- 1Department of Gynaecology, Obstetrics and Reproductive Medicine, Saarland University Hospital, Homburg, Saarland, Germany
- 2Department of Gynaecology and Obstetrics, Klinikum Bremen-Nord, Bremen, Germany
- 3Department of Otorhinolaryngologie and Head and Neck Surgery, Saarland University Hospital, Homburg, Germany
- 4Department of Pathology, Saarland University Hospital, Homburg, Germany
- 5Institute of Medical Biometry, Epidemiology and Medical Informatics, Saarland University Hospital, Homburg, Saarland, Germany
With approximately 220,000 newly diagnosed cases per year, ovarian cancer is among the most frequently occurring cancers among women and the second leading cause of death from gynecological malignancies worldwide. About 70% of these cancers are diagnosed in advanced stages (FIGO IIB–IV), with a 5-year survival rate of 20–30%. Due to the poor prognosis of this disease, research has focused on its pathogenesis and the identification of prognostic factors. One possible approach for the identification of biological markers is the identification of tumor entity-specific genetic “driver mutations”. One such mutation is 3q26 amplification in the tumor driver SEC62, which has been identified as relevant to the pathogenesis of ovarian cancer. This study was conducted to investigate the role of SEC62 in ovarian malignancies. Patients with ovarian neoplasias (borderline tumors of the ovary and ovarian cancer) who were treated between January 2007 and April 2019 at the Department of Gynecology and Obstetrics, Saarland University Hospital, were included in this retrospective study. SEC62 expression in tumor tissue samples taken during clinical treatment was assessed immunohistochemically, with the calculation of immunoreactivity scores according to Remmele and Stegner, Pathologe, 1987, 8, 138–140. Correlations of SEC62 expression with the TNM stage, histological subtype, tumor entity, and oncological outcomes (progression-free and overall survival) were examined. The sample comprised 167 patients (123 with ovarian cancer and 44 with borderline tumors of the ovary) with a median age of 60 (range, 15–87) years. At the time of diagnosis, 77 (46%) cases were FIGO stage III. All tissue slides showed SEC62 overexpression in tumor cells and no SEC62 expression in other cells. Median immunoreactivity scores were 8 (range, 2–12) for ovarian cancer and 9 (range, 4–12) for borderline tumors of the ovary. Patients with borderline tumors of the ovary as well as patients with ovarian cancer and an immunoreactive score (IRS) ≤ 9 showed an improved overall survival compared to those presenting with an IRS score >9 (p = 0.03). SEC62 seems to be a prognostic biomarker for the overall survival of patients with ovarian malignancies.
1 Introduction
With approximately 220,000 newly diagnosed cases per year, ovarian cancer is among the most frequently occurring cancers in women; worldwide, it is the eighth leading cause of cancer-related death and the second leading cause of death from gynecological malignancies (Jayson et al., 2014; Siegel et al., 2016; Webb and jordan., 2017). Given the lack of early detection and screening options and their subtle presentation, 70–80% of ovarian carcinomas are diagnosed in advanced stages [International Federation of Gynecology and Obstetrics (FIGO) stages IIB–IV], with a 5-year survival rate of 20–30% (Buys et al., 2011; Torre et al., 2018). For this reason, and given the lack of tailored therapy despite the optimization of standard chemotherapeutic regimens, treatment options are limited. According to international guidelines, the standard treatment for ovarian cancer is maximal cytoreductive surgery followed by combination chemotherapy for many decades (Benedet et al., 2000). Ovarian cancer survival rates have hitherto been known to depend directly on the extent of surgical debulking, notably the achievement of complete cytoreduction (R0 resection), and on the amount of tumor remaining postoperatively (Bristow et al., 2002; Elattar et al., 2011). Due to the poor prognosis of this disease, recent research on ovarian cancer has focused on its pathogenesis, the identification of prognostic factors, and the development of precise therapeutic options based thereon.
A possible way of identifying these biologic markers is the identification of tumor-specific genetic tumor-driver mutations. Amplifications of the long arm of chromosome 3 (3q26 region) have been shown to be tumor-driver mutations, with high incidences in patients with head and neck, lung, and cervical cancers (Cancer Genome Atlas Research Network et al., 2008; Grobner et al., 2018). These alterations have also been observed with a frequency of 43.7% in patients with ovarian cancer (Hagerstrand et al., 2013). Given the potential impact of 3q amplifications on the pathogenesis of ovarian cancer, numerous studies have focused on the identification of 3q-oncogenes, such as PIK3CA, p63, nCLAPM1, and FXR1, but none of these genes has shown a functional correlation with ovarian tumorigenesis (Woenckhaus et al., 2002; Comtesse et al., 2007). Hagerstrand et al. conducted a systematic analysis of genes, which most frequently showed amplification in the 3q26 region and identified SKIL and SEC62 as tumor-driver-genes for ovarian cancer development (Hagerstrand et al., 2013). SEC62 encodes a transmembrane protein of the endoplasmic reticulum (Sec62). The precise physiological functions of the protein are not completely understood, but it has been shown to play roles in intracellular protein transport, ER-phagy to counteract cellular stress, and intracellular calcium homeostasis (Lakkaraju et al., 2012; Lang et al., 2012; Linxweiler et al., 2013; Fumagalli et al., 2016). SEC62 overexpression in tumor tissue compared with tumor-free tissue has been observed for lung, prostate, and cervical cancers at the protein and mRNA levels, and high SEC62 expression has been found to correlate with lymph node metastasis and poorer overall prognosis (Linxweiler et al., 2012, 2013, 2016; Wemmert et al., 2016). These results suggest that SEC62 plays a role in the pathogenesis of these tumor entities and may be an important tumor-driver oncogene. Although details of the molecular mechanisms responsible for these functions are poorly understood, in-vitro experiments conducted with lung cancer cell lines have revealed increased stress tolerance and enhanced migration, transition, and proliferation of SEC62-overexpressing cells (Greiner et al., 2011).
In light of the findings of Hagerstrand et al. who identified SEC62 as a potential tumor-driver-gene for the development of ovarian cancer and the prognostic impact of SEC62 in other tumor entities (Hagerstrand et al., 2013), the aim of this study was to assess the role of SEC62 as a possible prognostic marker in patients with ovarian neoplasia.
