- 1Department of Physician Assistant Studies, Clarkson University, Potsdam, NY, United States
- 2Developmental Integrative Biology, Department of Biological Sciences, University of North Texas, Denton, TX, United States
- 3Department of Biology, SUNY Potsdam, Potsdam, NY, United States
- 4Green Godwit Consulting, Cleveland, OH, United States
- 5FUJIFILM Diosynth Biotechnologies Texas, College Station, TX, United States
Thyroid hormones are key regulators of development and metabolism in vertebrates. During the nestling period, young of altricial species transition from an ectothermic phenotype to an endothermic phenotype. Red-winged blackbirds are an altricial species that exhibit an increase in plasma 3,3’, 5-triiodo-L-thyronine (T3) levels during the first 5 days post-hatch (dph), begin to develop endothermic metabolic responses by 7 dph, and fledge within 10 days of hatching. We propose that thyroid hormones play an important role in regulating development of endothermy during the nestling period in altricial birds. To better understand the effects of thyroid hormones on endothermic metabolic development in an altricial species, we treated nestling red-winged blackbirds on 2, 3, and 5 dph with either methimazole (MMI) to induce hypothyroidism or supplemental T3 to induce hyperthyroidism. We then measured on 5, 7, and 9 dph morphology and whole animal O2 consumption (
Introduction
Red-winged blackbirds (Agelaius phoenicius) are altricial birds that hatch with an ectothermic-like phenotype, naked without insulation and poor motor coordination, leaving them unable to effectively thermoregulate early in nestling development. During the first days of nestling life, energy is devoted towards growth, and hatchlings increase in body mass by 9–10 times their initial body mass, resulting in one of the fastest measured avian nestling growth rates (Olson, 1992). During these first days, nestlings allocate most of their energy towards growth and structural development and depend on their parents for thermoregulation (Olson, 1992; Olson et al., 1999). Red-winged blackbird nestlings gain greater endothermic capacity—observed as a metabolic thermogenic response to cold challenges—between 5 and 9 days post hatching (Olson, 1992; Sirsat et al., 2016). During this transition from incipient to fully mature endothermy, various systems such as whole-body metabolism, body temperature regulation, pulmonary ventilation, organ masses, and skeletal mitochondrial function undergo drastic changes (Sirsat et al., 2016; Price and Dzialowski, 2018).
Thyroid hormones play a prominent role in regulating metabolism and energy expenditure of birds (Decuypere et al., 2005) and exert effects on key physiological functions involved in energy use of tissues (Yen, 2001), including development of body systems and obligatory metabolic heat production of endothermic species (Breall et al., 1984; McNabb, 2007; Little and Seebacher, 2014). During cold exposure, endotherms increase their metabolic rates and heat production to thermoregulate within a given range of body temperatures. The capacity for adult metabolic responses are mediated in part by thyroid hormones action on skeletal muscle and brown adipose tissue (Iwen et al., 2018).
Thyroid hormones are important for a number of developmental processes in birds during hatching and nestling development (De Groef et al., 2013). A number of recent studies examined the influence of maternal thyroid hormones passed on in the egg on subsequent development in altricial species development (Hsu et al., 2020; Sarraude et al., 2020; Cossin-Sevrin et al., 2022). Elevating egg yolk thyroid hormones resulted in earlier hatching and larger hatchlings in collared flycatchers (Ficedula albicollis) (Hsu et al., 2019) but had no effect in the great tit hatchling (Parus major) (Cossin-Sevrin et al., 2022). The effect on the collared flycatcher disappeared quickly in the developing nestling. Alternatively, inhibition of thyroid hormones during the nestling period of the zebra finch (Taeniopygia guttata) resulted in decreased growth and altered behavior (Rainwater et al., 2008). Based on these studies, we predict that thyroid hormones produced by the nestling are more important regulators of metabolic development than those supplied in the yolk by the mother.
Thyroid levels and hypothalamic-pituitary-thyroid (HPT) axis control in altricial species like the red-winged blackbird increase in parallel with development of endothermic capacity (Olson et al., 1999). Red-winged blackbird nestling T3 plasma levels peak at 4 days post-hatch (dph) and remain elevated through day 8 of post hatching life. This developmental trajectory for plasma T3 levels is consistent with documented rises in plasma T3 in other altricial species such as ring-necked doves, great tits, and European starlings (McNabb and Cheng, 1985; Schew et al., 1996; Silverin and Rudas, 1996; Výboh et al., 1996). Red-winged blackbird T4 plasma concentration similarly peaks around 4 dph and remains elevated through 10 dph. These studies in altricial species, as well as those concerned with precocial species of birds (Sirsat and Dzialowski, 2020), suggest that functional maturation of the HPT axis is critical to the ontogeny of endothermic thermoregulatory development in birds (Olson et al., 1999). Nonetheless, only a few studies have manipulated thyroid hormones during the nestling period to test this hypothesis.
In the present study, we investigated how altering plasma T3 levels during the first 5 days of post-hatching life influenced development and growth of red-winged blackbird nestlings up to fledging. We pharmacologically altered T3 plasma levels during the first 5 days of post-hatching life to manipulate the timing of the increase in plasma T3 levels in relation to the normal developmental trajectory of plasma T3 levels (Olson et al., 1999). This manipulation allowed us to examine the role of circulating plasma T3 on nestling growth and development of endothermic capacity. Additionally, we measured how development of ventilatory control associated with the cold-induced metabolic response responded to altered thyroid hormone levels. We predicted that in response to early nestling hypothyroid conditions the development of endothermy, measured as a strong whole animal metabolic rate and ventilation response to cooling, would occur later in the nestling period. In contrast, we expected nestling hyperthyroid conditions to accelerate the development of endothermy to earlier in the nestling development.
Materials and methods
Animals and collection sites
Red-winged blackbird nestlings were collected between May and July of 2014 and 2015 from the Lewisville Aquatic Ecosystem Research Facility (LAERF) and two retention ponds on the University of North Texas campus in Denton County in north Texas (USFWS Permit No. MB02732B-2, TPWS permit #SPR-0214-034 to E. M. Dzialowski). Nests were located and monitored daily in the morning or early afternoon to determine timing of egg laying and hatching sequence and to assess nestling status throughout the nesting period. Sample sizes were determined by the number of successful nests found in our study population.
Experimental design and drug administration
Experiments were conducted over two consecutive years. During the first year, nestlings were orally treated with either methimazole (MMI), a thyroperoxidase inhibitor, or water as a control. During the second year, nestlings were orally treated with supplemental 3,3’, 5- Triiodo-L- thyronine (T3) or water as control. We treat each year as a separate experiment.
Each year, nests containing at least three eggs were randomly assigned into one of the two groups. In 2014, nests were assigned either a hypothyroid treatment (10 mg MMI/kg nestling mass, MP Biomedicals, Solon, Ohio) or control (equal volume of water as of the methimazole treatment). In 2015, nests were assigned either a hyperthyroid treatment (5 mg T3/kg nestling mass, Sigma-Aldrich, St Louis, MO, United States) or control (equal volume of water as of the T3 treatment). The T3 solution was prepared by dissolving 3,3’, 5- Triiodo-L- thyronine in sterile 0.2M NaOH and diluted with 0.9% NaCl sterile saline. The MMI solution was prepared by dissolving methimazole in 0.9% NaCl sterile saline. Nestlings were left in their nest and raised and fed by their parents in the nest until the day they were to be exposed to the cold challenge in the lab. In the field, nestlings were weighed daily after hatching and received an oral dose with a volume in microliters equal to their mass in grams by Gilson pipette of their respective treatments on 2-, 3- and 5-day post-hatch (dph).
Nestlings on 5, 7, or 9 dph were collected from their nest in the field in the morning and transported to the University of North Texas (32 km) and used within 2–4 h of arrival in the laboratory. Nestlings were kept in a bench top incubator at 35°C (model 1602N, Hova-bator G.Q.F. manufacturing, Savannah, GA) and fed roach nymphs (Blaptica dubia) ad libitum until experiments began.
A subset of nestlings were brought to the laboratory on 4 dph, dosed with either T3 or saline solution, and blood samples were collected at 2, 8, 12, 18 and 24 h intervals after drug administration to determine the rate of plasma T3 metabolism after T3 supplementation.
