- Retired, Graz, Austria
Non-communicable diseases, like diabetes, cardiovascular diseases, cancer, stroke, chronic obstructive pulmonary disease, osteoporosis, arthritis, Alzheimer’s disease and other more are a leading cause of death in almost all countries. Lifestyle factors, especially poor diet and tobacco consumption, are considered to be the most important influencing factors in the development of these diseases. The Western diet has been shown to cause a significant distortion of normal physiology, characterized by dysregulation of the sympathetic nervous system, renin-angiotensin aldosterone system, and immune system, as well as disruption of physiological insulin and oxidant/antioxidant homeostasis, all of which play critical roles in the development of these diseases. This paper addresses the question of whether the development of smoking-related non-communicable diseases follows the same pathophysiological pattern. The evidence presented shows that exposure to cigarette smoke and/or nicotine causes the same complex dysregulation of physiology as described above, it further shows that the factors involved are strongly interrelated, and that all of these factors play a key role in the development of a broad spectrum of smoking-related diseases. Since not all smokers develop one or more of these diseases, it is proposed that this disruption of normal physiological balance represents a kind of pathogenetic “basic toolkit” for the potential development of a range of non-communicable diseases, and that the decision of whether and what disease will develop in an individual is determined by other, individual factors (“determinants”), such as the genome, epigenome, exposome, microbiome, and others. The common pathophysiological pattern underlying these diseases may provide an explanation for the often poorly understood links between non-communicable diseases and disease comorbidities. The proposed pathophysiological process offers new insights into the development of non-communicable diseases and may influence the direction of future research in both prevention and therapy.
1 Introduction
Non-communicable disorders, like type 2 diabetes, cardiovascular diseases (CVD), cancer, stroke, chronic obstructive pulmonary disease (COPD), arthritis, mental health issues and other more are a major cause of death in almost every country. Lifestyle factors, especially poor diet and tobacco use, are considered to be the main influencing factors in the development of these diseases.
The tobacco epidemic is one of the major public health threats. Cigarette smoking is the most common form of tobacco use and a leading cause of preventable death worldwide. Despite numerous efforts to curb the spread of tobacco use, about six million people worldwide die each year as a result of tobacco smoking (Commar et al., 2015). According to WHO estimates, the global mortality rate from tobacco use (active and passive) is about 7.2 million per year (Lelieveld et al., 2020). Cigarette smoking has been linked to the development of CVD, COPD, hypertension, cancer, as well as many chronic systemic diseases with inflammatory components such as atherosclerosis, Crohn’s disease, rheumatoid arthritis, psoriasis, Graves’ ophthalmopathy and type 2 diabetes (Centers for Disease Control and Prevention, 2010; Hunter and Reddy, 2013). The mechanisms by which cigarette smoking induces and promotes these diseases are complex and interconnected and still not completely understood.
As outlined in a recent article by Kopp (2019), the Western diet causes a dysregulation of several important physiological factors that play a crucial role in the development of various non-communicable diseases like atherosclerosis, cancer, type 2 diabetes, neurodegenerative diseases, and many more. Development of oxidative stress (OxS), hyperinsulinemia and insulin resistance, subclinical inflammation, and dysregulation of the sympathetic nervous system (SNS), renin-angiotensin aldosterone system (RAAS), and immune system have been identified as major alterations in this context.
This paper addresses the question of whether cigarette smoking causes the same pathophysiological changes as described above and if the development of smoking-related diseases therefore follows the same pathogenetic pattern.
2 Oxidative stress, hyperinsulinemia and insulin resistance, subclinical inflammation, and dysregulation of the SNS, the RAAS and the immune system: Influence of tobacco smoking
2.1 Smoking and OxS
In the cell, mitochondrial respiration is one of the main sources of reactive oxygen species (ROS). ROS are also generated by non-mitochondrial sources such as the nicotinamide adenine dinucleotide phosphate oxidase (NOX) and NOX homologs, ROS-generating enzymes, and the β-oxidation of fatty acids in the peroxisome. In addition to ROS, organisms also generate reactive nitrogen species (RNS) (Shields et al., 2021). In the physiological state, the level of cellular ROS is stable in a dynamic equilibrium because eukaryotic cells have multiple antioxidant defense mechanisms. ROS and RNS play a dual role: on the one hand, they regulate biological and physiological processes as signaling molecules on the other hand, a disturbed balance between the oxidative and antioxidant systems of cells and tissues, resulting in excess ROS and RNS, can damage macromolecular targets such as lipids, proteins, and nucleic acids and disrupt redox signaling (Gough and Cotter, 2011; Forman and Zhang, 2021). OxS and nitrosative stress play an essential role in the pathogenesis of many chronic diseases, including, (but not limited to) atherosclerosis, COPD, type 2 diabetes, neurodegenerative diseases, and cancer (Forman and Zhang, 2021; Yoon et al., 2021).
Tobacco smoke contains a complex mixture of toxic chemicals in the particulate and gaseous phases, e.g., high concentrations of a host of ROS and RNS, like superoxide, nitric oxide (NO), and peroxynitrite. Exposure to cigarette smoke therefore leads to significant OxS (Caliri et al., 2021). In a human biomarker study, Jansen et al. (2014) investigated the influence of smoking on biomarkers of OxS, redox and antioxidant status in a healthy male population. The study confirmed that smokers have increased levels of OxS biomarkers and impaired antioxidant status. An animal study in Wistar rats demonstrated that relatively stable oxidants in the gaseous phase of cigarette smoke can pass through the pulmonary alveolar wall into the blood and increase systemic OxS (Yamaguchi et al., 2007). As shown in an in vivo human study by Padmavathi et al. (2018), smoking increased OxS by decreasing antioxidant status in both erythrocytes and platelets and higher nitrite/nitrate levels in erythrocytes. Using an in vitro mesencephalic cell model, Barr et al. (2007) showed that nicotine induces dose-dependent ROS concentrations that lead to activation of the stress-dependent NF-κB pathway in mesencephalic cells.
In addition to exogenous ROS, smoking causes mitochondrial dysfunction, which - in the form of a vicious circle - leads to an endogenous increase in ROS production and OxS (Miró et al., 1999; Boukhenouna et al., 2018). Furthermore, smoking contributes to an imbalance of the oxidant/antioxidant system, resulting in significantly lower levels of antioxidant enzymes (Al-Bashaireh et al., 2018; Boukhenouna et al., 2018). The number of cigarettes smoked daily has an impact on the degree of oxidative damage and the reduction of antioxidant defenses (Kamceva et al., 2016).
2.2 Smoking and insulin resistance
Insulin is a hormone that has a key function in cell metabolism. In healthy people, proper insulin secretion and the sensitivity of peripheral tissues to the effects of the hormone keep glucose levels within the normal range. Insulin resistance is manifested by impaired glucose uptake and oxidation, impaired ability to suppress lipid oxidation, and a decrease in glycogen synthesis. Hyperinsulinemia can be both a result and a driver of insulin resistance (Nolan and Prentki, 2019).
Numerous studies have demonstrated negative, apparently dose-dependent effects of cigarette smoking on peripheral insulin action (Facchini et al., 1992; Śliwińska-Mossoń and Milnerowicz, 2017). Smoking is associated with insulin resistance and hyperinsulinemia. Compared to non-smokers, chronic cigarette smokers are insulin resistant, hyperinsulinemic and dyslipidemic. Serum insulin concentrations have been shown to be higher in smokers than in non-smokers, even after adjusting for factors that influence insulin resistance (Facchini et al., 1992; Kim K.W. et al., 2017). A human clinical trial by Attvall et al. (1993), which examined the short-time effect of smoking on insulin sensitivity in a group of 7 healthy habitual smokers, showed that smoking acutely impairs insulin effects and leads to insulin resistance and compensatory hyperinsulinemia. The degree of insulin resistance was found to be related to the number of cigarettes/day (Eliasson et al., 1994). In another human clinical trial involving 20 chronic smokers and 20 age-, sex-, and BMI-matched healthy subjects, cigarette smoking was shown to acutely worsen glucose tolerance in both healthy non-smokers and habitual tobacco smokers after smoking as few as 3 cigarettes (Frati et al., 1996). Nicotine produced insulin resistance through ROS-induced downregulation of Nrf2 activity in cardiomyocytes in vitro (Li et al., 2019). In an animal study, ApoE gene knockout mice were exposed to electronic cigarettes, e-cigarettes without nicotine, or conventional cigarettes for 18 weeks. A control group received fresh air. The result of the study showed a significant decrease in insulin tolerance in the e-cigarette, e-cigarette without nicotine, and cigarette groups compared to the control group (Lan et al., 2020). In addition, chronic cigarette smoking appears to significantly worsen insulin resistance in patients with type 2 diabetes (Śliwińska-Mossoń and Milnerowicz, 2017). Eliasson et al. (1996) found in a clinical study that long-term use of nicotine gum was associated with insulin resistance and hyperinsulinemia, suggesting that nicotine is a component of cigarette smoke that leads to insulin resistance.
2.3 Smoking, SNS and RAAS
The RAAS and the SNS are major physiological systems which play an important role in normal physiology as well as in pathologic conditions. The SNS, one of the two divisions of the autonomic nervous system, regulates the function of virtually all human organ systems. The catecholamine neuroeffectors norepinephrine (NE) and epinephrine act as both neurotransmitters and circulating hormones. While NE is released primarily from the sympathetic nerve terminals, epinephrine is mainly secreted from the adrenal medulla (Benarroch, 2020).
Nicotine and particulate matter in tobacco smoke lead to a rapid increase in SNS activity. This is reflected in increased plasma catecholamine levels and is maintained by a positive feedback loop between SNS activity and ROS (Middlekauff et al., 2014; Xiao et al., 2019).
The RAAS plays a central role in the regulation of vascular tone and salt and fluid balance. In addition to the classical circulating RAAS with angiotensin II (ANG II) as the main effector, there is also a local or tissue RAAS that operates independently of the circulating RAAS mainly at the cellular level via local ANG peptide synthesis, and plays a role in tissue physiology and homeostasis. ANG II, produced from Ang I by an angiotensin-converting enzyme (ACE), binds to ANG II type 1 receptor (AT1R) and ANG II type 2 receptor. ACE homolog ACE 2 antagonizes the functions of ANG II by degrading ANG II to its biologically active product ANG (1-7), which acts via the Mas receptor (Santos et al., 2018; Nehme et al., 2019). In addition to the more direct effects of ANG II on the cardiovascular system, the RAAS promotes inflammation and tissue injury (Patel et al., 2017; Nehme et al., 2019). Nicotine and/or cigarette smoke have been reported to impair RAAS homeostasis in multiple organ systems by upregulating the ACE/ANG-II/AT1R axis and downregulating the compensatory ACE2/ANG-(1-7)/Mas receptor axis (Oakes et al., 2018). In an animal study on hamsters exposed to cigarette smoke, Wang et al. (2010) showed that cigarette smoke increases tissue ANG II levels, likely by increasing chymase activity. Similarely, a 6-month exposure of Sprague-Dawley rats to cigarette smoke significant increased ANG II levels and ACE protein expression and decreased ACE2 expression in lung tissue (Yuan et al., 2015). A prospective study on 108 smokers with non-diabetic chronic kidney disease treated with ACE inhibitor therapy showed that smoking attenuated the renal protection achieved by ACE inhibition (Roehm et al., 2017).
2.4 Smoking, immune system and inflammation
Subclinical inflammation is the result of release of pro-inflammatory cytokines from immune-related cells as part of a widespread activation of the innate immune system. When activated, cells of the innate immune system produce proinflammatory cytokines that coordinate local and systemic inflammatory responses as part of a defense mechanism designed to eliminate harmful stimuli and promote tissue repair. Chronic (subclinical) inflammation causes OxS, whereas the OxS response can stimulate the release of proinflammatory cytokines and trigger subclinical inflammation, suggesting that inflammation and OxS are pathophysiological events that are closely linked in a vicious cycle (Liguori et al., 2018; Valacchi et al., 2018). Several important functions of the immune system are mediated by toll-like receptors (TLR) and nuclear factor-κB (NF-κB). TLRs are important effectors of innate immunity, and their activation is one of the first defense mechanisms, leading to an immune response through the production of proinflammatory cytokines, type I interferons, and other inflammatory mediators. There is growing evidence that TLRs play an important role in promoting inflammation, OxS, and endothelial dysfunction (Hovland et al., 2015; Salvador et al., 2016; Lazaridis et al., 2021). Their expression is not limited to innate immune cells and immune tissues, but is found in all tissues, including cardiovascular, liver, pancreas, colon, small intestine, lung, kidney, ovary, placenta, testis, prostate, skeletal muscle, and brain (El-Zayat et al., 2019; Lazaridis et al., 2021). All TLR signaling pathways culminate in the activation of NF-κB, which plays an important regulatory role in the inflammatory immune responses (Kawai and Akira, 2007; El-Zayat et al., 2019). Dysregulation of TLRs and NF-κB activation disrupts immune homeostasis and leads to an exaggerated inflammatory response through excessive and sustained production of proinflammatory cytokines and chemokines (Gao et al., 2017; Liu et al., 2017).
Although the exact mechanisms underlying smoking-related immunopathology are not fully understood, there is ample evidence that smoking impairs both innate and adaptive immunity. It disrupts immunological homeostasis and plays a dual role in regulating immunity, both increasing pathological immune responses and weakening the normal defensive function of the immune system (Kianoush et al., 2017; Qiu et al., 2017). Cigarette smoking causes dysregulation of TLRs and NF-κB (Yang et al., 2006; Semlali et al., 2012), which plays an important role in the pathogenesis of various diseases, including atherosclerosis (Hovland et al., 2015; Salvador et al., 2016), type 2 diabetes (Jialal et al., 2014; Aly et al., 2020), hypertension (Lazaridis et al., 2021), and others more.
3 OxS, hyperinsulinemia and insulin resistance, subclinical inflammation and dysregulation of the SNS, the RAAS and the immune system: Interrelations
As shown in Figure 1, these factors are strongly interrelated, suggesting that dysregulation of one of them may cause imbalance in others.
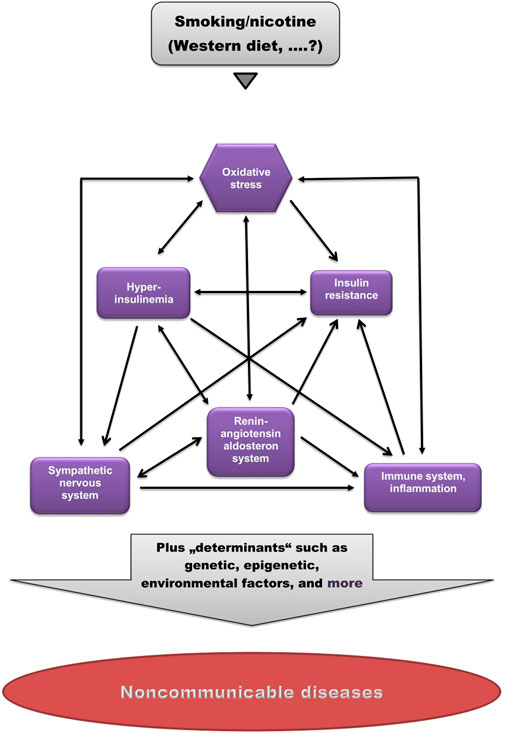
FIGURE 1. Pathogenetic model of (smoking-related) non-communicable diseases. Smoking/nicotine can cause non-physiological activation of each of these factors. Note the high degree of interrelations. (Modified from Kopp, 2019).
