
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 20 April 2022
Sec. Exercise Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.833809
This article is part of the Research Topic Biomechanics, Aging, Exercise and Other Interventions View all 17 articles
Background: The effect of resistance exercise on the autonomic nervous system of patients with hypertension has not been identified.
Objective: To explore a suitable resistance training method for hypertension patients to regulate blood pressure (BP) and autonomic nervous system function.
Method: Forty-five hypertension patients aged between 55 and 70 years were randomly equally divided into three groups: the high-intensity resistance exercise (HE) group, the low-intensity resistance exercise combined with blood flow restriction (LE-BFR) group, and the low-intensity resistance exercise (LE) group. All patients performed quadriceps femoris resistance exercise. The exercise intensity of HE, LE-BFR and LE group was 65, 30 and 30% of one repetition maximum (1RM), respectively. The LE-BFR group used pressure cuffs to provide 130% of systolic pressure to the patient’s thighs during resistance exercise. The training program was 20 times/min/set with a 1-min break after each set, and was conducted five sets/day and 3 days/week, lasting for 12 weeks. The heart rate (HR), BP, root-mean-square of difference-value of adjacent RR intervals (RMSSD), low frequency (LF) and high frequency (HF) were evaluated before and after the first training and the last training.
Result: Significant differences in HR were observed in both recovery states after the first and last training (p < 0.01). After 12 weeks of training, the recovery speed of HR in the LE-BFR group increased significantly (p < 0.01). The systolic blood pressures in the HE and LE-BFR group were significantly reduced (p < 0.05 and p < 0.01), and the differences among groups were significant (p < 0.01). In the last recovery state, the RMSSD of the LE group was significantly lower than that in the first recovery state (p < 0.01). The LF/HF ratios of the HE and LE groups in the resting and recovery states were increased significantly (all p < 0.01). LF/HF ratios in the LE-BFR group in the resting and recovery state were decreased significantly (both p < 0.01).
Conclusion: Compared to HE and LE, LE-BFR could effectively decrease systolic pressure and regulate the autonomic nervous system function in hypertension patients.
Hypertension is an important public health problem worldwide and is considered the main risk factor for cardiovascular diseases (Mozaffarian et al., 2015). The autonomic nervous system (ANS) plays an important role in regulating blood pressure (BP). Studies have found that imbalance and dysregulation of the ANS, manifested as enhanced sympathetic activity (Li et al., 2020; Esler, 2011), precede the occurrence of hypertension and develop concurrently with hypertension (Hering et al., 2016; Mancia and Grassi, 2014; Zubcevic et al., 2019). Therefore, it is important to modify the imbalance and dysregulation of the ANS for hypertension patients with sympathetic predominant states.
Exercise helps to lower BP, but patients with arterial hypertension were advised to avoid high levels of acute cardiovascular stress (Vale et al., 2018) because of the sympathetic predominance. Even though aerobic exercise was demonstrated to be able to inhibit sympathetic predominance (Esler, 2011), the effect of resistance exercise on decreasing sympathetic activity has not yet been identified in previous studies (Trevizani et al., 2018).
A transient research has found high-intensity resistance exercise (HE) can lead to a significant increase in systolic blood pressure (SBP) after the exercise, while low-intensity resistance exercise (LE) cannot, but LE can lead to a significant increase in the low frequency/high frequency (LF/HF) ratio (Vale et al., 2018). Hence, it remains unclear whether high-intensity and low-intensity exercise are unfavorable to patients with hypertension, and the exploration of resistance exercise suitable for hypertensive patients is required to reduce BP and avoid cardiovascular disease risks.
Low-intensity resistance exercise combined with blood flow restriction (LE-BFR) has been widely implemented to investigate its cardiovascular effect, but most of the research explored the cardiovascular effect on healthy adults. Early et al. found LE-BFR could lower SBP in healthy young adults (Early et al., 2020). However, studies on LE-BFR in hypertension patients are rare (Cerqueira et al., 2021).
This study aimed to analyze and compare the impacts of HE, LE, and LE-BFR on BP and ANS in hypertension patients, and to provide the most efficacious resistance exercise procedure that could reduce BP and avoid cardiovascular disease risks for hypertension patients.
Forty-five hypertension patients aged between 55 and 70 years, including 16 men and 29 women, were voluntarily included. Patients with the following features were excluded: 1) body mass index (BMI) > 28; 2) abnormal electrocardiogram in the resting state and after exercise; 3) with musculoskeletal disorders; 4) with physical exercises more than twice a week; 5) SBP ≥180 mmHg and/or diastolic blood pressure (DBP) ≥ 100 mmHg in resting state; 6) special diet control. Patients were included in this study only when the clinical evaluation showed that they had no restrictions on participating in physical exercise. A written informed consent was obtained from each patient after they understood the detailed description of all procedures. This study was approved by the Ethics Committee for Human Experiments of Rehabilitation hospital of Huishan in Wuxi, Jiangsu, China (ID: HK-LLWYH-202002).