2 Materials and methods
2.1 Patients and tissue samples
All patients with primary ovarian cancer and borderline tumors of the ovary who were treated at the Department of Gynecology and Obstetrics, Saarland University Hospital, Homburg, Germany, between January 2007 and April 2019 were screened for enrollment in this retrospective study. The study protocol was approved by the Saarland Institutional Review Board (reference no. 207/11). The inclusion criteria were the availability of formalin-fixed, paraffin-embedded (FFPE) tissue samples and complete data on clinical parameters (including follow-up). Exclusion criteria were missing tissue samples and incomplete clinical information or follow-up. Data on patient and tumor characteristics were obtained by clinical chart review. Platinum sensitivity and resistance were defined as progression-free intervals of ≥6 and <6 months, respectively, after the completion of adjuvant platinum-based chemotherapy (Davis et al., 2014). SEC62 expression was analyzed in the whole cohort and in patients with borderline ovarian tumors and ovarian cancer, respectively, and correlated with overall survival (OS) and progression-free survival (PFS).
2.2 Immunohistochemical analysis of Sec62
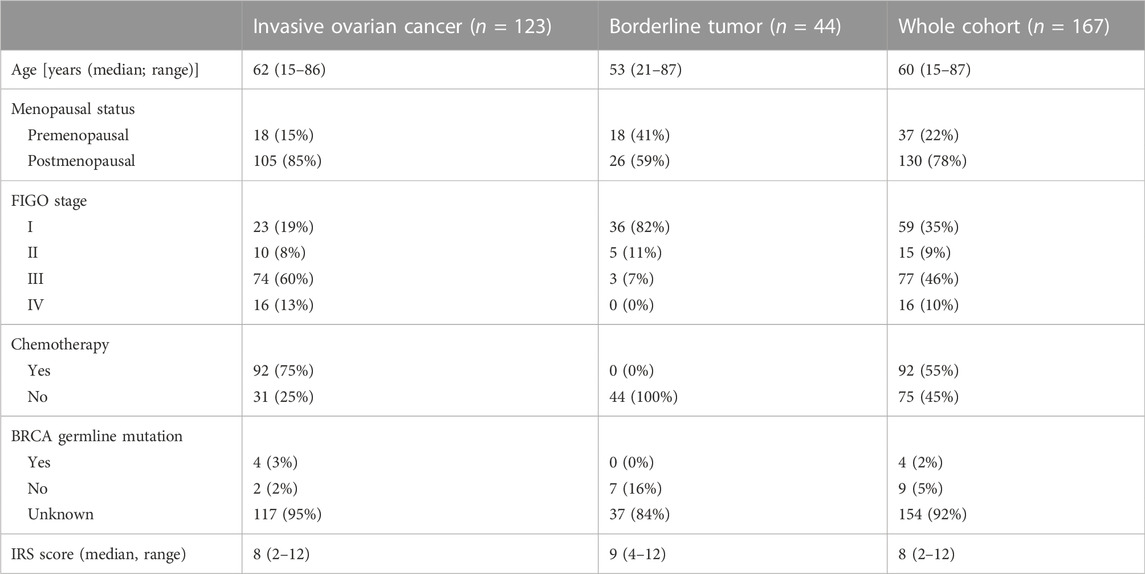
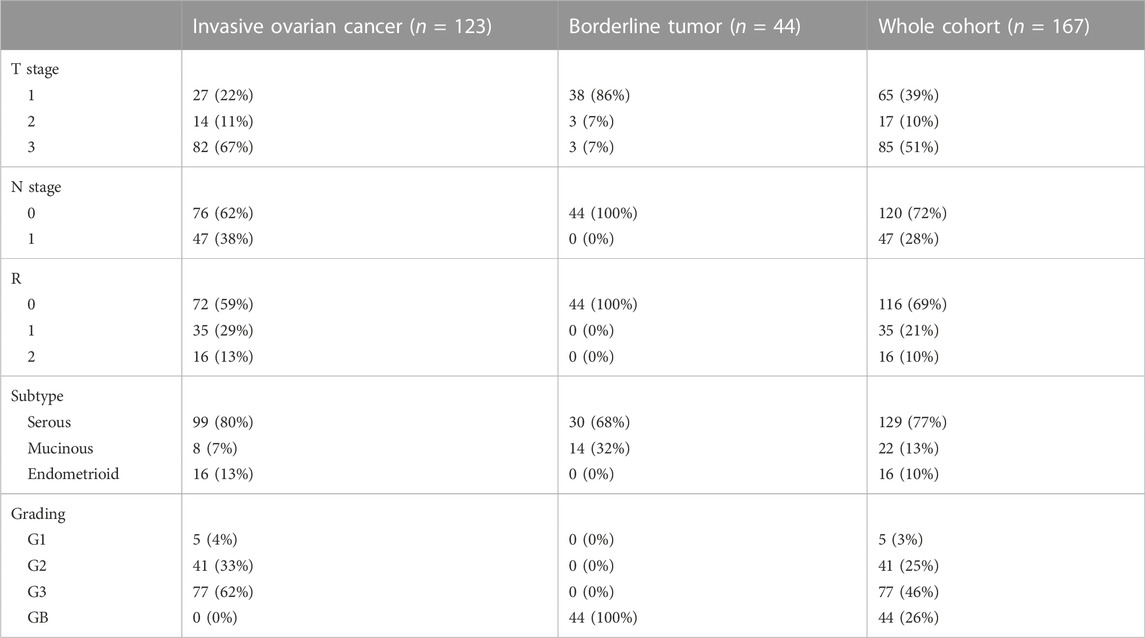
A pathologist evaluated hematoxylin-stained tissue samples taken from representative FFPE blocks of the primary tumor specimens (definitive pathological specimens obtained during surgery) and histologically tumor-free ovarian tissue. The first three 10-µm sections of each sample were discarded, and 3-µm sections were then cut using a rotary microtome (RM 2235; Leica Microsystems, Wetzlar, Germany), transferred onto Superfrost Ultra Plus Microscope slides (Menzel-Gläser, Braunschweig, Germany), and dried overnight in an incubator at 37°C. After deparaffinization, heat-induced epitope retrieval was performed in retrieval solution (Dako S1699; Agilent Technologies, Santa Clara, CA, United States of America) and non-specific protein binding sites were blocked by incubation in a 3% bovine serum albumin (BSA)–phosphate buffered saline (PBS) solution (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) for 30 min at room temperature. Subsequently, primary antibody incubation was performed with a 1:800 solution (diluted in 1% BSA–PBS) of a specific SEC62 affinity-purified polyclonal rabbit antipeptide antibody directed against the C terminus of human SEC62 (made in house) for 1 h at room temperature (Takacs et al., 2019). The rabbit antibody was directed against the COOH terminal undecapeptide of human Sec62 protein plus an