
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 05 January 2022
Sec. Cardiac Electrophysiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.807545
This article is part of the Research Topic Transcription Factors and Arrhythmogenesis View all 5 articles
A correction has been applied to this article in:
Corrigendum: Association of ZFHX3 genetic polymorphisms and extrapulmonary vein triggers in patients with atrial fibrillation who underwent catheter ablation
 Inseok Hwang
Inseok Hwang Oh-Seok Kwon
Oh-Seok Kwon Myunghee Hong
Myunghee Hong Song-Yi Yang
Song-Yi Yang Je-Wook Park
Je-Wook Park Hee Tae Yu
Hee Tae Yu Tae-Hoon Kim
Tae-Hoon Kim Jae-Sun Uhm
Jae-Sun Uhm Boyoung Joung
Boyoung Joung Moon-Hyoung Lee
Moon-Hyoung Lee Hui-Nam Pak*
Hui-Nam Pak*
Background: The ZFHX3 gene (16q22) is the second most highly associated gene with atrial fibrillation (AF) and is related to inflammation and fibrosis. We hypothesized that ZFHX3 is associated with extra-pulmonary vein (PV) triggers, left atrial (LA) structural remodeling, and poor rhythm outcomes of AF catheter ablation (AFCA).
Methods: We included 1,782 patients who underwent a de novo AFCA (73.5% male, 59.4 ± 10.8 years old, 65.9% paroxysmal AF) and genome-wide association study and divided them into discovery (n = 891) and replication cohorts (n = 891). All included patients underwent isoproterenol provocation tests and LA voltage mapping. We analyzed the ZFHX3, extra-PV trigger-related factors, and rhythm outcomes.
Result: Among 14 single-nucleotide polymorphisms (SNPs) of ZFHX3, rs13336412, rs61208973, rs2106259, rs12927436, and rs1858801 were associated with extra-PV triggers. In the overall patient group, extra-PV triggers were independently associated with the ZFHX3 polygenic risk score (PRS) (OR 1.65 [1.22–2.22], p = 0.001, model 1) and a low LA voltage (OR 0.74 [0.56–0.97], p = 0.029, model 2). During 49.9 ± 40.3 months of follow-up, clinical recurrence of AF was significantly higher in patients with extra-PV triggers (Log-rank p < 0.001, HR 1.89 [1.49–2.39], p < 0.001, model 1), large LA dimensions (Log-rank p < 0.001, HR 1.03 [1.01–1.05], p = 0.002, model 2), and low LA voltages (Log-rank p < 0.001, HR 0.73 [0.61–0.86], p < 0.001, model 2) but not the ZFHX3 PRS (Log-rank p = 0.819).
Conclusion: The extra-PV triggers had significant associations with both ZFHX3 genetic polymorphisms and acquired LA remodeling. Although extra-PV triggers were an independent predictor of AF recurrence after AFCA, the studied AF risk SNPs intronic in ZFHX3 were not associated with AF recurrence.
Catheter ablation of atrial fibrillation (AF) is the most effective rhythm control method for AF patients (Hindricks et al., 2021). Circumferential pulmonary vein isolation (CPVI) is the most important procedure during AF catheter ablation (AFCA), and the efficiency of a long-lasting CPVI is improving with the catheter technology (Peyvandi et al., 2016; Reddy et al., 2020; Pak et al., 2021). However, AF is a progressive degenerative disease and shows a constant rate of recurrence despite an effective CPVI (Park et al., 2019). In particular, the rhythm outcome of a second ablation is poorer in patients with a well-maintained de novo PVI, suggesting the role of extra-PV triggers in the mechanism of AF recurrence (Kim et al., 2017; Park et al., 2020). In patients with significant atrial substrate remodeling, isoproterenol provocation-induced extra-PV triggers are more common and the post-AFCA recurrence rate is higher in an extra-PV trigger group compared with a non-extra-PV trigger group, and an extra-PV trigger ablation lowers the recurrence rate (Lee et al., 2018; Kim et al., 2021). However, little is known about the mechanism and appropriate techniques for the mapping and ablation of extra-PV triggers of AF. AF is a heritable disease influenced by more than 100 genes (Roselli et al., 2018). Among them, the ZFHX3 gene is the second most highly associated with AF and affects inflammation, fibrosis, matrix deposition, and atrial structural changes (Nojiri et al., 2004; Park et al., 2013; Liu et al., 2014; Tomomori et al., 2018; Alí et al., 2019). Therefore, in this study, we explored whether extra-PV triggers, which are found frequently in patients with acquired atrial structural remodeling, are influenced by the genetic background. In addition, since the ZFHX3 gene can affect not only atrial remodeling, but also the reaction to catheter ablation lesions, we evaluated how it affects the AFCA outcome.
