments on cells’ cultures exposed to trophoblastic cell’s microenvironment showed a selective antiviral activity against different viruses like togavirus, varicella zoster and HIV. This action seems to be ineffective on other pathogens like L. monocytogenes and Toxoplasma gondii (Bayer et al., 2015). Human trophoblasts are resistant to different viruses by the intervention of a huge variety of cells, including miRNAs, located on chromosome 19, which are exclusively expressed in the placenta (Bortolin-Cavaille et al., 2009). Their targets include different types of non-trophoblastic cells like fibroblasts, Hofbauer cells, maternal and fetal cells. These miRNAs send silencing signals to nearby and distant cells through a non-hormonal mechanism (Schwartz, 2017). These findings support the hypothesis that, in some conditions, the trophoblastic cells and the miRNAs are able to inhibit the viral entrance in the cell and the subsequent perinatal infection. The largest published study, prospectively included 427 pregnant women with confirmed SARS-CoV-2 infection and 244 newborns. Among the babies, 12 had confirmed SARS-CoV-2 infection, but only six of them within 12 h after birth. The mode of delivery did not influence the viral transmission, as three infants born by elective cesarean section had evidence of both viral RNA in nasopharyngeal swabs and positive IgM circulating antibodies (Knight et al., 2020). The observation that several neonates born to positive mothers have IgM antibodies confirms the fetal viral infection, as IgM antibodies do not cross the placenta (Kotlyar et al., 2021). However, an early postnatal infection cannot be excluded.
By contrast, Yan et al., did not report any vertical transmission, confirmed by the absence of SARS-CoV-2 RNA in amniotic fluid, cord blood and pharyngeal swabs of all the newborns of 116 affected women (Yan et al., 2020). In addition, in a study on 38 pregnant women with SARS-CoV-2, no cases of intrauterine transmission were found, despite the presence of several neonatal complications, such as severe neonatal respiratory distress syndrome, pneumothorax, asphyxia, stillbirth or small fetus for gestational age (Schwartz, 2020). An overview of the evidence concerning SARS-CoV2 vertical transmission regardless of the mode of delivery is shown in Table 2.
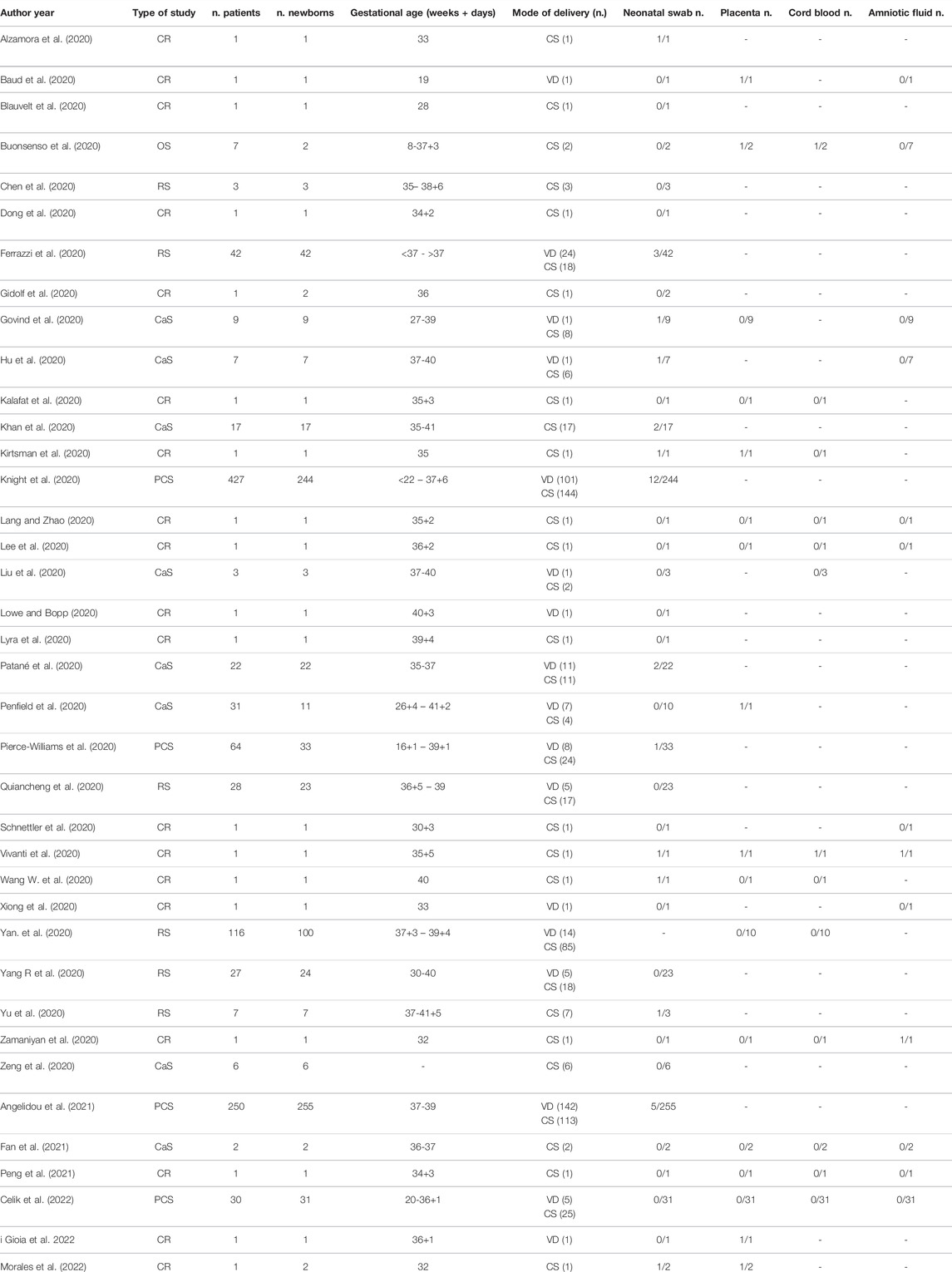
TABLE 2. Studies on the vertical transmission of Sars-CoV-2. CR, case report; OS, observational study; PCS, prospective cohort study; RS, retrospective study; CaS, case series; VD, vaginal delivery; CS, cesarean section.
