- 1Animal and Fish Production Department, College of Agricultural and Food Sciences, King Faisal University, Al Hofuf, Saudi Arabia
- 2Fish and Animal Production Department, Faculty of Agriculture (Saba Basha), Alexandria University, Alexandria, Egypt
- 3National Institute of Oceanography and Fisheries (NIOF), Cairo, Egypt
The current study examines the effect of dietary supplementation of ethanolic extract of Arthrospira platensis NIOF17/003, which is mainly natural astaxanthins (97.50%), on the growth performance, feed utilization, bacterial abundance, and immune-related and antioxidant gene expressions of the Pacific white leg shrimp, Litopenaeus vannamei. A total of 360 healthy L. vannamei postlarvae (0.19 ± 0.003 g) were divided into four groups (0, 2, 4, and 6 g natural astaxanthins/kg diet) each in three replicates, at an initial density of 30 PLs per tank (40 L capacity). The shrimp were fed the tested diets three times a day at a rate of 10% of their total body weight for 90 days. Diets supplemented with different astaxanthin levels significantly improved shrimp growth performance and feed conversion ratio compared to the control diet. No significant differences were observed in survival rates among all experimental groups. The immune-related genes (prophenoloxidase, lysozyme, beta-glucan binding protein, transglutaminase, and crustin) mRNA levels were significantly upregulated in groups fed with different concentrations of the natural astaxanthins in a dose-dependent manner. The prophenoloxidase gene is the highest immune-upregulated gene (14.71-fold change) in response to astaxanthin supplementation. The superoxide dismutase mRNA level was significantly increased with increasing dietary astaxanthin supplementation. In addition, increasing astaxanthin supplementation levels significantly reduced the count of heterotrophic bacteria and Vibrio spp. in the culture water and shrimp intestine. Overall, the current results concluded that diet supplementation with natural astaxanthin, extracted from Arthrospira platensis, enhanced the growth performance, immune response, and antioxidant status of L. vannamei.
Introduction
Shrimp is currently one of the most important aquatic animals worldwide. Due to the increase in global demand, shrimp culture has developed intensively and has priority among the leading aquaculture sectors in many countries (Lukwambe et al., 2019; Abbas et al., 2020). Among all penaeid shrimp species, the Pacific white leg shrimp, Litopenaeus vannamei, is the widest species being extensively cultured (Sharawy et al., 2022), accounting for more than 70% of the global shrimp production (Li et al., 2018). However, there are numerous barriers to sustaining aquaculture development globally, including the feed industry, pollution, low survival rates, climatic changes, and poor water quality (Abo-Taleb et al., 2020; Alprol et al., 2021a; Alprol et al., 2021c; Hassan et al., 2021). To cope with the global increase of intensive shrimp farming, the shrimp feed industry has been developed using several strategies. Among these strategies, feed additive supplementation is one of the most important industries that has gained great importance for several shrimp species as growth promoters, immunity enhancers, and an alternative strategy for disease-fighting (Sharawy et al., 2020; Sharawy et al., 2022).
Recently, due to their high concentration of natural bioactive compounds, algal cells (microalgae and seaweeds) have attract great attention for utilization as feed additives, showing improvement in growth performance, feed utilization, and immunity stimulation of cultured animal species, besides improving the water quality (Ashour et al., 2020; Ashour et al., 2021; Mabrouk et al., 2021; Zaki et al., 2021; Mansour et al., 2022). Depending on the algal strain, algal cells contain protein with high essential amino acid content, lipids with high unsaturated fatty acid levels, and carbohydrates (polysaccharides, etc.), which are necessary compounds in shrimp feeding, growth, and metamorphosis (Liñán-Cabello et al., 2003; Wade et al., 2017). Among all the microalgae strains, Arthrospira (a filamentous cyanobacterium) is the richest microalgae species in many phytochemicals (Mansour et al., 2021). Arthrospira platensis contains high levels of essential amino acids, fatty acids, minerals, and pigments like phycocyanin and astaxanthin, which have important biological functions and serve in several industries (Madkour et al., 2012; El-Shouny et al., 2015; Osman et al., 2016). It could be used for the replacement of fishmeal in the diet of Pacific white shrimp, Litopenaeus vannamei, and the obtained results did not show any significant differences with partial or total replacements in the growth performance and feed utilization levels. In addition, the PUFAs was increased significantly with A. platensis treatments and the survival of A. platensis supplemented groups was significantly increased under hypoxia challenge (Pakravan et al., 2017). The hot-water extract of A. platensis improved the growth, genes expression, immune response, and resistance of L. vannamei against Vibrio alginolyticus (Lin et al., 2010; Tayag et al., 2010). Besides phycocyanin, astaxanthin is the main carotenoid that exists in A. platensis (Gouveia et al., 2003; An et al., 2017).
Astaxanthin, a xanthophyll carotenoid, is a fat-soluble red pigment that has more significant biological activities than other carotenoids (Lim et al., 2018). Astaxanthins are extensively used as feed additives in diets of juveniles and adults of several shrimp species. It could be resulting in improved growth performance, survival, feed utilization, immunity responses, digestive enzyme activities, body composition, reproductive performance, spermatophore, egg, and larval qualities, and overcoming the pigment deficiency of Pacific white leg shrimp, L. vannamei (Niu et al., 2009; Pei et al., 2009; Chuchird et al., 2015), kuruma shrimp, Marsupenaeus japonicus (Wang et al., 2019), Penaeus monodon (Chien et al., 2003), red cherry shrimp, Neocaridina davidi (Tomas et al., 2020). In addition, astaxanthin dietary supplementation has positive effects on growth, molting cycle, free radical scavenging capacity, and nitrite stress tolerance of Penaeus japonicus postlarvae (Petit et al., 1997) and Pleoticus muelleri (Díaz et al., 2014). Furthermore, natural astaxanthins derived from the green seaweed, Enteromorpha intestinalis were used as feed additives to increase astaxanthin content in shrimp, Penaeus monodon, muscles (Mondal et al., 2015). In the present study, astaxanthin was extracted from local strain of A. platensis NIOF17/003 as available, cheap, and sustainable source that can be used in a commercial scale as feed additives in shrimp diets. Recently, A. platensis was used as an efficient source for astaxanthin that can be increased via environment conditions manipulation (Moradi et al., 2021) or inducing mutation (An et al., 2017).
The immune systems of crustaceans depend on innate immunity, that is, mediated by cellular and humoral effectors, which recognize invading microorganisms and trigger various defense mechanisms to eliminate pathogens (Söderhäll and Cerenius, 1992; Mansour et al., 2021; Sharawy et al., 2022). Humoral effectors include the prophenoloxidase system (ProPO), hemolymph clotting mechanism, melanization, and antimicrobial immune response (Jiravanichpaisal et al., 2006; Cerenius et al., 2008). The high immune surveillance of invertebrates could be associated with high amounts of hemolymph carotenoids, which could regulate genes expression of several immune-related genes, in particular, the ProPO gene (Cornet et al., 2007; Babin et al., 2010), CuZn superoxide dismutase (SOD) gene (Han et al., 2016), and other immune genes. Dietary astaxanthin could partially alleviate oxidative stress via inducing relatively higher gene expression levels of antioxidant enzymes in L. vannamei (Zhang et al., 2013). Meanwhile, the commercially farmed crustaceans did not have the internal mechanism for the de novo synthesis of astaxanthin and did not have the access to obtain different carotenoid sources from the environment (Higuera-Ciapara et al., 2006; Seabra and Pedrosa, 2010). Accordingly, dietary supplementation with astaxanthin is a necessity in the formulated diet. Therefore, the current study aimed to investigate the effects of increasing supplementation levels of the acetonic extract of the A. platensis NIOF17/003, which mainly consists of natural astaxanthins, as a feed additive on the growth performance, feed utilization, immune-related genes expression, and bacterial abundance of Pacific white leg shrimp.
Materials and Methods
Arthrospira platensis NIOF17/003
Cyanobacterium, Arthrospira platensis NIOF17/003 (GenBank accession number: MW396472), isolated from the El-Khadra saline-alkaline Lake, Wadi El-Natrun, Egypt, was molecularly identified, and cultivated, as well as its potential applications in different fields, were determined as described previously (Alprol et al., 2021b; Hassan et al., 2021; Mabrouk et al., 2021; Zaki et al., 2021).
