
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 01 December 2022
Sec. Invertebrate Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.1039401
This article is part of the Research Topic Cephalopods in the Anthropocene: Multiple Challenges in a Changing Ocean View all 16 articles
 Lloyd A. Trueblood1*
Lloyd A. Trueblood1* Kirt Onthank2
Kirt Onthank2 Noah Bos2
Noah Bos2 Lucas Buller2
Lucas Buller2 Arianna Coast1,2
Arianna Coast1,2 Michael Covrig2
Michael Covrig2 Ethan Edwards2
Ethan Edwards2 Stefano Fratianni2
Stefano Fratianni2 Matthew Gano2
Matthew Gano2 Nathaniel Iwakoshi2
Nathaniel Iwakoshi2 Eden Kim2
Eden Kim2 Kyle Moss2
Kyle Moss2 Chantel Personius2
Chantel Personius2 Stephanie Reynoso2
Stephanie Reynoso2 Cheyne Springbett2
Cheyne Springbett2Elevated atmospheric CO2 as a result of human activity is dissolving into the world’s oceans, driving a drop in pH, and making them more acidic. Here we present the first data on the impacts of ocean acidification on a bathyal species of octopus Muusoctopus leioderma. A recent discovery of a shallow living population in the Salish Sea, Washington United States allowed collection via SCUBA and maintenance in the lab. We exposed individual Muusoctopus leioderma to elevated CO2 pressure (pCO2) for 1 day and 7 days, measuring their routine metabolic rate (RMR), critical partial pressure (Pcrit), and oxygen supply capacity (α). At the time of this writing, we believe this is the first aerobic metabolic data recorded for a member of Muusoctopus. Our results showed that there was no change in either RMR, Pcrit or α at 1800 µatm compared to the 1,000 µatm of the habitat where this population was collected. The ability to maintain aerobic physiology at these relatively high levels is discussed and considered against phylogeny and life history.
Presently atmospheric CO2 levels are over 400 ppm (Dunn et al., 2020) well above pre industrial levels of approximately 275 ppm (Macfarling et al., 2006). The world’s oceans absorb as much as one-third of annual anthropogenic CO2 (Doney et al., 2009), causing an increase in oceanic partial pressure of CO2 (pCO2). The increased pCO2 drives a decrease in pH, causing oceanic pH to decline from its pre-industrial revolution level of 8.2 to a current average below 8.1, in a process termed ocean acidification (OA) (Caldeira and Wickett, 2003).
Initial studies of the impact of OA on marine organisms focused largely on challenges faced by calcifying organisms (Fabry et al., 2008). However more recently there has been a number of studies that examine how OA impacts the physiology of an array of organisms. Changes in pH have been shown to impact respiratory physiology (Miller, 1985; Bridges, 1995; Widdicombe and Spicer, 2008; Seibel, 2016). Negative impacts to respiratory physiology make it more difficult to obtain oxygen from the environment and may limit aerobic energy production. This has been shown in crab, squid, fish, and sipunculids (Portner and Zielinski, 1998; Langenbuch and Portner, 2002; Metzger et al., 2007; Rosa and Seibel, 2008; Munday et al., 2009; Walther et al., 2009).
Studies within cephalopods have shown various, and sometimes conflicting, responses to environmental hypercapnia and the resulting low pH. At environmentally relevant ranges of 700–1700 μatm adult Cuttlefish Sepia officinalis show no change in aerobic metabolic rate (Gutowska et al., 2008) whereas embryonic S. officinalis showed an increase in routine metabolism (Rosa et al., 2013). In squid, the vertically migrating epipelagic squid Dosidicus gigas showed conflicting responses with some showing metabolic depression (Rosa and Seibel, 2008) and others showing no effect (Birk et al., 2018). Sepioteuthis lessoniana had no aerobic metabolic response below 2000 µatm pCO2 (Hu et al., 2014). Alternatively the benthic, inter- and sub-tidal octopus Octopus rubescens had a short term (1 day) increase in routine metabolic rate (RMR) which then returned to pre-exposure levels within 1 week. Additionally, critical oxygen partial pressure (Pcrit) was significantly higher after long term exposure to elevated pCO2 (Onthank et al., 2021).
In studies where pCO2 is pushed above 2,000 µatm there is a continued variation in response. Cuttlefish increase calcification of their cuttlebone (Gutowska et al., 2008; Gutowska et al., 2010), whereas squid statoliths are malformed and more porous (Kaplan et al., 2013). Above 4,000 µatm squid continue to show a decrease in aerobic metabolic rate (Hu et al., 2014).
The majority of studies which examine the impact of OA on cephalopods have focused primarily on cuttlefish and squid. As of this writing there has only been one other study we are aware of that explored how OA affects octopus and no studies on bathyal occurring species of octopus. Here, we examine the impact of OA on the Smoothskin octopus Muusoctopus leioderma (Berry, 1911). Like all other members of Family Enteroctopodidae, M. leioderma is a deep living species. It can be found in the Northern Pacific from the Sea of Okhotsk off Siberia to California and are reported to live on muddy or silty bottoms at meso and bathyal depths ranging from 250 to 1400 m (Conners et al., 2016). This range overlaps with an oxygen minimum zone that has both low oxygen levels as well as pCO2 levels in excess of 2000 µatm (Kamykowski and Zentara, 1990; Paulmier et al., 2011). The greatest frequency of occurrence for M. leioderma has been reported between 450–650 m (Conners et al., 2016). However, it has previously been reported as shallow as 70 m (Hochberg, 1998). Recently a population has been found at depths reachable by SCUBA (10–15 m) in Burrows Bay, Skagit County, Washington, United States, the shallowest record for any individuals in the genus Muusoctopus, a major deep water octopus genus with 28 recognized species. Morphological and genetic data were used to confirm the species identity of this population as Muusoctopus leioderma (Onthank unpublished data) (Figure 1).
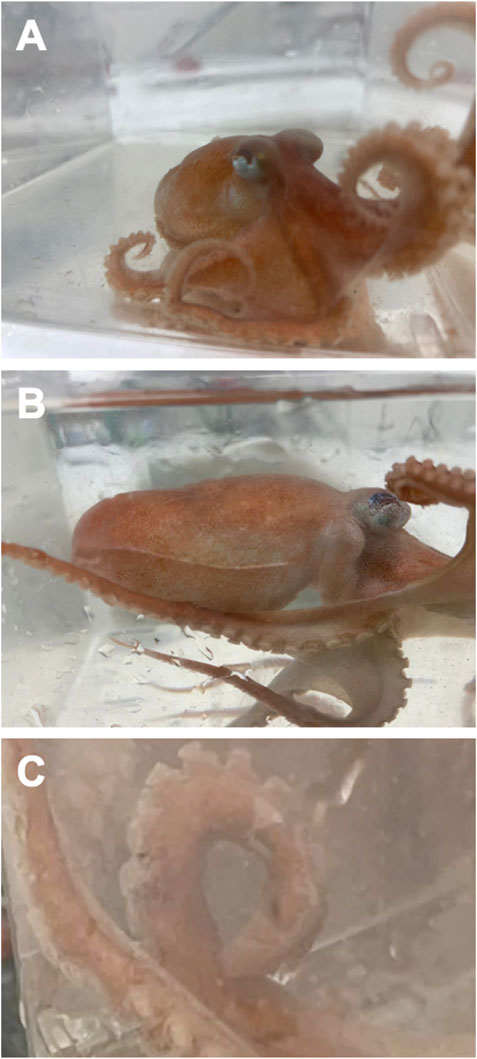
FIGURE 1. Muusoctopus leioderma collected at Burrows Bay, Anacortes Washington, United States (A). Note the well-developed ridge of skin on the periphery mantle (B) and hectocotylized arm on third armright (C).