Patients were randomly equally divided into three groups: HE, LE-BFR, and LE groups.
The one repetition maximum (1RM) of quadriceps femoris was evaluated by the Isokinetic Muscle Strength Evaluation Training System (SYSTEM4, BIODEX Co., Ltd., New Jersey, United States). Before the evaluation, each patient underwent a 15 minutes of lower limb muscle stretching as warm-up and then sat on the chair of the isokinetic dynamometer with one lower leg perpendicular to the ground. Before the evaluation, all patients participated in an adaptation session. When doing adaptation exercises, the patients performed ten contractions of quadriceps femoris to move the knee joint from 90° flexion to 0° extension without load. During the evaluation, the patients performed three contractions of quadriceps femoris to move the knee joint from 90° flexion to 0° extension. The instrument calculated the 1RM by the strength of contraction. The 1RM of both legs was evaluated, and the higher value was selected as the patient’s evaluation result. An assessment was conducted every 4 weeks to help the subjects adjust their exercise intensity.
The autonomic modulation index of the cardiovascular system was obtained by FIRSTBEAT (Version 4.7.3.1, Firstbeat Technologies Ltd., Jyväskylä, Finland). Time domain variables examined included the mean heart rate (HR) and the root-mean-square of difference-value of adjacent RR intervals (RMSSD). Frequency domain indicators examined included low frequency (LF; 0.04–0.15 Hz) and high frequency (HF; 0.15–0.50 Hz) values. In addition, the LF/HF ratio was calculated to represent the sympathovagal balance (Task Force, 1996).
Participants were required to avoid drinking any liquids containing caffeine and/or alcohol within 2 h before the assessment. All evaluations were performed at least 2 h after a meal. The index of ANS in the resting states were obtained 0–15 min before the first and the last trainings. In order to get the indicators of ANS in the recovery state on the same time period, RMSSD, LF and HF were obtained 10–20 min after the first and the last trainings. HR in the recovery state was collected 10–30 min after the training, as it turned stable 10 min after the training.
Blood pressures were measured 0–15 min before the first and last trainings in the resting state. For patients in the LE-BFR group, inflatable pressure cuffs (KAATSUMASTER, Kaatsu Japan Co., Ltd., Tokyo, Japan) were used to wrap around the upper third of both thighs to restrict blood flow. When patients performed exercises, the pressure cuffs were inflated and the pressure was 130% of the subjects’ SBP (Zhao et al., 2021). The pressure cuffs were removed after each set of exercise.
The resistance exercises in each group were conducted by contracting quadriceps femoris, leading to the extension of knee joints on the isokinetic dynamometer. The exercise intensity of patients in the HE group was 65% of 1RM, and that in the LE group and LE-BFR group was 30% of 1RM. During exercises, patients in the LE-BFR group underwent blood occlusions by inflating the pressure cuffs. The resistance exercise program was 20 times/min/set, with a 1-min break after each set and a number of five sets/day. The exercise frequency was 3 days/week, for a total of 12 weeks. Each repetition was completed within 3 s, with the rhythm controlled by a metronome. Both legs of the patients were trained and their daily physical activities were not intervened.
The measurement data were expressed as mean ± standard deviation (SD). Differences within each group were analyzed using the paired t test. Differences among three groups were analyzed by one-way ANOVA, and the LSD method was used for multiple comparisons. The p-value reported was two-sided, and the differences with a p-value <0.05 was considered statistically significant, while p-value <0.01 was considered highly statistically significant. All statistical analyses were performed using SPSS software (version 13.0, SPSS, Chicago, IL, United States).
Before the training started, there were no significant differences among groups in terms of age, height, body mass, and other characteristics (Table 1).
After 12 weeks of training, HR in the resting state didn’t vary significantly in the three groups (p > 0.05) (Figure 1A).
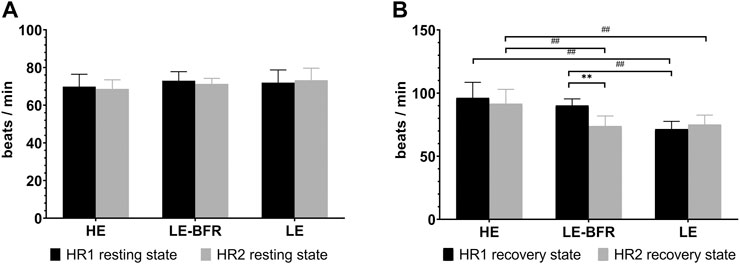
FIGURE 1. (A) HR in the resting state before the first and last training. (B) HR in the recovery state after the first and last training. HR1 resting state, HR in the resting state before the first training. HR2 resting state, HR in the resting state before the last training. HR1 recovery state, HR in the recovery state after the first training. HR2 recovery state, HR in the recovery state after the last training. Data are presented as the mean ± standard deviation (SD). **p < 0.01, significantly different from baseline. ##p < 0.01, significantly different among groups.
