
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 28 February 2022
Sec. Cardiac Electrophysiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.818122
This article is part of the Research Topic Clinical Relevance of the Zebrafish Heart as a Model for Human Cardiac Arrhythmogenesis and Cardiomyopathies View all 6 articles
 Matthew R. Stoyek1†
Matthew R. Stoyek1† Eilidh A. MacDonald1,2†
Eilidh A. MacDonald1,2† Melissa Mantifel1†
Melissa Mantifel1† Jonathan S. Baillie1†
Jonathan S. Baillie1† Bailey M. Selig1
Bailey M. Selig1 Roger P. Croll1
Roger P. Croll1 Frank M. Smith3
Frank M. Smith3 T. Alexander Quinn1,4*
T. Alexander Quinn1,4*
Cardiac excitation originates in the sinoatrial node (SAN), due to the automaticity of this distinct region of the heart. SAN automaticity is the result of a gradual depolarisation of the membrane potential in diastole, driven by a coupled system of transarcolemmal ion currents and intracellular Ca2+ cycling. The frequency of SAN excitation determines heart rate and is under the control of extra- and intracardiac (extrinsic and intrinsic) factors, including neural inputs and responses to tissue stretch. While the structure, function, and control of the SAN have been extensively studied in mammals, and some critical aspects have been shown to be similar in zebrafish, the specific drivers of zebrafish SAN automaticity and the response of its excitation to vagal nerve stimulation and mechanical preload remain incompletely understood. As the zebrafish represents an important alternative experimental model for the study of cardiac (patho-) physiology, we sought to determine its drivers of SAN automaticity and the response to nerve stimulation and baseline stretch. Using a pharmacological approach mirroring classic mammalian experiments, along with electrical stimulation of intact cardiac vagal nerves and the application of mechanical preload to the SAN, we demonstrate that the principal components of the coupled membrane- Ca2+ pacemaker system that drives automaticity in mammals are also active in the zebrafish, and that the effects of extra- and intracardiac control of heart rate seen in mammals are also present. Overall, these results, combined with previously published work, support the utility of the zebrafish as a novel experimental model for studies of SAN (patho-) physiological function.
In mammals, electrical excitation of the heart, which is responsible for its contraction and pumping action, originates in a distinct “pacemaker” region of the right atrium known as the sinoatrial node (SAN) (Gaskell, 1882; Keith and Flack, 1907). Cardiomyocytes in the SAN exhibit automaticity, due to a gradual depolarisation of their membrane potential (Vm) in diastole (diastolic depolarisation, DD) that eventually reaches the threshold for action potential (AP) firing, whose frequency determines heart rate (HR) (Irisawa et al., 1993; Jansen et al., 2018; Quinn and Magder, 2021). While the relative importance of individual subcellular mechanisms of SAN automaticity have been intensely debated (DiFrancesco and Noble, 2012; Maltsev and Lakatta, 2012; Rosen et al., 2012), it is now generally accepted that pacemaker activity is driven by a robust system involving multiple overlapping contributors that maintain cardiac rhythm even when individual mechanisms are impeded (Lakatta and DiFrancesco, 2009; Noble et al., 2010).
Mammalian studies have demonstrated that SAN automaticity is driven primarily by two systems of cellular mechanisms, commonly referred to as the membrane and Ca2+ “clocks” (consisting of transarcolemmal ionic currents and intracellular Ca2+ cycling, respectively). These two “clocks” form a system of tightly coupled oscillators (Lakatta et al., 2010) that are mutually entrained by intracellular regulatory mechanisms (MacDonald et al., 2020b). In the initial phase of diastole, DD in the SAN is caused by the opening of hyperpolarisation-activated cyclic nucleotide-gated (HCN) channels, which pass an inward cation current known as the “funny” current (If) (DiFrancesco, 2010). As Vm becomes more positive, HCN channels begin to close, If decreases, and voltage-gated Ca2+ channels 3.1 (CaV3.1) and 1.3 (CaV1.3) begin to open, generating the depolarising transient (T-type) current (ICa,T) and the CaV1.3 component of the long-lasting (L-type) Ca2+ current (ICa,L) that result in the continuation of DD (Mesirca et al., 2015). As diastole progresses, local release of Ca2+ from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR; known as “Ca2+ sparks”), occurring both spontaneously and being triggered by CaV1.3 Ca2+ influx (Torrente et al., 2016), results in an increase in cytosolic Ca2+. A portion of this Ca2+ is returned to the SR by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), with the remaining Ca2+ being extruded from the cell by the Na+—Ca2+ exchanger (with 3 Na+ ions entering the cell for every Ca2+ ion exiting), which generates an electrogenic, depolarising current (Lakatta et al., 2008). Ultimately, Vm reaches threshold for CaV1.2 channel opening (∼ −40 mV), generating additional ICa,L (Mesirca et al., 2015), which is responsible for the upstroke of the SAN AP and results in SAN excitation. Repolarisation of Vm then occurs through opening of rapid and slow delayed rectifier K+ currents, which causes the re-activation of If and the beginning of a new phase of DD (Mangoni and Nargeot, 2008). Importantly, DD is not prevented by inwardly rectifying K+ channels (which stabilise and maintain the negative resting Vm of working cardiomyocytes), as those channels are minimally expressed or absent in SAN myocytes (Bartos et al., 2015).
Sinoatrial node automaticity is strongly influenced by extracardiac and intracardiac factors, which alter transarcolemmal ion flux and intracellular Ca2+ handling, resulting in adaptation of HR to physiological changes. The principal extracardiac control of SAN function is via the central nervous system (CNS), acting through autonomic innervation of the heart (Ardell and Armour, 2016). Postganglionic sympathetic neurons project from the spinal cord and paravertebral ganglia directly to the cells of the heart (including in the SAN), where they release norepinephrine, which stimulates β-adrenergic receptors. The resulting Gαs-coupled intracellular signalling increases HCN, L-type Ca2+, and delayed rectifier K+ channel activity, as well as SR Ca2+ release, which increases the slope of DD, and thus the frequency of SAN firing and HR (MacDonald et al., 2020b). In contrast, preganglionic parasympathetic neurons project from the brainstem to stimulate postganglionic intracardiac neurons, a portion of which project directly to SAN cells. There they release acetylcholine, which stimulates muscarinic receptors, resulting in Gαi/o-coupled intracellular signalling (as well as Gβγ-mediated activation of G-protein-coupled inward rectifying K+ channels), which reduces DD slope and thus HR (MacDonald et al., 2020b). While much of the neuronal input to the heart originates outside the organ, there is growing evidence for significant intracardiac, autoregulatory neurohormonal control of cardiac function (including SAN automaticity) by the intracardiac nervous system (IcNS) (Stoyek et al., 2021a). The IcNS is composed of a complex network of afferent, efferent, and interneurons (forming local circuits), which are embedded within the tissues of the heart. While the IcNS has traditionally been viewed as simply a “relay station” for extracardiac neuronal input from the CNS, it is now appreciated that the IcNS may in fact perform local processing and integration of CNS signals, as well as form intracardiac autoregulatory reflex loops (Armour, 2008). SAN automaticity is also affected by a host of additional extra- and intracardiac factors, including other neurotransmitters, neuropeptides, and circulating and locally released autocrine, paracrine, and endocrine factors. Interestingly, direct electrical stimulation of CNS inputs to the SAN, or pharmacologic activation of autonomic receptors on SAN cells not only affect HR, but also the spatial nature of SAN excitation, causing shifts in the intra-nodal site of initial excitation (“leading pacemaker site”) (Opthof, 1988; Boyett et al., 2000; Lang and Glukhov, 2021).
The predominant intracardiac factor governing SAN automaticity, however, is tissue stretch. In a wide variety of mammals (except for mice), stretch of the SAN results in a nearly instantaneous increase in HR (Quinn and Kohl, 2012; Quinn et al., 2011a). This occurs through acute feedback of the SAN’s mechanical state to its electrical activity via subcellular mechano-electric coupling (or mechano-electric feedback) mechanisms (Quinn et al., 2014; Quinn and Kohl, 2021). SAN mechano-sensitivity has long been known to affect HR, and the rapid response of the SAN to stretch is critical for its adaptation to constantly changing physiological conditions. In particular, the SAN stretch response allows for the beat-by-beat matching of cardiac output to physiological fluctuations in venous return and for preventing excessive bradycardia and over-distension of the right atrium by opposing the baroreceptor response when both venous return and arterial pressure are increased (Quinn, 2015; MacDonald and Quinn, 2021). Diastolic mechanical load (i.e., preload) of the SAN may also be important for the regularity of baseline rhythm. During atrial diastole, the right atrium and SAN are stretched by both right atrial filling and contraction of the ventricles, which pulls the atrio-ventricular valve-plane apically. Peak SAN stretch levels coincide with the period of DD, as Vm is moving toward the threshold for AP generation, such that subthreshold stretch-induced depolarisation may mechanically “prime” SAN cells for excitation, facilitating their steady firing. At the same time, stretch-induced depolarisation may help normalise electrical activity across the node, by the electrical entrainment of SAN cells. Combined, these effects may act to specifically enhance DD and “smooth out” differences in automaticity between cells across the SAN, thus stabilising cardiac rhythm (MacDonald and Quinn, 2021). Overall, this has led some to propose that the SAN stretch response represents a third primary driver of pacemaker activity, which may be considered the mechanics “clock” (Quinn and Kohl, 2012; MacDonald and Quinn, 2021).
The zebrafish is an increasingly important experimental model for the study of cardiac (patho-) physiology (Gut et al., 2017; Stoyek and Quinn, 2018; Echeazarra et al., 2021). The zebrafish has a fully sequenced genome, which may be altered using standard genetic techniques (Rafferty and Quinn, 2018; Stoyek et al., 2021b), and almost every cardiac gene has a human ortholog with analogous function (Howe et al., 2013). Functionally, the zebrafish heart has HR, AP morphologies, ion channels (Vornanen and Hassinen, 2016; Ravens, 2018), and Ca2+-handling proteins (Rayani et al., 2018; van Opbergen et al., 2018) that are generally more similar to human than are rodent characteristics. There are also differences between the zebrafish and human heart to be considered, including the zebrafish heart having two (rather than four) chambers, relatively low pressures (Hu et al., 2001), a degree of genetic redundancy due to genome duplications (Howe et al., 2013), and a greater dependency of contraction on transarcolemmal Ca2+ flux than SR Ca2+ release (Rayani et al., 2018; van Opbergen et al., 2018), which may limit its use for some applications.
The function and genetics of the zebrafish SAN is similar to that of human. In zebrafish, the SAN is found in a ring-like structure at the venous pole of the heart (the border between the venous sinus and the atrium), embedded within the leaflets of the sinoatrial valve (Arrenberg et al., 2010; Tessadori et al., 2012; Stoyek et al., 2015, 2016; Abu Nahia et al., 2021). Its development parallels that of the mammalian SAN, occurring from isl1-, tbx-, bmp4-, and hcn4-expressing cells under the control of shox2 and facilitated by the suppression of nkx2.5 through Wnt signalling (Burkhard et al., 2017; Martin and Waxman, 2021; Minhas et al., 2021). The first indication of the currents involved in zebrafish SAN automaticity came from the discovery of a mutation (slow mo) that caused a reduction in HR by affecting a hyperpolarisation-activated inward current with similar properties to If (Baker et al., 1997; Warren et al., 2001). More recently, the general involvement of the membrane and Ca2+ “clocks” in SAN automaticity were investigated using pharmacological interventions, which confirmed the role of If (block of HCN channels reduced HR by ∼65%) and demonstrated a role for intracellular Ca2+ cycling (block of SR Ca2+ release with ryanodine, combined with block of SR Ca2+ reuptake with thapsigargin reduced HR by ∼40%) (Marchant and Farrell, 2019). Yet, the potential involvement of other membrane and Ca2+ “clock” mechanisms (i.e., ICa,T, ICa,L) and the relative importance of these two pacemaker systems for SAN automaticity in the zebrafish has not been explored.
In terms of the extra- and intracardiac control of SAN automaticity, it has been shown that the response of HR to: (i) direct electrical stimulation of cardiac vagal nerves (Stoyek et al., 2016, 2018, 2021a); (ii) pharmacologic activation of autonomic receptors on SAN cells (Schwerte et al., 2006; Stoyek et al., 2016); (iii) local paracrine signalling (e.g., natriuretic peptides (Bendig et al., 2006; Becker et al., 2014; MacDonald et al., 2017); or (iv) tissue stretch (Werdich et al., 2012; MacDonald et al., 2017) is also similar to human and other mammals. Yet, aspects related to pacemaker shift with autonomic input and the importance of mechanical preload for the regularity of baseline rhythm have yet to be explored.
In the present study, we sought to determine the drivers of SAN automaticity in zebrafish, by mirroring classic pharmacological experiments used for their determination in mammals (and in particular rabbit), combined with confocal anatomical imaging and immunofluorescence assessment of the presence and distribution of key proteins for the membrane and Ca2+ “clocks” in the zebrafish SAN. This was followed by an investigation of open questions related to the extra- and intracardiac control of HR by vagal nerve stimulation and SAN stretch, specifically whether nerve stimulation leads to intra-nodal pacemaker shift (as seen in rabbits and other mammals) and whether mechanical preload stabilises cardiac rhythm (with matching rabbit experiments, for a mammalian comparison). Our results demonstrate that the principal components of the coupled membrane-Ca2+ oscillator system that drives automaticity in mammals are also active in the zebrafish SAN, and that the effects of extra- and intracardiac control of HR seen in mammals are also present in the zebrafish, supporting the utility of the zebrafish as an experimental model for studies of SAN pathophysiological function.
All experimental procedures were approved by the Dalhousie University Committee for Laboratory Animals and followed the guidelines of the Canadian Council on Animal Care. Details of experimental protocols have been reported following the Minimum Information about a Cardiac Electrophysiology Experiment (MICEE) reporting standard (Quinn et al., 2011b).