This study was conducted as a single-center retrospective study. The study protocol followed the principles of the Declaration of Helsinki, and it was approved by the Institutional Review Board at Yonsei University Health System. All patients provided written consent for inclusion in the Yonsei AF Ablation Cohort prior to the study (ClinicalTrials.gov identifier: NCT02138695). A total of 1,782 patients underwent a de novo AF catheter ablation from March 2009 to January 2021 and post-procedural isoproterenol provocation tests (Figure 1). Among those patients, 221 had extra-PV triggers. The study exclusion criteria were (1) permanent AF refractory to electrical cardioversion, (2) AF with valvular disease grade > 2; (3) prior cardiac surgery with concomitant AF surgery, and (4) patients who did not undergo an isoproterenol provocation test or (5) genome-wide association study (GWAS).
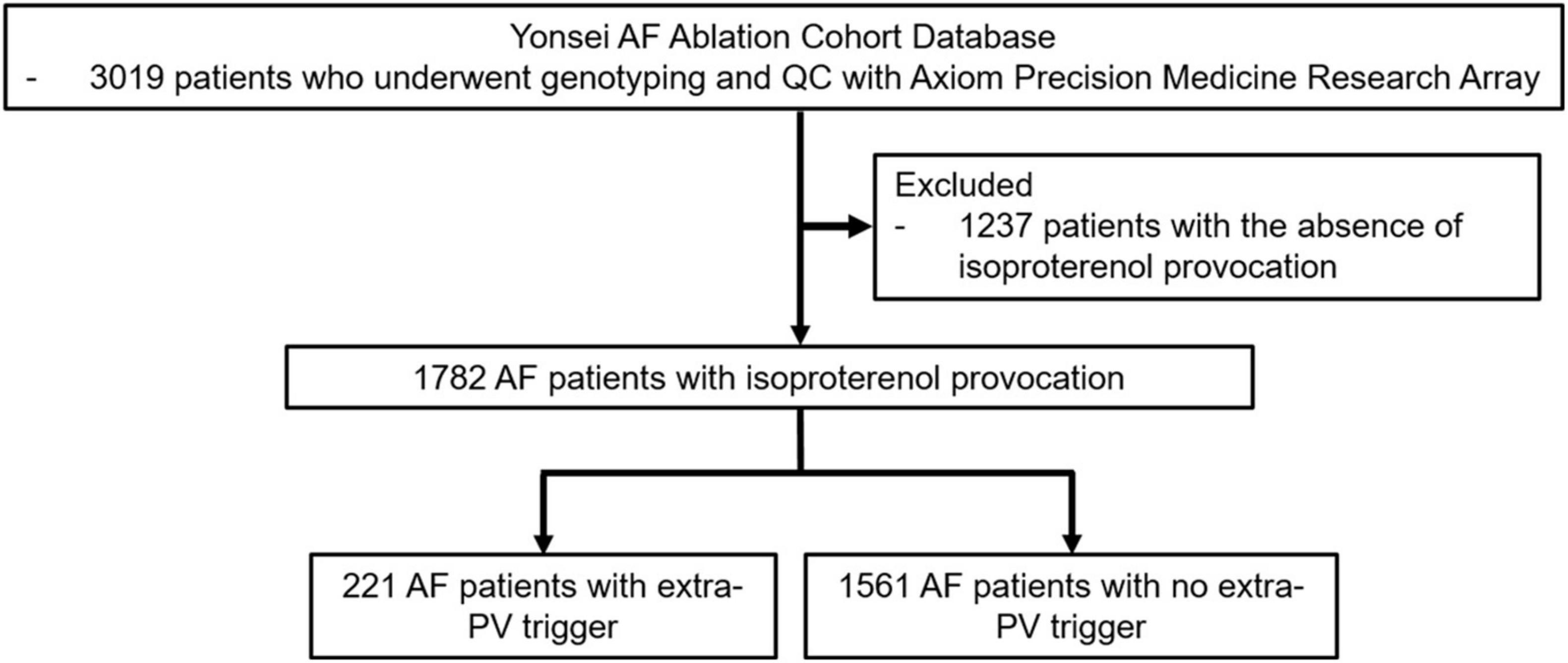
Figure 1. Flow chart of the enrollment and analysis of the study population. The 221 AF patients who underwent de novo AFCA procedures with extra-PV triggers and 1561 AF patients without extra-PV triggers were included in the study population.
Genomic DNA was extracted from peripheral blood samples using the QuickGene DNA whole blood kits with a QuickGene mini 80 (KURABO, Osaka, Japan). DNA genotyping data were obtained using the Axiom Precision Medicine Research Array (PMRA, Thermo Fisher Scientific, MA, United States). This DNA chip array consisted of more than 900,000 markers to assist in the translation of the research results to clinical insight.