After the first training, HR in the recovery state in the LE group (71.5 ± 6.2 b⋅min−1) was significantly lower than that in the LE-BFR (90.2 ± 5.2 b⋅min−1) and HE groups (96.3 ± 12.3 b⋅min−1) (both p < 0.01). After 12 weeks of training, HR in the recovery state in the LE-BFR group decreased significantly (p < 0.01), and HR in the HE group (91.6 ± 11.4 b⋅min−1) was significantly higher than that in the LE-BFR (73.9 ± 8.0 b⋅min−1) and LE groups (75.1 ± 7.5 b⋅min−1) (both p < 0.01) (Figure 1B).
Before the training, there were no significant differences in BP among groups (p > 0.05). After 12 weeks of training, SBP in the HE (148.1 ± 11.4 vs. 142.9 ± 4.9 mmHg, p < 0.05) and LE-BFR groups (144.7 ± 7.9 vs. 129.7 ± 7.6 mmHg, p < 0.01) were significantly decreased compared to that before resistance training. SBP in the LE-BFR group was significantly lower than that in the HE (148.1 ± 11.4 mmHg) and LE groups (140.9 ± 5.2 mmHg) (both p < 0.01) (Figure 2A). There were no significant differences in DBP among groups before and after the trainings (p > 0.05) (Figure 2B).
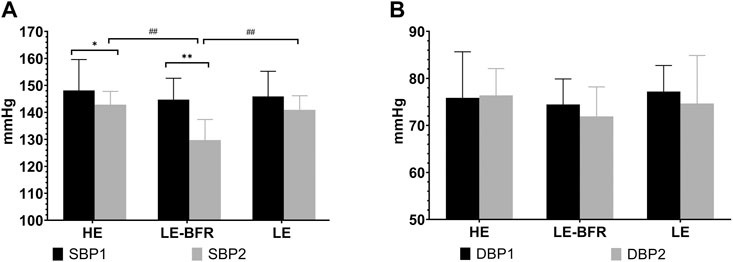
FIGURE 2. (A) SBP in the resting state before the first and the last training. (B) DBP in the resting state before the first and the last training. SBP1, SBP before the first training. SBP2, SBP before the last training. DBP1, DBP before the first training. DBP2, DBP before the last training. Data are presented as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, significantly different from baseline. ##p < 0.01, significantly different among groups.
In terms of RMSSD, there were no significant differences among groups in the resting states before the training and 12 weeks after the training or in the recovery states after the first training (p > 0.05) (Figures 3A, B). After 12 weeks of training, RMSSD in the LE group in the recovery state was decreased significantly (20.4 ± 3.2 vs. 16.7 ± 4.5 m, p < 0.01). Comparing the recovery state after the first and last training, there was no significant decrease of RMSSD in the HE group (17.7 ± 11.0 vs. 15.3 ± 5.3 m, p > 0.05), and no significant increase of RMSSD in the LE-BFR group (19.1 ± 5.9 vs. 19.9 ± 4.9 m, p > 0.05). However, there was significant difference in RMSSD in the recovery state between the HE and LE-BFR groups after the last training (p < 0.05) (Figures 3B, D).
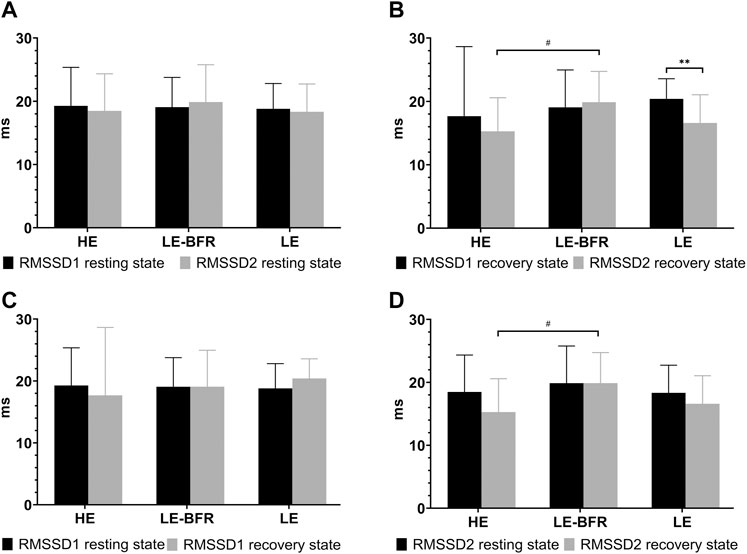
FIGURE 3. (A) RMSSD in the resting state before the first and the last training. (B) RMSSD in the recovery state after the first and the last training. (C) RMSSD before and after the first training. (D) RMSSD before and after the last training. RMSSD1 resting state, RMSSD in the resting state before the first training. RMSSD2 resting state, RMSSD in the resting state before the last training. RMSSD1 recovery state, RMSSD in the recovery state after the first training. RMSSD2 recovery state, RMSSD in the recovery state after the last training. Data are presented as the mean ± standard deviation (SD). **p < 0.01, significantly different from baseline. #p < 0.05, significantly different among groups.
