
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 17 November 2022
Sec. Invertebrate Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.1024409
Bursaphelenchus xylophilu is a worldwide quarantine nematode, causing huge economic losses and ecological disasters in many countries. The sex ratio of B. xylophilus plays an important role in the nematode infestation. The laf-1-related genes are highly conserved in animals, playing crucial roles in sex determination. Therefore, we investigated the expression pattern and biological function of its orthologue, Bxy-laf-1 in B. xylophilus. Bxy-laf-1 has two typical conserved DNA-binding domains, DEAD and Helicase C. The real-time quantitative PCR data revealed that Bxy-laf-1 expression was required throughout the entire life of B. xylophilus, with the maximum expression in the J2 stage and the lowest expression in the adult stage. mRNA in situ hybridization showed that Bxy-laf-1 is mainly located in the cephalopharynx and reproductive organs of B. xylophilus. RNA interference (RNAi) indicated that the head swing frequency was dramatically decreased. The RNA interference results displayed that a significant reduction in motility was observed in the hatched larvae. The female to male sex ratio was also decreased in the F0 and F1 generations, but recovered in the F2 generation. The tail of female adults with eggs in the belly appeared deformities. This phenomenon appeared in the F0 and F1 generations, but recovered in the F2 generation. Bxy-laf-1 is a typical sex-determination gene with distinct expression patterns in males and females. As demonstrated in other species, the sex ratio was altered after knocking down Bxy-laf-1 expression. The results of this study contribute to our understanding of the molecular processes of Bxy-laf-1 in B. xylophilus, which may provide clues about how to control pine wilt disease by inhibiting ontogenic growth and reducing nematode fertility.
Bursaphelenchus xylophilus (pinewood nematode) is a global quarantine nematode that is originally from the United States (Wingfield et al., 1982) before spreading to Asia and Europe. It has now wreaked havoc on the invading region’s economy (Sun, 1982; Yi et al., 1989; Mota et al., 1999; Kikuchi et al., 2011; Robertson et al., 2011; Zhao et al., 2016). In B. xylophilus endemic locations, previous studies have demonstrated significant interspecific conflict between invading nematodes and native sister nematodes (Bursaphelenchus mucronatus), with B. xylophilus finally displacing native nematodes (Cheng et al., 2009). In recent studies, we discovered that the sex ratio (female-to-male) of the two nematodes increases dramatically in mixed cultures, suggesting that the shift in the sex ratio played a key role in species competition. As a result, altering the sex ratio is a new concept for controlling the pest’s invasion. The fundamental elements that determine the sex of pine worms are still unknown.
The laf-1 gene is a critical male sex determination gene in Caenorhabditis elegans (Haag, 2005), which favors male cell fate by negatively regulating the expression of the tra-2 gene in hermaphrodites and males, resulting in more male progeny (Hubert and Anderson, 2009). At all stages of development, it is expressed in the somatic cells of male gonads and hermaphrodite nematodes. The laf-1 heterozygous mutant in C. elegans causes XX animals to mature into females and partially feminizes XO somatic cells and germline (Doniach, 1986; Schedl and Kimble, 1988; Goodwin et al., 1997). In addition, laf-1 is required for embryonic development. A pure mutation in laf-1 causes the death of embryos or larval in C. elegans (Hubert and Anderson, 2009). Its significance in embryo and gonadal development has been confirmed. Therefore, it assumes that laf-1 may play a conserved role in determining sex fate in B. xylophilus. Regulating sex ratios in nematode populations may provide a promising clue for managing nematodes. However, no research on Bxy-laf-1 has been reported in B. xylophilus yet.
Bxy-laf-1 gene was cloned to investigate the expression pattern and biological function in B. xylophilus. In this work, sequence alignment, phylogenetic analysis and 3D-struction predication were performed and real-time quantitative PCR (RT-qPCR), whole-mount in situ hybridization and RNA interference (RNAi) were used to examine the expression levels at different stages of B. xylophilus development and biological functions of Bxy-laf-1. This is the first study to show that Bxy-laf-1 is required for the mobility, early development, reproduction and mating behavior of B. xylophilus.
The B. xylophilus NXY61 nematode population was received from the Chinese Academy of Forestry’s Research Institute of Forest Protection in Beijing, China, and was first isolated from sick Pinus massoniana in Ningbo, Zhejiang province, China. Botrytis cinerea, a fungal food source, was supplied by a Zhejiang University laboratory. Nematodes were cultivated in Petri dishes with potato dextrose agar (PDA) media at 25°C in the dark on a lawn of B. cinerea (Mamiya, 1975; Wang, 2007).
The isolated mixed stage nematodes were transferred to a fresh Petri dish containing sterile distilled water and allowed to lay embryos for 1 h at 25°C in the dark to collect synchronized B. xylophilus samples (Futai, 1980). The synchronized embryos were collected after the mixed stage nematodes were removed. The recovered synchronous embryos were cultured for 24 h in the dark at 25°C to produce synchronous second-stage juveniles (J2) (Wang et al., 2005). Following that, the J2 larvae were centrifuged at 3,000 rpm for 3 min, the supernatant was removed, and they were put on B. cinerea for 29, 48, and 72 h at 25°C in the dark, separately (Zhu et al., 2016). The Bellman funnel method was used to capture synchronized juveniles from J2 to the fourth stage (J4) and adults (Viglierchio and Schmitt, 1983).
TRIZOL Reagent (Thermo Fisher Scientific, China) was used to extract total RNA from B. xylophilus at various developmental stages, according to the manufacturer’s instructions. After that, the RNAs were processed with RNase-free DNase I. (Thermo Fisher Scientific). A NanoDrop® ND-2000 spectrophotometer was used to determine RNA concentration and purity (Thermo Fisher Scientific). By reverse transcription with PrimeScript™ RT reagent Kit with gDNA Eraser, first-strand cDNA was generated from 0.5 g of total RNA using a combination of oligo dT primers and random hexamers (Takara, China).
To amplify laf-1 in B. xylophilus, a set of specialized primers (Table 1) were created. 100 ng of cDNA, 10 μL 2 × Ex Taq Polymerase Premix (TaKaRa Biotechnology Co. Ltd., Dalian, China), 1 μL of each primer (10 pmol L−1), and sterile distilled water were used in a 20 μL reaction. The following was the amplification procedure: 5 min at 94°C, then 35 cycles of 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C, followed by a 5-min extension step at 72°C. The amplified products were then cloned into a pGEM®-T Easy vector for sequencing (Promega, Madison, WI, United States).
