
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 08 November 2022
Sec. Exercise Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.1030568
This article is part of the Research TopicExercise intervention for Prevention, Management of and Rehabilitation from COVID-19View all 10 articles
 Nuttawut Sittichai1
Nuttawut Sittichai1 Nichapa Parasin2
Nichapa Parasin2 Surasak Saokaew3,4,5
Surasak Saokaew3,4,5 Sukrit Kanchanasurakit3,4,6
Sukrit Kanchanasurakit3,4,6 Nuttawan Kayod7
Nuttawan Kayod7 Ketnapa Praikaew7
Ketnapa Praikaew7 Pochamana Phisalprapa8*
Pochamana Phisalprapa8* Mujalin Prasannarong7*
Mujalin Prasannarong7*Purpose: This systematic review and meta-analysis investigated the association between Physical activity (PA) before Coronavirus Disease 2019 (COVID-19) infection and the severity of illness and mortality in COVID-19 patients.
Methods: A comprehensive search was undertaken to identify retrospective and nonrandomized controlled trial studies comparing the severity and mortality of COVID-19 infection among COVID-19 patients who had previously reported their participation in PA with those who had not. The databases searched were PubMed, Cochrane Library, Scopus, Science Direct, EMBASE, OPENGREY.EU, and ClinicalTrials.gov. The risk of bias was assessed using the Newcastle-Ottawa Scale. A random-effects model was used for determining pairwise meta-analyses. The protocol was registered with PROSPERO (CRD42021262548).
Results: Eighteen studies met the inclusion criteria (5 cross-sectional, 12 cohort, and 1 case-control studies). All 1 618 680 subjects were adults. PA significantly decreased the risk of death in COVID-19 patients (odds ratio [OR] 0.34; 95% confidence interval [CI], 0.19–0.62; p < 0.001) and the risk of severe outcomes (OR 0.60; 95% CI, 0.48–0.76; p < 0.001). Subgroup analysis showed that PA for ≥150 min/wk at a moderate intensity or ≥75 min/wk at a vigorous intensity reduced the risks of severity and mortality. Vigorous PA reduced mortality risk, whereas moderate to vigorous PA reduced the risks of severity and mortality.
Conclusion: PA before infection might reduce severity and mortality in COVID-19 patients, especially PA ≥ 150 min/wk of moderate activity or ≥75 min/wk of vigorous activity. However, careful interpretations should be considered due to the difference in PA patterns and severity definitions among included studies. This finding implies that engaging in regular PA, even in different patterns, has beneficial effects on the severity and mortality of COVID-19 patients.
Coronavirus disease 2019 (COVID-19) is an infectious respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Lai et al., 2020). The World Health Organization declared COVID-19 a global pandemic on March 11, 2020 (World Health Organization, 2020). As at September 2022, over 600 million confirmed cases and over six million deaths were attributed to the virus worldwide (World Health Organization, 2022a). Transmission of the virus can occur through direct contact with the respiratory droplets of an infected person (generated through coughing and sneezing). Individuals can also be infected by touching surfaces contaminated with the virus and then touching their face (eg, the eyes, nose, or mouth) (Halperin, 2021). Approximately 80% of cases are asymptomatic or have mild symptoms, whereas the remainder can be severe and critical, leading to death (Verity et al., 2020) or persistent long COVID (Fahriani et al., 2021; Fajar et al., 2021). During the virus’s rapid spread in the absence of a COVID-19 vaccine, many countries implemented restrictive policies (eg, stay-at-home orders and the closures of parks, gymnasiums, and recreation centers). These policies were highly influential in containing the number of COVID-19 infections and preventing healthcare systems from being overwhelmed (Kharroubi and Saleh, 2020). However, the policies led to a significant increase in physical inactivity (PiA) (Stockwell et al., 2021), which has been considered a risk factor for developing COVID-19 severity and mortality (Centers for Disease Control and Prevention, 2020).
Physical activity (PA) has been defined as any bodily movement produced by skeletal muscle function resulting in energy expenditure (Caspersen et al., 1985). PA in daily life can be categorized as structured and incidental activities (Strath et al., 2013). Structured activities are bodily movements that usually occur during free time to promote health and fitness, such as weight training, jogging, and swimming (Strath et al., 2013). Incidental activities are bodily movements that occur during daily living, such as walking to school, gardening, and washing a car (Strath et al., 2013). The PA Guidelines for Americans recommend that all adults between 18 and 65 years of age engage in at least 30 min of moderate-intensity aerobic PA a day for 5 days a week or at least 20 min of high-intensity aerobic PA a day for 3 days a week (Piercy et al., 2018). Regular PA is recommended as a supplement to help strengthen the immune system to defend against COVID-19 infection (da Silveira et al., 2020). In addition, PA counteracts some noncommunicable diseases, such as obesity, diabetes mellitus, and arterial hypertension that increase the likelihood of COVID-19 patients experiencing severe outcomes (Chandrasekaran and Ganesan, 2020). However, there is insufficient scientific evidence supporting the recommendations of the PA Guidelines for Americans. A previous systematic review and meta-analysis of Rahmati et al. (2022) reported that PA decreased hospitalization, intensive care unit (ICU) admissions, and mortality rates of patients with COVID-19 in all study types. COVID-19 patients with a history of resistance and endurance exercises experience a lower rate of hospitalization and mortality, respectively. Meta-analysis results from a few studies showed lower mortality in low and moderate-vigorous PA (Rahmati et al., 2022). Only one study (Lee et al., 2021) was included to analyze the effects of PA level on ICU admission. It is suggested that further study should be recommended due to the limited number of studies.