Discussion
Most available information on SARS-CoV-2 infection comes from adults and the effect of the infection on newborns is still controversial. During pregnancy, several complications were reported with a high rate of preterm birth, fetal malformations, intrauterine growth restriction, hypertensive disorders, and new onset gestational diabetes (Dashraath et al., 2020; Thomas et al., 2020; Timircan et al., 2021; Jamieson and Rasmussen, 2022). These outcomes are not always related to the severity of the maternal disease, since they may occur in the absence of symptoms. It was shown that ACE2 and TMPRSS2 receptors, the target sites for SARS-CoV-2 invasion, are expressed in the placenta (Jamieson and Rasmussen, 2022). Their expression varies in the different trimesters of pregnancy. Placental infection is responsible for circulatory abnormalities, which may provoke thrombosis of vessels and ischemic lesions, resulting in a chronic fetal hypoxia. In addition, the vascular damage and the inflammatory environment seem to be involved in preterm birth and preeclampsia. Despite the huge knowledge on maternal complications, there is fragmentary data on fetal and neonatal infection and on the biological mechanisms involved. Vertical transmission was observed in some cases but the majority of studies did not find the virus in the newborn. In some cases, the virus was detected in nasopharyngeal swabs, in others, circulating IgM were found, even in the presence of negative swabs, showing that the fetus was infected (Kotlyar et al., 2020). The absence of viral RNA in nasopharyngeal swabs raises the question whether it is appropriate to limit SARS-CoV-2 testing in infants to routine respiratory samples or, instead, it is necessary to examine samples from different sites. In fact, fetal infection could not be easily detected by pharyngeal swabs, since the virus mostly dwells in the gastrointestinal tract. Therefore, rectal swabs analysis at birth should always be performed. In addition, Liguoro et al. argue that newborns are often tested for SARS-CoV-2 only if symptomatic (Liguoro et al., 2021); this might reduce the possibility of detecting a vertical transmission. Indeed, the evidence on the tissue tropism of SARS-CoV-2 suggests the potential infection of other organs and tissues rather than the respiratory tract in infants, although the clinical outcomes are still unconfirmed.
The absence of a neonatal infection could be also due to the neutralizing effect of maternal immunity against the virus: in fact, studies on influenza confirmed the efficacy of the maternal humoral response in reducing the infection in infants (Puck et al., 1980; Zaman et al., 2008). The immune response could be related to the severity of disease; Szymczak reported that the production of anti-N antibodies is strongly associated with maternal severe symptoms and a rise in body temperature. Therefore, the more aggressive the disease is, the more effective the antibody response is (Szymczak et al., 2021). Moreover, it is often impossible to determine the exact time of the maternal infection, making it challenging to obtain data on SARS-CoV-2 prevalence in newborns.
There are several limitations in our review principally due to the quality of the available literature, as they are mainly case reports, case series or low-sample populations studies. Moreover, in most cases there is a lack of data on placenta, amniotic fluid and cord blood samples analysis. Lastly, the analysis of specific IgG and IgM in newborns’ blood, which are of paramount importance, are rarely reported.
To date, this narrative review includes the most recent and thorough evidence regarding the mechanisms underlying the possible SARS-CoV-2 vertical transmission. The long-term implications of the virus infections remain to be investigated. A better knowledge of the trophoblastic immune response and of the virus cell entry pathways is necessary to fully understand the mechanisms involved in susceptibility to SARS-CoV-2 infection. More research studies on larger cohorts of pregnant women, as well as longitudinal studies on their newborns, will be necessary to understand whether SARS-CoV-2 mother-to-child transmission could cause long-term consequences on maternal and fetal health.
Author Contributions
MP and RS have given substantial contributions to study conception, RB, GT, and MP to data acquisition, analysis and interpretation, MV, LM, SF, and GB to manuscript writing, MP, RS, EA, and RB to manuscript critical revision. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Raya B., Madhi S. A., Omer S. B., Amirthalingam G., Giles M. L., Flanagan K. L., et al. (2021). Global Perspectives on Immunization against SARS-CoV-2 during Pregnancy and Priorities for Future Research: An International Consensus Paper from the World Association of Infectious Diseases and Immunological Disorders. Front. Immunol. 12, 808064. doi:10.3389/fimmu.2021.808064
Ahlberg M., Neovius M., Saltvedt S., Söderling J., Pettersson K., Brandkvist C., et al. (2020). Association of SARS-CoV-2 Test Status and Pregnancy Outcomes. JAMA 324, 1782–1785. doi:10.1001/jama.2020.19124
Akhtar H., Patel C., Abuelgasim E., Harky A. (2020). COVID-19 (SARS-CoV-2) Infection in Pregnancy: A Systematic Review. Gynecol. Obstet. Invest. 85, 295–306. doi:10.1159/000509290
Alzamora M. C., Paredes T., Caceres D., Webb C. M., Valdez L. M., La Rosa M. (2020). Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol 37, 861–865. doi:10.1055/s-0040-1710050
American College of Obstetricians and Gynecologists (2018). ACOG Committee Opinion No. 741. Maternal Immunization. Obstet. Gynecol. 131, 214–217. doi:10.1097/AOG.0000000000002662
Angelidou A., Sullivan K., Melvin P. R., Shui J. E., Goldfarb I. T., Bartolome R., et al. (2021). Association of Maternal Perinatal SARS-CoV-2 Infection with Neonatal Outcomes during the COVID-19 Pandemic in Massachusetts. JAMA Netw. Open 4, e217523. doi:10.1001/jamanetworkopen.2021.7523