To analyze the sequence of Bxy-laf-1, the cloned fragment obtained via the above process was used to BLAST our recently sequenced transcriptome. Additional protein sequences for alignments were obtained from Genbank (www.ncbi.nlm.nih.gov/genbank/). DNAMAN software (Lynnon Biosoft) was used to perform multiple sequence alignments, and MEGAX was used to create a phylogenetic tree using the neighbour-joining technique (Sudhir et al., 2018). The nucleotide sequences of cloned genes were used to determine the protein sequences of Bxy-laf-1 of B. xylophilus. Genbank (www.ncbi.nlm.nih.gov/genbank/) was also utilized to get protein sequences for additional species used in alignments. TMHMM server 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) and SignaIP 5.0 (http://www.cbs.dtu.dk/services/SignaIP/) were used to predict Bxy-laf-1 transmembrane helices and signal sequences, respectively. ExPASy ProtParam (https://web.expasy.org/protparam/) was used to evaluate the physicochemical parameters of the inferred amino acid sequence of Bxy-laf-1, such as amino acid composition, molecular weight, isoelectric point, and instability (Gasteiger et al., 2003).
The predicted three-dimensional structure of Bxy-LAF-1 was modeled with AlphaFold2 (John et al., 2021). The structure visualization was created in PyMOL program (Schrödinger, LLC) (Rigsby and Parker, 2016).
RT-qPCR technique was used to examine the expression profile of Bxy-laf-1 in B. xylophilus at various developmental stages (embryo, J2, J3, J4, and adult). The same procedure was used to extract total RNA and convert it to cDNA as previously reported. The qTOWER 2.2 qPCR System (Analytik JenaAG) was used to perform RT-qPCR with TB Green® Premix Ex Taq IITM (TaKaRa, TliRNaseH Plus). Internal loading controls included the stable reference genes β-actin (GenBank accession number EU100952.1) and tbb-2 (tubulin beta-2 chain, GenBank accession number MT769316). The 2−ΔΔCT approach was used to determine the relative expression level of Bxy-laf-1 (Livak and Schmittgen, 2001). Beacon Designer software was used to create primers for the Bxy-laf-1 and reference genes, following the manufacturer’s instructions (Table 1) (Brenda and Chhandak, 2015). Melt curves and standard curves were used to confirm the primers’ specificity and amplification efficiency, respectively.
mRNA in situ hybridization was done at different developmental stages (embryo, J2, J3, J4, female adult, and male adult) of B. xylophilus to investigate the temporal and spatial expression patterns of Bxy-laf-1, based on the procedure of Motohashi et al. in C. elegans with minimal modifications (Motohashi et al., 2006; Tang et al., 2020). Probes were made from the cloned fragment (1,085 bp) acquired using the aforementioned procedure. A DIG RNA labeling kit was used to produce and label antisense and sense RNA probes (Roche Gmbh, Mannheim, Germany). A ZEISS Inverted Observer light microscope was used to make the final observations (Carl Zeiss, Germany). As a negative control, the sensing probe was employed.
The roles of Bxy-laf-1 in B. xylophilus were investigated using RNAi. The dsRNA fragments for Bxy-laf-1 and gfp were synthesized using a MEGAscript® T7 High Yield Transcription Kit (Thermo Fisher Scientific Inc.) and the primers as directed by the manufacturer (Table 1). A NanoDrop®ND-2000 spectrophotometer (Thermo Fisher Scientific, United States) was used to assess the quality of produced dsRNAs, which were then visualized in a 1.5% agarose gel.
The RNAi efficiency was determined using specific Bxy-laf-1 primer sets (Table 1). Three parallel sets of experiments were run, with four replicate wells set up each time, for a total of three biological replicates.
Synchronized embryos and J2 (approximately 20,000 individuals of each stage) were washed and soaked for 24 h in the dark at 25°C in dsRNA (0.5 μg m L−1) with the soaking buffer (0.05 percent gelatin, 5.5 mM KH2PO4, 2.1 mM NaCl, 4.7 mM NH4Cl, 3 mM spermidine). As a control, the same number of nematodes were soaked in the soaking buffer without dsRNA or gfp dsRNA. Each treatment was replicated three times. Following treatment, the different developmental stages were cultured separately on a B. cinerea lawn in the PDA plate to adulthood (F0) at 25°C (Tabara et al., 1998). After they gave birth to F1 embryos, collect 1,000 adult F0 nematodes and the results of sex ratio was statistically analyzed. Then the F1 generation embryos were cultured to adulthood, and the same method was used to collect, count and analyze the sex ratio of the F1 generation and F2 generation adults.
The movement of these nematodes was captured using an Axio Cam Mrm camera and Zeiss Axio Vision software (Carl Zeiss) at 25°C. Thirty-second films were acquired for each position. A successful head thrash was defined as a reorientation of more than 120° in a single head swing. Each replicate used thirty synchronized J2, J3 nematodes, and the experiment was repeated three times. The Nikon NI-SS microscope was used to examine morphological phenotypes (Nikon, Japan). Nikon DS-Ri2 camera was used to capture differential interference contrast (DIC) images.
To explore the influence of Bxy-laf-1 on progeny production, virgin adults were grown and employed in the study. J2 were soaked in the buffer with dsRNA (0.5 mg L−1) and without dsRNA for 24 h to improve mating efficiency and increase the possibilities of mating observation, and J4 was obtained by synchronous culture for 24 h. Synchronized male and female J4 were cultivated separately for 24 h to yield virgin mature males and females. There are four cross mating combinations (CK ♀ + CK ♂, RNAi ♀ + CK ♂, CK ♀ + RNAi ♂, RNAi ♀ + RNAi ♂), CK means control. (Li et al., 2020). Under a microscope, a virgin male and virgin female were placed in a drop of water on a concave glass slide. In a humidifier, the slides were stored in the dark at 25°C (Zhou et al., 2020). A zoom stereomicroscope (Zeiss, Thornwood, United States) was used to investigate the mating behavior of male and female adults. The percentage of error positioning ratio (males who did not correctly locate the female vulva) and average number of offspring was calculated. Each experimental treatment had three sets of 30 pairs of nematodes (30 males and 30 females), and the experiment was performed three times.
All data, including head thrashing frequency, improper positioning ratio, and sex ratios, were presented as mean standard error. SPSS 22.0 was used to do statistical analysis and calculations based on the independent sample t-test and one-way ANOVA (SPSS Inc., United States). The results were deemed statistically significant when p < 0.05 was used. All RT-qPCRs were performed in triplicate.