The present study aimed to systematically review all available evidence and pooled odds ratios (ORs) adjusted by confounding factors to determine whether regular PA before COVID-19 infection affects the severity of illness and mortality in COVID-19 patients.
This investigation was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for healthcare applications (Page et al., 2021). The protocol was prospectively registered with PROSPERO (www.crd.york.ac.uk/PROSPERO; reference CRD42021262548).
The PubMed, Cochrane Library, Scopus, Science Direct, and EMBASE databases were systematically searched through 15 June 2022. The search keywords were “COVID*,” “exercise,” “physical activity,” “mortality,” and “severity” (Supplementary Table S1). In addition, grey literature was searched in OPENGREY.EU and ClinicalTrials.gov through 18 September 2022. No language restrictions were applied. Reference lists were also manually reviewed to find citations for additional pertinent meta-analyses and reviews.
Each study identified by the search was reviewed (by NS, NK, and KP in the authors’ list) to determine if it had information on the PA or exercise habits of COVID-19 patients before their infection. The PA definition follows WHO (World Health Organization, 2022b), such as regular movement during leisure time, moderate- and vigorous-intensity PA, and active activity (i.e., walking, cycling, wheeling, sports, active recreation, and play). The comparators (physical inactivity; PiA) were activities or habits that were not classified as PA definition of WHO. The primary outcomes of interest were the severity and mortality of COVID-19. Severe COVID-19 follows WHO severity definitions (World Health Organization, 2022c), including severe and critical COVID-19. Articles that reported ORs of severely symptomatic patients admitted to a hospital under treatment (in the general ward and ICU) were included in this study. Non-severe COVID-19 was patients who had an absence of any sign of severe or critical COVID-19. Mortality was confirmed deaths from COVID-19, both inpatient and outpatient, reported in included studies. Moreover, case reports, case series, letters, and studies without interesting outcomes or group comparisons were excluded.
Two investigators (NK and KP in the authors’ list) independently screened the titles and abstracts of the retrieved studies. Full texts were reviewed as necessary. Trials were determined to be eligible for this study based on the inclusion and exclusion criteria mentioned above. A third investigator (MP in the authors’ list) resolved disagreements.
Two investigators (NS and KP in the authors’ list) independently extracted details from the selected studies and recorded them in a Microsoft Excel spreadsheet. A third investigator (MP in the authors’ list) resolved disagreements. The data extracted were related to the setting, study design, sample size, patient demographics, PA or exercise details, comparators, number of mortalities, and number of patients at each severity level. The authors of the retrieved studies were contacted for missing data by email.
Two investigators (NP and MP in the authors’ list) independently evaluated the risk of bias in the included cohort, cross-sectional, and case-control studies using the Newcastle-Ottawa Scale (NOS) (Stang, 2010). A third investigator (SK in the authors’ list) resolved disagreements. The NOS contains three domains: quality of selection, comparability, and outcome. Each study was defined as low, moderate, and high quality when scores were 0–3, 4–6, and 7–10, respectively.
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to rate the quality of evidence of estimates (Guyatt et al., 2008). This study used GRADE to determine the quality of evidence for outcome according to five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The levels of evidence can be categorized into four levels: high, moderate, low, and very low.
Analyses were conducted using Stata Statistical Software, release 14.1 (StataCorp LLC, College Station, TX, United States). A random-effects model was used for determining pairwise meta-analyses. The results are reported as odds ratios (ORs) and 95% confidence intervals (CIs). Heterogeneity in each pairwise comparison was estimated using the I2 statistic. Publication bias was assessed using a funnel plot, and Egger’s tests were employed to assess the funnel plot asymmetry. Sensitivity analysis and subgroup analysis by level of PA were performed to evaluate the robustness of the results in determining the severity and mortality of illness in COVID-19 patients.
The literature search process is illustrated in Figure 1. The search strategies identified 11 418 articles from five databases and one article from two grey literature databases. Duplicates accounted for 1131 articles and were eliminated. After screening the titles and abstracts, an additional 10 246 articles were excluded for various reasons. Twenty-five articles were excluded from the study after assessment for eligibility (Supplementary Table S2). The meta-analysis was carried out on the remaining 18 eligible original articles.
Table 1 summarizes the core characteristics and outcomes of the selected studies. In all, 1 618 680 subjects were adults. Five studies used cross-sectional designs (Halabchi et al., 2021; Tavakol et al., 2021; Yuan et al., 2021; Tret’yakov et al., 2020; Latorre-Román et al., 2021), 12 had cohort study designs (Sallis et al., 2021; Cho et al., 2021; Lee et al., 2021; Hamrouni et al., 2021; Ahmadi et al., 2021; Hamer et al., 2020; Maltagliati et al., 2021; Malisoux et al., 2022; Baynouna Alketbi et al., 2021; Rowlands et al., 2021; Salgado-Aranda et al., 2021; Pinto et al., 2021), and only one employed a case-control design (Ekblom-Bak et al., 2021). We discovered four studies in the United Kingdom: Hamrouni et al., 2021; Ahmadi et al., 2021; Hamer et al., 2020; and Rowlands et al., 2021. Meanwhile, four studies were conducted in Europe: Latorre-Román et al., 2021; Salgado-Aranda et al., 2021; Ekblom-Bak et al., 2021; and Malisoux et al., 2022. In the meantime, six studies were carried out in Asia (Halabchi et al., 2021; Tavakol et al., 2021; Cho et al., 2021; Lee et al., 2021; Yuan et al., 2021; Baynouna AlKetbi et al., 2021). Besides that, three studies were discovered in the United States (Sallis et al., 2021), Brazil (Pinto et al., 2021), and Russia (Tret’yakov et al., 2020).