Baergen R. N., Heller D. S. (2020). Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 23, 177–180. doi:10.1177/1093526620925569
Bahadur G., Homburg R., Yoong W., Singh C., Bhat M., Kotabagi P., et al. (2020). Adverse Outcomes in SAR-CoV-2 (COVID-19) and SARS Virus Related Pregnancies with Probable Vertical Transmission. Jbra 24, 351–357. doi:10.5935/1518-0557.20200057
Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., et al. (2020). Second-Trimester Miscarriage in a Pregnant Woman with SARS-CoV-2 Infection. JAMA 323, 2198–2200. doi:10.1001/jama.2020.7233
Bayer A., Delorme-Axford E., Sleigher C., Frey T. K., Trobaugh D. W., Klimstra W. B., et al. (2015). Human Trophoblasts Confer Resistance to Viruses Implicated in Perinatal Infection. Am. J. Obstet. Gynecol. 212, e1–71. doi:10.1016/j.ajog.2014.07.060
Beesley M., Davidson J., Panariello F., Shibuya S., Scaglioni D., Jones B., et al. (2022). COVID‐19 and Vertical Transmission: Assessing the Expression of ACE2/TMPRSS2 in the Human Fetus and Placenta to Assess the Risk of SARS‐CoV‐2 Infection. BJOG 129, 256–266. doi:10.1111/1471-0528.16974
Beharier O., Plitman Mayo R., Raz T., Nahum Sacks K., Schreiber L., Suissa-Cohen Y., et al. (2021). Efficient Maternal to Neonatal Transfer of Antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 Vaccine. J. Clin. Invest. 131. doi:10.1172/JCI150319
Blauvelt C. A., Chiu C., Donovan A. L., Prahl M., Shimotake T. K., George R. B., et al. (2020). Acute Respiratory Distress Syndrome in a Preterm Pregnant Patient with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 136, 46–51. doi:10.1097/AOG.0000000000003949
Bortolin-Cavaille M.-L., Dance M., Weber M., Cavaille J. (2009). C19MC microRNAs Are Processed from Introns of Large Pol-II, Non-protein-coding Transcripts. Nucleic Acids Res. 37, 3464–3473. doi:10.1093/nar/gkp205
Buonsenso D., Raffaelli F., Tamburrini E., Biasucci D. G., Salvi S., Smargiassi A., et al. (2020). Clinical Role of Lung Ultrasound for Diagnosis and Monitoring of COVID‐19 Pneumonia in Pregnant Women. Ultrasound Obstet. Gynecol. 56, 106–109. doi:10.1002/uog.22055
Bwire G. M., Njiro B. J., Mwakawanga D. L., Sabas D., Sunguya B. F. (2021). Possible Vertical Transmission and Antibodies against SARS‐CoV‐2 Among Infants Born to Mothers with COVID‐19: A Living Systematic Review. J. Med. Virol. 93, 1361–1369. doi:10.1002/jmv.26622
Cascella M., Rajnik M., Aleem A., Dulebohn S. C., Di Napoli R. (2022). Features, Evaluation, and Treatment of Coronavirus (COVID-19). Treasure Island, FL: StatPearls Publishing.
CDC COVID-19 Vaccine Breakthrough Case Investigations Team (2021). COVID-19 Vaccine Breakthrough Infections Reported to CDC - United States. Morbidity mortality weekly Rep. 70, 792–793. January 1-April 30, 2021. doi:10.15585/mmwr.mm7021e3
Celik E., Vatansever C., Ozcan G., Kapucuoglu N., Alatas C., Besli Y., et al. (2022). Placental Deficiency during Maternal SARS-CoV-2 Infection. Placenta 117, 47–56. doi:10.1016/j.placenta.2021.10.012
Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. (2020). Clinical Characteristics and Intrauterine Vertical Transmission Potential of COVID-19 Infection in Nine Pregnant Women: a Retrospective Review of Medical Records. The Lancet 395, 809–815. doi:10.1016/S0140-6736(20)30360-3
Collier A.-r. Y., McMahan K., Yu J., Tostanoski L. H., Aguayo R., Ansel J., et al. (2021). Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. Jama 325, 2370–2380. doi:10.1001/jama.2021.7563
Conde-Agudelo A., Romero R. (2022). SARS-CoV-2 Infection during Pregnancy and Risk of Preeclampsia: a Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 226, 68–89. doi:10.1016/j.ajog.2021.07.009
Coopersmith C. M., Antonelli M., Bauer S. R., Deutschman C. S., Evans L. E., Ferrer R., et al. (2021). The Surviving Sepsis Campaign: Research Priorities for Coronavirus Disease 2019 in Critical Illness. Crit. Care Med. 49, 598–622. doi:10.1097/CCM.0000000000004895
Cui D., Liu Y., Jiang X., Ding C., Poon L. C., Wang H., et al. (2021). Single‐cell RNA Expression Profiling of SARS‐CoV‐2‐related ACE2 and TMPRSS2 in Human Trophectoderm and Placenta. Ultrasound Obstet. Gynecol. 57, 248–256. doi:10.1002/uog.22186
Dashraath P., Wong J. L. J., Lim M. X. K., Lim L. M., Li S., Biswas A., et al. (2020). Coronavirus Disease 2019 (Covid-19) Pandemic and Pregnancy. Am. J. Obstet. Gynecol. 222, 521–531. doi:10.1016/j.ajog.2020.03.021
Delahoy M. J., Whitaker M., O'Halloran A., Chai S. J., Kirley P. D., Alden N., et al. (2020). Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19 - COVID-NET, 13 States, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 69, 1347–1354. doi:10.15585/mmwr.mm6938e1
Di Gioia C., Zullo F., Bruno Vecchio R. C., Pajno C., Perrone G., Galoppi P., et al. (2022). Stillbirth and Fetal Capillary Infection by SARS-CoV-2. Am. J. Obstet. Gynecol. MFM 4, 100523. doi:10.1016/j.ajogmf.2021.100523
Dick A., Rosenbloom J. I., Gutman-Ido E., Lessans N., Cahen-Peretz A., Chill H. H. (2022). Safety of SARS-CoV-2 Vaccination during Pregnancy- Obstetric Outcomes from a Large Cohort Study. BMC Pregnancy Childbirth 22, 166. doi:10.1186/s12884-022-04505-5
Dong L., Tian J., He S., Zhu C., Wang J., Liu C., et al. (2020). Possible Vertical Transmission of SARS-CoV-2 from an Infected Mother to Her Newborn. JAMA 323, 1846–1848. doi:10.1001/jama.2020.4621
Druckmann R., Druckmann M.-A. (2005). Progesterone and the Immunology of Pregnancy. J. Steroid Biochem. Mol. Biol. 97, 389–396. doi:10.1016/j.jsbmb.2005.08.010
Eberle C., James-Todd T., Stichling S. (2021). SARS-CoV-2 in Diabetic Pregnancies: a Systematic Scoping Review. BMC Pregnancy Childbirth 21, 573. doi:10.1186/s12884-021-03975-3
European Center for Disease Prevention and Control (2020). Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients with Suspected or Confirmed COVID-19. Stockholm: ECDC.