Full length of the orthologue of laf-1, Bxy-laf-1, was determined, which contained an open reading frame (ORF) of 2,163 bp and encoded 720 amino acids in B xylophilus. This gene has one copy number and no other isoforms in the genome. The estimated molecular formula of the Bxy-laf-1 protein was C3512H5548N1038O1068S29 with a theoretical equivalent pI value of 8.67, a molecular weight of 80.33 kDa and an atomic number of 11,195. It is speculated that Bxy-laf-1 may be an unstable protein with a calculated instability index (Ⅱ) of 43.75. Protein sequence of LAF-1 alignment clearly revealed that BXY-LAF-1 had two conserved domains, DEAD and Helicase C (Figure 1). These two domains belong to an ATP-dependent RNA helicase required during sperm development and is essential for the integrity of the reproductive system (Lu et al., 2020). In addition, Bxy-LAF-1 of B. xylophilus had the highest level of similarities with Bmu-LAF-1 of B. mucronatus. To uncover the evolutionary relationship between the Bxy-laf-1 gene and the laf-1 gene of other species, the phylogenetic tree of LAF-1 was constructed, showing that Bxy-LAF-1 and Bmu-LAF-1 formed one branch, and further produced a big node with other LAF-1 proteins in nematodes, such as LAF-1 of C. elegans (Figure 2).
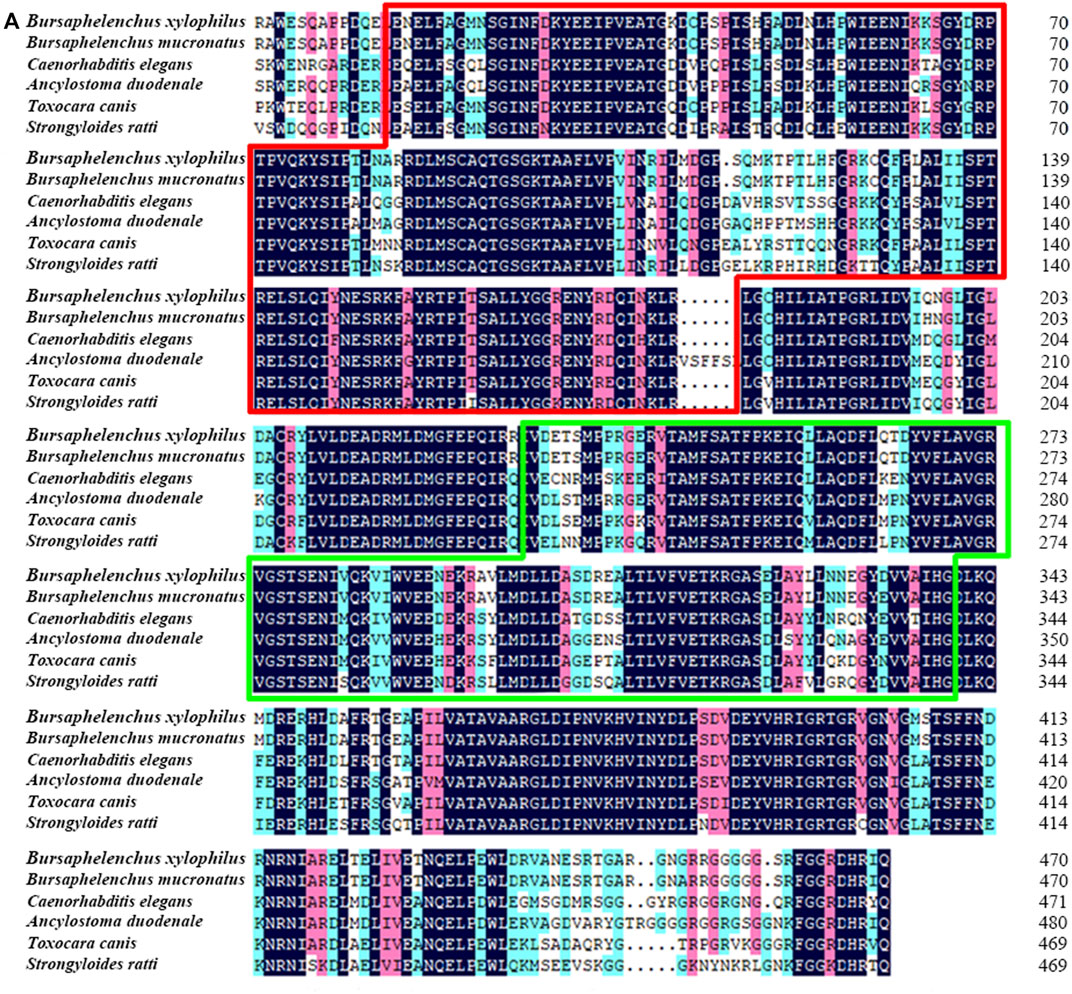
FIGURE 1. Sequence analysis of Bxy-laf-1. (A) Multiple amino acid sequence alignments. B. xylophilus (CAD5234552.1), B. mucronatus (OM210018), Caenorhabditis elegans (NP 001254859.1), Ancylostoma duodenale (KIH65785.1), Toxocara canis (KHN84719.1) and Strongyloides ratti (XP 024501163.1). Black, red and blue shading indicates invariant, highly conserved and moderately conserved amino acid residues, respectively. The two conserved DNA-binding motifs of LAF-1, DEAD and Helicase C, are enclosed by the red and green boxes, respectively.
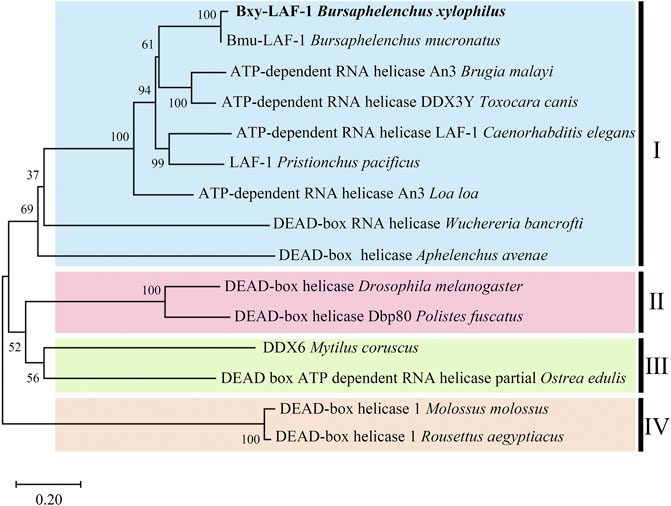
FIGURE 2. Phylogenetic analysis of LAF-1s from different species. The scale bar indicates evolutionary distance (0.20 substitutions per position). Ⅰ (LAF-1s in Nematoda): Bursaphelenchus xylophilus (CAD5234552.1), Bursaphelenchus mucronatus (OM210018), Brugia malayi (XP 042930654.1), Toxocara canis (KHN84719.1), Caenorhabditis elegans (NP 001254859.1), Pristionchus pacificus (KAF8381410.1), Loa loa (XP 003140162.1), Wuchereria bancrofti (EJW85565.1), Aphelenchus avenae (KAH7731651.1); Ⅱ (LAF-1s in Arthropoda): Drosophila melanogaster (AAC23709.1), Polistes fuscatus (XP 043504272.1); Ⅲ (LAF-1s in Molluscs): Mytilus coruscus (CAC5359041.1), Ostrea edulis (AFJ91775.1); Ⅳ (LAF-1s in Chordata): Molossus (KAF6429176.1), Rousettus aegyptiacus (KAF6446427.1).