Transthoracic echocardiography (Sonos 5500, Philips Medical System, Andover, MA, United States or Vivid 7, GE Vingmed Ultrasound, Horten, Norway) was used to measure the cardiac chamber size, left ventricular ejection fraction, trans-mitral Doppler flow velocity, and ratio of the early diastolic peak mitral inflow velocity to the early diastolic mitral annular velocity (E/Em). The procedure was in accordance with the American Society of Echocardiography guidelines (Nagueh et al., 2009).
We used the Prucka CardioLab™ Electrophysiology system (General Electric Medical Systems, Inc., Milwaukee, WI, United States) to record the intracardiac electrograms and generated 3D electroanatomical maps (NavX, Abbott, Inc., Chicago, IL, United States; CARTO system, Biosense Webster, Diamond Bar, CA, United States) using a circumferential PV-mapping catheter through a long sheath. The 3D electroanatomical maps were merged with 3D spiral CT images. We conducted transseptal punctures and obtained multi-view pulmonary venograms. Systemic anticoagulation was conducted with intravenous heparin to maintain an activated clotting time of 350–400 s during the procedure. An open-irrigated tip catheter (30–60 W; 47°C) was utilized for the AFCA. The CPVI was conducted during the de novo procedure with bidirectional block. Cavotricuspid isthmus block was generated for most patients during the procedure unless there was an atrioventricular conduction disease. We performed an additional empirical linear ablation including a roof line, posterior interior line (posterior box lesion), anterior line, left lateral isthmus ablation, right atrial ablation, or complex fractionated electrogram ablation at the operator’s discretion.
After completing the protocol-based AF ablation procedure, AF or atrial tachycardia (AT) was induced by high-current ramp pacing (250–120 ms, 10 mA, pulse width 5 ms, Bloom Associates, Denver, CO, United States) from the high right atrial (RA) electrodes as previously described (Kim et al., 2021). We infused isoproterenol (5∼20 μg/min depending on β-blocker use with a target heart rate of 120 bpm) for at least 3 min before induction and maintained this for 3 min after the induction of AF or AT. Then, internal cardioversion was conducted utilizing a biphasic shock (2–20 J) with R wave synchronization (Lifepak12, Physiocontrol Ltd., Redmond, WA, United States). Electrical cardioversion was performed under induced deep sedation, while all the other procedures were conducted under conscious sedation. After successful electrical cardioversion, we stopped the isoproterenol infusion and waited for 10 min to detect any immediate recurrence of AF or AT originating from the extra-PV trigger. The existence of extra-PV triggers was defined by the immediate recurrence of AF or AT within the 10 min of an isoproterenol cool-down after cardioversion. If further AF triggers were observed under the isoproterenol effect, we determined the potential location of the extra-PV triggers based on the contact bipolar electrograms and conducted a quick and detailed 3D-activation mapping with a multielectrode catheter. Based on 3D mapping of the non-PV triggers, we ablated those triggers with 35–50 W for 10 s for each lesion until the elimination.
After AFCA, the patients were discharged without taking any anti-arrhythmic drugs, with the exception of those who had recurrent extra-PV triggers, symptomatic frequent atrial premature beats, non-sustained atrial tachycardia, or an early recurrence of AF during admission. The patients visited the outpatient clinic at 1, 3, 6, and 12 months and every 6 months thereafter or whenever symptoms occurred. All patients underwent electrocardiography (ECG) at every visit. Twenty-four hour Holter monitoring was also conducted at 3 and 6 months, annually for 2–5 years, and then biannually after 5 years. Holter and event monitor recordings were obtained when patients experienced palpitations suggestive of an arrhythmia recurrence. We defined AF recurrence as any episode of AF or atrial tachycardia (AT) lasting at least 30 s in duration. Any ECG documentation of an AF recurrence within a 3-month blacking period was classified as an early recurrence, whereas AF recurrence after 3 months was diagnosed as a clinical recurrence.
Continuous variables were summarized as the mean ± SD and compared by either an independent Student’s t-test or Mann-Whitney U-test as appropriate. Categorical variables were summarized as the number (percentage of the total group) and compared by either a chi-square test or Fisher’s exact test as appropriate. A multivariable logistic regression analysis was used to identify the independent predictors of the existence of extra-PV triggers. A multivariable Cox regression analysis was conducted to identify the independent predictors of an AF clinical recurrence. A Kaplan-Meier analysis with a log-rank test was conducted to calculate the AF recurrence-free survival over time and AF recurrence rates according to the clinical and genetic factors. A P-value less than 0.05 was considered statistically significant. We constructed a polygenic risk score (PRS) using two SNPs (rs1336412 and rs61208973) and excluded three (linkage disequilibrium, r2 > 0.2). We calculated the PRS using a β-coefficient (effect size) and the number of effect alleles. The PRS was divided into quartiles for a statistical analysis performed using SPSS software (SPSS Inc., Chicago, IL, United States).