In terms of focus, 10 studies investigated the impacts of PA on mortality (Ahmadi et al., 2021; Baynouna AlKetbi et al., 2021; Cho et al., 2021; Ekblom-Bak et al., 2021; Halabchi et al., 2021; Hamrouni et al., 2021; Lee et al., 2021; Pinto et al., 2021; Sallis et al., 2021; Yuan et al., 2021), and 14 studies reported the severity of the disease (Tavakol et al., 2021; Yuan et al., 2021; Tret’yakov et al., 2020; Sallis et al., 2021; Lee et al., 2021; Hamer et al., 2020; Maltagliati et al., 2021; Latorre-Román et al., 2021; Malisoux et al., 2022; Ekblom-Bak et al., 2021; Baynouna AlKetbi et al., 2021; Rowlands et al., 2021; Salgado-Aranda et al., 2021; Pinto et al., 2021). Variations in PA or exercise intervention were observed across the 18 studies. Fourteen studies involved PA. In 1 of these 14 studies, one group of participants performed inconsistent activity for between 11 and 149 min/wk, while another group undertook consistent PA for at least 150 min/wk (Sallis et al., 2021). The participants in the 13 other studies engaged in a moderate to high PA level (Hamer et al., 2020; Ahmadi et al., 2021; Baynouna AlKetbi et al., 2021; Cho et al., 2021; Ekblom-Bak et al., 2021; Hamrouni et al., 2021; Latorre-Román et al., 2021; Maltagliati et al., 2021; Pinto et al., 2021; Rowlands et al., 2021; Salgado-Aranda et al., 2021; Tavakol et al., 2021; Malisoux et al., 2022). As for the type of PA, one study reported regular sports, including bodybuilding, team sports, combat sports, and individual sports (Halabchi et al., 2021). However, only three studies showed exercise intervention, including aerobic and muscle strengthening (Yuan et al., 2021; Tret’yakov et al., 2020; Lee et al., 2021).
According to the NOS, all studies were rated between 7 and 10 stars. Therefore, all studies were high quality (Table 2). However, the incomplete comparability data in six studies were inadequately explained and not appropriately addressed.
A pooled OR model was used to evaluate two sets of studies. One set investigated the effects of PA on mortality, and the other set explored PA’s impact on the severity of illness in COVID-19 patients (Figure 2).
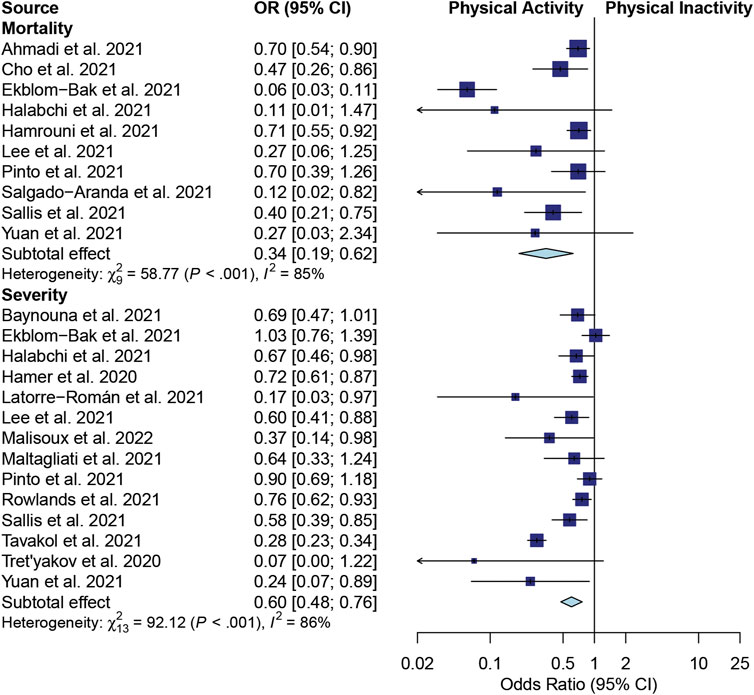
FIGURE 2. A forest plot of the association between physical activity and mortality and severity in COVID-19 patients.
Regarding the mortality outcome, 10 studies were meta-analyzed (Ahmadi et al., 2021; Cho et al., 2021; Ekblom-Bak et al., 2021; Halabchi et al., 2021; Hamrouni et al., 2021; Lee et al., 2021; Pinto et al., 2021; Salgado-Aranda et al., 2021; Sallis et al., 2021; Yuan et al., 2021). Significant heterogeneity was found among these articles (I2 = 85%; p < 0.001). The effects shown as pooled ORs suggested that PA significantly reduced the risk of death in COVID-19 patients compared with PiA (OR 0.34; 95% CI, 0.19–0.62; p < 0.001) (Figure 2). A statistical study of the publication bias showed no obvious bias or significant difference in the publications, which was confirmed by a funnel plot (Supplementary Figure S1). The evidence of Egger’s test for bias showed p = 0.083 (Supplementary Figure S2).