The three-dimensional structure prediction of Bxy-laf-1 protein of B. xylophilus was modeled by AlphaFold2, using 6KUX as the template (Figure 3). Bxy-laf-1 exhibits two conserved structural domains, DEAD box helicase and Helicase conserved C-terminal domain (Figure 3A). The molecular surface of Bxy-laf-1 was also calculated using the PyMOL program (Figure 3B), the variable region and conserved region were colored from blue to purple.
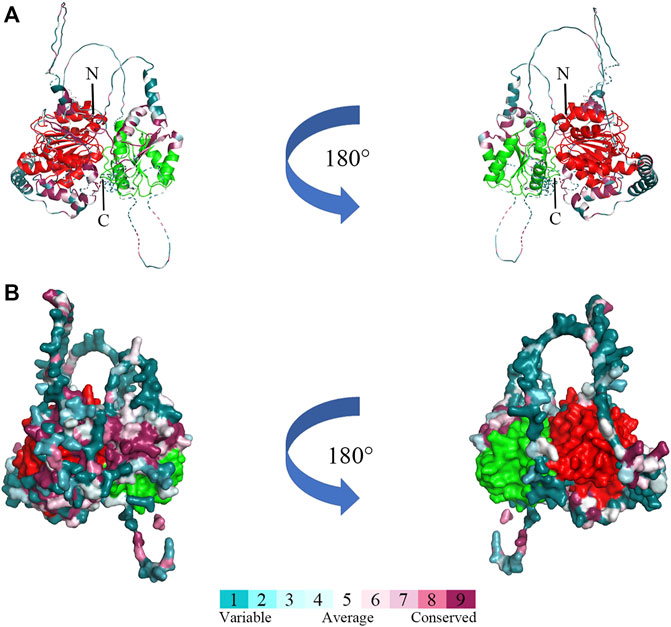
FIGURE 3. Predicted three-dimensional structure of Bxy-laf-1. (A) Cartoon representative of Bxy-LAF-1. N, N-terminal; C, C-terminal. (B) The Bxy-LAF-1 protein in surface representation. The right model is rotated along the long axis from the left model. The coloring gradient ranges from variable region to conserved regions, and the two conserved DNA-binding motifs are colored by red and green. The red area is DEAD box helicase, and the green is Helicase conserved C-terminal domain.
The expression profiles of Bxy-laf-1 were investigated at different stages including embryonic stages, juveniles (J2, J3, J4), and adult stages in B. xylophilus. In the early ontogenesis of B. xylophilus, the expression level of Bxy-laf-1 increased, and reached its highest level at the J2 stage (Figure 4). Subsequently, the expression level of Bxy-laf-1 gradually decreased from the J3 stage and reached its lowest level at the adult stage, the gene expression level of males was significantly higher than that of females, and the gene expression pattern of females and males was different. These results suggest that Bxy-laf-1 gene may play an important role in embryonic and J2 stages of B. xylophilus.
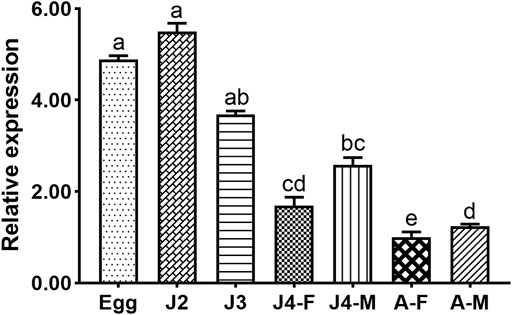
FIGURE 4. Bxy-laf-1 expression levels in B. xylophilus at various developmental stages were investigated using real-time quantitative PCR. The tbb-2 and β-actin endogenous reference genes were used to standardize the data. Adults were judged to have a 100% expression level. The same letter indicates that the difference between two groups is not significant, whereas distinct letters indicate that the difference between two groups has reached a significant level (p < 0.05). The standard error of the mean is shown by the error bars. Adult, sexually mature worms; Egg, the embryo-stage; J2, the second-stage juveniles; J3, the third-stage juveniles; J4-F, the fourth-stage of female juveniles; J4-M, the fourth-stage of male juveniles; A-F, the adult stage of female juveniles; A-M, the adult stage of male juveniles.
Whole-mount in situ hybridization revealed that digoxigenin-labeled antisense probe staining was found from the early embryo to the adult, which was compatible with RT-qPCR findings. Single-ended staining is the expression pattern in the embryonic stage (Figures 5A,B). The stained region expanded as embryos developed. At the J2 and J3 stages, the staining began to centralize to the head region and tail region, which may be related to its motor activity (Figures 5D,E). The hybrid signal was observed in the gonads of male and female worms, as well as the portions that would develop as external sexual organs during the stage of sex differentiation (J4) (Ye and Feng, 1993) (Figures 5F,G). The hybridization signals were seen in the gonads and other reproductive organs of both male and female worms in the sexually mature adult stage. The staining in female adults was mostly located in the vulva, whereas the signal in male adults was mostly found around the tail spicules (Figures 5H,I). It is speculated that Bxy-laf-1 may have an effect on the sex determination and mating behavior of B. xylophilus. In summary, it is speculated that Bxy-laf-1 was predominantly expressed in the head, gonads, and sex organs of B. xylophilus.

FIGURE 5. Localization of Bxy-laf-1 mRNA in B. xylophilus via in situ hybridization. Hybridization with the digoxigenin-labelled specific probe in embryo (A,B), no staining was observed in embryo (C), second-stage juvenile (D), third-stage juvenile (E), female fourth-stage juvenile (F), male fourth-stage juvenile (G), female adult (H), and male adult (I). No staining was observed in male adult (J) with control sense probes. a, anterior; p, posterior; m, metacorpus; an, anus; v, vulva; sp, spicules.
Three groups including the no probe control, Bxy-laf-1 dsRNA treated, and foreign gfp dsRNA treated were utilized in this experiment. There was no statistically significant difference between the no probe control and gfp dsRNA-treated group (Figure 6). When compared to the control group, the relative expression levels of embryonic, J2, J3 and J4 stage synchronous B. xylophilus were down-regulated by Bxy-laf-1 dsRNA interference, attaining a significant level difference (p < 0.05).