Astaxanthins Extraction, Preparation, and Analysis
Natural astaxanthin, a carotenoid pigment, was extracted from the blue-green alga A. platensis NIOF17/003, according to Ju et al. (2009) with some modifications. Briefly, 1 kg of A. platensis fine dried powder was soaked in 100% acetone (10% w: v) and extracted three times on a rotary shaker for 72 h at 200 rpm in the dark at room temperature. The extracts were combined and filtered through filter paper (Whatman No. 1). Then the filtrates were concentrated using a rotary evaporator at 40°C under reduced pressure (Elshobary et al., 2020). The crude extract yield was weighed and calculated as a percentage of the initial sample weight. The yield of crude extract was stored at −20°C until further application. To determine the phytochemical profile of A. platensis crude extract, GC-Mass Spectrophotometry analysis was performed as previously described by Ashour et al. (2020). The unknown phytochemical compounds were identified based on comparing the obtained mass spectra with those available in the NIST library (National Institute of Standards and Technology, United States).
Experimental Animals
Pacific white leg shrimp, L. vannamei, postlarvae (PLs) were brought from a private commercial shrimp hatchery located in Borg El-Arab, Alexandria City, Egypt, to the indoor facilities of the Suez Branch, National Institute of Oceanography and Fisheries (NIOF-Suez). Firstly, the PLs were acclimated for 2 weeks in fiberglass tanks (500 L) under the same controlled conditions of the feeding trial (26–28°C, 31–32 ppt, and continuous aeration). During the acclimated period, PLs were fed a commercial diet (Aller-Aqua, Giza Governorate, Egypt). The compliance with ethical standards in the experimental setup and fish handling was approved by the Research Committee of the NIOF, Egypt.
Experimental Facilities and Design
According to a completely randomized design, the current feeding trial was performed in three replicates for each treatment. After 2 weeks of acclimation, 360 healthy PLs (0.19 ± 0.003 g) at an initial stocking density of 30 PLs per tank were handed out into 12 tanks (40 L capacity). During the experimental period (90 days), all PLs were fed three times a day (6:00, 12:00, and 18:00 h) at a rate of 10% of their total body weight. Every day before the first feeding, all tanks were siphoning to clean and remove the accumulated excreta and unconsumed feed. As a result of the siphoning process, 10% water was replaced daily with filtered, oxygenated seawater (Sharawy et al., 2020).
Water Quality Parameters
During the feeding trial, water quality parameters were checked, twice a week, for alkalinity (mg/L), NH3 (mg/L), PO4 (mg/L), NO3 (mg/L), and NO2 (mg/L) as described by APHA (2005). In addition, to maintain the water quality values as recommended for shrimp, the temperature (°C, using a mercury thermometer suspended at a depth of 30 cm), pH (using a pH meter, Orion, United States), and salinity (ppt, using a refractometer, United States) were investigated daily (1.00 p.m.) (Boyd and Tucker, 2012).
Diets Preparation
In the current feeding trial, four dietary supplementation treatments were performed for 90 days: D1, a control diet, that the shrimp was fed a commercial diet (Aller-Aqua, Giza Governorate, Egypt, as presented in Table 1). The other three diets (D2, D3, and D4) were fed diets supplemented with different levels of the natural astaxanthins (2, 4, and 6 g/kg diet, respectively), as a crude extract of A. platensis NIOF17/003. The addition of natural astaxanthins was performed as described by Mehrabi et al. (2012) with some modifications. The control diet was powdered and divided into four equal portions. The specific quantities of the natural astaxanthins (0, 2, 4, and 6 g/kg diet, respectively) were suspended well in 50 ml of corn oil and then sprayed well over the three powdered diets and mixed well. For the control diet (D1), the same volume of corn oil was sprayed without astaxanthins. Then, all four diets were re-pelletized in a pellet mill to obtain the proper diameter, dried at room temperature with forced air, and stored in plastic bags at 4°C until use.
Measured Parameters
Growth Performance and Nutrient Utilization Indices
At the end of the experiment, the PLs weights were recorded to determine the final body weight (FBW, g). Moreover, to determine the growth performance of Pacific white leg shrimp, L. vannamei, the weight gain (WG), feed conversion ratio (FCR), survival (%), and specific growth rate (SGR) were calculated using the following formulas:
where: Ln FBW and Ln IBW are the natural logarithm of final body weight (g) and initial body weight (g); while t is the time in days.
Whole-Body Proximate Chemical Analysis
At the end of the experiment, to determine the whole-body proximate chemical composition, five shrimp from each replicate were selected randomly and homogenized by a blender, dried in an oven, ground, and stored at −20°C for subsequent analysis. Both biochemical analyses of shrimp and diet were applied as described by the standard methods of AOAC (2003). The shrimp, dry matter, crude protein, crude fat, and crude ash were determined, while for diets, crude protein, crude fat, crude ash, and fibre were determined, and the nitrogen-free extract was calculated.
Bacterial Abundance Assessment
The bacterial abundance of water and shrimp intestines was performed according to APHA (2005). At the end of the experiment, three shrimp samples were chosen randomly from each replicate, and the intestines were aseptically extracted to estimate the bacterial count as described by Sharawy et al. (2020). The outwardly surface bacteria were removed by washing each gut three times with sterile distilled water. After that, they were washed in ethanol 96% and homogenized in a mortar separately. At the end of the experiment, samples of culture water (1 ml) and intestines (1 g) were taken from each treatment (three replicates) and supplied with sterile distilled water (9 ml). Later, make dilutions (1:10) and transferred 1–10 ml TSA (Trypticase soy agar) and TCBS (Thiosulphate-Citrate-Bile salts) agar plates and incubated at 37°C for TSA and 28°C TCBS (Sharawy et al., 2020). After 24 h, the colonies in each plate of the TSA and TCBS were counted, and the colonies of Vibrio spp. were confirmed using the 0129 test (Thermo Scientific™ Oxoid™ 0129 Discs) (Xie et al., 2020).
Immune-Related Gene Expressions Analysis
Triplicate samples of the shrimp abdominal muscles from each replicate were directly excised with fully sterile dissecting tools under cold conditions. The samples were kept at −80°C until gene expression analysis. Total RNA was extracted from the samples using the TRIzol method (easy-RED, iNtRON Biotechnology) as directed by the manufacturer. The OD ratio at 260/280 nm of RNA purity was determined using a NanoDrop system (BioDrop), and the samples with the highest ratio (A260/A280 1.8) were used for cDNA synthesis (1 ng/µl) for each reaction. Total RNA was treated with DNase I (NEB, United States) as the template for the synthesis of first-strand cDNA using reverse transcriptase (RT-PCR beads, Enzynomics, Korea), and the reaction was carried out using PCR amplification (Applied Biosystems Veriti 96-Well Thermal Cycler, United States) under the manufacturer’s conditions. The following cDNA was used in the Real-Time PCR reaction (Bico, Thermo-Fisher): initial denaturation at 95°C for 15 min, 40 cycles with the following parameters (95°C, 10 s; 58–62°C, 20 s; and 72°C, 30 s). Unique and specific products were seen as a melting curve at the end of the last cycle when the temperature increased from (58–62–95°C) in increments of 0.5°C. The studied immune-related genes were prophenoloxidase (proPO), lysozyme (Lys), beta-glucan binding protein (Bgp), superoxide dismutase (SOD), transglutaminase (TGase), and crustin (Crus), and their primers were presented in Table 2. The housekeeping gene (β-actin) was used to measure gene expression or fold shift of the target genes (Yang et al., 2013). The values give n-fold difference relative to the calibrator (control) when the 2ΔΔCt method is applied in normalizing the critical threshold (Ct) quantities of target genes with quantities β-actin (Livak and Schmittgen, 2001).
Statistical Analysis
The experiment was performed in triplicates and the results of growth performances were presented as the means ± standard deviation (SD). The normality and homogeneity assumptions were confirmed before the statistical analysis of the data. Before analysis, all results in percentages were arc-sin transformed (Zar, 1984). Using the IBM SPSS Statistics software (IBM, v.23), statistical analysis was performed by the One-Way Analysis of Variance (ANOVA), followed by Duncan’s post-hoc test, at a significant p ≤ 0.05.
Results
Astaxanthin of A. platensis NIOF17/003
The yield of crude extract of A. platensis NIOF17/003 was weighed and calculated as a percentage of the initial weight. The calculated final yield concentration was 27 g/kg (2.7%). The GC-MS analysis of the crude extract of A. platensis NIOF17/003 shows three main phytochemical compounds belonging to three retention times (Table 3). These different bioactive compounds were astaxanthin (C40H52O4, exact molecular weight: 596.38) with the highest peak area (97.50%) and the highest probability (21.40%). The peak area and probability of the other two bioactive compounds (C35H42N6O2 and C34H44ClN5O2) were 0.38%, and 0.65%, respectively, and the probability was 7.07%, and 6.60%, respectively (Table 3). The chemical structure of these three phytochemicals were identified using the NIST library as shown in Figure 1.