3.1 OxS, immune system and inflammation
OxS plays a critical role in the development and maintenance of subclinical inflammation (Biswas, 2016; Forrester et al., 2018), while inflammation causes OxS, suggesting that inflammation and OxS are closely related pathophysiological events (Biswas, 2016).
3.2 OxS and RAAS
A very close relationship exists between OxS and RAAS overactivation. OxS and ANG II signaling mutually regulate each other by multiple mechanisms. Activation of the RAAS induces OxS, for instance in the vascular system or the kidney while OxS can activate the RAAS (Ramalingam et al., 2017). In vitro studies have shown that Ang II stimulates superoxide anion production in mesangial cells (Jaimes et al., 1998), whereas OxS was shown to be a direct stimulator of AT1R expression in an animal study (Banday and Lokhandwala, 2008), indicating a positive feedback loop.
3.3 OxS and insulin
Increased ROS production and OxS caused insulin resistance in vitro and in animal studies (Fazakerley et al., 2018). Exposure of ß-cells to mono-oleoyl glycerol induced insulin hypersecretion through the production of ROS, whereas ROS scavengers abolished the secretion (Saadeh et al., 2012). Chronic insulin treatment resulted in a significant increase in intracellular formation of superoxide anions, hydrogen peroxide, and hydroxyl radicals in 3T3-L1 fat cells, leading to the development of insulin resistance (Ge et al., 2008).
3.4 OxS and SNS
There is a positive feedback loop between sympathetic nerve activity and OxS (Middlekauff et al., 2014). OxS was shown to stimulate sympathetic nervous system activity in several experimental models of hypertension (Campese et al., 2005; Ye et al., 2006; Oliveira-Sales et al., 2008) while injection of antioxidants resulted in a decrease in sympathetic nervous system activity (Ye et al., 2006). Otherwise, NE increased superoxide production in human peripheral blood mononuclear cells via NOX activation and ROS production (Deo et al., 2013).
3.5 RAAS and immune system
ANG-II can cause dysregulations of the adaptive immune system through the stimulation of macrophages and other immune cells mediated by signalling through AT1R (Bernstein et al., 2018). In addition, Ang II can trigger activation of TLR4 via AT1R in various cell types, such as the kidney, vasculature, and central nervous system, resulting in an inflammatory immune response (Biancardi et al., 2017).
3.6 RAAS and insulin resistance
ANG II causes hyperinsulinemia and insulin resistance (Zhou et al., 2012; Mori et al., 2013). In an (ex vivo) hypertrophy model, hearts from ANG-II-treated mice exhibited decreased insulin sensitivity with markedly reduced glucose oxidation rates (Mori et al., 2013), whereas clinical trials in healthy subjects using the hyperinsulinemic euglycemic clamp technique showed that acute and chronic hyperinsulinemia increases RAAS activation (Rooney et al., 1991; Perlstein et al., 2007).
3.6 SNS and insulin resistance
Acute increases in plasma insulin (within the physiological range) stimulated a dose-dependent increase in SNS activity in normal subjects, as demonstrated by increased NE plasma concentrations and microneurographic studies (Rowe et al., 1981), whereas clinical trials in normal volunteer subjects showed that NE infusions cause insulin resistance (Lembo et al., 1994; Khoury and McGill, 2011), indicating the existence of a negative feedback loop (Kishi and Hirooka, 2013).
3.7 Hyperinsulinemia, insulin resistance and inflammation
In vitro studies showed that hyperinsulinemia causes insulin resistance via downregulation of insulin receptor phosphorylation (Catalano et al., 2014). Hyperinsulinemia has been shown to promote adipose tissue inflammation in mice, whereas a decrease in circulating insulin levels lead to a decrease in the expression of pro-inflammatory markers and macrophage content in adipose tissue (Pedersen et al., 2015). Hyperinsulinemia and hyperglycemia were (independently) shown to induce inflammatory responses in human chondrocytes in vitro, namely by activating NF-κB (Rufino et al., 2017).
Inflammation, particularly long-term chronic inflammation, may play important roles in development and progression of insulin resistance through multiple pathways (Chen et al., 2015; Pedersen et al., 2015; Wu and Ballantyne, 2020). Finally, insulin resistance generates compensatory hyperinsulinemia (Reaven and Tsao, 2003).
3.8 SNS and immune system
The SNS regulates many immune system functions (Nance and Sanders, 2007; Padro and Sanders, 2014) and is causally related to the development of chronic subclinical inflammation (Karakas et al., 2018).
3.9 SNS and RAAS
The SNS and the RAAS interact with each other in the form of a positive feedback loop: NE activates ANG II production by stimulating renin secretion, whereas circulating ANG II interacts with the SNS and potentiates NE release from sympathetic nerve terminals (Saxena, 1992; Grassi, 2001).
4 Non-communicable diseases caused by smoking: Dysregulation of the SNS, RAAS, and immune system, and disruption of physiological insulin and oxidant/antioxidant homeostasis as common underlying causative factors
4.1 Cardiovascular diseases
CVDs is a general term for a wide range of diseases, including diseases of the heart muscle and the vascular system that supplies the heart, brain, and other vital organs.
4.1.1 Atherosclerosis
Atherosclerosis, the underlying cause of most CVDs, is the most important source of morbidity and mortality in the world (Barquera et al., 2015). Atherosclerosis is an inflammatory disease of the arterial wall, characterized by chronic inflammation and altered immune response. The pathogenesis of atherosclerosis involves at least three serious aspects: endothelial dysfunction, inflammation, and alterations in lipid metabolism. The early phase of atherosclerosis is associated with dyslipidemia and endothelial dysfunction. The endothelium is an active inner layer of the blood vessels and an important regulator of vascular tone by the production of NO. It also prevents leukocyte adhesion and platelet aggregation and maintains vascular health. Endothelial dysfunction is characterized by an imbalance between vasodilation and vasoconstriction, a deficiency of NO, platelet aggregation, thrombus formation, and increased vascular endothelial permeability. Vascular inflammation is involved in all stages of the atherosclerotic process, from lesion formation to plaque rupture and thrombus formation (Park and Park, 2015; Poznyak et al., 2020). Atherogenic dyslipidemia, defined as elevated triglyceride levels, high levels of small low-density lipoprotein cholesterol (LDL) and low levels of high-density lipoprotein cholesterol, also plays a pivotal role in CVD development (Borén and Williams, 2016). In a situation of increased endothelial permeability, high plasma LDL levels result in an increased rate of entry into the intima, and consequently, higher intimal LDL deposition. Deposition of cholesterol in the subendothelial area with subsequent sequestration and oxidation leads to activation of the innate and adaptive immune system and chronic vascular inflammation. Oxidized LDL, one of the major autoantigens in atherosclerosis, leads to the formation of foam cells and fatty streaks in the vessel wall, a hallmark of the onset of atherosclerosis (Boren et al., 2020; Poznyak et al., 2020). Various cell types, including macrophages, lymphocytes, endothelial cells, and smooth muscle cells, are involved in atherosclerotic lesion formation (Falk, 2006). Activation, proliferation and migration of vascular smooth muscle cells are crucial in both early and late stages of atherosclerosis. Vascular smooth muscle cells invade the early atherosclerotic lesion from the media and enlarge the lesions, but also form a protective fibrous cap (Chistiakov et al., 2015; Grootaert and Bennett, 2021). As the disease progresses, rupture or erosion of an atherosclerotic plaque, with subsequent thrombus formation and occlusion of the artery, may occur (Poznyak et al., 2020).
Vascular OxS (Poznyak et al., 2020), hyperinsulinemia (Arcaro et al., 2002), and dysregulation of the SNS (Grassi, 2001; Karakas et al., 2018), the systemic and local vascular RAAS (Pacurari et al., 2014), and the immune system (Hovland et al., 2015; Salvador et al., 2016) play key roles in the developmental process.
NOXs, an important source of cellular ROS, are involved in a variety of processes of atherogenesis. Numerous studies have shown that the upregulation of NOXs in vascular tissues and cell types such as endothelial cells, smooth muscle cells, fibroblasts, and others plays a key role in atherogenesis by promoting endothelial dysfunction and vascular inflammation. Of the 4 NOX isoforms expressed in human vascular tissue, NOX1 and especially NOX2 have been shown to promote atherogenicity (Kuntic et al., 2020; Poznyak et al., 2020). An in vivo study in NOX2 knockout mice has shown that knocking out NOX2 completely prevents the cardiovascular and cerebral side effects of e-cigarette smoking. In addition, in vitro studies using acrolein have replicated much of the NOX-2-associated vascular dysfunction caused by electronic cigarettes (Kuntic et al., 2020).
There is increasing evidence for an interaction between ROS-generating enzymes, such as mitochondrial oxidases, and NOXs as important sources of vascular ROS production (Poznyak et al., 2020). Also, ANG II-mediated OxS contributes to the initiation and maintenance of endothelial dysfunction, vascular inflammation, and vascular remodeling (Ekholm and Kahan, 2021; Poznyak et al., 2021). In addition, the RAAS stimulates accumulation of low-density lipoproteins, especially the oxidatively modified form, while (oxidized) lipid accumulation enhances the expression of RAAS components in blood vessels. This cross-talk between dyslipidemia and RAAS plays an important role in the atherosclerotic process (Singh and Mehta, 2003). As shown in a human clinical trial, even moderate hyperinsulinemia, which is comparable to fasting hyperinsulinemia in insulin resistance, can cause severe endothelial dysfunction in large conduit arteries (Arcaro et al., 2002). Insulin resistance may also alter systemic lipid metabolism and lead to the development of atherogenic dyslipidemia (Ormazabal et al., 2018). Persistent SNS overactivity induces functional and structural changes in various organs, like heart and blood vessels (Borchard, 2001).
Smoking is the most preventable cause of CVD and is involved in all steps of the above cascade (Roy et al., 2017). Exposure to cigarette smoke (Kim et al., 2014), its major constituent nicotine (Petsophonsakul et al., 2021), and electronic cigarettes (El-Mahdy et al., 2022) causes vascular OxS that is mediated, at least in part, by NOXs. Smoking-induced OxS decreases NO bioavailability, leading to endothelial dysfunction and vascular remodeling, the development and progression of vascular inflammation, and oxidation of lipoproteins. Further, in vitro cellular studies have shown that constituents of cigarette smoke lead to depletion of the cofactor tetrahydrobiopterin in vascular endothelial cells, which is essential for endothelial NO synthase function, resulting in uncoupling and dysfunction of endothelial NO synthase and endothelial dysfunction (Abdelghany et al., 2018). In addition, smoking-derived superoxide anions react preferentially with NO rather than with its endogenous scavenger superoxide dismutase, promoting the formation of peroxynitrite. Peroxynitrite causes uncoupling and dysfunction of endothelial NO synthase and endothelial dysfunction, as demonstrated in vivo studies in smokers and in vitro studies in bovine aortic endothelial cells (Peluffo et al., 2009). Finally, peroxynitrite inactivates prostacyclin synthase by activating NF-κB and increasing nitric oxide synthase expression in endothelial cells in vitro, which also leads to endothelial dysfunction (Cooke and Davidge, 2002). As shown in in vitro studies of human gingival epithelial cells (Semlali et al., 2012) and macrophages (Yang et al., 2006), smoking leads to dysregulation of TLRs and NF-κB, which contribute to atherogenesis by promoting OxS, inflammation, and endothelial dysfunction (Hovland et al., 2015; Lazaridis et al., 2021). Clinical and experimental data suggest that platelets and the coagulation system also play an important role in atherogenesis and atherothrombosis, and that exposure to cigarette smoke has prothrombotic effects by impairing the functions of endothelial cells, platelets, fibrinolytic factors, and coagulation factors (Barua and Ambrose, 2013; Gutowska et al., 2019). Nicotine-mediated impairment of RAAS homeostasis causes endothelial dysfunction, vascular inflammation, vascular remodeling and increased arterial stiffness (Oakes et al., 2018). Finally, smoking causes insulin resistance, hyperinsulinemia and atherogenic dyslipidemia (Facchini et al., 1992; Frati et al., 1996; Li et al., 2019; Lan et al., 2020). (Limitation: see discussion).
4.1.2 Hypertension
Essential hypertension (EH), one of the most common chronic diseases, is a major risk factor for stroke, myocardial infarction and heart failure and the leading cause of morbidity and mortality worldwide (Mills et al., 2020). It is characterized by significant and persistent elevations in arterial pressure. The etiology of EH is complex and multifactorial and still not fully understood. Genetic (familial predisposition), environmental and behavioral factors (such as high salt intake, psychological stress, obesity, gut dysbiosis) are known hypertensinogenic factors (Carretero and Oparil, 2000; Harrison et al., 2021). Abnormalities of cardiac, vascular, and renal function are central to the pathophysiology of EH. The balance between cardiac output and peripheral vascular resistance is an important factor in maintaining normal blood pressure. In most patients with EH, there is an imbalance between cardiac output and peripheral vascular resistance in favor of increased peripheral resistance caused mainly by persistent smooth muscle constriction in the small arterioles with subsequent thickening of the wall and narrowing of the lumen. In adaptation to the increased peripheral resistance, hypertrophy of the cardiac musculature develops. The kidney plays a key role in the development of EH. This is reflected in the fact that “blood pressure goes with the kidney”, as cross-transplantation experiments and findings from kidney transplantation in humans have shown. On the one hand, kidney dysfunction leads to an increase in blood pressure, on the other hand, high blood pressure accelerates the loss of function of the kidney (Carretero and Oparil, 2000; Harrison et al., 2021).