A group of AF patients [73.5% male, 59.4 ± 10.8 years old, and 65.9% paroxysmal AF (PAF)] who received a trigger provocation was included in our study. All patients underwent a genetic analysis by a GWAS. Table 1 summarizes the characteristics of the patients with extra-PV triggers and those without. To find the extra-PV trigger-associated SNPs, we divided the included patient population into cohort 1 (73.7% male, 59.3 ± 10.8 years old, and 65.3% PAF) and cohort 2 (73.2% male, 59.4 ± 10.7 years old, and 66.4% PAF, Supplementary Table 1). Logistic regression analyses showed that 9 ZFHX3 SNPs were associated with extra-PV triggers in cohort 1 and 10 SNPs in cohort 2 (Supplementary Table 2). Among them, the 5 SNPs, rs13336412, rs61208973, rs2106259, rs12927436, and rs1858801, were associated with extra-PV triggers (p < 0.05, Table 2). The 5 AF risk SNPs evaluated in this study were located in intronic regions of the ZFHX3 gene on chromosome 16. We displayed the individual position and risk/ref allele, risk allele frequency (RAF) for replicated SNPs in Table 2. In 1,782 patients, 1,733 (97.3%) had ZFHX3-associated SNPs.
Table 1 compares 221 patients with extra-PV triggers and 1,561 patients without. In patients with extra-PV triggers, the AF duration was longer (p < 0.001) and mean left atrial (LA) voltage lower (p = 0.004). The risk allele frequencies of the rs13336412 (85.5 vs. 77.5%, p = 0.006), rs2106259 (61.1 vs. 53.1%, p = 0.025), rs12927436 (60.2 vs. 51.3%, p = 0.014), rs1858801 (69.7 vs. 61.3%, p = 0.016), and PRS (0.04 ± 0.12 vs. -0.03 ± 0.11, p < 0.001) were significantly higher in the extra-PV trigger group than in those without (Figure 2). In the multivariate logistic regression analyses, for model 1, female (OR 0.73, [0.53–1.00], p = 0.049), no pre-existing vascular disease (OR 0.47, [0.26–0.85], p = 0.013), and PRS for ZFHX3 (odds ratio [OR] 1.65, [1.22–2.22], p = 0.001, Model 1) and a lower LA voltage (OR 0.74, [0.56–0.97], p = 0.029, Model 2) were independently associated with extra-PV triggers, but each of 5 SNPs was not independently associated with extra-PV trigger in the multivariate logistic regression analyses (Table 3). A regional specificity of extra-PV triggers was observed in the superior vena cava (12.9 vs. 29.6%, p = 0.039) in patients with rs13336412 (Supplementary Table 3).
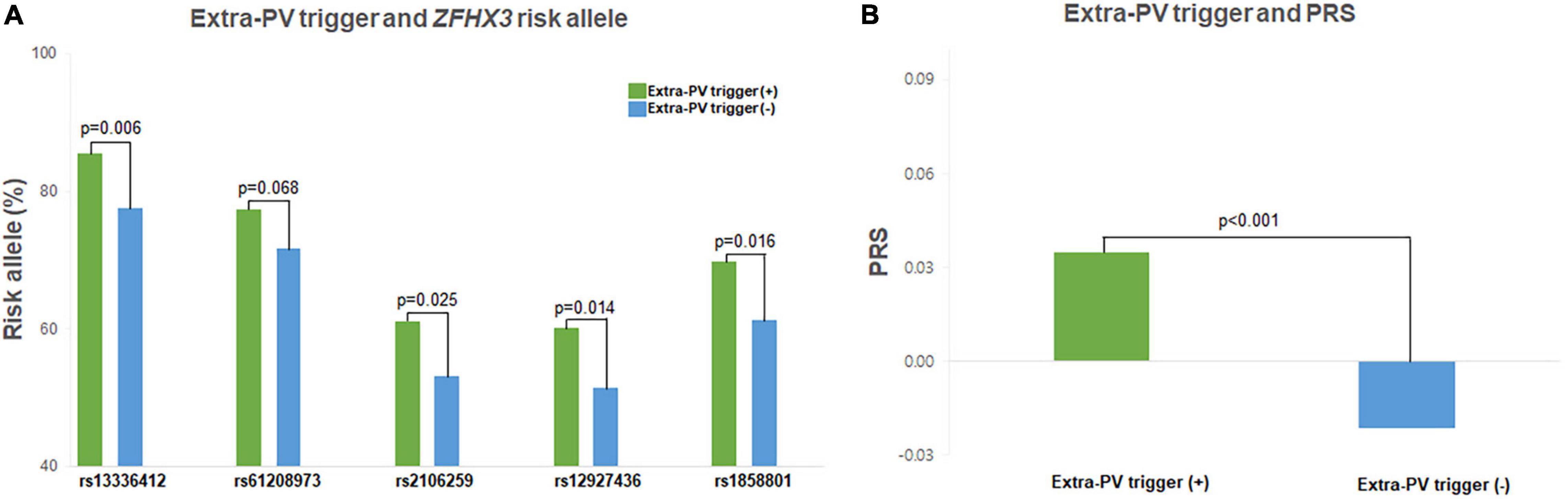
Figure 2. Characteristics of the extra-PV triggers. The extra-PV trigger risk allele frequency and PRS were higher in all 5 SNPs (A) and AF patients with extra-PV triggers (B).