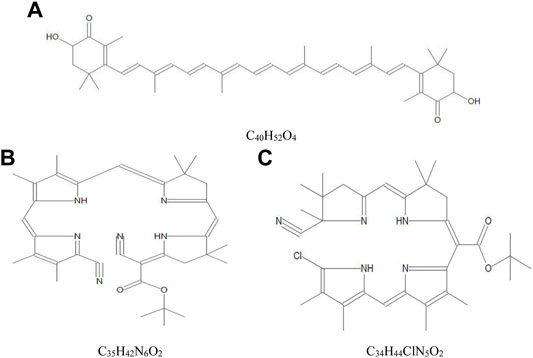
FIGURE 1. Mass spectra and chemical structure of the three phytochemical compounds identified in crude acetonic extract of Arthrospira platensis NIOF17/003. (A): Astaxanthin; (B) Cyano 5-{5-[5-(5-cyano-3,4-dimethylpyrrol-2-ylidenemethyl)-3,4-dimethyl-1H-pyrrol-2-ylmethylene]-4,4-dimethyl-4,5-dihydro-3H-pyrro-2-ylmethylene}-4,4-dimethylpyrrolidin-2-ylene-acetic acid, tert.-butyl ester; and (C): [5-(5-Chloro-3,4-dimethyl-1H-pyrrol-2-ylmethylene)-3,4-dimethyl-5H-pyrrol-2-yl]-[5-(5-cyano-4,4,5-trimethyl-4,5-dihydro-3H-pyrrol-2-ylmethylene)-4,4-dimethylpyrrolidin-2-ylene]-acetic acid, tert.-butyl ester.
Water Quality Parameters
Table 4 shows the water quality parameters during the experiments. The results revealed that all recorded water quality conditions (°C, pH, salinity, alkalinity, NH3, PO4, NO3, and NO2) were in the recommended ranges for shrimp culture. No significant difference was observed among fish fed the control diet and the diets supplemented with different concentrations of astaxanthins.
Growth Performance and Nutrient Utilization Indices
Table 5 shows the effect of dietary supplementation of astaxanthin on the growth performance and feed utilization of L. vannamei juveniles. Diets supplemented with different concentrations of astaxanthins (D2, D3, and D4) experienced a significant (p < 0.05) improvement of FW, WG, and FCR compared to the control diet. On the other hand, no significant differences (p < 0.05) were obtained in survival or SGR among the diets supplemented with astaxanthins (D2, D3, and D4) and the control. While, the response of shrimp, in terms of WG and FCR to increasing inclusion levels of dietary astaxanthin supplementation showed a linear regression pattern with a strong correlation for WG (r2 = 0.9112) and a moderate correlation for FCR (r2 = 0.6867), as presented in Figure 2.
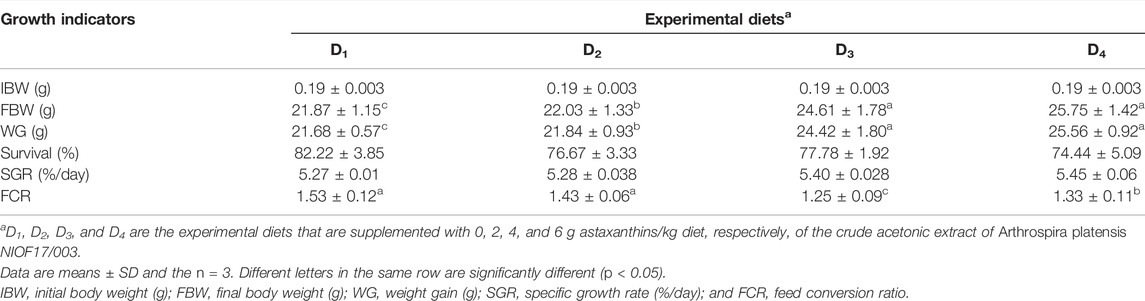
TABLE 5. Growth performance and feed utilization of Pacific white leg shrimp, Litopenaeus vannamei, fed experimental diets for 90 days.
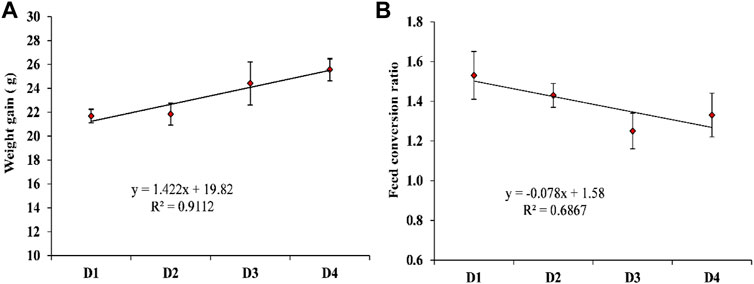
FIGURE 2. The appropriate regression models of the increasing inclusion levels of dietary astaxanthin supplementation for the (A) weight gain; (B) food conversion ratio of L. vannamei. D1, D2, D3, and D4 are the experimental diets that are supplemented with 0, 2, 4, and 6 g astaxanthin/kg diet, respectively, of the natural astaxanthins of the crude acetonic extract of Arthrospira platensis NIOF17/003. Data are means ± SD and the n = 3.
Body Proximate Analysis
As presented in Table 6, there are significant differences (p > 0.05) that were reported in the whole-body chemical composition (dry matter, protein, fat, and ash content) of shrimp L. vannamei. The control group had the highest significant (p < 0.05) values of dry matter and crude protein content. While D4 showed the highest significant (p < 0.05) impacts on fat and ash content (Table 6).
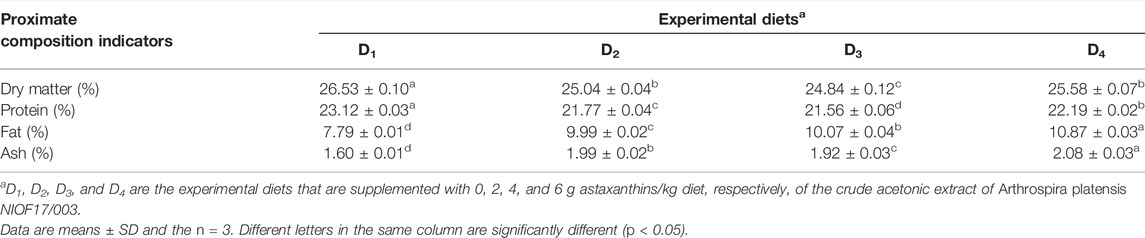
TABLE 6. Proximate whole-body proximate analysis (% of wet weight) of Pacific white leg shrimp, Litopenaeus vannamei, fed experimental diets for 90 days.
Bacterial Abundance Investigations
The effects of experimental diets supplemented with astaxanthin on both total heterotrophic bacteria (THB) and total Vibrio spp. count (TVC) in the water and intestine of L. vannamei are shown in Table 7. It was observed that the degradative heterotrophic bacteria were more abundant in the intestine than in water. Concerning pathogenic bacteria, the genus Vibrio spp. was chosen as an indicator for the pathogenicity of shrimp, and the count was lower than heterotrophic bacteria. However, when compared to the control, the THB count in the water and intestine decreased gradually as astaxanthin supplementation levels increased.
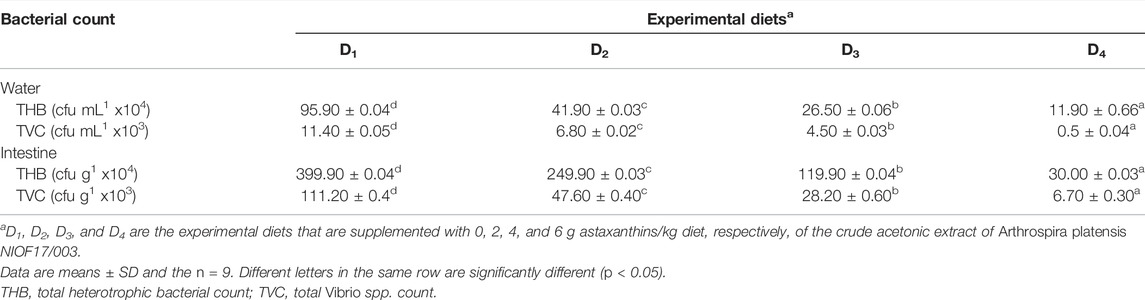
TABLE 7. Bacterial abundance in the culture water and intestine of Pacific white leg shrimp, Litopenaeus vannamei, fed experimental diets for 90 days.