As part of a marine environmental physiology course at the Rosario Beach Marine Lab (RBML) Muusoctopus leioderma were collected and held at 1,000 or 1800 µatm for 1 day and 7 days. Routine metabolic rate, critical partial pressure (Pcrit) and oxygen supply capacity (α) were recorded for each treatment. The Salish Sea, where the RBML is located, is a unique location for OA studies as CO2-rich water from the California Undercurrent wells up into this shallow basin producing persistent hypercapnic conditions (Murray et al., 2015). The pCO2 regularly reaches 1,000 µatm (Onthank et al., 2021). Habitats such as the Salish Sea will experience accentuated acidification due to local hypoxia and eutrophication (Cai et al., 2011; Melzner et al., 2013). The goal of this study was to examine the impacts of short-term and prolonged exposure to hypercapnia on aerobic metabolism of a bathyal associated species of benthic octopus. The population of M. leioderma we used for this study was found at depths, and locations near populations of O. rubescens, and likely experiences similar environmental conditions. Because of this we hypothesized that M. leioderma would show a similar response to elevated pCO2 as O. rubescens having a short term increase in RMR after 24h exposure, and a return to pre-exposure RMR and elevated Pcrit with a prolonged 7-day exposure to hypercapnic conditions. This is the first publication of aerobic metabolic rate for any species in the genus Muusoctopus. This is also the first study of effects of ocean acidification in any deep water living species of octopus.
This research was carried out at the Rosario Beach Marine Laboratory in Anacortes, Washington United States. Water samples were taken at the octopus collection site in Burrows Bay. Samples were taken at depth where the octopus were collected via SCUBA. A 50 ml high-density polyethylene sample container filled with air was opened at depth and filled with a water sample, excluding all air bubbles. A screw top lid was used to cap the sample. All water samples were immediately transported to the RBML where pH on the total scale (pHT) was measured using the m-cresol purple spectrophotometric method (Dickson et al., 2007) within 3 h. Alkalinity was determined by open-cell titration (Dickson et al., 2007), and alkalinity values were calculated from titration data using the at() function in the “seacarb” package version 3.2.14 in R (Gattuso et al., 2015). The resulting measured alkalinity and pH were used to calculate the pCO2 using the carb() function in the “seacarb” package in R. Samples from the collection site had pCO2 ∼1,000 µatm.
Seventeen Muusoctopus leioderma (mass = 2.5–70.0 g) were collected in June through August of 2021 from Burrows Bay, Skagit County, Washington State, United States by SCUBA at depths of 10–20 m (Figure 2). Octopuses were found on the sediment bottom during night dives. Individuals were placed in plastic resealable bags for transport to RMBL. At RMBL octopuses were placed in holding aquaria with sediment that had previously been collected from Burrows Bay. The holding aquaria were supplied with unmodified seawater directly from the lab seawater system which uses seawater pumped from Rosario Bay.
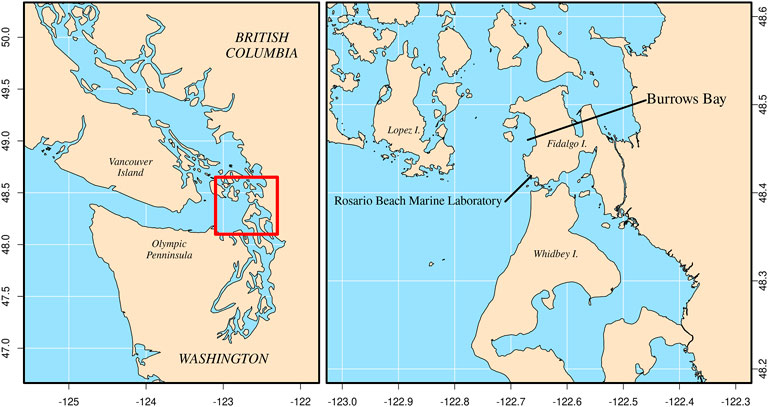
FIGURE 2. Location of Burrows Bay, Anacortes, Washington, United States where Muusoctopus leioderma were collected for this study.
The Salish Sea has persistent hypercapnic conditions that reach 1,000 µatm CO2 (Murray et al., 2015) and in the future may increase by an additional 800 µatm (Barry et al., 2010; Cai et al., 2011; Melzner et al., 2013; Bianucci et al., 2018). At the time of octopus collection, water samples from Burrows Bay were approximately 1,000 µatm, which is consistent with values recorded previously by Onthank et al. (2021) at surface and 15 m depth. The selected pCO2 range allowed us to examine whether the Burrows Bay population that regularly experiences 1,000 µatm CO2 would be impacted by the predicted increased CO2 levels resulting in 1800 µatm CO2 locally.
After being held in aquaria for at least 1 day, octopuses were transferred to treatment tanks as previously described (Culler-Juarez and Onthank, 2021). Each tank (113.5 L) was made using an insulated cooler with an overhead window in the lid to allow observation of octopus. The tank system included an Active Aqua AACH10HP chiller to maintain temperature, and a venturi injector to keep tank water oxygenated. Each tank had a slow constant water exchange from the lab sea water system which flowed fresh seawater into the tank at ∼100 ml min−1 and drained from an overflow port. This prevented building up waste in each tank without having to perform large, full-tank water changes. Temperature and pCO2 of each tank was controlled with a pH-stat system (https://open-acidification.github.io/) which received temperature input from a PT-100 temperature probe and pH input from a single junction glass pH electrode. The pH of aquaria was measured daily using the m-cresol purple spectrographic method (Dickson et al., 2007), and that measured aquarium pH used to perform a one-point calibration of the pH electrode. Temperature probes were calibrated using a 0.1°C resolution alcohol thermometer. To modify pCO2 pure gaseous CO2 was slowly bubbled into the tank by a solenoid controlled via the Open Acidification Tank Controller. Temperature was controlled by powering on/off a chiller controlled by the same Tank Controller.
In addition to pH measurements, the total alkalinity (AT) was measured weekly using a modified open cell titration based on Dickson et al. (2007). Off gassing time was increased from 6 to 10 min with vigorous stirring via a stir bar and motor. Titrations were verified against certified reference material (CRM, supplied by Andrew Dickson, Scripps Institution of Oceanography, San Diego, CA, United States). The alkalinity values were calculated from titration data using the “seacarb” package in R (Gattuso et al., 2015). The measured alkalinity and desired pCO2 of each tank were used to calculate pH setpoints and tank pH set points were updated weekly. The pH was calculated from raw spectrographic data using the specpH(), function in the OTools package in R (https://github.com/KirtOnthank/OTools), alkalinity and PCO2 were determined using the at() function and carb function respectively in the seacarb package in R (https://CRAN.R-project.org/package=seacarb).
After at least 1 day acclimating in holding aquaria, octopus were transferred to treatment tanks. One octopus was placed in a treatment tank and held at either 1,000 µatm or 1800 µatm for 1 day and then its RMR and Pcrit were measured (methods described below) and it was then returned to the same treatment tank and held for additional 6 days, for a total of 7 days exposure to its treatment pCO2. Routine metabolic rate and Pcrit were then measured again after the 7th day of exposure. During treatment feeding was done by placing purple shore crabs in the treatment tank after the first 1 day RMR data collection and removed 1 day prior to RMR and Pcrit measurements on day 7.