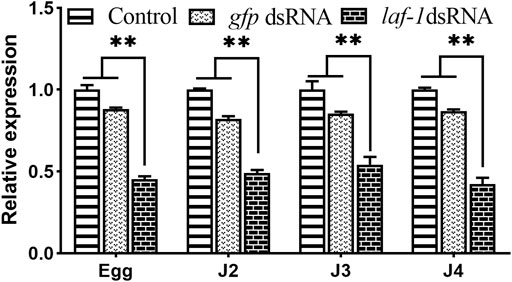
FIGURE 6. Real-time quantitative PCR analysis of Bxy-laf-1 expression levels after RNAi soaking at different developmental stages in B. xylophilus. Egg, the embryo-stage; J2, the second-stage juveniles; J3, the third-stage juveniles; J4, the forth-stage juveniles. Control, the no probe control, means that nematodes were treated with soaking buffer alone; gfp dsRNA, the negative control means that nematodes were treated with gfp dsRNA; Bxy-laf-1 dsRNA, means that nematodes were treated with Bxy-laf-1 dsRNA; Data were normalized to those of endogenous reference genes tbb-2 and β-actin. Expression level of the control groups was considered as 100%. Asterisks indicate a significant difference between two groups (*means p < 0.05, **means p < 0.01). Error bars represent standard error of mean.
The abnormal in sinusoidal locomotion was observed in all treated B. xylophilus larvae. In terms of movement morphology, the body of the J2 hatched from dsRNA-treated embryos curled up. The head slightly swayed, and the nematode lost the ability to move. The same phenomenon appeared in J2 and J3 larvae when treated with Bxy-laf-1 dsRNA. This suggests that Bxy-laf-1 may regulate the motor nerves of larvae.
In addition, a significant decrease in head swing frequency was observed in the larvae of all three stages mentioned above. In the Bxy-laf-1 dsRNA treatment group, the head swing frequency of J2 hatched from the treated embryos was 0.33 ± 0.12 times 30 s−1 (p < 0.0005), and the treated J2 was 0.07 ± 0.06 times 30 s−1(p < 0.0005). J3 was 0.33 ± 0.12 times 30 s−1 (p < 0.0005). For the control groups, it was 10.10 ± 0.45 times 30 s−1, 10.60 ± 0.42 times 30 s−1, 21.13 ± 0.49 times 30 s−1, respectively (Figure 7). The frequency of head swing of B. xylophilus treated with gfp dsRNA was similar to that of the control treated with non-dsRNA. This demonstrates the importance of Bxy-laf-1 in larval motility.
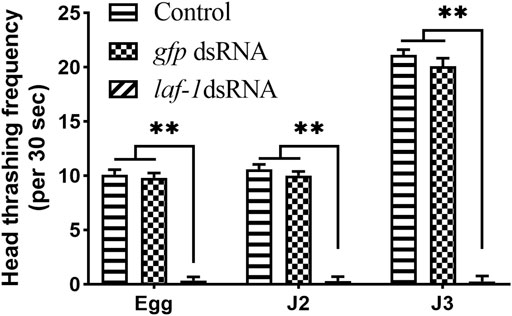
FIGURE 7. Head swing frequency of B. xylophilus after RNAi soaking at different developmental stages. Egg, larvae hatch from the treated embryo-stage; J2, the second-stage juveniles after 24 h treatment; J3, the third-stage juveniles after 24 h treatment. Control, the no probe control, means that nematodes were treated with soaking buffer alone; gfp dsRNA, the negative control means that nematodes were treated with gfp dsRNA; Bxy-laf-1 dsRNA, means that nematodes were treated with Bxy-laf-1 dsRNA. Asterisks indicate a significant difference between two groups (p < 0.05). Error bars represent standard error of mean.
After treating embryos, J2, J3 and J4 with Bxy-laf-1 dsRNA, the sex ratios of female to male were calculated. RNAi results showed that the F0 generation sex ratio was 1.63 ± 0.03 when treated during embryogenesis, and 2.07 ± 0.02 when treated at J2 compared to 2.7 around for the normal sex ratio. Statistical analysis also showed that both of them reached significant levels (p < 0.05). However, the sex ratio was 2.18 ± 0.03 when treated at J3 stage, or 2.47 ± 0.02 when treated at J4 stage.
The nematodes from the two stages in which the sex ratio decreased significantly were further cultivated to adults, and the sex ratio of the offspring was calculated. According to the RNAi results, the sex ratios of adult nematodes after embryonic and J2 treatments of B. xylophilus decreased to varying degrees in both the F0 and F1 generations, while the sex ratio of the F2 generation recovered to the normal level (Table 2). In order to verify the above results, the collected adult nematodes were tested for RNA interference efficiency. RT-qPCR results showed that the expression of Bxy-laf-1 in F0 and F1 nematodes after RNAi was inhibited by Bxy-laf-1 dsRNA to varying degrees (Figures 8A,B). These results indicated that knockdown of Bxy-laf-1 has a certain degree of influence on the sex ratio of B. xylophilus and can last for one generation.
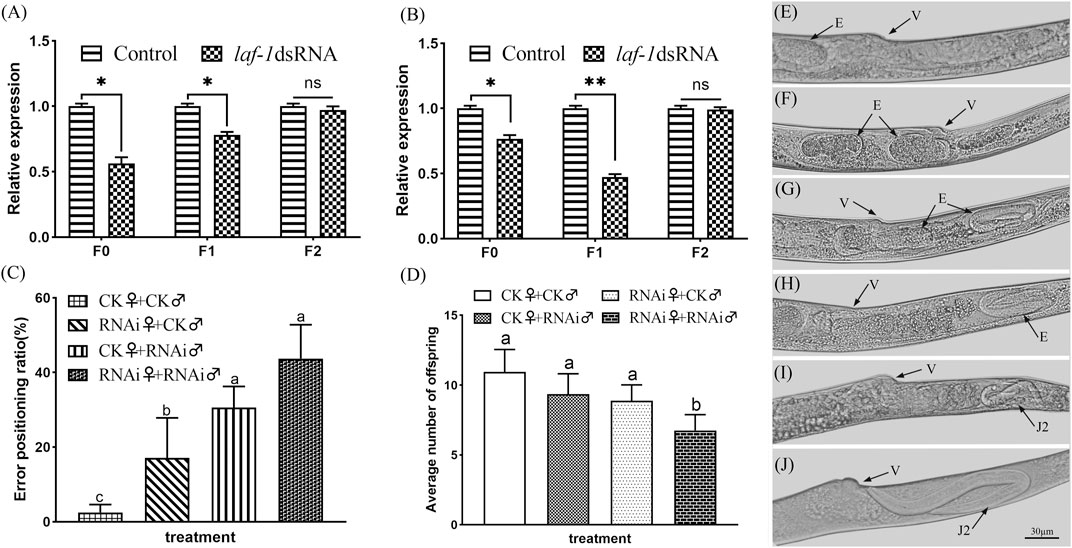
FIGURE 8. RNAi soaking by Bxy-laf-1. Real-time quantitative PCR analysis of Bxy-laf-1 expression levels after RNAi soaking at embryos (A) and J2 (B). Asterisks indicate a significant difference between two groups (*means p < 0.05, **means p < 0.01). (C) Error positioning ratio of B. xylophilus virgin adults. (D) Average number of offspring of B. xylophilus. The same letter indicates that the difference is not significant, and different letters indicate that the difference has reached a significant level between two groups (p < 0.05). (E,F) Position of embryos in the control group in vivo. (G,H) Embryos that have moved to the tail after Bxy-laf-1 dsRNA treatment. (I,J) J2 larvae that have hatched in the tail. Error bars represent standard error of mean. v, vulva; E, embryo; J2, second-stage juveniles. Scale bar: 30 μm (E–J).