Before the training, there were no significant differences in LF/HF ratio among groups (p > 0.05). After 12 weeks of training, in terms of LF/HF ratio in resting state, significant increases were observed in the HE (1.5 ± 0.3 vs. 2.8 ± 0.5, p < 0.01) and LE groups (1.5 ± 0.3 vs. 1.7 ± 0.4, p < 0.01), whereas significant decrease was observed in the LE-BFR group (1.5 ± 0.2 vs. 0.7 ± 0.1, p < 0.01). Moreover, there were significant differences in LF/HF ratio among groups (p < 0.01) (Figure 4A). In the recovery state after the first training, LF/HF ratio in the HE group was significantly higher than that in the LE-BFR and LE groups (both p < 0.01) (Figure 4B). In the recovery state after the last training, significant increases in LF/HF ratio were observed in the HE (2.5 ± 0.4 vs. 5.4 ± 1.0, p < 0.01) and LE groups (1.6 ± 0.3 vs. 1.8 ± 0.3, p < 0.01), while significantly decreased LF/HF ratio was observed in the LE-BFR group (1.8 ± 0.3 vs. 0.7 ± 0.1, p < 0.01). There were significant differences in LF/HF ratio among groups (p < 0.01) (Figure 4B).
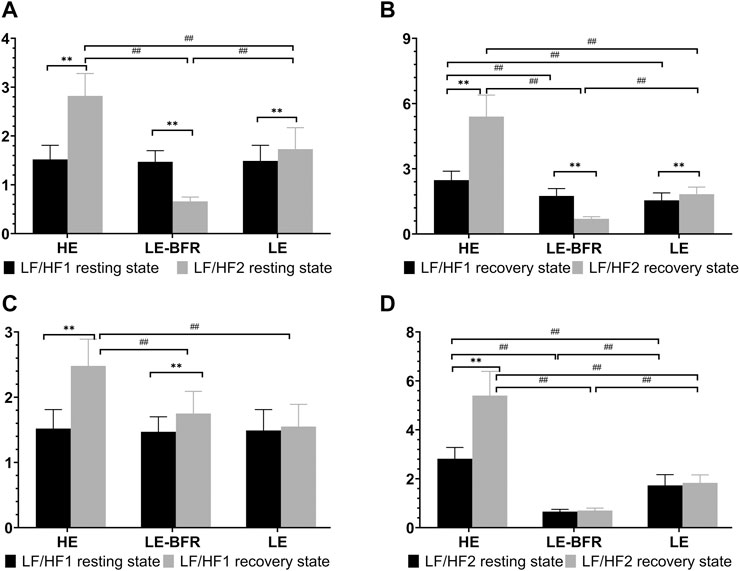
FIGURE 4. (A) LF/HF in the resting state. (B) LF/HF in the recovery state. (C) LF/HF before and after the first training. (D) LF/HF before and after the last training. LF/HF1 resting state, LF/HF in the resting state before the first training. LF/HF2 resting state, LF/HF in the resting state before the last training. LF/HF1 recovery state, LF/HF in the recovery state after the first training. LF/HF2 recovery state, LF/HF in the recovery state after the last training. Data are presented as the mean ± standard deviation (SD). **p < 0.01, significantly different from baseline. ##p < 0.01, significantly different among groups.