aminoterminal cysteine (peptide sequence in single letter code: CGETPKSSHEKS). Commercial anti-Sec62 antibodies were described elsewhere as suitable alternatives (Liu et al., 2021). Each staining series included positive and negative (without primary antibody) controls. Visualization was performed using the Dako real detection system (Agilent Technologies) according to the manufacturer’s instructions, and the slides were counterstained with hematoxylin (Dako; Agilent Technologies, Glostrup, Denmark). Three independent examiners (one pathologist and two gynecologists) with wide experience in immunohistochemical (IHC) evaluation characterized SEC62 immunoreactivity using Remmele and Stegner’s immunoreactive score (IRS), a well-established and unbiased semiquantitative validation system for the IHC assessment of estrogen receptor detection in breast cancer (Remmele and Stegner., 1987). In this system, staining intensity is classified as none (0), weak (1), intermediate (2), and strong (3). The percentage of stained cells is classified as none (0), <10% (1), 10–50% (2), 51–80% (3), and >80% (4). The IRS is the product of the staining intensity and stained cell percentage scores. We defined the samples’ Sec62 protein contents as low (IRS 0–8), and high (IRS 9–12), as in previous studies (Linxweiler et al., 2012; Wemmert et al., 2016) (Figure 1), and compared this content between tumor/borderline tumor tissue and histologically tumor-free tissue from the same patients (Figure 2).
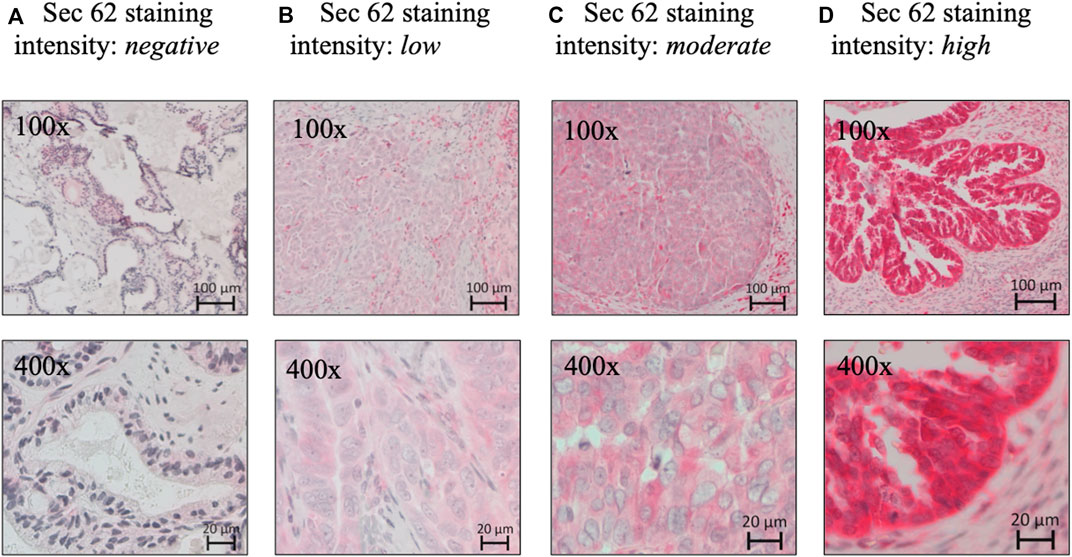
FIGURE 1. Sec62 immunohistochemistry. (A) Negative SEC62 expression in normal ovarian tissue, as well as low (B), moderate (C), and high immunostaining intensity (D) in serous ovarian cancer. SEC62 expression is indicated by a red signal, counterstaining with hematoxylin (blue).
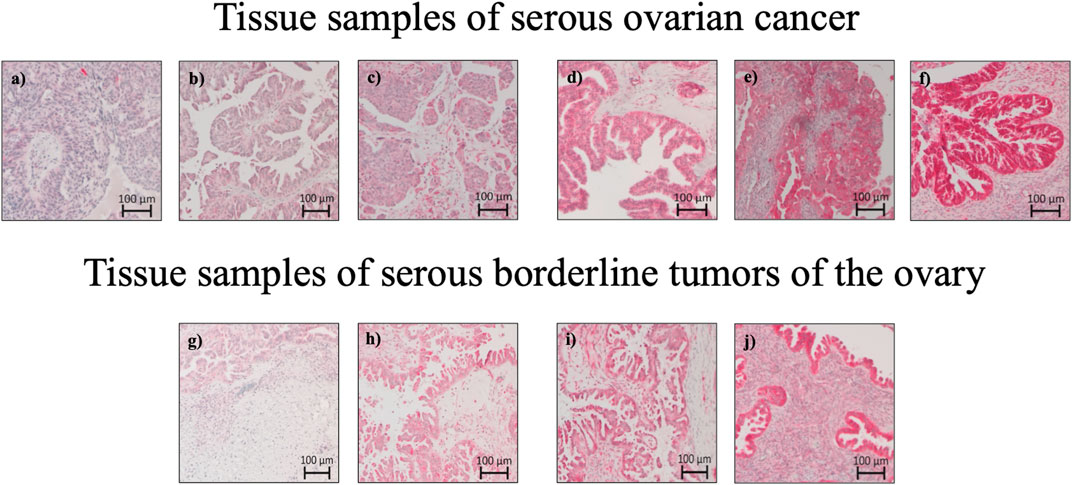
FIGURE 2. Sec62 immunohistochemistry stainings: Tissue samples of serous ovarian cancer and serous borderline tumors of the ovary. (A) Serous ovarian cancer with Sec62 immunoreactive score (IRS) 3, (B) serous ovarian cancer with Sec62 immunoreactive score (IRS) 4, (C) serous ovarian cancer with Sec62 immunoreactive score (IRS) 8, (D,E) serous ovarian cancer with Sec62 immunoreactive score (IRS) 9, (F) serous ovarian cancer with Sec62 immunoreactive score (IRS) 12, (G) serous borderline tumors of the ovary with Sec62 immunoreactive score (IRS) 4, (H) serous borderline tumors of the ovary with Sec62 immunoreactive score (IRS) 6, (I) serous borderline tumors of the ovary with Sec62 immunoreactive score (IRS) 8, (J) serous borderline tumors of the ovary with Sec62 immunoreactive score (IRS) 9.