As for the severity outcome, 14 studies estimated the effects of PA on the severity of disease in patients with COVID-19 (Halabchi et al., 2021; Tavakol et al., 2021; Yuan et al., 2021; Tret’yakov et al., 2020; Sallis et al., 2021; Lee et al., 2021; Hamer et al., 2020; Maltagliati et al., 2021; Latorre-Román et al., 2021; Malisoux et al., 2022; Ekblom-Bak et al., 2021; Baynouna AlKetbi et al., 2021; Rowlands et al., 2021; Pinto et al., 2021). Significant heterogeneity was observed among these articles (I2 = 86%; p < 0.001). The overall effects of the pooled ORs demonstrated a significantly decreased risk of severe disease in COVID-19 patients with PA compared with PiA (OR 0.60; 95% CI, 0.48–0.76; p < 0.001) (Figure 2). As assessed by the funnel plot (Supplementary Figure S1), there was no apparent systematic bias (Egger’s test for bias: p = 0.472; Supplementary Figure S2).
The quality of evidence of the observational studies included in the meta-analysis is determined in Table 3. For each outcome, the observational studies were rated very low on the GRADE scale. Since the included studies were noted to have high heterogeneity, we decided to rate down for the inconsistency. The interventions were delivered in different activities among these studies. Thus, we downgraded the rating of indirectness.
A sensitivity analysis was undertaken to investigate the robustness of the effect estimates. The different models (fixed-effects and random-effects) did not significantly modify the pooled ORs for all outcomes. However, as there were studies on PA at different intensity levels, we performed a subgroup analysis by moderate and high/vigorous intensity. Moreover, some studies identified a sufficient PA level as a PA of ≥150 min/wk of moderate activity or ≥75 min/wk of vigorous activity (Table 4). A subgroup analysis was therefore performed by using sufficient PA. The results showed that PA for ≥150 min/wk at a moderate intensity or ≥75 min/wk at a vigorous intensity reduced the risks of severity and mortality. High or vigorous PA levels reduced the mortality risk, whereas moderate to high/vigorous PA reduced the risks of severity and mortality in COVID-19 patients.
This systematic review and meta-analysis revealed an association between regular PA and severity and mortality in COVID-19 patients. We included adjusted ORs that considered confounding factors possibly related to the outcomes. The major finding was that regular PA reduced patients’ risks of severity and mortality.
SARS-CoV-2 enters the host cell via the angiotensin-converting enzyme (ACE) two receptor. The targeted cells, especially lung parenchyma, have an off-balance between the ACE2/Ang (1–7)/Mas axis and ACE/AngII/AT1R axis that aggravates tissue injury (Yan et al., 2020). Previous studies reported that treatments with ACE inhibitors and angiotensin II receptor blockers were associated with a lower risk of death in COVID-19 patients (Kai and Kai, 2020; Yan et al., 2020; Zhang et al., 2020). Tavakol et al. (2021) reported a reverse correlation between increased PA and COVID-19 disease severity. Our study found a significantly lower risk of mortality with high levels of PA than with PiA. Moderate to high intensity tended to reduce the risk. Cho et al. (2021) reported that dose-dependent exercise reduced infection risk and mortality in COVID-19 patients. They found that vigorous and moderate intensities of exercise training (>1000 metabolic equivalent of task (MET)-min/wk) could reduce the risk of COVID-19 infection and mortality (Cho et al., 2021).
However, excessive exercise has been reported to increase the risk of respiratory infection. Long-term hyperventilation in untrained subjects may involve physical damage to bronchial and alveolar epithelial cells (Nieman, 2000; Murphy et al., 2008). The soluble ACE2 (sACE2) and the transmembrane ACE2 (tACE2) competitively bind with the SARS-CoV-2 virus. Therefore, the probability of a virus entering the targeted cells may be reduced (Yan et al., 2020). However, COVID-19 severity has an opposite response to moderate- and high-intensity exercise. Moderate-intensity exercise increases sACE2, whereas high-intensity exercise increases tACE2 (Hagiu, 2021). Our study found a reduced severity risk in patients who previously had sufficient PA (≥150 min/wk of moderate activity or ≥75 min/wk of vigorous activity) or moderate to high-intensity PA.
Regular exercise can improve the systemic immune response and reduce infection. The ratio of natural killer (NK) cells is modified, and changes in T helper cell expression and function due to exercise may improve the body’s resistance to respiratory infections (Timmons and Cieslak, 2008; Nieman and Wentz, 2019). Moderate exercise increases NK-cell activity (Nieman et al., 1990; Nieman, 2000). In addition, T helper (Th) cells (Th1 and Th2) and T-cell function in the upper respiratory tract mucosa are improved (Timmons and Cieslak, 2008; Nieman and Wentz, 2019). Moreover, McFarlin et al. (2005) reported that exercise training improves NK-cell activity. PA might reduce the cytokine storm following infection with SARS-CoV-2. Regular PA or exercise facilitates the release of anti-inflammatory myokines (Marino et al., 2022); reduces chemokines, pro-inflammatory cytokines, and the pathogen load (Lowder et al., 2006; Sim et al., 2009; Warren et al., 2015); and promotes the balance of pro- and anti-inflammatory cytokines via renin-angiotensin system (RAS) modulation (Agarwal et al., 2011). Increased vagal tone resulting from high levels of PA also reduces pro-inflammatory cytokines (Tracey, 2009). In addition, aerobic exercise training decreases tissue inflammation by increasing antioxidant enzyme levels (superoxide dismutase and glutathione peroxidase) and mitochondrial biogenesis (Toledo et al., 2012).