In the recovery state after the first training, compared to the resting state, LF/HF ratios in the HE and LE-BFR groups were increased significantly (both p < 0.01). Moreover, LF/HF ratio in the HE group was higher than that in the LE-BFR and LE groups (both p < 0.01) (Figure 4C). In the resting state before the last training, LF/HF ratio was increased in the HE group compared to the LE group (p < 0.01), and in the LE group compared to the LE-BFR group (p < 0.01). In the recovery state after the last training, compared to the resting state before the last training, LF/HF ratio in the HE group was increased significantly (p < 0.01), but no significant difference in LF/HF ratio between LE-BFR and LE groups were observed (p > 0.05). There were significant differences in LF/HF ratio among the three groups (p < 0.01) (Figure 4D). As shown in Table 2, HF in the HE group was decreased very significantly (p < 0.01) and HF in the LE-BFR group was increased very significantly (p < 0.01) after the training, which led to the highly significant change of LF/HF ratios among the groups.
This study aimed to find out the resistance exercise type that was beneficial for regulating BP and ANS, and that could avoid higher cardiovascular risk. It was suggested that compared to HE and LE, LE-BFR could more effectively regulate SBP and the imbalance and dysregulation of the ANS in hypertension patients. It was also indicated that when hypertension patients used resistant exercise to decrease SBP in the resting state, LE-BFR was the most effective exercise type compared to HE and LE, leading to less cardiovascular risk.
Both high BP and high HR are risk factors of cardiovascular, however, this research didn’t find the decrease of SBP related to HR in the resting states. In this research, there was no significant difference between the resting HR before and after the training.
HR recovery rate reflects the balance of the autonomic nervous system (Silva et al., 2021). Previous studies have found slow HR recovery rate after exercise is associated with increased risk for arrhythmia and other cardiovascular morbidities and mortality, while increased HR recovery rate is associated with improved prognosis and lower mortality related to cardiovascular disease (Heffernan et al., 2007; Smith et al., 2005; Stein et al., 2005; Task Force, 1996; Tsuji et al., 1996; Zhang et al., 2016). Cardiovascular and neurovegetative adaptations to exercise training through alteration in sympathovagal balance lead to changes in HR recovery (Suzic et al., 2017). After the first training, the HR recovery rate of the HE and LE-BFR groups was slower than that of the LE group, implying that for hypertension patients without exercise habits, the cardiovascular risk caused by temporary HE and LE-BFR was higher than that by LE. After 12 weeks of training, the HR recovery rate of the LE-BFR group was significantly improved, and there was no difference in this value between the LE and LE-BFR group. In addition, the HR of HE group was even higher than that of the LE-BFR and LE groups. This finding indicated that LE-BFR could result in better adaptability of the cardiovascular function, thereby lowering cardiovascular risk. However, HE could cause higher cardiovascular risks compared to LE-BFR and LE.
High SBP is the leading cause of death and disability worldwide (Lim et al., 2012). The meta-analysis by Naci et al. (2019) pointed out that middle to high intensity resistance exercises could effectively reduce BP. Consistent with their research, the present study suggested that HE could effectively decrease SBP. Furthermore, our research found LE-BFR could also decrease SBP, and the effect of LE-BFR was more significant compared to the HE and LE modes. These results demonstrated that LE-BFR could exert better antihypertensive effects in subjects compared to HE. For people who are not able to perform high intensity resistant exercises, they can reduce their BP by LE-BFR training.
RMSSD is a parameter to evaluate parasympathetic activity. In the present study, RMSSD in the resting state didn’t change significantly. This result was similar to the findings from several previous studies. The study by Takahashi et al. (2009) included 17 healthy old men who conducted high intensity resistant exercises for 12 weeks and showed that RMSSD in the resistant exercise group didn’t change significantly. Millar et al. (2013) suggested similar results of RMSSD in the resting state with our findings when they inspected elderly hypertension patients who performed low intensity resistant exercises for 12 weeks. The findings about RMSSD by Gerage et al. (2013) also consistent with our results. The difference between us is their subjects were elderly healthy women but our subjects were elderly hypertension patients. However, the conclusions of the study by Caruso et al. (2015) on RMSSD in the resting state in elderly patients with coronary heart disease was different with our results. They showed that RMSSD in resting state had increased after low intensity resistant exercises for 8 weeks. The cause of the difference might be the different exercise forms, exercise volume and detection time.
In the present study, the RMSSD in the recovery state after the first training was the highest in the LE group, and the lowest in the HE group. Although there were no significant differences among groups, the trend was consistent with the result of the transient study by Okuno et al. (2014), which covered about 20–29 years healthy men. In their study, there were significant differences among groups. The possible reason of significant differences in their study was that the exercise intensity of their subjects was 10% higher than ours, and the subjects must exercise to exhaustion in the last set. The article by Isidoro et al. (2017) on healthy men aged 20–35 years also showed that transient high intensity resistant exercises could reduce RMSSD significantly. The difference between their and our results might come from the fact that all limbs of subjects in their research should exercise and the total exercise volume was different. Taken together, it was suggested that RMSSD could not be used as an independent indicator to evaluate cardiovascular risk. RMSSD might indicate an increase in cardiovascular risk only when other parameters of the ANS prompted this risk with decreasing RMSSD.