Dysregulation of multiple homeostatic systems, with damage to the vascular system, heart, and kidneys, are involved in the developmental process (Oparil et al., 2018; Harrison et al., 2021). OxS (Poznyak et al., 2020; Harrison et al., 2021), insulin resistance (Mancusi et al., 2020), dysregulation of the systemic and local vascular RAAS (Manrique et al., 2009), the SNS (Tsioufis et al., 2011; Harrison et al., 2021) and the immune system (Harrison et al., 2021; Lazaridis et al., 2021) are key factors in the developmental process and cause endothelial dysfunction, vasoconstriction, vascular inflammation and vascular remodeling, hallmarks of EH (Oparil et al., 2018; Lazaridis et al., 2021). Impaired endothelium-dependent relaxation and structural changes in arteries and arterioles lead to stiffening of these vessels and an increase in peripheral vascular resistance and arterial pressure. Subsequently, damage to the heart and kidneys may occur, leading to dysfunction of these organs and further progression of the disease (Oparil et al., 2018; Harrison et al., 2021). OxS plays an important role in renal dysfunction and cardiovascular injury. Studies have shown that patients with essential hypertension have excessive ROS levels associated with reduced antioxidant capacity. NOXs of the vessels, heart, kidneys and immune system are a major source of ROS and OxS in EH (Touyz et al., 2020). There is increasing evidence that mitochondrial OxS is also invoved (Harrison et al., 2021). Fasting and postprandial insulin levels are higher in untreated patients with EH compared with normotensive controls, and there is a direct relationship between plasma insulin concentration and blood pressure. Insulin resistance and compensatory hyperinsulinemia contribute to EH through multiple mechanisms, including OxS, increased tissue ANG II and aldosterone activity, and increased activation of the SNS (Mancusi et al., 2020).
While the results of epidemiological studies examining the effects of smoking on the development of EH are controversial (reviewed in Samadian et al., 2016), there are several lines of evidence supporting a causal relationship between smoking and EH: an in vivo mouse model provided convincing evidence that chronic cigarette smoking causes hypertension and cardiac remodeling (Talukder et al., 2011). Exposure to cigarette smoke (Kim et al., 2014), its major constituent nicotine (Petsophonsakul, 20121), and electronic cigarettes (El-Mahdy et al., 2022) causes vascular OxS mediated at least in part by NOXs, leading to endothelial dysfunction, vascular remodeling, and vascular inflammation, key factors in the development process (Doonan et al., 2010; Golbidi et al., 2020). As shown in in vitro studies of human gingival epithelial cells (Semlali et al., 2012) and macrophages (Yang et al., 2006), smoking leads to dysregulation of TLRs and NF-κB. TLRs are expressed not only on immune cells, but also on non-immune cells such as vascular endothelial and smooth muscle cells, as well as on cells of the kidneys and nervous system, which are the three major target organs in hypertension. TLR activation on immune and vascular cells leads to activation of downstream signaling pathways that trigger an immune response with upregulation of proinflammatory cytokines and activation of NOXs, which contribute to the development of hypertension by promoting OxS, inflammation, endothelial dysfunction, smooth muscle cell migration and proliferation, and ultimately organ damage (Hovland et al., 2015; Lazaridis et al., 2021). Impairment of RAAS homeostasis by chronic smoking or nicotine exposure leads to endothelial dysfunction, vascular inflammation, vascular remodeling, and increased arterial stiffness (Oakes et al., 2018). Exposure to cigarette smoke (Middlekauff et al., 2014; Xiao et al., 2019) and e-cigarette smoke (Dimitriadis et al., 2022) acutely exerts a hypertensive effect, mainly through its powerful sympathetic excitatory effect. (Limitation: see discussion).
4.2 Cancer
Cancer is a complex, multifactorial disease involving genetics, environment and lifestyle. Lung cancer is the leading cause of cancer-related deaths worldwide (Sung et al., 2021). An estimated 90% of lung cancer deaths are due to tobacco smoking. In addition, there is sufficient evidence of a causal relationship between tobacco smoking and an increased risk of cancer of the upper digestive tract, esophagus, stomach, bladder, kidney, colon, prostate, pancreas, and acute myeloid leukemia. Data on an association between tobacco smoking and liver, cervical, brain, gallbladder, Hodgkin’s disease, non-Hodgkin’s lymphoma, and hematologic malignancies are inconsistent and require further investigation (Khani et al., 2018). There is a strong dose-response relationship between duration and intensity of cigarette smoking and many cancers (Inoue-Choi et al., 2018).
Tobacco smoke is a toxic mixture of thousands of chemicals, at least 70 of which are carcinogenic. The most important carcinogens in humans include nicotine, nitrosamines and polycyclic aromatic hydrocarbons. On the one hand, carcinogens such as tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons trigger cancer by causing DNA mutations and/or DNA and protein adducts; on the other hand, nicotine and nitrosamines promote cancer progression through receptor-mediated effects, like activation of non-neuronal nicotinic acetylcholine receptors and β-adrenergic receptors. Upon receptor activation, a wide range of signal transduction pathways is activated, which promote cancer cell growth, angiogenesis, migration, and invasion, and inhibit apoptosis (Xue et al., 2014; Nooshinfar et al., 2017).
Metabolically activated tobacco smoke carcinogens directly cause mutations observed in tumor suppressor genes and oncogenes (Hecht, 2012). In addition to smoke-related carcinogens, OxS, hyperinsulinemia and insulin resistance, inflammation, the SNS and the RAAS were identified as important causal factors for cancer development and progression. OxS and inflammation play an essential role in tumorigenesis by promoting multiple oncogenic events, including cell proliferation, angiogenesis, migration, metabolic reprogramming, and evasion of apoptosis in cancer cells (Hayes et al., 2020; Vaidya et al., 2020). Inflammation predisposes to the development of cancer and plays an important role in all stages of tumor development; cancer cells, together with their surrounding stromal and inflammatory cells, form an inflammatory tumor microenvironment which contributes to proliferation, angiogenesis, metastasis and more (Greten and Grivennikov, 2019). A growing body of evidence suggests that dysregulation of TLRs plays a critical role in cancer development and progression by modulating the inflammatory microenvironment (Mokhtari et al., 2021). Elevated insulin levels have been associated with increased cancer risk and progression in epidemiological studies. Both insulin itself and insulin-like growth factors 1 and 2 are potent mitogens that play important roles in promoting cell proliferation, differentiation, metastasis, and inhibition of apoptosis (Gallagher and LeRoith, 2020; Yee et al., 2020). Insulin receptors are normally downregulated in response to hyperinsulinemia. In cancer cells, however, insulin receptors may be over-expressed 2–6 fold which further exposes them to growth stimulation by insulin (Milazzo et al., 1992). In addition, hyperinsulinemia leads to a decrease in sex hormone-binding globulin, which can increase the availability of free sex hormones and promote the development of sex hormone-dependent cancers such as breast, prostate, and endometrial cancers (Arcidiacono et al., 2012). The RAAS also plays a crucial role in cancer biology and affects development and maintenance of cancer directly and indirectly by remodeling the tumor microenvironment. It regulates almost all hallmarks of cancer including tumorigenesis, proliferation, angiogenesis, cell migration and metastasis (Pinter and Jain, 2017; Rasha et al., 2019). Finally, β-adrenergic signaling has been shown to promote several cellular processes that contribute to cancer initiation, progression, and metastasis, including proliferation, inflammation, angiogenesis, immune escape, epithelial-mesenchymal transition and invasion of the extracellular matrix (Mravec et al., 2020; Conceição et al., 2021). (Limitation: see discussion).
4.3 Type 2 diabetes
Diabetes is one of the most common chronic metabolic disorder caused by an interplay of genetic, environmental and lifestyle factors. It is characterized by defective glucose metabolism as a result of impaired pancreatic β-cell function and insulin resistance (Galicia-Garcia et al., 2020). Many epidemiological studies have shown that cigarette smoking is one of the most important modifiable risk factors for type 2 diabetes, and most studies showed a dose-response relationship between smoking and diabetes (Śliwińska-Mossoń and Milnerowicz, 2017). In addition, cigarette smoking worsens metabolic control in diabetic patients. In a cross-sectional clinical study, smokers had on average 15%–20% higher insulin requirements and higher serum triglyceride concentrations compared with non-smokers, increasing to 30% in heavy smokers (Madsbad et al., 1980).
Normal glucose homeostasis requires fine tuning of insulin secretion by pancreatic β-cells in response to changes in blood glucose levels. Type 2 diabetes is characterized by insulin resistance, impaired hepatic glucose homeostasis and dysregulated insulin secretion. Initially, insulin resistance is compensated by increased insulin secretion, but over time, ß-cell dysfunction and diabetes can develop (Galicia-Garcia et al., 2020). Mitochondrial OxS plays a critical role in the development of insulin resistance (Fazakerley et al., 2018) and insulin hypersecretion (Saadeh et al., 2012). It further can induce β-cell dysfunction due to the low antioxidant capacity of beta cells (Eguchi et al., 2021). In vitro studies in ß-cells/islets and in vivo animal studies showed that ANG II causes β-cell inflammation and β-cell dysfunction through induction of endoplasmic reticulum stress (Chan et al., 2017). NOX-induced vascular OxS is also associated with the development and progression of the major vascular complications of diabetes (Urner et al., 2020). In the Atherosclerosis Risk in Communities Study, which enrolled more than 8,000 non-diabetic middle-aged adults, SNS overactivity nearly doubled the risk of type 2 diabetes during an eight-year follow-up period (Carnethon et al., 2003). Smoking-related dysregulation of TLRs and NF-κB (Yang et al., 2006; Semlali et al., 2012) may play a role in the development of type 2 diabetes and its complications. TLRs, particularly TLR2 and TLR4, are involved in the development and progression of insulin resistance and diabetic complications such as diabetic nephropathy and vascular damage (Jialal et al., 2014; Aly et al., 2020). A clinical study in type 2 diabetic patients with or without renal insufficiency showed that the expression of TLR2 and TLR4 was higher in patients with renal insufficiency than in patients without renal insufficiency or in normal subjects and correlated positively with the degree of insulin resistance and negatively with the degree of insulin sensitivity (Aly et al., 2020). Nicotine also may be at least partially responsible for the development of diabetes. On the one hand, long-term use of nicotine gum is associated with insulin resistance and hyperinsulinemia (Eliasson et al., 1996). In addition, nicotine can affect insulin secretion via neuronal nicotinic acetylcholine receptors expressed on beta cells. Acute nicotine exposure at concentrations greater than 1 μmol/L inhibited high glucose-induced insulin release in isolated human islet cells in vitro (Yoshikawa et al., 2005). (Limitation: see discussion).
4.4 Osteoporosis
Osteoporosis is a chronic metabolic disease of the skeleton characterized by loss of bone mass and deterioration of bone quality, leading to increased susceptibility to fractures. Osteoblasts and osteoclasts are the main cells responsible for bone remodeling. Their activity is regulated by various factors, including the RANKL-RANK-OPG signaling pathway, estradiol, various cytokines and calciotropic hormones (Al-Bashaireh et al., 2018).
Cigarette smoking creates an imbalance in bone turnover, which leads to lower bone mass and decreased bone mineral density and makes bones prone to osteoporosis and fractures. Smoking was therefore classified as a risk factor for osteoporosis and fractures and included in the Fracture Risk Assessment Tool (Al-Bashaireh et al., 2018). The effects of tobacco smoke on bone health are complex, and several mechanisms are thought to play a role, including alterations in the metabolism of calciotropic hormones and intestinal calcium absorption, disruptions in the production and metabolism of sex hormones, alterations in the hormonal metabolism of the adrenal cortex and in the system of receptor activators of NF-κB and osteoprotegerin (Yoon et al., 2012; Al-Bashaireh et al., 2018). Chronic exposure to cigarette smoke caused enhanced osteoclast function by upregulating the RANKL/OPG ratio in an in vitro bone co-culture system, resulting in an osteoporotic microenvironment (Zhu et al., 2020). In postmenopausal women, bone mineral density was significantly lower in smokers than in non-smokers in a human clinical study. An additional animal study showed that administration of nicotine to wild-type mice decreased bone mass (Kiyota et al., 2020).
In a human cross-sectional study on 612 participants, smoking was associated with lower serum levels of vitamin D than non-smokers, with a dose-response pattern (Jiang et al., 2016). The exact mechanisms by which smoking affects vitamin D metabolism are still unclear. It is worth noting, however, that high insulin levels— common in smokers (Eliasson et al., 1994)—can lower vitamin D levels (De Pergola et al., 2013). Also, chronic overstimulation of the RAAS is thought to result in lower vitamin D levels (Ferder et al., 2013). Smoking cessation was associated with a significant increase in bone mineral density within a short period of time in a human clinical trial and in an animal study, as reflected by a significant decrease in serum levels of TRAP5b, a marker of bone resorption, and an increase in levels of osteocalcin and non-carboxylated osteocalcin (Kiyota et al., 2020).
In addition to the above factors, other factors play important roles in osteoporosis. OxS influences the bone remodeling process and causes an imbalance between osteoclast and osteoblast activity (Callaway and Jiang, 2015). Smoking-related OxS can increase bone resorption and contribute to lower bone mass (Kohler et al., 2021). Subclinical inflammation and SNS and RAAS imbalances are also associated with bone resorption and osteoporosis. Clinical and molecular evidence suggests that subclinical inflammation has a significant impact on bone turnover and the development of osteoporosis (Ginaldi et al., 2009). Chronic subclinical inflammation is an important trigger for osteoclast differentiation, which increases bone resorption (Kalyan et al., 2017). OxS and the formation of advanced glycation end products act as the link between inflammation and bone resorption (Pietschmann et al., 2016). There is ample evidence that the SNS is closely linked to bone remodeling. Increased SNS activity leads to bone loss through increased bone resorption and decreased bone formation mediated by β2-adrenergic influence on osteoblastic and osteoclastic cells (Farr et al., 2012; Kim et al., 2017). In a human clinical study of 23 women (10 premenopausal, 13 postmenopausal), sympathetic activity measured by microneurography was inversely related to bone volume and trabecular microstructure. Moreover, sympathetic activity in a subgroup of postmenopausal women correlated negatively with serum levels of procollagen type I N-peptide, a marker of bone formation in osteoporosis (Farr et al., 2012). Dysregulation of the RAAS has also been implicated in the development of osteoporosis. Ang II accelerates osteoporosis by activating osteoclasts and inhibiting osteoblast differentiation and bone formation (Chen, et al., 2017; Mo et al., 2020). (Limitation: see discussion).
4.5 Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic disorders in premenopausal women. It can affect the endocrine, reproductive, metabolic, and psychological health of women beginning at puberty. The etiology of this syndrome remains largely unknown, but there is growing evidence that a genetic predisposition, as well as epigenetic and environmental factors, including diet and lifestyle, are involved in the developmental process. A recently published meta-analysis of Mendelian randomization studies has shown that a genetic predisposition to smoking (smoking initiation or lifetime smoking) is a causal risk factor for PCOS (Larsson and Burgess, 2022).
PCOS is associated with abnormal hormone production and metabolism, with elevated androgen levels and a high ratio of luteinizing hormone to follicle-stimulating hormone being among the fundamental alterations. Hyperandrogenemia is considered the main clinical hallmark of PCOS. The pathogenesis of PCOS is complex and not yet fully understood. Hyperinsulinemia and insulin resistance play a key role in the pathogenesis of PCOS. Insulin stimulates the theca cells of the ovary to produce excess testosterone (mainly attributed to a steroidogenic defect in theca cells) and suppresses the level of sex hormone-binding globulin, resulting in an increase in free testosterone (Sanchez-Garrido and Tena-Sempere, 2020; Rostamtabar et al., 2021).