2.3 Analysis of The Cancer Genome Atlas data
A dataset from The Cancer Genome Atlas (TCGA) was analyzed using publicly available sequencing data from the National Cancer Institute’s GDC data portal. The analysis was performed on 13 July 2022 and included 86,046 cases from 67 different primary tumor sites.
2.4 Statistical analysis
The SPSS software (v. 27; IBM Corporation, Armonk, NY, United States of America) was used for the statistical analysis. Qualitative and quantitative data are presented as absolute and relative frequencies and medians and ranges, respectively. For categorical variables (i.e., IRSs), we used Pearson’s chi-squared test for group comparison. The Kaplan–Meier method was used for the univariate analysis of PFS and OS durations (in months). Survival curves were compared using the log-rank test. Multivariate binary logistic regression analysis with stepwise forward and backward selection of factors associated with SEC62 expression was conducted. Covariates for the multivariate analysis were selected based on the univariate findings and clinically relevant factors, such as the TNM and FIGO stages, histopathological subtype, platinum sensitivity or resistance, and tumor entity. Two-sided p values <0.05 were considered to be significant.
3 Results
To address the role of SEC62 as a possible prognostic marker in patients with ovarian neoplasia, 171 patients with primary ovarian cancer and borderline tumors of the ovary, who were treated at the Department of Gynecology and Obstetrics, Saarland University Hospital, Homburg, Germany, were assessed for eligibility. Four of them were excluded due to insufficient slide quality and the lack of residual material for repeat staining. Thus, the analyses were conducted with samples from 167 patients (123 with invasive ovarian cancer and 44 with borderline tumors of the ovary). The median ages at the time of diagnosis were 62 (range, 15–86) years in the cohort of patients with invasive ovarian cancer and 53 (range, 21–78) years in the cohort of patients with borderline tumors (Table 1). At the time of diagnosis, 46% (n = 77) of patients had FIGO stage III G3 tumors and 77% (n = 129) of histopathological subtypes were serous (Table 2). The median PFS duration was 14 (range, 0–68) months and the median OS duration was 25 (range, 0–109) months (Table 3). Detailed oncologic outcomes are shown in Table 3.
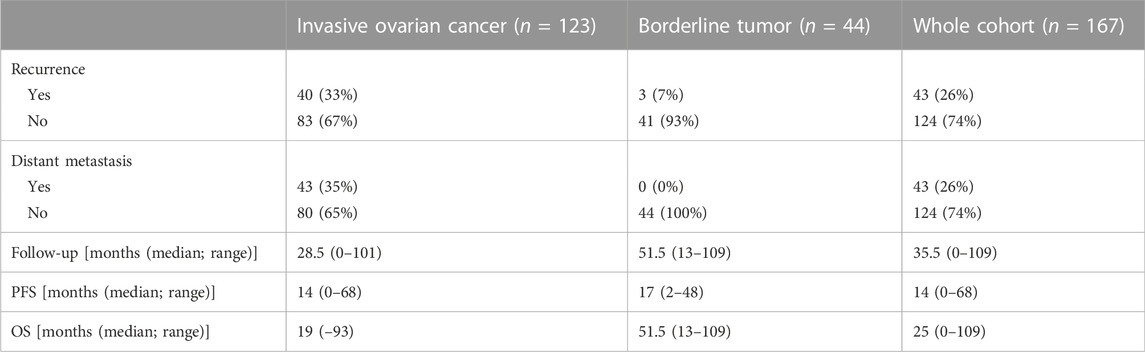
TABLE 3. Oncologic outcomes for patients with invasive ovarian cancer, borderline tumors of the ovary and the whole cohort (PFS = progression-free survival, OS = overall survival, n = 167).
3.1 Immunohistochemical (IHC) analysis of Sec62
Immunohistochemical analysis was carried out according to an established protocol and employed an anti-Sec62 antibody, which is directed against the COOH terminal undecapeptide of human Sec62 protein and was already successfully used in various immunohistochemical analyses of human patient tissue (Greiner et al., 2011; Linxweiler et al., 2012; Bochen et al., 2017). Furthermore, the antibody had previously been shown to be specific for Sec62 under denaturing as well as native conditions, i.e. Western blot and fluorescence microscopy-signals were quenched after silencing of the SEC62 gene in human cells (Greiner et al., 2011). All slides analyzed showed SEC62 overexpression. We observed cytoplasmic Sec62 positivity in all ovarian cancer and borderline ovarian tumor cells, but not in physiological ovarian tissue cells. The median IRSs for ovarian cancer and borderline tumor samples [8 (range, 2–12) and 9 (range, 4–12), respectively] did not differ significantly (Table 1). No correlation between progression-free survival and Sec62 expression was detected when including the whole cohort, the cohort of patients with ovarian cancer or the cohort of patients with borderline tumors of the ovary respectively (whole cohort p = 0.15; ovarian cancer p = 0.13; borderline tumors of the ovary p = 0.74) (Table 4; Figure 3). Analyzing correlations between Sec62 expression scores and overall survival, we observed a median overall survival of 91 months (range 79–103) in the whole cohort consisting of patients with ovarian cancer and borderline tumor of the ovary with an IRS ≤9, while it amounted to 36 months (range 11–61) in patients with an IRS >9 (p = 0.03) Table 4, Figure 3A). For the cohort of patients with ovarian cancer, a median overall survival of 49 months (range 26–72) for the subgroup of IRS ≤9 vs. 20 months (range 12–28) for the subgroup of IRS >9 was observed (p = 0.02) Table 4, Figure 3B). No correlation between overall survival and Sec62 expression was detected in the borderline ovarian tumor cohort (p = 1.00, Table 4, Figure 3C).