LF is a parameter to express sympathetic and vagal modulations simultaneously, and this index represents sympathetic modulations best. HF is an indicator of vagal modulation. LF/HF ratio is a parameter to evaluate the sympathovagal balance (Task Force, 1996). Enhanced sympathetic and weakened parasympathetic predominance regulations are associated with increased cardiovascular risk (Mourot et al., 2004; Seiler et al., 2007; Lima et al., 2011). In the present study, after 12 weeks of training, both in the resting state and the recovery state, LF/HF ratio in the HE and LE groups increased significantly, whereas this value in the LE-BFR group decreased significantly. This indicated that patients in the LE-BFR group had significantly improved ANS, while those in the HE and LE groups didn’t show this advantage. The study of Lima et al. (2011) about HE only also showed that LF/HF ratio increased after HE. However, the study by Bellavere et al. (2018) showed that after HE for 4 months, the LF/HF ratio of subjects with type II diabetes decreased. The transient high-intensity resistance training proposed by Isidoro et al. (2017) also showed a decreased LF/HF ratio. The difference might come from different modes of exercise, which was supported by the study by Morishima et al. (2018). They compared low-intensity high-repetition and high-intensity low-repetition resistance exercises, and the results verified that the intensity, number of repetition, and intermittent resting time were important factors affecting BP and endothelial function after exercise. However, their study didn’t involve the indicators for ANS. Moreover, the difference in detection time might also affect the results.
Although LF/HF ratios in both HE and LE groups increased significantly, there was also difference between them. LF increased significantly in the LE group while HF decreased significantly in the HE group after 12 weeks of training. The LF/HF ratio in HE group increased greatly (1.52–2.82 in the resting state; 2.48 to 5.40 in the recovery state), but the LF/HF ratio in LE group was still in normal range (1.49–1.73 in the resting state; 1.55 to 1.83 in the recovery state). Because the long-term follow-up study about heart rate variability and mortality (Kuo et al., 2018) demonstrated high LF/HF ratio was an independent risk factor for mortality and LF/HF ratio >2.6 was associated with a higher mortality, our research indicates LE and LE-BFR was safer than HE.
This study was the first to compare BP and ANS in hypertension patients after performing three types of resistant exercises for 12 weeks. Those are different from others. We found LE-BFR had the most obvious effect on reducing SBP and adjusting sympathetic-parasympathetic balance, and was associated with lower cardiovascular risk compared to HE and LE. There are some limitations in the present study. For example, BP in the recovery state wasn’t measured. Additionally, although the effects of 12 weeks of training was examined, the long-term effects of resistance exercise needed to be further observed. Furthermore, no biochemical indicators related to ANS were detected, making it difficult to identify the causes of the changes in RMSSD, LF and HF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee for Human Experiments of Rehabilitation hospital of Huishan. The patients/participants provided their written informed consent to participate in this study.
AL and MZ designed the study; YZ wrote the manuscript; YCZ, XM, and LQ performed the experiment; AL, MZ, and YZ critically revised the manuscript; All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Grant number 81101456); the Nanjing Medical Science and Technique Development Foundation (Grant number YKK19073); the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant number 18KJA320002) and research project of kinesiology laboratory of Nanjing Sport Institute (Grant number SYS202105).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bellavere F., Cacciatori V., Bacchi E., Gemma M. L., Raimondo D., Negri C., et al. (2018). Effects of Aerobic or Resistance Exercise Training on Cardiovascular Autonomic Function of Subjects with Type 2 Diabetes: A Pilot Study. Nutr. Metab. Cardiovasc. Dis. 28, 226–233. doi:10.1016/j.numecd.2017.12.008
Caruso F. R., Arena R., Phillips S. A., Bonjorno J. C., Mendes R. G., Arakelian V. M., et al. (2015). Resistance Exercise Training Improves Heart Rate Variability and Muscle Performance: a Randomized Controlled Trial in Coronary Artery Disease Patients. Eur. J. Phys. Rehabil. Med. 51, 281–289.