Systemic and local ovarian SNS hyperactivity has been linked to the pathogenesis of PCOS, although the exact mechanism is not fully understood (Davis et al., 2019). Direct intraneural recordings of sympathetic nerve activity in the muscle vascular bed in women with PCOS showed that PCOS is associated with increased sympathetic nerve activity (Sverrisdottir et al., 2008). Increased sympathetic tone is associated with elevated androgen levels, anovulation, and menstrual irregularity and may play a role in the pathogenesis of the disease (Sverrisdottir et al., 2008; Li et al., 2014; Davis et al., 2019).
In recent years, a growing number of studies suggest that PCOS is a (pro)inflammatory disease and that chronic, low-grade inflammation is an important factor in ovarian dysfunction and the development of associated metabolic abnormalities. OxS plays an important role in chronic low-grade inflammation and is considered a potential triggering factor in the pathogenesis of PCOS. Markers of OxS and inflammation correlate strongly with circulating androgens (González, 2012; Rostamtabar et al., 2021). ROS play an important role in regulating ovulation and follicular dynamics. Increased ROS production may stimulate inflammatory signaling pathways, leading to ovarian cyst dysfunction and disruption of normal ovulation (Boots and Jungheim, 2015). CYP17, an important component of the androgen synthesis pathway, was upregulated in interstitial theca cells by proinflammatory stimuli and inhibited by resveratrol in vitro (Ortega et al., 2014). (Limitation: see discussion).
4.6 Chronic obstructive pulmonary disease
COPD is the third leading cause of death globally. COPD is characterized by chronic airway inflammation, emphysema and bronchiolar obstruction, with impaired lung function. Cigarette smoking is the major risk factor for COPD (Hikichi et al., 2019; Agusti et al., 2020). Exposure to tobacco and/or e-cigarette/nicotine vapor causes significant OxS and inflammation (Zuo et al., 2014). OxS (Boukhenouna et al., 2018; Barnes, 2022) and increased activation of the RAAS (Tan et al., 2018; Mei et al., 2020) are essential driving mechanisms in the pathogenesis of COPD, predominantly due to their inflammatory potential. There is growing evidence that COPD is associated with lung-specific and systemic immune dysfunction that triggers chronic inflammation and subsequent tissue destruction (Rovina et al., 2013; Bhat et al., 2015). Numerous recent data support an important role for TLRs in the initiation and development of the inflammatory process in COPD (Sidletskaya et al., 2020). Hyperinsulinemia and insulin resistance have also been implicated in the developmental process of COPD. In a human clinical trial, hyperinsulinemia and insulin resistance were demonstrated in COPD patients compared to healthy controls (Ruan et al., 2019). Hyperinsulinemia contributes to the inflammatory process and has adverse effects on the structure and function of the airways (Singh et al., 2016; Ruan et al., 2019). Experimental data show that insulin exerts a number of remodeling effects on airway smooth muscle cells, including the ability to increase proliferation of primary human airway smooth muscle cells, thereby affecting lung structure and function (Sagun et al., 2015). Furthermore, insulin can lower vitamin D levels (De Pergola et al., 2013), a factor that has been linked to the developmental process of COPD (Janssens et al., 2011). Finally, human clinical studies revealed that COPD is associated with increased sympathetic nervous system activity, which may contribute to progression and adverse outcome of the disease (Heindl et al., 2001; Andreas et al., 2014). (Limitation: see discussion).
4.7 Psoriasis
Psoriasis is a chronic, systemic immune-mediated disease with skin and joint manifestations. It is associated with hyperproliferation and dysfunctional differentiation of keratinocytes. Both genetic and environmental factors are involved in the developmental process. Smoking has been associated with the onset of psoriasis and has also been linked with the severity of the disease (Naldi, 2016; Zhou et al., 2020). A recently published review and meta-analysis of sixteen case-control studies found that heavy smokers and those who had smoked for a long time were particularly at increased risk for developing psoriasis (Zhou et al., 2020).
Insulin sensitivity has been reported to be significantly lower in patients with psoriasis compared to control subjects (Gyldenløve et al., 2015). In a large cohort study, including 21,789 postmenopausal women, insulin resistance was significantly associated with an increased risk of psoriasis during a cumulative 21-year follow-up (Chan et al., 2022). Several human clinical trials found a significant positive correlation of serum insulin levels and insulin resistance indices with psoriasis severity (Napolitano et al., 2015).
Also, overactivation of the local RAAS as a result of increased ACE expression (Silva et al., 2020), and smoking-related OxS and inflammation (Armstrong et al., 2011; Pleńkowska et al., 2020) were found to play important roles in the pathogenesis of the disease. There is growing evidence that TLRs participate in the pathogenesis of various inflammatory skin diseases, including psoriasis. Abnormal activation of TLRs leads to an exaggerated autoimmune response with increased production of cytokines such as IL17A and IL22, which are responsible for hyperproliferation and abnormal differentiation of keratinocytes, ultimately leading to the formation of psoriatic plaques (Sun et al., 2019). (Limitation: see discussion).
4.8 Multiple sclerosis
Multiple sclerosis (MS) is a chronic, potentially disabling disease of the SNS, characterized by inflammation, demyelination and axonal degeneration. Many genetic and environmental factors have been shown to contribute to its development, including cigarette smoking, Epstein-Barr virus infections, vitamin D deficiency, and obesity (Rosso and Chitnis, 2020).
OxS has been suggested to play a key role in the development of demyelination and axonal damage in both MS and its animal models (Ohl et al., 2016). In the acute phase OxS triggers inflammatory processes, in the chronic phase it maintains neurodegeneration. OxS was found to be associated with mitochondrial dysfunction, dysregulation of axonal bioenergetics and iron accumulation in the brain (Adamczyk and Adamczyk-Sowa, 2016). Recent research has shown that TLRs play a crucial role in the pathogenesis and progression of neuroimmune diseases by triggering an inflammatory response (Li et al., 2021). The expression of TLR3 and TLR4 was significantly increased in the center of MS lesions and around inflamed vessels in human postmortem brain tissues (Bsibsi et al., 2002). In addition, overactivation of the RAAS was found to play a pivotal role in the autoimmune inflammatory process. In a model of experimental autoimmune encephalomyelitis, quantitative RT-PCR analyses revealed upregulation of renin, angiotensin-converting enzyme, and AT1R in the inflamed spinal cord and immune system during autoimmune inflammation (Stegbauer et al., 2009). Insulin resistance and hyperinsulinemia are also common in patients with MS, as has been demonstrated in a human clinical trials (Penesova et al., 2015; Ruiz-Argüelles et al., 2018) and are thought to contribute to metabolic complications and overall impairment (Limitation: see discussion).
4.9 Crohn’s disease
Crohn’s disease is an idiopathic, chronic inflammatory process that can affect any part of the gastrointestinal tract, but often affects the colon and the terminal ileum. Cigarette smoking is a major risk factor for Crohn’s disease (Berkowitz et al., 2018; Papoutsopoulou et al., 2020). OxS, inflammation, hyperactivation of the immune system and upregulation of the RAAS are involved in the developmental process. Chronic inflammation and immune system hyperactivation are associated with OxS, and OxS is thought to play an important role in the development and maintenance of inflammation and abnormal immune responses (Alzoghaibi, 2013; Luceri et al., 2019). Recently, there has been increasing evidence that immune dysfunction, particularly TLR-mediated dysfunction of the innate immune system, plays a central role in the pathogenesis of Crohn’s disease. As has been shown, most members of the TLR family are involved in the progression of this disease (Lu et al., 2018). Activation of the local mucosal RAAS may promote development of Crohn’s disease and inflammation. In a transgenic mouse model that overproduces active renin, overactivation of the RAAS was shown to promote colitis by stimulating intestinal epithelial cell apoptosis and mucosal TH17 responses (Shi et al., 2016). Renin expression was enhanced in colon biopsies from patients with Crohn’s disease, and renin and ANG II levels in the colon were markedly increased in a TNBS-induced experimental colitis model (He et al., 2019). Insulin hypersecretion was found in patients with Crohn’s disease in a human clinical study by Bregenzer et al. (2006), likely caused by an upregulated enteropancreatic axis. (Limitation: see discussion).
4.10 Rheumatoid arthritis
Rheumatoid arthritis (RA) is a autoimmune disease that is characterized by chronic inflammation, synovial hyperplasia and cartilage and bone destruction in multiple joints and is influenced by both genetic and environmental factors (Torres et al., 2017).
Smoking is considered one of the best-known environmental risk factors for the development and severity of RA. A large proportion of patients have a history of smoking, and in addition, smokers are at increased risk for more severe rheumatoid arthritis. Smoking is associated with the pathogenesis of RA primarily through promotion of OxS, impairment of the immune response, and likely through epigenetic changes (Ishikawa, and Terao, 2020; Alemany-Cosme et al., 2021). Human clinical trials showed that patients with active disease have high levels of OxS, which translates into increased lipid peroxidation, protein oxidation, and DNA damage. Impairment of the body’s enzymatic and non-enzymatic antioxidant defense systems contributes to tissue damage and thus, chronicity of the disease (Mateen et al., 2016; Alemany-Cosme et al., 2021). It has been established that a dysfunctional TLR-mediated response is characteristic of RA patients and contributes to the development of a chronic inflammatory state (Arleevskaya et al., 2020). In addition, insulin resistance and dysregulation of the RAAS are associated with disease development. Insulin acts as a critical modulator of the inflammatory response by regulating intracellular and intercellular signaling pathways in immune cells, cartilage and synovial tissue. The increased prevalence of insulin resistance in patients with rheumatoid arthritis correlates with disease activity and disease-specific factors such as chronic systemic inflammation (Tripolino et al., 2021). Both classical (Moreira et al., 2021) and local RAAS activation (Cobankara et al., 2005) play important roles in the pathogenesis of rheumatoid arthritis. Ang II is considered a potent proinflammatory mediator, and overexpression of AT2R has been demonstrated in vitro in the inflamed synovial tissue of RA patients (Terenzi et al., 2017). (Limitation: see discussion).
4.11 Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder that affects tens of millions of people worldwide (Alzheimer’s Association, 2020). Epidemiological studies, meta-analyses, and case-control studies show that cigarette smoking is associated with a significantly increased risk of neurodegenerative diseases such as Alzheimer’s disease and dementia (Cataldo et al., 2010; Rusanen et al., 2011). AD is a multifactorial disorder, and the multiple mechanisms associated with the disease are not completely clear. It is driven by the production and deposition of amyloid β peptide and intracellular accumulation of neurofibrillary tangles of hyperphosphorylated τ protein (Cheignon et al., 2018; Abeysinghe et al., 2020).
Cigarette smoking was associated with risk biomarkers for Alzheimer’s disease characterized by excessive OxS, neuroinflammation, and elevated β-amyloid 42 levels in a case-control study in humans (Liu et al., 2020). Also, another human clinical study showed that exposure to cigarette smoke produces significant OxS in the central nervous system (Durazzo et al. (2016). OxS plays an important role in the developmental process of AD (Verdile et al., 2015; Misrani et al., 2021). On the one hand, the brain is particularly susceptible to oxidative damage due to its high oxygen consumption, high content of polyunsaturated fatty acids, and relatively high content of redox transition metal ions; on the other hand, the level of antioxidants in the brain is very low. Numerous research studies have shown that lipid peroxidation is greatly increased in AD. The accumulation of amyloid-β protein triggered by ROS leads to the degradation of the lysosomal membrane and eventually contributes to the death of neurons (Verdile et al., 2015; Misrani et al., 2021). Dysregulation of TLRs plays an important role in the development of AD, particularly in the early stages of the disease, by affecting synaptic plasticity, microglial activity, τ phosphorylation, and inflammatory responses (Momtazmanesh et al., 2020). The RAAS is involved in the development and progression of AD by increasing amyloid-β production, OxS and inflammation, and decreasing the release of acetylcholine (Gebre et al., 2018). Emerging evidence from human clinical trials and animal studies suggests that hyperinsulinemia and brain insulin resistance also are involved in the developmental process through multiple pathways, including a decreased clearance of amyloid-β-peptide and phosphorylation of τ protein, hallmarks of AD, and through effects on vasoreactivity, lipid metabolism, and inflammation (Fishel, et al., 2005; Verdile et al., 2015; Kellar and Craft, 2020). Finally, overactivation of the SNS has been implicated in the development of AD. NE is thought to be functionally largely opposed to the neuromodulators serotonin, dopamine, acetylcholine and melatonin, which are distributed throughout the brain and modulate many processes in the pathophysiology underlying AD (Fitzgerald, 2021). (Limitation: see discussion).
This list could be continued, but would go beyond the scope of this publication.
5 Smoking and microbiome
The intestinal microbiome plays an important role in human health and also in the development of disease due to its interactions with the immune system. Dysbiosis of the gut microbiome has been linked to several diseases, including asthma, COPD, Crohn’s disease, ulcerative colitis, CVD, obesity, rheumatoid arthritis, systemic lupus erythematosus, central nervous system diseases and cancer. A growing number of human clinical trials and animal studies have shown that smoking alters the composition of the gut microbiome. However, the underlying mechanisms of how smoking affects the microbiome are still largely unknown (Gui et al., 2021; Martinez et al., 2021).
6 Discussion
Cigarette smoking is the leading cause of preventable deaths worldwide. Cigarette smoking has been implicated in the pathogenesis of a host of chronic non-communicable diseases. The mechanisms by which cigarette smoking induces and promotes these diseases are still under debate. This review shows that smoking, like the Western diet (Kopp, 2019), causes a significant distortion of physiological balance, characterized by dysregulation of the SNS, the RAAS and the immune system, as well as disruption of physiological insulin and oxidant/antioxidant homeostasis, manifested as OxS and insulin resistance. As shown in Figure 1, all of these factors are strongly interrelated, suggesting that dysregulation of one of them may cause imbalance in others.
The evidence presented further shows that these factors play a key role in the development of a broad spectrum of smoking-related diseases, including CVDs, COPD, cancer, type 2 diabetes, Crohn’s disease, rheumatoid arthritis, psoriasis, PCOS, osteoporosis, MS and AD.
The dysregulation of the above factors may affect other physiological factors as well, which then also contribute to disease development. For example, overactivation of the RAAS (Singh and Mehta, 2003) and insulin resistance (Ormazabal et al., 2018) alter systemic lipid metabolism, leading to the development of atherogenic dyslipidemia. Chronic overstimulation of the RAAS (Ferder et al., 2013) and hyperinsulinemia (De Pergola et al., 2013; Cooper et al., 2020) were found to cause low vitamin D levels. Increasing epidemiological and laboratory diagnostic evidence suggests that vitamin D deficiency is associated with the onset and progression of numerous chronic non-communicable diseases (Wang et al., 2017). Hyperinsulinemia may increase the availability of free sex hormones, thereby promoting the development of sex hormone-dependent cancers (Arcidiacono et al., 2012).