Routine metabolic rate were measured after fasting for 24 h. Octopus were placed in 1 L or 120 ml as body size required, flow through water-jacketed respirometers. Experimental temperatures were between 13°C and 14°C. The same type of pH-stat system that was used in the treatment tanks was used to adjust the pH of the seawater for the flow through respirometry. PyroScience Firesting or Presense O2 flow-through optode cells and robust temperature probes were placed on the incurrent and excurrent channel of each respirometer. A peristaltic pump was used to cycle water through the system. Flow rates for each respirometer were measured at the start and end of each respirometry run by measuring output of water mL for 1 minute. Flow rates for 1 L respirometers had a mean rate of 30 ml min-1 and 120 ml respirometers had a mean flow rate of 7 ml min-1. Octopuses were placed in the respirometers for 24 h and aerobic metabolic rates were measured throughout; during analysis, the first 3 hours of each RMR run was trimmed out to account for any handling stress on metabolism. Because the octopus were able to spontaneously move in their respirometer, though they typically did not, we termed our metabolic rates routine metabolic rate instead of standard metabolic rates. After RMR and Pcrit experiments were completed, the octopuses were removed and oxygen consumption was measured in the empty respirometer to determine back-ground respiration. After the first eight runs, background respiration was consistently 5% or less of octopus respiration, and was therefore not recorded for the remainder of the study. After background respiration was measured, inflow and outflow optodes were connected immediately in series to evaluate drift. Throughout all experiments no drift was detectable. RMR was calculated from raw oxygen data using the resp.open() function in the OTools package in R (https://github.com/KirtOnthank/OTools). In brief, this function calculates RMR by subtracting the oxygen concentration of the outflow water from the oxygen concentration of the inflow water, multiplying this by the flow rate, and dividing by the mass of the octopus.
Following 24 h RMR measurements, the respirometer was closed by connecting the inflow of the respirometer to the outflow. Oxygen concentration in the respirometer was allowed to fall to at least 50 µmol O2 l−1. Oxygen supply capacity (α) and Pcrit was determined from aerobic metabolic rate (R) as function of oxygen partial pressure (PO2). We used the calc_alpha function to determine α and determined the Pcrit using the α-method described in Seibel et al. (2021) using the calc_pcrit() function in the “respirometry” package in R (https://CRAN.R-project.org/package=respirometry).
The effects of pCO2 exposure on the log of RMR, α, and Pcrit were examined using repeated-measures linear mixed effect models with log of mass, pCO2 and duration in treatment included as fixed factors and octopus ID as a random factor using the lme() function in the “nlme” package in R (Pinheiro et al., 2022). Estimated marginal means (covariate-corrected means, in this case, mass) were determined for each pCO2 category using the emmeans package in R (Lenth, 2018).
All of the raw datasets, data ID files, and R scripts underlying all statistical analysis and figures presented in this study can be found at the Zenodo online repository: https://doi.org/10.5281/zenodo.7058934.
Control treatments had a measured pCO2 of 1,083 ± 48 µatm; high CO2 treatments had a measured pCO2 of 1767 ± 94. (Table 1).
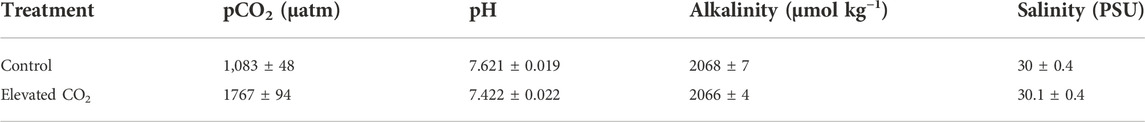
TABLE 1. Carbonate system parameters of control and experimental tanks. The pH was measured daily for each of the four control and four experimental tanks, (n = 29 per tank), Alkalinity and salinity were measured weekly including day one and the last day of the experiments (n = 6 per tank). Means are presented with ± as standard error.
There was a significant effect of mass (linear mixed-effects model, x2 = 5.84, df = 1, p = 0.01565), but not of pCO2 (linear mixed-effects model, x2 = 0.19, df = 1, p = 0.6621) nor day of measurement (linear mixed-effects model, x2 = 2.18, df = 1, p = 0.13939) on the RMR of Muusoctopus leioderma. Routine metabolic rate for 1 day exposure showed an estimated marginal mean (EMM) at 1,000 µatm pCO2 = 2.60 µmol O2 g−1 hr−1 and at 1800 µatm pCO2 = 2.79 µmol O2 g−1 hr−1 and for 7 days exposure RMR 1000 µatm pCO2 = 2.64 µmol O2 g−1 hr−1 and at 1800 µatm pCO2 = 2.84 µmol O2 g−1 hr−1 (Table 2; Figure 3).
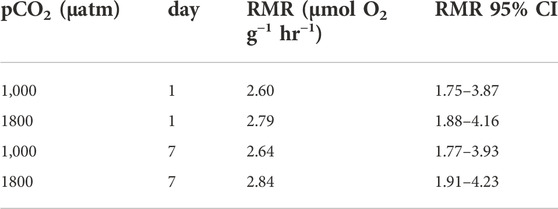
TABLE 2. Estimated marginal means for routine metabolic rate of Muusoctopus leioderma held at either 1,000 or 1800 µatm pCO2 for 1 day and 7 days. These values are corrected for mass of 18.3 g, the value that corresponds to the mean of the logged masses from the linear mixed effects analysis. For all treatments n = 8.
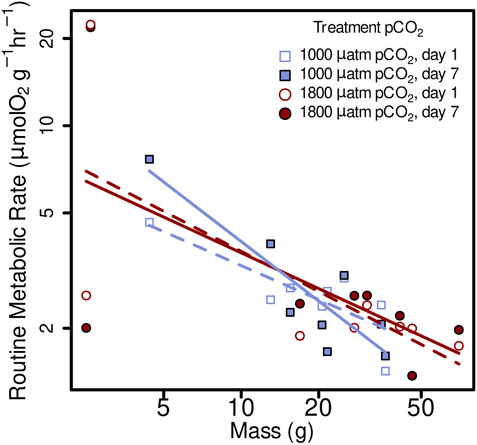
FIGURE 3. Routine metabolic rate (RMR) from Muusoctopus leioderma in Burrows Bay, Anacortes Washington held for 1 day (open symbols) and 7 days (closed symbols) at 1,000 µatm (purple squares) or 1800 µatm (maroon circles) pCO2 expressed as a function of body mass (M). Routine metabolic rate was significantly affected by body mass (M), decreasing mass specific RMR with increased body mass in all four treatments as follows: 1,000 µatm 1 day RMR = 8.06 M−0.39, 1,000 µatm 7 day RMR = 19.17 M−0.68, 1800 µatm 1 day RMR = 10.61 M−0.46, and 1800 7 day RMR = 9.36 M−0.41. Note that axes are plotted on a log scale.
Routine metabolic rate was significantly affected by body mass (M), decreasing mass specific RMR with increased body mass in all four treatments as follows: 1,000 µatm 1 day RMR = 8.06 M−0,39, 1,000 µatm 7 day RMR = 19.17 M−0.68, 1800 µatm 1 day RMR = 10.61 M−0.46, and 1800 7 day RMR = 9.36 M−0.41 (Figure 3; linear mixed-effects model, x2 = 5.84, p = 0.0157).