During 49.9 ± 40.3 months of follow-up, clinical recurrence was significantly higher in patients with extra-PV triggers (Log-rank p < 0.001, Figure 3A), a bigger LA size (Log-rank p < 0.001, Figure 3B), and a lower LA voltage (n = 1,322, Log-rank p < 0.001, Figure 3C). In the Cox regression analysis, extra-PV trigger (hazard ratio [HR] 1.89, [1.49–2.39], p < 0.001), a bigger LA size (HR 1.04, [1.02–1.05], p < 0.001, Model 1), and a lower LA voltage [HR 0.73, (0.61–0.86) p < 0.001, Model 2] were independently associated with clinical recurrence of AF after AFCA (Table 4). However, neither a Kaplan-Meier (Figure 3D) nor Cox regression (Table 4) analysis showed a relationship between the ZFHX3 PRS and AF recurrence after catheter ablation.
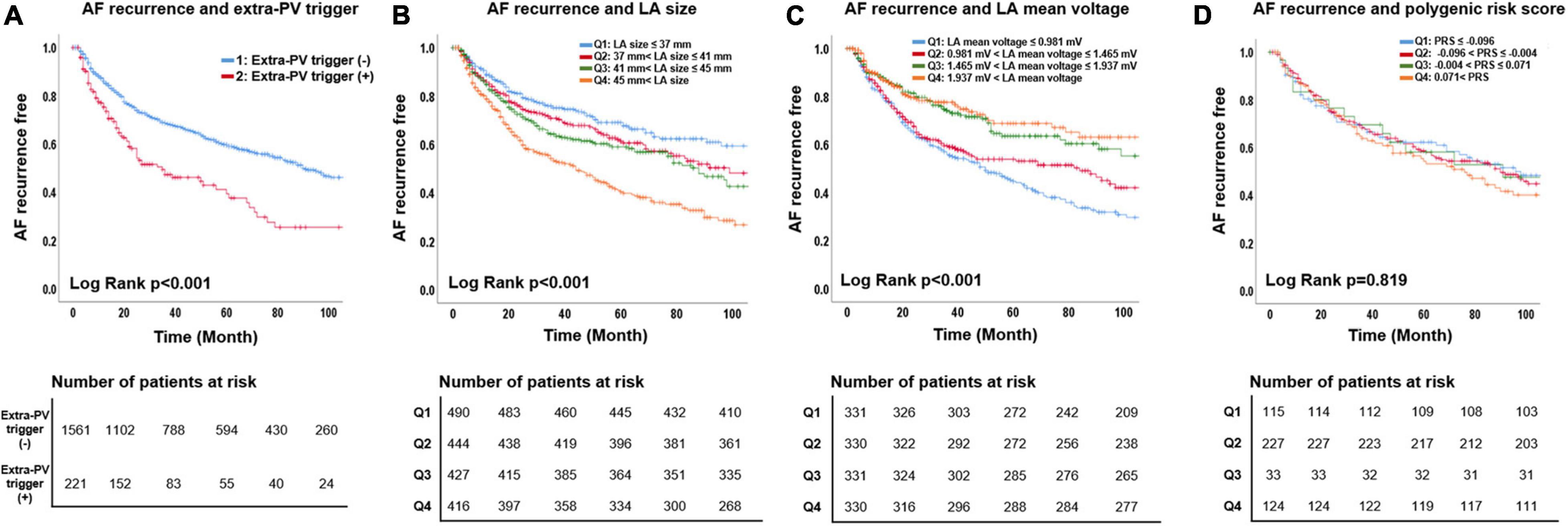
Figure 3. Association of AF recurrence with the extra-PV triggers, LA mean voltage, LA size, and polygenic risk score. The AF recurrence rate was higher for the extra-PV trigger (A), larger LA size (B), and low LA mean voltage (C) groups. The PRS for extra-PV triggers was not associated with AF recurrence (D).
The post-AFCA recurrence rate was significantly higher in patients with extra-PV triggers and the ZFHX3 risk allele than in those with extra-PV triggers and no ZFHX3 risk allele (Log-rank p = 0.024) or those without extra-PV triggers (p < 0.001, Supplementary Figure 1A). We also compared AF recurrence between extra-PV trigger (+) ZFHX3 (–) group and extra-PV trigger (–) group and both of groups did not have statistical difference (p = 0.623, Supplementary Figure 1A). In patients without extra-PV triggers, the presence of a ZFHX3 risk allele did not affect the AF recurrence after AFCA (Log-rank p = 0.749, Supplementary Figure 1B).