Whole-animal metabolic rate and body temperature
Oxygen consumption rate (
Where
Along with measuring
Pulmonary ventilation
Estimates of tidal volumes in the thermoneutral zone and during cooling were made using a barometric technique described in Sirsat and Dzialowski (2016), Sirsat and Dzialowski (2020). Changes in pressure associated with breathing were measured with a spirometer (ADIstruments) connected in-line with each respirometry chamber (Menna and Mortola, 2003). Volume calibration was conducted in each respirometry chamber after each trial by injecting known volumes of air (Vcal) into the system by Hamilton syringe (Hamilton, Reno, NV). The corresponding change in pressure (Pcal) was used to calibrate the system (K = Vcal/Pcal as in (Szdzuy and Mortola, 2008)). Each respirometry chamber’s relative humidity was measured using humidity sensors (HIH 4021, Honeywell, Minneapolis, MN) and included to estimate chamber water vapor pressure. Tidal volume estimates were calculated from K, chamber temperature, Tb, water vapor pressure, and measured pressure changes. Breathing frequency (ƒ, breaths/min) was recorded from the pressure waves using the cycle measurement function in LabChart 7 software (ADInstruments). Minute ventilation (ml min−1) was calculated by multiplying tidal volume by ventilation frequency. For two 7 dph nestlings in 2014 (one control and one MMI), the temperature probe did not function resulting in no body temperature data for calculation of tidal volume. For these two 7 dph nestlings with missing Tb, we carried out mean imputations of Tb using the Tb from 7 dph nestlings within the given treatment to calculate tidal volume and minute ventilation.
Blood collection and morphometrics
After metabolic rate and pulmonary ventilation measurements, the animals were anesthetized with isoflurane. Blood (approximately 0.5–1 ml) was collected from anesthetized animals in heparinized 1 ml plastic syringes from the atria of the heart by cardiac puncture. Blood was immediately transferred into a 1.5 ml plastic vial and centrifuged (Model 16K, Bio-Rad, Hercules, CA) at 3,000 rpm for 10 min at 4°C. Plasma was aliquoted and stored at −20°C for later plasma T3 analysis. Animals were euthanized by decapitation under isoflurane anesthesia and dissected. Wet masses of whole body, yolk mass, cardiac ventricles, liver, heart, both kidneys, and both lungs were measured (±0.1mg, Item no. E12140, Ohaus Corp., Pine brook, NJ). To examine structural size, lengths of head to beak (from the posterior end of the supraoccipital bone to the tip of the beak, with the skin intact), femur, tarsus, and wing cord were measured using calipers (±0.01 mm Traceable® Fisher Scientific, Pittsburgh, PA).
Total plasma T3 concentrations were measured in duplicate as in Sirsat and Dzialowski (2020), using an AccuBind T3 ELISA (125-300 Monobind Inc., Lake Forest, CA, United States).
Data analysis
We examined responses using a measure of standardized effect size, Cohen’s d with 95% confidence interval, because we were interested in the magnitude of the effect of altering plasma T3 hormone status on the various measured parameters within each nestling age (Nakagawa and Cuthill, 2007; Sneddon et al., 2017). When assessing responses to altering T3 levels with Cohen’s d effect sizes we used the following ranges for small (0.49 > d > 0.2), moderate (0.79 > d > 0.5), large (1.19 > d > 0.8), or very large (>1.2) effects. Body mass was analyzed by ANOVA with age and treatment as factors. We used an ANCOVA to obtain estimated marginal means accounting for body mass as a covariate of heart ventricle, liver, lungs, and kidney and lengths of the femur, tarsus, wing cord, and head-to-beak. Body mass was included as a covariate to account for differences in body masses between treatments. For all morphological measures, the effect size was determined for the control compared with the treated response at each age as Cohen’s d ± 95% CI from the ANCOVA estimated marginal means (Nakagawa and Cuthill, 2007). Morphometry data is presented as the ANCOVA estimated marginal means ±95% CI accounting for mass as the covariate. Oxygen consumption, ventilation frequency, tidal volume, minute ventilation, and Tb were all analyzed by two-way repeated measured ANOVA with thyroid treatment and ambient temperature as factors. When ambient temperature was significant in the model, we separated the data by treatment to determine the ambient temperature at which differences first appeared in the treatments (Dzal and Milsom, 2021). We did this by looking at paired Cohen’s d effect sizes ±95% CI to determine at what temperatures there was a large or greater effect (>0.8) of the treatment on the parameter in question. We considered Cohen’s d values of importance when their ±95% CI did not encompass 0. Statistical analyses were performed in Jamovi running R version 4.0.2 (https://www.jamovi.org/) and Prism 9.
Results
Plasma T3
Treatment with MMI on days 2, 3 and 5 post-hatch had a large effect on the plasma T3 levels of nestlings (Figure 1A, Figure 2A; treatment effect F1, 45 = 11.06, p = 0.001). On day 5 post-hatch, MMI treatment during the first half of the nestling period had a large negative effect on the nestling’s plasma T3 levels that disappeared by 9 dph (Figure 2A).
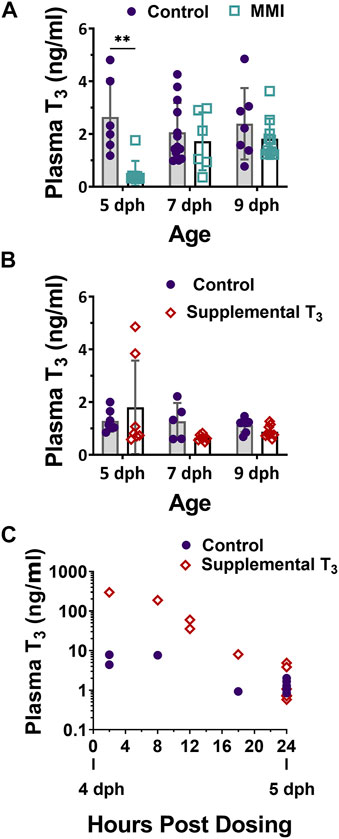
FIGURE 1. Plasma 3,3′, 5- Triiodo-L- thyronine (T3) levels in 5, 7, and 9 dph red-winged blackbird nestlings after (A) Methimazole (MMI) or (B) T3 treatment on 2, 3, and 5 dph. (A) After treatment with MMI, plasma T3 levels were lower than controls but recovered to control levels by 9 dph. Control n—5dph = 6, 7 dph = 13, 9 dph = 7; MMI n—5 dph = 9, 7 dph = 6, 9 dph = 10. (B) Treatment with T3 did not produce a large effect in plasma T3 levels at any age. Contol n—5 dph = 7; 7 dph = 5; 9 dph = 7; T3 n—5 dph = 7; 7 dph = 7; 9 dph = 9. (C) 4 dph nestling plasma T3 increased within 2-h of oral T3 dosing and returned to control levels within 24-h after oral dosing. Data presented as mean ± standard deviation. ** indicates significant difference between control and treatment within an age at p < 0.001 and a very large Cohen’s d effect size.
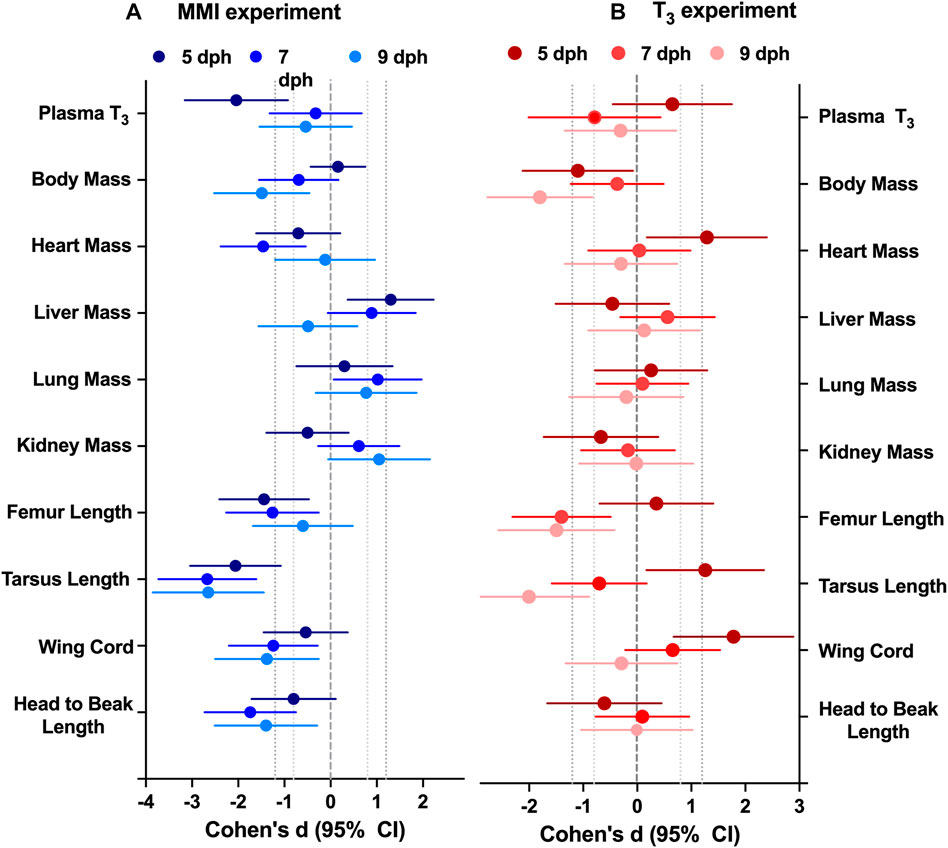
FIGURE 2. Effect of altering thyroid plasma levels during the first half of nestling period on morphology differed between (A) hypothyroid and (B) hyperthyroid treatments. Data presented as the effect size estimate Cohen’s d ± 95% confidence intervals comparing the effect of the treatment with control values within an experimental year. Cohen’s d effect size are calculated from the ANCOVA estimated marginal means with body mass as a covariate to remove the effect of body mass. n = 7–10 per treatment.