Critical oxygen partial pressure was not significantly affected by elevated pCO2, in 1 day treatments (1,000 µatm Pcrit = 3.64 kPa, 1800 µatm Pcrit = 3.14 kPa) or 7 day (1,000 µatm Pcrit = 5.00, 1800 µatm Pcrit = 4.50) (Figure 4; linear mixed-effects model, x2 = 0.4646, p = 0.4954). However, it did show a significant increase from day 1 and day 7 within both 1,000 µatm and 1800 µatm pCO2 treatments (Figure 4; linear mixed-effects model, x2 = 10.53, p = 0.001).
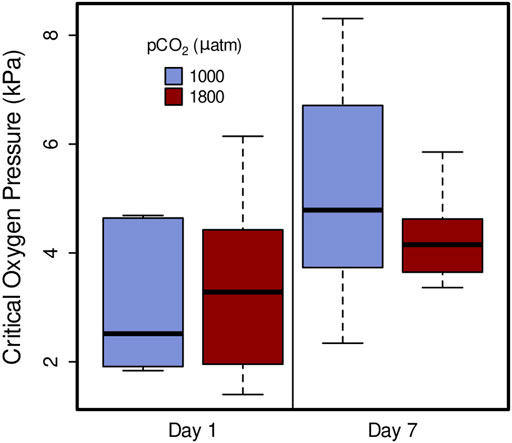
FIGURE 4. Critical oxygen pressure (Pcrit) of Muusoctopus leioderma after 1 day and 7 day exposure to carbon dioxide partial pressure (pCO2) of 1,000 µatm (purple, Day 1 n = 7, Day 7 n = 7) and 1800 µatm (maroon, Day 1 n = 8, Day 7 n = 7). Critical oxygen pressures are not significantly different between pCO2 treatments after long-term exposure, however, they did show an increase from day 1 and day 7 within both 1,000 µatm and 1800 µatm pCO2 treatments (linear mixed-effects model, x2 = 10.53, p = 0.001).
Oxygen supply capacity was not significantly affected by elevated pCO2, in 1 day treatments (1,000 µatm α= 0.80 µmol O2 g−1 hr−1 kPa−1, 1800 µatm α = 0.89 µmol O2 g−1 hr−1 kPa−1) or 7 day (1,000 µatm α = 0.53 µmol O2 g−1 hr−1 kPa−1, 1800 µatm α = 0.46 µmol O2 g−1 hr−1 kPa−1) (Figure 5; linear mixed-effects model, x2 = 0.06, p = 0.8080). However, it did show a decrease from day 1 and day 7 within both 1,000 µatm and 1800 µatm pCO2 treatments (Figure 5; linear mixed-effects model, x2 = 17.35, p = 0.000031). There was an effect of mass on oxygen supply capacity in all four treatments as follows: 1,000 µatm 1 day α = 1.47 + (−0.025M), 1,000 µatm 7 day α = 1.09 + (−0.021M), 1800 µatm 1 day α = 1.29 + (−0.015M), and 1800 7 day α = 0.39 + 0.003M (Figure 5; linear mixed-effects model, x2 = 9.84, p = 0.00171).
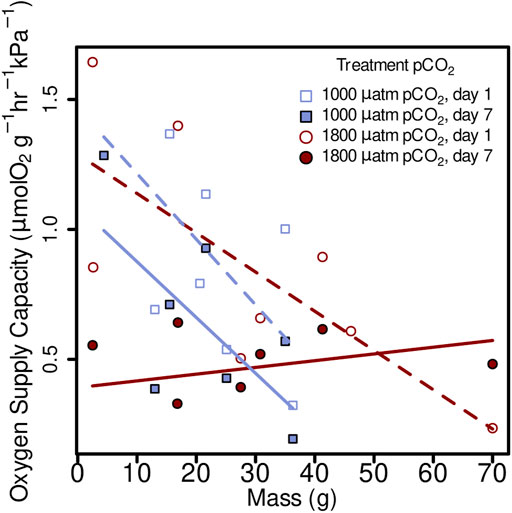
FIGURE 5. Oxygen supply capacity (α) from M. leioderma in Burrows Bay, Anacortes Washington held for 1 day (open symbols) and 7 days (closed symbols) at 1,000 µatm (purple squares) or 1,800 µatm (maroon circles) pCO2 expressed as a function of body mass (M). Oxygen supply capacity was significantly affected by body mass (M), decreasing α with increased body mass in three of four treatments as follows: 1,000 µatm 1 day α = 1.47 + (−0.025)M, 1,000 µatm 7 day α = 1.09 + (−0.021)M, 1800 µatm 1 day α = 1.29 + (−0.015)M, and 1800 7 day α = 0.39 + 0.003M.
This study is the first to investigate the effects of near-future ocean acidification on the physiology of a deep-water octopus. The Salish Sea is an excellent location for studying the effects of ocean acidification. It has historically had a persistent elevated pCO2, attributed in part to upwelling from the California Undercurrent (Murray et al., 2015). It is common to see pCO2 above 1,200 µatm which exceeds all but the most extreme atmospheric pCO2 projections for the end of this century (Onthank et al., 2021). Organisms that live in the Salish sea are persistently exposed to elevated pCO2 throughout their life history, making them excellent study subjects to explore the impact OA may have on such organisms after many generations.
Previous work on cephalopods has shown conflicting outcomes, with squid having a decrease in RMR and increase in Pcrit when exposed to pCO2 above 1,000 µatm (Rosa and Seibel, 2008; Rosa and Seibel, 2010; Hu et al., 2014; Seibel, 2016), as well as no effect (Birk et al., 2018). The observed negative effect on aerobic metabolism has been postulated to be the result of challenges to respiratory physiology. Cephalopods rely on hemocyanin as a respiratory pigment which has a pronounced Bohr effect, resulting in a decrease in oxygen affinity in areas of low pH. Because of this, it has previously been predicted that cephalopods would not tolerate large changes in environmental pH (Portner and Zielinski, 1998; Rosa and Seibel, 2008; Seibel, 2016). However recent work by Birk et al. (2018) suggests that epipelagic squid blood oxygen carrying capacity is minimally impacted by changes in environmental pH. Yet recent work in octopus suggests this may not be valid for all cephalopods. Octopus rubescens exposed to elevated pCO2 shows a short-term elevation in RMR, however there was no change in RMR after 5 weeks and a significant increase in Pcrit at the same time point, indicating a reduction in oxygen supply capacity resulting in decreased hypoxia tolerance (Onthank et al., 2021). This deviation from the prediction of Birk and Seibel’s model may be linked to the difference in Bohr coefficient found in some species of octopus as compared to those found in squid. In their proposed model squid were assigned a Bohr coefficient of −1.5 as being a worst-case scenario for considering impacts of OA on cephalopods. However octopus hemocyanin has an even larger Bohr effect with Bohr coefficients of −1.7 in Enteroctopus dofleini (Miller, 1985) and −1.99 in Octopus macropus (Lykkeboe and Johansen, 1982). This suggests that elevated pCO2 may have a greater effect on octopus blood oxygen binding and aerobic metabolism.