Immune-Related Gene Expressions
Dietary inclusion of astaxanthin enhanced the expression of all studied genes: Bgp, Lys, proPO, TGases, Crus, and SOD in the muscle tissue of L. vannamei at the end of the feeding trial (Figure 3). The expression of the Bgp gene was significantly increased in the dietary supplemented treatment with astaxanthin at a level of 4 g/kg compared to the control group and D2 even though the expression in D3 was higher than D4. Generally, gene expression of Lys, proPO, TGases, Crus, and SOD was significantly upregulated (p < 0.05) with increasing the concentration level of natural astaxanthins in the diet compared to the control. The proPO gene expression was the most upregulated gene among the other genes, and the relative fold change was 14.71 compared to the control group.
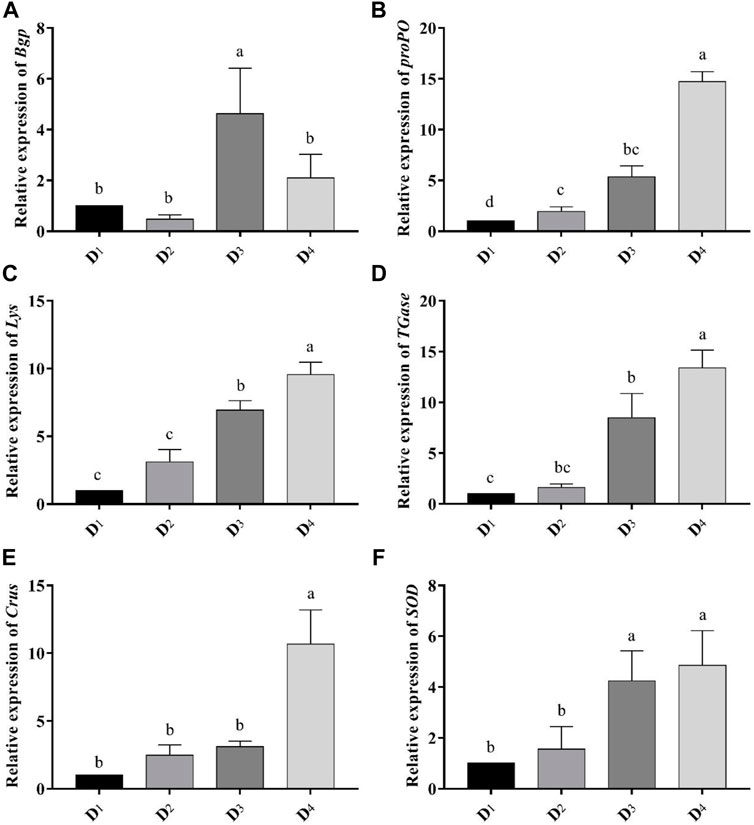
FIGURE 3. Analysis of immune-related gene-expressions [Bgp (A), proPO (B), Lys (C), TGase (D), Crus (E), and SOD (F)] comparing to the expression of β-actin gene in the different inclusion levels of astaxanthin dietary supplementation. D1, D2, D3, and D4 are the experimental diets that are supplemented with 0, 2, 4, and 6 g astaxanthins/kg diet, respectively, of the crude acetonic extract of Arthrospira platensis NIOF17/003. The data are means ± SD and the n = 9. Different letters for the same gene indicated a significantly differences (p < 0.05).
Discussion
Recently, with the growing development of aquaculture, feed additives demand is globally increased, resulting in an enormous space for the industrial application of natural astaxanthin (Lu et al., 2021). Generally, natural astaxanthins are extracted from different aquatic organisms such as shrimp (Scurria et al., 2020), soft coral (Metwally et al., 2020), fish (Yu and Liu, 2020), seaweeds (Teramukai et al., 2020), and microalgae (Molino et al., 2018). Among all aquatic organisms, microalgae have the highest ability to produce astaxanthin than any aquatic animal (Gallego et al., 2019). Microalgae are high productive microorganisms, carbon captures, and oxygen producers. Therefore, the reliance on the production of microalgae-based astaxanthin has positive environmental impacts in mitigating the effects of global warming and creating environmental benefits (Lee and Ding, 1994; Wu et al., 2017).
In the present study, the final yield of crude extract, mainly astaxanthin, of A. platensis was 2.7%. Compared to other microalgal species, Haematococcus pluvialis is considered the most widespread species that produces natural astaxanthin with a high final yield ranging from 3.8%–4% (Lee and Ding, 1994; Aflalo et al., 2007; Ranga Rao et al., 2010). Meanwhile, the final yield of natural astaxanthin in the present study exceeded that reported in several microalgae species, such as Chlorella zofingiensis, Neochloris wimmeri, Chlorococcum sp., Botryococcus braunii, Tetraselmis sp., and Scenedesmus obliquus, which produced 0.68% (Orosa et al., 2001), 0.60% (Orosa et al., 2000), 0.20% (Zhang et al., 1997), 0.01% (Grung and Metzger, 1994), 0.23% (Lim et al., 2018), and 0.30% (Qin et al., 2008), respectively. The yields of astaxanthin among different microalgae species vary due to several reasons, such as strain, extraction solvent, and extraction conditions. Accordingly, A. platensis NIOF17/003 could be considered as a source for producing natural astaxanthin.
Astaxanthin has been used as a dietary supplementation for aquatic animals (Niu et al., 2009; Lim et al., 2018). In the current study, the FW and WG, were significantly increased with the increase of astaxanthin supplementation levels compared to the control. In accordance with the present findings, Flores et al. (2007) reported that the inclusion of 80 mg/kg of synthetic astaxanthin enhanced the growth, survival, and molting frequency of P. vannamei. In addition, the inclusion of natural astaxanthin extracted from H. pluvialis significantly improves the growth performance, pigmentation, and antioxidant capacity of the white prawn Exopalaemon carinicauda (Zhang et al., 2021). Astaxanthins extracted from H. pluvialis and A. platensis strain Pacifica improved shrimp kuruma and M. Japonicus growth and hypoxia stress resistance (Chien and Shiau, 2005).
The maintenance of high immune surveillance is one of the crucial measures of successful aquatic cultured animals (Mansour et al., 2018; Mansour et al., 2020). In crustaceans, the innate immune system is the main animal defence to any pathogen. This defence is mediated by several kinds of cells, enzymes, and antimicrobial peptides (Huang et al., 2020). The present finding revealed an upregulation of the Bgp gene in shrimp fed 4 and 6 g astaxanthin/kg diets, especially with the 4 g/kg diet. The Bgp gene works as a vital factor for activation of the proPO system, coagulation progression, and expression of antimicrobial peptides after recognizing the microbial components (Goncalves et al., 2012). The Bgp gene showed a delayed upregulation in L. vannamei fed immunostimulant b-1, 3-glucan from Schizophyllum commune daily for a 1-week feeding trial (Wang et al., 2008). This contrast with the present findings could be due to the short treatment period with b-1,3-glucan than astaxanthin and the different mode of action of both treatments.
In addition, the proPO gene expression was significantly increased in shrimp fed 4 and 6 g/kg astaxanthin supplemented diets compared to the control group, and it was approximately 14-fold higher than the control, in the present study. Furthermore, the expression of proPO is the highest among all studied immune-related genes in the present study with increasing the concentration of astaxanthin (6 g/kg diet). Whereas, one of the most important components of the shrimp immune system is prophenoloxidase (Sritunyalucksana and Söderhäll, 2000).