Facchetti F., Bugatti M., Drera E., Tripodo C., Sartori E., Cancila V., et al. (2020). SARS-CoV2 Vertical Transmission with Adverse Effects on the Newborn Revealed through Integrated Immunohistochemical, Electron Microscopy and Molecular Analyses of Placenta. EBioMedicine 59, 102951. doi:10.1016/j.ebiom.2020.102951
Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., et al. (2021). Perinatal Transmission of 2019 Coronavirus Disease-Associated Severe Acute Respiratory Syndrome Coronavirus 2: Should We Worry? Clin. Infect. Dis. 72, 862–864. doi:10.1093/cid/ciaa226
Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., et al. (2020). Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 296, E115–E117. doi:10.1148/radiol.2020200432
Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S., et al. (2020). Vaginal Delivery in SARS‐CoV‐2‐infected Pregnant Women in Northern Italy: a Retrospective Analysis. Bjog: Int. J. Obstet. Gy 127, 1116–1121. doi:10.1111/1471-0528.16278
Filardo S., Di Pietro M., Mastromarino P., Sessa R. (2020). Therapeutic Potential of Resveratrol against Emerging Respiratory Viral Infections. Pharmacol. Ther. 214, 107613. doi:10.1016/j.pharmthera.2020.107613
Gargani L., Soliman-Aboumarie H., Volpicelli G., Corradi F., Pastore M. C., Cameli M. (2020). Why, when, and How to Use Lung Ultrasound during the COVID-19 Pandemic: Enthusiasm and Caution. Eur. Heart J. Cardiovasc. Imaging 21, 941–948. doi:10.1093/ehjci/jeaa163
Gidlöf S., Savchenko J., Brune T., Josefsson H. (20202020). COVID‐19 in Pregnancy with Comorbidities: More liberal Testing Strategy Is Needed. Acta Obstet. Gynecol. Scand. 99, 948–949. doi:10.1111/aogs.13862
Godoy H., Vieyra D., Sanchez H., Loyo M., Reyes G., Rojas F., et al. (2022). Vertical Transmission: Evidence of COVID-19 in a Twin Pregnancy. JBRA Assist. Reprod. 26, 153–157. doi:10.5935/1518-0557.20210058
Govind A., Essien S., Karthikeyan A., Fakokunde A., Janga D., Yoong W., et al. (2020). Re: Novel Coronavirus COVID-19 in Late Pregnancy: Outcomes of First Nine Cases in an Inner City London Hospital. Eur. J. Obstet. Gynecol. Reprod. Biol. 251, 272–274. doi:10.1016/j.ejogrb.2020.05.004
Gray K. J., Bordt E. A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. (2021). Coronavirus Disease 2019 Vaccine Response in Pregnant and Lactating Women: A Cohort Study. Am. J. Obstet. Gynecol. 225, e1–303. e1–.e17. doi:10.1016/j.ajog.2021.03.023
Guo C. C., Mi J. Q., Nie H. (2020). Seropositivity Rate and Diagnostic Accuracy of Serological Tests in 2019-nCoV Cases: A Pooled Analysis of Individual Studies. Eur. Rev. Med. Pharmacol. Sci. 24, 10208–10218. doi:10.26355/eurrev_202010_23243
Hall O. J., Klein S. L. (2017). Progesterone-based Compounds Affect Immune Responses and Susceptibility to Infections at Diverse Mucosal Sites. Mucosal Immunol. 10, 1097–1107. doi:10.1038/mi.2017.35
Hantoushzadeh S., Shamshirsaz A. A., Aleyasin A., Seferovic M. D., Aski S. K., Arian S. E., et al. (2020). Maternal Death Due to COVID-19. Am. J. Obstet. Gynecol. 223, 109–e16. doi:10.1016/j.ajog.2020.04.03010.1016/j.ajog.2020.04.030
Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271–280. doi:10.1016/j.cell.2020.02.052
Hosier H., Farhadian S. F., Morotti R. A., Deshmukh U., Lu-Culligan A., Campbell K. H., et al. (2020). SARS-CoV-2 Infection of the Placenta. J. Clin. Invest. 130, 4947–4953. doi:10.1172/JCI139569
Hu X., Gao J., Luo X., Feng L., Liu W., Chen J., et al. (2020). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vertical Transmission in Neonates Born to Mothers with Coronavirus Disease 2019 (COVID-19) Pneumonia. Obstet. Gynecol. 136, 65–67. doi:10.1097/AOG.0000000000003926
Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. The Lancet 395, 497–506. doi:10.1016/S0140-6736(20)30183-5
Jamieson D. J., Rasmussen S. A. (2022). An Update on COVID-19 and Pregnancy. Am. J. Obstet. Gynecol. 226, 177–186. doi:10.1016/j.ajog.2021.08.054
Jering K. S., Claggett B. L., Cunningham J. W., Rosenthal N., Vardeny O., Greene M. F., et al. (2021). Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth with and without COVID-19. JAMA Intern. Med. 181, 714–717. doi:10.1001/jamainternmed.2020.9241
Jorgensen S. C. J., Burry L., Tabbara N. (2022). Role of Maternal COVID‐19 Vaccination in Providing Immunological protection to the Newborn. Pharmacotherapy 42, 58–70. doi:10.1002/phar.2649
Kalafat E., Yaprak E., Cinar G., Varli B., Ozisik S., Uzun C., et al. (2020). Lung Ultrasound and Computed Tomographic Findings in Pregnant Woman with COVID‐19. Ultrasound Obstet. Gynecol. 55, 835–837. doi:10.1002/uog.22034