Hearts were isolated from wild-type (AB) adult zebrafish (6–12 months post fertilisation), as previously described (Stoyek et al., 2015, 2016, 2017, 2018; MacDonald et al., 2017). Zebrafish were euthanised with tricaine (1.5 mM MS-222; E10521, Sigma-Aldrich, Oakville, ON, Canada) in Tris-buffered (pH 7.4; BP152, Fisher Scientific, Ottawa, ON, Canada) room temperature tank water, until they showed no opercular respiration movements and lacked a locomotor response to fin pinch (∼2 min). They were then placed in a Sylgard-lined petri dish (DC 170, Dow Corning, Midland, MI, United States) filled with modified Krebs—Henseleit (KH) solution containing (in mM): 120 NaCl; 4.7 KCl; 26 NaHCO3; 1.4 NaH2PO4; 1.0 MgCl2; 1.8 CaCl2; 5.0 Glucose, with an osmolality of 290 ± 5 mOsm/kg and a pH of 7.40 ± 0.05 when bubbled with carbogen (95% O2, 5% CO2) at 28°C (additional details provided in Supplementary Table 1). A ventral midline incision was made through the body wall and pericardium to expose the heart. The heart was removed via incisions at the venous sinus (venous pole) and the ventral aorta (arterial pole) and placed in a separate Sylgard-lined petri dish and with 15 mL of the same modified KH solution bubbled with carbogen but maintained at 28.0 ± 0.5°C (physiological zebrafish temperature) with a temperature-controlled (TC-344C, Warner Instruments, Hamden, CT, United States) warmed platform (WP-16, Warner Instruments, Hamden, CT, United States). The heart was pinned in the dish with two needle electrodes (12 mm, 29 gauge; MLA1213, ADInstruments, Colorado Springs, CO, United States) at the arterial and venous poles of the heart, with a third reference electrode at the edge of bath. The electrodes were connected to an ECG amplifier (Animal Bio Amp, ADInstruments, Colorado Springs, CO, United States) and bath temperature was monitored using a thermocouple (T-type Pod, ADInstruments, Colorado Springs, CO, United States). Temperature and ECG signals were continuously recorded for the entire experiment at 2 kHz using a data acquisition device (PowerLab, ADInstruments, Colorado Springs, CO, United States) controlled by LabChart (ADInstruments, Colorado Springs, CO, United States). All experiments were performed in spontaneously beating hearts.
After a 10 min acclimatisation period, antagonists to the known principal components of the coupled membrane-Ca2+ oscillator pacemaker system in mammals were applied to the heart via the addition of stock solutions at the edge of the bath, followed by mixing via aspiration of solution with a transfer pipette. Concentrations and time of application were in the range previously used for rabbit, unless otherwise noted (additional details provided in Supplementary Table 2): (i) ivabradine hydrochloride for intracellular block of HCN channels (3 μM, 40 min) (Thollon et al., 1994, 2007; Bucchi et al., 2007; Yaniv et al., 2012, 2013, 2014); (ii) cesium chloride (CsCl) for extracellular block of HCN channels (3 mM, 7 min) (Noma et al., 1983; Dennis and Williams, 1986; Denyer and Brown, 1990; Leitch et al., 1995; Nikmaram et al., 1997; Zhang and Vassalle, 2000; Bogdanov et al., 2006; Thollon et al., 2007); (iii) nickel(II) chloride (NiCl2) for block of T-type Ca2+ channels (165 μM, 15 min; previous studies in rabbit used 40–100 μM (Hagiwara et al., 1988; Satoh, 1995), however previous work in zebrafish involving current measurements by patch clamp used a higher concentration (Nemtsas et al., 2010), which preliminary experiments demonstrated was more effective); (iv) ryanodine for block of RyR (1 μM, 15 min) (Hata et al., 1996; Satoh, 1997; Bogdanov et al., 2001, 2006; Vinogradova et al., 2002; Bucchi et al., 2003, 2007; Lancaster et al., 2004; Lyashkov et al., 2007); (v) BAPTA-AM for chelation of cytosolic Ca2+ (5 μM, 30 min exposure, followed by 30 min in modified KH alone to allow for de-esterification of the AM group) (Vinogradova et al., 2000; Bogdanov et al., 2006); and (vi) nifedipine for block of L-type Ca2+ channels (0.2 μM, 15 min) (Kawai et al., 1981; Senges et al., 1983; Satoh and Tsuchida, 1993; Masumiya et al., 1997; Budriesi et al., 1998; Bogdanov et al., 2006). Controls included: (i) 60 min with modified KH alone (time control); (ii) 30 min with 0.05% dimethyl sulfoxide (DMSO, followed by 30 min in modified KH alone for comparison with BAPTA-AM); and (iii) 15 min with 0.05% ethanol. HR (in beats per minute, bpm) was calculated from the R-waves of the ECG using custom routines in Matlab (MathWorks, Natick, MA, United States) and averaged over 3 min immediately before drug application and 3 min after the end of the drug incubation period. Stability of HR was assessed as the inter-beat variability of cycle length (CL) by calculating the root mean square of its successive differences (RMSSDCL) over each 3 min period.
As in previous work (Stoyek et al., 2015, 2016, 2018; MacDonald et al., 2017), to expose the SAN, the ventricle was removed via resection at the atrioventricular junction, the atrium cut open, laid epicardial side down, and pinned flat. For functional experiments, a suction microelectrode, composed of a pulled glass capillary (1.00/0.58 mm outer/inner diameter; 1B100, World Precision Instruments, Sarasota, FL, United States) in a microelectrode holder (MPH6R10, World Precision Instruments, Sarasota, FL, United States) connected to the ECG amplifier, was positioned with a three-axis rack and pinion stage (62041, Edmond Optics, Barrington, IL, United States) at the edge of the atrium and suction applied with a 1 ml syringe to measure the local ECG, in combination with a 26-27 gauge polytetrafluoroethylene coated silver wire (AGT1510, World Precision Instruments, Sarasota, FL, United States) reference electrode positioned at the edge of the bath.
For immunofluorescence assessment of the presence and distribution of HCN channels and RyR in the zebrafish SAN, atria were isolated from zebrafish expressing eGFP under the myocyte-specific myl7 promoter [tg(myl7:eGFP)] for visualisation of the cardiac musculature. As previously described (Stoyek et al., 2015, 2016, 2018), the atria were fixed overnight in 2% paraformaldehyde (PFA; RT-15710, Electron Microscopy Sciences, Hatfield, PA, United States) with 1% DMSO (BP231-1, Sigma-Aldrich, Oakville, ON, Canada) in phosphate-buffered saline (PBS, P3813, Sigma-Aldrich, Oakville, ON, Canada). The atria were then rinsed three times for 15 min each in PBS and transferred to a solution containing 0.1% Triton X-100 (PBS-T; T9284, Sigma-Aldrich, Oakville, ON, Canada) in PBS with primary antibodies for HCN4 channels (1:50; APC-052, Alomone Labs, Jerusalem, Israel) and RyR (1:100; MA3-916, Fisher Scientific) and incubated for 3–5 days with agitation at 4°C (additional details for antibodies and solutions provided in Supplementary Tables 3, 4). Tissues were rinsed three times for 15 min each in PBS-T and transferred to PBS-T containing the appropriate secondary antibody (1:300, AlexaFluor555 or AlexaFluor647; A-21429 or A-21236, Fisher Scientific – additional details provided in Supplementary Table 3) for 3–5 days with agitation at 4°C. Final rinsing was done in PBS and specimens were placed in Scale CUBIC-R1 clearing solution (Susaki et al., 2014; additional details provided in Supplementary Table 4) overnight at room temperature with gentle agitation. Atria were then mounted on glass slides in CUBIC-R1 for confocal microscopy.
Processed specimens were examined as whole-mounts using an LSM 510 confocal microscope (Carl Zeiss, Toronto, ON, Canada) with a plan-apochromat 10 × , 0.45 NA (SF25, Carl Zeiss) or 40 × , 0.80 NA (LCI Plan-Neofluar, Carl Zeiss) objective. Preparations were epi-illuminated with a 488 nm argon laser and 543 nm and 605 nm helium-neon lasers reflected by a 488/543/633 nm dichroic mirror (HFT 488/543/633; Carl Zeiss). Emitted fluorescence was collected using 480-520 nm and 530-585 nm band-pass filters and a 615 nm long-pass filter (Carl Zeiss). Confocal image Z-stacks were acquired and processed using Zeiss Zen2009 software from regions of interest surrounding immunoreactive tissues (omission of primary or secondary antibodies resulted in absence of immunoreactivity), which ranged from 50–350 μm in depth, including a region of 10–25 μm above and below the region of interest to ensure that all relevant structures were captured, while limiting issues of light scattering associated with deeper tissue scans. Figure plates were constructed from images processed with Photoshop (CS6, Adobe, Mountain View, CA, United States). Brightness and contrast of some images were adjusted to ensure panel-to-panel consistency in each figure.
Voltage optical mapping was performed to image excitation of the zebrafish SAN, as previously described (Stoyek et al., 2016, 2018). Isolated atria were left to acclimatise for 30 min, during the last 10 min of which a voltage-sensitive dye was added to the bath (10 μM, di-4-ANBDQPQ; provided by Leslie Loew, Richard D. Berlin Center for Cell Analysis and Modeling, University of Connecticut Health Center, Farmington, CT, United States). The atria were then washed with fresh modified KH solution containing the excitation-contraction uncoupler ( ± )-blebbistatin (10 μM; 13186, Cayman Chemical, Ann Arbor, MI, United States) to eliminate contraction-induced artifacts in optical recordings and left for an additional 30 min to allow blebbistatin to act. Preparations were epi-illuminated through a macro zoom microscope (MVX10, Olympus, Toronto, ON, Canada) with a mercury lamp (U-HGLGPS, Olympus, Toronto, ON, Canada) passed through a 630-650 nm band-pass filter (D640/20X, Chroma Technology, Bellows Falls, VT, United States) and reflected by a 685 nm dichroic mirror (FF85-Di02, Semrock, Rochester, NY, United States). Emitted fluorescence was collected with a 2 × , 0.50 NA objective (MV PLAPO 2XC, Olympus, Toronto, ON, Canada) at 4-6.3 × magnification through a 700 nm long-pass filter (ET700LP, Chroma Technology, Bellows Falls, VT, United States). Recordings were captured by a 128 × 128 pixel, 16-bit electron-multiplying charge-coupled device camera (Cascade 128+, Photometrics, Tucson, AZ, United States) at 511 frames/s using MultiRecorder software (provided by Johannes Schröder—Schetelig and Stefan Luther, Max Planck Institute for Dynamics and Self-Organization, Göttingen, Germany). To identify the site of initial SAN excitation, binary images were created for three successive cardiac cycles and the initiation site averaged using custom Matlab routines. An oval was overlaid on the image to fit the SAN and the binary images were mapped to a brightfield exemplar image of the SAN by rigid transformation using Fiji software (Schindelin et al., 2012).
To check for possible effects of blebbistatin on zebrafish electrophysiology (which has been shown in embryonic isolated hearts to have no effects; Jou et al. (2010), but has not been tested in adult hearts), intracellular microelectrode recordings of SAN Vm in isolated atrium (as well as recording of Vm in the atrium and ventricle of the whole heart) were acquired as previously described (Kopton et al., 2018), with and without application of blebbistatin. Microelectrodes made from borosilicate glass capillaries (1.0/0.5 mm outer/inner diameter, with internal filament; type BF/100/50/10, Sutter Instruments; Novato, AZ, United States) were pulled on a Brown/Flaming micropipette puller (Model P97, Sutter Instruments) to tip diameters resulting in a 40–60 MΩ resistance when filled with 3 M KCl. Electrodes were coupled to the headstage of an amplifier (Model 1600 Neuroprobe Amplifier, A/M Systems; Everett, WA, United States) operated in current clamp mode with an electrode holder (ESW-M10N, Warner Instruments, Hamden, CT, United States). Electrodes were advanced with a mechanical manipulator (MX/4, Narishige Group, Amityville, NY, United States) into the myocardium. Before cell penetration, the tip potential of the electrode was nulled using the bridge controls of the intracellular amplifier with the electrode tip in the bath. At the end of a recording, the microelectrode was withdrawn from the cell, the null potential was checked, and the previous data adjusted if necessary. Vm was taken as the difference between the potential measured in the bath with a silver/silver-chloride lead and the intracellular potential. Successful impalement was signalled by a sudden step of the electrode potential to a negative value. Vm was recorded at 10 kHz with the PowerLab data acquisition device controlled by LabChart (ADInstruments, Colorado Springs, CO, United States). From the Vm recordings, characteristics of the SAN, atrial, and ventricular cell AP were calculated, using custom routines in Matlab following previously described algorithms (Bucchi et al., 2007; Logantha et al., 2019). For the SAN, these included: (i) maximum diastolic potential (MDP), defined as the most negative Vm reached during AP repolarisation; (ii) slope of DD, defined as the change in Vm over the time between the point of MDP and the initiation of the AP, which was determined as the point at which the rate of change of Vm was greater than 500 mV/s; (iii) maximum rate of change of Vm during the upstroke (dV/dtmax); (iv) maximum Vm during the AP; (v) AP amplitude, defined as the change in Vm between the initiation and peak of the AP; and (vi) AP duration at 50% (APD50) and 80% (APD80) repolarisation, defined as the time between the initiation of the AP and the restoration of Vm from peak to 50% or 80% of MDP. For the atrium and ventricle, resting Vm was calculated as the average value over the 25 ms immediately preceding the initiation of the AP.
Vagal nerve stimulation was performed as previously described (Stoyek et al., 2016, 2017, 2018). Approximately 1 mm of the rami of the cardiac branches of the left and right vagal nerves were exposed in the walls of the ducts of Cuvier. Bipolar silver wire electrodes were positioned on each with three-axis rack and pinion stages (MM-3, Narishige Group) and connected to constant current isolation units (PSIU6, Grass Instruments, Quincy, MA, United States) driven by a dual output square pulse stimulator (S88, Grass Instruments). The left or right vagal nerves were stimulated with trains of rectangular pulses (300 μA, 15 Hz, 0.5 ms pulse width, 10 s train duration). The site of initial SAN excitation was determined as baseline, during vagal nerve stimulation, and during vagal nerve stimulation after 15 min of exposure to the nicotinic receptor antagonist hexamethonium bromide (10 μM; H0879, Sigma-Aldrich, Oakville, ON, Canada).
Sinoatrial node were isolated from hearts of female rabbits (New Zealand White, 2.1 ± 0.2 kg), as previously described (MacDonald et al., 2020a). Rabbits were euthanised by ear vein injection of pentobarbital (140 mg/kg), followed by rapid heart excision, aortic cannulation, and Langendorff perfusion (20 mL/min) with modified KH solution containing (in mM): 120 NaCl; 4.7 KCl; 24 NaHCO3; 1.4 NaH2PO4; 1.0 MgCl2; 1.8 CaCl2; 10.0 glucose, with an osmolality of 300 ± 5 mOsm/kg and a pH of 7.40 ± 0.05 when bubbled with carbogen at 37 ± 0.5°C (additional details provided in Supplementary Table 1). The atria were removed by cutting along the atrio-ventricular valve plane and placed in a Sylgard-lined dish filled with the same modified KH solution at 37°C and bubbled with carbogen. The edge of each atrial appendage was pinned down with the bottom of the atrial appendages (which normally rest against the ventricles) face up, taking care to not strain tissue beyond its inherent dimensions. The endocardial side of the SAN was exposed by making incisions along the front surface of the superior and inferior Venae cavae (SVC and IVC). Excess fat and pericardial tissue were carefully removed from the underside of the preparation, without pulling or stretching the tissue. The rabbit SAN was then dissected along the medial edge of the crista terminalis and the interatrial septum.