Here we found, unlike the decreased RMR reported in squid (Rosa and Seibel, 2008) or the short term elevated RMR in response to hypercapnia observed in Octopus rubescens (Onthank et al., 2021), Muusoctopus leioderma RMR was not significantly impacted when exposed to 1800 µatm. Additionally there was no significant change in α or Pcrit as a result of exposure to hypercapnia. This does not follow most of the trends observed in other octopus or squid species previously studied. Our results do however show a change in both α and Pcrit in both CO2 treatments with day 7 Pcrit values being higher than those recorded for 1 day (Figure 4). It could be that these changes are the result of repeated exposure to low oxygen in subsequent Pcrit measurements, however, this would be contrary to previous work showing a decrease in Pcrit with repeated hypoxia exposure in octopus (Onthank, 2008). The combination of no change in RMR, indicating the animals are not significantly stressed, with changes to α and Pcrit are particularly puzzling. This octopus species spends a considerable proportion of time in subsurface sediment burrows, which, as discussed below, would likely expose them to regular bouts of hypoxia. This would likely drive increases in supply capacity, such as elevating the amount of respiratory pigments to meet metabolic demands. This hypothesis is supported by our finding the oxygen supply capacity in M. leioderma (α = 0.80 µmol O2 g-1 hr-1 kPa-1 under control conditions) is substantially higher than other shallow living octopus species for which α is known (Octopus vulgaris: 0.30 µmol O2 g-1 h−1 kPa−1, Octopus bimaculoides: 0.34 µmol O2 g−1 hr−1 kPa−1, Octopus rubescens: 0.32 µmol O2 g−1 hr−1 kPa−1, as well as the deep sea octopus Octopus californicus: 0.23 µmol O2 g−1 hr−1 kPa−1 (Table 3) (Seibel and Childress, 2000; Valverde and García, 2005; Seibel and Deutsch, 2020; Onthank et al., 2021). However, similar to how terrestrial organisms decrease hematocrit as they acclimate to elevated oxygen pressure at lower elevations (Keys et al., 1986; Zubieta-Calleja et al., 2007; Borras et al., 2010), it is possible that the introduction of these octopuses to the well-oxygenated tanks in the lab initiated acclimation processes which lower oxygen supply capacity, such as a reduction of blood hemocyanin concentration or a reduction in hemocyanin oxygen affinity, while not impacting RMR. Such a decrease in oxygen affinity of hemocyanin when exposed to elevated oxygen concentrations has been demonstrated in the shore crab Carcinus maenas (Lallier et al., 1987). Unlike those in other treatments, octopuses in 1800 µuatm pCO2 at 7 days showed a positive relationship between oxygen supply capacity and mass. The reasons for this are unclear, but the unusual responses at the extremes of octopus body masses may be driving this. The largest individual (70 g) in 1800 µatm pCO2 is the only one that showed an increase in oxygen supply capacity, while the smallest individual (2.5 g) in 1800 µatm pCO2 showed the largest decrease in oxygen supply capacity in the study. Combined, this suggests a mass specific and pCO2 specific response in alpha to time in treatment, but more data would be required to make a firm conclusion.
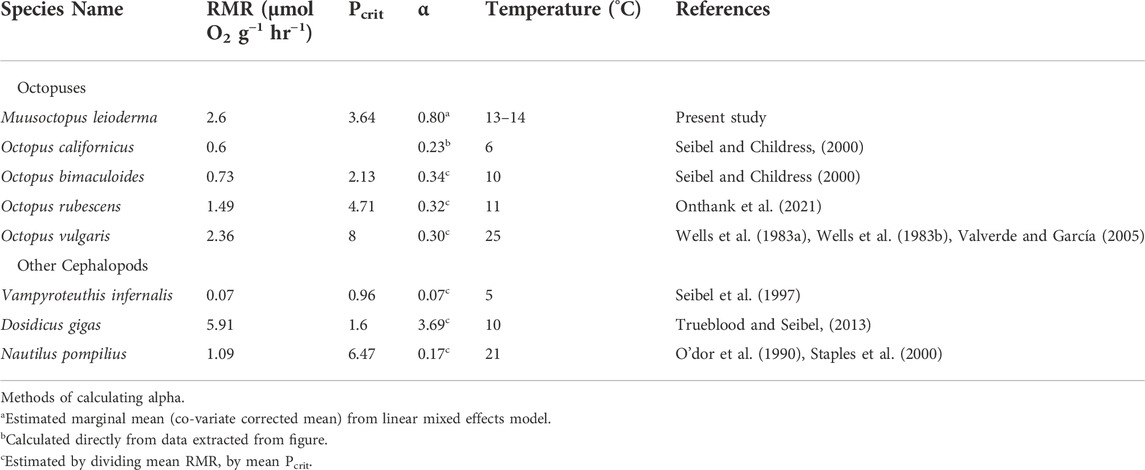
TABLE 3. Comparative routine metabolic rate (RMR), critical partial pressure (Pcrit) and oxygen supply capacity (α) for species of cephalopods from the literature compared to those recorded for Muusoctopus leioderma in the present study.
Muusoctopus leioderma from this study lives in close geographic proximity to Octopus rubescens, and as such they encounter similar abiotic environmental factors. It would be reasonable to assume then these two species should have a similar response to elevated pCO2, yet they do not. The observed variation in responses to elevated pCO2 may be linked to differences in phylogenies and behavior between the two species.
Muusoctopus leioderma has historically been considered a meso to bathyal benthic species, residing between 200–1,500 m depth, with a greatest occurrence between 400–650 m (Conners et al., 2016). This species is part of the Family Enteroctopodidae which includes three genera and a total of ∼33 species (Ibáñez et al., 2016; Jereb et al., 2016; Sanchez et al., 2018). Of these, all are deep living species with very few exceptions such as most of the species of genus Enteroctopus which are found as deep as 1,500 m, but which can also be found in shallow water. There is one instance of Muusoctopus eureka found at 20 m (Laptikhovsky et al., 2011) and Muusoctopus leioderma at 70 m (Hochberg, 1998) and at 10 m in the present study. Otherwise, all other species occupy depth ranges between 200 and 1,500 m. Along the west coast of North America there is a pronounced oxygen minimum zone, marked by low oxygen and high levels of CO2 above 2000 µatm (Paulmier et al., 2011) that occurs between approximately 300–800 m (Kamykowski and Zentara, 1990). These depths overlap with the depth of greatest abundance of M. leioderma (Conners et al., 2016). The population of M. leioderma used in this study is the shallowest occurrence recorded for any species in the genus Muusoctopus that we are aware of. Yet this population of M. leioderma presents classic deep sea features, having no ink sac, minimal chromatic change, large eyes, and can only be found out of its burrows in the dark. As a member of Family Enteroctopodidae, there is a long evolutionary history of deep occurrence. It is logical that as M. leioderma seemingly has adapted anatomy for the deep sea it’s physiology would have as well.
Conversely, Octopus rubescens, a member of family Octopodidae, is the most abundant shallow water octopus along the west coast of North America. Its distribution ranges from the mouth of the Gulf of California, Mexico to the Gulf of Alaska. It is common in the intertidal and has been found as deep as 300 m (Hochberg, 1998). These depths are shallower than the depths of OMZs along the west coast of North America typically occur (Kamykowski and Zentara, 1990). Like other shallow water species O. rubescens has an ink sac, performs dynamic skin color and texture change, (Packard and Hochberg, 1977), and is most active during the day (Humbert et al., 2022). This species and its shallow water congeners have not experienced the same environmental hypoxia and hypercapnia of species who live at depths corresponding to OMZs and thus may not have the same physiological adaptations.