The error positioning ratio was significantly affected after being treated with Bxy-laf-1 dsRNA. The error positioning ratio during the 8 h observation time was 3.33 ± 1.60% and 38.89 ± 3.96% for group of CK♀ + CK♂ and group of RNAi♀ + RNAi♂ respectively (p < 0.01) (Figure 8C). After the females or males were treated by Bxy-laf-1 dsRNA, the males could not accurately locate the female vulva, resulting in an increase in the error positioning ratio. Subsequently, we placed nematodes in each group for 24 h after the end of observation, and counted the amount of eggs in each group. The average number of offspring after the 24 h was 10.94 ± 1.61 and 6.75 ± 1.14 for group of CK♀ + CK♂ and group of RNAi♀ + RNAi♂ respectively (p < 0.01) (Figure 8D). Based on the above phenomena, we observed and counted the characters of vulva parts of female worms after RNAi, and found that the embryos of adult female treated with Bxy-laf-1 dsRNA did not emerge from the vulva but gradually moved through the vulva to the tail. The embryos located in the tail cannot be discharged from the body, and then hatched into J2, swimming in the female body, causing the female to die. This phenomenon occurred in both the F0 and F1 generations, with 14.3% of the F0 generation and 10.9% of the F1 generation affected.
The laf-1 gene is a member of the DEAD box helicase family, that is highly consered from nematode to human. It plays an important role in sex determination and differentiation. This article introduces the functional and expression properties of the sequence.
Bxy-laf-1 is evolutionarily conserved. Phylogenetic analysis showed that Bxy-laf-1 and Bmu-LAF-1 clustered on the same branch and had similar homology with the model organism C. elegans LAF-1. Based on sequence alignment, three-dimensional structure and domains, it is further confirmed that Bxy-laf-1 is evolutionarily conserved among species in different phyla.
RT-PCR data showed that a basic level of Bxy-laf-1 expression was needed throughout the life cycle of B. xylophilus. Bxy-laf-1 was found in B. xylophilus embryos, tails of J2 and J3 stages (probably the portions that would develop into gonads), and gonads of J4 and adults, according to the results of mRNA in situ hybridization. In C. elegans, laf-1 is an ortholog of Bxy-laf-1. It is expressed in the gonads of male C. elegans adults, which is quite similar to our findings in B. xylophilus. However, unlike C. elegans, laf-1 is expressed at the highest level in the embryonic stage of C. elegans (Hubert and Anderson, 2009), while the highest level in B. xylophilus occurs in J2. Although the apparent structure of C. elegans and B. xylophilus is somewhat different, the expression pattern of laf-1 in C. elegans is basically consistent with that of Bxy-laf-1 in B. xylophilus.
Successful knockdown of Bxy-laf-1 in B. xylophilus embryos and J2 resulted in obvious abnormal phenotypes. Impairment in locomotion indicates possible deficits in the muscle or neurons. laf-1 gene belongs to DEAD-box protein family. Coincidentally, related genes of the DEAD-box protein family have been shown to regulate motor functions in insects and vertebrates. For instance, the loss of Gemin3 in the muscles of Drosophila larvae can cause the flight muscles to degenerate and lose the ability to fly (Cauchi et al., 2008; Shpargel et al., 2009). The mutation of ddx39 ab in zebrafish can cause trunk muscular dystrophy (Zhang et al., 2018). We therefore speculate that Bxy-laf-1 is related to larval muscle development and affects the movement of B. xylophilus larvae.
The lack of Bxy-laf-1 significantly reduced the female to male sex ratios of B. xylophilus. Related genes of the DEAD-box family are involved in sex differentiation in C. elegans (Hubert and Anderson, 2009), zebrafish (Sone et al., 2020) and channel catfish (Tian et al., 2017). Our results found that after Bxy-laf-1 dsRNA interfered with B. xylophilus, the ratio of female to male in F0 and F1 generations was significantly reduced, indicating that Bxy-laf-1 is involved in the development of sexual differentiation in B. xylophilus. In C. elegans, the sex determination pathway is regulated by multiple genes (Simone et al., 2012), the laf-1 gene is located upstream of tra-2 promoting female development (Doniach, 1986). It affects the export of tra-2 mRNA from the nucleus by encoding DEAD-box RNA helicase, thereby promoting the ontogeny of C. elegans males. Our results indicate that the laf-1 gene promotes the female ontogeny of B. xylophilus, which is contrary to the results of the model organism C. elegans. Further studies have shown that there is no homologous tra-2 gene in B xylophilus. From this we speculate that Bxy-laf-1 directly suppresses the expression of fem promoting male development (Doniach and Hodgkin, 1984) genes downstream of tra-2, thereby promoting female ontogeny. Here, we preliminarily speculated the function of Bxy-laf-1 in the sex determination pathway of B xylophilus. The relationship between Bxy-laf-1 and other sex determination genes requires further research.
After RNAi, the number of eggs laid by the females decreased significantly, about 14% and 11% of female’s embryos in F0 and F1 moved to the tail and could not be layed successfully. Studies have shown that mutations in genes in the DEAD-box protein family in C. elegans can cause the gonads to shrink (Ryuji et al., 2009). At the same time, it regulates the development of the gonads in both Romanomermis wuchangensis and Ovomermis sinensis (Duan et al., 2017; Tao et al., 2018). In addition, related genes in the DEAD-box family have similar regulatory effects on insects and mammals. For example, the knockdown of DDX3 and DDX6 in L. migratoria can impair ovarian development and oocyte maturation (Wang et al., 2021a; Wang et al., 2021b). In Drosophila, 12 genes are known to encode DEAD-box protein (Consortium, 2003), and the functions of bel and vasa are necessary for female fertility (Hay et al., 1988; Lasko and Ashburner, 1988; Styhler et al., 1998; Johnstone et al., 2005). We speculate that the knockdown of the Bxy-laf-1 may cause abnormal ovarian development in B. xylophilus females, causing the embryos to move abnormally to the tail. In addition, in our search results, the positioning error rate of males in mating behavior increased sharply after interference. The lack of Dhh1 in Saccharomyces cerevisiae directly leads to serious mating defects (Ka et al., 2008). Therefore, it can be inferred that Bxy-laf-1 also has an effect on mating behavior.