Zebrafish SAN stretch was performed as previously described (MacDonald et al., 2017). Two custom-made micro-sized hooks (∼100 μm in diameter), created from glass capillaries (2.00/1.12 mm outer/inner diameter; 1B200F, World Precision Instruments, Sarasota, FL, United States), were positioned opposing each other in the long-axis direction inside the SAN ring under an upright microscope (BX63, Olympus, Toronto, ON, Canada) with a 5 × , 0.15 NA objective (MPlanFL N, Olympus, Toronto, ON, Canada), such that the SAN tissue rested in the loops of the hooks without tissue puncture or damage. The hooks were held by microelectrode holders (MPH110, World Precision Instruments, Sarasota, FL, United States) that were coupled to three-axis hydraulic micromanipulators for fine positioning (MHW-103, Narishige International, East Meadow, NY, United States). One micromanipulator was mounted to a piezoelectric linear translator (P-621.1CD, Physik Instrumente, Auburn, AL, United States) for application of nm resolution computer-controlled movement (USB-6361, operated by custom routines developed in LabView, National Instruments, Austin, TX, United States), while the other remained stationary and was attached to an isometric force transducer (PY2 72-4491, Harvard Apparatus, Saint-Laurent, QC, Canada). Both units were fixed to stages on a rack and pinion track for coarse positioning (56798, Edmond Optics, Barrington, IL, United States). The ECG of the slack SAN was recorded, followed by application of ∼5 mg of tension to the tissue by slowly separating the hooks with the piezoelectric translator. HR and the RMSSDCL were calculated from the ECG signal for the 30 s immediately before and the 30 s immediately after the application of mechanical preload using custom routines in Matlab.
Rabbit SAN stretch was performed as previously described (MacDonald et al., 2020a). Following SAN isolation, insect pins were woven through the cut edges of the SVC and IVC above and below the SAN tissue. A clip hanging from the isometric force transducer was attached to the SVC pin. A clip coupled to a computer-controlled linear DC-servomotor (LM 1247-02-01; FAULHABER MICROMO, Clearwater, FL, United States) was attached to and supported the IVC pin with bipolar electrodes at either end of the pin connected to the ECG amplifier. The entire SAN was immersed in a water-jacketed chamber containing the modified KH solution at 37°C and bubbled with carbogen. The ECG of the slack SAN was recorded, followed by application of ∼300 mg of tension to the tissue by slowly lowering the IVC pin with the DC-servomotor. As for the zebrafish, HR and RMSSDCL were calculated from the ECG signal for the 30 s immediately before and the 30 s immediately after the application of mechanical preload using custom routines in Matlab.
Data are shown as mean ± standard error of the mean (SEM). Group means were compared either by paired or unpaired, two-tailed, Student’s t-tests or one-way, unpaired ANOVA and Tukey post-hoc tests, with a significance value of p < 0.05.
Figures 1A,B show the effects of pharmacological block of membrane “clock” components on HR and RMSSDCL in the isolated zebrafish heart (measured values are provided in Supplementary Table 5). Both If antagonists caused a decrease in HR (ivabradine: −168 ± 15 bpm or −86 ± 3%, n = 7, p < 0.001; cesium: −140 ± 15 bpm or −73 ± 4%, n = 6, p < 0.001) and an irregular inter-beat interval, so were associated with an increase in RMSSDCL (ivabradine: +104 ± 25 ms, p = 0.006; cesium: +41 ± 13 ms, p = 0.023). The antagonist for ICa,T (nickel) also caused a decrease in HR (−18 ± 6 bpm or −10 ± 3%, n = 7, p = 0.016), but without a change in RMSSDCL (p = 0.100). There was no change HR or RMSSDCL in the time (Figures 1E,F; n = 7, p = 0.24, and p = 0.34) or vehicle (DMSO: n = 6, p = 0.30, and p = 0.66; ethanol: n = 6, p = 0.24, and p = 0.95) controls. There was no relationship between RMSSDCL and the underlying HR for any of the pharmacologic agents used to block components of the membrane “clock.”
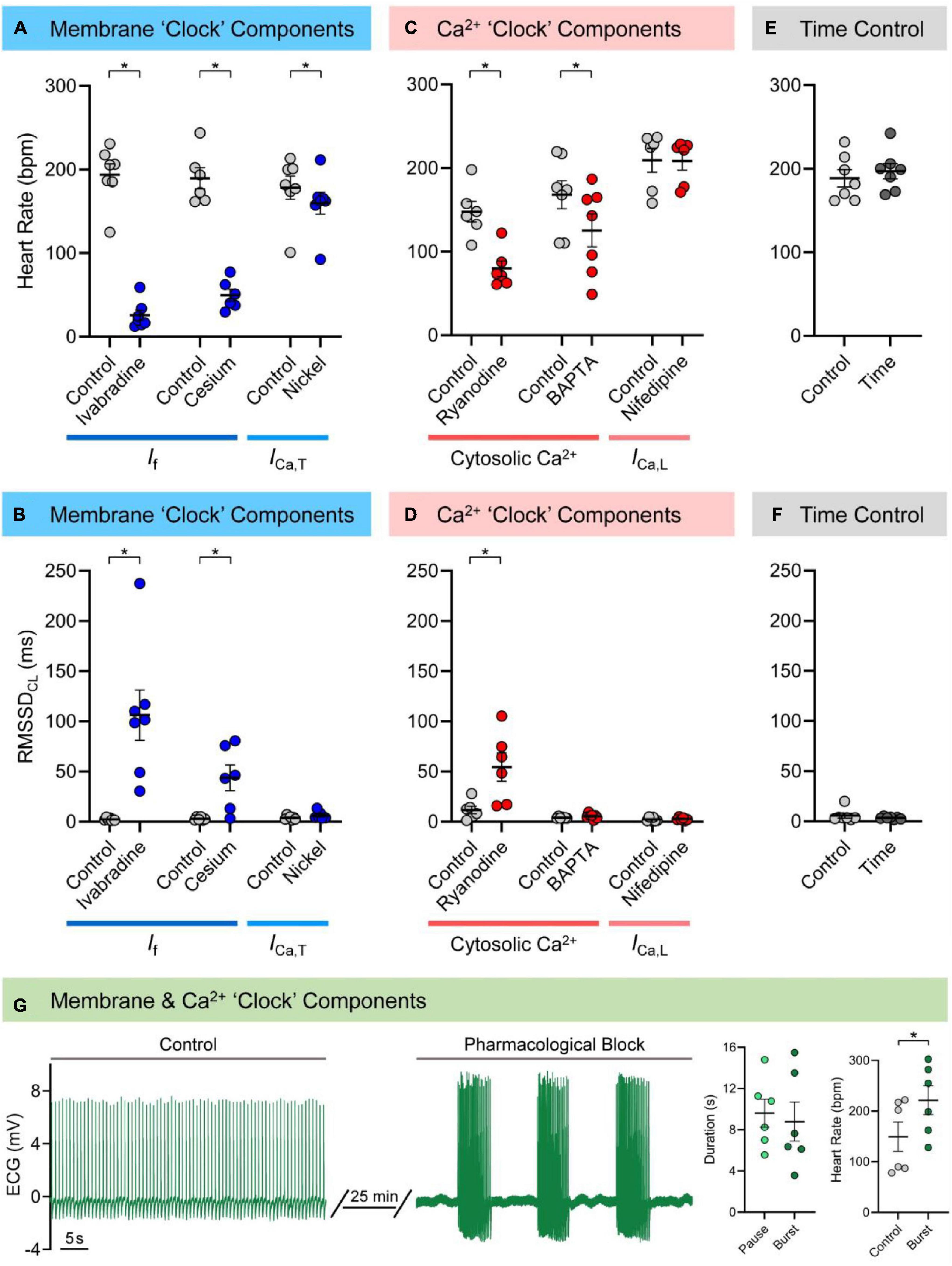
Figure 1. Mechanisms of zebrafish sinoatrial node (SAN) automaticity. Effects of pharmacological block of membrane (40 min of 3 μM ivabradine hydrochloride for intracellular block of “funny” current, If, n = 7; 7 min of 3 mM cesium chloride for extracellular block of If, n = 6; 15 min of 165 μM nickel(II) chloride for block of T-type Ca2+ current, ICa,T, n = 7) or Ca2+ (15 min of 1 μM ryanodine for block of ryanodine receptors, n = 6; 30 min exposure of 5 μM BAPTA-AM, followed by 30 min to allow for de-esterification of the AM group, for chelation of cytosolic Ca2+, n = 7; 15 min of 0.2 μM nifedipine for block of L-type Ca2+ current, ICa,L, n = 6) “clock” components, as well as matched time controls (60 min, n = 7) on heart rate (A,C,E) and the inter-beat variability of cycle length (measured as the root mean square of its successive differences, RMSSDCL; B,D,F), in the isolated zebrafish heart. Representative electrocardiogram (ECG) recording of zebrafish cardiac rhythm in control and after combined pharmacological block of membrane and Ca2+ “clock” components (3 mM cesium chloride, 165 μM nickel(II) chloride, and 1 μM ryanodine, n = 6) and measurements of the resulting SAN pause and burst duration and control and burst heart rate (G). Data shown as mean ± SEM. Means compared by paired, two-tailed, Student’s t-tests; *p < 0.05.
Figures 1C,D show the effects of pharmacological block of Ca2+ “clock” components on HR and RMSSDCL in the isolated zebrafish heart (measured values are provided in Supplementary Table 5). Block of RyR with ryanodine decreased HR (−68 ± 14 bpm or −45 ± 6%, n = 6, p = 0.005) and resulted in an irregular inter-beat interval, so was associated with an increase in RMSSDCL (+43 ± 15 ms, p = 0.036). Exposure to BAPTA to chelate cytosolic Ca2+ also decreased HR (−43 ± 15 bpm or −26 ± 8%, n = 7, p = 0.029), but did not affect RMSSDCL (p = 0.198). In contrast, block of ICa,L with nifedipine had no effect on HR (p = 0.813) or RMSSDCL (p = 0.626). There was no relationship between RMSSDCL and the underlying HR for any of the pharmacologic agents used to block components of the Ca2+ “clock.”
Figure 1G shows the effects of the combined pharmacological block of membrane and Ca2+ “clock” components (If, ICa,T, and RyR with cesium, nickel, and ryanodine) on SAN automaticity. The combined effect took between 10–25 min to stabilise, at which point there were long pauses in beating, followed by periods of a similar duration in which there were bursts of heart beats (9.6 ± 1.4 vs. 8.8 ± 1.9 s; p = 0.628). The HR of the bursts was greater than the baseline HR before pharmacological block (221 ± 28 vs. 149 ± 29 bpm; p = 0.048). Due to the nature of the heart rhythm after simultaneous pharmacological block of membrane and Ca2+ “clock” components (involving sinus pauses and bursts), RMSSDCL was not measured in this case.
Figure 2 shows the detailed structure of the zebrafish SAN (Figures 2A–D) and the expression of HCN4 channels and RyR, acquired by confocal imaging (Figures 2E–G). The genetically encoded eGFP, under the control of the myocyte-specific myl7 promoter (tg(myl7:eGFP)), revealed a ring of cells at the venous pole of the heart completely surrounding the sinoatrial valve opening (Figure 2B). Cells within this ring were identified by a distinct spindle-shaped cellular morphology that varied with depth in the SAN and showed a reduced (but variable) expression of contractile apparatus (Figure 2C), distinct from myocytes in the atrial wall (Figure 2D). Immunofluorescence showed the highest HCN4 immunoreactivity in SAN cells, with minimal immunoreactivity observed beyond the proximal ends of atrial trabeculae connecting to the SAN (Figure 2E). Immunoreactivity of RyR was also highest in the SAN, but still observed at lower levels in the atrium (Figure 2F). In general, there was high cellular co-expression of HCN4 and RyR (Figure 2G).
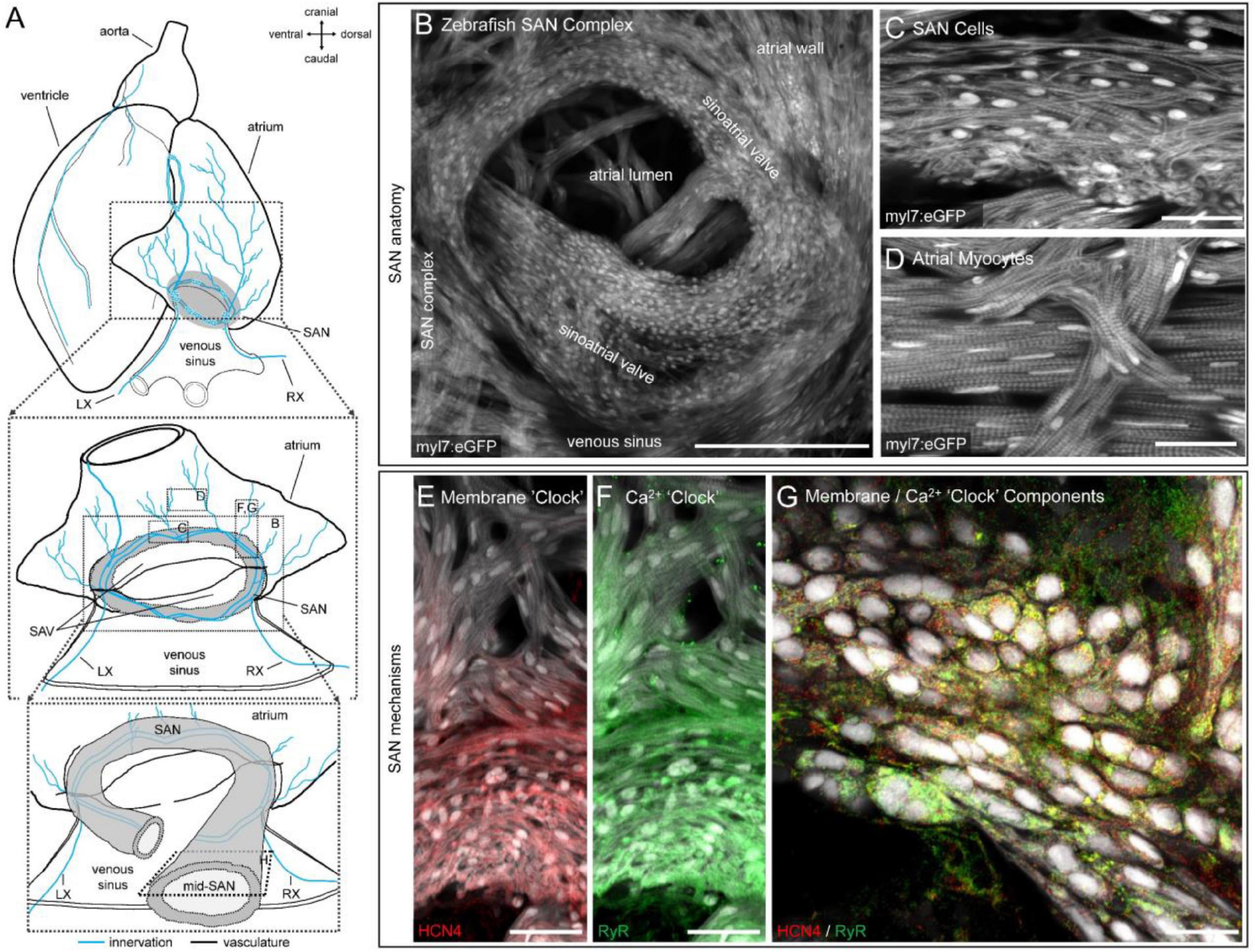
Figure 2. Structure and channel expression in the zebrafish sinoatrial node (SAN). Schematic of the zebrafish heart illustrating the location of the images in B-H (A). Overview of the zebrafish SAN complex visualised by eGFP fluorescence in myl7-expressing cardiomyocytes (B). Morphology of SAN cells (C) and atrial myocytes (D) in a 5 μm confocal z-projection. Immunofluorescence of hyperpolarisation-activated cyclic nucleotide-gated channel-4 (HCN4, red; E) and ryanodine receptor (RyR, green; F) expression in a 50 μm confocal z-projection through the SAN and proximal atrial myocardium. Cellular distribution of RyR and HCN4 in a single optical plane from approximately the mid-SAN (G). Scale bars (in μm): (B): 200 μm; (C,D): 25 μm; (E,F): 40 μm; (G): 25 μm. LX, left cardiac branch of the vagal nerve; RX, right cardiac branch of the vagal nerves; SAV, sinoatrial valve.