Karimi-Zarchi M., Neamatzadeh H., Dastgheib S. A., Abbasi H., Mirjalili S. R., Behforouz A., et al. (2020). Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr. Pathol. 39, 246–250. doi:10.1080/15513815.2020.1747120
Khalil A., von Dadelszen P., Draycott T., Ugwumadu A., O’Brien P., Magee L. (2020). Change in the Incidence of Stillbirth and Preterm Delivery during the Covid-19 Pandemic. JAMA 324, 705–706. doi:10.1001/jama.2020.12746
Khan S., Peng L., Siddique R., Nabi G., Nawsherwan M., Xue M., et al. (2020). Impact of COVID-19 Infection on Pregnancy Outcomes and the Risk of Maternal-To-Neonatal Intrapartum Transmission of COVID-19 during Natural Birth. Infect. Control. Hosp. Epidemiol. 41, 748–750. doi:10.1017/ice.2020.84
Khoury R., Bernstein P. S., Debolt C., Stone J., Sutton D. M., Simpson L. L., et al. (2020). Characteristics and Outcomes of 241 Births to Women with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection at Five New York City Medical Centers. Obstet. Gynecol. 136, 273–282. doi:10.1097/AOG.0000000000004025
Kirtsman M., Diambomba Y., Poutanen S. M., Malinowski A. K., Vlachodimitropoulou E., Parks W. T., et al. (2020). Probable Congenital SARS-CoV-2 Infection in a Neonate Born to a Woman with Active SARS-CoV-2 Infection. CMAJ 192, E647–E650. doi:10.1503/cmaj.200821
Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. (2020). Characteristics and Outcomes of Pregnant Women Admitted to Hospital with Confirmed SARS-CoV-2 Infection in UK: National Population Based Cohort Study. BMJ 369, m2107. doi:10.1136/bmj.m2107
Kotlyar A. M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., et al. (2021). Vertical Transmission of Coronavirus Disease 2019: a Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 224, 35–53. doi:10.1016/j.ajog.2020.07.049
Kumar M., Al Khodor S. (2020). Pathophysiology and Treatment Strategies for COVID-19. J. Transl. Med. 18, 353. doi:10.1186/s12967-020-02520-8
Kyle M. H., Hussain M., Saltz V., Mollicone I., Bence M., Dumitriu D. (2022). Vertical Transmission and Neonatal Outcomes Following Maternal SARS-CoV-2 Infection during Pregnancy. Clin. Obstet. Gynecol. 65, 195–202. doi:10.1097/GRF.0000000000000667
Lang G.-j., Zhao H. (2020). Can SARS-CoV-2-Infected Women Breastfeed after Viral Clearance? J. Zhejiang Univ. Sci. B 21, 405–407. doi:10.1631/jzus.B2000095
Lee D. H., Lee J., Kim E., Woo K., Park H. Y., An J. (2020). Emergency Cesarean Section Performed in a Patient with Confirmed Severe Acute Respiratory Syndrome Coronavirus-2 -a Case Report-. Korean J. Anesthesiol 73, 347–351. doi:10.4097/kja.20116
Li M., Chen L., Zhang J., Xiong C., Li X. (2020). The SARS-CoV-2 Receptor ACE2 Expression of Maternal-Fetal Interface and Fetal Organs by Single-Cell Transcriptome Study. PLoS One 15, e0230295. doi:10.1371/journal.pone.0230295
Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., et al. (2020). Maternal and Neonatal Outcomes of Pregnant Women with Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin. Infect. Dis. 71, 2035–2041. doi:10.1093/cid/ciaa352
Liguoro I., Pilotto C., Bonanni M., Ferrari M. E., Pusiol A., Nocerino A., et al. (2021). Correction to: SARS-COV-2 Infection in Children and Newborns: a Systematic Review. Eur. J. Pediatr. 180, 2343. doi:10.1007/s00431-021-03961-z
Liu H., Wang L.-L., Zhao S.-J., Kwak-Kim J., Mor G., Liao A.-H. (2020). Why Are Pregnant Women Susceptible to COVID-19? an Immunological Viewpoint. J. Reprod. Immunol. 139, 103122. doi:10.1016/j.jri.2020.103122
London V., McLaren R., Atallah F., Cepeda C., McCalla S., Fisher N., et al. (2020). The Relationship between Status at Presentation and Outcomes Among Pregnant Women with COVID-19. Am. J. Perinatol 37, 991–994. doi:10.1055/s-0040-1712164
Lowe B., Bopp B. (2020). COVID‐19 Vaginal Delivery - A Case Report. Aust. N. Z. J. Obstet. Gynaecol. 60, 465–466. doi:10.1111/ajo.13173
Lyra J., Valente R., Rosário M., Guimarães M. (2020). Cesarean Section in a Pregnant Woman with COVID-19: First Case in Portugal. Acta Med. Port 33, 429–431. doi:10.20344/amp.13883
Mahyuddin A. P., Kanneganti A., Wong J. J. L., Dimri P. S., Su L. L., Biswas A., et al. (2020). Mechanisms and Evidence of Vertical Transmission of Infections in Pregnancy Including SARS‐CoV ‐2s. Prenatal Diagn. 40, 1655–1670. doi:10.1002/pd.5765
Maltepe E., Fisher S. J. (2015). Placenta: the Forgotten Organ. Annu. Rev. Cel Dev. Biol. 31, 523–552. doi:10.1146/annurev-cellbio-100814-125620