In this retrospective analysis of a single-center cohort study, we found an association between the extra-PV triggers and genetic variants of ZFHX3 for the mechanism of AF among the patients who underwent AFCA. The extra-PV triggers were independently associated with both the genetic background, including the ZFHX3, and acquired atrial remodeling. During 49.9 ± 40.3 months of follow-up, the existence of extra-PV triggers and LA remodeling were independently associated with a clinical recurrence of AF after AFCA but not with the ZFHX3. Finding suggested that ZFHX3 SNPs were associated with the extra-PV trigger, but not AF recurrence after AFCA.
The extra-PV triggers are a major factor of AF recurrence after AFCA (Lee et al., 2018; Kim et al., 2021). Extra-PV triggers are associated with a low atrial voltage, being female, lower body mass index, absence of hypertension, and ventricular diastolic dysfunction in patients with paroxysmal AF (Kawai et al., 2019; Watanabe et al., 2021). Kim et al. (2021) recently reported that the existence of extra-PV triggers in repeat ablation procedures was related to women, diabetes, previous empirical extra-PV ablation, and cardiac parasympathetic nerve activity. Although an extra-PV trigger ablation mapped after an isoproterenol provocation significantly lowered the AF recurrence, it was still worse than that in patients without extra-PV triggers, even after an extra-PV trigger ablation (Kim et al., 2016; Lee et al., 2018).
The ZFHX3, located on chromosome 16q22, is significantly associated with the mechanism of AF (Gudbjartsson et al., 2009) and is the second most highly AF associated genome in the multiethnic GWAS consortium (Roselli et al., 2018). The function of ZFHX3 is related to the myogenic and neuronal differentiation by regulating the signal transducers and activators of transcription 3 (STAT3) (Nojiri et al., 2004; Liu et al., 2014). ZFHX3 interacts with the protein inhibitor of activated STAT3 (PIAS3) (Liu et al., 2014; Tomomori et al., 2018), which regulates paracrine circuits. Increased expression of STAT3 contributes to cell proliferation, transformation, inflammation, angiogenesis, migration, matrix deposition, and eventual structural changes in AF (Nojiri et al., 2004; Park et al., 2013; Liu et al., 2014; Tomomori et al., 2018; Alí et al., 2019). Although the roles of ZFHX3 in extra-PV triggers after AFCA have never been evaluated, we identified 5 SNPs of ZFHX3 associated with extra-PV triggers, which are a significant predictor of clinical recurrence of AF after catheter ablation. Therefore, extra-PV triggers appear to be influenced by both acquired and genetic factors and are more commonly found in structurally remodeled AF (Gabbiani et al., 1978; Park et al., 2013; Alí et al., 2019). Although ZFHX3 plays negative roles in structural remodeling and AF progression (Lubitz et al., 2014; Zaw et al., 2017), it can play a positive role in contributing to permanent conduction block by scar maturation at PV isolation lesion sites (Tomomori et al., 2018).
AF is a heritable disease, and more than 100 AF-associated genetic loci have been demonstrated through GWAS (Roselli et al., 2018). In studies observing the clinical outcome of AF according to the genetics, the top AF-associated gene PITX2 affects the anti-arrhythmic drug response and response to electrical cardioversion (Parvez et al., 2012, 2013). Husser et al. (2010) and Shoemaker et al. (2013) reported that AF genetic loci had a significant effect on the rhythm outcome after AFCA in small studies. On the other hand, Choi et al. (2015) reported that AF-associated genetic loci did not affect the rhythm outcome after AFCA in a study of 1,068 AF patients. These discordant results might be due to the ethnic difference, possibility of proxy SNPs, and sample sizes (Choi et al., 2015). In this study, we showed that the second most highly AF associated gene, ZFHX3, had a significant relationship with extra-PV triggers but not with the rhythm outcome of AFCA.
This study had several limitations. First, it was a single-center, retrospective, observational cohort study that might have had a selection bias. The extra-PV trigger-associated SNPs were replicated internally. Second, we only included patients who underwent an isoproterenol provocation test and excluded those who did not undergo a provocation test or GWAS. Third, although we used a consistent isoproterenol provocation protocol, it was not standardized globally or reproducibly. Fourth, we tried to eliminate extra-PV triggers as much as we could during the procedure, but the actual trigger might have existed in the epicardial layer or deep inside the septum.
The second most highly AF associated gene, ZFHX3 and acquired LA remodeling were associated with extra-PV trigger. However, extra-PV triggers were independent predictor of AF recurrence after AFCA, the ZFHX3 did not show genetic effects on AF recurrence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Review Board at Yonsei University Health System. The patients/participants provided their written informed consent to participate in this study.