Lysozyme can hydrolyze bacterial cell walls and operates as a non-specific innate defense molecule against bacterial infections. It has been demonstrated that it activates in penaeid shrimp in response to Vibrio, and its gene has been cloned and characterized in L. vannamei and M. japonicas (Hikima et al., 2003). In the present study, Lys gene expression in L. vannamei was increased gradually in the shrimps that fed with the three levels of astaxanthin supplemented diets. It was about 9-fold higher in the D4 treatment (6 g/kg diet) than in the control group. Kuruma shrimp fed a diet supplemented with astaxanthin experienced higher lysozyme activity, and total hemocyte count and improved the survival of shrimp against low salinity levels (Wang et al., 2019). Transglutaminase is recognized as an invertebrate defense mechanism. TGase gene silencing has previously been demonstrated to make shrimp susceptible to both bacterial and viral infections, indicating that TGase is an important component of the shrimp immune system (Fagutao et al., 2012). In this study, supplemented diets with natural astaxanthin influenced TGase gene expression and showed a significant upregulation in the fish fed 6 g/kg astaxanthin supplemented diet compared to the control group. In the same manner, Crust gene expression was improved with dietary supplementation of astaxanthin in a dose-dependent manner. Crustin is one of the antimicrobial peptides in penaeid shrimps hemolymph. After oral treatment for 7 days with peptidoglycan, a significant increase in crustin mRNA levels in M. japonicas was reported (Rattanachai et al., 2004). In the same line, Pacific white shrimp L. vannamei, fed diet supplemented with 80 mg astaxanthin/kg diet significantly improved serum phenoloxidase activity serum bacteriolytic activity, total haemocyte counts, and phagocytic activity (Wang et al., 2015). In addition, the antioxidant prosperities of astaxanthin (Figure 3F) could directly participate in the immune enhancement in L. vannamei. However, the immune-stimulating activity of astaxanthin in crustaceans still needs more investigation to better understand its mode of action.
Superoxide dismutase (SOD) is one of the main antioxidant enzymes responsible for scavenging reactive oxygen species and is considered a safeguarding mechanism inside the tissue that could be damaged by oxidation processes and phagocytosis (Chien et al., 2003). Because of its unique chemical structure, astaxanthin has the potential to has antioxidant effects, including free radicals scavenging and activating the expression and activities of several antioxidant enzymes (Eren et al., 2019; Yu et al., 2021). In our study, the expression of SOD was significantly upregulated in shrimp fed astaxanthins at levels of 4 and 6 g/kg compared to the control group. In line with the current findings, astaxanthin supplementation increased the expression levels of Cyt-Mn SOD, CAT, and GPx genes (Liu et al., 2018) and SOD activity in L. vannamei (Chuchird et al., 2015). Whereas, astaxanthins as a carotenoids reported to protect white blood cells from oxidative damage, enhancing cell-mediated, and humoral immune responses of vertebrates and invertebrates (Song et al., 2020). This refers to the antioxidant activity of carotenoids that may be involved in the immunomodulatory action by quenching singlet oxygen and free radicals (Cvetkovic et al., 2013).
Generally, the contents of the gut microbiota have a strong influence on the health of aquatic organisms (Sharawy et al., 2020), such as digestion, nutrient absorption, immunity responses, and biological antibiosis (Li et al., 2018). The intestinal bacteria respond quickly to changes in food consumption, diet composition, and ingredients (Ringø et al., 2016). In the current study, the counts of THB and TVC were significantly (p > 0.05) decreased with increasing the inclusion levels of astaxanthin, compared to the control diet. Whereas, the bacterial abundance of Vibrio spp. was decreased in all astaxanthin supplemented diets compared to the control. Chuchird et al. (2015) reported an increase in the survival, growth, and resistance to V. parahaemolyticus of L. vannamei fed an astaxanthin supplemented diet. Furthermore, shrimp-fed diets supplemented with astaxanthin had significantly lower total intestinal bacteria and Vibrio spp. counts (Chuchird et al., 2015). The mode of action by which astaxanthin affected the bacterial population still not clear and could need more investigation. All these indications are in line with our findings, which revealed that astaxanthin is a promising substance for controlling the pathogenic bacteria load during the whole culture process of shrimp. These results were attributed to the high biological activities of acetonic extract of A. platensis NIOF17/003, mainly astaxanthin, which make it a wonderful, sustainable, and eco-friendly feed additive for aquaculture applications.
Conclusion
From the current findings, it could be concluded that the Arthrospira platensis NIOF17/003 strain (Accession GenBank number: MW396472) is a good source of astaxanthin with a high final yield of about 2.7%. Dietary supplementation with natural astaxanthin enhanced the growth, feed utilization, and chemical composition of Pacific white leg shrimp, Litopenaeus vannamei. In addition, astaxanthin proved a powerful immune stimulant, antioxidant, and antibacterial substance for L. vanami. More research is needed to determine the mechanism of astaxanthin’s immunostimulant effects in shrimp, including cytokines mediated humoral and cellular innate immunity.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Ethics Statement
The animal study was reviewed and approved by the Research Committee of the National Institute of Oceanography and Fisheries, Egypt.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. GRANT797).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas E. M., Ali F. S., Desouky M. G., Ashour M., El-Shafei A., Maaty M. M., et al. (2020). Novel Comprehensive Molecular and Ecological Study Introducing Coastal Mud Shrimp (Solenocera Crassicornis) Recorded at the Gulf of Suez, Egypt. J. Mar. Sci. Eng. 9 (1), 9. doi:10.3390/jmse9010009
Abo-Taleb H., Ashour M., El-Shafei A., Alataway A., Maaty M. M. (2020). Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf). Water 12 (3), 656. doi:10.3390/w12030656
Aflalo C., Meshulam Y., Zarka A., Boussiba S. (2007). On the Relative Efficiency of Two- vs. One-Stage Production of Astaxanthin by the Green algaHaematococcus pluvialis. Biotechnol. Bioeng. 98 (1), 300–305. doi:10.1002/bit.21391
Alprol A. E., Ashour M., Mansour A. T., Alzahrani O. M., Mahmoud S. F., Gharib S. M. (2021a). Assessment of Water Quality and Phytoplankton Structure of Eight Alexandria Beaches, Southeastern Mediterranean Sea, Egypt. J. Mar. Sci. Eng. 9 (12), 1328. doi:10.3390/jmse9121328
Alprol A. E., Heneash A. M. M., Ashour M., Abualnaja K. M., Alhashmialameer D., Mansour A. T., et al. (2021b). Potential Applications of Arthrospira Platensis Lipid-free Biomass in Bioremediation of Organic Dye from Industrial Textile Effluents and its Influence on Marine Rotifer (Brachionus plicatilis). Materials 14 (16), 4446. doi:10.3390/ma14164446
Alprol A. E., Heneash A. M. M., Soliman A. M., Ashour M., Alsanie W. F., Gaber A., et al. (2021c). Assessment of Water Quality, Eutrophication, and Zooplankton Community in Lake Burullus, Egypt. Diversity 13 (6), 268. doi:10.3390/d13060268
An J., Gao F., Ma Q., Xiang Y., Ren D., Lu J. (2017). Screening for Enhanced Astaxanthin Accumulation Among Spirulina Platensis Mutants Generated by Atmospheric and Room Temperature Plasmas. Algal Res. 25, 464–472. doi:10.1016/j.algal.2017.06.006
AOAC (2003). Official Methods of Analysis. The Association of Official Analytical Chemists, Vol.I.17thed. Washington, DC, USA.
APHA (2005). Standard Methods for the Examination of Water and Wastewater. Washington, DC, USA: American Public Health Association.
Ashour M., Mabrouk M. M., Abo-Taleb H. A., Sharawy Z. Z., Ayoub H. F., Van Doan H., et al. (2021). A Liquid Seaweed Extract (TAM) Improves Aqueous Rearing Environment, Diversity of Zooplankton Community, whilst Enhancing Growth and Immune Response of Nile tilapia, Oreochromis niloticus, Challenged by Aeromonas Hydrophila. Aquaculture 543, 736915. doi:10.1016/j.aquaculture.2021.736915
Ashour M., Mabrouk M. M., Ayoub H. F., El-Feky M. M. M. M., Zaki S. Z., Hoseinifar S. H., et al. (2020). Effect of Dietary Seaweed Extract Supplementation on Growth, Feed Utilization, Hematological Indices, and Non-specific Immunity of Nile Tilapia, Oreochromis niloticus Challenged with Aeromonas Hydrophila. J. Appl. Phycol. 32 (5), 3467–3479. doi:10.1007/s10811-020-02178-1
Babin A., Biard C., Moret Y. (2010). Dietary Supplementation with Carotenoids Improves Immunity without Increasing its Cost in a Crustacean. Am. Nat. 176 (2), 234–241. doi:10.1086/653670
Boyd C. E., Tucker C. S. (2012). Pond Aquaculture Water Quality Management. Springer Science & Business Media.