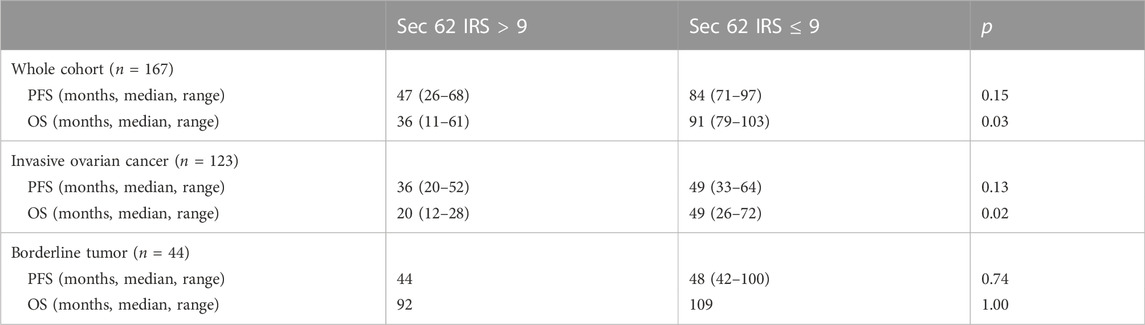
TABLE 4. Oncologic outcomes in correlation with immunohistochemical SEC 62 expression (PFS = progression-free survival, OS = overall survival, IRS = immunoreactive score).
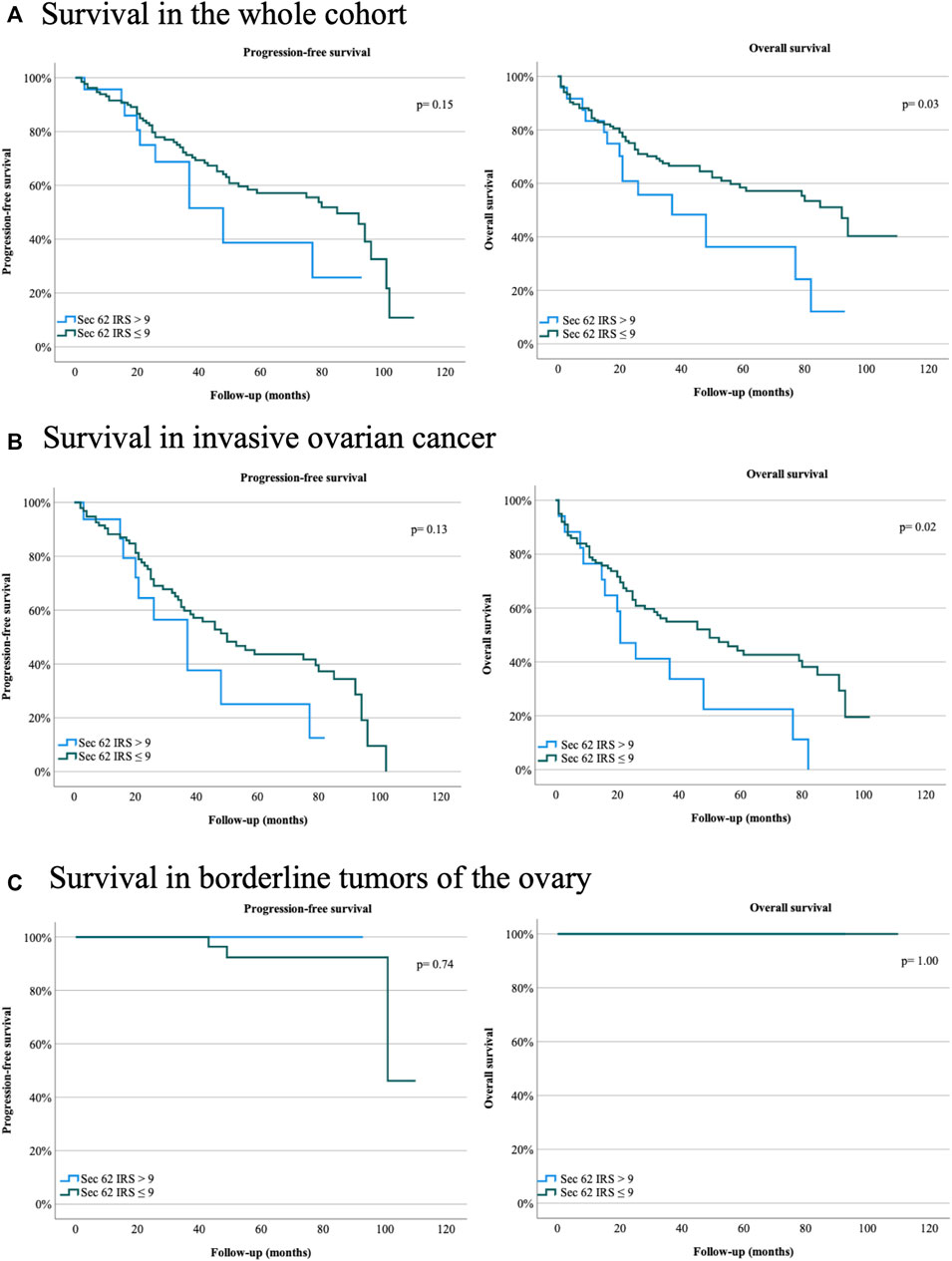
FIGURE 3. Survival rates for ovarian cancer patients and patients with borderline tumors of the ovary of the Department of Gynecology and Obstetrics, Saarland University Hospital, Homburg, Germany, between January 2007 and April 2019. (A) Progression-free and overall survival in the whole cohort. (B) Progression-free and overall survival in invasive ovarian cancer. (C) Progression-free and overall survival in borderline tumors of the ovary. Sec62 immunoreactive score (IRS) > 9, Sec62 IRS ≤9. Two-sided p values are indicated, values <0.05 were considered to be significant.