IH contributed to data anylsis and wrote the manuscript. O-SK was involved in data anaylsis and interpretation. MH was participated in genetic anaylsis. S-YY was participated in data acquisition. J-WP contributed to clinical data acquisition and statistical interpretation. HY and T-HK contributed to clinical data acquisition and clinical interpretation. J-SU and BJ contributed to data acquisition, clinical, and statistical interpretation. M-HL was involved in overall clinical data acquisition and data review. H-NP contributed to the study design, study protocol design, data review, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants (HI19C0114 and HI21C0011) from the Ministry of Health and Welfare and by a grant (NRF-2020R1A2B5B01001695) from the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (MSIP).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank John Martin for his linguistic assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.807545/full#supplementary-material
Alí, A., Boutjdir, M., and Aromolaran, A. S. (2019). Cardiolipotoxicity, Inflammation, and Arrhythmias: role for Interleukin-6 Molecular Mechanisms. Front. Physiol. 9:1866. doi: 10.3389/fphys.2018.01866
Choi, E. K., Park, J. H., Lee, J. Y., Nam, C. M., Hwang, M. K., Uhm, J. S., et al. (2015). Korean Atrial Fibrillation (AF) Network: genetic Variants for AF Do Not Predict Ablation Success. J. Am. Heart Assoc. 4:e002046. doi: 10.1161/JAHA.115.002046
Gabbiani, G., Chaponnier, C., and Hüttner, I. (1978). Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J. Cell Biol. 76, 561–568. doi: 10.1083/jcb.76.3.561
Gudbjartsson, D. F., Holm, H., Gretarsdottir, S., Thorleifsson, G., Walters, G. B., Thorgeirsson, G., et al. (2009). A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat. Genet. 41, 876–878. doi: 10.1038/ng.417
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., et al. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498.
Husser, D., Adams, V., Piorkowski, C., Hindricks, G., and Bollmann, A. (2010). Chromosome 4q25 Variants and Atrial Fibrillation Recurrence After Catheter Ablation. J. Am. Coll. Cardiol. 55, 747–753. doi: 10.1016/j.jacc.2009.11.041
Kawai, S., Mukai, Y., Inoue, S., Yakabe, D., Nagaoka, K., Sakamoto, K., et al. (2019). Non-Pulmonary Vein Triggers of Atrial Fibrillation Are Likely to Arise from Low-Voltage Areas in the Left Atrium. Sci. Rep. 9:12271. doi: 10.1038/s41598-019-48669-1
Kim, D., Hwang, T., Kim, M., Yu, H. T., Kim, T.-H., Uhm, J.-S., et al. (2021). Extra-Pulmonary vein triggers at de novo and the repeat atrial fibrillation catheter ablation. Front. Cardiovasc. Med. 8:759967. doi: 10.3389/fcvm.2021.759967
Kim, I.-S., Yang, P.-S., Kim, T.-H., Park, J., Park, J.-K., Uhm, J. S., et al. (2016). Clinical Significance of Additional Ablation of Atrial Premature Beats after Catheter Ablation for Atrial Fibrillation. Yonsei Med. J. 57, 72–80. doi: 10.3349/ymj.2016.57.1.72
Kim, T.-H., Park, J., Uhm, J.-S., Joung, B., Lee, M.-H., and Pak, H.-N. (2017). Pulmonary vein reconnection predicts good clinical outcome after second catheter ablation for atrial fibrillation. EP Eur. 19, 961–967. doi: 10.1093/europace/euw128
Lee, K.-N., Roh, S.-Y., Baek, Y.-S., Park, H.-S., Ahn, J., Kim, D.-H., et al. (2018). Long-Term Clinical Comparison of Procedural End Points After Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 11:e005019. doi: 10.1161/CIRCEP.117.005019
Liu, Y., Ni, B., Lin, Y., Chen, X.-G., Fang, Z., Zhao, L., et al. (2014). Genetic polymorphisms in ZFHX3 are associated with atrial fibrillation in a Chinese Han population. PLoS One 9:e101318. doi: 10.1371/journal.pone.0101318
Lubitz, S. A., Lunetta, K. L., Lin, H., Arking, D. E., Trompet, S., Li, G., et al. (2014). Novel Genetic Markers Associate With Atrial Fibrillation Risk in Europeans and Japanese. J. Am. Coll. Cardiol. 63, 1200–1210. doi: 10.1016/j.jacc.2013.12.015
Nagueh, S. F., Appleton, C. P., Gillebert, T. C., Marino, P. N., Oh, J. K., Smiseth, O. A., et al. (2009). Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 10, 165–193.