Treatment of nestlings with T3 on days 2, 3, and 5 post-hatch had little effect on the nestling plasma T3 levels on 5, 7 and 9 dph (Figure 1B, Figure 2B; treatment effect F1, 39 = 0.01, p = 0.091). There was a moderate elevated effect on 5 dph plasma levels in some animals, although samples were quite variable. Nestlings that had received supplemental T3, trended lower plasma T3 levels than controls on 7 dph, but were similar to controls on 9 dph. To ensure our method of supplementing T3 led to an elevation in plasma T3 during the first half of the nestling period, additional animals were dosed on 4 dph and their plasma T3 levels measured at 2, 8, 12, 18, and 24 h post-dosing (Figure 1C). At 2 h, plasma T3 levels were elevated above the controls and returned to control levels by 24 h post exposure.
Morphology
Treatment with MMI produced a very large effect on the growth trajectories of the morphological parameters examined (Figure 2; Table 1). Treatment with MMI had a very large negative effect on body mass on 9 dph, 4 days after the last dose of MMI. Induction of early hypothyroidism had a very large negative effect on heart mass and large positive effects on liver, lung, and kidney mass (Figure 2A). Structurally, exposure to MMI had a very large negative effect on size of the nestlings (Figure 2A). MMI treated nestlings tended to have shorter femurs, tarsus, wing cord, and head to beak length than controls (Figure 2A).
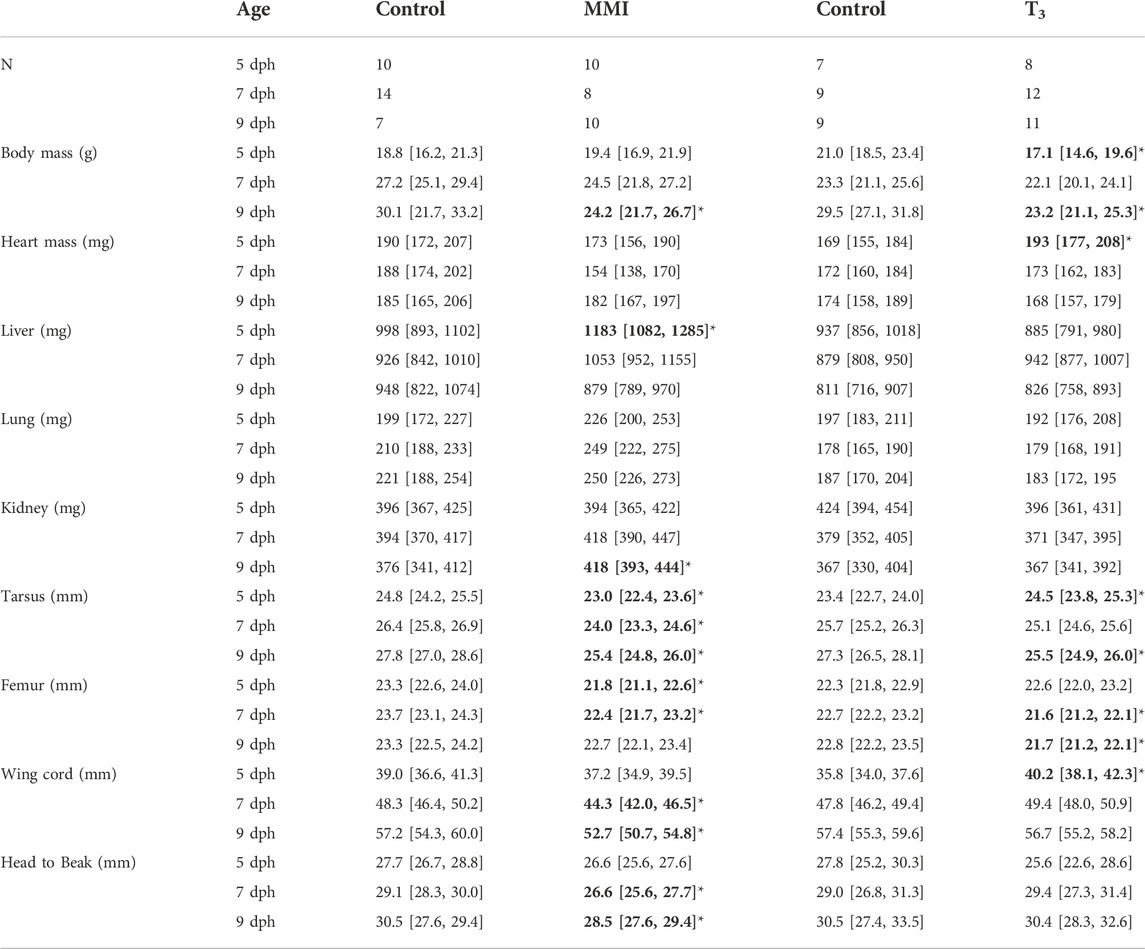
TABLE 1. Nestling red-winged blackbird body masses and ANCOVA estimated marginal means of morphometric measures with body mass as a covariate from MMI treatment experiment and T3 treatment experiment. Data presented as mean [95% CI]. Values in bold with an asterisks indicate very large effects (Cohen’s d > 1.2) of treatment on the parameter in comparison to the control.
Treatment with supplemental T3 had a large to very large negative effect on body mass of 5 and 9 dph nestlings compared with controls (Figure 2B; Table 1). Initially, on 5 dph, there was a very large positive effect of supplemental T3 on heart mass that disappeared by 7 dph. There was little effect of supplemental T3 on the other organ masses on 5, 7 and 9 dph (Figure 2). Structurally, T3 treatment initially had very large positive effect on tarsus length and wing cord compared with controls. By 9 dph the effect of supplemental T3 became a very large negative effect on tarsus length and no effect on wing cord lengths. Supplemental T3 also had a very large negative effect on femur length on 7 and 9 dph.