3.2 TCGA data analysis
We observed SEC62 alterations in 2,922 cases from TGCA, with a predominance of gene amplifications (Figure 4A). Thereby, five gynaecologic malignancies were ranked under the top eight tumor entities with the highest frequency of SEC62 gene alterations (ovarian cancer (39% of cases), cervical cancer (35% of cases), endometrial cancer (30% of cases), uterine endometrioid carcinoma (25% of cases), and breast cancer (15% of cases)) (Figure 4A). Overall survival across all cancer entities recorded in the TCGA atlas was shorter in patients with SEC62 alteration compared to patients without SEC62 alteration (p < 0.01; Figure 4B).
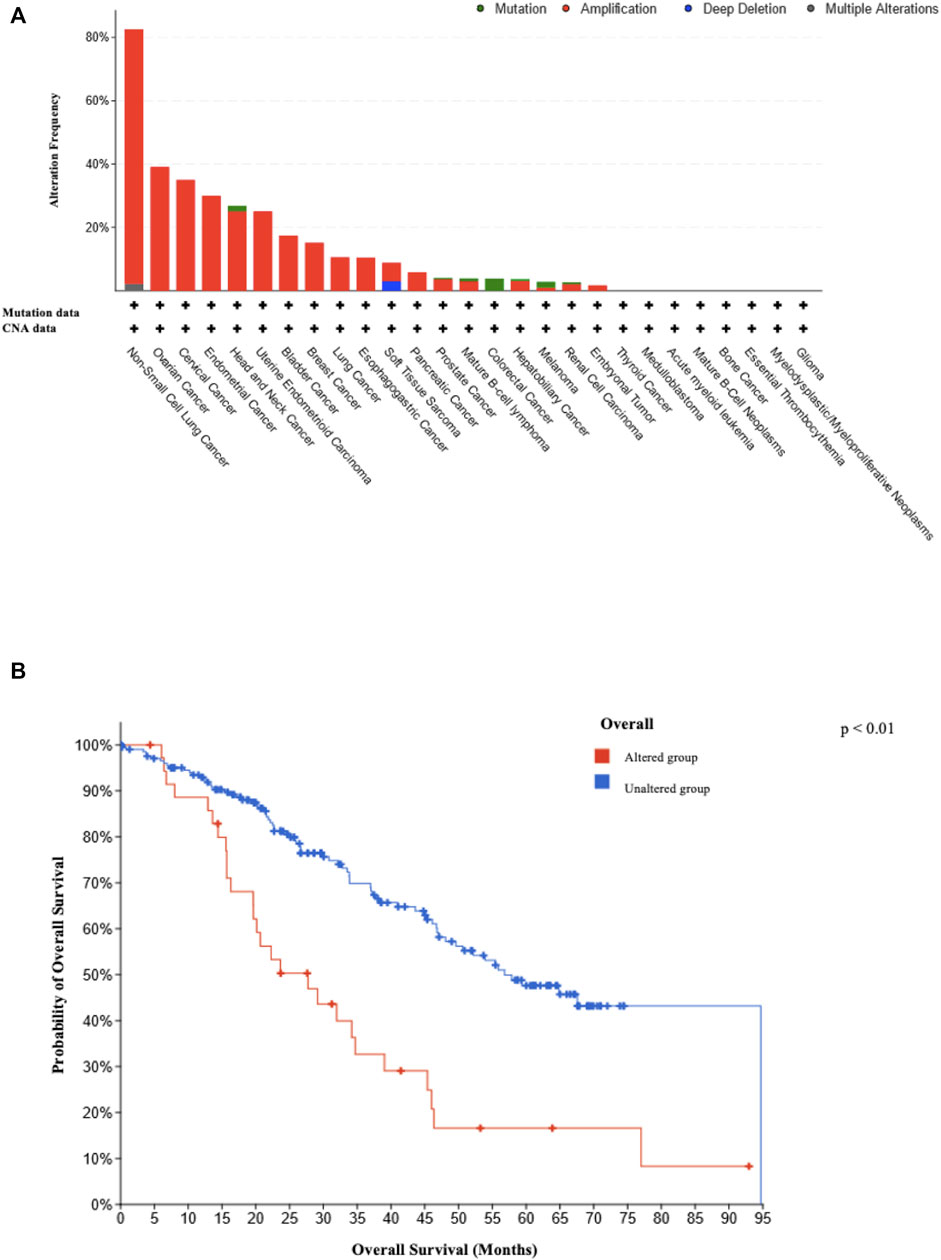
FIGURE 4. Type and frequency of reported SEC62 gene alterations and related survival rates. (A) Type and frequency of SEC62 gene alterations recorded in the TCGA atlas from the National Cancer Institute GDC Data Portal. The analysis was performed in 86,046 cases overall from 67 different primary tumor sites on 13 July 2022. CNA = copy number alteration (B) Overall survival across all cancer entities recorded in the TCGA atlas depending on SEC62 alteration. The analysis was performed on 13 July 2022. Two-sided p values are indicated, values <0.05 were considered to be significant.
4 Discussion
In this study, we found that SEC62 is overexpressed in ovarian cancer cells, SEC62 expression can serve as a prognostic marker for patients with ovarian cancer or borderline tumor of the ovary and SEC62 is a potential tumor-driver gene accounting for 3q amplification in ovarian cancer. For the prognostic role of SEC62, we could identify an IRS of >9 being associated with a shortened overall survival for patients with ovarian cancer, as well as for patients with borderline tumors of the ovary.
To our knowledge, this study is the first to address the prognostic relevance of SEC62 and the correlation between SEC62 overexpression and the poorer OS of patients with ovarian cancer. Our findings are in line with those for other tumor entities. Liu et al. observed a significant correlation of SEC61G overexpression with poor prognosis based on statistical analysis of data from the Cancer Genome Atlas cohort and the Chinese Glioma Genome Atlas cohort in patients with glioblastoma multiforme (Liu et al., 2019). Besides Bochen et al. found a high expression level of SEC62, defined as an IRS >0, to be significantly correlated with a shorter overall survival in IHC analyses of tissue specimens from 65 head and neck squamous cell carcinomas patients and 29 patients with cervical cancer of unknown primary (CUP) (Bochen et al., 2017). Overall survival was also shown to be significantly worse in a cohort of 53 patients with breast cancer, whose SEC62 overexpression was assessed by Takacs et al. by also evaluating their Sec62 staining intensity by IHC analyses of tissue samples (Takacs et al., 2019). Takacs and colleagues defined an IRS cut-off of 8 for prognostic relevance, whereas we found a cut-off of 9 to represent overexpression and be prognostically relevant for OS (Takacs et al., 2019). This slight difference in cut-off scores seems to be of statistical interest and can be waived in terms of oncological outcomes, underlining the observation that IRS >9 seem to be associated with poorer overall survival in at least two gynecological cancer entities.