Cerqueira M. S., Costa E. C., Santos Oliveira R., Pereira R., Brito Vieira W. H. (2021). Blood Flow Restriction Training: To Adjust or Not Adjust the Cuff Pressure over an Intervention Period? Front. Physiol. 12, 678407. doi:10.3389/fphys.2021.678407
Early K. S., Rockhill M., Bryan A., Tyo B., Buuck D., McGinty J. (2020). Effect of Blood Flow Restriction Training on Muscular Performance, Pain and Vascular Function. Intl J. Sports Phys. Ther. 15, 892–900. doi:10.26603/ijspt20200892
Esler M. (2011). The Sympathetic Nervous System through the Ages: from Thomas Willis to Resistant Hypertension. Exp. Physiol. 96, 611–622. doi:10.1113/expphysiol.2011.052332
Gerage A., Forjaz C. L., Nascimento M., Januário R. S., Polito M., Cyrino E. (2013). Cardiovascular Adaptations to Resistance Training in Elderly Postmenopausal Women. Int. J. Sports Med. 34, 806–813. doi:10.1055/s-0032-1331185
Heffernan K. S., Fahs C. A., Shinsako K. K., Jae S. Y., Fernhall B. (2007). Heart Rate Recovery and Heart Rate Complexity Following Resistance Exercise Training and Detraining in Young Men. Am. J. Physiology-Heart Circulatory Physiol. 293, H3180–H3186. doi:10.1152/ajpheart.00648.2007
Hering D., Kara T., Kucharska W., Somers V. K., Narkiewicz K. (2016). Longitudinal Tracking of Muscle Sympathetic Nerve Activity and its Relationship with Blood Pressure in Subjects with Prehypertension. Blood Press. 25, 184–192. doi:10.3109/08037051.2015.1121708
Isidoro N. J., Santana M. D. R., Valenti V. E., Garner D. M., de Abreu L. C. (2017). Cardiac Autonomic Recovery after Strength Exercise in Lower and Upper Limbs. Acta Cardiologica 72, 467–473. doi:10.1080/00015385.2017.1335454
Kuo G., Chen S.-W., Huang J.-Y., Wu C.-Y., Fu C.-M., Chang C.-H., et al. (2018). Short-term Heart Rate Variability as a Predictor of Long-Term Survival in Patients with Chronic Hemodialysis: A Prospective Cohort Study. J. Formos. Med. Assoc. 117, 1058–1064. doi:10.1016/j.jfma.2018.09.006
Li Y., Wei B., Liu X., Shen X. Z., Shi P. (2020). Microglia, Autonomic Nervous System, Immunity and Hypertension: Is There a Link? Pharmacol. Res. 155, 104451. doi:10.1016/j.phrs.2019.104451
Lim S. S., Vos T., Flaxman A. D., Danaei G., Shibuya K., Adair-Rohani H., et al. (2012). A Comparative Risk Assessment of burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990-2010: a Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. doi:10.1016/S0140-6736(12)61766-8
Lima A. H. R. d. A., Forjaz C. L. d. M., Silva G. Q. d. M., Menêses A. L., Silva A. J. M. R., Ritti-Dias R. M. (2011). Efeito agudo da intensidade Do exercício de força na modulação autonômica cardíaca pós-exercício. Arq. Bras. Cardiol. 96, 498–503. doi:10.1590/s0066-782x2011005000043
Mancia G., Grassi G. (2014). The Autonomic Nervous System and Hypertension. Circ. Res. 114, 1804–1814. doi:10.1161/circresaha.114.302524
Millar P. J., Levy A. S., McGowan C. L., McCartney N., MacDonald M. J. (2013). Isometric Handgrip Training Lowers Blood Pressure and Increases Heart Rate Complexity in Medicated Hypertensive Patients. Scand. J. Med. Sci. Sports 23, 620–626. doi:10.1111/j.1600-0838.2011.01435.x
Morishima T., Tsuchiya Y., Iemitsu M., Ochi E. (2018). High-intensity Resistance Exercise with Low Repetitions Maintains Endothelial Function. Am. J. Physiology-Heart Circulatory Physiol. 315, H681–H686. doi:10.1152/ajpheart.00281.2018
Mourot L., Bouhaddi M., Tordi N., Rouillon J. D., Regnard J. (2004). Short- and Long-Term Effects of a Single Bout of Exercise on Heart Rate Variability: Comparison between Constant and Interval Training Exercises. Eur. J. Appl. Physiol. 92, 508–517. doi:10.1007/s00421-004-1119-0
Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., et al. (2015). American Heart Association Statistics & S. Stroke StatisticsHeart Disease and Stroke Statistics--2015 Update: a Report from the American Heart Association. Circulation 131, e29–322. doi:10.1161/cir.0000000000000152