Oxygen consumption
Treatment with MMI resulted in altered developmental trajectories in the response of
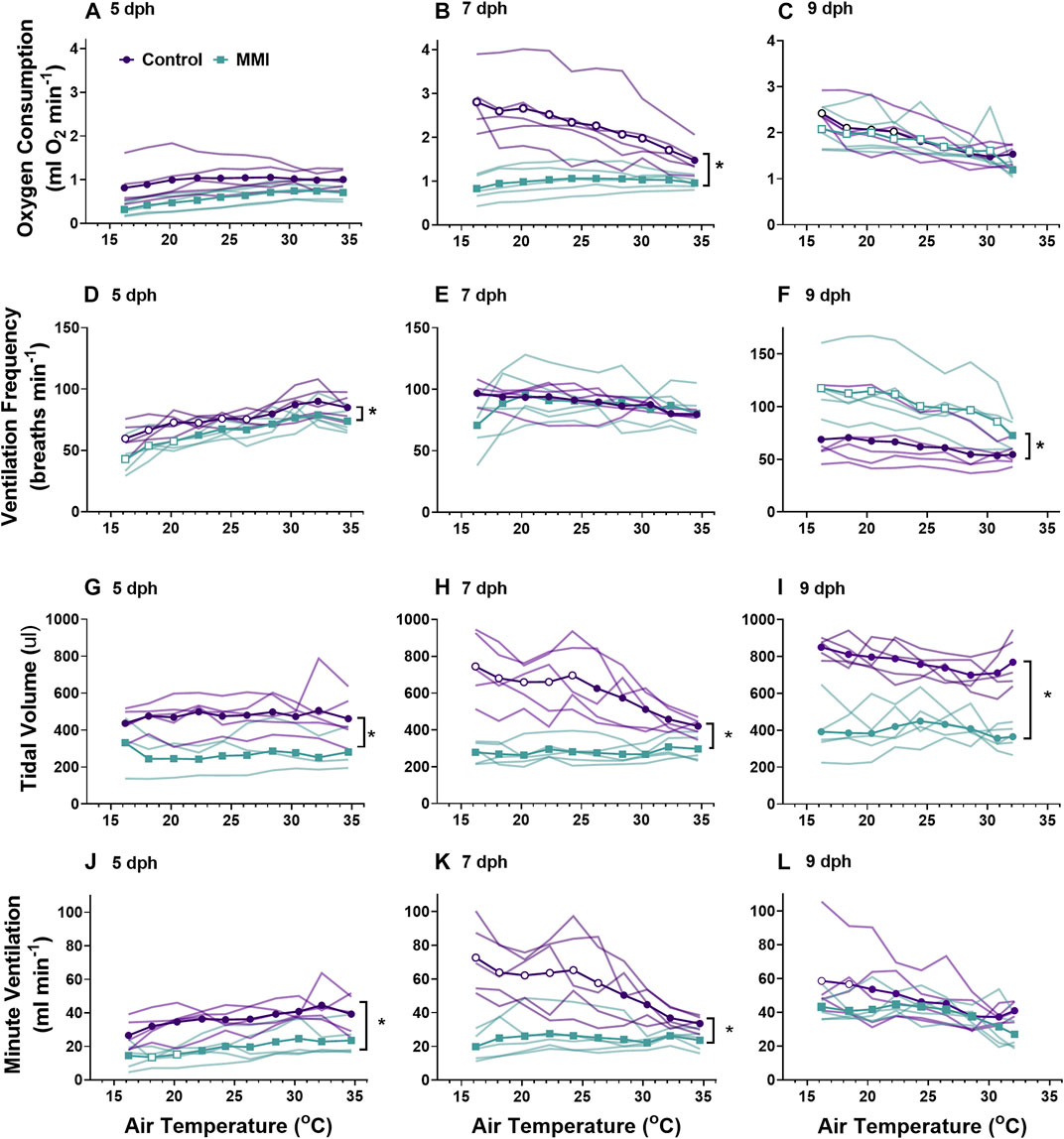
FIGURE 3. The ontogeny of oxygen consumption (A–C), ventilation frequency (D–F), tidal volume (G–I), and minute ventilation (I–K) were affected by treatment with MMI. Data presented as means and individual responses. * indicates a significant treatment effect at p < 0.05. Within a treatment, open symbols represent large or greater effects of temperature based on Cohen’s d effect size >0.8 compared with the thermal neutral value. n = 5 per treatment.
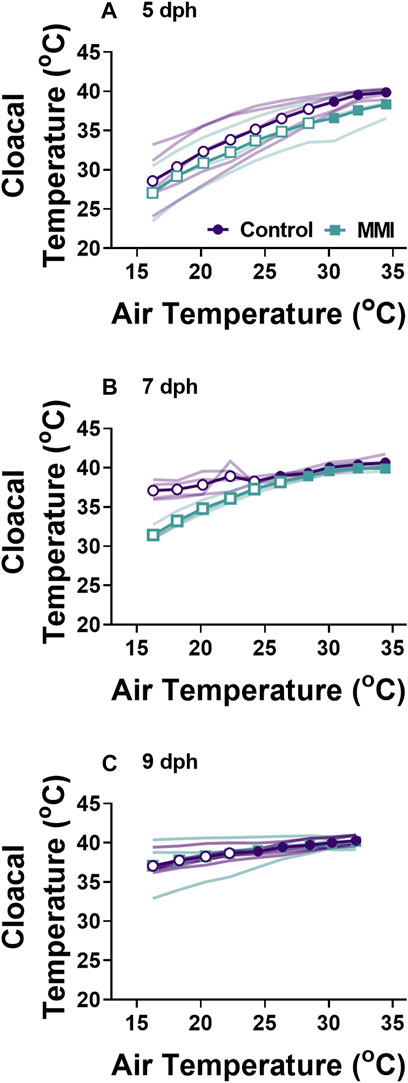
FIGURE 4. Changes in body temperature during cooling for control and MMI treated nestlings. Data presented as means individual responses. Within a treatment, open symbols represent large effect of temperature based on Cohen’s d effect sizes >0.8 compared with the thermal neutral value. n = 5 for 5 dph control and 9 dph control and MMI, n = 3 for 5 dph MMI, and n = 4 for control and MMI 7 dph.
Oxygen consumption rates from control and supplemental T3 nestlings followed the same developmental trajectories (Figures 5A–C). There was no change in
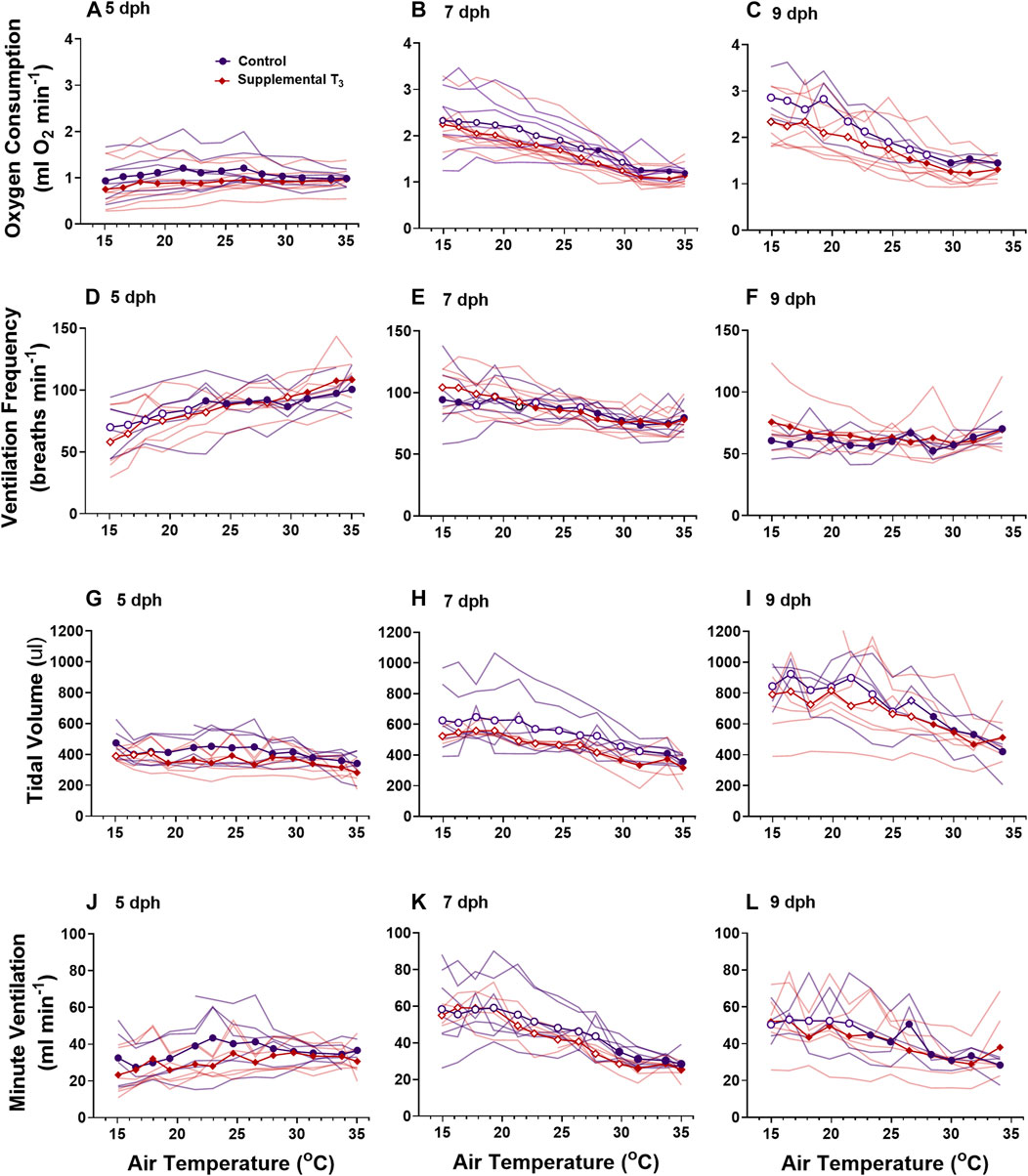
FIGURE 5. The ontogeny of oxygen consumption (A–C), ventilation frequency (D–F), tidal volume (G–I), and minute ventilation (I–K) were not affected by supplemental T3. There was no effect of T3 treatment on metabolism or ventilation. Data presented as means plus individual responses. Within a treatment, open symbols represent large or greater effects of temperature based on Cohen’s d effect sizes >0.8 compared with the thermal neutral value. n = 5 per treatment.