COVID-19 patients had changes in the alveolar-capillary membrane (ACM) (Yan et al., 2020), resulting in an impairment of the diffusion capacity of the lungs (Yan et al., 2020). Aerobic exercise training improves the diffusing capacity for carbon monoxide during resting and exercise by modifying the ACM. Enhancing the density and affinity of adrenoreceptors in respiratory muscles through aerobic exercise may improve respiratory muscle function, bronchodilation, and airway secretion production, thereby improving lung function (Tret’yakov et al., 2020). The more preserved lung function and the sparing use of respiratory regulatory elements during stress increase the ability to mobilize efficiently when needed.
A strategy for COVID-19 treatment may be attenuating the risk of comorbidities associated with severity and mortality, such as obesity, diabetes mellitus, stroke, and coronary artery disease. Regular exercise increases maximal oxygen uptake, shifting high-risk patients into low-risk patients (Ahmed, 2020). The metabolic dysfunction, impaired immune response, and increased adipose-tissue inflammatory cytokine secretion associated with obesity can increase the risk of severe pneumonia in COVID-19 patients (Cai et al., 2020; Popkin et al., 2020). Aerobic exercise improves metabolic function and the immune response and decreases obesity, especially central obesity. Disruptions of the ACM in the lung tissues of obese and diabetic patients were observed (Foster et al., 2010). The increased thickness and lowered elasticity of the ACM impaired the diffusion capacity of the lungs (Foster et al., 2010). Reducing the risk factors associated with COVID-19 infection and severity may reduce mortality.
This study has several strengths. First, the systematic review and meta-analysis followed a standard protocol (Page et al., 2021). Second, the included studies were of high quality, denoted by their high NOS scores for quality assessment. Third, the included factors’ adjusted ORs are likely to be involved in the severity and mortality of infection in COVID-19 patients, for example, demographic variables, comorbidities, medical history, smoking status, and alcohol consumption.
There are some limitations to this study. First, it included only retrospective, cross-sectional, case-control, and nonrandomized controlled trials. Because COVID-19 is a newly emerging disease, studying the effects of regular PA requires long-term monitoring. Moreover, using a randomized controlled trial may be ethically inappropriate. Second, severity definitions were different among studies. Some included studies did not mention, some defined patients admitted to ICU and some represented hospitalization. Third, there are different patterns of PA, and exercise training was observed. Some of the included studies failed to report full details of the PA or exercise, for example, type, intensity, frequency, and time to perform an activity. This may be the source of the high heterogeneity in severity (I2 = 86%) and mortality (I2 = 85.0%) found in our study.
On the other hand, the forest plots showed that the studies agreed that PA might induce less severe illness and lower mortality in COVID-19 patients than PiA. This finding implies that engaging in regular PA, even in different patterns, has beneficial effects on the severity and mortality of COVID-19 patients. More studies on the impact of regular PA or exercise training are needed to draw definitive conclusions. Finally, the levels of PA and exercise were evaluated by self-reporting questionnaires, which may not directly reflect the actual performance of the patients. Therefore, for clinical application, the results should be carefully interpreted. Nevertheless, this study provided knowledge and understanding to medical professionals about the effects of regular PA on severity and mortality in COVID-19 patients.
A previous meta-analysis from a few studies showed that low and moderate-vigorous PA lowered mortality in COVID-19 patients (Rahmati et al., 2022). However, only one study (Lee et al., 2021) was included to show the lower ICU admission. The researchers suggested that levels of PA should be recommended. A recent study of pooled ORs adjusted by confounding factors concluded that PA ≥ 150 min/wk of moderate activity or ≥75 min/wk of vigorous activity showed a reduction in severity and mortality risk in COVID-19 patients. A strategy for COVID-19 treatment has been reported to prevent infection by SARS-CoV-2. The strategy involves preventing viruses from entering the body; blocking the binding between the virus and host cells; preventing new viral replication; and improving the immune response, anti-inflammation, antioxidants, and body functions. Possible explanations for why PA and exercise training reduce severity and mortality in COVID-19 patients include improvements in the systemic immune response, inflammatory cytokines, antioxidants, and lung function. Moreover, PA may reduce viruses entering the cells and lower some risk factors (eg, obesity, diabetes mellitus, and arterial hypertension) associated with severe outcomes for COVID-19 patients. However, lockdowns and restrictions due to the COVID-19 pandemic might increase barriers to engaging in regular PA. A tele-exercise program may be a strategy for promoting PA to prevent infection and reduce the severity and mortality of illness in COVID-19 patients.