In contrast to others, we found no direct correlation in multivariate or Kaplan–Meier analyses between SEC62 expression and the response to platinum-containing chemotherapy. In an analysis of 102 colorectal cancer tissue microarrays, Liu et al. found that SEC62 promoted chemoresistance (Liu et al., 2021). SEC62 seems to play a role in the response to chemotherapy, although only tendencies have been observed to date; further studies are needed to clarify this correlation.
Our analyses of the TCGA data, which showed alterations of this gene (mostly amplifications) in 39% of all cases, underlines the potential role of SEC62 as a key tumor-driver gene in ovarian cancer. Hagerstrand et al. (2013) managed to expose the pathomechanism behind this tumor-driver gene, as these authors found SEC62 overexpressing tumor cells to be characterized by increased proliferation and migratory as well as invasive potential, three hallmarks of cancer cells (Hanahan, 2022). The shortened overall survival across all tumor entities detected for the SEC62 altered cohort emphasizes the prognostic effect of SEC62. These findings are well in line with other studies, in which SEC62 was identified as an oncogene for lung cancer, prostate cancer and head and neck cancer and in which high SEC62 expressions were correlated with a significant shorter disease-free and overall-survival (Greiner et al., 2011; Linxweiler et al., 2012 and 2016; Bochen et al., 2016).
With the identification of Sec62 as a potential prognostic marker for OS in patients with ovarian cancer, questions remain for further study. Due to the possible role of SEC62 as a predictor of the response of ovarian cancer to platinum-based chemotherapy, prospective in vitro and in vivo studies are needed to move toward the implementation of this knowledge in daily clinical routine.
Limitations of this study are the lack of statistically evaluating interobserver variability, as interobserver variability is a known source of error in immunohistochemical studies. Besides, further studies using a second evidence tool, such as qPCR, should be implemented, to confirm immunoreactivity scores on the RNA level.
Future work with ovarian cancer cell lines will need to address the question of whether or not SEC62 overexpression is associated with increased ER stress tolerance as well as increased migratory and invasive potential, three hallmarks of cancer that had been observed for various other SEC62 overexpressing tumor cells (reviewed in this Research Topic by Zimmermann et al., 2022). Only if these in vitro experiments demonstrate a causative effect of SEC62 overexpression on these three hallmarks in ovarian cancer cells, future in vivo experiments will address Sec62 as a potential therapeutic target for this tumor entity (also reviewed in this Research Topic by Zimmermann et al., 2022).
5 Conclusion
This study revealed an increased incidence of SEC62 alterations and a correlation between high SEC62 expression and worse OS in patients with ovarian cancer. These findings indicate that SEC62 may play an oncogenic role in the pathogenesis of ovarian cancer.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Saarland Institutional Review Board (application number: 207/11).
Author contributions
JR: project development, data collection, data analysis (including IHC analysis), manuscript writing, manuscript editing. MK, ML, ZT, MR, AK, RB: data analysis, manuscript editing. A-CS, BL: data collection, data analysis, manuscript editing. MW: data collection, data analysis (including IHC analysis), manuscript editing. GW: data analysis, manuscript editing. E-FS: project development, manuscript editing. JZ: data collection, data analysis (including IHC analysis), data management, manuscript writing, manuscript editing. All authors contributed to the manuscript and agreed to the final version of the manuscript.
Funding
JZ was supported by HOMFOR (Homburger Forschungsföderungsprogramm, Saarland University, Homburg, Germany; grant number: T201000878). We acknowledge support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and Saarland University within the Open Access Publication Funding programme.
Acknowledgments
The authors are grateful to Prof. Martin Jung (Medical Biochemistry and Molecular Biology, Saarland University, Homburg, Germany) for providing the self-made and affiniti-purified anti-Sec62 antibody.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Benedet J. L., Bender H., Jones H., Ngan H. Y., Pecorelli S. (2000). FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int. J. Gynaecol. Obstet. 70, 209–262. doi:10.1016/S0020-7292(00)90001-8
Bochen F., Adisurya H., Wemmert S., Lerner C., Greiner M., Zimmermann R., et al. (2017). Effect of 3q oncogenes SEC62 and SOX2 on lymphatic metastasis and clinical outcome of head and neck squamous cell carcinomas. Oncotarget 8, 4922–4934. doi:10.18632/oncotarget.13986
Bristow R. E., Tomacruz S. R., Armstrong D. K., Trimble E. L., Montz F. J. (2002). Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 20, 1248–1259. doi:10.1200/JCO.2002.20.5.1248
Buys S. S., Partridge E., Black A., Johnson C. C., Lamerato L., Isaacs C., et al. PLCO Project Team (2011). Effect of screening on ovarian cancer mortality: The prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA 305, 2295–2303. doi:10.1001/jama.2011.766
Cancer Genome Atlas Research Network (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068. doi:10.1038/nature07385
Comtesse N., Keller A., Diesinger I., Bauer C., Kayser K., Huwer H., et al. (2007). Frequent overexpression of the genes FXR1, CLAPM1 and EIF4G located on amplicon 3q26-27 in squamous cell carcinoma of the lung. Int. J. Cancer 120, 2538–2544. doi:10.1002/ijc.22585