It has been suggested that sex determination of Bursaphelenchus nematodes may not be genome-base or environmental-base (Shinya et al., 2022). In recent years, the sex-determining genes of B. xylophilus have been extensively studied. For example, mab-3 is essential for spermatogenesis in B. xylophilus (Zhou et al., 2020). In addition, more and more evidence indicates that the sex determination mechanism of B. xylophilus is also affected by environmental factors, such as nutrient conditions (Wei et al., 2021) and volatile substances concentration (Cui et al., 2018). Therefore, there may be multiple factors in the sex determination mechanism of B. xylophilus, which need to be further explored.
It is well known that C. elegans laf-1 is required for somatic sex determination. Our results show that B. xylophilus laf-1 was also required for sex differentiation. In two generations, knocking down Bxy-laf-1 expression resulted in a drop in the sex ratio of female to male and impacted female ovarian development. In addition, Bxy-laf-1 played an important role in mating behavior. The findings may contribute to a better understanding of the molecular mechanisms of sex determination and differentiation in this invasive worm, as well as give hopeful ideas for controlling pine wilt disease by preventing ontogenesis and limiting nematode reproduction.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OM210018.
JH, HY, and LZ conceived of and designed the experiments. SS, JC, and JW performed the experiments. JC, WL, and CW analyzed the data. JH, SS, and WL discussed results. SS, LZ, and HY wrote the paper.
This study was funded by the National Natural Science Foundation of China (31870633, 31670652).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Brenda T., Chhandak B. (2015). Rapid and simple method of qPCR primer design. Methods Mol. Biol. 1275, 173–179. doi:10.1007/978-1-4939-2365-6_13
Cauchi R. J., Davies K. E., Liu J. (2008). A motor function for the DEAD-box RNA helicase, Gemin3, in Drosophila. PLoS Genet. 4, e1000265. doi:10.1371/journal.pgen.1000265
Cheng X., Xie P., Cheng F., Xu R., Xie B. (2009). Competitive displacement of the native species Bursaphelenchus mucronatus by an alien species Bursaphelenchus xylophilus (Nematoda: Aphelenchida: Aphelenchoididae): A case of successful invasion. Biol. Invasions 11, 205–213. doi:10.1007/s10530-008-9225-2
Consortium T. F. (2003). The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 31, 172–175. doi:10.1093/nar/gkg094
Cui J., Li Y.-X., Zhang W., Wang X., Pan L., Feng Y.-Q., et al. (2018). The population structure and sex ratios of Bursaphelenchus xylophilus under α-pinene stress. J. For. Res. (Harbin). 31, 921–926. doi:10.1007/s11676-018-0847-7
Doniach T. (1986). Activity of the sex-determining gene tra-2 is modulated to allow spermatogenesis in the C. elegans hermaphrodite. Genetics 114, 53–76. doi:10.1093/genetics/114.1.53
Doniach T., Hodgkin J. (1984). A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev. Biol. 106, 223–235. doi:10.1016/0012-1606(84)90077-0
Duan M., Xiong J., Lu D., Wang G., Ai H. (2017). Transcriptome sequencing analysis and functional identification of sex differentiation genes from the mosquito parasitic nematode, Romanomermis wuchangensis. PLoS ONE 11, e0163127. doi:10.1371/journal.pone.0163127
Futai K. (1980). Development rate and population growth of Bursaphelenchus lignicolus (Nematoda : Aphelenchoididae) and B. mucronatus. Appl. Entomol. Zool. 15, 115–122. doi:10.1303/aez.15.115
Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R. D., Bairoch A. (2003). ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788. doi:10.1093/nar/gkg563
Goodwin E. B., Hofstra K., Hurney C. A., Mango S., Kimble J. (1997). A genetic pathway for regulation of tra-2 translation. Dev. Camb. Engl. 124, 749–758. doi:10.1242/dev.124.3.749
Haag E. S. (2005). WormBook : The online review of C. elegans biology.The evolution of nematode sex determination: C. elegans as a reference point for comparative biology
Hay B., Jan L. Y., Jan Y. N. (1988). A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 55, 577–587. doi:10.1016/0092-8674(88)90216-4
Hubert A., Anderson P. (2009). The C. elegans sex determination gene laf-1 encodes a putative DEAD-box RNA helicase. Dev. Biol. 330, 358–367. doi:10.1016/j.ydbio.2009.04.003
John J., Richard E., Alexander P., Tim G., Michael F., Olaf R., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. doi:10.1038/s41586-021-03819-2
Johnstone O., Deuring R., Bock R., Linder P., Fuller M. T., Lasko P. (2005). Belle is a Drosophila DEAD-box protein required for viability and in the germ line. Dev. Biol. 277, 92–101. doi:10.1016/j.ydbio.2004.09.009
Ka M., Park Y., Kim J. (2008). The DEAD-box RNA helicase, Dhh1, functions in mating by regulating Ste12 translation in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 367, 680–686. doi:10.1016/j.bbrc.2007.12.169
Kikuchi T., Cotton J. A., Dalzell J. J., Koichi H., Natsumi K., Paul M., et al. (2011). Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 7, e1002219. doi:10.1371/journal.ppat.1002219
Lasko P. F., Ashburner M. (1988). The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335, 611–617. doi:10.1038/335611a0
Li Y., Gao M., Liu B., Wang X., Hu J., Liu Z., et al. (2020). Mating and reproductive characteristics of the pathogenic nematode Bursaphelenchus xylophilus. J. For. Res. (Harbin). 32, 1281–1286. doi:10.1007/s11676-020-01150-6
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
Lu S., Wang J., Chitsaz F., Derbyshire M. K., Geer R. C., Gonzales N. R., et al. (2020). CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 48, D265-D268. doi:10.1093/nar/gkz991
Mamiya Y. (1975). The life history of the pine wood nematode, Bursaphelenchus lignicolus. Jpn. J. Nematology 5, 16–25.