Since not all smokers develop one or more of these diseases, it is proposed that this disruption of physiological balance represents a kind of pathogenetic “basic toolkit” for the potential development of a range of non-communicable diseases, and that the decision of whether and what disease will develop in an individual is determined by other, individual factors (“determinants”), such as the genome, epigenome, exposome, microbiome, and others (Figure 1).
Often, more than one chronic disease develops in an individual, which is referred to as comorbidity (Valderas et al., 2009). The pathophysiological pattern common to these diseases may provide an explanation for the often poorly understood links between non-communicable diseases and disease comorbidities, like for instance CVDs and type 2 diabetes (Einarson et al., 2018), CVD, COPD and cancer (Buddeke et al., 2019), CVDs and osteoporosis (Farhat and Cauley, 2008), CVDs and AD (Stampfer, 2006), COPD and osteoporosis (Sarkar et al., 2015), CVDs and psoriasis (Hu and Cheng-Che, 2017), psoriasis and AD (Kim et al., 2020), and psoriasis with CVDs, cancers, type 2 diabetes and Crohn’s disease (Takeshita et al., 2017), to name but a few.
A limitation of this work is that the evidence that the development of the diseases described is due to smoking-induced dysregulation of the above physiological factors is partly indirect. This is because there is a partial lack of studies describing the direct smoking-related influence of these factors on disease development. In these cases, the evidence relies on a combination of studies showing the dysregulatory effect of smoking and/or cigarette smoke constituents on the above factors and studies showing the pathogenetic effect of these factors on various non-communicative diseases.
7 Summary and conclusion
The aim of this work was to address the question of whether the pathogenesis of smoking-related and diet-related non-communicable diseases follows the same pattern. The evidence presented shows that both cause a significant distortion of physiological balance characterized by dysregulation of a group of important, strongly interrelated physiological factors that play key roles in the developmental process of many (most?) non-communicable diseases.
The proposed pathophysiological process offers new insights into the development of non-communicable diseases and may influence the direction of future research in both prevention and therapy.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE, angiotensin-converting enzyme; AD, Alzheimer’s disease; ANG, angiotensin; AT1R, ANG II type 1 receptor; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; EH, essential hypertension; LDL, low-density lipoprotein cholesterol; MS, multiple sclerosis; NE, norepinephrine; NF-κB, nuclear factor-κB; NO, nitric oxide; NOX, nicotinamide adenine dinucleotide phosphate oxidase; OPG, osteoprotegerin; OxS, oxidative stress; PCOS, polycystic ovary syndrome; RA, rheumatoid arthritis; RAAS, renin-angiotensin aldosterone system; RANKL, receptor activator of nuclear factor (NF)-kB-ligand; RNS, nitrogen species; ROS, reactive oxygen species; RT-PCR, reverse transcription polymerase chain reaction; SNS, sympathetic nervous system; TLR, toll-like receptor.
References
Abdelghany T. M., Ismail R. S., Mansoor F. A., Zweier J. R., Lowe F., Zweier J. L. (2018). Cigarette smoke constituents cause endothelial nitric oxide synthase dysfunction and uncoupling due to depletion of tetrahydrobiopterin with degradation of GTP cyclohydrolase. Nitric Oxide 76, 113–121. doi:10.1016/j.niox.2018.02.009
Abeysinghe A. A. D. T., Deshapriya R. D. U. S., Udawatte C. (2020). Alzheimer's disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 256, 117996. doi:10.1016/j.lfs.2020.117996
Adamczyk B., Adamczyk-Sowa M. (2016). New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid. Med. Cell. Longev. 2016, 1973834. doi:10.1155/2016/1973834
Agusti A., Vogelmeier C., Faner R. (2020). Copd 2020: Changes and challenges. Am. J. Physiol. Lung Cell. Mol. Physiol. 319 (5), L879–L883. doi:10.1152/ajplung.00429.2020
Al-Bashaireh A. M., Haddad L. G., Weaver M., Chengguo X., Kelly D. L., Yoon S. (2018). The effect of tobacco smoking on bone mass: An overview of pathophysiologic mechanisms. J. Osteoporos. 2018, 1206235. doi:10.1155/2018/1206235
Alemany-Cosme E., Sáez-González E., Moret I., Mateos B., Iborra M., Nos P., et al. (2021). Oxidative stress in the pathogenesis of Crohn’s disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants 10 (1), 64. doi:10.3390/antiox10010064
Aly R. H., Ahmed A. E., Hozayen W. G., Rabea A. M., Ali T. M., El Askary A., et al. (2020). Patterns of toll-like receptor expressions and inflammatory cytokine levels and their implications in the progress of insulin resistance and diabetic nephropathy in type 2 diabetic patients. Front. Physiol. 11, 609223. doi:10.3389/fphys.2020.609223
Alzheimer’s Association (2020). Alzheimer’s disease facts and figures. Physician 16, 391–460. doi:10.1002/alz.12068
Alzoghaibi M. A. (2013). Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 19 (39), 6540–6547. doi:10.3748/wjg.v19.i39.6540
Andreas S., Haarmann H., Klarner S., Hasenfuß G., Raupach T. (2014). Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung 192 (2), 235–241. doi:10.1007/s00408-013-9544-7
Arcaro G., Cretti A., Balzano S., Lechi A., Muggeo M., Bonora E., (2002). Insulin causes endothelial dysfunction in humans: Sites and mechanisms. Circulation 105 (5), 576–582. doi:10.1161/hc0502.103333
Arcidiacono B., Iiritano S., Nocera A., Possidente K., Nevolo M. T., Ventura V., et al. (2012). Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 789174. doi:10.1155/2012/789174
Arleevskaya M. I., Larionova R. V., Brooks W. H., Bettacchioli E., Renaudineau Y. (2020). Toll-like receptors, infections, and rheumatoid arthritis. Clin. Rev. Allergy Immunol. 58 (2), 172–181. doi:10.1007/s12016-019-08742-z
Armstrong A. W., Armstrong E. J., Fuller E. N., Sockolov M. E., Voyles S. V. (2011). Smoking and pathogenesis of psoriasis: A review of oxidative, inflammatory and genetic mechanisms. Br. J. Dermatol. 165 (6), 1162–1168. doi:10.1111/j.1365-2133.2011.10526.x
Attvall S., Fowelin J., Lager I., Von Schenck H., Smith U. (1993). Smoking induces insulin resistance—A potential link with the insulin resistance syndrome. J. Intern. Med. 233 (4), 327–332. doi:10.1111/j.1365-2796.1993.tb00680.x
Banday A. A., Lokhandwala M. F. (2008). Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am. J. Physiol. Ren. Physiol. 295 (3), F698–F706. doi:10.1152/ajprenal.90308.2008
Barnes P. J. (2022). Oxidative stress in chronic obstructive pulmonary disease. Antioxidants 11 (5), 965. doi:10.3390/antiox11050965
Barquera S., Pedroza-Tobías A., Medina C., Hernández-Barrera L., Bibbins-Domingo K., Lozano R., et al. (2015). Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 46 (5), 328–338. doi:10.1016/j.arcmed.2015.06.006
Barr J., Sharma C. S., Sarkar S., Wise K., Dong L., Periyakaruppan A., et al. (2007). Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol. Cell. Biochem. 297 (1), 93–99. doi:10.1007/s11010-006-9333-1
Barua R. S., Ambrose J. A. (2013). Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler. Thromb. Vasc. Biol. 33 (7), 1460–1467. doi:10.1161/ATVBAHA.112.300154
Benarroch E. E. (2020). Physiology and pathophysiology of the autonomic nervous system. Continuum 26 (1), 12–24. doi:10.1212/CON.0000000000000817
Berkowitz L., Schultz B. M., Salazar G. A., Pardo-Roa C., Sebastián V. P., Álvarez-Lobos M. M., et al. (2018). Impact of cigarette smoking on the gastrointestinal tract inflammation: Opposing effects in Crohn’s disease and ulcerative colitis. Front. Immunol. 9, 74. doi:10.3389/fimmu.2018.00074
Bernstein K. E., Khan Z., Giani J. F., Cao D. Y., Bernstein E. A., Shen X. Z. (2018). Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 14 (5), 325–336. doi:10.1038/nrneph.2018.15
Bhat T. A., Panzica L., Kalathil S. G., Thanavala Y. (2015). Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 12 (2), S169–S175. doi:10.1513/AnnalsATS.201503-126AW
Biancardi V. C., Bomfim G. F., Reis W. L., Al-Gassimi S., Nunes K. P. (2017). The interplay between Angiotensin II, TLR4 and hypertension. Pharmacol. Res. 120, 88–96. doi:10.1016/j.phrs.2017.03.017
Biswas S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 5698931. doi:10.1155/2016/5698931
Boots C. E., Jungheim E. S. (2015). Inflammation and human ovarian follicular dynamics. Semin. Reprod. Med. 33 (4), 270–275. doi:10.1055/s-0035-1554928
Borchard U. (2001). The role of the sympathetic nervous system in cardiovascular disease. J. Clin. Basic Cardiol. 4 (3), 175–177. doi:10.1016/j.ccl.2013.09.010
Boren J., Chapman M. J., Krauss R. M., Packard C. J., Bentzon J. F., Binder C. J., et al. (2020). Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European atherosclerosis society consensus panel. Eur. Heart J. 41 (24), 2313–2330. doi:10.1093/eurheartj/ehz962
Borén J., Williams K. J. (2016). The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 27 (5), 473–483. doi:10.1097/MOL.0000000000000330
Boukhenouna S., Wilson M. A., Bahmed K., Kosmider B. (2018). Reactive oxygen species in chronic obstructive pulmonary disease. Oxid. Med. Cell. Longev. 2018, 5730395. doi:10.1155/2018/5730395
Bregenzer N., Hartmann A., Strauch U., Schölmerich J., Andus T., Bollheimer C. L. (2006). Increased insulin resistance and β cell activity in patients with Crohn's disease. Inflamm. Bowel Dis. 12 (1), 53–56. doi:10.1097/01.mib.0000195975.97673.f5
Bsibsi M., Ravid R., Gveric D., van Noort J. M. (2002). Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 61 (11), 1013–1021. doi:10.1093/jnen/61.11.1013
Buddeke J., Bots M. L., Van Dis I., Visseren F. L., Hollander M., Schellevis F. G., et al. (2019). Comorbidity in patients with cardiovascular disease in primary care: A cohort study with routine healthcare data. Br. J. Gen. Pract. 69 (683), e398–e406. doi:10.3399/bjgp19X702725
Caliri A. W., Tommasi S., Besaratinia A. (2021). Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 787, 108365. doi:10.1016/j.mrrev.2021.108365
Callaway D. A., Jiang J. X. (2015). Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Min. Metab. 33 (4), 359–370. doi:10.1007/s00774-015-0656-4
Campese V. M., Shaohua Y., Huiquin Z. (2005). Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 46, 533–539. doi:10.1161/01.HYP.0000179088.57586.26
Carnethon M. R., Golden S. H., Folsom A. R., Haskell W., Liao D. (2003). Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: The atherosclerosis risk in Communities study, 1987–1998. Circulation 107 (17), 2190–2195. doi:10.1161/01.CIR.0000066324.74807.95
Carretero O. A., Oparil S. (2000). Essential hypertension: Part I: Definition and etiology. Circulation 101 (3), 329–335. doi:10.1161/01.CIR.101.3.329
Catalano K. J., Maddux B. A., Szary J., Youngren J. F., Goldfine I. D., Schaufele F. (2014). Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS One 9 (9), e108693. doi:10.1371/journal.pone.0108693
Cataldo J. K., Prochaska J. J., Glantz S. A. (2010). Cigarette smoking is a risk factor for Alzheimer's disease: An analysis controlling for tobacco industry affiliation. J. Alzheimers Dis. 19 (2), 465–480. doi:10.3233/JAD-2010-1240
Centers for Disease Control and Prevention (2010). How tobacco smoke causes disease: The biology and behavioural basis for smoking-attributable disease 2010. A report of the surgeon general. Available: https://www.ncbi.nlm.nih.gov/books/NBK53017/pdf/Bookshelf_NBK53017.pdf (Accessed August 15, 2022).
Chan A. A., Li H., Li W., Pan K., Yee J. K., Chlebowski R. T., et al. (2022). Association between baseline insulin resistance and psoriasis incidence: the Women’s Health Initiative. Archives of dermatological research 314 (9), 869–880. doi:10.1007/s00403-021-02298-9
Chan S. M., Lau Y. S., Miller A. A., Ku J. M., Potocnik S., Ye J. M., et al. (2017). Angiotensin II causes β-cell dysfunction through an ER stress-induced proinflammatory response. Endocrinology 158 (10), 3162–3173. doi:10.1210/en.2016-1879
Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. (2018). Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 14, 450–464. doi:10.1016/j.redox.2017.10.014
Chen C. I., Yeh J. S., Tsao N. W., Lin F. Y., Shih C. M., Chiang K. H., et al. (2017). Association between renin–angiotensin–aldosterone system blockade and future osteoporotic fracture risk in hypertensive population: A population-based cohort study in taiwan. Medicine 96 (46), e8331. doi:10.1097/MD.0000000000008331
Chen L., Chen R., Wang H., Liang F. (2015). Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 508409. doi:10.1155/2015/508409
Chistiakov D. A., Orekhov A. N., Bobryshev Y. V. (2015). Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 214 (1), 33–50. doi:10.1111/apha.12466
Cobankara V., Öztürk M. A., Kiraz S., Ertenli I., Haznedaroglu I. C., Pay S., et al. (2005). Renin and angiotensin-converting enzyme (ACE) as active components of the local synovial renin-angiotensin system in rheumatoid arthritis. Rheumatol. Int. 25 (4), 285–291. doi:10.1007/s00296-004-0564-8
Commar A., Prasad V., Tursan d’Espaignet E. (2015). WHO global report on trends in prevalence of tobacco smoking 2015. Geneva: World Health Organization. Available at: https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition (Accessed August 15, 2022).