Nojiri, S., Joh, T., Miura, Y., Sakata, N., Nomura, T., Nakao, H., et al. (2004). ATBF1 enhances the suppression of STAT3 signaling by interaction with PIAS3. Biochem. Biophys. Res. Commun. 314, 97–103. doi: 10.1016/j.bbrc.2003.12.054
Pak, H.-N., Park, J.-W., Yang, S.-Y., Kim, T.-H., Uhm, J.-S., Joung, B., et al. (2021). Cryoballoon Versus High-Power, Short-Duration Radiofrequency Ablation for Pulmonary Vein Isolation in Patients With Paroxysmal Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 14:e010040. doi: 10.1161/CIRCEP.121.010040
Park, J. H., Pak, H.-N., Lee, S., Park, H. K., Seo, J.-W., and Chang, B.-C. (2013). The clinical significance of the atrial subendocardial smooth muscle layer and cardiac myofibroblasts in human atrial tissue with valvular atrial fibrillation. Cardiovasc. Pathol. 22, 58–64. doi: 10.1016/j.carpath.2012.05.001
Park, J.-W., Yu, H. T., Kim, T.-H., Uhm, J.-S., Kim, J.-Y., Joung, B., et al. (2019). Trends and Outcome of Catheter Ablation of Atrial Fibrillation Over 9 Years— Focus on Empirical Extra-Pulmonary Vein Ablation. Circ. J. 83, 304–312.
Park, J.-W., Yu Hee, T., Kim, T.-H., Uhm, J.-S., Joung, B., Lee, M.-H., et al. (2020). Mechanisms of Long-Term Recurrence 3 Years After Catheter Ablation of Atrial Fibrillation. JACC Clin. Electrophysiol. 6, 999–1007. doi: 10.1016/j.jacep.2020.04.035
Parvez, B., Shoemaker, M. B., Muhammad, R., Richardson, R., Jiang, L., Blair, M. A., et al. (2013). Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm 10, 849–855. doi: 10.1016/j.hrthm.2013.02.018
Parvez, B., Vaglio, J., Rowan, S., Muhammad, R., Kucera, G., Stubblefield, T., et al. (2012). Symptomatic Response to Antiarrhythmic Drug Therapy Is Modulated by a Common Single Nucleotide Polymorphism in Atrial Fibrillation. J. Am. Coll. Cardiol. 60, 539–545. doi: 10.1016/j.jacc.2012.01.070
Peyvandi, F., Mannucci, P. M., Garagiola, I., El-Beshlawy, A., Elalfy, M., Ramanan, V., et al. (2016). A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. New Engl. J. Med. 374, 2054–2064. doi: 10.1056/NEJMoa1516437
Reddy, V. Y., Anter, E., Rackauskas, G., Peichl, P., Koruth, J. S., Petru, J., et al. (2020). Lattice-Tip Focal Ablation Catheter That Toggles Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 13:e008718. doi: 10.1161/CIRCEP.120.008718
Roselli, C., Chaffin, M. D., Weng, L.-C., Aeschbacher, S., Ahlberg, G., Albert, C. M., et al. (2018). Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 50, 1225–1233. doi: 10.1038/s41588-018-0133-9
Shoemaker, M. B., Muhammad, R., Parvez, B., White, B. W., Streur, M., Song, Y., et al. (2013). Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. Heart Rhythm 10, 394–400. doi: 10.1016/j.hrthm.2012.11.012
Tomomori, S., Nakano, Y., Ochi, H., Onohara, Y., Sairaku, A., Tokuyama, T., et al. (2018). Maintenance of low inflammation level by the ZFHX3 SNP rs2106261 minor allele contributes to reduced atrial fibrillation recurrence after pulmonary vein isolation. PLoS One 13:e0203281. doi: 10.1371/journal.pone.0203281
Watanabe, K., Nitta, J., Inaba, O., Sato, A., Inamura, Y., Kato, N., et al. (2021). Predictors of non-pulmonary vein foci in paroxysmal atrial fibrillation. J. Intervent. Cardiac. Electrophysiol. 61, 71–78. doi: 10.1007/s10840-020-00779-x
Keywords: atrial fibrillation, ZFHX3, genetic polymorphism, extra-pulmonary vein, recurrence, catheter ablation
Citation: Hwang I, Kwon O-S, Hong M, Yang S-Y, Park J-W, Yu HT, Kim T-H, Uhm J-S, Joung B, Lee M-H and Pak H-N (2022) Association of ZFHX3 Genetic Polymorphisms and Extra-Pulmonary Vein Triggers in Patients With Atrial Fibrillation Who Underwent Catheter Ablation. Front. Physiol. 12:807545. doi: 10.3389/fphys.2021.807545
Received: 02 November 2021; Accepted: 09 December 2021;
Published: 05 January 2022.
Edited by:
Yi-Jen Chen, Taipei Medical University, TaiwanReviewed by:
Minglong Chen, The First Affiliated Hospital of Nanjing Medical University, ChinaCopyright © 2022 Hwang, Kwon, Hong, Yang, Park, Yu, Kim, Uhm, Joung, Lee and Pak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Nam Pak, aG5wYWtAeXVocy5hYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.