Figure 3 shows the initial site(s) of SAN excitation and the effect of left or right vagal nerve stimulation determined by voltage optical mapping. At baseline, the site of initial excitation varied between subjects, and was multi-focal in some cases (n = 2/8; Figures 3B,E). During left (LVNS; Figure 3C) or right (RVNS; Figure 3F) vagal nerve stimulation, there was a change in the location of initial excitation in each subject, with a greater incidence of multi-focal activity (left: n = 4/8; right: n = 6/8). After application of the nicotinic receptor blocker hexamethonium, the effect of vagal nerve stimulation on the initial site of SAN excitation was abolished.
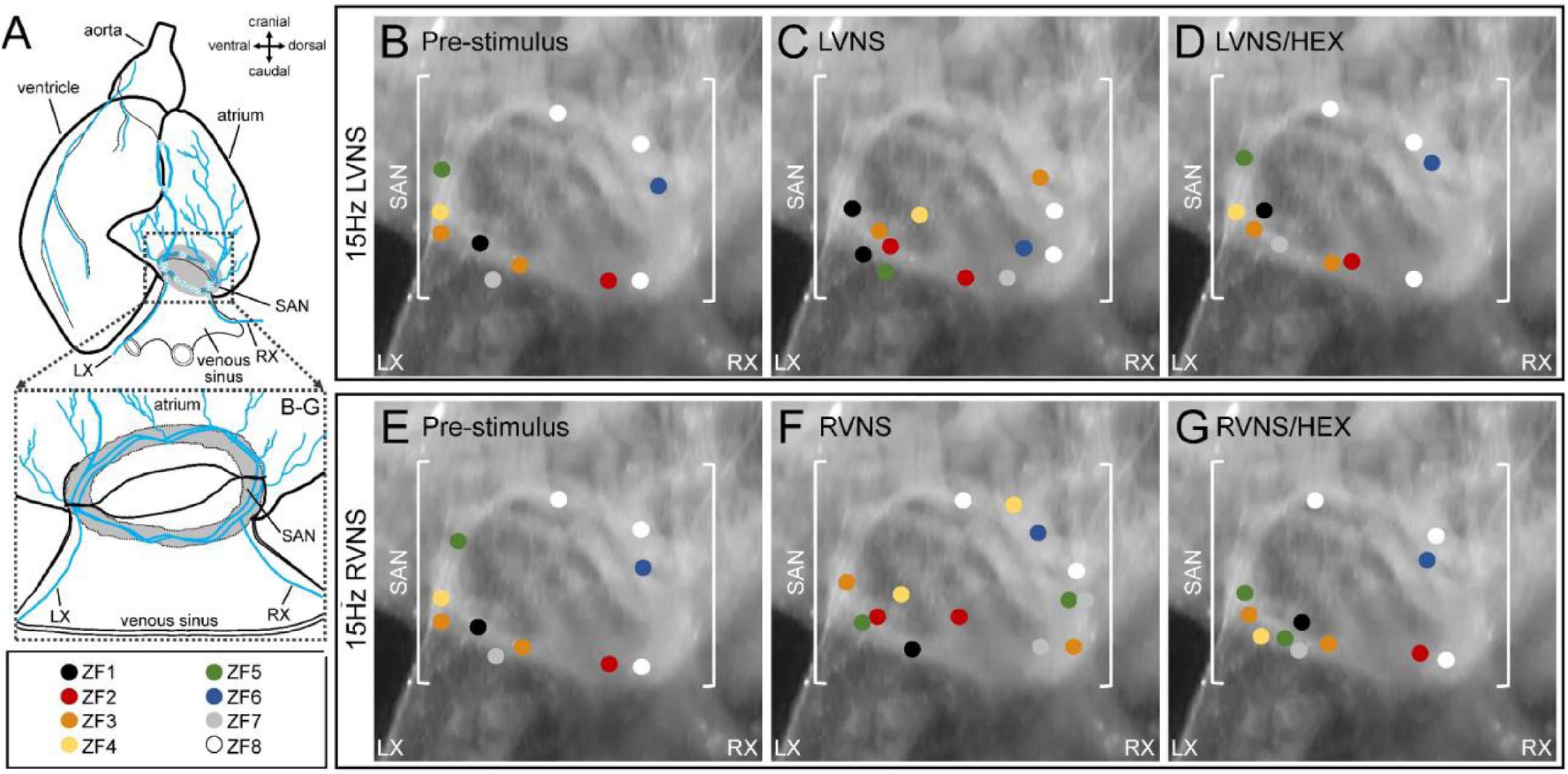
Figure 3. Effect of vagal nerve stimulation on leading pacemaker site of the zebrafish sinoatrial node (SAN). Schematic of the zebrafish isolated heart showing the location of the SAN and the intact left (LX) and right (RX) cardiac branches of the vagal nerve (A). The leading pacemaker site(s) measured in individual SAN before nerve stimulation (B,E), during left (LVNS, C) or right (RVNS, F) vagal nerve stimulation at 15 Hz, and during LVNS and RVNS after application of the nicotinic receptor blocker hexamethonium (D,G), projected onto a representative SAN image, with individual subjects differentiated by colour (n = 8).
Figure 4 shows AP characteristics from cells within the SAN, atrium, and ventricle with and without blebbistatin (measured values are provided in Supplementary Table 6). SAN cells had a less negative minimum Vm than atrial and ventricular cells, a lower dV/dtmax, lower AP amplitude and max Vm, and a shorter APD50 and APD80 (Figures 4F–K). Atrial cells had a shorter APD50 and APD80 than ventricular cells (Figures 4J,K). In the SAN, blebbistatin resulted in a reduction of the slope of DD (−39%, p = 0.028; Figure 4E), however the effect was not large enough to cause a reduction in HR (p = 0.463; although HR did become more variable, Figure 4D). In the atrium, blebbistatin resulted in an increase of APD50 and APD80 (+13% and +9%, p = 0.006 and p = 0.012; Figures 4J,K) and in the ventricle a decrease of APD80 (−9%, p = 0.003; Figure 4K). No other AP parameters were affected by blebbistatin (Figures 4F–K).
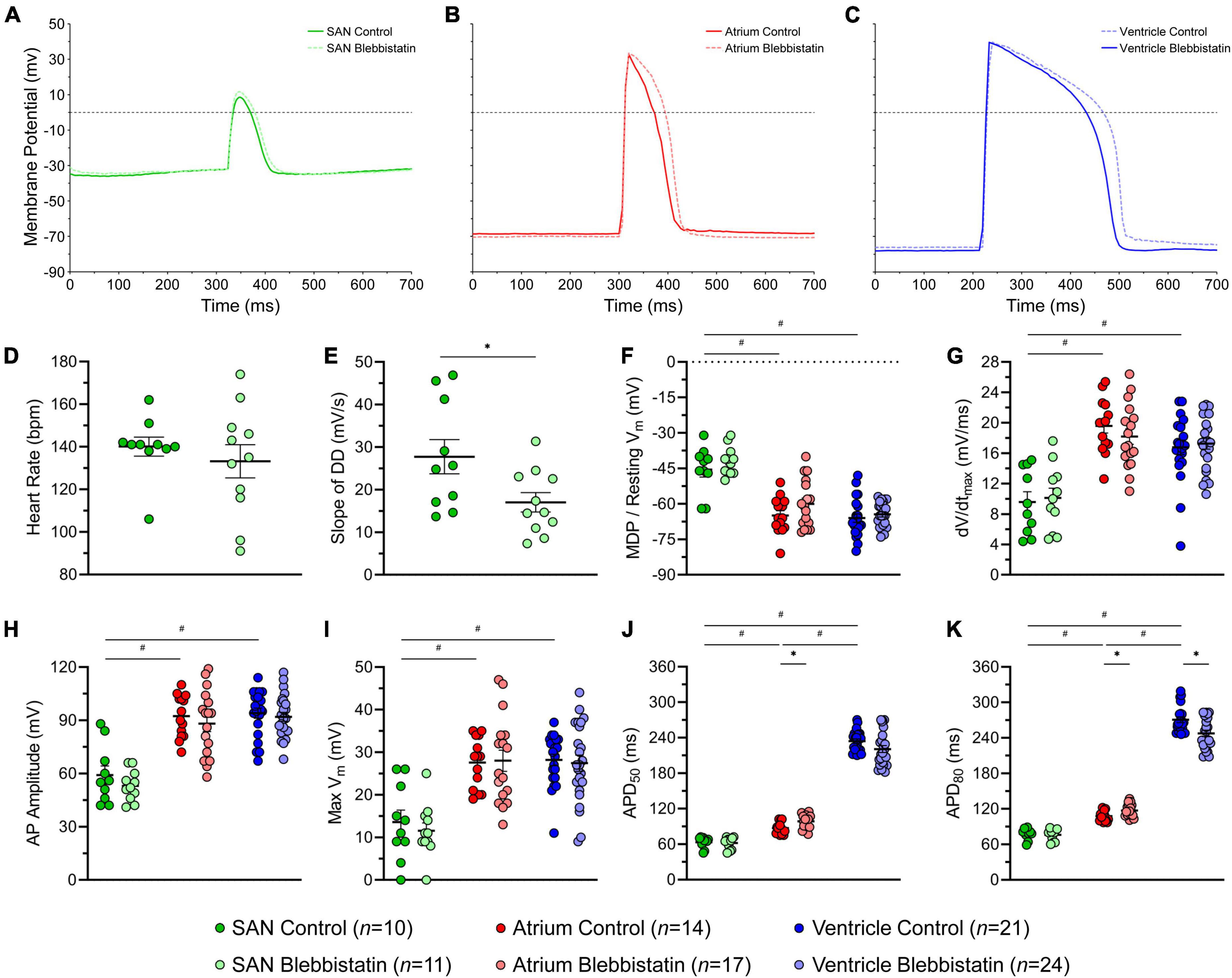
Figure 4. Effects of blebbistatin on the zebrafish sinoatrial node (SAN), atrial, and ventricular action potential (AP). Representative AP (A–C) and measured parameters (D–K) in the SAN (green), atrium (red), and ventricle (blue) with (dark) and without (light) blebbistatin (10 μM for 30 min). APD50 and APD80, AP duration at 50% and 80% repolarisation; DD, diastolic depolarisation; dV/dtmax, maximum rate of change of membrane potential during the AP upstroke; MDP, maximum diastolic potential; Vm, membrane potential. Data shown as mean ± SEM. SAN, atrium, and ventricle compared by unpaired, one-way ANOVA and Tukey post hoc tests; #p < 0.05. Control and blebbistatin compared by unpaired, two-tailed, Student’s t-tests; *p < 0.05. n indicates number of impalements, which were acquired in N = 6 hearts.
Figure 5 shows the HR and RMSSDCL of the slack or mechanically preloaded isolated zebrafish (n = 8) and rabbit (n = 8) SAN. When slack, the beating of the SAN in both species was irregular, as seen from the ECG traces (Figures 5A,D) and the beat-by-beat measurements of HR (Figures 5B,E). With the application of a static preload (zebrafish: 5.0 ± 0.7 mg; rabbit: 307.3 ± 49.3 mg), beating became much more rhythmic. This was associated with an increase in HR of the zebrafish (132 ± 12 vs. 166 ± 8 bpm; 30 ± 9%, p = 0.003; Figure 5C) but not the rabbit (195 ± 11 vs. 196 ± 8 bpm; p = 0.915; Figure 5F) SAN, while RMSSDCL decreased in both species (74.9 ± 15.1 vs. 10.1 ± 2.2 ms; −78 ± 7%, p = 0.004 and 9.9 ± 3.0 vs. 1.4 ± 0.3 ms; −64 ± 14%, p = 0.031; Figures 5C,F).