Conceição F., Sousa D. M., Paredes J., Lamghari M. (2021). Sympathetic activity in breast cancer and metastasis: Partners in crime. Bone Res. 9 (1), 9–11. doi:10.1038/s41413-021-00137-1
Cooke C. L. M., Davidge S. T. (2002). Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am. J. Physiol. Cell Physiol. 282 (2), C395–C402. doi:10.1152/ajpcell.00295.2001
Cooper I. D., Crofts C. A., DiNicolantonio J. J., Malhotra A., Elliott B., Kyriakidou Y., et al. (2020). Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: Rationale for clinical management. Open Heart 7 (2), e001356. doi:10.1136/openhrt-2020-001356
Davis S. E., Hendryx J., Bouwer S., Menezes C., Menezes H., Patel V., et al. (2019). Correlation between physiologic and osteopathic measures of sympathetic activity in women with polycystic ovary syndrome. J. Am. Osteopath. Assoc. 119 (1), 7–17. doi:10.7556/jaoa.2019.004
De Pergola G., Nitti A., Bartolomeo N., Gesuita A., Giagulli V. A., Triggiani V., et al. (2013). Possible role of hyperinsulinemia and insulin resistance in lower vitamin D levels in overweight and obese patients. Biomed. Res. Int. 2013, 921348. doi:10.1155/2013/921348
Deo S. H., Jenkins N. T., Padilla J., Parrish A. R., Fadel P. J. (2013). Norepinephrine increases NADPH oxidase-derived superoxide in human peripheral blood mononuclear cells via α-adrenergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305 (10), R1124–R1132. doi:10.1152/ajpregu.00347.2013
Dimitriadis K., Narkiewicz K., Leontsinis I., Konstantinidis D., Mihas C., Andrikou I., et al. (2022). Acute effects of electronic and tobacco cigarette smoking on sympathetic nerve activity and blood pressure in humans. Int. J. Environ. Res. Public Health 19 (6), 3237. doi:10.3390/ijerph19063237
Doonan R. J., Hausvater A., Scallan C., Mikhailidis D. P., Pilote L., Daskalopoulou S. S. (2010). The effect of smoking on arterial stiffness. Hypertens. Res. 33 (5), 398–410. doi:10.1038/hr.2010.25
Durazzo T. C., Korecka M., Trojanowski J. Q., Weiner M. W., O’Hara R., Ashford J. W., et al. (2016). Active cigarette smoking in cognitively-normal elders and probable Alzheimer’s disease is associated with elevated cerebrospinal fluid oxidative stress biomarkers. J. Alzheimers Dis. 54 (1), 99–107. doi:10.3233/JAD-160413
Eguchi N., Vaziri N. D., Dafoe D. C., Ichii H. (2021). The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int. J. Mol. Sci. 22 (4), 1509. doi:10.3390/ijms22041509
Einarson T. R., Acs A., Ludwig C., Panton U. H. (2018). Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 17 (1), 83–19. doi:10.1186/s12933-018-0728-6
Ekholm M., Kahan T. (2021). The impact of the renin-angiotensin-aldosterone system on inflammation, coagulation, and atherothrombotic complications, and to aggravated COVID-19. Front. Pharmacol. 12, 640185. doi:10.3389/fphar.2021.640185
El-Mahdy M. A., Ewees M. G., Eid M. S., Mahgoup E. M., Khaleel S. A., Zweier J. L. (2022). Electronic cigarette exposure causes vascular endothelial dysfunction due to NADPH oxidase activation and eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 322 (4), H549–H567. doi:10.1152/ajpheart.00460.2021
El-Zayat S. R., Sibaii H., Mannaa F. A. (2019). Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 43 (1), 187. doi:10.1186/s42269-019-0227-2
Eliasson B., Attvall S., Taskinen M. R., Smith U. (1994). The insulin resistance syndrome in smokers is related to smoking habits. Arterioscler. Thromb. 14 (12), 1946–1950. doi:10.1161/01.atv.14.12.1946
Eliasson B. R., Taskinen M. R., Smith U. (1996). Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation 94 (5), 878–881. doi:10.1161/01.CIR.94.5.878
Facchini F. S., Hollenbeck C. B., Jeppesen J., Chen Y. D. I., Reaven G. M. (1992). Insulin resistance and cigarette smoking. Lancet 339 (8802), 1128–1130. doi:10.1016/0140-6736(92)90730-q
Falk E. (2006). Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 47 (8), C7–C12. doi:10.1016/j.jacc.2005.09.068
Farhat G. N., Cauley J. A. (2008). The link between osteoporosis and cardiovascular disease. Clin. Cases Min. Bone Metab. 5 (1), 19–34.
Farr J. N., Charkoudian N., Barnes J. N., Monroe D. G., McCready L. K., Atkinson E. J., et al. (2012). Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J. Clin. Endocrinol. Metab. 97 (11), 4219–4227. doi:10.1210/jc.2012-2381
Fazakerley D. J., Minard A. Y., Krycer J. R., Thomas K. C., Stöckli J., Harney D. J., et al. (2018). Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 293 (19), 7315–7328. doi:10.1074/jbc.RA117.001254
Ferder M., Inserra F., Manucha W., Ferder L. (2013). The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am. J. Physiol. Cell Physiol. 304 (11), C1027–C1039. doi:10.1152/ajpcell.00403.2011
Fishel M. A., Watson G. S., Montine T. J., Wang Q., Green P. S., Kulstad J. J., et al. (2005). Hyperinsulinemia provokes synchronous increases in central inflammation and β-amyloid in normal adults. Arch. Neurol. 62 (10), 1539–1544. doi:10.1001/archneur.62.10.noc50112
Fitzgerald P. J. (2021). Norepinephrine may oppose other neuromodulators to impact Alzheimer’s disease. Int. J. Mol. Sci. 22 (14), 7364. doi:10.3390/ijms22147364
Forman H. J., Zhang H. (2021). Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20 (9), 689–709. doi:10.1038/s41573-021-00233-1
Forrester S. J., Kikuchi D. S., Hernandes M. S., Xu Q., Griendling K. K. (2018). Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122 (6), 877–902. doi:10.1161/CIRCRESAHA.117.311401
Frati A. C., Iniestra F., Ariza C. R. (1996). Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes care 19 (2), 112–118. doi:10.2337/diacare.19.2.112
Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K. B., et al. (2020). Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 21 (17), 6275. doi:10.3390/ijms21176275
Gallagher E. J., LeRoith D. (2020). Hyperinsulinaemia in cancer. Nat. Rev. Cancer 20 (11), 629–644. doi:10.1038/s41568-020-0295-5
Gao W., Xiong Y., Li Q., Yang H. (2017). Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to nano therapeutics. Front. Physiol. 8, 508. doi:10.3389/fphys.2017.00508
Ge X., Yu Q., Qi W., Shi X., Zhai Q. (2008). Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic. Res. 42 (6), 582–591. doi:10.1080/10715760802158448
Gebre A. K., Altaye B. M., Atey T. M., Tuem K. B., Berhe D. F. (2018). Targeting renin–angiotensin system against Alzheimer’s disease. Front. Pharmacol. 9, 440. doi:10.3389/fphar.2018.00440
Ginaldi L., Mengoli L. P., De Martinis M. (2009). “Osteoporosis, inflammation and ageing,” in Handbook on immunosenescence (Dordrecht: Springer), 1329–1352. doi:10.1186/1742-4933-2-14
Golbidi S., Edvinsson L., Laher I. (2020). Smoking and endothelial dysfunction. Curr. Vasc. Pharmacol. 18 (1), 1–11. doi:10.2174/1573403X14666180913120015
González F. (2012). Inflammation in polycystic ovary syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 77 (4), 300–305. doi:10.1016/j.steroids.2011.12.003
Gough D. R., Cotter T. G. (2011). Hydrogen peroxide: A jekyll and hyde signalling molecule. Cell Death Dis. 2 (10), e213. doi:10.1038/cddis.2011.96
Grassi G., West I. C., Wilkinson R., Thomas T. H. (2001). Renin–angiotensin–sympathetic crosstalks in hypertension: Reappraising the relevance of peripheral interactions. J. Hypertens. 19 (10), 485–493. doi:10.1097/00004872-200103000-00017
Greten F. R., Grivennikov S. I. (2019). Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 51 (1), 27–41. doi:10.1016/j.immuni.2019.06.025
Grootaert M. O., Bennett M. R. (2021). Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 117 (11), 2326–2339. doi:10.1093/cvr/cvab046
Gui X., Yang Z., Li M. D. (2021). Effect of cigarette smoke on gut microbiota: State of knowledge. Front. Physiol. 12, 673341. doi:10.3389/fphys.2021.673341
Gutowska K., Formanowicz D., Formanowicz P. (2019). Selected aspects of tobacco-induced prothrombotic state, inflammation and oxidative stress: Modeled and analyzed using petri nets. Interdiscip. Sci. 11 (3), 373–386. doi:10.1007/s12539-018-0310-7
Gyldenløve M., Storgaard H., Holst J. J., Vilsbøll T., Knop F. K., Skov L. (2015). Patients with psoriasis are insulin resistant. J. Am. Acad. Dermatol. 72 (4), 599–605. doi:10.1016/j.jaad.2015.01.004
Harrison D. G., Coffman T. M., Wilcox C. S. (2021). Pathophysiology of hypertension: The mosaic theory and beyond. Circ. Res. 128 (7), 847–863. doi:10.1161/CIRCRESAHA.121.318082
Hayes J. D., Dinkova-Kostova A. T., Tew K. D. (2020). Oxidative stress in cancer. Cancer cell 38 (2), 167–197. doi:10.1016/j.ccell.2020.06.001
He L., Du J., Chen Y., Liu C., Zhou M., Adhikari S., et al. (2019). Renin-angiotensin system promotes colonic inflammation by inducing TH17 activation via JAK2/STAT pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 316 (6), G774–G784. doi:10.1152/ajpgi.00053.2019
Hecht S. S. (2012). Lung carcinogenesis by tobacco smoke. Int. J. Cancer 131 (12), 2724–2732. doi:10.1002/ijc.27816
Heindl S., Lehnert M., Criee C. P., Hasenfuss G., Andreas S. (2001). Marked sympathetic activation in patients with chronic respiratory failure. Am. J. Respir. Crit. Care Med. 164 (4), 597–601. doi:10.1164/ajrccm.164.4.2007085
Hikichi M., Mizumura K., Maruoka S., Gon Y. (2019). Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 11 (17), S2129–S2140. doi:10.21037/jtd.2019.10.43
Hovland A., Jonasson L., Garred P., Yndestad A., Aukrust P., Lappegård K. T., et al. (2015). The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis 241 (2), 480–494. doi:10.1016/j.atherosclerosis.2015.05.038
Hu S. C. S., Cheng-Che E., (2017). Psoriasis and cardiovascular comorbidities: Focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int. J. Mol. Sci. 18 (10), 2211. doi:10.3390/ijms18102211
Hunter D. J., Reddy K. S. (2013). Noncommunicable diseases. N. Engl. J. Med. 369 (14), 1336–1343. doi:10.1056/NEJMra1109345
Inoue-Choi M., Hartge P., Liao L. M., Caporaso N., Freedman N. D. (2018). Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the national institutes of health-AARP cohort. Int. J. Cancer 142 (2), 271–280. doi:10.1002/ijc.31059
Ishikawa Y., Terao C. (2020). The impact of cigarette smoking on risk of rheumatoid arthritis: A narrative review. Cells 9 (2), 475. doi:10.3390/cells9020475
Jaimes E. A., Galceran J. M., Raij L. (1998). Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 54 (3), 775–784. doi:10.1046/j.1523-1755.1998.00068.x
Jansen E., Beekhof P., Ruskovska T. (2014). The effect of smoking on biomarkers of (anti) oxidant status. J. mol. biomark. diagn. 5 (6), 1–4. doi:10.4172/2155-9929.1000207
Janssens W., Mathieu C., Boonen S., Decramer M. (2011). Vitamin D deficiency and chronic obstructive pulmonary disease: A vicious circle. Vitam. Horm. 86, 379–399. doi:10.1016/B978-0-12-386960-9.00017-4
Jialal I., Kaur H., Devaraj S. (2014). Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 99 (1), 39–48. doi:10.1210/jc.2013-3092
Jiang C. Q., Chan Y. H., Xu L., Jin Y. L., Zhu T., Zhang W. S., et al. (2016). Smoking and serum vitamin D in older Chinese People: Cross-sectional analysis based on the guangzhou biobank cohort study. BMJ open 6 (6), e010946. doi:10.1136/bmjopen-2015-010946
Kalyan S., Patel M. S., Kingwell E., Côté H. C., Liu D., Prior J. C. (2017). Competing factors link to bone health in polycystic ovary syndrome: Chronic low-grade inflammation takes a toll. Sci. Rep. 7 (1), 3432–3438. doi:10.1038/s41598-017-03685-x
Kamceva G., Arsova-Sarafinovska Z., Ruskovska T., Zdravkovska M., Kamceva-Panova L., Stikova E. (2016). Cigarette smoking and oxidative stress in patients with coronary artery disease. Open Access Maced. J. Med. Sci. 4 (4), 636–640. doi:10.3889/oamjms.2016.117
Karakas M., Haase T., Zeller T. (2018). Linking the sympathetic nervous system to the inflammasome: Towards new therapeutics for atherosclerotic cardiovascular disease. Eur. Heart J. 39 (1), 70–72. doi:10.1093/eurheartj/ehx374
Kawai T., Akira S. (2007). Signaling to NF-kappaB by toll-like receptors. Trends Mol. Med. 13 (11), 460–469. doi:10.1016/j.molmed.2007.09.002
Kellar D., Craft S. (2020). Brain insulin resistance in Alzheimer's disease and related disorders: Mechanisms and therapeutic approaches. Lancet. Neurol. 19 (9), 758–766. doi:10.1016/S1474-4422(20)30231-3
Khani Y., Pourgholam-Amiji N., Afshar M., Otroshi O., Sharifi-Esfahani M., Sadeghi-Gandomani H., et al. (2018). Tobacco smoking and cancer types: A review. Biomed. Res. Ther. 5 (4), 2142–2159. doi:10.15419/bmrat.v5i4.428
Khoury N., McGill J. B. (2011). Reduction in insulin sensitivity following administration of the clinically used low-dose pressor, norepinephrine. Diabetes. Metab. Res. Rev. 27 (6), 604–608. doi:10.1002/dmrr.1212
Kianoush S., Yakoob M. Y., Al-Rifai M., DeFilippis A. P., Bittencourt M. S., Duncan B. B., et al. (2017). Associations of cigarette smoking with subclinical inflammation and atherosclerosis: ELSA-brasil (the Brazilian longitudinal study of adult health). J. Am. Heart Assoc. 6 (6), e005088. doi:10.1161/JAHA.116.005088
Kim B. J., Kwak M. K., Ahn S. H., Kim H., Lee S. H., Song K. H., et al. (2017). Lower bone mass and higher bone resorption in pheochromocytoma: Importance of sympathetic activity on human bone. J. Clin. Endocrinol. Metab. 102 (8), 2711–2718. doi:10.1210/jc.2017-00169
Kim M., Han C. H., Lee M. Y. (2014). NADPH oxidase and the cardiovascular toxicity associated with smoking. Toxicol. Res. 30 (3), 149–157. doi:10.5487/TR.2014.30.3.149
Kim M., Park H. E., Lee S. H., Han K., Lee J. H. (2020). Increased risk of Alzheimer’s disease in patients with psoriasis: A nationwide population-based cohort study. Sci. Rep. 10 (1), 6454–6457. doi:10.1038/s41598-020-63550-2
Kishi T., Hirooka Y. (2013). Sympathoexcitation associated with Renin-Angiotensin system in metabolic syndrome. Int. J. Hypertens. 2013, 406897. doi:10.1155/2013/406897
Kiyota Y., Muramatsu H., Sato Y., Kobayashi T., Miyamoto K., Iwamoto T., et al. (2020). Smoking cessation increases levels of osteocalcin and uncarboxylated osteocalcin in human sera. Sci. Rep. 10 (1), 16845–16849. doi:10.1038/s41598-020-73789-4
Kohler J. B., Junqueira J. J. M., da Silva T. C. M., Filho M. A. G. P., Iolanda De Fátima L. C., Lopes F. D. T., et al. (2021). Smoking-induced oxidative stress in bone: The effects on bone turnover. J. Orthop. Orthop. Surg. 2 (2), 14–23. doi:10.29245/2767-5130/2021/2.1138
Kopp W. (2019). How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 12, 2221–2236. doi:10.2147/DMSO.S216791
Kuntic M., Oelze M., Steven S., Kröller-Schön S., Stamm P., Kalinovic S., et al. (2020). Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: Evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur. Heart J. 41 (26), 2472–2483. doi:10.1093/eurheartj/ehz772
Lan K., Zhang G., Liu L., Guo Z., Luo X., Guan H., et al. (2020). Electronic cigarette exposure on insulin sensitivity of ApoE gene knockout mice. Tob. Induc. Dis. 18, 68. doi:10.18332/tid/125399
Larsson S. C., Burgess S. (2022). Appraising the causal role of smoking in multiple diseases: A systematic review and meta-analysis of mendelian randomization studies. EBioMedicine 82, 104154. doi:10.1016/j.ebiom.2022.104154