Cerenius L., Lee B. L., Söderhäll K. (2008). The proPO-System: Pros and Cons for its Role in Invertebrate Immunity. Trends Immunol. 29 (6), 263–271. doi:10.1016/j.it.2008.02.009
Chien Y.-H., Pan C.-H., Hunter B. (2003). The Resistance to Physical Stresses by Penaeus monodon Juveniles Fed Diets Supplemented with Astaxanthin. Aquaculture 216 (1-4), 177–191. doi:10.1016/S0044-8486(02)00056-X
Chien Y.-H., Shiau W.-C. (2005). The Effects of Dietary Supplementation of Algae and Synthetic Astaxanthin on Body Astaxanthin, Survival, Growth, and Low Dissolved Oxygen Stress Resistance of Kuruma Prawn, Marsupenaeus japonicus Bate. J. Exp. Mar. Biol. Ecol. 318 (2), 201–211. doi:10.1016/j.jembe.2004.12.016
Chuchird N., Rorkwiree P., Rairat T. (2015). Effect of Dietary Formic Acid and Astaxanthin on the Survival and Growth of Pacific White Shrimp (Litopenaeus Vannamei) and Their Resistance to Vibrio parahaemolyticus. SpringerPlus 4 (1), 440–512. doi:10.1186/s40064-015-1234-x
Cornet S., Biard C., Moret Y. (2007). Is There a Role for Antioxidant Carotenoids in Limiting Self-Harming Immune Response in Invertebrates? Biol. Lett. 3 (3), 284–288. doi:10.1098/rsbl.2007.0003
Cvetkovic D., Fiedor L., Fiedor J., Wiśniewska-Becker A., Markovic D. (2013). Molecular Base for Carotenoids Antioxidant Activity in Model and Biological Systems: The Health-Related Effects. NY, USA: Nova Science Publishers: Hauppauge.
Díaz A. C., Velurtas S. M., Espino M. L., Fenucci J. L. (2014). Effect of Dietary Astaxanthin on Free Radical Scavenging Capacity and Nitrite Stress Tolerance of Postlarvae Shrimp, Pleoticus Muelleri. J. Agric. Food Chem. 62 (51), 12326–12331. doi:10.1021/jf503754q
El-Shouny W., Sharaf M., Abomohra A., Abo-Eleneen M. (2015). Production Enhancement of Some Valuable Compounds of Arthrospira Platensis. J. Basic Environ. Sci. 2, 74–83.
Elshobary M. E., El-Shenody R. A., Ashour M., Zabed H. M., Qi X. (2020). Antimicrobial and Antioxidant Characterization of Bioactive Components from Chlorococcum Minutum. Food Biosci. 35, 100567. doi:10.1016/j.fbio.2020.100567
Eren B., Tuncay Tanrıverdi S., Aydın Köse F., Özer Ö. (2019). Antioxidant Properties Evaluation of Topical Astaxanthin Formulations as Anti‐aging Products. J. Cosmet. Dermatol 18 (1), 242–250. doi:10.1111/jocd.12665
Fagutao F. F., Maningas M. B. B., Kondo H., Aoki T., Hirono I. (2012). Transglutaminase Regulates Immune-Related Genes in Shrimp. Fish shellfish Immunol. 32 (5), 711–715. doi:10.1016/j.fsi.2012.01.018
Flores M., Díaz F., Medina R., Re A. D., Licea A. (2007). Physiological, Metabolic and Haematological Responses in White Shrimp Litopenaeus Vannamei (Boone) Juveniles Fed Diets Supplemented with Astaxanthin Acclimated to Low-Salinity Water. Aquac. Res. 38 (7), 740–747. doi:10.1111/j.1365-2109.2007.01720.x
Gallego R., Bueno M., Herrero M. (2019). Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-By-Products, Seaweeds and Microalgae - an Update. TrAC Trends Anal. Chem. 116, 198–213. doi:10.1016/j.trac.2019.04.030
Goncalves P., Vernal J., Rosa R. D., Yepiz-Plascencia G., de Souza C. R. B., Barracco M. A., et al. (2012). Evidence for a Novel Biological Role for the Multifunctional β-1,3-glucan Binding Protein in Shrimp. Mol. Immunol. 51 (3-4), 363–367. doi:10.1016/j.molimm.2012.03.032
Gouveia L., Rema P., Pereira O., Empis J. (2003). Colouring Ornamental Fish (Cyprinus carpio and Carassius auratus ) with Microalgal Biomass. Aquac. Nutr. 9 (2), 123–129. doi:10.1046/j.1365-2095.2003.00233.x
Grung M., Metzger P., Liaaen-Jensen S. v. (1994). Algal Carotenoids 53; Secondary Carotenoids of Algae 4; Secondary Carotenoids in the Green Alga Botryococcus Braunii, Race L, New Strain. Biochem. Syst. Ecol. 22 (1), 25–29. doi:10.1016/0305-1978(94)90111-2
Han J., Lu Y., Zheng H., Liu H., Deng H., Zhang B. (2016). Differential Expression of CuZnSOD Gene under Low Temperature Stress in Noble Scallop Chlamys Nobilis with Different Carotenoid Content. Fish shellfish Immunol. 54, 30–39. doi:10.1016/j.fsi.2016.03.160
Hassan S. M., Ashour M., Sakai N., Zhang L., Hassanien H. A., Gaber A., et al. (2021). Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis Sativus). Agriculture 11 (4), 320. doi:10.3390/agriculture11040320
Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F. M. (2006). Astaxanthin: a Review of its Chemistry and Applications. Crit. Rev. food Sci. Nutr. 46 (2), 185–196. doi:10.1080/10408690590957188
Hikima S., Hikima J.-i., Rojtinnakorn J., Hirono I., Aoki T. (2003). Characterization and Function of Kuruma Shrimp Lysozyme Possessing Lytic Activity against Vibrio Species. Gene 316, 187–195. doi:10.1016/S0378-1119(03)00761-3
Huang Z., Aweya J. J., Zhu C., Tran N. T., Hong Y., Li S., et al. (2020). Modulation of Crustacean Innate Immune Response by Amino Acids and Their Metabolites: Inferences from Other Species. Front. Immunol. 11 (2812). doi:10.3389/fimmu.2020.574721
Jiravanichpaisal P., Lee B. L., Söderhäll K. (2006). Cell-mediated Immunity in Arthropods: Hematopoiesis, Coagulation, Melanization and Opsonization. Immunobiology 211 (4), 213–236. doi:10.1016/j.imbio.2005.10.015
Ju Z. Y., Forster I. P., Dominy W. G. (2009). Effects of Supplementing Two Species of Marine Algae or Their Fractions to a Formulated Diet on Growth, Survival and Composition of Shrimp (Litopenaeus Vannamei). Aquaculture 292 (3-4), 237–243. doi:10.1016/j.aquaculture.2009.04.040
Lee Y.-K., Ding S.-Y. (1994). Cell Cycle and Accumulation of Astaxanthin in Haematococcus Lacustris (Chlorophyta)1. J. Phycol. 30 (3), 445–449. doi:10.1111/j.0022-3646.1994.00445.x
Li E., Xu C., Wang X., Wang S., Zhao Q., Zhang M., et al. (2018). Gut Microbiota and its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus Vannamei. Rev. Fish. Sci. Aquac. 26 (3), 381–399. doi:10.1080/23308249.2018.1440530
Lim K. C., Yusoff F. M., Shariff M., Kamarudin M. S. (2018). Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquacult 10 (3), 738–773. doi:10.1111/raq.12200