Regular PA can reduce the severity and mortality risk of COVID-19 patients, especially with ≥150 min/wk of moderate activity or ≥75 min/wk of vigorous activity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
NS, NP, SS, NK, KP, MP, and PP took part in designing the study, the selection process, and the writing of the first manuscript draft; NS, NP, SS, and MP took part in designing the study; NS, NP, SS, SK, NK, KP, and MP took part in the selection process and statistical analyses; NS, NP, NK, KP, MP, and PP wrote sections of the manuscript; all authors read and approved the final version of the manuscript and agreed with the order of presentation of the authors.
This work was partially supported by the Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN) [grant number: FF65-UoE005], School of Pharmaceutical Sciences, University of Phayao. The funding source had no role in the study design, the collection, analysis, and interpretation of the data.
The authors are indebted to David Park for the English-language editing of this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.1030568/full#supplementary-material
Supplementary Figure S1 | Assessing publication bias by funnel plot: (A) effects of physical activity on mortality; (B) effects of physical activity on severity.
Supplementary Figure S2 | Assessing publication bias by Egger’s test: (A) effects of physical activity on mortality; (B) effects of physical activity on severity. Abbreviations: CI, confidence interval; SND, standard.
Supplementary Table S1 | Data search algorithm.
Supplementary Table S2 | Excluded studies after assessment for eligibility.
Agarwal D., Welsch M. A., Keller J. N., Francis J. (2011). Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res. Cardiol. 106 (6), 1069–1085. doi:10.1007/s00395-011-0231-7
Ahmadi M. N., Huang B. H., Inan-Eroglu E., Hamer M., Stamatakis E. (2021). Lifestyle risk factors and infectious disease mortality, including COVID-19, among middle aged and older adults: Evidence from a community-based cohort study in the United Kingdom. Brain Behav. Immun. 96, 18–27. doi:10.1016/j.bbi.2021.04.022
Ahmed I. (2020). COVID-19 – does exercise prescription and maximal oxygen uptake (VO2 max) have a role in risk-stratifying patients? Clin. Med. 20 (3), 282–284. doi:10.7861/clinmed.2020-0111
Baynouna AlKetbi L. M., Nagelkerke N., Abdelbaqi H., Alblooshi F., AlSaedi M., Almansoori S., et al. (2021). Risk factors for SARS-CoV-2 infection severity in Abu Dhabi. J. Epidemiol. Glob. Health 11 (4), 344–353. doi:10.1007/s44197-021-00006-4
Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., et al. (2020). Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 43 (7), 1392–1398. doi:10.2337/dc20-0576
Caspersen C. J., Powell K. E., Christenson G. M. (1985). Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 100 (2), 126–131. doi:10.2307/20056429
Centers for Disease Control and Prevention (2020). People with certain medical conditions centers for disease control and prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (Accessed Mar 28, 2020).
Chandrasekaran B., Ganesan T. B. (2020). Sedentarism and chronic disease risk in COVID 19 lockdown – A scoping review. Scott. Med. J. 66 (1), 3–10. doi:10.1177/0036933020946336
Cho D. H., Lee S. J., Jae S. Y., Kim W. J., Ha S. J., Gwon J. G., et al. (2021). Physical activity and the risk of COVID-19 infection and mortality: A nationwide population-based case-control study. J. Clin. Med. 10 (7), 1539. doi:10.3390/jcm10071539
da Silveira M. P., da Silva Fagundes K. K., Bizuti M. R., Starck É., Rossi R. C., de Resende e Silva D. T. (2020). Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 21 (1), 15–28. doi:10.1007/s10238-020-00650-3
Ekblom-Bak E., Väisänen D., Ekblom B., Blom V., Kallings L. V., Hemmingsson E., et al. (2021). Cardiorespiratory fitness and lifestyle on severe COVID-19 risk in 279, 455 adults: A case control study. Int. J. Behav. Nutr. Phys. Act. 18 (1), 135. doi:10.1186/s12966-021-01198-5
Fahriani M., Ilmawan M., Fajar J. K., Maliga H. A., Frediansyah A., Masyeni S., et al. (2021). Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - a systematic review and meta-analysis. Narra J. 1 (2), 36. doi:10.52225/narraj.v1i2.36
Fajar J. K., Ilmawan M., Mamada S., Mutiawati E., Husnah M., Yusuf H., et al. (2021). Global prevalence of persistent neuromuscular symptoms and the possible pathomechanisms in COVID-19 recovered individuals: A systematic review and meta-analysis. Narra J. 1 (3), 48. doi:10.52225/narra.v1i3.48
Foster D. J., Ravikumar P., Bellotto D. J., Unger R. H., Hsia C. C. W. (2010). Fatty diabetic lung: Altered alveolar structure and surfactant protein expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 298 (3), L392–L403. doi:10.1152/ajplung.00041.2009
Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi:10.1136/bmj.39489.470347.AD
Hagiu B. A. (2021). Moderate exercise may prevent the development of severe forms of COVID-19, whereas high-intensity exercise may result in the opposite. Med. Hypotheses 157, 110705. doi:10.1016/j.mehy.2021.110705