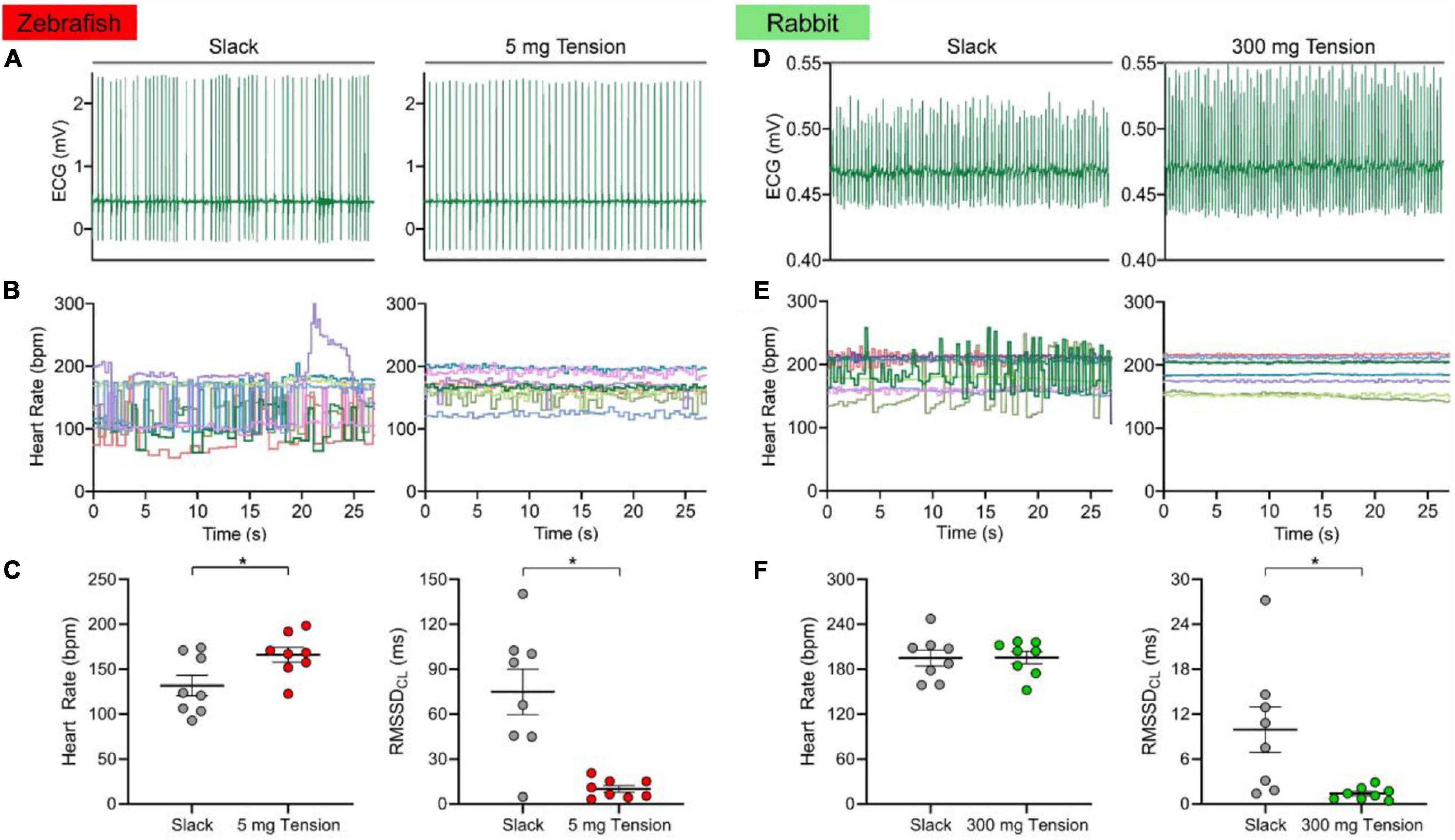
Figure 5. Effect of mechanical preload on zebrafish and rabbit sinoatrial node automaticity. Representative electrocardiogram (ECG) recording of zebrafish (A) and rabbit (D) sinoatrial node rhythm under unloaded (slack) or mechanically preloaded (5 mg or 300 mg) conditions. Heart rate over time of individual zebrafish (B, n = 8) and rabbit (E, n = 8) sinoatrial nodes when mechanically unloaded or preloaded. Average heart rate and the inter-beat variability of cycle length (measured as the root mean square of its successive differences, RMSSDCL) of the zebrafish (C) and rabbit (F) sinoatrial node when mechanically unloaded or preloaded. Data shown as mean ± SEM. Means compared by paired, two-tailed, Student’s t-tests; *p < 0.05.
In this study, we explored open questions related to SAN automaticity in the zebrafish. Through pharmacological experiments based on those previously performed in mammals, we have demonstrated that the cellular mechanisms responsible for SAN automaticity are similar in the zebrafish (see summary in Figure 6). Further, we have shown that the principal extracardiac (e.g., autonomic nervous system) and intracardiac (e.g., mechanical preload) mechanisms controlling HR in mammals operate in a similar manner in the zebrafish SAN, resulting in a shift in the leading pacemaker site and a stabilisation of cardiac rhythm, respectively. Overall, these results add further support to previously published work suggesting the zebrafish as an experimental model for studies of SAN (patho-) physiological function.
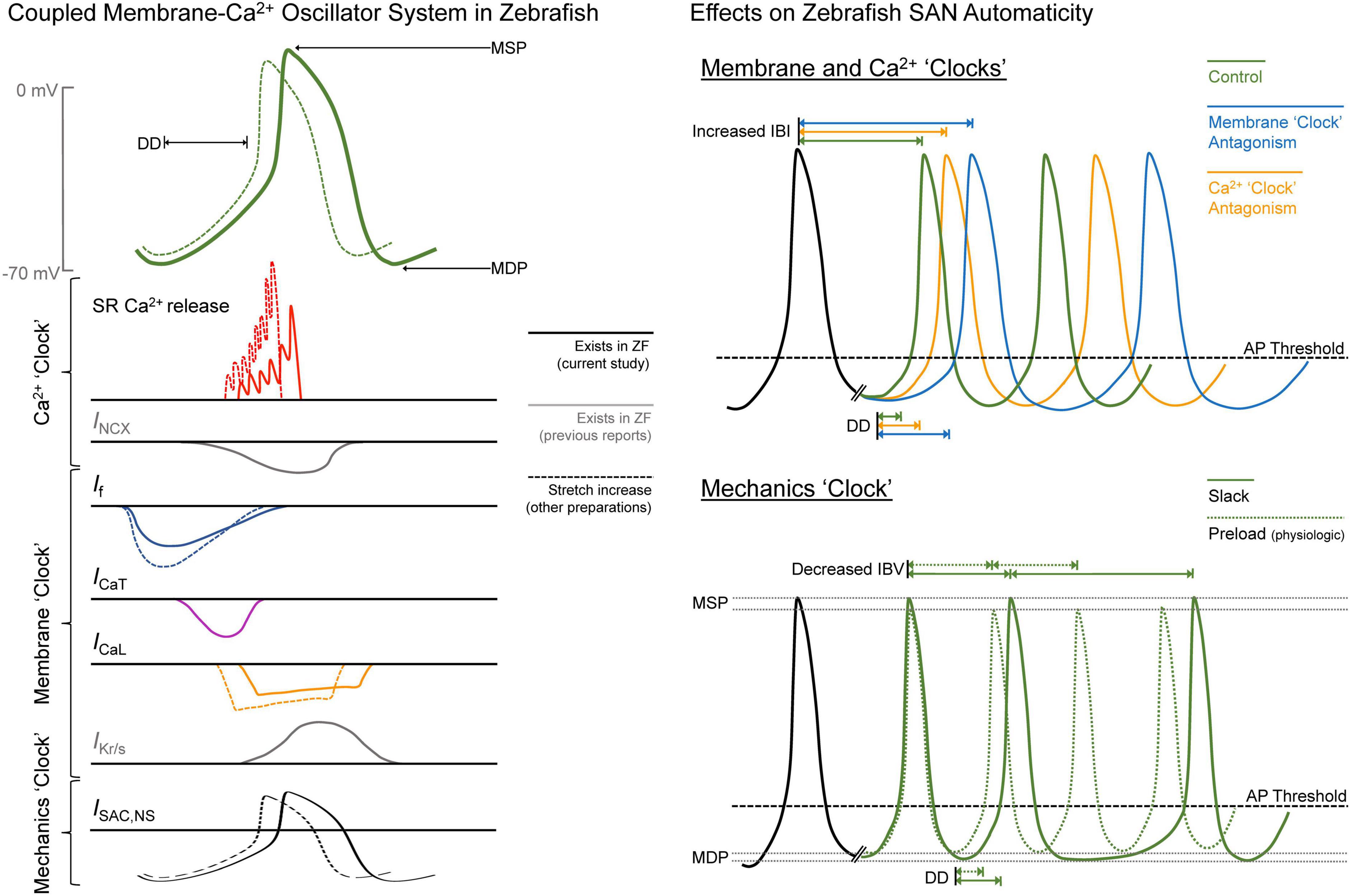
Figure 6. Summary of the drivers of zebrafish sinoatrial node (SAN) automaticity. AP, action potential; DD, diastolic depolarisation; ICa,L, L-type Ca2+ current; ICa,T, T-type Ca2+ current; IBI, inter-beat interval; IBV, inter-beat variation; If, “funny” current; IKr/s, rapid/slow delayed rectifier K+ current; INCX, Na+-Ca2+ exchanger current; ISAC,NS, cation non-selective stretch-activated channels; MDP, maximum diastolic potential; MSP, maximum systolic potential; RyR, ryanodine receptors; SR, sarcoplasmic reticulum; ZF, zebrafish.
In our experiments, changes in HR with pharmacological antagonism of mechanisms known to be critical for SAN automaticity in mammals suggest that similar pacemaker mechanisms are present in the zebrafish heart (Figure 6). We found that block of If with ivabradine or cesium resulted in a large decrease in HR (−86 or −73%). Block of RyR with ryanodine, chelation of cytosolic Ca2+ with BAPTA, or block of ICa,T with nickel resulted in smaller decreases in HR (−45, −26, or −10%), while block of ICa,L with nifedipine had no effect (Figure 1). Previous experiments using rabbit isolated SAN tissue and cells also demonstrated decreases in HR with similar drug concentrations (a comparison, with relevant references, is provided in Table 1). While the decrease in HR with block of ICa,T, RyR, or chelation of cytosolic Ca2+ in the current experiments were in the same range as previous reports in rabbit, the change in HR with If block was much larger, and we saw no change with block of ICa,L, while others have shown a large decrease. This difference suggests that there is a different balance in the contribution of the various components of the membrane-Ca2+ oscillator system to SAN automaticity in the zebrafish compared to mammals, such that If plays a more prominent role, and the CaV1.3 component of ICa,L is less important.
The apparent critical contribution of If to SAN automaticity in the zebrafish is supported by genetic studies in which a mutation causing a reduction in a hyperpolarisation-activated inward current with similar properties to If resulted in bradycardia (38% decrease in HR in embryos and 28% in surviving adults; Baker et al., 1997; Warren et al., 2001). More recent studies using a similar pharmacological approach as in our experiments have also shown that block of If (with 10 μM ivabradine or intraperitoneal injection of 4 μg/g zatebradine) causes a large decrease in zebrafish HR (60 – 65%; Lin et al., 2014; Marchant and Farrell, 2019).
Past studies also support the importance of RyR and intracellular Ca2+ cycling for zebrafish SAN automaticity shown in our study, as ryanodine (intraperitoneal injection of 50 ng/g), combined with thapsigargin (1.3 μg/g, to block SR Ca2+ reuptake) reduced HR by ∼40% (Marchant and Farrell, 2019). A similar result has been shown in another teleost (the trout), in which ryanodine (10 μM) and thapsigargin (1 μM) resulted in a 44% decrease in HR (Haverinen and Vornanen, 2007). In both zebrafish (Bovo et al., 2013) and trout (Llach et al., 2011), it has also been shown that Ca2+ sparks are present in atrial and ventricular myocytes. In zebrafish, however, their amplitude is smaller and duration greater compared to rabbit, due in part to a lower sensitivity of RyR to Ca2+ (Bovo et al., 2013). The lower sensitivity of RyR to Ca2+ in zebrafish may in part explain the smaller decrease in HR with ICa,L block seen in our experiments, as Ca2+ sparks triggered by CaV1.3-mediated Ca2+ influx may play a less prominent role in SAN automaticity compared to mammals. Yet, while CaV1.3-mediated Ca2+ sparks may not be present in zebrafish, and spontaneous Ca2+ sparks in zebrafish have characteristics differing from those in rabbit, RyR block still resulted in a similar decrease in HR (see Table 1). This could be due to a greater relative RyR expression, and thus total Ca2+ spark-induced Ca2+ efflux in SAN cells of zebrafish compared to rabbit or may be supported by the relatively higher Na+-Ca2+ exchanger activity seen in teleosts (as demonstrated in trout; Hove-Madsen et al., 2003) due to unique exchanger paralogs (Marshall et al., 2005).
The particular importance of If and (at least spontaneous) Ca2+ release via RyR to pacemaking in the zebrafish is also supported by the high expression of HCN and RyR protein shown in the SAN by immunofluorescence in our study (Figure 2), as well as the increase in the instability of cardiac rhythm with their block (Figure 1), as has been shown to also occur in rabbit (Yaniv et al., 2014). This instability may, however, partly be driven by the decrease in HR, which itself has been shown to increase HR variability (Rocchetti et al., 2000).
In regards to the contribution of ICa,T to SAN automaticity shown in our study, a robust ICa,T has been demonstrated in both the atrium and ventricle of the zebrafish heart (Nemtsas et al., 2010; Alday et al., 2014), so is presumably also present in the zebrafish SAN (T-type Ca2+ channels in the adult mammalian heart are in fact generally restricted to the SAN; Mesirca et al., 2015). Importantly, in studies in which HR was reduced with mutation of If channels it was shown that ICa,T was still present and unaffected (Baker et al., 1997), so would have been a contributor to the continued pacemaking in those animals.
It has been shown that compared to other regions of the heart, the zebrafish SAN overexpresses several signature mammalian pacemaker genes, including those encoding HCN and Ca2+- and K+-gated channels (von der Heyde et al., 2020; Minhas et al., 2021). In the context of SAN function, it has also been shown that knock down of the large-conductance Ca2+-activated K+ channel (KCa1.1) in zebrafish, which is strongly expressed in the human SAN, results in sinus bradycardia (Pineda et al., 2021), so it may play an important role in zebrafish SAN automaticity through its contribution to repolarisation.
Interestingly, combined pharmacological antagonism of If, ICa,T, and RyR did not completely block beating but instead resulted in long SAN pauses interspersed by bursts of a higher frequency than the HR before pharmacological block. This suggests that when these primary molecular mechanisms of SAN automaticity in the zebrafish are inhibited, SAN excitation is prevented until other mechanisms overcome this inhibition and cause the heart to beat (for instance, mechano-electric coupling effects driven by SAN stretch, or large, sudden releases of Ca2+ from the SR due to severe Ca2+ overload), reflecting the overall complexity of the coupled membrane-Ca2+ oscillator pacemaker system. The mechanisms responsible for this finding warrant further investigation.