Mota M. M., Braasch H., Bravo M. A., Penas A. C., Burgermeister W., Metge K., et al. (1999). First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1, 727–734. doi:10.1163/156854199508757
Motohashi T., Tabara H., Kohara Y. (2006). Protocols for large scale in situ hybridization on C. elegans larvae. WormBook, 1–8. doi:10.1895/wormbook.1.103.1
Rigsby R. E., Parker A. B. (2016). Using the PyMOL application to reinforce visual understanding of protein structure. Biochem. Mol. Biol. Educ. 44, 433–437. doi:10.1002/bmb.20966
Robertson L., Arcos S. C., Escuer M., Merino R. S., Esparrago G., Abelleira A., et al. (2011). Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner Buhrer, 1934 (Nickle, 1970) in Spain, 13.Nematology
Ryuji M., Alessandro P., Adrian S. (2009). The DEAD-box protein MEL-46 is required in the germ line of the nematode Caenorhabditis elegans. BMC Dev. Biol. 9, 35. doi:10.1186/1471-213X-9-35
Schedl T., Kimble J. (1988). fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119, 43–61. doi:10.1093/genetics/119.1.43
Shinya R., Sun S., Dayi M. (2022). Possible stochastic sex determination in Bursaphelenchus nematodes.
Shpargel K. B., Praveen K., Rajendra T. K., Matera A. G. (2009). Gemin3 is an essential gene required for larval motor function and pupation in Drosophila. Mol. Biol. Cell. 20, 90–101. doi:10.1091/mbc.e08-01-0024
Simone Z., Sonja G., Marco M., Marco B., Samantha R., Pamela B., et al. (2012). The sperm-oocyte switch in the C. elegans hermaphrodite is controlled through steady-state levels of the fem-3 mRNA. RNA (New York, N.Y.) 18, 1385–1394. doi:10.1261/rna.031237.111
Sone R., Taimatsu K., Ohga R., Nishimura T., Tanaka M., Kawahara A. (2020). Critical roles of the ddx5 gene in zebrafish sex differentiation and oocyte maturation. Sci. Rep. 10, 14157. doi:10.1038/s41598-020-71143-2
Styhler S., Nakamura A., Swan A., Suter B., Lasko P. (1998). Vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Dev. Camb. Engl. 125, 1569–1578. doi:10.1242/dev.125.9.1569
Sudhir K., Glen S., Michael L., Christina K., Koichiro T. (2018). Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi:10.1093/molbev/msy096
Sun Y. (1982). The pine wilt nematode (Bursaphelenchus xylophilus) detected at Sun Yet-sen's mausoleum in. Nanjing.
Tabara H., Grishok A., Mello C. C. (1998). RNAi in C. elegans: Soaking in the genome sequence. Science 282, 430–431. doi:10.1126/science.282.5388.430
Tang J., Ma R., Zhu N., Guo K., Guo Y., Bai L., et al. (2020). Bxy-fuca encoding α-L-fucosidase plays crucial roles in development and reproduction of the pathogenic pinewood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 76, 205–214. doi:10.1002/ps.5497
Tao S., Jiao Z., Wen G., Zhang L., Wang G. (2018). Cloning and expression analysis of the DEAD-box/RNA helicase Oslaf-1 in Ovomermis sinensis. PloS one 13, e0192101. doi:10.1371/journal.pone.0192101
Tian C., Tan S., Bao L., Zeng Q., Liu S., Yang Y., et al. (2017). DExD/H-box RNA helicase genes are differentially expressed between males and females during the critical period of male sex differentiation in channel catfish. Comp. Biochem. Physiol. Part D. Genomics Proteomics 22, 109–119. doi:10.1016/j.cbd.2017.02.008
Viglierchio D. R., Schmitt R. V. (1983). On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 15, 438–444.
Wang J., Li T., Deng S., Ma E., Zhang J., Xing S. (2021a). DDX6 is essential for oocyte development and maturation in Locusta migratoria. Insects 12, 70. doi:10.3390/insects12010070
Wang J., Li T., Deng S., Ma E., Zhang J., Xing S. (2021b). The RNA helicase DDX3 is required for ovarian development and oocyte maturation in Locusta migratoria. Arch. Insect Biochem. Physiol. 106, e21775. doi:10.1002/arch.21775
Wang S. (2007). Artificial breeding and the study of RNA interference of the pine wood nematode (Bursaphelenchusxylophilus). Hangzhou: Zhejiang Forestry College. master’s thesis master’s thesis.
Wang Y., Yamada T., Sakaue D., Suzuki K. (2005). Variations in life history parameters and their influence on rate of population increase of different pathogenic isolates of the pine wood nematode, Bursaphelenchus xylophilus. Nematology 7, 459–467. doi:10.1163/156854105774355545
Wei P., Li Y., Zhang W., Gao M., Zhenkai L., Xingyao Z. (2021). Factors affecting the ratio of female to male of pinewood nematodes Bursaphelenchus xylophilus (steiner buhrer) nickle, 12. Nanjing: Forests.
Wingfield M. J., Blanchette R. A., Nicholls T. H., Robbins K. (1982). The pine wood nematode: A comparison of the situation in the United States and Japan. Can. J. For. Res. 12, 71–75. doi:10.1139/x82-010
Ye W., Feng Z. (1993). Study on the ontogeny of pine wood nematode. Guangzhou: Journal of South China Agricultural University, 78–83.
Yi C. K., Byun B. H., Park J. D., Yang S. I., Chang K. H. (1989). First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Seoul: research reports of the forestry research institute.
Zhang L., Yang Y., Li B., Scott I. C., Lou X. (2018). The DEAD-box RNA helicase Ddx39ab is essential for myocyte and lens development in zebrafish, 145. Cambridge, England): Development, dev161018.
Zhao L., Zhang X., Wei Y., Zhou J., Zhang W., Qin P., et al. (2016). Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nat. Commun. 7, 12341. doi:10.1038/ncomms12341
Zhou L., Ma X., Zhu N., Zou Q., Guo K., Bai L., et al. (2020). The role of mab-3 in spermatogenesis and ontogenesis of pinewood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 77, 138–147. doi:10.1002/ps.6001
Keywords: Bursaphelenchus xylophilus, LAF-1, RNA interference, RT-qPCR, spatial-temporal expression
Citation: Sun S, Wang J, Liu W, Chen J, Zhou L, Wu C, Yu H and Hu J (2022) Regulatory roles of Bxy-laf-1 in reproductive behaviour of Bursaphelenchus xylophilus. Front. Physiol. 13:1024409. doi: 10.3389/fphys.2022.1024409
Received: 25 August 2022; Accepted: 02 November 2022;
Published: 17 November 2022.
Edited by:
Wei Dou, Southwest University, ChinaReviewed by:
Bo Liu, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2022 Sun, Wang, Liu, Chen, Zhou, Wu, Yu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongshi Yu, ODQ0Mjc5MzE1QHFxLmNvbQ==; Jiafu Hu, aHVqaWFmdTIwMDBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.