Lazaridis A., Gavriilaki E., Douma S., Gkaliagkousi E. (2021). Toll-like receptors in the pathogenesis of essential hypertension. A forthcoming immune-driven theory in full effect. Int. J. Mol. Sci. 22 (7), 3451. doi:10.3390/ijms22073451
Lelieveld J., Pozzer A., Pöschl U., Fnais M., Haines A., Münzel T. (2020). Loss of life expectancy from air pollution compared to other risk factors: A worldwide perspective. Cardiovasc. Res. 116 (11), 1910–1917. doi:10.1093/cvr/cvaa025
Lembo G., Capaldo B., Rendina V., Iaccarino G., Napoli R., Guida R., et al. (1994). Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am. J. Physiol. 266 (2), E242–E247. doi:10.1152/ajpendo.1994.266.2.E242
Li H., Liu S., Han J., Li S., Gao X., Wang M., et al. (2021). Role of toll-like receptors in neuroimmune diseases: Therapeutic targets and problems. Front. Immunol. 12, 777606. doi:10.3389/fimmu.2021.777606
Li W., Chen Y., Xu L. (2014). Association of sympathetic nervous system activity with polycystic ovarian syndrome. Clin. Exp. Obstet. Gynecol. 41 (5), 499–506. doi:10.12891/ceog16592014
Li Z., Xu W., Su Y., Gao K., Chen Y., Ma L., et al. (2019). Nicotine induces insulin resistance via downregulation of Nrf2 in cardiomyocyte. Mol. Cell. Endocrinol. 495, 110507. doi:10.1016/j.mce.2019.110507
Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., et al. (2018). Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 757–772. doi:10.2147/CIA.S158513
Liu T., Zhang L., Joo D., Sun S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2 (1), 17023–17029. doi:10.1038/sigtrans.2017.23
Liu Y., Li H., Wang J., Xue Q., Yang X., Kang Y., et al. (2020). Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw. Open 3 (10), e2018777. doi:10.1001/jamanetworkopen.2020.18777
Lu Y., Li X., Liu S., Zhang Y., Zhang D. (2018). Toll-like receptors and inflammatory bowel disease. Front. Immunol. 9, 72. doi:10.3389/fimmu.2018.00072
Luceri C., Bigagli E., Agostiniani S., Giudici F., Zambonin D., Scaringi S., et al. (2019). Analysis of oxidative stress-related markers in Crohn’s disease patients at surgery and correlations with clinical findings. Antioxidants 8 (9), 378. doi:10.3390/antiox8090378
Madsbad S., McNair P., Christensen M. S., Christiansen C., Faber O. K., Binder C., et al. (1980). Influence of smoking on insulin requirement and metbolic status in diabetes mellitus. Diabetes care 3 (1), 41–43. doi:10.2337/diacare.3.1.41
Mancusi C., Izzo R., di Gioia G., Losi M. A., Barbato E., Morisco C. (2020). Insulin resistance the hinge between hypertension and type 2 diabetes. High. Blood Press. Cardiovasc. Prev. 27 (6), 515–526. doi:10.1007/s40292-020-00408-8
Manrique C., Lastra G., Gardner M., Sowers J. R. (2009). The renin angiotensin aldosterone system in hypertension: Roles of insulin resistance and oxidative stress. Med. Clin. North Am. 93 (3), 569–582. doi:10.1016/j.mcna.2009.02.014
Martinez J. E., Kahana D. D., Ghuman S., Wilson H. P., Wilson J., Kim S. C., et al. (2021). Unhealthy lifestyle and gut dysbiosis: A better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front. Endocrinol. 12, 667066. doi:10.3389/fendo.2021.667066
Mateen S., Moin S., Khan A. Q., Zafar A., Fatima N. (2016). Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PloS one 11 (4), e0152925. doi:10.1371/journal.pone.0152925
Mei D., Tan W. D., Liao W., Heng C. M., Wong W. F. (2020). Activation of angiotensin II type-2 receptor protects against cigarette smoke-induced COPD. Pharmacol. Res. 161, 105223. doi:10.1016/j.phrs.2020.105223
Middlekauff H. R., Park J., Moheimani R. S. (2014). Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: Mechanisms and implications for cardiovascular risk. J. Am. Coll. Cardiol. 64 (16), 1740–1750. doi:10.1016/j.jacc.2014.06.1201
Milazzo G., Giorgino F., Damante G., Sung C., Stampfer M. R., Vigneri R., et al. (1992). Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 52 (14), 3924–3930.
Mills K. T., Stefanescu A., He J. (2020). The global epidemiology of hypertension. Nat. Rev. Nephrol. 16 (4), 223–237. doi:10.1038/s41581-019-0244-2
Miró O., Alonso J. R., Jarreta D., Casademont J., Urbano-Márquez A., Cardellach F., et al. (1999). Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis 20 (7), 1331–1336. doi:10.1093/carcin/20.7.1331
Misrani A., Tabassum S., Yang L. (2021). Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front. Aging Neurosci. 13, 617588. doi:10.3389/fnagi.2021.617588
Mo C., Ke J., Zhao D., Zhang B. (2020). Role of the renin–angiotensin–aldosterone system in bone metabolism. J. Bone Min. Metab. 38, 772–779. doi:10.1007/s00774-020-01132-y
Mokhtari Y., Pourbagheri-Sigaroodi A., Zafari P., Bagheri N., Ghaffari S. H., Bashash D. (2021). Toll-like receptors (TLRs): An old family of immune receptors with a new face in cancer pathogenesis. J. Cell. Mol. Med. 25 (2), 639–651. doi:10.1111/jcmm.16214
Momtazmanesh S., Perry G., Rezaei N. (2020). Toll-like receptors in Alzheimer's disease. J. Neuroimmunol. 348, 577362. doi:10.1016/j.jneuroim.2020.577362
Moreira F. R. C., de Oliveira T. A., Ramos N. E., Abreu M. A. D., Simões e Silva A. C. (2021). The role of renin angiotensin system in the pathophysiology of rheumatoid arthritis. Mol. Biol. Rep. 48 (9), 6619–6629. doi:10.1007/s11033-021-06672-8
Mori J., Alrob O. A., Wagg C. S., Harris R. A., Lopaschuk G. D., Oudit G. Y. (2013). ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: A critical role of PDK4. Am. J. Physiol. Heart Circ. Physiol. 304 (8), H1103–H1113. doi:10.1152/ajpheart.00636.2012
Mravec B., Horvathova L., Hunakova L. (2020). Neurobiology of cancer: The role of β-adrenergic receptor signaling in various tumor environments. Int. J. Mol. Sci. 21 (21), 7958. doi:10.3390/ijms21217958
Naldi L. (2016). Psoriasis and smoking: Links and risks. PsoriasisAuckl. NZ) 6, 65–71. doi:10.2147/PTT.S85189
Nance D. M., Sanders V. M. (2007). Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 21 (6), 736–745. doi:10.1016/j.bbi.2007.03.008
Napolitano M., Megna M., Monfrecola G. (2015). Insulin resistance and skin diseases. ScientificWorldJournal. 2015, 479354. doi:10.1155/2015/479354
Nehme A., Zouein F. A., Deris Zayeri Z., Zibara K. (2019). An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 6 (2), 14. doi:10.3390/jcdd6020014
Nolan C. J., Prentki M. (2019). Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 16 (2), 118–127. doi:10.1177/1479164119827611
Nooshinfar E., Bashash D., Abbasalizadeh M., Safaroghli-Azar A., Sadreazami P., Akbari M. E. (2017). The molecular mechanisms of tobacco in cancer pathogenesis. Iran. J. Cancer Prev. 10 (1), e7902. doi:10.17795/ijcp-7902
Oakes J. M., Fuchs R. M., Gardner J. D., Lazartigues E., Yue X. (2018). Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315 (5), R895–R906. doi:10.1152/ajpregu.00099.2018
Ohl K., Tenbrock K., Kipp M. (2016). Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 277, 58–67. doi:10.1016/j.expneurol.2015.11.010
Oliveira-Sales E. B., Dugaich A. P., Carillo B. A., Abreu N. P., Boim M. A., Martins P. J., et al. (2008). Oxidative stress contributes to renovascular hypertension. Am. J. Hypertens. 21, 98–104. doi:10.1038/ajh.2007.12
Oparil S., Acelajado M. C., Bakris G. L., Berlowitz D. R., Cífková R., Dominiczak A. F., et al. (2018). Hypertension. Nat. Rev. Dis. Prim. 4, 18014. doi:10.1038/nrdp.2018.14
Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F. A. (2018). Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17 (1), 122. doi:10.1186/s12933-018-0762-4
Ortega I., Villanueva J. A., Wong D. H., Cress A. B., Sokalska A., Stanley S. D., et al. (2014). Resveratrol potentiates effects of simvastatin on inhibition of rat ovarian theca-interstitial cells steroidogenesis. J. Ovarian Res. 7 (1), 21–28. doi:10.1186/1757-2215-7-21
Pacurari M., Kafoury R., Tchounwou P. B., Ndebele K. (2014). The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflam. 2014, 689360. doi:10.1155/2014/689360
Padmavathi P., Raghu P. S., ReddyBulle S., Marthadu S. B., Maturu P., et al. (2018). Chronic cigarette smoking-induced oxidative/nitrosative stress in human erythrocytes and platelets. Mol. Cell. Toxicol. 14 (1), 27–34. doi:10.1007/s13273-018-0004-6
Padro C. J., Sanders V. M. (2014). Neuroendocrine regulation of inflammation. Semin. Immunol. 26 (5), 357–368. doi:10.1016/j.smim.2014.01.003
Papoutsopoulou S., Satsangi J., Campbell B. J., Probert C. S. (2020). Review article: Impact of cigarette smoking on intestinal inflammation - direct and indirect mechanisms. Aliment. Pharmacol. Ther. 51 (12), 1268–1285. doi:10.1111/apt.15774
Park K. H., Park W. J. (2015). Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J. Korean Med. Sci. 30 (9), 1213–1225. doi:10.3346/jkms.2015.30.9.1213
Patel S., Rauf A., Khan H., Abu-Izneid T. (2017). Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 94, 317–325. doi:10.1016/j.biopha.2017.07.091
Pedersen D. J., Guilherme A., Danai L. V., Heyda L., Matevossian A., Cohen J., et al. (2015). A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol. Metab. 4 (7), 507–518. doi:10.1016/j.molmet.2015.04.003
Peluffo G., Calcerrada P., Piacenza L., Pizzano N., Radi R. (2009). Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: Studies in cultured cells and smokers. Am. J. Physiol. Heart Circ. Physiol. 296 (6), H1781–H1792. doi:10.1152/ajpheart.00930.2008
Penesova A., Vlcek M., Imrich R., Vernerova L., Marko A., Meskova M., et al. (2015). Hyperinsulinemia in newly diagnosed patients with multiple sclerosis. Metab. Brain Dis. 30 (4), 895–901. doi:10.1007/s11011-015-9665-1
Perlstein T. S., Gerhard-Herman M., Hollenberg N. K., Williams G. H., Thomas A. (2007). Insulin induces renal vasodilation, increases plasma renin activity, and sensitizes the renal vasculature to angiotensin receptor blockade in healthy subjects. J. Am. Soc. Nephrol. 18 (3), 944–951. doi:10.1681/ASN.2006091026
Petsophonsakul P., Burgmaier M., Willems B., Heeneman S., Stadler N., Gremse F., et al. (2021). Nicotine promotes vascular calcification via intracellular Ca2+-mediated, Nox5-induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells. Cardiovasc. Res. 118, 2196–2210. cvab244. doi:10.1093/cvr/cvab244
Pietschmann P., Mechtcheriakova D., Meshcheryakova A., Föger-Samwald U., Ellinger I. (2016). Immunology of osteoporosis: A mini-review. Gerontology 62 (2), 128–137. doi:10.1159/000431091
Pinter M., Jain R. K. (2017). Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 9 (410), eaan5616. doi:10.1126/scitranslmed.aan5616
Pleńkowska J., Gabig-Cimińska M., Mozolewski P. (2020). Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int. J. Mol. Sci. 21 (17), 6206. doi:10.3390/ijms21176206
Poznyak A. V., Bharadwaj D., Prasad G., Grechko A. V., Sazonova M. A., Orekhov A. N. (2021). Renin-angiotensin system in pathogenesis of atherosclerosis and treatment of CVD. Int. J. Mol. Sci. 22 (13), 6702. doi:10.3390/ijms22136702
Poznyak A. V., Grechko A. V., Orekhova V. A., Khotina V., Ivanova E. A., Orekhov A. N. (2020). NADPH oxidases and their role in atherosclerosis. Biomedicines 8 (7), 206. doi:10.3390/biomedicines8070206
Qiu F., Liang C. L., Liu H., Zeng Y. Q., Hou S., Huang S., et al. (2017). Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 8 (1), 268–284. doi:10.18632/oncotarget.13613
Ramalingam L., Menikdiwela K., LeMieux M., Dufour J. M., Kaur G., Kalupahana N., et al. (2017). The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Biophys. Acta. Mol. Basis Dis. 1863 (5), 1106–1114. doi:10.1016/j.bbadis.2016.07.019
Rasha F., Ramalingam L., Gollahon L., Rahman R. L., Rahman S. M., Menikdiwela K., et al. (2019). Mechanisms linking the renin-angiotensin system, obesity, and breast cancer. Endocr. Relat. Cancer 26 (12), R653–R672. doi:10.1530/ERC-19-0314
Reaven G., Tsao P. S. (2003). Insulin resistance and compensatory hyperinsulinemia: The key player between cigarette smoking and cardiovascular disease? J. Am. Coll. Cardiol. 41 (6), 1044–1047. doi:10.1016/s0735-1097(02)02982-0
Roehm B., Simoni J., Pruszynski J., Wesson D. E. (2017). Cigarette smoking attenuates kidney protection by angiotensin-converting enzyme inhibition in nondiabetic chronic kidney disease. Am. J. Nephrol. 46 (4), 260–267. doi:10.1159/000481206
Rooney D. P., Edgar J. D. M., Sheridan B., Atkinson A. B., Bell P. M. (1991). The effects of low dose insulin infusions on the renin angiotensin and sympathetic nervous systems in normal man. Eur. J. Clin. Invest. 21 (4), 430–435. doi:10.1111/j.1365-2362.1991.tb01391.x
Rosso M., Chitnis T. (2020). Association between cigarette smoking and multiple sclerosis: A review. JAMA Neurol. 77 (2), 245–253. doi:10.1001/jamaneurol.2019.4271
Rostamtabar M., Esmaeilzadeh S., Tourani M., Rahmani A., Baee M., Shirafkan F., et al. (2021). Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J. Cell. Physiol. 236 (2), 824–838. doi:10.1002/jcp.29912
Rovina N., Koutsoukou A., Koulouris N. G. (2013). Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 413735. doi:10.1155/2013/413735
Rowe J. W., Young J. B., Minaker K. L., Stevens A. L., Pallotta J., Landsberg L. (1981). Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes 30 (3), 219–225. doi:10.2337/diab.30.3.219
Roy A., Rawal I., Jabbour S., Prabhakaran D. (2017). Tobacco and cardiovascular disease: A summary of evidence. Cardiovasc. Respir. Relat. Disord. 1, 1. doi:10.1596/978-1-4648-0518-9_ch4
Ruan W., Yan C., Zhu H., Wang S., Jia X., Shao L., et al. (2019). Downregulated level of insulin in COPD patients during AE; role beyond glucose control? Int. J. Chron. Obstruct. Pulmon. Dis. 14, 1559–1566. doi:10.2147/COPD.S197164
Rufino A. T., Ribeiro M., Pinto Ferreira J., Judas F., Mendes A. F. (2017). Hyperglycemia and hyperinsulinemia-like conditions independently induce inflammatory responses in human chondrocytes. J. Funct. Morphol. Kinesiol. 2 (2), 15. doi:10.3390/jfmk2020015
Ruiz-Argüelles A., Méndez-Huerta M. A., Lozano C. D., Ruiz-Argüelles G. J. (2018). Metabolomic profile of insulin resistance in patients with multiple sclerosis is associated to the severity of the disease. Mult. Scler. Relat. Disord. 25, 316–321. doi:10.1016/j.msard.2018.08.014
Rusanen M., Kivipelto M., Quesenberry C. P., Zhou J., Whitmer R. A. (2011). Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch. Intern. Med. 171 (4), 333–339. doi:10.1001/archinternmed.2010.393
Saadeh M., Ferrante T. C., Kane A., Shirihai O., Corkey B. E., Deeney J. T. (2012). Reactive oxygen species stimulate insulin secretion in rat pancreatic islets: Studies using mono-oleoyl-glycerol. PloS one 7 (1), e30200. doi:10.1371/journal.pone.0030200
Sagun G., Gedik C., Ekiz E., Karagoz E., Takir M., Oguz A. (2015). The relation between insulin resistance and lung function: A cross sectional study. BMC Pulm. Med. 15 (1), 139. doi:10.1186/s12890-015-0125-9
Salvador B., Arranz A., FranciscoCordoba L., Punzon C., Llamas M. Á., et al. (2016). Modulation of endothelial function by Toll like receptors. Pharmacol. Res. 108, 46–56. doi:10.1016/j.phrs.2016.03.038
Samadian F., Dalili N., Jamalian A. (2016). Lifestyle modifications to prevent and control hypertension. Iran. J. Kidney Dis. 10 (5), 237–263.