We found that the site of initial SAN excitation varied between individual zebrafish and was in some cases multi-focal. With vagal nerve stimulation there was a shift in the location of these sites and a greater incidence of multi-focal excitation. Effects differed with left vs. right nerve stimulation and were abolished by nicotinic receptor blockade, which prevents signal transmission from pre- to postganglionic neurons. It has been shown previously in zebrafish (Stoyek et al., 2016), as well as in carp (Saito and Tenma, 1976), that left or right vagal nerve stimulation leads to bradycardia (due to parasympathetic activation), followed by a post stimulation tachycardia (due to sympathetic activation), as in mammals. Simultaneous left-right nerve stimulation results in a summative HR effect, as the left and right vagal nerves innervate distinct regions of the SAN, proximal to their entry into the heart (Stoyek et al., 2015). In fact, approximately 90% of all intracardiac neurons in the zebrafish heart are located within the SAN region, with terminals concentrated near intracardiac ganglia adjacent to cells expressing HCN4 (the principal HCN isoform contributing to SAN automaticity in zebrafish and mammals; Stoyek et al., 2015), as well as adrenergic and cholinergic receptors (Stoyek et al., 2015). It has also been demonstrated that excessive vagal nerve stimulation can silence the SAN (presumably due to activation of the acetylcholine-activated K+ current, which is present in zebrafish; Nemtsas et al., 2010), causing a shift in the origin of heart excitation toward the atrioventricular node (Stoyek et al., 2016).
A shift in the leading pacemaker site with electrical stimulation of extracardiac inputs or during pharmacologic activation of autonomic receptors has been demonstrated in a variety of mammals (Opthof, 1988; Boyett et al., 2000; Lang and Glukhov, 2021), including rabbit (West, 1955; Toda and West, 1967; Bouman et al., 1968; Toda, 1968; Toda and Shimamoto, 1968; Spear et al., 1979; Mackaay et al., 1980; Shibata et al., 2001; Lang et al., 2011). In general, sympathetic stimulation tends to shift the leading pacemaker site superiorly, while parasympathetic simulation results in an inferior shift, with the distance of shift scaling with the associated change in HR. These shifts have been attributed primarily to regional heterogeneity across the SAN, in terms of the balance of pacemaker currents in SAN cells and their responsiveness to autonomic stimulation, either due to differences in local innervation or receptor density (Opthof, 1988; Boyett et al., 2000; Lang and Glukhov, 2021). In the current study, the multi-focal nature of the initial site of SAN excitation, combined with its change during vagal nerve stimulation, suggest that there may be a similar heterogeneity in the zebrafish SAN.
The diameter of the SAN in mature zebrafish (6–12 months post fertilisation) has been reported to range between 200–600 μm, depending on subject-specific size and age (Stoyek et al., 2015, 2016; MacDonald et al., 2017). Based on nuclei counts from confocal z-projections of the SAN, the number of pacemaker cells is estimated to be in the low-to-mid thousands. In the current study we observed morphological differences in SAN cells across the node. In terms of functional heterogeneity, microelectrode recordings of SAN cell AP have shown variation in measured parameters (e.g., slope of DD, MDP, dV/dtmax, AP amplitude, APD), however findings are too preliminary to make strong conclusions about the spatial heterogeneity of electrical properties. In terms of the currents underlying pacemaker activity, to our knowledge no study has investigated differences in current densities or protein or gene expression across the SAN, which is an interesting topic for future experimental work. Non-uniform local innervation of the zebrafish SAN has already been demonstrated (Stoyek et al., 2015), such that the distinct regions of SAN innervation from the left and right vagal nerves may explain the differences in pacemaker shift observed during their individual stimulation. The potential for heterogeneity of pacemaker currents and autonomic receptors across the SAN, however, remains to be investigated, although it appears there are regional differences in SAN automaticity from the earliest stages of zebrafish development (Arrenberg et al., 2010).
We found that HR of the slack zebrafish SAN in isolated atria was irregular, and that rhythm was stabilised by static stretch of the SAN to a mechanical preload (Figure 6). This finding was corroborated in rabbit isolated SAN. Sustained stretch of the SAN has long been known to cause an acute increase in HR (Quinn et al., 2011a; Quinn and Kohl, 2012), including in rabbit (Quinn and Kohl, 2016; MacDonald et al., 2020a) and zebrafish (MacDonald et al., 2017). This chronotropic effect is thought to occur through activation of cation non-selective stretch-activated channels, and perhaps also through mechano-sensitive components of the coupled membrane-Ca2+ oscillator pacemaker system (Quinn and Kohl, 2012, 2021). The critical nature of a “minimal” diastolic tension for the generation and stabilisation of rhythmic SAN excitation was first suggested by experiments in cat (Lange et al., 1966), dog (Brooks et al., 1966) and rabbit (Ushiyama and Brooks, 1977) isolated SAN, in which stretch either restored regular rhythm in slack SAN with irregular activity or resulted in spontaneous excitation in previously quiescent SAN. In such cases, it is apparent that instability or SAN quiescence is due to the failure of other pacemaker mechanisms to sustain DD, which is restored by a sufficient mechanical preload, along with a positive shift in the maximum diastolic potential towards AP threshold. In fact, the importance of an adequate preload for SAN pacemaking may be present from the very first heartbeat during embryonic development, as fluid pressure build-up in the quiescent cardiac tube appears to be a pre-requirement for the initiation of spontaneous cardiac excitation during ontogenesis (Rajala et al., 1976, 1977; Chiou et al., 2016). Our current experiments demonstrate that the critical nature of a mechanical preload for rhythmic SAN firing (MacDonald and Quinn, 2021) applies to both zebrafish and rabbit hearts.
We found that the application of blebbistatin to the isolated zebrafish heart resulted in some changes to its electrophysiology. While HR and most measured AP characteristics were unaffected, blebbistatin caused a decrease in the slope of DD in the SAN, an increase in APD in the atrium, and a decrease of APD in the ventricle. Reports of the effects of blebbistatin on cardiac electrophysiology in mammals have been variable. In isolated rabbit hearts, right ventricular and atrial tissue, and in rat ventricular myocytes, it was first shown that blebbistatin has no effect on SAN activity or on cardiomyocyte APD (Fedorov et al., 2007). Since that time, studies using rabbit hearts have confirmed a lack of an effect of blebbistatin on APD, while later studies using rabbit and pig hearts have demonstrated an increase (Swift et al., 2021). The increase in APD that we observed in the zebrafish atrium agrees with these later findings. It is unclear whether this increase in APD is due to non-specific interactions of blebbistatin with cardiac ion channels and/or transporters or is instead a secondary effect of the loss of contraction caused by excitation-contraction uncoupling, for instance due to a loss of mechano-electric coupling effects or to a decrease in sarcolemmal ATP-sensitive K+ channel current caused by a decrease in metabolic demand (Swift et al., 2021). The other electrophysiological findings in our study (decreased DD slope in the SAN and increased APD in the ventricle) suggest there may be direct effects of blebbistatin on ion channels and/or transporters, warranting further investigations to determine the specific mechanism(s) involved. It is worth noting that blebbistatin may also affect other aspects of cardiac electrophysiology (e.g., conduction, refractoriness, APD restitution, arrhythmia threshold), as well as Ca2+ handling (e.g., cytosolic Ca2+ levels, Ca2+ transient amplitude and duration), important for the function of the SAN and working myocardium (Swift et al., 2021), which deserve consideration in future studies using zebrafish.
The principal limitation of our approach is the use of pharmacological interventions to determine the relative importance of the various components of the coupled membrane-Ca2+ oscillator pacemaker system. While our results show in principle which components are relevant for SAN automaticity in the zebrafish, the differences in HR change with If and ICa,L block compared to rabbit could reflect differences in the level or specificity of block, or the presence of unappreciated channel paralogs in zebrafish not found in mammals (Jackson et al., 2007), rather than reflecting a relative difference in their contribution. This effect could be compounded by the use of drug concentrations previously used in mammals, but generally not corroborated in zebrafish. Future studies involving genetic manipulations of the relevant genes may help further define the particular importance of each of the mechanisms contributing to SAN automaticity in the zebrafish.
When considering the regional excitation of the SAN, its shift with vagal nerve stimulation, or the effects of mechanical preload, it is important to consider that the zebrafish SAN is a ring-shaped structure, as opposed to the sheet-like structure of the mammalian SAN, and that the vagal nerve carries both parasympathetic and sympathetic inputs (Stoyek et al., 2015). This will affect source-sink relations important for SAN excitation as well as the regional distribution of applied stretch and resulting tissue tension.
Finally, in the zebrafish experiments of the current study sex of the animals was not considered, while in the rabbit experiments SAN were isolated only from female animals. Although we are not aware of any reported differences in male versus female zebrafish or rabbit SAN, sex differences in the function and molecular mechanisms of the SAN have been described in rat (Doris et al., 2019), so should be considered in future studies.
Here we have demonstrated that the cellular mechanisms responsible for SAN automaticity in zebrafish, as well as critical aspects of the control of SAN automaticity by the autonomic nervous system and tissue stretch, are in general similar to those in mammalian hearts (summarised in Figure 6). These findings accord with previous work considering other aspects of zebrafish SAN structure, genetics, composition, and function, thus further supporting the use of zebrafish as an experimental model for studies relating to SAN (patho-) physiology. The zebrafish has several other advantages as an experimental model for cardiac investigations, including its well-defined genome, ease of genetic manipulation, high fecundity, and relatively fast generation time, as well as the external development of nearly optically transparent embryos and relatively thin cardiac tissue that allow for cell-level measurement and manipulation of structure and function in vivo during development and in situ in the isolated heart. Combined, these aspects make the zebrafish particularly useful for genetic studies and studies involving advanced light-based techniques, such as live fluorescence imaging and optogenetic manipulations (Baillie et al., 2021). Its use therefore represents an exciting opportunity for innovative, cell-specific investigations into unexplored aspects of (patho-)physiological SAN function and control. In fact, the zebrafish has recently been utilised to explore the impact of ageing on the function and control of the SAN (Stoyek et al., 2018) and to model age-related sinus arrest and sick sinus syndrome (Yan et al., 2020). Similar studies in the future hold the promise of providing further clinically relevant novel insight into the essential contribution of the SAN to cardiac function.
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
All experimental procedures were approved and reviewed by the Dalhousie University Committee for Laboratory Animals and followed the guidelines of the Canadian Council on Animal Care.
MS, EM, JB, RC, FS, and TQ: conception and design of experiments. MS, EM, MM, JB, and BS: data collection. MS, EM, MM, JB, BS, and TQ: data analysis. MS, EM, and TQ: drafting of manuscript. All authors approved the final manuscript.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2016-04879 to TQ, PGS-D to MS and EM, and USRA to MM and BS), the Heart and Stroke Foundation of Canada (G-18-0022185 to TQ), and the Canadian Institutes of Health Research (MOP 342562 to TQ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the members of the Zebrafish Core Facility and Cellular and Molecular Digital Imaging Facility at Dalhousie University for their support of our work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.818122/full#supplementary-material
Abu Nahia, K., Migdal, M., Quinn, T. A., Poon, K. L., Lapinski, M., Sulej, A., et al. (2021). Genomic and physiological analyses of the zebrafish atrioventricular canal reveal molecular building blocks of the secondary pacemaker region. Cell. Mol. Life Sci. 78, 6669–6687. doi: 10.1007/s00018-021-03939-y
Alday, A., Alonso, H., Gallego, M., Urrutia, J., Letamendia, A., Callol, C., et al. (2014). Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol. Res. 84, 26–31. doi: 10.1016/j.phrs.2014.03.011
Ardell, J. L., and Armour, J. A. (2016). Neurocardiology: structure-based function. Compr. Physiol. 15, 1635–1653. doi: 10.1002/cphy.c150046
Armour, J. A. (2008). Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 93, 165–176. doi: 10.1113/expphysiol.2007.041178
Arrenberg, A. B., Stainier, D. Y., Baier, H., and Huisken, J. (2010). Optogenetic control of cardiac function. Science 330, 971–974.
Baillie, J. S., Stoyek, M. R., and Quinn, T. A. (2021). Seeing the light: the use of zebrafish for optogenetic studies of the heart. Front. Physiol. 12:748570. doi: 10.3389/fphys.2021.748570
Baker, K., Warren, K. S., Yellen, G., and Fishman, M. C. (1997). Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. U.S.A. 94, 4554–4559. doi: 10.1073/pnas.94.9.4554
Bartos, D. C., Grandi, E., and Ripplinger, C. M. (2015). Ion channels in the heart. Compr. Physiol. 5, 1423–1464. doi: 10.1002/cphy.c140069
Becker, J. R., Chatterjee, S., Robinson, T. Y., Bennett, J. S., Panakova, D., Galindo, C. L., et al. (2014). Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development 141, 335–345. doi: 10.1242/dev.100370
Bendig, G., Grimmler, M., Huttner, I. G., Wessels, G., Dahme, T., Just, S., et al. (2006). Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes. Dev. 20, 2361–2372. doi: 10.1101/gad.1448306
Bogdanov, K. Y., Maltsev, V. A., Vinogradova, T. M., Lyashkov, A. E., Spurgeon, H. A., Stern, M. D., et al. (2006). Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ. Res. 99, 979–987. doi: 10.1161/01.RES.0000247933.66532.0b
Bogdanov, K. Y., Vinogradova, T. M., and Lakatta, E. G. (2001). Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: Molecular partners in pacemaker regulation. Circ. Res. 88, 1254–1258. doi: 10.1161/hh1201.092095
Bouman, L. N., Gerlings, E. D., Biersteker, P. A., and Bonke, F. I. (1968). Pacemaker shift in the sino-atrial node during vagal stimulation. Pflugers Arch. 302, 255–267. doi: 10.1007/BF00586730
Bovo, E., Dvornikov, A. V., Mazurek, S. R., de Tombe, P. P., and Zima, A. V. (2013). Mechanisms of Ca2+ handling in zebrafish ventricular myocytes. Pflugers. Arch. 465, 1775–1784. doi: 10.1007/s00424-013-1312-2
Boyett, M., Honjo, H., and Kodama, I. (2000). The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc. Res. 47, 658–687. doi: 10.1016/s0008-6363(00)00135-8
Brooks, C. M., Lu, H. H., Lange, G., Mangi, R., Shaw, R. B., and Geoly, K. (1966). Effects of localized stretch of the sinoatrial node region of the dog heart. Am. J. Phys. 211, 1197–1202. doi: 10.1152/ajplegacy.1966.211.5.1197
Bucchi, A., Baruscotti, M., Robinson, R. B., and DiFrancesco, D. (2003). If-dependent modulation of pacemaker rate mediated by camp in the presence of ryanodine in rabbit sino-atrial node cells. J. Mol. Cell. Cardiol. 35, 905–913. doi: 10.1016/s0022-2828(03)00150-0
Bucchi, A., Baruscotti, M., Robinson, R. B., and DiFrancesco, D. (2007). Modulation of rate by autonomic agonists in SAN cells involves changes in diastolic depolarization and the pacemaker current. J. Mol. Cell. Cardiol. 43, 39–48. doi: 10.1016/j.yjmcc.2007.04.017
Budriesi, R., Aicardi, G., Campana, G., Spampinato, S., Zaza, A., Bisi, A., et al. (1998). Selective cardiodepressant activity of fluodipine, a fluorenone-1,4-dihydropyridine derivative. Eur. J. Pharmacol. 359, 161–170. doi: 10.1016/s0014-2999(98)00654-2
Burkhard, S., van Eif, V., Garric, L., Christoffels, V. M., and Bakkers, J. (2017). On the evolution of the cardiac pacemaker. J. Cardiovasc. Dev. Dis. 4:4.