Marín Gabriel M. Á., Malalana Martínez A. M., Marín Martínez M. E., Anel Pedroche J. (2020). Negative Transmission of SARS-CoV-2 to Hand-Expressed Colostrum from SARS-CoV-2-Positive Mothers. Breastfeed. Med. 15, 492–494. doi:10.1089/bfm.2020.0183
Mendoza M., Garcia‐Ruiz I., Maiz N., Rodo C., Garcia‐Manau P., Serrano B., et al. (2020). Pre‐eclampsia‐like Syndrome Induced by Severe COVID‐19: a Prospective Observational Study. Bjog: Int. J. Obstet. Gy. 127, 1374–1380. doi:10.1111/1471-0528.16339
Miranda J., Martín-Tapia D., Valdespino-Vázquez Y., Alarcón L., Espejel-Nuñez A., Guzmán-Huerta M., et al. (2019). Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability. Cells 8, 1174. doi:10.3390/cells8101174
Mithal L. B., Otero S., Shanes E. D., Goldstein J. A., Miller E. S. (2021). Cord Blood Antibodies Following Maternal Coronavirus Disease 2019 Vaccination during Pregnancy. Am. J. Obstet. Gynecol. 225, 192–194. doi:10.1016/j.ajog.2021.03.035
Moore K. M., Suthar M. S. (2021). Comprehensive Analysis of COVID-19 during Pregnancy. Biochem. Biophysical Res. Commun. 538, 180–186. doi:10.1016/j.bbrc.2020.12.064
Moro F., Buonsenso D., Moruzzi M. C., Inchingolo R., Smargiassi A., Demi L., et al. (2020). How to Perform Lung Ultrasound in Pregnant Women with Suspected COVID‐19. Ultrasound Obstet. Gynecol. 55, 593–598. doi:10.1002/uog.22028
Ouyang Y., Bagalkot T., Fitzgerald W., Sadovsky E., Chu T., Martínez-Marchal A., et al. (2021). Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin. MSphere 6, e00250–21. doi:10.1128/mSphere.00250-21
Patanè L., Morotti D., Giunta M. R., Sigismondi C., Piccoli M. G., Frigerio L., et al. (2020). Vertical Transmission of Coronavirus Disease 2019: Severe Acute Respiratory Syndrome Coronavirus 2 RNA on the Fetal Side of the Placenta in Pregnancies with Coronavirus Disease 2019-positive Mothers and Neonates at Birth. Am. J. Obstet. Gynecol. MFM 2, 100145. doi:10.1016/j.ajogmf.2020.100145
Patberg E. T., Adams T., Rekawek P., Vahanian S. A., Akerman M., Hernandez A., et al. (2021). Coronavirus Disease 2019 Infection and Placental Histopathology in Women Delivering at Term. Am. J. Obstet. Gynecol. 224, e1–382. doi:10.1016/j.ajog.2020.10.020
Penfield C. A., Brubaker S. G., Limaye M. A., Lighter J., Ratner A. J., Thomas K. M., et al. (2020). Detection of Severe Acute Respiratory Syndrome Coronavirus 2 in Placental and Fetal Membrane Samples. Am. J. Obstet. Gynecol. MFM 2, 100133. doi:10.1016/j.ajogmf.2020.100133
Peng L., Khan S., Ali A., Ahmed S., Ali L., Han G., et al. (2021). Vertical Transmission Potential of SARS-CoV-2 from Infected Mother to Twin Neonates. Future Virol. 16–382379. doi:10.2217/fvl-2020-0324
Pereira L. (2018). Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 5, 273–299. doi:10.1146/annurev-virology-092917-043236
Piccinni M.-P., Romagnani S. (1996). Regulation of Fetal Allograft Survival by Hormone-Controlled Th1- and Th2-type Cytokines. Immunol. Res. 15, 141–150. doi:10.1007/BF02918503
Pierce-Williams R. A. M., Burd J., Felder L., Khoury R., Bernstein P. S., Avila K., et al. (2020). Clinical Course of Severe and Critical Coronavirus Disease 2019 in Hospitalized Pregnancies: a United States Cohort Study. Am. J. Obstet. Gynecol. MFM 2, 100134. doi:10.1016/j.ajogmf.2020.100134
Porpora M. G., Merlino L., Masciullo L., D’Alisa R., Brandolino G., Galli C., et al. (2021). Does Lung Ultrasound Have a Role in the Clinical Management of Pregnant Women with SARS COV2 Infection? Ijerph 18, 2762. doi:10.3390/ijerph18052762
Puck J. M., Glezen W. P., Frank A. L., Six H. R. (1980). Protection of Infants from Infection with Influenza A Virus by Transplacentally Acquired Antibody. J. Infect. Dis. 142, 844–849. doi:10.1093/infdis/142.6.844
Qiancheng X., Jian S., Lingling P., Lei H., Xiaogan J., Weihua L., et al. (2020). Coronavirus Disease 2019 in Pregnancy. Int. J. Infect. Dis. 95, 376–383. doi:10.1016/j.ijid.2020.04.065
Ramsey P. S., Ramin K. D. (2001). Pneumonia in Pregnancy. Obstet. Gynecol. Clin. North America 28, 553–569. doi:10.1016/s0889-8545(05)70217-5
Rottenstreich A., Zarbiv G., Oiknine-Djian E., Vorontsov O., Zigron R., Kleinstern G., et al. (2022). The Effect of Gestational Age at BNT162b2 mRNA Vaccination on Maternal and Neonatal SARS-CoV-2 Antibody Levels. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. America, ciac135. doi:10.1093/cid/ciac135
Rubino F., Amiel S. A., Zimmet P., Alberti G., Bornstein S., Eckel R. H., et al. (2020). New-Onset Diabetes in Covid-19. N. Engl. J. Med. 383, 789–790. doi:10.1056/NEJMc2018688