Ventilation
We observed differential responses to cooling in ventilation parameters based on age and MMI treatment (Figure 3). On day 5 post-hatching, control nestlings’ ventilation frequency (Figure 3D; F1, 8 = 13.45, p = 0.0063), tidal volume (Figure 3G; F1,8 = 9.20, p = 0.0162), and minute ventilation (Figure 3J; F1,8 = 19.44, p = 0.0023) were all larger than those of MMI treated nestlings, but the response to cooling was similar between the two treatments. Comparing 7 dph control and MMI treated nestlings, there were treatment effects on tidal volume (Figure 3H; F1,8 = 25.8, p = 0.001) and minute ventilation (Figure 3K; F1,8 = 14.73, p = 0.005) with control nestling levels being higher than MMI treated nestlings. In control 7 dph nestlings, the very large increase in minute ventilation associated with the decreasing ambient temperature was accomplished by a very large increase in tidal volume with no change in ventilation frequency (Figures 3E,H,K). Minute ventilation, tidal volume and ventilation frequency remained unchanged during cooling in the 7 dph MMI treated nestlings. In 9 dph nestlings, both control and MMI treated nestlings increased minute ventilation but did so in different ways (Figures 3F,I,L). Control nestlings had a higher tidal volume than MMI treated nestlings (Figure 3I; F1, 8 = 80.0, p < 0.0001) and small increases in both tidal volume and frequency combined to increase minute ventilation (Figures 3F,I; Cohen’s d < 0.8) while MMI treated nestlings had a smaller tidal volume than controls and had very large increases in ventilation frequency in response to cooling (Figure 3F).
Supplementing nestlings with T3 early in the nestling period had no effect on the development of ventilation or ventilatory responses to cooling at any age (Figure 5).
Discussion
The major question addressed in this study was whether altering thyroid hormone levels during the first 5 days post hatching would influence the growth and metabolic developmental trajectories of altricial red-winged blackbird nestlings from day 5 through 9 dph. Collectively, our data indicate that circulating thyroid hormones are important regulators of the development of endothermy and pulmonary ventilation in this species. Larger developmental effects in both morphology and physiology were observed in response to a hypothyroid condition when compared with the responses in control and hyperthyroid conditions.
Plasma T3 levels
Altricial nestlings exhibit a rise in plasma T3 levels during the first few days of post-hatching life followed by a plateau and then decline to adult levels by fledging (McNabb and Cheng, 1985; McNabb, 2006; De Groef et al., 2013). In red-winged blackbird nestlings, plasma T3 levels initially rose at hatching, plateaued around 4 dph, and remained elevated through 10 dph (Olson et al., 1999). Similar plasma T3 developmental trajectories have been observed in ring doves (McNabb and Cheng, 1985), European starlings (Schew et al., 1996; Výboh et al., 1996), great tit (Silverin and Rudas, 1996), zebra finch (Yamaguchi et al., 2017), and semi-altricial king penguin (Cherel et al., 2004). On day 5 post-hatching, plasma T3 levels in control nestlings appear to have reached the plateau as observed in other altricial and semi-altricial hatchlings (Figure 1).
In both years’ experiments, nestlings were dosed on days 2, 3, and 5 post-hatching in order to alter the natural developmental trajectory of plasma T3 levels. In 2014, treatment with MMI on 2, 3, and 5 dph resulted in a large decrease in plasma T3 levels on 5 dph compared to the control (Figure 1A). MMI primarily accumulates in the thyroid gland and lowers the production of T4 by inhibiting thyroid peroxidase (Vickers et al., 2012; Herck et al., 2013). Although plasma T4 was not measured in the current study, we expect plasma T4 decreased leading to a decreased production of T3 and the subsequent lower plasma levels of T3 in 5 dph MMI treated group. After cessation of MMI administration on 5 dph, plasma T3 levels began to increase by 7 dph. The 7 dph and older nestlings in the current study were presumably able to elevate T3 levels because of the eventual metabolism of MMI and subsequent production of T4 and T3.
Based on T3 plasma levels measured on 5 dph, supplemental T3 had little effect on nestling plasma T3 levels. However, upon halting the supplemental T3 after 5 dph, there was a trend for plasma T3 levels to be lower on 7 dph in supplemented vs. control nestlings which suggests that elevation of plasma T3 on 2, 3, and 5 dph may have inhibited the development of the thyroid gland. This lower T3 after removal of supplemental T3 may be due to reflex inhibition by exogenous T3 on the hypothalamic pituitary thyroid axis (McNabb, 2007). We expect that the supplemental T3 elevated plasma T3 on days 2, 3, and 5 dph as we found with our time course in the 4 dph nestlings. Since the 5 dph animals in which plasma T3 was measured were not dosed in the 18 h prior to their plasma being sampled (Figure 1C), we did not observe an increase in their plasma T3 at the 5 dph timepoint. In nestlings dosed on 5 dph in the field, their plasma levels were expected to have risen upon dosing and fallen back to control levels by 6 dph. The rapid elevation of plasma T3 followed over the next 24 h by a rapid decrease down to control group’s plasma levels (Figure 1C) suggests that nestlings rapidly metabolized the supplemental T3, allowing for rapid plasma clearance. The rate of T3 metabolism is not surprising, given this species has one of the highest postnatal growth rates recorded in birds (Olson, 1992).
Morphology
Altering the plasma T3 trajectory during the first 5 days of post-hatch life altered nestling growth. Newborn altricial red-winged blackbird hatchlings allocate energy towards rapid growth during the first week of nestling development before they develop proficient endothermic capacity towards the end of nestling period (Olson, 1992). In Texas, we found our nestlings fledge rapidly within 10 days of hatching. The response of nestling morphology on days 5–9 was altered by both hyperthyroid and hypothyroid conditions during the first 5 days as a nestling. The T3 hyperthyroid treatment resulted in significant decrease in body mass at 5 and 9 dph (Figure 2). Similarly, the MMI hypothyroid treatment resulted in significant decrease in nestling body mass at 7 and 9 dph, compared to control groups in red-winged blackbirds. Body mass did not differ between 5 dph MMI hypothyroid nestlings and control nestlings, but MMI treated nestling body mass began to lag behind controls after 5 dph. Similar differences in body mass have been observed previously where pharmacologically inducing hypothyroidism decreased body mass in altricial zebra finch nestlings (Rainwater et al., 2008), precocial Barnacle geese (Deaton et al., 1998) and broiler chicken (Decuypere et al., 1987) hatchlings. Prolonged treatment with 6-n- propyl-2-thiouracil (PTU), another thyroperoxidase inhibitor with similar action to that of MMI, from 1 to 5 weeks after hatch, resulted in hindered growth of Muscovy ducklings (Rey et al., 2010). These observations of slower growth both in hyper- and hypothyroidism indicate the need for maintaining optimum levels of plasma T3 levels during the rapid growth period in red-winged blackbird nestlings.
Along with mass, structural size of the nestlings was influenced by the thyroid plasma levels experienced during the first 5 days of the nestling phase. Hypothyroid conditions during the first 5 dph resulted in decreased structural size compared with controls (Figure 2). Similar decreases in structural size were observed in Zebra finch nestlings treated with perchlorate, an iodine transport inhibitor (Rainwater et al., 2008). In T3 supplemented nestlings, structural sizes did not begin to decrease compared with controls until after 5 dph when the T3 levels were dropping in the hyperthyroid nestlings. Hypothyroidism decreased tibiotarsal length in altricial zebra finch nestlings possibly due to growth plate dysgenesis as observed in mammals (Harvey et al., 2002; Rainwater et al., 2008). It is unclear if these differences in structural size would translate into differences in flight capacity of adults.
Hypothyroidism and hyperthyroidism differentially influenced the growth of tissues such as the heart and liver within the nestling. Hearts from MMI treated nestlings were smaller on 5 and 7 dph compared with the controls; the same organs were larger in T3 treated nestlings at 5 dph. Cardiac mass of 1 dph (Sirsat and Dzialowski, 2020) and 8 weeks Pekin duck (Bishop et al., 2000) were similarly decreased by MMI treatment. Deaton et al. (1998) also reported lower relative ventricle mass in hypothyroid barnacle geese and suggested the effect could be due to reduction of cardiac protein synthesis resulting in lower cardiac muscle mass. Hearts from 1 dph Pekin duck treated with T3 during late incubation were not different in mass from the controls either during external pipping or 1 dph (Sirsat and Dzialowski, 2020).