Chiou, K. K., Rocks, J. W., Chen, C. Y., Cho, S., Merkus, K. E., Rajaratnam, A., et al. (2016). Mechanical signaling coordinates the embryonic heartbeat. Proc. Natl. Acad. Sci. U.S.A. 113, 8939–8944. doi: 10.1073/pnas.1520428113
Dennis, P. D., and Williams, E. M. (1986). Further studies of alinidine induced bradycardia in the presence of caesium. Cardiovasc. Res. 20, 375–378. doi: 10.1093/cvr/20.5.375
Denyer, J. C., and Brown, H. F. (1990). Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current If. J. Physiol. 429, 401–409. doi: 10.1113/jphysiol.1990.sp018264
DiFrancesco, D. (2010). The role of the funny current in pacemaker activity. Circ. Res. 106, 434–446. doi: 10.1161/CIRCRESAHA.109.208041
DiFrancesco, D., and Noble, D. (2012). The funny current has a major pacemaking role in the sinus node. Heart Rhythm. 9, 299–301.
Doris, U., Kharche, S., Petkova, M., Borbas, B., Logantha, S. J., Fedorenko, O., et al. (2019). A sexy approach to pacemaking: differences in function and molecular make up of the sinoatrial node. Histol. Histopathol. 34, 1255–1268. doi: 10.14670/HH-18-115
Echeazarra, L., Hortigón-Vinagre, M. P., Casis, O., and Gallego, M. (2021). Adult and developing zebrafish as suitable models for cardiac electrophysiology and pathology in research and industry. Front. Physiol. 11:607860. doi: 10.3389/fphys.2020.607860
Fedorov, V. V., Lozinsky, I. T., Sosunov, E. A., Anyukhovsky, E. P., Rosen, M. R., Balke, C. W., et al. (2007). Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 4, 619–626. doi: 10.1016/j.hrthm.2006.12.047
Gaskell, W. H. (1882). Preliminary observations on the innervation of the heart of the tortoise. J. Physiol. 3, 369–379. doi: 10.1113/jphysiol.1882.sp000110
Gut, P., Reischauer, S., Stainier, D. Y. R., and Arnaout, R. (2017). Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 97, 889–938. doi: 10.1152/physrev.00038.2016
Hagiwara, N., Irisawa, H., and Kameyama, M. (1988). Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 395, 233–253. doi: 10.1113/jphysiol.1988.sp016916
Hata, T., Noda, T., Nishimura, M., and Watanabe, Y. (1996). The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessels. 11, 234–241. doi: 10.1007/BF01746203
Haverinen, J., and Vornanen, M. (2007). Temperature acclimation modifies sinoatrial pacemaker mechanism of the rainbow trout heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1023–R1032. doi: 10.1152/ajpregu.00432.2006
Hove-Madsen, L., Llach, A., Tibbits, G. F., and Tort, L. (2003). Triggering of sarcoplasmic reticulum Ca2+ release and contraction by reverse mode Na+/Ca2+ exchange in trout atrial myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1330–R1339. doi: 10.1152/ajpregu.00404.2002
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. doi: 10.1038/nature12111
Hu, N., Yost, H. J., and Clark, E. B. (2001). Cardiac morphology and blood pressure in the adult zebrafish. Anat. Rec. 264, 1–12. doi: 10.1002/ar.1111
Irisawa, H., Brown, H. F., and Giles, W. (1993). Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 73, 197–227. doi: 10.1152/physrev.1993.73.1.197
Jackson, H. A., Marshall, C. R., and Accili, E. A. (2007). Evolution and structural diversification of hyperpolarization-activated cyclic nucleotide-gated channel genes. Physiol. Genomics. 29, 231–245. doi: 10.1152/physiolgenomics.00142.2006
Jansen, H. J., Quinn, T. A., and Rose, R. A. (2018). “Cellular sinoatrial node and atrioventricular node activity in the heart,” in Encyclopedia of Cardiovascular Research and Medicine, eds R. S. Vasan and D. B. Sawyer (Amsterdam: Elsevier), 576–592. doi: 10.1016/b978-0-12-809657-4.99759-x
Jou, C. J., Spitzer, K. W., and Tristani-Firouzi, M. (2010). Blebbistatin effectively uncouples the excitation-contraction process in zebrafish embryonic heart. Cell. Physiol. Biochem. 25, 419–424. doi: 10.1159/000303046
Kawai, C., Konishi, T., Matsuyama, E., and Okazaki, H. (1981). Comparative effects of three calcium antagonists, diltiazem, verapamil and nifedipine, on the sinoatrial and atrioventricular nodes. Experimental and clinical studies. Circulation 63, 1035–1042. doi: 10.1161/01.cir.63.5.1035
Keith, A., and Flack, M. (1907). The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J. Anat. Physiol. 41, 172–189.
Kopton, R. A., Baillie, J. S., Rafferty, S. A., Moss, R., Zgierski-Johnston, C. M., Prykhozhij, S. V., et al. (2018). Cardiac electrophysiological effects of light-activated chloride channels. Front. Physiol. 9:1806. doi: 10.3389/fphys.2018.01806
Lakatta, E. G., and DiFrancesco, D. (2009). What keeps us ticking: a funny current, a calcium clock, or both? J. Mol. Cell. Cardiol. 47, 157–170. doi: 10.1016/j.yjmcc.2009.03.022
Lakatta, E. G., Maltsev, V. A., and Vinogradova, T. M. (2010). A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 106, 659–673. doi: 10.1161/CIRCRESAHA.109.206078
Lakatta, E. G., Vinogradova, T. M., and Maltsev, V. A. (2008). The missing link in the mystery of normal automaticity of cardiac pacemaker cells. Ann. N. Y. Acad. Sci. 1123, 41–57. doi: 10.1196/annals.1420.006
Lancaster, M. K., Jones, S. A., Harrison, S. M., and Boyett, M. R. (2004). Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: heterogeneity of role and control. J. Physiol. 556, 481–494. doi: 10.1113/jphysiol.2003.057372
Lang, D., and Glukhov, A. V. (2021). Cellular and molecular mechanisms of functional hierarchy of pacemaker clusters in the sinoatrial node: new insights into sick sinus syndrome. J. Cardiovasc. Dev. Dis. 8, 43. doi: 10.3390/jcdd8040043
Lang, D., Petrov, V., Lou, Q., Osipov, G., and Efimov, I. R. (2011). Spatiotemporal control of heart rate in a rabbit heart. J. Electrocardiol. 44, 626–634. doi: 10.1016/j.jelectrocard.2011.08.010
Lange, G., Lu, H. H., Chang, A., and Brooks, C. M. (1966). Effect of stretch on the isolated cat sinoatrial node. Am. J. Phys. 211, 1192–1196. doi: 10.1152/ajplegacy.1966.211.5.1192
Leitch, S. P., Sears, C. E., Brown, H. F., and Paterson, D. J. (1995). Effects of high potassium and the bradycardic agents ZD7288 and cesium on heart rate of rabbits and guinea pigs. J. Cardiovasc. Pharmacol. 25, 300–306. doi: 10.1097/00005344-199502000-00016
Lin, E., Ribeiro, A., Ding, W., Hove-Madsen, L., Sarunic, M. V., Beg, M. F., et al. (2014). Optical mapping of the electrical activity of isolated adult zebrafish hearts: acute effects of temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R823–R836. doi: 10.1152/ajpregu.00002.2014
Llach, A., Molina, C. E., Alvarez-Lacalle, E., Tort, L., Benítez, R., and Hove-Madsen, L. (2011). Detection, properties, and frequency of local calcium release from the sarcoplasmic reticulum in teleost cardiomyocytes. PLoS One 6:e23708. doi: 10.1371/journal.pone.0023708
Logantha, S. J. R. J., Kharche, S. R., Zhang, Y., Atkinson, A. J., Hao, G., Boyett, M. R., et al. (2019). Sinus node-like pacemaker mechanisms regulate ectopic pacemaker activity in the adult rat atrioventricular ring. Sci. Rep. 13:11781. doi: 10.1038/s41598-019-48276-0
Lyashkov, A. E., Juhaszova, M., Dobrzynski, H., Vinogradova, T. M., Maltsev, V. A., Juhasz, O., et al. (2007). Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ. Res. 100, 1723–1731. doi: 10.1161/CIRCRESAHA.107.153676
MacDonald, E. A., and Quinn, T. A. (2021). What keeps us ticking? Sinoatrial node mechano-sensitivity: the grandfather-clock of cardiac rhythm. Biophys. Rev. 13, 707–716. doi: 10.1007/s12551-021-00831-8
MacDonald, E. A., Rose, R. A., and Quinn, T. A. (2020b). Neurohumoral control of sinoatrial node activity and heart rate: experimental insight and findings from human. Front. Physiol. 11:170. doi: 10.3389/fphys.2020.00170
MacDonald, E. A., Madl, J., Greiner, J., Ramadan, A. F., Wells, S. M., Torrente, A. G., et al. (2020a). Sinoatrial node structure, mechanics, electrophysiology and the chronotropic response to stretch in rabbit and mouse. Front. Physiol. 11:809. doi: 10.3389/fphys.2020.00809
MacDonald, E. A., Stoyek, M. R., Rose, R. A., and Quinn, T. A. (2017). Intrinsic regulation of sinoatrial node function and the zebrafish as a model of stretch effects on pacemaking. Prog. Biophys. Mol. Biol. 130, 198–211. doi: 10.1016/j.pbiomolbio.2017.07.012
Mackaay, A. J., Opthof, T., Bleeker, W. K., Jongsma, H. J., and Bouman, L. N. (1980). Interaction of adrenaline and acetylcholine on cardiac pacemaker function. Functional inhomogeneity of the rabbit sinus node. J. Pharmacol. Exp. Ther. 214, 417–422.
Maltsev, V. A., and Lakatta, E. G. (2012). The funny current in the context of the coupled-clock pacemaker system. Heart Rhythm. 9, 302–307. doi: 10.1016/j.hrthm.2011.09.022
Mangoni, M. E., and Nargeot, J. (2008). Genesis and regulation of the heart automaticity. Physiol. Rev. 88, 919–982. doi: 10.1152/physrev.00018.2007
Marchant, J. L., and Farrell, A. P. (2019). Membrane and calcium clock mechanisms contribute variably as a function of temperature to setting cardiac pacemaker rate in zebrafish Danio rerio. J. Fish Biol. 95, 1265–1274. doi: 10.1111/jfb.14126
Marshall, C. R., Fox, J. A., Butland, S. L., Ouellette, B. F., Brinkman, F. S., and Tibbits, G. F. (2005). Phylogeny of Na+/Ca2+ exchanger (NCX) genes from genomic data identifies new gene duplications and a new family member in fish species. Physiol. Genomics 21, 161–173. doi: 10.1152/physiolgenomics.00286.2004
Martin, K. E., and Waxman, J. S. (2021). Atrial and sinoatrial node development in the zebrafish heart. J. Cardiovasc. Dev. Dis. 8:15. doi: 10.3390/jcdd8020015
Masumiya, H., Tanaka, H., and Shigenobu, K. (1997). Effects of Ca2+ channel antagonists on sinus node: prolongation of late phase 4 depolarization by efonidipine. Eur. J. Pharmacol. 17, 15–21. doi: 10.1016/s0014-2999(97)01150-3
Mesirca, P., Torrente, A. G., and Mangoni, M. E. (2015). Functional role of voltage gated Ca2+ channels in heart automaticity. Front. Physiol. 6:19. doi: 10.3389/fphys.2015.00019
Minhas, R., Loeffler-Wirth, H., Siddiqui, Y. H., Obrêbski, T., Vashisht, S., Nahia, K. A., et al. (2021). Transcriptome profile of the sinoatrial ring reveals conserved and novel genetic programs of the zebrafish pacemaker. BMC Genomics 22:715. doi: 10.1186/s12864-021-08016-z
Nemtsas, P., Wettwer, E., Christ, T., Weidinger, G., and Ravens, U. (2010). Adult zebrafish heart as a model for human heart? An electrophysiological study. J. Mol. Cell. Cardiol. 48, 161–171. doi: 10.1016/j.yjmcc.2009.08.034
Nikmaram, M. R., Boyett, M. R., Kodama, I., Suzuki, R., and Honjo, H. (1997). Variation in effects of Cs+, UL-FS-49, and ZD-7288 within sinoatrial node. Am. J. Physiol. 272, H2782–H2792. doi: 10.1152/ajpheart.1997.272.6.H2782
Noble, D., Noble, P. J., and Fink, M. (2010). Competing oscillators in cardiac pacemaking. Circ. Res. 106, 1791–1797. doi: 10.1161/CIRCRESAHA.110.218875
Noma, A., Morad, M., and Irisawa, H. (1983). Does the “pacemaker current” generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 397, 190–194. doi: 10.1007/BF00584356
Opthof, T. (1988). The mammalian sinoatrial node. Cardiovasc. Drugs Ther. 1, 573–597. doi: 10.1007/bf02125744
Pineda, S., Nikolova-Krstevski, V., Leimena, C., Atkinson, A. J., Altekoester, A. K., Cox, C. D., et al. (2021). Conserved role of the large conductance calcium-activated potassium channel, KCa1.1, in sinus node function and arrhythmia risk. Circ. Genom. Precis. Med. 14:e003144. doi: 10.1161/CIRCGEN.120.003144
Quinn, T. A. (2015). Cardiac mechano-electric coupling: a role in regulating normal function of the heart? Cardiovasc. Res. 108, 1–3. doi: 10.1093/cvr/cvv203
Quinn, T. A., and Kohl, P. (2012). Mechano-sensitivity of cardiac pacemaker function: Pathophysiological relevance, experimental implications, and conceptual integration with other mechanisms of rhythmicity. Prog. Biophys. Mol. Biol. 110, 257–268. doi: 10.1016/j.pbiomolbio.2012.08.008
Quinn, T. A., and Kohl, P. (2016). Rabbit as a model for studies of cardiac mechano-electric and mechano-mechanical coupling. Prog. Biophys. Mol. Biol. 121, 110–122. doi: 10.1016/j.pbiomolbio.2016.05.003
Quinn, T. A., and Kohl, P. (2021). Cardiac mechano-electric coupling: acute effects of mechanical stimulation on heart rate and rhythm. Physiol. Rev. 101, 37–92. doi: 10.1152/physrev.00036.2019
Quinn, T. A., and Magder, S. (2021). “Physiology of heart rate,” in Cardiopulmonary Monitoring: Basic Physiology, Tools, and Bedside Management For The Critically Ill, eds S. Magder, A. Malhotra, K. A. Hibbert, and C. C. Hardin (Berlin: Springer), 87–106.