Lin Y.-C., Tayag C. M., Huang C.-L., Tsui W.-C., Chen J.-C. (2010). White Shrimp Litopenaeus Vannamei that Had Received the Hot-Water Extract of Spirulina Platensis Showed Earlier Recovery in Immunity and Up-Regulation of Gene Expressions after pH Stress. Fish shellfish Immunol. 29 (6), 1092–1098. doi:10.1016/j.fsi.2010.09.002
Liñán-Cabello M. A., Paniagua-Michel J., Zenteno-Savín T. (2003). Carotenoids and Retinal Levels in Captive and Wild Shrimp,Litopenaeus Vannamei. Aquac. Nutr. 9 (6), 383–389. doi:10.1046/j.1365-2095.2003.00267.x
Liu X., Wang B., Li Y., Wang L., Liu J. (2018). Effects of Dietary Botanical and Synthetic Astaxanthin on E/Z and R/S Isomer Composition, Growth Performance, and Antioxidant Capacity of White Shrimp, Litopenaeus Vannamei, in the Nursery Phase. Invertebr. Surviv. J. 15 (1), 131–140. doi:10.25431/1824-307X/isj.v15i1.131-140
Livak K. J., Schmittgen T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25 (4), 402–408. doi:10.1006/meth.2001.1262
Lu Q., Li H., Zou Y., Liu H., Yang L. (2021). Astaxanthin as a Microalgal Metabolite for Aquaculture: A Review on the Synthetic Mechanisms, Production Techniques, and Practical Application. Algal Res. 54, 102178–178. doi:10.1016/j.algal.2020.102178
Lukwambe B., Nicholaus R., Zhang D., Yang W., Zhu J., Zheng Z. (2019). Successional Changes of Microalgae Community in Response to Commercial Probiotics in the Intensive Shrimp (Litopenaeus Vannamei Boone) Culture Systems. Aquaculture 511, 734257. doi:10.1016/j.aquaculture.2019.734257
Mabrouk M. M., Ashour M., Labena A., Zaki M. A. A., Abdelhamid A. F., Gewaily M. S., et al. (2021). Nanoparticles of Arthrospira Platensis Improves Growth, Antioxidative and Immunological Responses of Nile tilapia ( Oreochromis niloticus ) and its Resistance to Aeromonas Hydrophila. Aquac. Res. 53, 125–135. doi:10.1111/are.15558
Madkour F. F., Kamil A. E.-W., Nasr H. S. (2012). Production and Nutritive Value of Spirulina Platensis in Reduced Cost Media. Egypt. J. Aquatic Res. 38 (1), 51–57. doi:10.1016/j.ejar.2012.09.003
Mansour A. T., Alsaqufi A. S., Alkhamis Y. A., Al-Gazar F. F., Zaki M. A., Nour A. A. M., et al. (2021). The Evaluation of Arthrospira Platensis Bioactivity and Their Dietary Supplementation to Nile tilapia Vegetarian Diet on Growth Performance, Feed Utilization, Body Composition and Hemato-Biochemical Parameters. Ann. Anim. Sci. 21 (3), 1061–1080. doi:10.2478/aoas-2021-0003
Mansour A. T., Ashour M., Alprol A. E., Alsaqufi A. S. (2022). Aquatic Plants and Aquatic Animals in the Context of Sustainability: Cultivation Techniques, Integration, and Blue Revolution. Sustainability 14, 3257. doi:10.3390/su14063257
Mansour A. T., Espinosa C., García-Beltrán J. M., Miao L., Ceballos Francisco D. C., Alsaqufi A. S., et al. (2020). Dietary Supplementation of Drumstick Tree, Moringa Oleifera, Improves Mucosal Immune Response in Skin and Gills of Seabream, Sparus Aurata, and Attenuates the Effect of Hydrogen Peroxide Exposure. Fish. Physiol. Biochem. 46 (3), 981–996. doi:10.1007/s10695-020-00763-2
Mansour A. T., Miao L., Espinosa C., García-Beltrán J. M., Ceballos Francisco D. C., Esteban M. Á. (2018). Effects of Dietary Inclusion of Moringa Oleifera Leaves on Growth and Some Systemic and Mucosal Immune Parameters of Seabream. Fish. Physiol. Biochem. 44 (4), 1223–1240. doi:10.1007/s10695-018-0515-z
Mehrabi Z., Firouzbakhsh F., Jafarpour A. (2012). Effects of Dietary Supplementation of Synbiotic on Growth Performance, Serum Biochemical Parameters and Carcass Composition in Rainbow Trout (Oncorhynchus mykiss) Fingerlings. J. Animal Physiology Animal Nutr. 96 (3), 474–481. doi:10.1111/j.1439-0396.2011.01167.x
Metwally A., El-Naggar H., El-Damhougy K., Bashar M., Ashour M., Abo-Taleb H. (2020). GC-MS Analysis of Bioactive Components in Six Different Crude Extracts from the Soft Coral (Sinularia Maxim) Collected from Ras Mohamed, Aqaba Gulf, Red Sea, Egypt. Egypt. J. Aquatic Biolo. Fish. 24 (6), 425–434. doi:10.21608/ejabf.2020.114293
Molino A., Rimauro J., Casella P., Cerbone A., Larocca V., Chianese S., et al. (2018). Extraction of Astaxanthin from Microalga Haematococcus pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 283, 51–61. doi:10.1016/j.jbiotec.2018.07.010
Moradi Z., Madadkar Haghjou M., Zarei M., Colville L., Raza A. (2021). Synergy of Production of Value-Added Bioplastic, Astaxanthin and Phycobilin Co-products and Direct Green 6 Textile Dye Remediation in Spirulina Platensis. Chemosphere 280, 130920. doi:10.1016/j.chemosphere.2021.130920
Niu J., Tian L.-X., Liu Y.-J., Yang H.-J., Ye C.-X., Gao W., et al. (2009). Effect of Dietary Astaxanthin on Growth, Survival, and Stress Tolerance of Postlarval Shrimp, Litopenaeus Vannamei. J. World Aquac. Soc. 40 (6), 795–802. doi:10.1111/j.1749-7345.2009.00300.x
Orosa M., Torres E., Fidalgo P., Abalde J. (2000). Production and Analysis of Secondary Carotenoids in Green Algae. J. Appl. Phycol. 12 (3), 553–556. doi:10.1023/A:1008173807143
Orosa M., Valero J. F., Herrero C., Abalde J. (2001). Comparison of the Accumulation of Astaxanthin in Haematococcus pluvialis and Other Green Microalgae under N-Starvation and High Light Conditions. Biotechnol. Lett. 23 (13), 1079–1085. doi:10.1023/A:1010510508384
Osman M. E. H., Abo-Shady A. M., El-Nagar M. M. F. (2016). Cyanobacterial Arthrospira (Spirulina Platensis) as Safener against Harmful Effects of Fusilade Herbicide on Faba Bean Plant. Rend. Fis. Acc. Lincei 27 (3), 455–462. doi:10.1007/s12210-015-0498-y
Pakravan S., Akbarzadeh A., Sajjadi M. M., Hajimoradloo A., Noori F. (2017). Partial and Total Replacement of Fish Meal by Marine Microalga Spirulina Platensis in the Diet of Pacific White Shrimp Litopenaeus Vannamei: Growth, Digestive Enzyme Activities, Fatty Acid Composition and Responses to Ammonia and Hypoxia Stress. Aquac. Res. 48, 5576–5586. doi:10.1111/are.13379
Pei S., Guan Y., Ma Y. (2009). Effects of Dietary Supplementation of Astaxanthin on Growth, Survival and Antioxidant Capacity of Pacific White Shrimp (Litopenaeus Vannamei). Fish. Sci. (Dalian) 28 (3), 126–129.