Sahin D., Tanacan A., Erol S. A., Anuk A. T., Yetiskin F. D. Y., Keskin H. L., et al. (2021). Updated Experience of a Tertiary Pandemic center on 533 Pregnant Women with COVID‐19 Infection: A Prospective Cohort Study from Turkey. Int. J. Gynecol. Obstet. 152, 328–334. doi:10.1002/ijgo.13460
Schnettler W. T., Al Ahwel Y., Suhag A. (2020). Severe Acute Respiratory Distress Syndrome in Coronavirus Disease 2019-infected Pregnancy: Obstetric and Intensive Care Considerations. Am. J. Obstet. Gynecol. MFM 2, 100120. doi:10.1016/j.ajogmf.2020.100120
Schwartz D. A. (2020). An Analysis of 38 Pregnant Women with COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 144, 799–805. doi:10.5858/arpa.2020-0901-SA
Schwartz D. A., Morotti D., Beigi B., Moshfegh F., Zafaranloo N., Patanè L. (2020). Confirming Vertical Fetal Infection with Coronavirus Disease 2019: Neonatal and Pathology Criteria for Early Onset and Transplacental Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 from Infected Pregnant Mothers. Arch. Pathol. Lab. Med. 144, 1451–1456. doi:10.5858/arpa.2020-0442-SA
Schwartz D. A. (2017). Viral Infection, Proliferation, and Hyperplasia of Hofbauer Cells and Absence of Inflammation Characterize the Placental Pathology of Fetuses with Congenital Zika Virus Infection. Arch. Gynecol. Obstet. 295, 1361–1368. doi:10.1007/s00404-017-4361-5
Seethy A. A., Singh S., Mukherjee I., Pethusamy K., Purkayastha K., Sharma J. B., et al. (2021). Potential SARS-CoV-2 Interactions with Proteins Involved in Trophoblast Functions - an In-Silico Study. Placenta 103, 141–151. doi:10.1016/j.placenta.2020.10.027
Shanes E. D., Mithal L. B., Otero S., Azad H. A., Miller E. S., Goldstein J. A. (2020). Placental Pathology in COVID-19. Am. J. Clin. Pathol. 154, 23–32. doi:10.1093/ajcp/aqaa089
Shek C. C., Ng P. C., Fung G. P. G., Cheng F. W. T., Chan P. K. S., Peiris M. J. S., et al. (2003). Infants Born to Mothers with Severe Acute Respiratory Syndrome. Pediatrics 112, e254. doi:10.1542/peds.112.4.e254
Shimabukuro T. T., Kim S. Y., Myers T. R., Moro P. L., Oduyebo T., Panagiotakopoulos L., et al. (2021). Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 384, 2273–2282. doi:10.1056/NEJMoa2104983
Sigo Position Paper (2021). Gravidanza e Vaccinazione anti COVID. Avaialable at: https://www.sigo.it/comunicati-covid-19/position-paper-gravidanza-e-vaccinazione-anti-covid (Accessed May 6th, 2021).
Smith V., Seo D., Warty R., Payne O., Salih M., Chin K. L., et al. (2020). Maternal and Neonatal Outcomes Associated with Covid-19 Infection: a Systematic Review. PLoS One 15, e0234187. doi:10.1371/journal.pone.0234187
Stiehm E. R., Keller M. A. (2002). Breast Milk Transmission of Viral Disease. Adv. Nutr. Res. 10, 105–122. doi:10.1007/978-1-4615-0661-4_5
Szymczak A., Jędruchniewicz N., Torelli A., Kaczmarzyk-Radka A., Coluccio R., Kłak M., et al. (2021). Antibodies Specific to SARS-CoV-2 Proteins N, S and E in COVID-19 Patients in the normal Population and in Historical Samples. J. Gen. Virol. 102, 001692. doi:10.1099/jgv.0.001692
Taglauer E. S., Wachman E. M., Juttukonda L., Klouda T., Kim J., Wang Q., et al. (2022). Acute Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy Is Associated with Placental Angiotensin-Converting Enzyme 2 Shedding. Am. J. Pathol. 192, 595–603. doi:10.1016/j.ajpath.2021.12.011
Thomas P., Alexander P. E., Ahmed U., Elderhorst E., El-Khechen H., Mammen M. J., et al. (2020). Vertical Transmission Risk of SARS-CoV-2 Infection in the Third Trimester: A Systematic Scoping Review. J. Maternal-Fetal Neonatal Med. 1, 1–8. doi:10.1080/14767058.2020.1786055
Timircan M., Bratosin F., Vidican I., Suciu O., Tirnea L., Avram V., et al. (2021). Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection. Medicina 57, 796. doi:10.3390/medicina57080796
Valdés G., Neves L. A. A., Anton L., Corthorn J., Chacón C., Germain A. M., et al. (2006). Distribution of Angiotensin-(1-7) and ACE2 in Human Placentas of normal and Pathological Pregnancies. Placenta 27, 200–207. doi:10.1016/j.placenta.2005.02.015
Villalaín C., Herraiz I., Luczkowiak J., Pérez-Rivilla A., Folgueira M. D., Mejía I., et al. (2020). Seroprevalence Analysis of SARS-CoV-2 in Pregnant Women along the First Pandemic Outbreak and Perinatal Outcome. PLoS ONE 15, e0243029. doi:10.1371/journal.pone.0243029
Villar J., Ariff S., Gunier R. B., Thiruvengadam R., Rauch S., Kholin A., et al. (2021). Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and without COVID-19 Infection. JAMA Pediatr. 175, 817–826. doi:10.1001/jamapediatrics.2021.1050