Liver mass was elevated in the MMI treated nestlings on days 5 and 7 post hatching and returned to control levels by 9 dph (Figure 2). Similar elevations in liver mass were observed in Zebra finch nestlings that had been treated with the perchlorate (Rainwater et al., 2008), pekin duck embryos (Sirsat and Dzialowski, 2020), and broiler chicken hatchlings (Decuypere et al., 1987) treated with MMI. Treatment with MMI in humans has been shown to produce hepatotoxicity (Gomez-Peralta et al., 2018). This increase in liver mass may be in response to the hepatotoxic MMI.
Whole-animal metabolic rate and body temperature
Red wing blackbird nestlings develop an endothermic response to gradual cooling around 7 dph (Olson, 1992). Prior to this time, they are able to maintain metabolic rate, but not elevate it. Tazawa et al. (2001) suggested that the maturation of an endothermic response to cooling correlated with the rise in thyroid hormones in developing birds. In the red-winged blackbird, T3 levels rise and plateau around 4-5 dph with plasma T4 levels peaking on 7 dph and both remain elevated through 9 dph (Figure 1A, (Olson et al., 1999)). Associated with this T3 plateau is the rise of an incipient endothermy where metabolic rate does not increase but is maintained over a relatively wide range of temperatures in 5 dph nestlings (Figure 3A; (Olson, 1992)), with a strong endothermic response by 7 dph (Figure 3B). The hyperthyroid nestlings show a similar endothermic developmental trajectory as control nestlings. Development of a strong endothermic response of hypothyroid nestlings was delayed compared with euthyroid nestlings (Figures 3A–H). This delay correlates well with the trajectory of plasma T3, being low in 5 dph nestlings and rising to a plateau by 7 dph (Figure 1A). Previous studies on nestling altricial birds observed that growth of the thyroid gland is highest just before the development of endothermy (Olson et al., 1999; McNabb, 2006). In nestling starlings, thyroid hormones plateau around 10 dph (McNabb, 2006) with the strongest endothermic metabolic response to cooling beginning on 12 dph (Clark, 1982). A similar relationship between thyroid hormone levels and the development of endothermy have been observed in a marsupial, the Tasmanian bettong (Rose and Kuswanti, 2004).
Thyroid hormones are known to be involved in an increase in skeletal muscle protein expression, Na+/K+ ATPase activity, sarcoplasmic reticulum calcium ATPase expression, changes in fatty acyl composition of the cell membrane, and β-adrenergic receptor density (Hulbert, 2000; Harper and Seifert, 2008). Thyroid hormones also stimulate maturation of actin and myosin isoforms resulting in maturation of skeletal muscle contractile function in developing chicken and turkey (Gardahaut et al., 1992; Maruyama et al., 1995). Thyroid hormone mediated maturation of skeletal muscle enables increased capacity for shivering thermogenesis, which is present early in the development of endothermy in altricial Red-winged blackbirds (Olson, 1994). Thyroid hormones also act directly on the central nervous system, specifically the hypothalamus, modulating sympathetic outflow signaling to skeletal muscle and heart (McNabb, 2007). Maturation of these processes in the nestling are required to exhibit a strong endothermic response to cooling in the form of increased metabolic rate.
Hyperthyroid conditions early in the nestling stage did not influence metabolism or the development of endothermy from 5 dph onward. It was predicted that moving the rise in T3 to earlier in the nestling period would accelerate the development of endothermy, such that 5 dph hyperthyroid animals would exhibit at least a moderate endothermic response to cooling. However, the metabolism and endothermic response of 5 dph hyperthyroid nestlings was similar to control nestlings. While hyperthyroid conditions produced an increase in resting metabolic rate of adult birds (Liu et al., 2006; Ruuskanen et al., 2021), the influence on birds just after hatching seems to be limited. During hatching, precocial Pekin ducks exposed to elevated plasma T3 levels had elevated resting metabolisms compared with euthyroid embryos (Sirsat and Dzialowski, 2020). Red-winged blackbird nestlings experiencing hyperthyroid during the first 5 nestling days exhibited similar metabolic responses to cooling on 5, 7, and 9 dph as those that had been euthyroid throughout the first 5 days as nestlings (Figure 5). Contrary to our prediction, the findings suggest that elevated thyroid hormones prior to the natural elevation in plasma T3 are unable to accelerate the developmental trajectory. This observation may be because during this phase of development, tissue T3 levels are already high enough to fully saturate the thyroid hormone receptors.
On 9 dph, MMI-treated nestlings showed endothermic metabolic responses similar to control and T3-supplemented animals (Figure 3C, Figure 5C). These results confirm that hypothyroid status decreased resting
Pulmonary ventilation
Development of ventilation responses to cooling in control nestlings develops along the same trajectory as that of metabolic rate. Control nestling red-winged blackbirds were able to increase minute ventilation in response to environmental cooling beginning on 7 dph. During cooling, 5 dph nestlings maintained tidal volume, but ventilation frequency decreased (Figures 3, 5). By day 9, nestlings increased minute ventilation with an increase in both tidal volume and breathing frequency in response to gradual cooling. Development of ventilation associated with an endothermic metabolic response has largely been examined in precocial birds (Sirsat and Dzialowski, 2016). In a similar fashion to the red-winged blackbird, the precocial pekin duck hatchling increased both tidal volume and breathing frequency to elevate minute ventilation during cooling (Sirsat and Dzialowski, 2016, Sirsat and Dzialowski, 2020). While an endothermic increase in ventilation does not occur until after 5 dph, chemosensitivity to oxygen and carbon dioxide develops well before this time in the red-winged blackbird (Dzialowski et al., 2016). Therefore, although nestlings at 5 dph have the capacity to finely regulate ventilation, they do not do so in response to cooling.
Altering the trajectory of the nestling plasma T3 levels had variable effects on the development of pulmonary ventilation and its regulation during gradual cooling. Elevating plasma T3 levels prior to the “normal” rise that occurs at 4-5 dph had no influence on the development of pulmonary ventilation. Ventilation control was altered when T3 levels were inhibited by administration of MMI during the first half of the nestling stage. Developing under hypothyroid conditions resulted in lower tidal volume, frequency, and ventilation at 5 dph and lower tidal volumes at rest in days 7 and day 9 nestlings (Figure 3). 9 dph hypothyroid animals were able to elevate ventilation frequency to offset the lower tidal volumes, resulting in similar ventilation as the control animals. On 7 dph, the MMI treated nestlings did not increase tidal volume like the control nestlings. This observation suggests a lack of T3 during this period of development may be influencing the development of the respiratory control network. It remains to be seen if hypothyroid conditions would also influence the development of the hypoxic and hypercapnic ventilatory responses (Dzialowski et al., 2016).
Precocial Pekin ducks exposed to hypothyroidism during the late stages of development exhibit similar changes in ventilation frequency, tidal volume, and minute ventilation (Sirsat and Dzialowski, 2020). Hypothyroidism in chicken embryos resulted in solid lung tissue on 19 days of incubation, a decrease in ventilation frequency, increase in thyroid receptor-β (TR), and attenuation of the natural increase in angiotensin converting enzyme (ACE) activity (Wittmann et al., 1987; Bjørnstad et al., 2016). Hypothyroidism in mammals resulted in decrease of central respiratory drive along with reduction in respiratory muscle strength (Milla and Zirbes, 2012). In the mammalian fetus, thyroid hormones play a role in pulmonary fluid resorption, increased expression of pulmonary β-adrenergic receptors, and increased Na+/K+ ATPase activity (Barker et al., 1990; Wilson et al., 2007; Das, 2013). In developing chicken, thyroid hormones affect lung blood flow, pulmonary vascular resistance through action on kallikrein-kinin, and ACE systems (Decuypere et al., 2005).
Delays in pulmonary ventilation caused by hypothyroidism have been observed in developing rats. Rat pups whose mothers were treated with MMI from initiation of pregnancy through 2 weeks post postpartum exhibited altered ventilation function (Rousseau et al., 2019). In both neonatal birds and mammals, hypothyroidism has a negative influence on ventilation development that may be due to alterations of the autonomic nervous system during development. Hypothyroid conditions delayed the maturation of the respiratory control network in rat pups (Rousseau et al., 2021). In the rat pups, there was a GABAergic inhibition that produced the abnormal respiratory responses. These same GABAergic pathways may play a role in the avian response observed here. Hypothyroid conditions in neonatal rats resulted in degradation and hypertrophy of the diaphragm muscles (Canavan et al., 1994). The mechanism by which hypothyroidism influences development of respiratory control of altricial birds remains to be seen.