Quinn, T. A., Bayliss, R. A., and Kohl, P. (2011a). “Mechano-electric feedback in the heart: effects on heart rate and rhythm,” in Heart Rate and Rhythm: Molecular Basis, Pharmacological Modulation and Clinical Implications, eds O. N. Tripathi, U. Ravens, and M. C. Sanguinetti (Berlin: Springer-Verlag), 133–151. doi: 10.1007/978-3-642-17575-6_7
Quinn, T. A., Granite, S., Allessie, M. A., Antzelevitch, C., Bollensdorff, C., Bub, G., et al. (2011b). Minimum information about a cardiac electrophysiology experiment (MICEE): standardised reporting for model reproducibility, interoperability, and data sharing. Prog. Biophys. Mol. Biol. 107, 4–10. doi: 10.1016/j.pbiomolbio.2011.07.001
Quinn, T. A., Kohl, P., and Ravens, U. (2014). Cardiac mechano-electric coupling research: fifty years of progress and scientific innovation. Prog. Biophys. Mol. Biol. 115, 71–75. doi: 10.1016/j.pbiomolbio.2014.06.007
Rafferty, S. A., and Quinn, T. A. (2018). A beginner’s guide to understanding and implementing the genetic modification of zebrafish. Prog. Biophys. Mol. Biol. 138, 3–19. doi: 10.1016/j.pbiomolbio.2018.07.005
Rajala, G. M., Kalbfleisch, J. H., and Kaplan, S. (1976). Evidence that blood pressure controls heart rate in the chick embryo prior to neural control. J. Embryol. Exp. Morpholog. 36, 685–695. doi: 10.1242/dev.36.3.685
Rajala, G. M., Pinter, M. J., and Kaplan, S. (1977). Response of the quiescent heart tube to mechanical stretch in the intact chick embryo. Dev. Biol. 61, 330–337. doi: 10.1016/0012-1606(77)90302-5
Ravens, U. (2018). Ionic basis of cardiac electrophysiology in zebrafish compared to human hearts. Prog. Biophys. Mol. Biol. 138, 38–44. doi: 10.1016/j.pbiomolbio.2018.06.008
Rayani, K., Lin, E., Craig, C., Lamothe, M., Shafaattalab, S., Gunawen, M., et al. (2018). Zebrafish as a model of mammalian cardiac function: optically mapping the interplay of temperature and rate on voltage and calcium dynamics. Prog. Biophys. Mol. Biol. 138, 69–90. doi: 10.1016/j.pbiomolbio.2018.07.006
Rocchetti, M., Malfatto, G., Lombardi, F., and Zaza, A. (2000). Role of the input/output relation of sinoatrial myocytes in cholinergic modulation of heart rate variability. J. Cardiovasc. Electrophysiol. 11, 522–530. doi: 10.1111/j.1540-8167.2000.tb00005.x
Rosen, M. R., Nargeot, J., and Salama, G. (2012). The case for the funny current and the calcium clock. Heart Rhythm. 9, 616–618. doi: 10.1016/j.hrthm.2011.10.008
Saito, T., and Tenma, K. (1976). Effects of left and right vagal stimulation on excitation and conduction of the carp heart (Cyprinus carpio). J. Comp. Physiol. 111, 39–53. doi: 10.1007/bf00691110
Satoh, H. (1995). Role of T-type Ca2+ channel inhibitors in the pacemaker depolarization in rabbit sino-atrial nodal cells. Gen. Pharmacol. 26, 581–587. doi: 10.1016/0306-3623(94)00214-8
Satoh, H. (1997). Electrophysiological actions of ryanodine on single rabbit sinoatrial nodal cells. Gen. Pharmacol. 28, 31–38. doi: 10.1016/s0306-3623(96)00182-6
Satoh, H., and Tsuchida, K. (1993). Comparison of a calcium antagonist, CD-349, with nifedipine, diltiazem, and verapamil in rabbit spontaneously beating sinoatrial node cells. J. Cardiovasc. Pharmacol. 21, 685–692. doi: 10.1097/00005344-199305000-00001
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi: 10.1038/nmeth.2019
Schwerte, T., Prem, C., Mairosl, A., and Pelster, B. (2006). Development of the sympatho-vagal balance in the cardiovascular system in zebrafish (Danio rerio) characterized by power spectrum and classical signal analysis. J. Exp. Biol. 209, 1093–1100. doi: 10.1242/jeb.02117
Senges, J., Rizos, I., Brachmann, J., Anders, G., Jauernig, R., Hamman, H. D., et al. (1983). Effect of nifedipine and AQ-A 39 on the sinoatrial and atrioventricular nodes of the rabbit and their antiarrhythmic action on atrioventricular nodal reentrant tachycardia. Cardiovasc. Res. 17, 132–144. doi: 10.1093/cvr/17.3.132
Shibata, N., Inada, S., Mitsui, K., Honjo, H., Yamamoto, M., Niwa, R., et al. (2001). Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp. Physiol. 86, 177–184. doi: 10.1113/eph8602100
Spear, J. F., Kronhaus, K. D., Moore, E. N., and Kline, R. P. (1979). The effect of brief vagal stimulation on the isolated rabbit sinus node. Circ. Res. 44, 75–88. doi: 10.1161/01.res.44.1.75
Stoyek, M. R., and Quinn, T. A. (2018). One fish, two fish, red fish, blue fish: zebrafish as a model for cardiac research. Prog. Biophys. Mol. Biol. 138, 1–2. doi: 10.1016/j.pbiomolbio.2018.11.003
Stoyek, M. R., Croll, R. P., and Smith, F. M. (2015). Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J. Comp. Neurol. 523, 1683–1700. doi: 10.1002/cne.23764
Stoyek, M. R., Hotells, L., and Quinn, T. A. (2021a). From mice to mainframes: experimental models for investigations of the intracardiac nervous system. J. Cardiovasc. Dev. Dis. 8:149. doi: 10.3390/jcdd8110149
Stoyek, M. R., Quinn, T. A., Croll, R. P., and Smith, F. M. (2016). Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am. J. Physiol. Heart Circ. Physiol. 311, H676–H688. doi: 10.1152/ajpheart.00330.2016
Stoyek, M. R., Rafferty, S. A., and Quinn, T. A. (2021b). “Genetically modified zebrafish as experimental model,” in Handbook of Molecular Biotechnology, ed. D. Liu (Boca Raton, FL: CRC Press).
Stoyek, M. R., Rog-Zielinska, E. A., and Quinn, T. A. (2018). Age-associated changes in electrical function of the zebrafish heart. Prog. Biophys. Mol. Biol. 138, 91–104. doi: 10.1016/j.pbiomolbio.2018.07.014
Stoyek, M. R., Schmidt, M. K., Wilfart, F. M., Croll, R. P., and Smith, F. M. (2017). The in vitro zebrafish heart as a model to investigate the chronotropic effects of vapor anesthetics. Am. J. Physiol. Reg. Integr. Comp. Physiol. 313, R669–R679. doi: 10.1152/ajpregu.00467.2016
Susaki, E. A., Tainaka, K., Perrin, D., Kishino, F., Tawara, T., Watanabe, T. M., et al. (2014). Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739. doi: 10.1016/j.cell.2014.03.042
Swift, L. M., Kay, M. W., Ripplinger, C. M., and Posnack, N. G. (2021). Stop the beat to see the rhythm: excitation-contraction uncoupling in cardiac research. Am. J. Physiol. Heart Circ. Physiol. 321, H1005–H1013. doi: 10.1152/ajpheart.00477.2021
Tessadori, F., van Weerd, J. H., Burkhard, S. B., Verkerk, A. O., de Pater, E., Boukens, B. J., et al. (2012). Identification and functional characterization of cardiac pacemaker cells in zebrafish. PLoS One 7:e47644. doi: 10.1371/journal.pone.0047644
Thollon, C., Bedut, S., Villeneuve, N., Cogé, F., Piffard, L., Guillaumin, J.-P., et al. (2007). Use-dependent inhibition of hHCN4 by ivabradine and relationship with reduction in pacemaker activity. Br. J. Pharmacol. 150, 37–46. doi: 10.1038/sj.bjp.0706940
Thollon, C., Cambarrat, C., Vian, J., Prost, J. F., Peglion, J. L., and Vilaine, J. P. (1994). Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br. J. Pharmacol. 112, 37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x
Toda, N. (1968). Cholinergic actions in the sinoatrial node of the reserpine-pretreated rabbit. J. Pharmacol. Exp. Ther. 159, 290–297.
Toda, N., and Shimamoto, K. (1968). The influence of sympathetic stimulation on transmembrane potentials in the s-a node. J. Pharmacol. Exp. Ther. 159, 298–305.
Toda, N., and West, T. C. (1967). Interaction between na, ca, mg, and vagal stimulation in the s-a node of the rabbit. Am. J. Physiol. 212, 424–430. doi: 10.1152/ajplegacy.1967.212.2.424
Torrente, A. G., Mesirca, P., Neco, P., Rizzetto, R., Dubel, S., Barrere, C., et al. (2016). L-type CaV1.3 channels regulate ryanodine receptor-dependent Ca2+ release during sino-atrial node pacemaker activity. Cardiovasc. Res. 109, 451–461. doi: 10.1093/cvr/cvw006
Ushiyama, J., and Brooks, C. M. C. (1977). Interaction of oscillators: effect of sinusoidal stretching of the sinoatrial node on nodal rhythm. J. Electrocardiol. 10, 39–44. doi: 10.1016/s0022-0736(77)80029-0
van Opbergen, C. J. M., van der Voorn, S. M., Vos, M. A., de Boer, T. P., and van Veen, T. A. B. (2018). Cardiac Ca2+ signalling in zebrafish: translation of findings to man. Prog. Biophys. Mol. Biol. 138, 45–58. doi: 10.1016/j.pbiomolbio.2018.05.002
Vinogradova, T. M., Bogdanov, K. Y., and Lakatta, E. G. (2002). Beta-adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ. Res. 90, 73–79. doi: 10.1161/hh0102.102271
Vinogradova, T. M., Zhou, Y. Y., Bogdanov, K. Y., Yang, D., Kuschel, M., Cheng, H., et al. (2000). Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase ii activation. Circ. Res. 87, 760–767. doi: 10.1161/01.res.87.9.760
von der Heyde, B., Emmanouilidou, A., Mazzaferro, E., Vicenzi, S., Höijer, I., Klingström, T., et al. (2020). Translating gwas-identified loci for cardiac rhythm and rate using an in vivo image- and crispr/cas9-based approach. Sci. Rep. 10:11831. doi: 10.1038/s41598-020-68567-1
Vornanen, M., and Hassinen, M. (2016). Zebrafish heart as a model for human cardiac electrophysiology. Channels (Austin) 10, 101–110. doi: 10.1080/19336950.2015.1121335
Warren, K. S., Baker, K., and Fishman, M. C. (2001). The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am. J. Physiol. Heart Circ. Physiol. 281, H1711–H1719. doi: 10.1152/ajpheart.2001.281.4.H1711
Werdich, A. A., Brzezinski, A., Jeyaraj, D., Khaled Sabeh, M., Ficker, E., Wan, X., et al. (2012). The zebrafish as a novel animal model to study the molecular mechanisms of mechano-electrical feedback in the heart. Prog. Biophys. Mol. Biol. 110, 154e165. doi: 10.1016/j.pbiomolbio.2012.07.006
West, T. C. (1955). Ultramicroelectrode recording from the cardiac pacemaker. J. Pharmacol. Exp. Ther. 115, 283–290.
Yan, J., Li, H., Bu, H., Jiao, K., Zhang, A. X., Le, T., et al. (2020). Aging-associated sinus arrest and sick sinus syndrome in adult zebrafish. PLoS One 15:e0232457. doi: 10.1371/journal.pone.0232457
Yaniv, Y., Lyashkov, A. E., Sirenko, S., Okamoto, Y., Guiriba, T.-R., Ziman, B. D., et al. (2014). Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to-beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells. J. Mol. Cell. Cardiol. 77, 1–10. doi: 10.1016/j.yjmcc.2014.09.008
Yaniv, Y., Maltsev, V. A., Ziman, B. D., Spurgeon, H. A., and Lakatta, E. G. (2012). The “funny” current (If) inhibition by ivabradine at membrane potentials encompassing spontaneous depolarization in pacemaker cells. Molecules 17, 8241–8254. doi: 10.3390/molecules17078241
Yaniv, Y., Sirenko, S., Ziman, B. D., Spurgeon, H. A., Maltsev, V. A., and Lakatta, E. G. (2013). New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: bradycardic effects of ivabradine are linked to suppression of intracellular Ca2 + cycling. J. Mol. Cell. Cardiol. 62, 80–89. doi: 10.1016/j.yjmcc.2013.04.026
Keywords: heart rate, voltage clock, calcium clock, mechanics clock, autonomic nervous system, stretch, leading pacemaker site, blebbistatin
Citation: Stoyek MR, MacDonald EA, Mantifel M, Baillie JS, Selig BM, Croll RP, Smith FM and Quinn TA (2022) Drivers of Sinoatrial Node Automaticity in Zebrafish: Comparison With Mechanisms of Mammalian Pacemaker Function. Front. Physiol. 13:818122. doi: 10.3389/fphys.2022.818122
Received: 19 November 2021; Accepted: 21 January 2022;
Published: 28 February 2022.
Edited by:
Ursula Ravens, Technical University Dresden, GermanyReviewed by:
Sunil Jit Ramamoorthy Jeewanlal Logantha, University of Liverpool, United KingdomCopyright © 2022 Stoyek, MacDonald, Mantifel, Baillie, Selig, Croll, Smith and Quinn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. Alexander Quinn, YWxleC5xdWlubkBkYWwuY2E=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.