Vivanti A. J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., et al. (2020). Transplacental Transmission of SARS-CoV-2 Infection. Nat. Commun. 11, 3572. doi:10.1038/s41467-020-17436-6
Wang M. J., Schapero M., Iverson R., Yarrington C. D. (2020). Obstetric Hemorrhage Risk Associated with Novel COVID-19 Diagnosis from a Single-Institution Cohort in the United States. Am. J. Perinatol 37, 1411–1416. doi:10.1055/s-0040-1718403
Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. (2020). Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 323, 1843–1844. doi:10.1001/jama.2020.3786
Wei S. Q., Bilodeau-Bertrand M., Liu S., Auger N. (2021). The Impact of COVID-19 on Pregnancy Outcomes: a Systematic Review and Meta-Analysis. CMAJ 193, E540–E548. doi:10.1503/cmaj.202604
Weinberger B. (2021). Vaccines and Vaccination against SARS-CoV-2: Considerations for the Older Population. Vaccines 9, 1435. doi:10.3390/vaccines9121435
Wong S. F., Chow K. M., Leung T. N., Ng W. F., Ng T. K., Shek C. C., et al. (2004). Pregnancy and Perinatal Outcomes of Women with Severe Acute Respiratory Syndrome. Am. J. Obstet. Gynecol. 191, 292–297. doi:10.1016/j.ajog.2003.11.019
World Health Organization (2020). Statement on the Second Meeting of the International Health Regulations Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Avaialable at: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (Accessed on January 31, 2022).
Xiong X., Wei H., Zhang Z., Chang J., Ma X., Gao X., et al. (2020). Vaginal Delivery Report of a Healthy Neonate Born to a Convalescent Mother with COVID-‐19. J. Med. Virol. 92, 1657–1659. doi:10.1002/jmv.25857
Yan J., Guo J., Fan C., Juan J., Yu X., Li J., et al. (2020). Coronavirus Disease 2019 in Pregnant Women: a Report Based on 116 Cases. Am. J. Obstet. Gynecol. 223, e1–111. doi:10.1016/j.ajog.2020.04.014
Yang H., Hu B., Zhan S., Yang L.-y., Xiong G. (2020). Effects of Severe Acute Respiratory Syndrome Coronavirus 2 Infection on Pregnant Women and Their Infants. Arch. Pathol. Lab. Med. 144, 1217–1222. doi:10.5858/arpa.2020-0232-SA
Yang R., Mei H., Zheng T., Fu Q., Zhang Y., Buka S., et al. (2020). Pregnant Women with COVID-19 and Risk of Adverse Birth Outcomes and Maternal-Fetal Vertical Transmission: a Population-Based Cohort Study in Wuhan, China. BMC Med. 18, 330. doi:10.1186/s12916-020-01798-1
Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., et al. (2020). Clinical Features and Obstetric and Neonatal Outcomes of Pregnant Patients with COVID-19 in Wuhan, China: a Retrospective, single-centre, Descriptive Study. Lancet Infect. Dis. 20, 559–564. doi:10.1016/S1473-3099(20)30176-6
Zaman K., Roy E., Arifeen S. E., Rahman M., Raqib R., Wilson E., et al. (2008). Effectiveness of Maternal Influenza Immunization in Mothers and Infants. N. Engl. J. Med. 359, 1555–1564. doi:10.1056/NEJMoa0708630
Zamaniyan M., Ebadi A., Aghajanpoor S., Rahmani Z., Haghshenas M., Azizi S. (2020). Preterm Delivery, Maternal Death, and Vertical Transmission in a Pregnant Woman with COVID‐19 Infection. Prenatal Diagn. 40, 1759–1761. doi:10.1002/pd.5713
Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., et al. (2020). Antibodies in Infants Born to Mothers with COVID-19 Pneumonia. JAMA 323, 1848–1849. doi:10.1001/jama.2020.4861
Keywords: SARS-CoV-2, coronavirus, placenta, pregnancy, vertical transmission, neonatal infection, pregnancy outcomes
Citation: Sessa R, Anastasi E, Brandolino G, Brunelli R, Di Pietro M, Filardo S, Masciullo L, Terrin G, Viscardi MF and Porpora MG (2022) What is the Hidden Biological Mechanism Underlying the Possible SARS-CoV-2 Vertical Transmission? A Mini Review. Front. Physiol. 13:875806. doi: 10.3389/fphys.2022.875806
Received: 14 February 2022; Accepted: 23 March 2022;
Published: 05 May 2022.
Edited by:
Giuseppe Ricci, University of Trieste, ItalyReviewed by:
Ran You, Nanjing Children’s Hospital, ChinaCopyright © 2022 Sessa, Anastasi, Brandolino, Brunelli, Di Pietro, Filardo, Masciullo, Terrin, Viscardi and Porpora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Grazia Porpora, bWFyaWFncmF6aWEucG9ycG9yYUB1bmlyb21hMS5pdA==
 Rosa Sessa
Rosa Sessa Emanuela Anastasi
Emanuela Anastasi Gabriella Brandolino
Gabriella Brandolino Roberto Brunelli
Roberto Brunelli Marisa Di Pietro
Marisa Di Pietro Simone Filardo
Simone Filardo Luisa Masciullo
Luisa Masciullo Gianluca Terrin
Gianluca Terrin Maria Federica Viscardi
Maria Federica Viscardi Maria Grazia Porpora
Maria Grazia Porpora