Conclusion
In the altricial red-winged blackbird nestling, the trajectory of thyroid hormone levels during early development influences growth and metabolic maturation. In this study, greater developmental changes were seen in response to hypothyroidism than hyperthyroidism. Decreased levels of plasma T3 in the current study showed, for the first time in wild altricial birds, delayed ontogeny of endothermic response. These findings are similar to delayed endothermic development observed in developing precocial Pekin duck in response to hypothyroidism (Sirsat and Dzialowski, 2020). The lack of metabolic and ventilatory changes in response to hyperthyroidism may be due to the already high levels of T3 during development, with further elevation having little effect as receptors may already be saturated.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by University of North Texas IACUC.
Author contributions
TS, SS, EP, and ED contributed to conception and design of the study. TS, SS, EP, MP, and ED contributed to collecting and processing the data. ED and TS performed the statistical analysis. TS, SS, and ED contributed to drafting the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This work was supported by the National Science Foundation (IOS 1145758 to ED).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barker P. M., Strang L. B., Walters D. V. (1990). The role of thyroid hormones in maturation of the adrenaline-sensitive lung liquid reabsorptive mechanism in fetal sheep. J. Physiol. 424, 473–485. doi:10.1113/jphysiol.1990.sp018078
Bishop C. M., McCabe C. J., Gittoes N. J., Butler P. J., Franklyn J. A. (2000). Tissue-specific regulation of thyroid hormone receptor mRNA isoforms and target gene proteins in domestic ducks. J. Endocrinol. 165, 607–615. doi:10.1677/joe.0.1650607
Bjørnstad S., Samara A., Erichsen A., Paulsen R. E., Glover J. C., Roald B. (2016). Hampered lung maturation in methimazole-induced hypothyroidism in fetal chicken: morphological and molecular correlates to human fetal development. Neonatology 110, 83–92. doi:10.1159/000444656
Breall J. A., Rudolph A. M., Heymann M. A. (1984). Role of thyroid hormone in postnatal circulatory and metabolic adjustments. J. Clin. Invest. 73, 1418–1424. doi:10.1172/JCI111346
Canavan J. P., Holt J., EastonSmith K., Goldspink D. F. (1994). Thyroid-induced changes in the growth of the liver, kidney, and diaphragm of neonatal rats. J. Cell. Physiol. 161, 49–54. doi:10.1002/jcp.1041610107
Cherel Y., Durant J. M., Lacroix A. (2004). Plasma thyroid hormone pattern in king penguin chicks: a semi-altricial bird with an extended posthatching developmental period. Gen. Comp. Endocrinol. 136, 398–405. doi:10.1016/j.ygcen.2004.02.003
Clark L. (1982). The development of effective homeothermy and endothermy by nestling starlings. Comp. Biochem. Physiology Part A Physiology 73, 253–260. doi:10.1016/0300-9629(82)90066-4
Cossin-Sevrin N., Hsu B.-Y., Marciau C., Viblanc V. A., Ruuskanen S., Stier A. (2022). Effect of prenatal glucocorticoids and thyroid hormones on developmental plasticity of mitochondrial aerobic metabolism, growth and survival: an experimental test in wild great tits. J. Exp. Biol. 225, jeb243414. doi:10.1242/jeb.243414
Das K. C. (2013). Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in mle-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria. PLoS ONE 8, e73358. doi:10.1371/journal.pone.0073358
De Groef B., Grommen S. V. H., Darras V. M. (2013). Hatching the cleidoic egg: the role of thyroid hormones. Front. Endocrinol. 4, 63. doi:10.3389/fendo.2013.00063
Deaton K. E., Bishop C. M., Butler P. J. (1998). Tissue-specific effects of hypothyroidism on postnatal muscle development in the barnacle goose. J. Exp. Biol. 201, 827–836. doi:10.1242/jeb.201.6.827
Decuypere E., Buyse J., Scanes C. G., Huybrechts L., Kühn E. R. (1987). Effects of hyper- or hypothyroid status on growth, adiposity and levels of growth hormone, somatomedin C and thyroid metabolism in broiler chickens. Reprod. Nutr. Dev. 27, 555–565. doi:10.1051/rnd:19870414
Decuypere E., Van As P., Van der Geyten S., Darras V. M. (2005). Thyroid hormone availability and activity in avian species: A review. Domest. Anim. Endocrinol. 29, 63–77. doi:10.1016/j.domaniend.2005.02.028
Dzal Y. A., Milsom W. K. (2021). Effects of hypoxia on the respiratory and metabolic responses to progressive cooling in newborn rodents that range in heterothermic expression. Exp. Physiol. 106, 1005–1023. doi:10.1113/EP089085
Dzialowski E. M., Sirsat T. S., Sirsat S. K., Price E. R. (2016). Breathing while altricial: the ontogeny of ventilatory chemosensitivity in red-winged blackbird (Agelaius phoeniceus) nestlings. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R1105–R1112. doi:10.1152/ajpregu.00273.2016
Gardahaut M. F., Fontaine-Perus J., Rouaud T., Bandman E., Ferrand R. (1992). Developmental modulation of myosin expression by thyroid hormone in avian skeletal muscle. Development 115, 1121–1131. doi:10.1242/dev.115.4.1121
Gomez-Peralta F., Velasco-Martínez P., Abreu C., Cepeda M., Fernández-Puente M. (2018). Hepatotoxicity in hyperthyroid patient after consecutive methimazole and propylthiouracil therapies. Endocrinol. Diabetes Metab. Case Rep. 2018, 17-0173. doi:10.1530/EDM-17-0173
Harper M.-E., Seifert E. L. (2008). Thyroid hormone effects on mitochondrial energetics. Thyroid 18, 145–156. doi:10.1089/thy.2007.0250
Harvey C. B., O’Shea P. J., Scott A. J., Robson H., Siebler T., Shalet S. M., et al. (2002). Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol. Genet. Metab. 75, 17–30. doi:10.1006/mgme.2001.3268
Herck S. L. J. V., Geysens S., Bald E., Chwatko G., Delezie E., Dianati E., et al. (2013). Maternal transfer of methimazole and effects on thyroid hormone availability in embryonic tissues. J. Endocrinol. 218, 105–115. doi:10.1530/JOE-13-0089
Hsu B.-Y., Doligez B., Gustafsson L., Ruuskanen S. (2019). Transient growth-enhancing effects of elevated maternal thyroid hormones at no apparent oxidative cost during early postnatal period. J. Avian Biol. 2019, e01919. doi:10.1111/jav.01919
Hsu B.-Y., Sarraude T., Cossin-Sevrin N., Crombecque M., Stier A., Ruuskanen S. (2020). Testing for context-dependent effects of prenatal thyroid hormones on offspring survival and physiology: an experimental temperature manipulation. Sci. Rep. 10, 14563. doi:10.1038/s41598-020-71511-y
Hulbert A. J. (2000). Thyroid hormones and their effects: a new perspective. Biol. Rev. Camb. Philos. Soc. 75, 519–631. doi:10.1017/s146479310000556x
Iwen K. A., Oelkrug R., Brabant G. (2018). Effects of thyroid hormones on thermogenesis and energy partitioning. J. Mol. Endocrinol. 60, R157–R170. doi:10.1530/JME-17-0319
Little A. G., Seebacher F. (2014). The evolution of endothermy is explained by thyroid hormone-mediated responses to cold in early vertebrates. J. Exp. Biol. 217, 1642–1648. doi:10.1242/jeb.088880
Liu J.-S., Chen Y.-Q., Li M. (2006). Thyroid hormones increase liver and muscle thermogenic capacity in the little buntings (Emberiza pusilla). J. Therm. Biol. 31, 386–393. doi:10.1016/j.jtherbio.2006.01.002
Maruyama K., Kanemaki N., May J. D. (1995). Thyroid hormones on embryo development and appearance of myosin heavy chain isoforms in turkeys (Meleagris gallopavo). Comp. Biochem. Physiology Part C Pharmacol. Toxicol. Endocrinol. 112, 109–117. doi:10.1016/0742-8413(95)02002-0
McNabb F. M. A. (2006). Avian thyroid development and adaptive plasticity. Gen. Comp. Endocrinol. 147, 93–101. doi:10.1016/j.ygcen.2005.12.011