Halabchi F., Mazaheri R., Sabeti K., Yunesian M., Alizadeh Z., Ahmadinejad Z., et al. (2021). Regular sports participation as a potential predictor of better clinical outcome in adult patients with COVID-19: A large cross-sectional study. J. Phys. Act. Health 18 (1), 8–12. doi:10.1123/jpah.2020-0392
Halperin D. T. (2021). Prevalence of asymptomatic SARS-CoV-2 infection. Ann. Intern. Med. 174 (2), 283. doi:10.7326/l20-1282
Hamer M., Kivimäki M., Gale C. R., Batty G. D. (2020). Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387, 109 adults in UK. Brain Behav. Immun. 87, 184–187. doi:10.1016/j.bbi.2020.05.059
Hamrouni M., Roberts M. J., Thackray A., Stensel D. J., Bishop N. (2021). Associations of obesity, physical activity level, inflammation and cardiometabolic health with COVID-19 mortality: A prospective analysis of the UK biobank cohort. BMJ Open 11 (11), e055003. doi:10.1136/bmjopen-2021-055003
Kai H., Kai M. (2020). Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—Lessons from available evidence and insights into COVID-19. Hypertens. Res. 43 (7), 648–654. doi:10.1038/s41440-020-0455-8
Kharroubi S., Saleh F. (2020). Are lockdown measures effective against COVID-19? Front. Public Health 8, 549692. doi:10.3389/fpubh.2020.549692
Lai C. C., Shih T. P., Ko W. C., Tang H. J., Hsueh P. R. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 55 (3), 105924. doi:10.1016/j.ijantimicag.2020.105924
Latorre-Román P. Á., Guzmán-Guzmán I. P., Delgado-Floody P., Herrador Sanchez J., Aragón-Vela J., García Pinillos F., et al. (2021). Protective role of physical activity patterns prior to COVID-19 confinement with the severity/duration of respiratory pathologies consistent with COVID-19 symptoms in Spanish populations. Res. Sports Med. 15, 1–12. doi:10.1080/15438627.2021.1937166
Lee S. W., Lee J., Moon S. Y., Jin H. Y., Yang J. M., Ogino S., et al. (2021). Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: A nationwide cohort study. Br. J. Sports Med. 56, 901–912. doi:10.1136/bjsports-2021-104203
Lowder T., Padgett D. A., Woods J. A. (2006). Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc. Immunol. Rev. 12, 97–111.
Malisoux L., Backes A., Fischer A., Aguayo G., Ollert M., Fagherazzi G. (2022). Associations between physical activity prior to infection and COVID-19 disease severity and symptoms: Results from the prospective predi-COVID cohort study. BMJ Open 12 (4), e057863. doi:10.1136/bmjopen-2021-057863
Maltagliati S., Sieber S., Sarrazin P., Cullati S., Chalabaev A., Millet G. P., et al. (2021). Muscle strength explains the protective effect of physical activity against COVID-19 hospitalization among adults aged 50 Years and older. medRxiv. 25, 21252451. doi:10.1101/2021.02.25.21252451
Marino F. E., Vargas N. T., Skein M., Hartmann T. (2022). Metabolic and inflammatory health in SARS-CoV-2 and the potential role for habitual exercise in reducing disease severity. Inflamm. Res. 71, 27–38. doi:10.1007/s00011-021-01517-3
McFarlin B. K., Flynn M. G., Phillips M. D., Stewart L. K., Timmerman K. L. (2005). Chronic resistance exercise training improves natural killer cell activity in older women. J. Gerontol. A Biol. Sci. Med. Sci. 60 (10), 1315–1318. doi:10.1093/gerona/60.10.1315
Murphy E. A., Davis J. M., Carmichael M. D., Gangemi J. D., Ghaffar A., Mayer E. P. (2008). Exercise stress increases susceptibility to influenza infection. Brain Behav. Immun. 22 (8), 1152–1155. doi:10.1016/j.bbi.2008.06.004
Nieman D. C., Nehlsen-Cannarella S. L., Fagoaga O. R., Henson D. A., ShannonM. , Hjertman J. M., et al. (2000). Immune function in female elite rowers and non-athletes. Br. J. Sports Med. 34 (3), 181–187. doi:10.1136/bjsm.34.3.181
Nieman D. C., Nehlsen-Cannarella S. L., Markoff P. A., Balk-Lamberton A. J., Yang H., Chritton D. B., et al. (1990). The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports Med. 11 (06), 467–473. doi:10.1055/s-2007-1024839
Nieman D. C., Wentz L. M. (2019). The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8 (3), 201–217. doi:10.1016/j.jshs.2018.09.009
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 372 (71), n71. doi:10.1136/bmj.n71
Piercy K. L., Troiano R. P., Ballard R. M., Carlson S. A., Fulton J. E., Galuska D. A., et al. (2018). The physical activity guidelines for americans. JAMA 320 (19), 2020–2028. doi:10.1001/jama.2018.14854
Pinto A. J., Goessler K. F., Fernandes A. L., Murai I. H., Sales L. P., Reis B. Z., et al. (2021). No independent associations between physical activity and clinical outcomes among hospitalized patients with moderate to severe COVID-19. J. Sport Health Sci. 10 (6), 690–696. doi:10.1016/j.jshs.2021.08.001
Popkin B. M., Du S., Green W. D., Beck M. A., Algaith T., Herbst C. H., et al. (2020). Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 21 (11), e13128. doi:10.1111/obr.13128
Rahmati M., Shamsi M. M., Khoramipour K., Malakoutinia F., Woo W., Park S., et al. (2022). Baseline physical activity is associated with reduced mortality and disease outcomes in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 32, e2349. doi:10.1002/rmv.2349