Petit H., Nègre-Sadargues G., Castillo R., Trilles J.-P. (1997). The Effects of Dietary Astaxanthin on Growth and Moulting Cycle of Postlarval Stages of the Prawn, Penaeus Japonicus (Crustacea, Decapoda). Comp. Biochem. Physiology Part A Physiology 117 (4), 539–544. doi:10.1016/S0300-9629(96)00431-8
Qin S., Liu G.-X., Hu Z.-Y. (2008). The Accumulation and Metabolism of Astaxanthin in Scenedesmus Obliquus (Chlorophyceae). Process Biochem. 43 (8), 795–802. doi:10.1016/j.procbio.2008.03.010
Ranga Rao A., Raghunath Reddy R. L., Baskaran V., Sarada R., Ravishankar G. A. (2010). Characterization of Microalgal Carotenoids by Mass Spectrometry and Their Bioavailability and Antioxidant Properties Elucidated in Rat Model. J. Agric. Food Chem. 58 (15), 8553–8559. doi:10.1021/jf101187k
Rattanachai A., Hirono I., Ohira T., Takahashi Y., Aoki T. (2004). Cloning of Kuruma Prawn Marsupenaeus japonicus Crustin-like Peptide cDNA and Analysis of its Expression. Fish. Sci. 70 (5), 765–771. doi:10.1111/j.1444-2906.2004.00869.x
Ringø E., Zhou Z., Vecino J. L. G., Wadsworth S., Romero J., Krogdahl Å., et al. (2016). Effect of Dietary Components on the Gut Microbiota of Aquatic Animals. A Never‐ending Story? Aquacult Nutr. 22 (2), 219–282. doi:10.1111/anu.12346
Scurria A., Fabiano Tixier A.-S., Lino C., Pagliaro M., D’Agostino F., Avellone G., et al. (2020). High Yields of Shrimp Oil Rich in Omega-3 and Natural Astaxanthin from Shrimp Waste. ACS omega 5 (28), 17500–17505. doi:10.1021/acsomega.0c01978
Seabra L. M. A. J., Pedrosa L. F. C. (2010). Astaxanthin: Structural and Functional Aspects. Rev. Nutr. 23 (6), 1041–1050. doi:10.1590/s1415-52732010000600010
Sharawy Z. Z., Abbas E. M., Abdelkhalek N. K., Ashry O. A., Abd El-Fattah L. S., El-Sawy M. A., et al. (2022). Effect of Organic Carbon Source and Stocking Densities on Growth Indices, Water Microflora, and Immune-Related Genes Expression of Litopenaeus Vannamei Larvae in Intensive Culture. Aquaculture 546, 737397–397. doi:10.1016/j.aquaculture.2021.737397
Sharawy Z. Z., Ashour M., Abbas E., Ashry O., Helal M., Nazmi H., et al. (2020). Effects of Dietary Marine Microalgae, Tetraselmis Suecica , on Production, Gene Expression, Protein Markers and Bacterial Count of Pacific White Shrimp Litopenaeus Vannamei. Aquac. Res. 51 (6), 2216–2228. doi:10.1111/are.14566
Söderhäll K., Cerenius L. (1992). Crustacean Immunity. Annu. Rev. Fish Dis. 2, 3–23. doi:10.1016/0959-8030(92)90053-Z
Song L., Yuan J., Ni S., Zhou Y., Wang X., Chen Y., et al. (2020). Enhancement of Adaptive Immune Responses of Aged Mice by Dietary Intake of β-glucans, with Special Emphasis on Anti-aging Activity. Mol. Immunol. 117, 160–167. doi:10.1016/j.molimm.2019.10.019
Sritunyalucksana K., Söderhäll K. (2000). The proPO and Clotting System in Crustaceans. Aquaculture 191 (1), 53–70. doi:10.1016/s0044-8486(00)00411-7
Subhra Bikash K. M., Bhattacharyya S. B., Mitra A. (2015). Seaweed Incorporated Diet Improves Astaxanthin Content of Shrimp Muscle Tissue. J Mar. Sci Res DevResearch Dev. 05 (2), 1. doi:10.4172/2155-9910.1000161
Tayag C. M., Lin Y.-C., Li C.-C., Liou C.-H., Chen J.-C. (2010). Administration of the Hot-Water Extract of Spirulina Platensis Enhanced the Immune Response of White Shrimp Litopenaeus Vannamei and its Resistance against Vibrio Alginolyticus. Fish shellfish Immunol. 28 (5-6), 764–773. doi:10.1016/j.fsi.2010.01.023
Teramukai K., Kakui S., Beppu F., Hosokawa M., Miyashita K. (2020). Effective Extraction of Carotenoids from Brown Seaweeds and Vegetable Leaves with Edible Oils. Innovative Food Sci. Emerg. Technol. 60, 102302. doi:10.1016/j.ifset.2020.102302
Tomas A. L., Sganga D. E., Marciano A., López Greco L. S. (2020). Effect of Diets on Carotenoid Content, Body Coloration, Biochemical Composition and Spermatophore Quality in the "red Cherry" shrimpNeocaridina davidi(Caridea, Atyidae). Aquacult Nutr. 26 (4), 1198–1210. doi:10.1111/anu.13076
Wade N. M., Gabaudan J., Glencross B. D. (2017). A Review of Carotenoid Utilisation and Function in Crustacean Aquaculture. Rev. Aquacult 9 (2), 141–156. doi:10.1111/raq.12109
Wang H., Dai A., Liu F., Guan Y. (2015). Effects of Dietary Astaxanthin on the Immune Response, Resistance to White Spot Syndrome Virus and Transcription of Antioxidant Enzyme Genes in Pacific White Shrimp, Litopenaeus Vannamei. Iran. J. Fish. Sci. 14 (3), 699–718. doi:10.22092/IJFS.2018.114476
Wang W., Ishikawa M., Koshio S., Yokoyama S., Dawood M. A. O., Hossain M. S., et al. (2019). Effects of Dietary Astaxanthin and Vitamin E and Their Interactions on the Growth Performance, Pigmentation, Digestive Enzyme Activity of Kuruma Shrimp (Marsupenaeus japonicus ). Aquac. Res. 50 (4), 1186–1197. doi:10.1111/are.13993
Wang Y.-C., Chang P.-S., Chen H.-Y. (2008). Differential Time-Series Expression of Immune-Related Genes of Pacific White Shrimp Litopenaeus Vannamei in Response to Dietary Inclusion of β-1,3-glucan. Fish Shellfish Immunol. 24 (1), 113–121. doi:10.1016/j.fsi.2007.09.008
Wu W., Wang P.-H., Lee D.-J., Chang J.-S. (2017). Global Optimization of Microalgae-To-Biodiesel Chains with Integrated Cogasification Combined Cycle Systems Based on Greenhouse Gas Emissions Reductions. Appl. Energy 197, 63–82. doi:10.1016/j.apenergy.2017.03.117
Xie J., Bu L., Jin S., Wang X., Zhao Q., Zhou S., et al. (2020). Outbreak of Vibriosis Caused by Vibrio Harveyi and Vibrio Alginolyticus in Farmed Seahorse Hippocampus kuda in China. Aquaculture 523, 735168. doi:10.1016/j.aquaculture.2020.735168
Yang S.-P., Wu Z.-H., Jian J.-C. (2013). Effect of Marine Red Yeast Rhodosporidium Paludigenum on Antioxidant-Related Gene Expression in Litopenaeus Vannamei. Israeli J. Aquaculture-Bamidgeh 65, 20665.
Yu W., Liu J. (2020). Astaxanthin Isomers: Selective Distribution and Isomerization in Aquatic Animals. Aquaculture 520, 734915. doi:10.1016/j.aquaculture.2019.734915
Yu W., Zhang M., Wang B., Xu R., Pang T., Liu J. (2021). Dietary Haematococcus pluvialis Powder Supplementation Affect Carotenoid Content, Astaxanthin Isomer, Antioxidant Capacity and Immune‐related Gene Expression in Pacific White Shrimp, Litopenaeus Vannamei. Aquac. Res. 52 (6), 2403–2414. doi:10.1111/are.15090
Zaki M. A., Ashour M., Heneash A. M. M., Mabrouk M. M., Alprol A. E., Khairy H. M., et al. (2021). Potential Applications of Native Cyanobacterium Isolate (Arthrospira Platensis NIOF17/003) for Biodiesel Production and Utilization of its Byproduct in Marine Rotifer (Brachionus plicatilis) Production. Sustainability 13 (4), 1769. doi:10.3390/su13041769
Zhang C., Jin Y., Yu Y., Xiang J., Li F. (2021). Effects of Natural Astaxanthin from Microalgae and Chemically Synthetic Astaxanthin Supplementation on Two Different Varieties of the Ridgetail White Prawn (Exopalaemon Carinicauda). Algal Res. 57, 102347. doi:10.1016/j.algal.2021.102347
Zhang D. H., Ng Y. K., Phang S. M. (1997). Composition and Accumulation of Secondary Carotenoids in Chlorococcum Sp. J. Appl. Phycol. 9 (2), 147–155. doi:10.1023/A:1007926528388
Keywords: astaxanthin, feed additives, immune response, antioxidant, bacterial abundance, marine shrimp
Citation: Mansour AT, Ashour M, Abbas EM, Alsaqufi AS, Kelany MS, El-Sawy MA and Sharawy ZZ (2022) Growth Performance, Immune-Related and Antioxidant Genes Expression, and Gut Bacterial Abundance of Pacific White Leg Shrimp, Litopenaeus vannamei, Dietary Supplemented With Natural Astaxanthin. Front. Physiol. 13:874172. doi: 10.3389/fphys.2022.874172
Received: 11 February 2022; Accepted: 01 June 2022;
Published: 23 June 2022.
Edited by:
Serhat Turkmen, University of Alabama at Birmingham, United StatesReviewed by:
Omid Safari, Ferdowsi University of Mashhad, IranPo-Tsang Lee, National Taiwan Ocean University, Taiwan
Copyright © 2022 Mansour, Ashour, Abbas, Alsaqufi, Kelany, El-Sawy and Sharawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdallah Tageldein Mansour, YW1hbnNvdXJAa2Z1LmVkdS5zYQ==, b3JjaWQub3JnLzAwMDAtMDAwMi01OTYzLTUyNzY=; Mohamed Ashour, bWljcm9hbGdhZV9lZ3lwdEB5YWhvby5jb20=, b3JjaWQub3JnLzAwMDAtMDAwMi0xNTk1LTExOTc=
 Abdallah Tageldein Mansour
Abdallah Tageldein Mansour Mohamed Ashour
Mohamed Ashour Eman M. Abbas3
Eman M. Abbas3 Zaki Z. Sharawy
Zaki Z. Sharawy