The Salish Sea receives water input from the upwelling of the California Undercurrent (Murray et al., 2015). As the CU upwells there is additional consumption of oxygen and production of CO2 from respiration in shelf sediments (Connolly et al., 2010; Bianucci et al., 2018) resulting in pCO2 levels in excess of 2,100 µatm and oxygen levels below 30% air saturation (Murray et al., 2015). It is probable that the population from this study was originated by individuals brought into the Salish Sea from CU upwelling. The Pcrit we recorded at 1,000 and 1800, (Figure 4), are close to Pcrit values recorded for other species of cephalopods who regularly experience enter OMZs, such as Dosidicus gigas (Trueblood and Seibel, 2013). While oxygen supply capacity is substantially lower than D. gigas, a transient of the OMZ, it is higher than that of Vampyroteuthis infernalis, a resident of the OMZ, and more than double that of shallow living octopods as discussed above (Table 3). This supports that M. leioderma may have adapted its oxygen supply capacity for the hypoxic waters like those of the CU. Similarly, the lack of significant change in RMR, Pcrit and α when exposed to 1800 µatm supports this population is adapted to life in environmental hypercapnia, similar to what is found in the CU. The response observed in O. rubescens by Onthank et al. (2021) at 1,500 µatm may reflect that this level of pCO2 is above historical levels this shallow living species has encountered, but is within the range it is able to acclimate to.
Octopus rubescens, like other species of octopus, spends the majority of each 1 day period in a den (Kayes, 1973; Mather, 1988; Humbert et al., 2022). While their natural dens in the wild have not been studied extensively, they are regularly observed using rocky outcroppings, large empty barnacle shells, beer bottles, and other hard containers of sufficient size (Anderson, 1997; Anderson et al., 1999). These containers allow for reasonable water exchange, and minimal background respiration besides that of the occupying octopus. As such, it is unlikely that O. rubescens would typically experience extreme hypoxia or hypercapnia while occupying its den.
Muusoctopus leioderma spend an appreciable amount of time in burrows as well. During this study we only observed them on silt bottoms at night, never during the day. Observations in the lab show their burrows to be small, not much larger than the volume of the individual’s body, and having a small narrow opening at least one mantle length below the surface of the substrate. The interior of silty coastal bottoms such as those found in Burrows Bay are often areas of low oxygen and elevated CO2 (Middelburg et al., 2005). By creating and occupying a small burrow and spending large portions of each day there, it is likely that M. leioderma regularly experiences hypoxia and hypercapnia and as a result has physiological adaptations for both.
In retrospect, it is unsurprising that our treatments resulted in no significant change in RMR or Pcrit given the typical high pCO2 of most of M. leioderma’s depth range. Despite being first described in 1911 (Berry, 1911), and collected frequently as bycatch in various trawl fisheries (Conners et al., 2016), this species has only recently been found as shallow at 10 m depth.
Depth of occurrence, and the resulting zonation of species has been attributed to environmental factors such as temperature, oxygen, and/or food availability, which impact the physiology of organisms, and competition (Rex, 1976; Collins et al., 1999; Carney, 2005; Yeh and Drazen, 2009). Deep-water animals living in higher pCO2 environments will likely be more robust to higher pCO2 than their shallow water counterparts. As pCO2 continues to rise in shallow waters, this could potentially shift balances that may have historically excluded deep-water animals from shallow-water environments. If this is true, the first locations you would expect to see such shallow-water invasions would be in areas that already experience relatively high pCO2, and recent anthropogenic inputs pushing CO2 levels even higher, such as the Salish Sea (Murray et al., 2015; Onthank et al., 2021). In addition, the first animals you would likely see invading into shallow waters would be, like octopuses, highly mobile and highly adaptable. Together, the finding that an octopus most commonly found in 450–650 m at the unprecedented depth of 10 m of water is relatively robust to elevated pCO2 could both partially explain its recent discovery in shallow water and also be a harbinger of future deep-to-shallow water invasions.
Our data is the first of its kind, examining the effects of OA on the aerobic metabolism of a bathyl species of octopus. We show that Muusoctopus leioderma maintained its RMR and Pcrit at elevated levels of 1800 µatm pCO2, even after 7 days of exposure. The ability to maintain aerobic physiology at these relatively high levels shows this species has physiological adaptations that are likely linked to its phylogeny and life history. It is unique to find this species in the shallow depths recorded in this study. However, it may be that their robust tolerance of elevated pCO2 has allowed them to survive in the hypercapnic shallow waters of the Salish Sea. Further research is needed to clarify the mechanism that drives this species tolerance to high environmental pCO2. Because of their resilience to acidification, and relative ease of collection and maintenance in the lab, Muusoctopus leioderma make an excellent model system to further study how some organisms may compensate for future levels of environmental pCO2.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.5281/zenodo.7058934 Zenodo.org.
Ethical review and approval was not required for the animal study because Cephalopods do not require an ethical review committee in the United States of America. Because of this our institutions do not require, nor conduct review of invertebrate subjects.
LT and KO contributed to conception and design of the study. All authors contributed to data collection. KO organized the data and performed the statistical analysis. LT wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
We would like to thank Jim Nestler, Alan Verde, Jesse Humbert, and Phoebe Churney for help collecting animals.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.1039401/full#supplementary-material
Supplementary Figure 1 | Flow chart of experimental procedures for examining the effect of elevated pCO2 on Muusoctopus leioderma from Burrows Bay, Washington United States.
Supplementary Figure 2 | Diagram of flow through respirometry system used for measuring routine metabolic rate (RMR) and critical partial pressure (Pcrit). A firesting optode system (Pyroscience GmbH Aachen Germany) was used to measure oxygen content of water at the inlet and outlet of the respirometer. A peristaltic pump was used to pull water through the system, blue arrows denote direction of water flow. Flow rates for each respirometer were measured at the start and end of each respirometry run by measuring output of water in mL for 1 min. The respirometer system was submerged in a water bath to maintain temperature. To measure Pcrit the system was closed by connecting the inlet and outlet ports.
Anderson R. C., Hughes P. D., Mather J. A., Steele C. W. (1999). Determination of the diet of Octopus rubescens Berry, 1953 (Cephalopoda: Octopodidae), through examination of its beer bottle dens in Puget Sound. Malacologia 41, 455–460.
Anderson R. (1997). in Workshop on the fishery and market potential of Octopus in California (Germany: Inter-Research Science Center), 141–149.
Barry J. P., Tyrell T., Hansson L., Plattner G.-K., Gattuso J.-P. (2010). Atmospheric CO2 targets for ocean acidification perturbation experiments. Luxembourg: Publications Office of the European Union.
Berry S. S. (1911). Preliminary notices of some new Pacific cephalopods. Proc. U. S. Natl. Mus. 40, 589–592. doi:10.5479/si.00963801.1838.589
Bianucci L., Long W., Khangaonkar T., Pelletier G., Ahmed A., Mohamedali T., et al. (2018). Sensitivity of the regional ocean acidification and carbonate system in Puget Sound to ocean and freshwater inputs. Elem. Sci. Anth. 6, 22. doi:10.1525/elementa.151
Birk M. A., McLean E. L., Seibel B. A. (2018). Ocean acidification does not limit squid metabolism via blood oxygen supply. J. Exp. Biol. 221, jeb187443. doi:10.1242/jeb.187443
Borras A., Cabrera J., Senar J. C. (2010). Hematocrit variation in response to altitude changes in wild birds: a repeated-measures design. Condor 112, 622–626. doi:10.1525/cond.2010.090113
Bridges C. R. (1995). Bohr and root effects in cephalopod haemocyanins-paradox or pressure in Sepia officinalis? Mar. Freshw. Behav. Physiol. 25, 121–130. doi:10.1080/10236249409378912
Cai W.-J., Hu X., Huang W.-J., Murrell M. C., Lehrter J. C., Lohrenz S. E., et al. (2011). Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 4, 766–770. doi:10.1038/ngeo1297
Caldeira K., Wickett M. E. (2003). Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365. doi:10.1038/425365a
Carney R. S. (2005). “Zonation of deep biota on continental margins,” in Oceanography and marine biology (CRC Press), 221–288.