Rowlands A. V., Dempsey P. C., Gillies C., Kloecker D. E., Razieh C., Chudasama Y., et al. (2021). Association between accelerometer-assessed physical activity and severity of COVID-19 in UK biobank. Mayo Clin. Proc. Innov. Qual. Outcomes 5 (6), 997–1007. doi:10.1016/j.mayocpiqo.2021.08.011
Salgado-Aranda R., Pérez-Castellano N., Núñez-Gil I., Orozco A. J., Torres-Esquivel N., Flores-Soler J., et al. (2021). Influence of baseline physical activity as a modifying factor on COVID-19 mortality: A single-center, retrospective study. Infect. Dis. Ther. 10 (2), 801–814. doi:10.1007/s40121-021-00418-6
Sallis R., Young D. R., Tartof S. Y., Sallis J. F., Sall J., Li Q., et al. (2021). Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 55 (19), 1099–1105. doi:10.1136/bjsports-2021-104080
Sim Y. J., Yu S., Yoon K. J., Loiacono C. M., Kohut M. L. (2009). Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J. Infect. Dis. 200 (9), 1434–1442. doi:10.1086/606014
Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Stockwell S., Trott M., Tully M., Shin J., Barnett Y., Butler L., et al. (2021). Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport Exerc. Med. 7 (1), e000960. doi:10.1136/bmjsem-2020-000960
Strath S. J., Kaminsky L. A., Ainsworth B. E., Ekelund U., Freedson P. S., Gary R. A., et al. (2013). Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American heart association. Circulation 128 (20), 2259–2279. doi:10.1161/01.cir.0000435708.67487.da
Tavakol Z., Ghannadi S., Tabesh M. R., Halabchi F., Noormohammadpour P., Akbarpour S., et al. (2021). Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. J. Public Health 4, 1–9. doi:10.1007/s10389-020-01468-9
Timmons B. W., Cieslak T. (2008). Human natural killer cell subsets and acute exercise: A brief review. Exerc. Immunol. Rev. 14, 8–23.
Toledo A. C., Magalhaes R. M., Hizume D. C., Vieira R. P., Biselli P. J. C., Moriya H. T., et al. (2012). Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur. Respir. J. 39 (2), 254–264. doi:10.1183/09031936.00003411
Tracey K. J. (2009). Reflex control of immunity. Nat. Rev. Immunol. 9 (6), 418–428. doi:10.1038/nri2566
Tret’yakov A. Y., Zakharchenko S. P., Romasenko L. V., Dyatlova A. V., Zhabskaya A. V., Ermilov O. V., et al. (2020). COVID-19 in individuals adapted to aerobic exercise. Pulʹmonologia. 30 (5), 553–560. doi:10.18093/0869-0189-2020-30-5-553-560
Verity R., Okell L. C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. (2020). Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet. Infect. Dis. 20 (6), 669–677. doi:10.1016/s1473-3099(20)30243-7
Warren K. J., Olson M. M., Thompson N. J., Cahill M. L., Wyatt T. A., Yoon K. J., et al. (2015). Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PLOS ONE 10 (6), e0129713. doi:10.1371/journal.pone.0129713
World Health Organization (2022a). Clinical management of COVID-19: Living guideline. Geneva: WHO. Licence: CC BY-NC-SA 3.0 IGO.
World Health Organization (2022b). Physical activity. [online] world health organization. Available at: https://www.who.int/news-room/fact-sheets/detail/physical-activity (Accessed Oct 8, 2022).
World Health Organization (2022c). WHO COVID-19 dashboard. [online] world health organization. Available at: https://covid19.who.int/ (Accessed September 13, 2022).
World Health Organization (2020). WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 World Health Organization. Available at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (Accessed Mar 28, 2022).
Yan T., Xiao R., Lin G. (2020). Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? FASEB J. 34, 6017–6026. doi:10.1096/fj.202000782
Yuan Q., Huang H., Chen X., Chen R., Zhang Y., Pan X., et al. (2021). Does pre-existent physical inactivity have a role in the severity of COVID-19? Ther. Adv. Respir. Dis. 15, 17534666211025221. doi:10.1177/17534666211025221
Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J., et al. (2020). Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 126 (12), 1671–1681. doi:10.1161/circresaha.120.317134
Keywords: exercise, physical activity, SARS-CoV-2, coronavirus, severity, mortality
Citation: Sittichai N, Parasin N, Saokaew S, Kanchanasurakit S, Kayod N, Praikaew K, Phisalprapa P and Prasannarong M (2022) Effects of physical activity on the severity of illness and mortality in COVID-19 patients: A systematic review and meta-analysis. Front. Physiol. 13:1030568. doi: 10.3389/fphys.2022.1030568
Received: 29 August 2022; Accepted: 24 October 2022;
Published: 08 November 2022.
Edited by:
Achraf Ammar, Johannes Gutenberg University Mainz, GermanyReviewed by:
Khaled Trabelsi, University of Sfax, TunisiaCopyright © 2022 Sittichai, Parasin, Saokaew, Kanchanasurakit, Kayod, Praikaew, Phisalprapa and Prasannarong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mujalin Prasannarong, bXVqYWxpbnBAZ21haWwuY29t; Pochamana Phisalprapa, Y29jb19hMTA1QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.