Naci H., Salcher-Konrad M., Dias S., Blum M. R., Sahoo S. A., Nunan D., et al. (2019). How Does Exercise Treatment Compare with Antihypertensive Medications? A Network Meta-Analysis of 391 Randomised Controlled Trials Assessing Exercise and Medication Effects on Systolic Blood Pressure. Br. J. Sports Med. 53, 859–869. doi:10.1136/bjsports-2018-099921
Okuno N. M., Pedro R. E., Leicht A. S., de Paula Ramos S., Nakamura F. Y. (2014). Cardiac Autonomic Recovery after a Single Session of Resistance Exercise with and without Vascular Occlusion. J. Strength Cond Res. 28, 1143–1150. doi:10.1519/jsc.0000000000000245
Seiler S., Haugen O., Kuffel E. (2007). Autonomic Recovery after Exercise in Trained Athletes. Med. Sci. Sports Exerc. 39, 1366–1373. doi:10.1249/mss.0b013e318060f17d
Silva É. P., Soares B. A., Reimberg M. M., Ritti-Dias R., Nascimento K. S., Anjos F. S., et al. (2021). Heart Rate Recovery in Asthmatic Children and Adolescents after Clinical Field Test. BMC Pulm. Med. 21, 61. doi:10.1186/s12890-020-01355-9
Smith L. L., Kukielka M., Billman G. E. (2005). Heart Rate Recovery after Exercise: a Predictor of Ventricular Fibrillation Susceptibility after Myocardial Infarction. Am. J. Physiology-Heart Circulatory Physiol. 288, H1763–H1769. doi:10.1152/ajpheart.00785.2004
Stein P. K., Domitrovich P. P., Huikuri H. V., Kleiger R. E., Cast I. (2005). Traditional and Nonlinear Heart Rate Variability Are Each Independently Associated with Mortality after Myocardial Infarction. J. Cardiovasc. Electrophysiol. 16, 13–20. doi:10.1046/j.1540-8167.2005.04358.x
Suzic Lazic J., Dekleva M., Soldatovic I., Leischik R., Suzic S., Radovanovic D., et al. (2017). Heart Rate Recovery in Elite Athletes: the Impact of Age and Exercise Capacity. Clin. Physiol. Funct. Imaging 37, 117–123. doi:10.1111/cpf.12271
Takahashi A. C. M., Melo R. C., Quitério R. J., Silva E., Catai A. M. (2009). The Effect of Eccentric Strength Training on Heart Rate and on its Variability during Isometric Exercise in Healthy Older Men. Eur. J. Appl. Physiol. 105, 315–323. doi:10.1007/s00421-008-0905-5
Task Force (1996). Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 17, 354–381.
Trevizani G. A., Seixas M. B., Benchimol-Barbosa P. R., Vianna J. M., da Silva L. P., Nadal J. (2018). Effect of Resistance Training on Blood Pressure and Autonomic Responses in Treated Hypertensives. J. Strength Cond Res. 32, 1462–1470. doi:10.1519/jsc.0000000000001995
Tsuji H., Larson M. G., Venditti F. J., Manders E. S., Evans J. C., Feldman C. L., et al. (1996). Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. Circulation 94, 2850–2855. doi:10.1161/01.cir.94.11.2850
Vale A. F., Carneiro J. A., Jardim P. C. V., Jardim T. V., Steele J., Fisher J. P., et al. (2018). Acute Effects of Different Resistance Training Loads on Cardiac Autonomic Modulation in Hypertensive Postmenopausal Women. J. Transl Med. 16, 240. doi:10.1186/s12967-018-1615-3
Zhang D., Shen X., Qi X. (2016). Resting Heart Rate and All-Cause and Cardiovascular Mortality in the General Population: a Meta-Analysis. Can. Med. Assoc. J. 188, E53–E63. doi:10.1503/cmaj.150535
Zhao Y., Lin A., Jiao L. (2021). Eight Weeks of Resistance Training with Blood Flow Restriction Improve Cardiac Function and Vascular Endothelial Function in Healthy Young Asian Males. Int. Health 13, 471–479. doi:10.1093/inthealth/ihaa089
Keywords: hypertension, autonomic nervous system, resistance exercise, blood flow restriction, blood pressure
Citation: Zhao Y, Zheng Y, Ma X, Qiang L, Lin A and Zhou M (2022) Low-Intensity Resistance Exercise Combined With Blood Flow Restriction is More Conducive to Regulate Blood Pressure and Autonomic Nervous System in Hypertension Patients—Compared With High-Intensity and Low-Intensity Resistance Exercise. Front. Physiol. 13:833809. doi: 10.3389/fphys.2022.833809
Received: 12 December 2021; Accepted: 02 March 2022;
Published: 20 April 2022.
Edited by:
Marcus Fraga Vieira, Universidade Federal de Goiás, BrazilReviewed by:
Ana Cristina Silva Rebelo, Universidade Federal de Goiás, BrazilCopyright © 2022 Zhao, Zheng, Ma, Qiang, Lin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aicui Lin, linaicui@126.com; Mo Zhou, zhoumo3480@126.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.