Collins M. A., Priede I. G., Bagley P. M. (1999). In situ comparison of activity in two deep-sea scavenging fishes occupying different depth zones. Proc. R. Soc. Lond. B 266, 2011–2016. doi:10.1098/rspb.1999.0879
Conners M. E., Aydin K. Y., Conrath C. L. (2016). Assessment of the octopus stock complex in the bering sea and aleutian islands. SAFE report NOAA.gov.
Connolly T. P., Hickey B. M., Geier S. L., Cochlan W. P. (2010). Processes influencing seasonal hypoxia in the northern California Current System. J. Geophys. Res. 115, C03021. doi:10.1029/2009JC005283
Culler-Juarez M. E., Onthank K. L. (2021). Elevated immune response in Octopus rubescens under ocean acidification and warming conditions. Mar. Biol. 168, 137. doi:10.1007/s00227-021-03913-z
Dickson A. G., Sabine C. L., Christian J. R. (2007). Guide to best practices for ocean CO2 measurements. Sidney. North Pacific Marine Science Organization.
Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A. (2009). Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. doi:10.1146/annurev.marine.010908.163834
Dunn R. J. H., Stanitski D. M., Gobron N., Willett K. M., Ades M., Adler R., et al. (2020). Global climate. Bull. Am. Meteorol. Soc. 101, S9–S128. doi:10.1175/bams-d-20-0104.1
Fabry V. J., Seibel B. A., Feely R. A., Orr J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432. doi:10.1093/icesjms/fsn048
Gattuso J. P., Epitalon J. M., Lavigne H., Orr J. C. (2015). seacarb: seawater carbonate chemistry. [WWW Document]. Available at: https://CRAN.R-project.org/package=seacarb.
Gutowska M. A., Pörtner H. O., Melzner F. (2008). Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. Mar. Ecol. Prog. Ser. 373, 303–309. doi:10.3354/meps7782
Gutowska M. A., Melzner F., Pörtner H. O., Meier S. (2010). Cuttlebone calcification increases during exposure to elevated seawater pCO2 in the cephalopod Sepia officinalis. Mar. Biol. 157, 1653–1663. doi:10.1007/s00227-010-1438-0
Hochberg F. G. (1998). “Class cephalopoda,” in Taxonomic attlas of the benthic fauna of the santa marina basin and the western santa barbara channel. Editors P. V. Scott, and J. A. Blake (Santa Barbara, CA: Santa Barbara Museum of Natural History), 175–235.
Hu M. Y., Guh Y.-J., Stumpp M., Lee J.-R., Chen R.-D., Sung P.-H., et al. (2014). Branchial NH4+-dependent acid–base transport mechanisms and energy metabolism of squid (Sepioteuthis lessoniana) affected by seawater acidification. Front. Zool. 11, 55–17. doi:10.1186/s12983-014-0055-z
Humbert J. W., Williams K., Onthank K. L. (2022). Den-associated behavior of Octopus rubescens revealed by a motion-activated camera trap system. Integr. Comp. Biol. 62, 1131–1143. doi:10.1093/icb/icac124
Ibáñez C. M., Pardo-Gandarillas M. C., Peña F., Gleadall I. G., Poulin E., Sellanes J. (2016). Phylogeny and biogeography of Muusoctopus (cephalopoda: Enteroctopodidae). Zool. Scr. 45, 494–503. doi:10.1111/zsc.12171
Jereb P., Roper C. F. E., Norman M. D., Finn J. K. (2016). “Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date,” in FAO species catalogue for fishery purposes. 2nd ed (Rome: FOA), Vol. 3. Octopods and Vampire Squids.
Kamykowski D., Zentara S. (1990). Hypoxia in the world ocean as recorded in the historical data set. Deep Sea Res. Part A. Oceanogr. Res. Pap. 37, 1861–1874. doi:10.1016/0198-0149(90)90082-7
Kaplan M. B., Mooney T. A., McCorkle D. C., Cohen A. L. (2013). Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PloS One 8, e63714. doi:10.1371/journal.pone.0063714
Kayes R. (1973). The daily activity pattern of Octopus vulgar is in a natural habitat. Mar. Behav. Physiol. 2, 337–343. doi:10.1080/10236247309386935
Keys G. C., Fleischer R. C., Rothstein S. I. (1986). Relationships between elevation, reproduction and the hematocrit level of brown-headed cowbirds. Comp. Biochem. Physiol. A Comp. Physiol. 83, 765–769. doi:10.1016/0300-9629(86)90725-5
Lallier F., Boitel F., Truchot J. (1987). The effect of ambient oxygen and temperature on haemolymph L-lactate and urate concentrations in the shore crab Carcinus maenas. Comp. Biochem. Physiol. Part A Physiol. 86, 255–260. doi:10.1016/0300-9629(87)90327-6
Langenbuch M., Portner H. O. (2002). Changes in metabolic rate and N excretion in the marine invertebrate sipunculus nudus under conditions of environmental hypercapnia: identifying effective acid—base variables. J. Exp. Biol. 205, 1153–1160. doi:10.1242/jeb.205.8.1153
Laptikhovsky V., Arkhipkin A., Brickle P., Hearne S., Neely K. (2011). Species range shifts due to environmental changes in scaled squid, Pholidoteuthis massyae and bathyal octopus, Muusoctopus eureka. Mar. Biodivers. Rec. 4, e34. doi:10.1017/s1755267210001053
Lenth R., 2018. emmeans: estimated marginal means, aka least-squares means. R package version1. Available at: https://CRAN.R-project.org/package=emmeans.
Lykkeboe G., Johansen K. (1982). A cephalopod approach to rethinking about the importance of the Bohr and Haldaneeffects. Pac. Sci. 36, 305–313.
Macfarling M., Etheridge D., Trudinger C., Steele P., Langenfelds R., Van Ommen T., et al. (2006). Law Dome CO2, CH4 and N2O ice core records extended to 2000 years BP. Geophys. Res. Lett. 33, L14810. doi:10.1029/2006gl026152
Melzner F., Thomsen J., Koeve W., Oschlies A., Gutowska M. A., Bange H. W., et al. (2013). Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. doi:10.1007/s00227-012-1954-1
Metzger R., Sartoris F. J., Langenbuch M., Pörtner H. O. (2007). Influence of elevated CO 2 concentrations on thermal tolerance of the edible crab Cancer pagurus. J. Therm. Biol. 32, 144–151. doi:10.1016/j.jtherbio.2007.01.010
Middelburg J. J., Duarte C. M., Gattuso J.-P. (2005). “Respiration in coastal benthic communities,” in Respiration in aquatic ecosystems (Oxford: Oxford University Press), 206–224.