McNabb F. M. A., Cheng M.-F. (1985). Thyroid development in altricial ring doves, Streptopelia risoria. Gen. Comp. Endocrinol. 58, 243–251. doi:10.1016/0016-6480(85)90340-5
McNabb F. M. A. (2007). The hypothalamic-pituitary-thyroid (HPT) axis in birds and its role in bird development and reproduction. Crit. Rev. Toxicol. 37, 163–193. doi:10.1080/10408440601123552
Menna T. M., Mortola J. P. (2003). Ventilatory chemosensitivity in the chick embryo. Respir. Physiol. Neurobiol. 137, 69–79. doi:10.1016/S1569-9048(03)00109-5
Milla C. E., Zirbes J. (2012). Pulmonary complications of endocrine and metabolic disorders. Paediatr. Respir. Rev. 13, 23–28. doi:10.1016/j.prrv.2011.01.004
Nakagawa S., Cuthill I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 82, 591–605. doi:10.1111/j.1469-185X.2007.00027.x
Olson J. M. (1992). Growth, the development of endothermy, and the allocation of energy in red-winged blackbirds (Agelaius phoeniceus) during the nestling period. Physiol. Zool. 65, 124–152. doi:10.1086/physzool.65.1.30158243
Olson J. M., McNabb F. M. A., Jablonski M. S., Ferris D. V. (1999). Thyroid development in relation to the development of endothermy in the red-winged blackbird (Agelaius phoeniceus). Gen. Comp. Endocrinol. 116, 204–212. doi:10.1006/gcen.1999.7363
Olson J. (1994). The ontogeny of shivering thermogenesis in the red-winged blackbird (Agelaius phoeniceus). J. Exp. Biol. 191, 59–88. doi:10.1242/jeb.191.1.59
Price E. R., Dzialowski E. M. (2018). Development of endothermy in birds: patterns and mechanisms. J. Comp. Physiol. B 188, 373–391. doi:10.1007/s00360-017-1135-0
Rainwater T. R., Wood M. B., Millam J. R., Hooper M. J. (2008). Effects of perchlorate on growth and behavior of a granivorous passerine, the zebra finch (Taeniopygia guttata). Arch. Environ. Contam. Toxicol. 54, 516–524. doi:10.1007/s00244-007-9045-x
Rey B., Roussel D., Romestaing C., Belouze M., Rouanet J.-L., Desplanches D., et al. (2010). Up-regulation of avian uncoupling protein in cold-acclimated and hyperthyroid ducklings prevents reactive oxygen species production by skeletal muscle mitochondria. BMC Physiol. 10, 5. doi:10.1186/1472-6793-10-5
Rose R. W., Kuswanti N. (2004). Thyroid function and the development of endothermy in a marsupial, the Tasmanian bettong, Bettongia gaimardi (Demarest 1822). Gen. Comp. Endocrinol. 136, 17–22. doi:10.1016/j.ygcen.2003.11.007
Rousseau J.-P., Tenorio-Lopes L., Ghio S. C., Desjardins P., Fournier S., Kinkead R. (2021). Thyroid hormones during the perinatal period are necessary to respiratory network development of newborn rats. Exp. Neurol. 345, 113813. doi:10.1016/j.expneurol.2021.113813
Rousseau J. P., Buteau-Poulin A., Kinkead R. (2019). Maternal thyroid hormone deficiency and cardiorespiratory disorder in rat pups. Exp. Neurol. 320, 112960. doi:10.1016/j.expneurol.2019.112960
Ruuskanen S., Hsu B.-Y., Nord A. (2021). Endocrinology of thermoregulation in birds in a changing climate. Mol. Cell. Endocrinol. 519, 111088. doi:10.1016/j.mce.2020.111088
Sarraude T., Hsu B.-Y., Groothuis T., Ruuskanen S. (2020). Testing the short-and long-term effects of elevated prenatal exposure to different forms of thyroid hormones. PeerJ 8, e10175. doi:10.7717/peerj.10175
Schew W. A., McNabb F. M. A., Scanes C. G. (1996). Comparison of the ontogenesis of thyroid hormones, growth hormone, and insulin-like growth factor-I in ad libitum and food-restricted (altricial) European starlings and (precocial) Japanese quail. Gen. Comp. Endocrinol. 101, 304–316. doi:10.1006/gcen.1996.0033
Silverin B., Rudas P. (1996). Thyroid hormones in nestling great tits (Parus major). Gen. Comp. Endocrinol. 103, 138–141. doi:10.1006/gcen.1996.0104
Sirsat S. K. G., Sirsat T. S., Crossley J. L., Sotherland P. R., Dzialowski E. M. (2016). The 12-day thermoregulatory metamorphosis of red-winged blackbirds (Agelaius phoeniceus). J. Comp. Physiol. B 186, 651–663. doi:10.1007/s00360-016-0978-0
Sirsat T. S., Dzialowski E. M. (2020). Manipulating plasma thyroid hormone levels at hatching alters development of endothermy and ventilation in Pekin duck (Anas platyrhynchos domestica). J. Exp. Biol. 223, jeb237701. doi:10.1242/jeb.237701
Sirsat T. S., Dzialowski E. M. (2016). Ventilation changes associated with hatching and maturation of an endothermic phenotype in the Pekin duck, Anas platyrhynchos domestica. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R766–R775. doi:10.1152/ajpregu.00274.2015
Sneddon L. U., Halsey L. G., Bury N. R. (2017). Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 220, 3007–3016. doi:10.1242/jeb.147058
Szdzuy K., Mortola J. P. (2008). Ventilatory chemosensitivity and thermogenesis of the chicken hatchling after embryonic hypercapnia. Respir. Physiol. Neurobiol. 162, 55–62. doi:10.1016/j.resp.2008.04.001
Tazawa H., Moriya K., Tamura A., Komoro T., Akiyama R. (2001). Ontogenetic study of thermoregulation in birds. J. Therm. Biol. 26, 281–286. doi:10.1016/S0306-4565(01)00031-6
Vickers A. E. M., Heale J., Sinclair J. R., Morris S., Rowe J. M., Fisher R. L. (2012). Thyroid organotypic rat and human cultures used to investigate drug effects on thyroid function, hormone synthesis and release pathways. Toxicol. Appl. Pharmacol. 260, 81–88. doi:10.1016/j.taap.2012.01.029
Výboh P., Zeman M., Juráni M., Buyse J., Decuypere E. (1996). Plasma thyroid hormone and growth hormone patterns in precocial Japanese quail and altricial European starlings during postnatal development. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 114, 23–27. doi:10.1016/0742-8413(95)02106-X
Wilson S. M., Olver R. E., Walters D. V. (2007). Developmental regulation of lumenal lung fluid and electrolyte transport. Respir. Physiol. Neurobiol. 159, 247–255. doi:10.1016/j.resp.2007.10.004
Withers P. C. (2001). Design, calibration and calculation for flow-through respirometry systems. Aust. J. Zool. 49, 445–461. doi:10.1071/zo00057
Wittmann J., Schmidt P., Schranner I. (1987). Activity of putative pulmonary ornithokallikrein and angiotensin-converting enzyme during the onset of lung respiration in the chick embryo. J. Exp. Zool. Suppl. 1, 219–226.
Yamaguchi S., Hayase S., Aoki N., Takehara A., Ishigohoka J., Matsushima T., et al. (2017). Sex differences in brain thyroid hormone levels during early post-hatching development in zebra Finch (Taeniopygia guttata). PLOS ONE 12, e0169643. doi:10.1371/journal.pone.0169643
Keywords: endothermy, thyroid hormone, ventilation, metabolic rate, altricial bird, Agelaius phoeniceus
Citation: Sirsat TS, Sirsat SKG, Price ER, Pineda M and Dzialowski EM (2022) Manipulating plasma thyroid hormone levels alters development of endothermy and ventilation in nestling red-winged blackbirds. Front. Physiol. 13:1027257. doi: 10.3389/fphys.2022.1027257
Received: 24 August 2022; Accepted: 14 November 2022;
Published: 29 November 2022.
Edited by:
Vincent Joseph, Laval University, CanadaReviewed by:
Cayleih E Robertson, McMaster University, CanadaLara Amaral-Silva, University of Missouri, United States
Copyright © 2022 Sirsat, Sirsat, Price, Pineda and Dzialowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edward M. Dzialowski, RWQuRHppYWxvd3NraUB1bnQuZWR1
†Present addresses: Tushar S. Sirsat, Department of Physician Assistant Studies, Clarkson University, Potsdam, NY, United States
Sarah K. Sirsat, Department of Biology, SUNY Potsdam, Potsdam, NY, United States
Edwan R. Price, Green Godwit Consulting, Cleveland, OH, United States
Megan Pineda, FUJIFILM Diosynth Biotechnologies Texas, College Station, TX, United States
 Tushar S. Sirsat
Tushar S. Sirsat Sarah K. G. Sirsat2,3†
Sarah K. G. Sirsat2,3† Edwan R. Price
Edwan R. Price Edward M. Dzialowski
Edward M. Dzialowski