Davis A., Tinker A. V., Friedlander M. (2014). Platinum resistant" ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 133, 624–631. doi:10.1016/j.ygyno.2014.02.038
Elattar A., Bryant A., Winter-Roach B. A., Hatem M., Naik R. (2011). Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 10, CD007565. doi:10.1002/14651858.CD007565.pub2
Fumagalli F., Noack J., Bergmann T., Cebollero E., Pisoni G. B., Fasana E., et al. (2016). Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat. Cell. Biol. 18, 1173–1184. doi:10.1038/ncb3423
Greiner M., Kreutzer B., Jung V., Grobholz R., Hasenfus A., Stöhr R., et al. (2011). Silencing of the SEC62 gene inhibits migratory and invasive potential of various tumor cells. Int. J. Cancer 128, 2284–2295. doi:10.1002/ijc.25580
Gröbner S. N., Worst B. C., Weischenfeldt J., Buchhalter I., Kleinheinz K., Rudneva V. A., et al. (2018). The landscape of genomic alterations across childhood cancers. Nature 555, 321–327. doi:10.1038/nature25480
Hagerstrand D., Tong A., Schumacher S. E., Ilic N., Shen R. R., Cheung H. W., et al. (2013). Systematic interrogation of 3q26 identifies TLOC1 and SKIL as cancer drivers. Cancer Discov. 3, 1044–1057. doi:10.1158/2159-8290.CD-12-0592
Hanahan D. (2022). Hallmarks of cancer: New dimensions. Cancer Discov. 12, 31–46. doi:10.1158/2159-8290.CD-21-1059
Jayson G. C., Kohn E. C., Kitchener H. C., Ledermann J. A. (2014). Ovarian cancer. Lancet 11, 1376–1388. doi:10.1016/S0140-6736(13)62146-7
Lakkaraju A. K. K., Thankappan R., Mary C., Garrison J. L., Taunton J., Strub K. (2012). Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol. Biol. Cell. 23, 2712–2722. doi:10.1091/mbc.E12-03-0228
Lang S., Benedix J., Fedeles S. V., Schorr S., Schirra C., Schäuble N., et al. (2012). Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells.-Sec62 and Sec63-depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J. Cell. Sci. 125, 1958–1969. doi:10.1242/jcs.096727
Linxweiler M., Schorr S., Jung M., Schäuble N., Linxweiler J., Langer F., et al. (2013). Targeting cell migration and the endoplasmic reticulum stress response with calmodulin antagonists: A clinically tested small molecule phenocopy of SEC62 gene silencing in human tumor cells. BMC Cancer 13, 574. doi:10.1186/1471-2407-13-574
Linxweiler M., Bochen F., Schick B., Wemmert S., Al Kadah B., Greiner M., et al. (2016). Identification of SEC62 as a potential marker for 3q amplification and cellular migration in dysplastic cervical lesions. BMC Cancer 16, 676. doi:10.1186/s12885-016-2739-6
Linxweiler M., Linxweiler J., Barth M., Benedix J., Jung V., Kim Y.-J., et al. (2012). Sec62 bridges the gap from 3q amplification to molecular cell biology in Non-Small Cell Lung Cancer. Am. J. Pathol. 180, 473–483. doi:10.1016/j.ajpath.2011.10.039
Liu B., Liu J., Liao Y., Jin C., Zhang Z., Zhao J., et al. (2019). Identification of SEC61G as a novel prognostic marker for predicting survival and response to therapies in patients with glioblastoma. Med. Sci. Monit. 25, 3624–3635. doi:10.12659/MSM.916648
Liu X., Su K., Sun X., Jiang Y., Wang L., Hu C., et al. (2021). Sec62 promotes stemness and chemoresistance of human colorectal cancer through activating Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res. 40, 132. doi:10.1186/s13046-021-01934-6
Remmele W., Stegner H. E. (1987). Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8, 138–140.
Siegel R. L., Miller K. D., Jemal A. (2016). Cancer statistics. Ca. Cancer J. Clin. 66, 7–30. doi:10.3322/caac.21442
Takacs F. Z., Radosa J. C., Linxweiler M., Kasohah M., Bohle R. M., Bochen F., et al. (2019). Identification of 3q oncogene SEC62 as a marker for distant metastasis and poor clinical outcome in invasive ductal breast cancer. Arch. Gynecol. Obstet. 299, 1405–1413. doi:10.1007/s00404-019-05081-4
Torre L. A., Trabert B., DeSantis C. E., Miller K. D., Samimi G., Runowicz C. D., et al. (2018). Ovarian cancer statistics. Ca. Cancer J. Clin. 68, 284–296. doi:10.3322/caac.21456
Webb P. M., Jordan S. J. (2017). Epidemiology of epithelial ovarian cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 41, 3–14. doi:10.1016/j.bpobgyn.2016.08.006
Wemmert S., Lindner Y., Linxweiler J., Wagenpfeil S., Bohle R., Niewald M., et al. (2016). Initial evidence for Sec62 as a prognostic marker in advanced head and neck squamous cell carcinoma. Oncol. Lett. 11, 1661–1670. doi:10.3892/ol.2016.4135
Woenckhaus J., Steger K., Werner E., Fenic I., Gamerdinger U., Dreyer T., et al. (2002). Genomic gain of PIK3CA and increased expression of p110alpha are associated with progression of dysplasia into invasive squamous cell carcinoma. J. Pathol. 198, 335–342. doi:10.1002/path.1207
Keywords: Sec62, prognostic, therapy, ovarian cancer, borderline tumors of the ovary, tumor driver mutation, 3q26 amplification
Citation: Radosa JC, Kasoha M, Schilz A-C, Takacs ZF, Kaya A, Radosa MP, Linxweiler B, Linxweiler M, Bohle RM, Wagner M, Wagenpfeil G, Solomayer E-F and Zimmermann JSM (2023) Effect of the 3q26-coding oncogene SEC62 as a potential prognostic marker in patients with ovarian neoplasia. Front. Physiol. 13:1054508. doi: 10.3389/fphys.2022.1054508
Received: 26 September 2022; Accepted: 03 December 2022;
Published: 04 January 2023.
Edited by:
Christoph Fahlke, Julich Research Centers (HZ), GermanyReviewed by:
Fabien Van Coppenolle, Université Claude Bernard Lyon 1, FranceRobert Blum, University Hospital Würzburg, Germany
Copyright © 2023 Radosa, Kasoha, Schilz, Takacs, Kaya, Radosa, Linxweiler, Linxweiler, Bohle, Wagner, Wagenpfeil, Solomayer and Zimmermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia C. Radosa, anVsaWEucmFkb3NhQHVrcy5ldQ==
 Julia C. Radosa
Julia C. Radosa Mariz Kasoha1
Mariz Kasoha1 Maximilian Linxweiler
Maximilian Linxweiler