Miller K. I. (1985). Oxygen equilibria of Octopus dofleini hemocyanin. Biochemistry 24, 4582–4586. doi:10.1021/bi00338a015
Munday P. L., Dixson D. L., Donelson J. M., Jones G. P., Pratchett M. S., Devitsina G. V., et al. (2009). Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. U. S. A. 106, 1848–1852. doi:10.1073/pnas.0809996106
Murray J. W., Roberts E., Howard E., O’Donnell M., Bantam C., Carrington E., et al. (2015). An inland sea high nitrate-low chlorophyll (HNLC) region with naturally high pCO2. Limnol. Oceanogr. 60, 957–966. doi:10.1002/lno.10062
O’dor R., Wells J., Wells M. (1990). Speed, jet pressure and oxygen consumption relationships in free-swimming Nautilus. J. Exp. Biol. 154, 383–396. doi:10.1242/jeb.154.1.383
Onthank K. L., Trueblood L. A., Schrock-Duff T., Kore L. G. (2021). Impact of short- and long-term exposure to elevated seawater Pco2 on metabolic rate and hypoxia tolerance in Octopus rubescens. Physiol. Biochem. Zool. 94, 1–11. doi:10.1086/712207
Onthank K. L. (2008). Aerobic metabolism and dietary ecology of Octopus rubescens. Masters Thesis. Walla: Walla University.
Packard A., Hochberg F. G. (1977). “Skin patterning in Octopus and other genera,” in Symp. Zool. Soc. Lond. 38, 191–231.
Paulmier A., Ruiz-Pino D., Garçon V. (2011). CO<sub>2</sub> maximum in the oxygen minimum zone (OMZ). Biogeosciences 8, 239–252. doi:10.5194/bg-8-239-2011
Pinheiro J., Bates D.R Core Team (2022). _nlme: Linear and nonlinear mixed effects models. R package version 3. Available at: https://CRAN.R-project.org/package=nlme, 1–159.
Portner H.-O., Zielinski S. (1998). Environmental constraints and the physiology of performance in squids. South Afr. J. Mar. Sci. 20, 207–221. doi:10.2989/025776198784126421
Rex M. A. (1976). “Biological accommodation in the deep-sea benthos: comparative evidence on the importance of predation and productivity,” in Presented at the deep sea research and oceanographic abstracts (Elsevier), 975–987.
Rosa R., Seibel B. A. (2008). Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc. Natl. Acad. Sci. U. S. A. 105, 20776–20780. doi:10.1073/pnas.0806886105
Rosa R., Seibel B. A. (2010). Metabolic physiology of the Humboldt squid, Dosidicus gigas: Implications for vertical migration in a pronounced oxygen minimum zone. Prog. Oceanogr. 86, 72–80. doi:10.1016/j.pocean.2010.04.004
Rosa R., Trübenbach K., Repolho T., Pimentel M., Faleiro F., Boavida-Portugal J., et al. (2013). Lower hypoxia thresholds of cuttlefish early life stages living in a warm acidified ocean. Proc. R. Soc. B. 280, 20131695.
Sanchez G., Setiamarga D. H., Tuanapaya S., Tongtherm K., Winkelmann I. E., Schmidbaur H., et al. (2018). Genus-level phylogeny of cephalopods using molecular markers: current status and problematic areas. PeerJ 6, e4331. doi:10.7717/peerj.4331
Seibel B. A., Childress J. J. (2000). Metabolism of benthic octopods (Cephalopoda) as a function of habitat depth and oxygen concentration. Deep Sea Res. Part I Oceanogr. Res. Pap. 47, 1247–1260. doi:10.1016/s0967-0637(99)00103-x
Seibel B. A., Deutsch C. (2020). Oxygen supply capacity in animals evolves to meet maximum demand at the current oxygen partial pressure regardless of size or temperature. J. Exp. Biol. 223, jeb210492. doi:10.1242/jeb.210492
Seibel B. A., Thuesen E. V., Childress J. J., Gorodezky L. A. (1997). Decline in Pelagic Cephalopod metabolism with habitat depth reflects differences in locomotory efficiency. Biol. Bull. 192, 262–278. doi:10.2307/1542720
Seibel B. A., Andres A., Birk M. A., Burns A. L., Shaw C. T., Timpe A. W., et al. (2021). Oxygen supply capacity breathes new life into critical oxygen partial pressure (Pcrit). J. Exp. Biol. 224, jeb242210. doi:10.1242/jeb.242210
Seibel B. A. (2016). Cephalopod susceptibility to asphyxiation via ocean incalescence, deoxygenation, and acidification. Physiology 31, 418–429. doi:10.1152/physiol.00061.2015
Staples J. F., Hershkowitz J. J., Boutilier R. G. (2000). Effects of ambient PO2 and temperature on oxygen uptake in Nautilus pompilius. J. Comp. Physiol. B 170, 231–236. doi:10.1007/s003600050280
Trueblood L. A., Seibel B. A. (2013). The jumbo squid, Dosidicus gigas (Ommastrephidae), living in oxygen minimum zones I: Oxygen consumption rates and critical oxygen partial pressures. Deep Sea Res. Part II Top. Stud. Oceanogr. 95, 218–224. doi:10.1016/j.dsr2.2012.10.004
Valverde J. C., García B. G. (2005). Suitable dissolved oxygen levels for common octopus (Octopus vulgaris cuvier, 1797) at different weights and temperatures: analysis of respiratory behaviour. Aquaculture 244, 303–314. doi:10.1016/j.aquaculture.2004.09.036
Walther K., Sartoris F.-J., Bock C., Pörtner H.-O. (2009). Impact of anthropogenic ocean acidification on thermal tolerance of the spider crab Hyas araneus. Biogeosciences 6, 2207–2215. doi:10.5194/bg-6-2207-2009
Wells M., O’dor R., Mangold K., Wells J. (1983a). Diurnal changes in activity and metabolic rate in Octopus vulgaris. Mar. Behav. Physiol. 9, 275–287. doi:10.1080/10236248309378598
Wells M., O’dor R., Mangold K., Wells J. (1983b). Feeding and metabolic rate in Octopus. Mar. Behav. Physiol. 9, 305–317. doi:10.1080/10236248309378600
Widdicombe S., Spicer J. I. (2008). Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J. Exp. Mar. Biol. Ecol. 366, 187–197. doi:10.1016/j.jembe.2008.07.024
Yeh J., Drazen J. C. (2009). Depth zonation and bathymetric trends of deep-sea megafaunal scavengers of the Hawaiian Islands. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 251–266. doi:10.1016/j.dsr.2008.08.005
Keywords: routine metabolic rate, aerobic metabolism, ocean acidfication, muusoctopus, critical oxygen partial pressure, bathyal, oxygen supply capacity
Citation: Trueblood LA, Onthank K, Bos N, Buller L, Coast A, Covrig M, Edwards E, Fratianni S, Gano M, Iwakoshi N, Kim E, Moss K, Personius C, Reynoso S and Springbett C (2022) Bathyal octopus, Muusoctopus leioderma, living in a world of acid: First recordings of routine metabolic rate and critical oxygen partial pressures of a deep water species under elevated pCO2. Front. Physiol. 13:1039401. doi: 10.3389/fphys.2022.1039401
Received: 08 September 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Rui Rosa, University of Lisbon, PortugalReviewed by:
Brad Seibel, University of Rhode Island, United StatesCopyright © 2022 Trueblood, Onthank, Bos, Buller, Coast, Covrig, Edwards, Fratianni, Gano, Iwakoshi, Kim, Moss, Personius, Reynoso and Springbett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lloyd A. Trueblood, TGxveWQuVHJ1ZWJsb29kQGxhc2llcnJhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.