Sanchez-Garrido M. A., Tena-Sempere M. (2020). Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 35, 100937. doi:10.1016/j.molmet.2020.01.001
Santos R. A. S., Sampaio W. O., Alzamora A. C., Motta-Santos D., Alenina N., Bader M., et al. (2018). The ACE2/Angiotensin-(1-7)/Mas axis of the renin-angiotensin system: Focus on Angiotensin-(1-7). Physiol. Rev. 98, 505–553. doi:10.1152/physrev.00023.2016
Sarkar M., Bhardwaj R., Madabhavi I., Khatana J. (2015). Osteoporosis in chronic obstructive pulmonary disease. Clin. Med. Insights. Circ. Respir. Pulm. Med. 9, 5–21. doi:10.4137/CCRPM.S22803
Saxena P. R. (1992). Interaction between the renin-angiotensin-aldosterone and sympathetic nervous systems. J. Cardiovasc. Pharmacol. 19, S80–S88. doi:10.1097/00005344-199219006-00013
Semlali A., Witoled C., Alanazi M., Rouabhia M. (2012). Whole cigarette smoke increased the expression of TLRs, HBDs, and proinflammory cytokines by human gingival epithelial cells through different signaling pathways. PloS one 7 (12), e52614. doi:10.1371/journal.pone.0052614
Shi Y., Liu T., He L., Dougherty U., Chen L., Adhikari S., et al. (2016). Activation of the renin-angiotensin system promotes colitis development. Sci. Rep. 6 (1), 27552. doi:10.1038/srep27552
Shields H. J., Traa A., Van Raamsdonk J. M. (2021). Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 9, 628157. doi:10.3389/fcell.2021.628157
Sidletskaya K., Vitkina T., Denisenko Y. (2020). The role of toll-like receptors 2 and 4 in the pathogenesis of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 1481–1493. doi:10.2147/COPD.S249131
Silva I. M. S., Assersen K. B., Willadsen N. N., Jepsen J., Artuc M., Steckelings U. M. (2020). The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp. Dermatol. 29 (9), 891–901. doi:10.1111/exd.14159
Singh B. M., Mehta J. L. (2003). Interactions between the renin-angiotensin system and dyslipidemia: Relevance in the therapy of hypertension and coronary heart disease. Arch. Intern. Med. 163 (11), 1296–1304. doi:10.1001/archinte.163.11.1296
Singh S., Bodas M., Bhatraju N. K., Pattnaik B., Gheware A., Parameswaran P. K., et al. (2016). Hyperinsulinemia adversely affects lung structure and function. Am. J. Physiol. Lung Cell. Mol. Physiol. 310 (9), L837–L845. doi:10.1152/ajplung.00091.2015
Śliwińska-Mossoń M., Milnerowicz H. (2017). The impact of smoking on the development of diabetes and its complications. Diab. Vasc. Dis. Res. 14 (4), 265–276. doi:10.1177/1479164117701876
Stampfer M. J. (2006). Cardiovascular disease and Alzheimer's disease: Common links. J. Intern. Med. 260 (3), 211–223. doi:10.1111/j.1365-2796.2006.01687.x
Stegbauer J., Lee D. H., Seubert S., Ellrichmann G., Manzel A., Kvakan H., et al. (2009). Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 106 (35), 14942–14947. doi:10.1073/pnas.0903602106
Sun L., Liu W., Zhang L. J. (2019). The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J. Immunol. Res. 2019, 1824624. doi:10.1155/2019/1824624
Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Sverrisdottir Y. B., Mogren T., Kataoka J., Janson P. O., Stener-Victorin E. (2008). Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am. J. Physiol. Endocrinol. Metab. 294 (3), E576–E581. doi:10.1152/ajpendo.00725.2007
Takeshita J., Grewal S., Langan S. M., Mehta N. N., Ogdie A., Van Voorhees A. S., et al. (2017). Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 76 (3), 377–390. doi:10.1016/j.jaad.2016.07.064
Talukder M. H., Johnson W. M., Varadharaj S., Lian J., Kearns P. N., El-Mahdy M. A., et al. (2011). Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 300 (1), H388–H396. doi:10.1152/ajpheart.00868.2010
Tan W. S. D., Liao W., Zhou S., Mei D., Wong W. S. F. (2018). Targeting the renin–angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 40, 9–17. doi:10.1016/j.coph.2017.12.002
Terenzi R., Manetti M., Rosa I., Romano E., Galluccio F., Guiducci S., et al. (2017). Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci. Rep. 7 (1), 13293. doi:10.1038/s41598-017-13746-w
Torres J., Mehandru S., Colombel J. F., Peyrin-Biroulet L. (2017). Crohn's disease. Lancet 389 (10080), 1741–1755. doi:10.1016/S0140-6736(16)31711-1
Touyz R. M., Rios F. J., Alves-Lopes R., Neves K. B., Camargo L. L., Montezano A. C. (2020). Oxidative stress: A unifying paradigm in hypertension. Can. J. Cardiol. 36 (5), 659–670. doi:10.1016/j.cjca.2020.02.081
Tripolino C., Ciaffi J., Pucino V., Ruscitti P., van Leeuwen N., Borghi C., et al. (2021). Insulin signaling in arthritis. Front. Immunol. 12, 672519. doi:10.3389/fimmu.2021.672519
Tsioufis C., Kordalis A., Flessas D., Anastasopoulos I., Tsiachris D., Papademetriou V., et al. (2011). Pathophysiology of resistant hypertension: The role of sympathetic nervous system. Int. J. Hypertens. 2011, 642416. doi:10.4061/2011/642416
Urner S., Ho F., Jha J. C., Ziegler D., Jandeleit-Dahm K. (2020). NADPH oxidase inhibition: Preclinical and clinical studies in diabetic complications. Antioxid. Redox Signal. 33 (6), 415–434. doi:10.1089/ars.2020.8047
Vaidya F. U., Chhipa A. S., Sagar N., Pathak C. (2020). Oxidative stress and inflammation can fuel cancer. Role Oxidative Stress Pathophysiol. Dis. 1, 229–258. doi:10.1007/978-981-15-1568-2_14
Valacchi G., Virgili F., Cervellati C., Pecorelli A. (2018). OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 9, 858. doi:10.3389/fphys.2018.00858
Valderas J. M., Starfield B., Sibbald B., Salisbury C., Roland M. (2009). Defining comorbidity: Implications for understanding health and health services. Ann. Fam. Med. 7 (4), 357–363. doi:10.1370/afm.983
Verdile G., Keane K. N., Cruzat V. F., Medic S., Sabale M., Rowles J., et al. (2015). Inflammation and oxidative stress: The molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediat. Inflamm. 2015, 105828. doi:10.1155/2015/105828
Wang H., Chen W., Li D., Yin X., Zhang X., Olsen N., et al. (2017). Vitamin D and chronic diseases. Aging Dis. 8 (3), 346–353. doi:10.14336/AD.2016.1021
Wang T., Han S. X., Zhang S. F., Ning Y. Y., Chen L., Chen Y. J., et al. (2010). Role of chymase in cigarette smoke-induced pulmonary artery remodeling and pulmonary hypertension in hamsters. Respir. Res. 11 (1), 36–10. doi:10.1186/1465-9921-11-36
Wu H., Ballantyne C. M. (2020). Metabolic inflammation and insulin resistance in obesity. Circ. Res. 126 (11), 1549–1564. doi:10.1161/CIRCRESAHA.119.315896
Xiao C., Wu M., Liu J., Gu J., Jiao X., Lu D., et al. (2019). Acute tobacco smoke exposure exacerbates the inflammatory response to corneal wounds in mice via the sympathetic nervous system. Commun. Biol. 2 (1), 33–18. doi:10.1038/s42003-018-0270-9
Xue J., Yang S., Seng S. (2014). Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers 6 (2), 1138–1156. doi:10.3390/cancers6021138
Yamaguchi Y., Nasu F., Harada A., Kunitomo M. (2007). Oxidants in the gas phase of cigarette smoke pass through the lung alveolar wall and raise systemic oxidative stress. J. Pharmacol. Sci. 103 (3), 275–282. doi:10.1254/jphs.fp0061055
Yang S. R., Chida A. S., Bauter M. R., Shafiq N., Seweryniak K., Maggirwar S. B., et al. (2006). Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 291 (1), L46–L57. doi:10.1152/ajplung.00241.2005
Ye S., Zhong H., Yanamadala S., Campese V. M. (2006). Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 48, 309–315. doi:10.1161/01.HYP.0000231307.69761.2e
Yee L. D., Mortimer J. E., Natarajan R., Dietze E. C., Seewaldt V. L. (2020). Metabolic health, insulin, and breast cancer: Why oncologists should care about insulin. Front. Endocrinol. 11, 58. doi:10.3389/fendo.2020.00058
Yoon S., Eom G. H., Kang G. (2021). Nitrosative stress and human disease: Therapeutic potential of denitrosylation. Int. J. Mol. Sci. 22 (18), 9794. doi:10.3390/ijms22189794
Yoon V., Maalouf N. M., Sakhaee K. (2012). The effects of smoking on bone metabolism. Osteoporos. Int. 23 (8), 2081–2092. doi:10.1007/s00198-012-1940-y
Yoshikawa H., Hellström-Lindahl E., Grill V. (2005). Evidence for functional nicotinic receptors on pancreatic β cells. Metabolism. 54 (2), 247–254. doi:10.1016/j.metabol.2004.08.020
Yuan Y. M., Luo L., Guo Z., Yang M., Ye R. S., Luo C. (2015). Activation of renin–angiotensin–aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J. Renin. Angiotensin. Aldosterone. Syst. 16 (2), 249–253. doi:10.1177/1470320315576256
Zhou H., Wu R., Kong Y., Zhao M., Su Y. (2020). Impact of smoking on psoriasis risk and treatment efficacy: A meta-analysis. J. Int. Med. Res. 48 (10), 1. doi:10.1177/0300060520964024
Zhou M. S., Schulman I. H., Zeng Q. (2012). Link between the renin–angiotensin system and insulin resistance: Implications for cardiovascular disease. Vasc. Med. 17 (5), 330–341. doi:10.1177/1358863X12450094
Zhu S., Häussling V., Aspera-Werz R. H., Chen T., Braun B., Weng W., et al. (2020). Bisphosphonates reduce smoking-induced osteoporotic-like alterations by regulating RANKL/OPG in an osteoblast and osteoclast co-culture model. Int. J. Mol. Sci. 22 (1), 53. doi:10.3390/ijms22010053
Keywords: epigenome and exposome, comorbidities, oxidative stress, hyperinsulinemia and insulin resistance, subclinical inflammation, microbiome, psoriasis, multiple sclerosis
Citation: Kopp W (2022) Pathogenesis of (smoking-related) non-communicable diseases—Evidence for a common underlying pathophysiological pattern. Front. Physiol. 13:1037750. doi: 10.3389/fphys.2022.1037750
Received: 06 September 2022; Accepted: 05 December 2022;
Published: 15 December 2022.
Edited by:
Fernanda Priviero, University of South Carolina, United StatesReviewed by:
Thomas Muenzel, University Medical Centre, Johannes Gutenberg University Mainz, GermanyFabiola Zakia Mónica, State University of Campinas, Brazil
Copyright © 2022 Kopp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolfgang Kopp, dy5rb3BwQHdlaXouY2M=
 Wolfgang Kopp
Wolfgang Kopp